Abstract
Liver dendritic cells (DCs), which may orchestrate the liver’s unique immunoregulatory functions, remain poorly characterized. We used a technique of overnight migration from pieces of normal human liver and skin to obtain tissue-derived DCs with minimal culture and no additional cytokine treatment. Liver and skin DCs had a monocyte-like morphology and a partially mature phenotype, expressing myeloid markers, MHCII, and co-stimulatory molecules; but only the skin DCs contained a population of CD1a+ cells. Overnight-migrated liver DCs activated naïve cord blood T cells efficiently. Liver DCs produced interleukin (IL)-10 whereas skin DCs failed to secrete IL-10 even after stimulation and neither skin nor liver-derived DCs secreted significant amounts of IL-12p70. Compared with skin DCs, liver DCs were less effective at stimulating T-cell proliferation and stimulated T cells to produce IL-10 and IL-4 whereas skin DCs were more potent stimulators of interferon-γ and IL-4. Monocyte-derived DCs were down-regulated after culture with liver-conditioned media, suggesting that local microenvironmental factors may be important. Thus we show for the first time clear tissue-specific differences in nonlymphoid DCs. Although it is not possible to conclude from our data whether liver DCs are more regulatory, or skin DCs more proimmunogenic, the ability of liver DCs to secrete IL-10 may be important for regulating local immune responses within the liver in the face of constant exposure to gut antigens.
The unique properties of dendritic cells (DCs) allow them to take up and process antigen from nonlymphoid tissues such as the liver, migrate to lymph nodes, and initiate primary immune responses.1 Since a method for generating DCs from human monocytes was established,2 the ability of these cells to initiate strong immune responses has been appreciated and exploited for instance in the treatment of malignancy.3 However it has been difficult to study DCs from normal human tissue where they are likely to play a crucial role in regulating local immune responses. This may be particularly true for the liver where there is a need to tightly control immune responses to the many antigens entering the liver from the gut via the portal vein.4
DCs freshly isolated from nonlymphoid organs are immature.5 They are unable to act as antigen-presenting cells and lack cell surface class II, which is found within the cell but not at the cell membrane.6 They do however display good antigen-capturing abilities, with high levels of expression of toll-like receptors, Fc receptors, complement and mannose receptors, and the ability to take up antigen by a number of mechanisms.7 In the normal state there is a steady movement of DCs from peripheral tissue via the lymph to the secondary lymphoid tissue,8 and this trafficking is markedly increased in the presence of an immunogenic stimulus.9 Migration of DCs from the liver to draining lymph nodes has been demonstrated in a rat model by following the movement of cells that take up colloidal carbon in the hepatic sinusoids and migrate via the portal tract to the lymphatics and draining nodes.10 It is clear from this elegant work, that immature liver DCs enter the liver from the circulation via the sinusoids where they take up antigens and express many pattern recognition receptors in common with macrophages, eg, CD14. On maturation they express chemokine receptors that direct them to the lymph vessels of the portal tracts, where they express more typical markers of DCs. These migratory cells when isolated from lymph have an intermediate phenotype, lacking phagocytic function but with less than maximum levels of class II expression. Similar cells have been identified migrating from sections of skin in culture.11
It is difficult to examine the function of normal tissue DCs in the steady state, but these cells, which continually traffic to the lymph nodes, may play a critical role in maintaining local tolerance. There is evidence that CD4 T-cell tolerance requires the presentation of antigen by a bone marrow-derived cell,12 and DCs have been identified as agents in the generation of T-cell tolerance in vivo.13–15 However, the study of such mechanisms is hindered by the difficulties in isolating cells in sufficient numbers from tissue and the comparative lack of specific markers to positively identify immature DCs in the human. Furthermore cells isolated directly from tissue require maturation before developing the ability to present antigen.16,17 To overcome some of these problems we have adapted a technique used for skin to isolate DCs migrating out of human liver tissue.
To determine whether differences in tissue-specific DCs contribute to organ-specific immune responses we compared the ability of DCs isolated using similar techniques from human skin and liver tissue to secrete cytokines and stimulate lymphocyte responses.
Materials and Methods
Human Liver and Skin Tissue
Normal liver tissue was obtained from liver available after reduction of donor liver for transplantation into children, after partial hepatectomy for colorectal metastases and benign focal disease: cystadenoma (n = 2), hemangioma (n = 1), follicular nodular hyperplasia (n = 2). Tissue used was at least 3 cm from tumor. Normal skin was obtained from tissue removed during plastic surgery. In some cases peripheral blood was collected during surgery and peripheral blood mononuclear cells (PBMCs) were isolated over Ficoll (Lymphoprep) gradients and cryopreserved. All tissue and blood samples were obtained with the informed consent of patients and after approval from the local ethics committee.
Migration and Isolation of Cells
Tissue (1- to 2-mm-thick wafers) were collected from the liver, and cut into 0.5-cm2 squares. At least 12 g of tissue were collected from each liver and after washing in phosphate-buffered saline were incubated in six-well plates and cultured overnight in complete media (RPMI 1640, 10% fetal calf serum, 2 mmol/L glutamine, 100 μg/ml streptomycin, and 100 U/ml penicillin). Human skin DCs were isolated as previously described18 by floating full thickness skin pieces on complete media. The skin tissue was cultured overnight in the same way as the liver DCs. Nonadherent cells that migrated out of the tissue into the media were collected. To study the result of further culture on maturation, the migrated cells were purified by isolation over a Ficoll (Lymphoprep metrizamide, Nygaard, Oslo, Norway) gradient, and cultured in smaller wells at 2 × 106 cells/ml, in complete media for a further 2 to 3 days. In all cases DCs were finally isolated by centrifugation over a 14.5% metrizamide gradient. Twelve percent Optiprep (Nycomed UK Ltd, Oxford, UK) in complete media was used for experiments at later time points.19 The yield was typically 0.5 × 105 cells/g of liver tissue. For assessment of function pure populations of DCs were obtained by negative selection. CD4, CD8, and CD15 antibodies were used to remove remaining lymphocytes and neutrophils by depletion using anti-Fc-conjugated Dynabeads (Dynal). Flow cytometry and immunocytochemistry of cytospins was used to confirm purity.
Monocyte-derived DCs (mDCs) were produced by isolating PBMCs from human peripheral blood using a Lymphoprep gradient and selecting CD14+ve cells using microbeads (Miltenyi Biotech) and columns. The cells were then cultured for 6 days in GM-CSF (800 U/ml) and interleukin (IL)-4 (500 U/ml) in complete media. The purity of these DCs was >95% by flow cytometry. These cells were used to compare the effects of media from cultured skin and liver on DC function. mDCs were cultured overnight in conditioned tissue culture media derived from 18-hour cultures of skin and liver tissue. Cells and debris were removed from the conditioned media by centrifugation. Fresh complete media was used as a control. The cells were then washed and compared in proliferation assays. The results were tested for significance by analysis of variance.
Reagents
The following unconjugated mouse anti-human antibodies were used to stain cells by flow cytometry and immunocytochemistry: UCHM-1 (CD14) and HB15A17.11 (CD83) from Serotec; 2LPM19c (CD11b), BU63 (CD86), UCHT-1 (CD3), MT310 (CD4), C8/144B (CD8), HD37 (CD19), EBM11 (CD68), DJ130c (CD16), and CR3/43 (HLA-DR) from DAKO; B-Ly6 (CD11c), HI149 (CD1a), and 5C3 (CD40) from Pharmingen; BDCA-2 and CD1c from Miltenyi Biotec; CD57 from Biogenics; 2G5 (CXCR4) and 120507 (DC-sign) from R&D; 7H12 (CCR7) kindly provided by Lijun Wu (Millenium Pharmaceuticals Inc., Cambridge, MA); 2D7 (CCR5) kindly provided by S. Qin (Millenium Pharmaceuticals Inc.), and for dual-color flow cytometry, phycoerythrin-conjugated L243 (HLA-DR) from Pharmingen. Mouse immunoglobulin isotype controls were obtained from Pharmingen.
For flow cytometry primary antibody staining was detected using fluorescein isothiocyanate-conjugated rabbit anti-mouse Fab from DAKO. For immunocytochemistry, primary antibodies were detected using rabbit anti-mouse immunoglobulin followed by mouse monoclonal alkaline phosphatase-anti-alkaline phosphatase (APAAP) (DAKO).
Immunostaining and Flow Cytometry
Cytospins and frozen sections were prepared and stored at −20°C until use. They were then fixed for 5 minutes in acetone and stained using a standard APAAP technique.20 Briefly, primary antibody was followed by rabbit anti-mouse monoclonal and mouse monoclonal APAAP (DAKO). The stain was developed with fast red and naphthol AS-MX phosphate substrate (Sigma).
Flow cytometry was used to assess phenotype of class II-positive cells. Cells were preincubated in human Ig to block Fc receptors, then stained with mouse anti-human primary antibody, followed by rabbit anti-mouse fluorescein isothiocyanate. Cells were incubated in 10% mouse serum before final incubation in phycoerythrin-class II antibody. For intracellular cytokine staining DCs were stimulated for 5 hours with 5 ng/ml of PMA and 500 ng/ml of ionomycin (Sigma), and cytokine export blocked with monensin (Pharmingen) used according to the manufacturer’s instructions. The cells were fixed using 4% paraformaldehyde and permeabilized with 0.5% saponin. Cell surface markers were stained, eg, CD11c followed by rabbit anti-mouse fluorescein isothiocyanate, before fixation and permeabilization, and phycoerythrin-conjugated antibodies to cytokine or the recommended control antibodies from Pharmingen were used to stain permeabilized cells.
Viability of cells was assessed using Viaprobe (7-amino-actinomycin D), Pharmingen. Analysis was performed on an Epics XL cytometer (Coulter). Data were processed using WinMDI version 2.4.
Electron Microscopy
After migration from liver and skin, cells were either cultured for 2 further days in complete media or were purified immediately. Cells were fixed in 2% glutaraldehyde, and sections prepared and viewed by the University of Birmingham Electron Microscopy Centre.
Mixed DC and Lymphocyte Response
The ability of the cells to activate naïve T cells was measured in a mixed DC/lymphocyte response (MLR). Cord blood was collected and the mononuclear cells isolated by separation over a Lymphoprep gradient. The antigen-presenting function of overnight-migrated DCs from normal liver was compared with PBMCs from the same patient. Graded numbers of irradiated (3000 rads) stimulator cells were added to 2 × 105 allogeneic cord blood mononuclear cells in 100 μl of complete media, in 96-well plates. The cells were cultured for 5 days and 1 μCi of 3H-thymidine was added for the last 18 hours. Thymidine uptake was counted on a β-plate counter and a mean of triplicate wells calculated (n = 3).
To compare the function of tissue-specific DCs, pure skin (n = 3) and liver DCs (n = 6) were isolated by negative selection and compared using a MLR; in this case, pure T cells were isolated by negative selection from adult blood, using CD19, CD11b, CD14, and CD56 antibodies and used as responders. DCs cultured overnight in skin or liver tissue conditioned media, were compared with DCs cultured in complete media using the same proliferation assay (n = 4). Data were compared by analysis of variance.
Detection of IL-10 and IL-12 by Enzyme-Linked Immunosorbent Assay
Overnight-migrated cells were cultured in 96-well plates at 106 cells/ml in complete media supplemented with 50 ng/ml of GM-CSF. In further experiments cells were stimulated with the addition of 10 ng/ml of interferon (IFN)-γ and 10 to 100 ng/ml of lipopolysaccharide (LPS) (Sigma) (higher concentrations were tested in experiments to detect IL-12p70), or IFN-γ and CD40 ligation. CD40L-transfected J558 cells21 were paraformaldehyde-fixed and washed before adding to the cells at a ratio of one cell to three DCs, for the IL-12 studies 1 μg/ml of soluble CD40L with enhancer (Alexis) was also tested. Media was saved and stored at −70°C. IL-10 and IL-12p70 were measured in the media using OPTeia kits produced by Pharmingen. The standard enzyme-linked immunosorbent assay protocol recommended by the company was followed. The results were compared by analysis of variance.
DC Stimulation of T Cells
A DC and T-cell MLR was used to study the T-cell responses generated by the DCs from skin and liver. Pure T cells were co-cultured in complete media for 5 days at 1:20 stimulator:responder ratios and the cells were then restimulated for 5 hours with anti-CD3-coated plates and soluble anti-CD28, in the presence of monensin. Pure T cells without co-culture with DCs were restimulated in the same way, and used as a control. The cells were stained for intracellular cytokine as described before. Statistical significance was assessed for each cytokine using a Mann-Whitney U-test and the difference in expression of all cytokines by each type of DC was assessed by analysis of variance.
Detection of IL-12 Expression by Real-Time Polymerase Chain Reaction (PCR)
Pure overnight-migrated DCs isolated as described above were stored at −70°C. RNA was isolated later using an RNeasy kit (Qiagen) including a DNase step to remove any contaminating genomic DNA.
The RNA was reverse-transcribed using superscript II reverse transcriptase (Invitrogen). The real-time PCR was set up using TaqMan Universal PCR Mastermix, a primer/probe mix specific to the gene of interest, and a primer/probe mix specific to 18S rRNA control reagent. The following primer/probe sequences were kindly designed by David Fitzpatrick, Immunex: IL-12 p35 forward primer 5′-gcc act cca gac cca gga a-3′, IL-12 p35 reverse primer 5′-gac ggc cct cag cag gtt-3′, IL-12 p35 probe ttc cca tgc ctt cac cac tcc ca, IL-12 p40 forward primer 5′-ctt cac cga caa gac ctc agc-3′, IL-12 p40 reverse primer 5′-aga tga gct ata gta gcg gtc ctg-3′, IL-12 p40 probe tca tct gcc gca aaa atg cca gc. The primer/probe mix for 18S was kindly supplied by Kai Toellner, Department of Immunology, University of Birmingham. The plate was run in the Applied Biosystems Prism 7700 sequence detector using Sequence Detection System analysis software. The PCR conditions were as follows: 50°C for 2 minutes, 95°C for 10 minutes, then 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. The software then analyzed the data and output a pair of cycle threshold (ct) values for each sample, one for the gene of interest (labeled FAM) and one for the 18S housekeeping control (labeled VIC). The ct values were then transported to a Microsoft Excel spreadsheet and analyzed to give a value representing the relative mRNA levels present for the gene of interest linearly as per the manufacturer’s instructions.
Results
Overnight-Migrated Cells Have a Partially Mature Phenotype and a Proportion Become Fully Mature in Culture
After a few hours in culture, cells that had migrated out of the tissue were seen in the media. The rate of emigration fell after 24 hours and we therefore used an overnight migration.
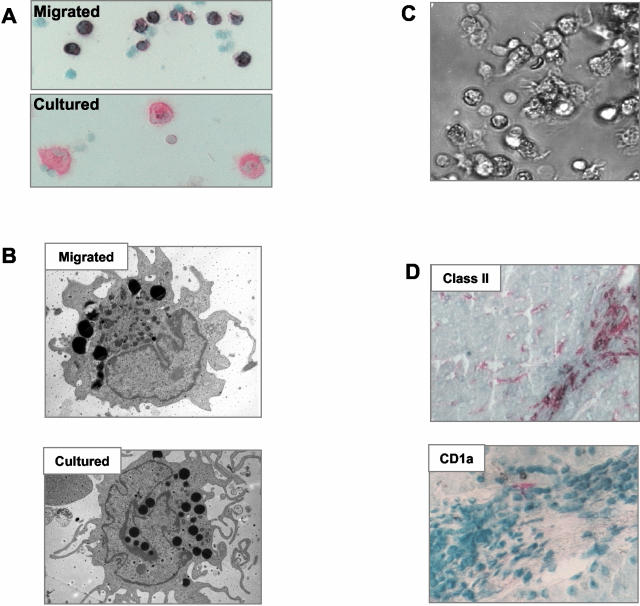
Cytospin preparations of overnight-migrated cells showed they were monocyte-like with an eccentric oval nucleus and more cytoplasm than lymphocytes (Figure 1A), whereas electron microscopy showed that the cells contained numerous vesicles, some electron opaque (Figure 1B). Using immunocytochemistry, they displayed strong staining for class II, CD86, and CD11b; weaker staining for CD11c, CD40, and ICAM-1; but little detectable CD83. Neither CD68 nor CD16 was present in DCs from normal liver. With further culture in complete media 25 to 50% of class II-positive cells from 3-day cultured samples increased in size and looked like typical mature DCs (Figure 1C), by electron microscopy the cells showed less dense cytoplasm, complex long processes/veils, and an open-lobed nucleus. The cultured cells had increased surface expression for class II and the co-stimulatory molecules compared with the overnight-migrated cells.
Figure 1.
Appearance of cultured liver DCs. A: Cells isolated after migration and stained using APAAP for class II expression, have a monocyte-like morphology. After further culture the cells acquire a more typical DC appearance. B: Transmission electron microscopy of DCs after overnight migration and after 2 days further culture. The migrating cells have an oval nucleus and dense cytoplasm, many of the cells had very dark staining vesicles in the cytoplasm. The cultured matured cells had a more complex structure of dendrites or veils and a more open and lobed nucleus. C: Cells migrated from normal liver and cultured for 2 to 3 days develop characteristic veils and a typical DC morphology. D: Class II-positive cells in the portal tract of normal liver have the appearance of DCs but are CD1a-negative. CD1a is not detected in normal liver and only very occasional CD1a cells are detected in inflamed liver (liver tissue is from a patient with chronic liver allograft rejection). Original magnifications: ×20 [A, D (bottom)]; ×5000 (B); ×10 (D, top).
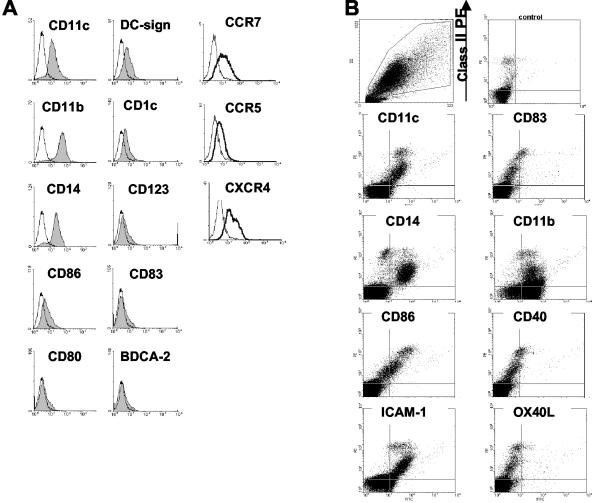
The cells were analyzed using dual-color flow cytometry (Figure 2, A and B, and Table 1) with DCs identified by surface class II staining. In general the surface staining confirmed the findings noted using immunocytochemistry. Class II-gated cells were CD16-, CD3-, CD19-, and CD57-negative, and they had a myeloid phenotype expressing CD11c, CD1c, low levels of CD123 and DC-SIGN, and no BDCA-2. Flow cytometry of cells cultured further shows the same heterogeneity as that seen with immunocytochemistry, with 25 to 50% of the class II cells developing a mature phenotype. These cells express increased levels of co-stimulatory molecules, CD86, CD40, and also CD83 (Figure 2B). Confirming the process of maturation, CD14 and CD11b, were detected on 50% and 90% of cells, respectively, after overnight migration but were down-regulated on class II high cells after further culture. Chemokine receptor expression by migrated cells was also assessed (Figure 2A). Most overnight-migrated liver DCs expressed CXCR4 whereas only those cells with high class II expression were CCR7+. A low level of CCR5 was detectable.
Figure 2.
Phenotype of liver DCs by flow cytometry. A: Overnight-migrated liver DCs were characterized by dual-color flow cytometry. Cells shown were gated on scatter characteristics and positive staining for class II. The control staining is shown as an unfilled histogram. B: Two-color plots show the emergence of mature high class II-expressing cells after culture in plain media for a further 2 days. The cells were isolated using a gradient only therefore some class II-/CD11b+ neutrophils are present.
Table 1.
Phenotype of Liver Dendritic Cells after Migration and after Maturation
| Overnight migrated | Class II high population after maturation | |
|---|---|---|
| Class II | ++++ | ++++ (Increased level of expression) |
| CD40 | ++ | +++ |
| CD86 | ++++ | ++++ (Increased level of expression) |
| ICAM | ++++ | ++++ |
| CD11c | ++++ | ++++ |
| CD11b | ++++ | ++ |
| CD14 | ++ | + |
| CD83 | + | +++ |
| CD16 | − | − |
| CD68 | − (Normal liver) | − |
| CD1c | ++ | ++ |
| CD1a | − | − |
| CD123 | + | + |
| BDCA-2 | − | − |
Assessment made using flow cytometry from at least five separate experiments. No expression, −; <20%, +; 20 to 50%, ++; 50 to 75%, +++; 75 to 100%, ++++.
Skin DCs isolated by migration have been previously characterized and our findings were consistent with published reports.11 The only notable difference between liver and skin DCs was in expression of CD1a. In our study 70% of the cells isolated from full thickness skin were CD1a+, whereas none of the liver DCs expressed CD1a. In addition none of the normal liver sections studied by immunohistochemistry contained any CD1a+ cells although very rare CD1a+ cells were detected in liver tissue from patients with inflammatory liver disease (Figure 1D).
Overnight-Migrated Cells Are Able to Activate Cord Blood T Cells, but Are Poor Stimulators in Comparison to Skin-Derived Cells
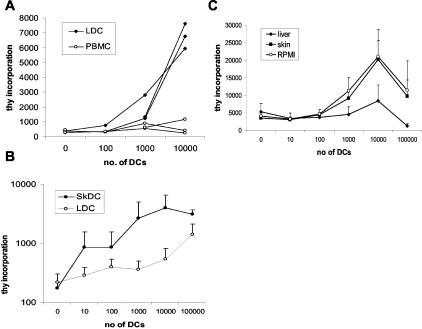
To confirm that the liver DCs were functional we compared the ability of overnight-migrated DCs and syngeneic PBMCs to stimulate T-cell proliferation in a modified MLR (Figure 3A). The stimulator cells were added to 1.5 × 105 allogeneic cord blood mononuclear cells. A peak response was elicited by liver-derived cells at a ratio of 1/10 to 20 stimulator cells/cord blood mononuclear cells in a 5-day assay whereas PBMCs were unable to elicit a proliferative response. However when purified liver DCs were compared with skin DCs in an alloproliferative response using pure adult T cells as responders the liver DCs were markedly less efficient at inducing T-cell proliferation, P < 0.001 (Figure 3B).
Figure 3.
Capacity of liver DCs to induce proliferation of allogeneic T cells. A: Migrated liver-derived DCs stimulate naïve T-cell proliferation. Proliferation of T cells in response to liver-derived DCs was measured in a thymidine incorporation assay. Varying numbers of stimulator cells, liver DCs, or PBMCs, were used to stimulate 2 × 105 cord blood mononuclear cells in a 5-day assay. Mean of triplicate wells is shown. Each line represents a different experiment with different DC preparations (n = 3). B: The ability of DCs from skin (n = 3) and liver (n = 5) to stimulate allogeneic T cells was tested in a modified MLR in which highly purified, negatively selected allogeneic T cells were used as responder cells and DCs that had been purified using negative selection as stimulators. The skin DCs were significantly better stimulators of allogeneic T cells than liver-derived DCs (P < 0.0001). C: The influence of soluble tissue factors to modify the response of DCs after overnight migration was tested using the media from these cultures. mDCs were cultured overnight in tissue-conditioned media or complete media, before comparison in a modified MLR using pure adult allogeneic T cells (n = 4). mDCs cultured overnight in liver tissue-conditioned media were poor stimulators of T-cell proliferation compared to normal mDCs (P < 0.001).
Soluble Tissue Factors from Liver Reduce the Ability of mDCs to Stimulate T-Cell Proliferation
To assess the factors involved in tissue-specific DC function, pure mDCs were cultured overnight in liver- or skin-conditioned media, or plain complete media. The ability of the DCs to stimulate the proliferation of negatively selected pure adult T cells was compared using the same proliferation assay as before. The ability of mDCs cultured in skin-conditioned media to stimulate T-cell activation was not different from those cultured in plain media (P = 0.65), whereas DCs cultured in liver-conditioned media had a significantly (P = 0.0007) reduced ability to stimulate T-cell proliferation (n = 4) (Figure 3C). This finding was not because of DC death as cells were counted before each assay using trypan blue, and cells were assessed for viability using flow cytometry at 24 hours. There was no difference, all cells showing >95% viability after culture, in conditioned or unconditioned media.
Cytokine Production by Skin and Liver DCs
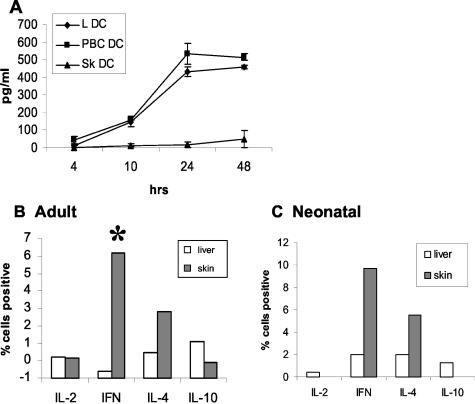
Migrated and purified liver DCs (n = 5) cultured with GM-CSF produced IL-10 (438 ± 45 pg/ml at 24 hours with GM-CSF) (Figure 4A), but not IL-12p70, although this cytokine was detected in the supernatant from IFN-γ- and LPS-stimulated PBMCs. In contrast under the same conditions of isolation and stimulation skin DCs (n = 3) produced no or greatly reduced levels of IL-10 (16 ± 16 pg/ml at 24 hours with GM-CSF), and again no IL-12p70. There was a significant difference in IL-10 production by liver and skin DCs (P < 0.0001), but no significant difference in production between normal and diseased liver DCs (P = 0.61). There was no significant difference in expression of IL-10 between unstimulated DCs and those in which further activation was provided by LPS or CD40 ligation.
Figure 4.
Function of liver-derived DCs. A: IL-10 expression was compared between DCs from normal liver (LDC, n = 5), diseased livers from patients with primary biliary cirrhosis (LDC PBC, n = 3), and normal skin (Sk DC, n = 3). Mean and SEM are shown. DCs from both normal and diseased liver secrete IL-10 whereas those from skin do not. Liver DCs produce significantly more IL-10 (P < 0.0001) than skin DCs, but a similar amount to diseased liver-derived DCs (P = 0.61). Highly pure, negatively selected liver and skin DCs were co-cultured with pure peripheral blood T cells (B) and pure cord blood T cells (C) in a modified MLR. The cells were then restimulated with CD28 and CD3 for 5 hours in the presence of monensin. Intracellular cytokine staining was used to characterize the T cells. The graph shows mean values for the percent positive cells compared to control T cells. Liver DCs (n = 5) are compared with skin DCs (n = 3). Whereas liver DCs stimulated T cells to express IL-10, but suppressed IFN-γ and did not effect IL-4, skin DCs failed to induce any T cells to express IL-10 but stimulated an average of 6% of T cells to express IFN-γ (*, P = 0.05). When expression of all cytokines was compared by analysis of variance there was a highly significant difference (P < 0.0005).
We were surprised by the lack of IL-12 p70 protein, and therefore went on to look for mRNA expression using real-time PCR. Although IL-12p70 was undetectable by enzyme-linked immunosorbent assay expression of both IL-12 p35 and p40 transcripts were detected by real-time PCR.
Liver DCs but Not Skin DCs Stimulate T Cells to Secrete IL-10
Pure T cells from adult peripheral blood were tested in an MLR experiment with pure DCs from skin and liver (Figure 4B). Skin DCs stimulated the T cells to secrete significantly more IFN-γ (P = 0.05) and IL-4, but no or few IL-10-positive T cells were detected (n = 3). In contrast liver DCs stimulated 2% of the T cells to secrete IL-10, and in some experiments IFN-γ secretion was suppressed compared to unstimulated T cells (n = 5). There was a significant difference in the cytokines secreted by the liver and skin DCs (P = 0.0004). The results were similar for parallel experiments using cord blood T cells as responders (Figure 4C).
Discussion
This study is the first detailed functional and phenotypic analysis of human liver-derived DCs, and one of the few to report functional data using human interstitial DCs.22 The cells were obtained by modifying a technique used to isolate skin DCs11 in which DCs are migrated out of tissue with minimal culture and no exogenous cytokines. Although the process of obtaining tissue at surgery might have resulted in a degree of activation when compared with resident DCs in the unperturbed normal liver we believe we have obtained relevant cells with which to study the role of DCs in noninflamed liver.11
We chose skin DCs for comparison because of the major differences between immune reactions in the skin and liver. For instance, skin allografts are more susceptible to rejection compared with liver allografts23 and whereas the liver is associated with reduced effector immune responses to the many harmless food antigens delivered via the portal vein,24 the skin represents a crucial barrier against the external environment, any breach of which requires a vigorous immune response. In addition the liver is a site of persistent viral infection. Differences between distinct subsets of DCs isolated from blood and lymphoid organs have been reported extensively but comparatively little is known about differences between myeloid-related DCs found in peripheral tissues.25 We believe that the current study is the first demonstration of important tissue-specific differences between human DCs derived from different nonlymphoid tissues.
The cells isolated by migration from the liver had a partially mature phenotype similar to that reported for migrated skin and gut DCs, with expression of DC-sign.26 With further culture the cells matured and expressed CD83, the most specific marker readily available for human DCs.27 In addition to phenotype, the cells were positively identified by function being able to stimulate naïve cord blood T cells in an MLR. The overnight-migrated liver DCs were able to stimulate naïve T-cell proliferation, albeit at a level below that seen with fully activated mature DCs. Little is known about the level of DC maturation before migration from normal tissue although previous studies of DCs derived from lymph show that lymph DCs are at a similar stage of maturation compared to the tissue-derived DCs reported in our study.
The DCs migrated from skin and liver were morphologically similar and expressed comparable levels of the myeloid markers CD11c and CD11b and MHC and co-stimulatory molecules. Although the method of isolation in each case was similar, it is possible that different pathways were used by DCs to migrate out of the skin and liver related to differences in anatomy and constitutive expression of chemokines or adhesion molecules. We therefore compared chemokine receptor levels on the two cell types and found that both populations were CXCR4+ and CCR5low consistent with the level of maturation established by the other phenotypic markers. In addition both populations contained a subpopulation of class IIhighCCR7+ cells. Only CD1a clearly differentiated between skin and liver cells and its absence from liver DCs was confirmed when we showed a lack of CD1a staining in normal liver and only very occasional positive cells in inflamed liver. The lack of CD1a also differentiates the liver-derived DCs from many other human DCs because monocyte and many cord blood progenitor-derived DC populations are CD1a+.2
Despite the modest differences in phenotype, we detected marked differences in function between liver- and skin-derived DCs. The liver-derived DCs were less effective stimulators of T-cell proliferation in an MLR. In addition, IL-10 was expressed by liver DCs but was undetectable or present at greatly reduced levels in media from skin DCs. Interestingly CD1a+ and CD1a− cells derived from human cord blood progenitors have the same difference in expression of IL-10.28 IL-10 is involved in the generation of regulatory T cells29 and has many other anti-inflammatory properties.30 Moreover DC-secreted IL-1031 has autocrine activity and prevents LPS- and CD40L-induced maturation. This could explain why treatment of liver DCs with CD40L or LPS had little effect on DC maturation or cytokine secretion in our study. IL-10-secreting DCs have been isolated from mouse Peyer’s patches32 and draining lymph nodes from mouse lung33 where these respiratory DCs were found to induce tolerance to inhaled antigen by induction of regulatory-type T cells. This finding suggests that the role of IL-10 production by interstitial tissue DCs is important for immunoregulation in a number of nonlymphoid organs.
In contrast IL-12p70 is important for the generation of Th1 responses34 and DC production of IL-12 is involved in Th1 cell differentiation.35 Regulation of production of the active heterodimer IL-12p70 is dependent on production of the two subunits IL-12p35 and p40, which are regulated independently.36 The undetectable protein expression of IL-12p70 by both skin- and liver-derived DCs in our study may reflect the relatively immature state of the cells because IL-12p70 production by DCs is tightly regulated and only occurs under specific conditions during DC maturation in response to multiple activation signals.37
To further demonstrate the differences in function between these tissue-derived DCs, we compared the T-cell responses stimulated by liver and skin DCs. The pattern of T-cell response we found has been demonstrated with human immature mDCs.38 Although the numbers of responding T cells were relatively small (up to 8%) we were using unstimulated DCs which are therefore not fully mature and the responding cells included both CD4 and CD8 T cells that had not undergone any previous stimulation or expansion in IL-2. Although the phenotype of the T cells generated by the skin DCs is consistent with strong Th1 immune responses, the ability of liver DCs to stimulate T-cell secretion of IL-10 suggests they may have a regulatory role.29
Functional differences between DCs in different tissues may develop as a consequence of several factors: the recruitment of different DC precursor subsets; the action of tissue-specific cytokines and molecules in the tissue microenvironment; and the interaction between tissue DCs and tissue-specific cells, eg, epithelial cells.39 Whereas our finding of CD1a+ve cells in skin but not in liver suggests the presence of different subsets of DCs, it is also likely that the microenvironment plays a role. We therefore compared the effects of culturing mDCs in liver- and skin-conditioned media and found that soluble tissue factors from the liver media dramatically reduced the ability of mDCs to stimulate T cells. This is surprising because the tissue injury involved in preparing the culture might be expected to release factors that would activate DCs. Many different factors have been shown to have a regulatory effect on DCs40,41 including IL-10, the secretion of which by liver DCs could be responsible for the differences observed.42
Our findings are of particular importance given the unique immunobiology of the liver. Recent evidence from several laboratories shows that although there is an efficient trafficking of DCs from sinusoidal blood via portal tracts to draining lymph nodes resulting in T-cell priming this local presentation of antigen frequently results in T-cell inactivation and tolerance rather than the development of effector responses. It has been suggested that this effect of the liver may be the result of the need to maintain immunological silence to harmless gut-derived antigens in food and may account for the enhanced survival of liver allografts and for the persistence of some liver pathogens.43
Previous reports in rodent models have shown relatively poor immunostimulatory capacity of tissue-derived DCs from liver, heart, and kidney,5,17,44,45 without further studies to compare these DCs, it is not possible to conclude from our data whether liver DCs are more regulatory, or skin DCs more proimmunogenic.
In conclusion we have shown clear tissue-specific differences between myeloid-type DCs of the same maturity from human liver and skin. The importance of such differences has recently been demonstrated by the finding that local DCs are responsible for generating lymphocytes with a tissue-specific homing potential.46 Our finding that liver-derived DCs spontaneously secrete IL-10 in the absence of IL-12 could be an important factor in the suppression of immune responses in the liver.
Acknowledgments
We thank Dr. Peter Lane for his ideas and guidance throughout the project, Professor Mike Salmon for constructive advice, Professor Chris Buckley for critically reviewing the manuscript, and Miss Waters and the Liver Unit Surgeons and theater staff for their invaluable help in obtaining liver and skin tissue.
Footnotes
Address reprint requests to Prof. David Adams, Liver Research Laboratories, MRC Centre for Immune Regulation, the University of Birmingham Institute of Clinical Science, Queen Elizabeth Hospital, Vincent’s Dr., Edgbaston, Birmingham B15 2TH. E-mail: d.h.adams@bham.ac.uk.
Supported by a training fellowship from the Medical Research Council and a Roche Organ Transplant Research Foundation 2-year grant.
References
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide or tumour lysate-pulsed dendritic cells. Nat Med. 1998;4:328–333. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- Austyn JM, Hankins DF, Larsen CP, Morris PJ, Rao AS, Roake JA. Isolation and characterization of dendritic cells from mouse heart and kidney. J Immunol. 1994;152:2401–2410. [PubMed] [Google Scholar]
- Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman RM, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells [see comments]. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c(−) type 2 dendritic cell precursors and CD11c(+) dendritic cells to produce type I IFN. J Immunol. 2001;66:2291–2295. doi: 10.4049/jimmunol.166.4.2291. [DOI] [PubMed] [Google Scholar]
- Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Ezaki T, Kudo S, Uehara Y. A life stage of particle-laden rat dendritic cells in vivo: their terminal division, active phagocytosis, and translocation from the liver to the draining lymph [see comments]. J Exp Med. 1996;183:1865–1878. doi: 10.1084/jem.183.4.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler AJ, Marsh DW, Yochum GS, Guzzo JL, Nigam A, Nelson WG, Pardoll DM. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J Exp Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, O’Sullivan B, Low P, Thomas R. Antigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory T cells secreting interleukin-10. Immunity. 2003;18:155–167. doi: 10.1016/s1074-7613(02)00503-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Woo J, Rao AS, Guzzo JL, Nigam A, Nelson WG, Pardoll DM, Watkins SC, Qian S, Starzl TE, Demetris AJ, Thomson AW. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J Exp Med. 1994;179:1823–1834. doi: 10.1084/jem.179.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Lu L, Rao AS, Li Y, Subbotin V, Starzl TE, Thomson AW. Isolation, phenotype, and allostimulatory activity of mouse liver dendritic cells. Transplantation. 1994;58:484–491. doi: 10.1097/00007890-199408270-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richters CD, Hoekstra MJ, van Baare J, Du PJ, Hoefsmit EC, Kamperdijk EW. Isolation and characterization of migratory human skin dendritic cells. Clin Exp Immunol. 1994;98:330–336. doi: 10.1111/j.1365-2249.1994.tb06146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AD, Starling GC, Hart DN. Isolation of human blood dendritic cells by discontinuous Nycodenz gradient centrifugation. J Immunol Methods. 1995;184:81–89. doi: 10.1016/0022-1759(95)00077-n. [DOI] [PubMed] [Google Scholar]
- Afford SC, Rhandawa S, Eliopoulos AG, Hubscher SG, Young LS, Adams DH. CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface FasL expression and amplifies Fas mediated hepatocyte death during allograft rejection. J Exp Med. 1999;189:441–446. doi: 10.1084/jem.189.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane P, Burdet C, McConnell F, Lanzavecchia A, Padovan E. CD40 ligand-independent B cell activation revealed by CD40 ligand-deficient T cell clones: evidence for distinct activation requirements for antibody formation and B cell proliferation. Eur J Immunol. 1995;25:1788–1793. doi: 10.1002/eji.1830250646. [DOI] [PubMed] [Google Scholar]
- Cremer I, Dieu-Nosjean MC, Marechal S, Dezutter-Dambuyant C, Goddard S, Adams D, Winter N, Menetrier-Caux C, Sautes-Fridman C, Fridman WH, Mueller CG. Long-lived immature dendritic cells mediated by TRANCE-RANK interaction. Blood. 2002;100:3646–3655. doi: 10.1182/blood-2002-01-0312. [DOI] [PubMed] [Google Scholar]
- Jones ND, Turvey SE, Van Maurik A, Hara M, Kingsley CI, Smith CH, Mellor AL, Morris PJ, Wood KJ. Differential susceptibility of heart, skin, and islet allografts to T cell-mediated rejection. J Immunol. 2001;166:2824–2830. doi: 10.4049/jimmunol.166.4.2824. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Hamashima T, Matsui S, Oka T. Prolongation of rat renal allograft survival by portal venous inoculation with donor lymphocytes. Transplant Proc. 1989;21:3268–3270. [PubMed] [Google Scholar]
- Patterson S. Flexibility and cooperation among dendritic cells. Nat Immunol. 2000;1:273–274. doi: 10.1038/83644. [DOI] [PubMed] [Google Scholar]
- Bell SJ, Rigby R, English N, Mann SD, Knight SC, Kamm MA, Stagg AJ. Migration and maturation of human colonic dendritic cells. J Immunol. 2001;166:4958–4967. doi: 10.4049/jimmunol.166.8.4958. [DOI] [PubMed] [Google Scholar]
- Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–3835. [PubMed] [Google Scholar]
- de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait-Yahia S, Banchereau J, Liu YJ, Lebecque S, Caux C. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160:1666–1676. [PubMed] [Google Scholar]
- Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, De Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal M, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–4318. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–396. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- Carra G, Gerosa F, Trinchieri G. Biosynthesis and posttranslational regulation of human IL-12. J Immunol. 2000;164:4752–4761. doi: 10.4049/jimmunol.164.9.4752. [DOI] [PubMed] [Google Scholar]
- Schulz O, Edwards DA, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha, 25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstein H, Morelli AE, Larregina AT, Ganster RW, Papworth GD, Logar AJ, Watkins SC, Falo LD, Thomson AW. Aspirin inhibits in vitro maturation and in vivo immunostimulatory function of murine myeloid dendritic cells. J Immunol. 2001;166:7053–7062. doi: 10.4049/jimmunol.166.12.7053. [DOI] [PubMed] [Google Scholar]
- Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- Saiki T, Ezaki T, Ogawa M, Matsuno K. Trafficking of host- and donor-derived dendritic cells in rat cardiac transplantation: allosensitisation in the spleen and hepatic nodes. Transplantation. 2001;71:1806–1815. doi: 10.1097/00007890-200106270-00017. [DOI] [PubMed] [Google Scholar]
- Klinkert WE, LaBadie JH, Bowers WE. Accessory and stimulating properties of dendritic cells and macrophages isolated from various rat tissues. J Exp Med. 1982;156:1–19. doi: 10.1084/jem.156.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]