Abstract
Commitment of the pulmonary epithelium to bronchial and bronchiolar airway lineages occurs during the transition from pseudoglandular to cannalicular phases of lung development, suggesting that regional differences exist with respect to the identity of stem and progenitor cells that contribute to epithelial maintenance in adulthood. We previously defined a critical role for Clara cell secretory protein-expressing (CE) cells in renewal of bronchiolar airway epithelium following injury. Even though CE cells are also the principal progenitor for maintenance of the bronchial airway epithelium, CE cell injury is resolved through a mechanism involving recruitment of a second progenitor cell population that we now identify as a GSI-B4 reactive, cytokeratin-14-expressing basal cell. These cells exhibit multipotent differentiation capacity as assessed by analysis of cellular phenotype within clones of LacZ-tagged cells. Clones were derived from K14-expressing cells tagged in a cell-type-specific fashion by ligand-regulable Cre recombinase-mediated genomic rearrangement of the ROSA26 recombination substrate allele. We conclude that basal cells represent an alternative multipotent progenitor cell population of bronchial airways and that progenitor cell selection is dictated by the type of airway injury.
Conducting airways of the lung are lined by a highly specialized epithelium whose composition and function varies along the proximal to distal axis. Despite a growing appreciation of mechanisms contributing to branching morphogenesis and lineage specification in the developing lung it is still unclear how a complex epithelium such as that present in the conducting airway is established and maintained through adulthood. Studies in the developing rat lung indicate that lineage restriction occurs during the transition from the pseudoglandular to cannalicular phases of lung development. At this time cells of the proximal airway lose the ability to respond to mesenchymally derived signals capable of inducing distal airway differentiation.1,2 Lineage restriction at this stage of embryonic lung development results in the establishment of tracheobronchial and bronchiolar airway lineages that differ according to cell types represented, the most notable example of which is the presence or absence, respectively, of cells with phenotypic properties of basal cells.3 Functional roles that have been proposed for basal cells in maintenance of bronchial airways include the tethering of columnar epithelial cells to the airway wall,4 in addition to functioning as a multipotent progenitor cell population.5–7
Progenitor cells responsible for maintenance of the airway epithelium have been identified in both the steady-state lung and following injury induced by mechanical or chemical disruption.8–14 These studies have identified multiple epithelial cell types with proliferative capacity and indicate that the pseudostratified epithelium of tracheobronchial airways are maintained by multiple progenitor cell populations that are distinct from those that sustain the simple epithelium of bronchioles.11,12 Progenitor cells of the bronchiolar conducting airway include Clara cell secretory protein (CCSP)-expressing (CE) cells in addition to a rare population of calcitonin gene-related peptide (CGRP)-expressing cells.11,14 Of these bronchiolar airway progenitor pools, CE cells represent a multipotent progenitor population.11 CCSP-expressing cells can be further subdivided according to their susceptibility to naphthalene; naphthalene-sensitive CE cells are more numerous and represent the classical nonciliated secretory (Clara) cell, whereas naphthalene-resistant CE cells represent a rare subpopulation of CE cells that localize to distinct airway microenvironments and have properties of tissue-specific stem cells.14–18 CGRP-expressing progenitor cells of the bronchiolar epithelium serve a self-renewing function but lack the potential for multipotent differentiation.16,19 In contrast, naphthalene-resistant CE cells are absolutely required for renewal of the bronchiolar epithelium.14,16
Bronchial airways also harbor at least two distinct progenitor cell populations. Both basal and nonciliated secretory cell types of bronchial airways have been shown to exhibit proliferative capacity.8,9,11,12,20,21,47 However, the relative contribution of secretory versus basal progenitor cell populations to epithelial maintenance and regeneration following injury remains controversial. Studies by Evans and colleagues12 involving injury targeted to ciliated cells indicated that nonciliated secretory cells represent the preferred progenitor cell type of the bronchial epithelium. The significant regenerative capacity of bronchial epithelial cells was highlighted in studies by Engelhardt and colleagues,22,23 who tagged isolated human bronchial epithelial cells with β-gal-expressing retroviruses and demonstrated that in some cases individually tagged cells give rise to clones harboring thousands of differentiated progeny when seeded into denuded tracheal xenografts. However, in contrast to findings by Evans et al12 indicating that secretory cells represented the principal progenitor cell in vivo, studies by Randell and colleagues7,24 exploring the differentiation potential of airway epithelial cell preparations enriched for basal cell populations suggest that basal cells may have the capacity for multipotent differentiation. This disparity could be accounted for by the existence of a hierarchical relationship between basal cells and secretory cells of the bronchial airway, a theory that we sought to explore in the present study.
Understanding mechanisms regulating the cellular composition and function of the conducting airway epithelium has the potential to provide insights into pathobiological mechanisms contributing to deteriorating lung function in chronic lung disease. Altered composition and function of the conducting airway epithelium is a central pathological feature of many chronic lung diseases such as asthma and chronic obstructive pulmonary disease (COPD).25,26 Moreover, the majority (approximately 90%) of primary lung tumors in humans arise from the tracheobronchial lining, even though this tissue covers less than 1% of the lung surface exposed to the atmosphere.27,28 We previously determined that ablation of CE cells of bronchial airways through ganciclovir (GCV) administration to transgenic mice (CCtk) expressing a CCSP promoter-driven Herpes simplex virus thymidine kinase transgene resulted in proliferation of a second uncharacterized progenitor cell pool.16 We hypothesized that basal cells represent a secondary population of multipotent progenitor cells of the bronchial epithelium that have the capacity to repair injured airways following Clara cell ablation. In the present study two different chemical ablation strategies were used to deplete Clara cells from airways of mice, one involving administration of GCV to CCtk transgenic mice and the other involving parenteral administration of high doses of the Clara cell-specific toxicant naphthalene. Through depletion of Clara cells in this manner it was possible to determine the contribution of alternative progenitor cell pools to the repair process. We identify cytokeratin 14-expressing basal cells as a second multipotent progenitor cell population of the bronchial epithelium. Preferential activation of basal cells following injury to CE cells suggests that the dynamics of progenitor cell utilization between steady-state and injury may serve to influence the lineage derivation and potentially the functional properties of the bronchial epithelium.
Materials and Methods
Animals
Mice were maintained as a specific pathogen-free in-house colony and representative animals were screened quarterly using a comprehensive 16-agent serological panel (Microbiological Associates, Rockville, MD). Mice were allowed food and water ad libitum and maintained on a 12-hour/day light/dark cycle. Animals used in this study were 2 to 4 months old.
CCtk Transgenic Mice and Ganciclovir Treatments
Generation and characterization of CCSP-HSVtk (CCtk) transgenic mice have been previously described.19 Transgenic mice (n = 4) were exposed to GCV delivered over a 24-hour period by miniosmotic pumps (ALZET, Palo Alto, CA) as described previously.16 To continuously label proliferating cells following GCV treatments in CCtk mice, 14-day miniosmotic pumps delivering 0.5 μl/hour of sterile 20 mg/ml bromodeoxyuridine (BrdU) in normal saline solution were implanted subcutaneously on recovery day 2. Animals were sacrificed on day 10 of recovery. Control CCtk transgenic mice (n = 4) were continuously administered with BrdU in the same manner for 8 days, without initial GCV treatment.
Generation of K14-CreERt Transgenic Mice and K14/RS Bitransgenic Mice
The K14-CreERt (K14) transgene construct was described previously.29 To facilitate purification of the full-length transgene an internal EcoRI site in the original plasmid was eliminated by partial digestion of the plasmid, filling in cohesive ends by Klenow polymerase and ligating the resulting blunt ends. The modified plasmid was digested to yield a 5.25-kb EcoRI/SphI fragment that was microinjected into the male pronucleus of FVB/n zygotes using standard techniques.30 Three K14 founders were identified by PCR analysis of tail DNA using primers specific for Cre recombinase coding sequences: 5′-GGACATGTTCAGGGATCGCCA-GGCG-3′ and 5′-GCATAACCAGTGAAACAGCATTGC-TG-3′.
Mice harboring the ROSA26-floxed Neo-LacZ recombination substrate (RS) transgene on a C57BL/6J background31 were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained by mating hemizygous individuals. Transgene positive mice were identified by PCR analysis of tail DNA using primers specific for the LacZ coding sequence: 5′-GTGGCAGCATCAGGGGAAAACCTT-3′ and 5′-GAATTCCGCCGATACT-GACG-GGCT-3′.
Bitransgenic K14/RS mice were generated by breeding homozygous or hemizygous K14 transgenic mice with hemizygous RS mice, and the progeny genotyped as detailed above.
β-Galactosidase Histochemistry
The cell type specificity and ligand-dependence of cre-mediated recombination was assessed for each K14 line by topical administration of 5 to 10 mg of Tamoxifen (Sigma, St. Louis, MO) in DMSO or DMSO alone to the dorsal skin29 and by systemic administration of Tamoxifen in corn oil (4 mg Tamoxifen/mouse i.p. on each of 5 consecutive days) to RS and K14/RS bitransgenic. Tissues, including trachea, lung, esophagus, tongue, toe, heart, and intestine, were harvested from Tamoxifen-treated mice, fixed in 4% paraformaldehyde for 20 minutes at room temperature, and stored in PBS at 4°C. For histochemical detection of Escherichia coli β-galactosidase enzymatic activity, tissues were incubated in X-gal staining solution [5 mmol/L K3Fe(CN)3, 5 mmol/L K4Fe(CN)6, 2 mmol/L MgCl2, 0.02% Nonidet P-40, 0.01% sodium deoxycholate, 1X PBS, and 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal)], for 4 to 5 hours at 37°C in the dark, washed in PBS, and postfixed in 4% paraformaldehyde at 4°C overnight.
Naphthalene and Tamoxifen Treatments
Mice were treated with naphthalene at a dose of 275 mg/kg body weight as previously described.18 Groups of 4 to 6 mice were recovered for 1, 1.5, 2, 3, 6, or 9 days and injected i.p. with 50 mg/kg body weight of BrdU (Sigma) 2 hours before sacrifice. Parenteral administration of Tamoxifen was used for conditional introduction of lineage tags within K14/RS bitransgenic mice. Tamoxifen (Sigma) was prepared as a 40 mg/ml suspension in Mazola corn oil and 100 μl of a 40 mg/ml solution injected i.p. each day from the second through the fourth postnaphthalene exposure days. Groups of naphthalene/Tamoxifen-treated mice were then sacrificed at day 4, 12, or 20 postnaphthalene exposure for evaluation of LacZ reporter gene expression (see below).
Tissue Collection
Animals were sacrificed with 100 mg/kg pentobarbital i.p. and exsanguinated. Lungs were inflation-fixed with 10% neutral-buffered formalin installed via a tracheal cannula and maintained at 10-cm water pressure for 10 minutes. The heart lung unit was immersed in the same fixative overnight and stored in PBS at 4°C. The caudal lobe was dehydrated, embedded in paraffin, and 5-μm sections, which contained the major axial pathway, 3 to 5 minor daughter airways, and numerous terminal bronchioles were mounted on microscope slides (VWR, West Chester, PA). Airway regions used for analysis included epithelium located between the extrapulmonary bronchus and the first two intrapulmonary branches of the main axial pathway.
GSI-B4 Lectin Histochemistry
Tissue sections were blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline containing 0.1 mmol/L CaCl2 and 0.1 mmol/L MgCl2 (LHB) for 30 minutes at room temperature and then incubated with 6.5 μg/ml biotinylated Griffonia (Bandeiraea) simplicifolia isolectin B4 (GSI-B4; Sigma)32,33 diluted in 3% BSA in LHB for 1 hour at room temperature. Sections were washed in LHB and then incubated with streptavidin-conjugated horseradish peroxidase (S-HRP) (Zymed, South San Francisco, CA) diluted in 0.5% BSA in LHB for 30 minutes at room temperature. Lectin binding sites were detected using a diaminobenzidine substrate kit (DAB, Vector, Burlingame, CA). Tongue tissue was used as positive control for GSI-B4 staining.
Immunohistochemistry
Adjacent serial sections were stained for CCSP, GSI-B4, cytokeratin 14 (K14), and BrdU to detect Clara cells, basal cells, and proliferating cells, respectively. Standard immunohistochemical techniques were used.34 Rabbit anti-rat CCSP was used as previously described.16 Mouse monoclonal K14 antibody (Clone LL002, 35) was purchased from NeoMarkers (Fremont, CA) and is specific for the highly conserved carboxy-terminal 16-amino acids of K14.36,37 The K14 antigen was retrieved by microwave treatment in citrate buffer. Antigen-antibody complexes were detected using biotinylated rabbit anti-mouse IgG3 (Zymed), and streptavidin-HRP. Sections of mouse tongue or trachea were included as positive controls. Nuclear BrdU was detected as previously described,16 except that proteinase K treatment was replaced by antigen retrieval with citrate buffer and sheep anti-BrdU was purchased from Biodesign (Saco, ME). Antigen-antibody complexes were detected with DAB and the sections were counterstained with hematoxylin. Images of representative fields were acquired using an Olympus Provis AX70 microscope (Olympus, Lake Success, NY) equipped with a Spot RT color digital camera (Diagnostic Instruments, Sterling Heights, MI) linked to a PC running Image-Pro Plus software (Media Cybernetics, Silver Spring, MD).
Dual Immunofluorescence
Cells coexpressing BrdU and CCSP, GSI-B4, or K14 were identified on adjacent serial sections using dual-immunofluorescence techniques. Primary antibodies were described above. Secondary antibodies were purchased from Molecular Probes (Eugene, OR) and the following combinations were used: Alexa Fluor 594 donkey anti-rabbit IgG + Alexa Fluor 488 donkey anti-goat IgG for CCSP and BrdU, streptavidin-conjugated Alexa Fluor 488 + Alexa Fluor 594 donkey anti-goat IgG for GSI-B4 and BrdU, and biotinylated rabbit anti-mouse IgG3 followed by streptavidin-conjugated Alexa Fluor 488 + Alexa Fluor 594 donkey anti-goat IgG for K14 and BrdU. Imaging was carried out as described above using a DAPI/Texas Red dual-optical excitation filter cube and an fluorescein isothiocyanate optical excitation filter cube (Olympus).
Dual-immunofluorescent analysis of CCSP and K14 used primary antibodies and antigen retrieval as described above. Sections were incubated with goat anti-CCSP and mouse anti-K14, and antigen/antibody complexes detected with Alexa Fluor 488 donkey anti-goat IgG and Alexa Fluor 594 goat anti-mouse IgG3. A Leica TCS SP confocal microscope equipped with argon and HeNe laser sources (Leica, Weizler, Germany) was used for analysis.
Morphometry
Lung tissue sections were immunostained for BrdU and CCSP, GSI-B4, or K14, and counterstained with hematoxylin. The length of the bronchial epithelium between lobar bronchus and the second intrapulmonary branch point was determined using the measurement function of Image-Pro Plus (Media Cybernetics, Silver Spring, MD). Cell density was determined by counting nucleated cells in this region and was expressed as cells per millimeter of basement membrane (BM). Data were collected from approximately 4.5 mm of BM in the bronchial zone indicated above. Density of specific cell types was determined by counting immunoreactive (IR) nucleated cells in the measured region and was reported as the number of IR cells per millimeter of BM. Labeling index was defined as the number of BrdU-labeled nuclei divided by the total number of nuclei and was presented as a percentage. The labeling index for each cell type was defined as the number of IR-BrdU dual-positive cells divided by the number of IR cells and was presented as a percentage. The proliferative fraction was defined as the number of IR-BrdU dual-positive cells divided by the total number of BrdU-labeled cells and was expressed as a percentage. The distribution of cells with characteristics of both Clara and basal cells was determined using sections dual-labeled for CCSP and a basal cell marker (K14) as described above. The number of dual-labeled cells (CCSP/K14) was divided by the total length of the BM and reported as a percentage. Statistical significance was determined by Student’s t-test.
Lineage Analysis in Naphthalene-Treated K14/RS Bitransgenic Mice
Groups of 4 to 6 adult K14/RS mice and RS mice were treated with naphthalene as described above on day 0, treated systemically with Tamoxifen as described above on days 2, 3, and 4, and sacrificed on days 4, 12, or 20. The distribution of cells harboring a recombined RS allele within the tracheal-lung unit was determined by detection of E. coli β-galactosidase enzymatic activity as described above. The lumen of the main axial intrapulmonary airway was exposed by microdissection and X-gal-stained cells were imaged using an Olympus AX70 microscope. To determine the phenotype of X-gal-stained cells within the bronchi, these regions were microdissected, embedded in paraffin and 5-μm sections were prepared. Sections containing stained cells were deparaffinized, hydrated, mounted with PBS and imaged. Tissue was then stained for either CCSP, GSI-B4, or K14 with HRP detection and re-imaged for colocalization of X-gal and reactivity toward phenotypic markers.
Results
Epithelial Renewal within the Bronchial Epithelium Following Secretory Cell Ablation
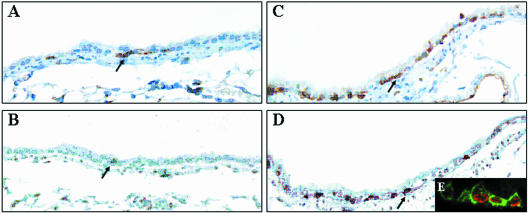
Nonciliated secretory (Clara) cells represent the preferred progenitor cell population contributing to maintenance of both bronchial and bronchiolar airways.11,12 We have previously demonstrated that ablation of the CE cell population, the principal secretory cell type of the rodent airway, results in a shift in progenitor cell utilization such that 45.8 ± 9.2% of BrdU-labeled airway cells were immunoreactive for the neuroendocrine cell marker CGRP and only 4.7 ± 2.8% of proliferating cells were immunoreactive for the Clara cell marker CCSP.16 To determine the phenotype of the remaining proliferative cells, CCSP-expressing cells were depleted by exposure of CCtk transgenic mice to GCV for 24 hours and dividing cells were marked by continuous infusion of the nucleoside analog BrdU between days 2 and 10 of the recovery period. Animals were sacrificed 10 days after GCV exposure and the contribution of basal cells to the proliferative population determined by colocalization of the basal cell-specific GSI-B4 lectin binding site and BrdU on serial adjacent sections. Analysis of control animals similarly exposed to BrdU for 8 days demonstrated that GSI-B4 reactive cells were infrequent constituents of the normal bronchial epithelium. These cells had no access to the airway lumen and exhibited flattened or elongated morphology typical of basal cells (Figure 1A). Proliferation of the bronchial epithelium in general and of GSI-B4 reactive cells in particular was rare as determined by the infrequent detection of BrdU-labeled cells (Figure 1B). In contrast, the injured bronchial epithelium of GCV-treated CCtk transgenic mice was characterized by hyperplasia of GSI-B4 reactive cells (Figure 1C) and increased proliferation as evidenced by the frequent occurrence of BrdU-labeled nuclei (Figure 1D). Dual-fluorescence detection of GSI-B4 binding site and BrdU and analysis by confocal microscopy demonstrated that hyperplastic GSI-B4 reactive cells were BrdU-labeled (Figure 1E). However, due to continuous labeling with BrdU in this experiment the precursor cell giving rise to this GSIB4/BrdU-labeled population could not be determined. These data indicate that depletion of the CE cell population results in hyperplasia of GSI-B4 reactive cells and suggest that basal cells may represent a major progenitor cell population that is activated in response to depletion of the secretory cell population. This finding identifies a fundamental distinction between progenitor cell populations of bronchial and bronchiolar airways with respect to their contribution to epithelial renewal. Unlike bronchiolar airways, which are completely dependent on CE cells for epithelial renewal, bronchial airways are maintained through participation of two distinct populations of multipotent progenitors. Dependence of bronchiolar regeneration on CE cells complicated further analysis of basal cell proliferation and differentiation capacity using the CCtk transgenic mouse model, as failure of bronchiolar regeneration led to declining lung function and death.16 As such, it was not possible to recover mice for the extended period of time necessary to fully assess whether proliferative basal cells exhibited the capacity for restoration of depleted CCSP-expressing cells.
Figure 1.
Proliferation and hyperplasia of basal cells in the bronchial epithelium of GCV-treated CCtk transgenic mice. CCtk mice were acutely exposed to vehicle (A and B) or 10 mg GCV (C and D), continuously exposed to BrdU during days 2 to 8 of the recovery period, and sacrificed on recovery day 10. Basal cells were detected by GSI-B4 lectin histochemistry (A and C) and S-phase cells were detected on adjacent serial sections by immunohistochemical detection of BrdU (C and D). Lectin and immune complexes were detected with diaminobenzidine (brown stain). Original magnification, ×400. Tissue sections from animals treated with GCV and recovered 3 days were analyzed by dual-immunofluorescence detection of GSI-B4 (green fluorescence) and BrdU (red fluorescence) and were analyzed by confocal microscopy (E). Original magnification, ×600.
Basal Cell Hyperplasia Following Naphthalene-Induced Clara Cell Injury
To assess regeneration of the bronchial epithelium following Clara cell depletion (in the context of these and our previous studies Clara cells are defined as a naphthalene-sensitive CE population), adult female FVB/n mice were challenged by parenteral administration of 275 mg naphthalene per kilogram of body weight. Mice were recovered for 1, 1.5, 2, 3, 6, or 9 days and injected with BrdU 2 hours before sacrifice. The cellularity and composition of the epithelium was determined at each recovery time point through enumeration of hematoxylin-stained nuclei and of immunohistochemically stained CCSP-, cytokeratin 14 (K 14)-, and GSI-B4 binding site-positive cells. Using this approach basal cells were identified either through their reactivity toward GSI-B4 lectin or through their expression of K 14, the obligate heterodimer of cytokeratin 5.48 In agreement with previous reports demonstrating a sexual dimorphism in the response to naphthalene,38 administration of 275 mg/kg naphthalene to female mice resulted in rapid necrosis and exfoliation of CCSP-immunoreactive (IR) Clara cells within all airway regions including the bronchial epithelium (Figure 2B). As a result, overall cell density in the bronchial epithelium was reduced to approximately 48.5% of control on days 1.5 and 2 (Figure 2M) and returned to normal by day 3 of recovery (Figure 2M).
Figure 2.
Cellular composition of the normal and regenerating bronchial epithelium. Lung tissue sections from untreated (A, E, and I) and naphthalene-treated mice that had recovered 3 days (B, F, and J), 6 days (C, G, and K), or 9 days (D, H, and L) were immunostained for CCSP (A to D), GSI-B4 (E to H), or K14 (I to L). Tissue was counterstained with hematoxylin and images of the lobar bronchus collected using standard light microscopy. Original magnification, ×400. Cellular composition (M) within the bronchial epithelium of control (Ctrl) or naphthalene-treated mice that had been recovered 1.5 to 9 days (d) was defined as the number of immunoreactive cells in each category divided by the length on the BM in millimeters. The mean ± SEM (n = 4 to 5) is presented for each group. *, P < 0.05 relative to values for untreated control mice.
The representation of CCSP-IR cells within the steady-state bronchial epithelium was 74.8 ± 6.7 cells per millimeter of BM (Figure 2M) and declined to 3.6 ± 2.6 cells per millimeter of BM 2 days after naphthalene treatment (Figure 2M). By recovery day 3, scattered CCSP-IR cells were observed in the bronchial epithelium (Figure 2B) and CCSP-IR cell density increased slightly to 3.9 ± 1.4 cells per millimeter of BM (Figure 2M). A dramatic increase in the number of CCSP-IR cells was observed on day 6 (53.7 ± 4.3 cells per millimeter of BM; Figure 2, C and M) and CCSP-IR cells returned to a nearly normal distribution by day 9 (67.2 ± 5.4 cells per millimeter of BM; Figure 2, D and M). Nascent CCSP-IR cells were uniformly distributed throughout the bronchial epithelium at all time points analyzed and a clear demarcation of regenerated regions in the bronchial epithelium and non-regenerated regions of the bronchiolar epithelium was noted. These observations demonstrate a pattern of CE cell regeneration in the bronchial epithelium that is distinct from the focal regeneration that we have previously reported for the bronchiolar15,14,18 epithelium following naphthalene-induced injury. However, occasional clusters of rapidly regenerating CE cells were observed at branchpoints of intrapulmonary bronchi, indicating the potential for overlap of bronchial (widespread) and bronchiolar (focal) regenerative pathways within the transitional zone.
GSI-B4-reactive basal cells were rare in the normal airway (9.8 ± 5.3 cells per millimeter of BM), present only in the bronchial regions of the intrapulmonary airways (Figure 2E), and were distinct from the CCSP-IR cell population (compare Figure 2, A and E). The number of GSI-B4-reactive basal cells did not differ from control on recovery days 1.5 and 2 (Figure 2M) but exhibited a marked 4.5-fold increase by recovery day 3 (Figure 2, F and M). These hyperplastic cells formed multiple layers within the epithelium (data not shown) and were limited to the bronchi and large bronchioles. The number of GSI-B4-reactive basal cells gradually declined after recovery day 3 (Figure 2, F,G, M) and was similar to that observed in the untreated bronchial epithelium on recovery day 9 (Figure 2, H and M). These data indicate that severe depletion of Clara cells (naphthalene-sensitive CE cells) in the bronchial epithelium was followed by a transient basal cell hyperplasia, establishment of which preceded restoration of the CE cell population.
K14 is a commonly used marker for basal cells within various stratified epithelia35; however, immunohistochemical analysis of the intrapulmonary bronchial epithelium failed to detect cells expressing this marker in normal murine airways (Figure 2, I and M). In contrast, starting at day 2 of recovery cells expressing K14 were detected in regions of the bronchial epithelium harboring GSI-B4-reactive basal cells (Figure 2, J, K, and M). Changes in number and distribution of K14-expressing cells followed that of GSI-B4-reactive basal cells during the recovery period (Figure 2M) and these cells reached a maximum representation within the bronchial epithelium on recovery days 3 to 6. To determine whether GSI-B4 and K14 are co-expressed by individual cells, expression of these markers was assessed by dual-fluorescence microscopy at each recovery time point. Double-fluorescent labeling of bronchial epithelium from mice on recovery day 3 revealed that approximately 84% of GSI-B4-reactive cells expressed K14 and all K14-expressing cells were positive for GSI-B4 (data not shown). These results indicate that a subset of GSI-B4-reactive basal cells up-regulate expression of K14 in response to naphthalene-mediated Clara cell injury.
Epithelial cells that were not identified as either secretory or basal cells using the immunophenotyping approaches outlined above varied from approximately 50% of total cells in control and 9-day recovery bronchi to over 90% at the 1.5-day time point following naphthalene exposure. These cells possessed cilia on their apical surface.
Progenitor Cell Populations of the Regenerating Bronchial Epithelium
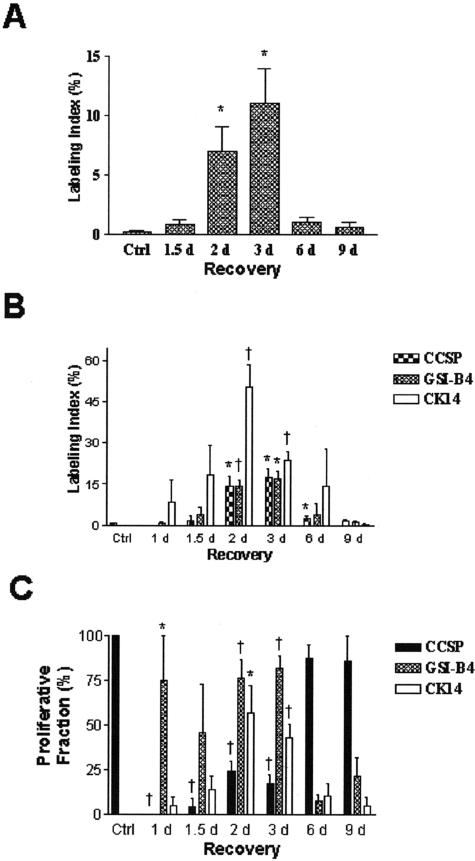
To identify progenitor cells responsible for renewal of the bronchial epithelium animals were treated with naphthalene as described above and injected with BrdU 2 hours before sacrifice. The labeling index defined as the number of BrdU-labeled cells divided by the total number of nucleated cells was 0.18 ± 0.11% in control animals (Figure 3A, F, and K and Figure 4A). This value was unchanged 1.5 days after naphthalene treatment (Figure 4A) but significantly increased to 6.95 ± 2.13% on day 2 of recovery (Figure 4A), peaked at 11.01 ± 2.94% on day 3 (Figure 3, C, H, and M and Figure 4A) decreased to 1.04 ± 0.39% by recovery day 6 (Figure 3, D, I, and N and Figure 4A) and was similar to control values on day 9 (Figure 3, E, J, and O and Figure 4A). These data indicate that the peak of cell proliferation precedes repopulation of the airway by CCSP-IR cells and suggest that a population of cells other than those defined by CCSP expression (CE) is responsible for regeneration of CE and ciliated cells in this region of the airway.
Figure 3.
Bronchial proliferation following naphthalene-induced Clara cell depletion. Corn oil (A, F, K) or naphthalene-treated mice were recovered 1.5 days (B, G, L), 3 days (C, H, M), 6 days (D, I, N), or 9 days (E, J, O) and injected with BrdU 2 hours before sacrifice. Adjacent serial sections from each experimental group were analyzed by dual immunofluorescence for CCSP (A to E, red) and BrdU (green), GSI-B4 binding site (F to J, green) and BrdU (red), or K14 (K to O, green) and BrdU (red).
Figure 4.
Labeling index and proliferation fraction of the bronchial epithelium. The total labeling index for the bronchial epithelium (A) was defined as the percentage of BrdU-labeled nuclei/total number of nuclei. The labeling index for each cell type (B) was defined as the percentage of dual-positive CCSP/BrdU, GSI-B4/BrdU, or K14/BrdU cells divided by the total number of cells in each category. The proliferative fraction (C) was defined as the percentage of BrdU-labeled cells of each cell type divided by the total number of BrdU-labeled nuclei. Each value was determined for control (Ctrl) and naphthalene-treated mice that had been recovered 1.5 to 9 days (d) and is reported as the mean ± SEM (n = 4 to 5 for each group). *, P < 0.05 and †, P < 0.01 relative to values in untreated control mice.
The contribution of CCSP-IR, GSI-B4-reactive, and K14-IR cells to the proliferative cell population was determined by dual-immunofluorescence analysis of these markers and BrdU at each recovery time point. In the steady-state bronchial epithelium, CCSP-IR cells represented nearly 100% of proliferative cells with 0.49 ± 0.12% of CCSP-IR cells actively synthesizing DNA (Figure 4, B and C). Following naphthalene treatment, proliferative CCSP-IR cells were rarely detected on recovery days 1 to 3 (Figure 3, B and C and Figure 4B). In contrast, CCSP-IR cells represented 87.5 ± 7.2% of the proliferative pool and 2.6 ± 0.7% of CCSP-IR were labeled with BrdU on recovery day 6 (Figure 3D and Figure 4, B and C). By recovery day 9 the contribution of CCSP-IR cells to the total proliferative pool (85.7 ± 14.3%, Figure 3E and Figure 4B) and the proliferative index among CCSP-IR cells (1.4 ± 0.7%) had returned to levels that were not significantly different from control (Figure 4C).
In contrast with the CCSP-IR cell population, GSI-B4 reactive cells were relatively quiescent in the steady-state bronchial epithelium (0.0 ± 0.0%, Figure 3F and Figure 4, B and C). However, within 1 day of naphthalene treatment, proliferation of GSI-B4 reactive cells increased to 75.0 ± 25.0% of all BrdU-labeled cells (Figure 4B) and 0.9 ± 0.4% of GSI-B4 reactive cells were labeled with BrdU (Figure 4C). The contribution of GSI-B4 reactive cells to the proliferative pool peaked at 82.1 ± 6.7% on recovery day 3 (Figure 3H and Figure 4B) and 16.8 ± 3.1% of this cell population was labeled (Figure 4C). By recovery day 6 proliferation of GSI-B4 reactive cells had declined to 7.5 ± 3.8% of total BrdU-labeled cells (Figure 3I and Figure 4B) with a proliferative index of 4.0 ± 3.8% within this cell population (Figure 4C). BrdU-labeled GSI-B4 reactive cells were rarely detected on recovery day 9 (Figure 3J and Figure 4B). These data indicate that the basal cell hyperplasia observed at days 3 and 6 of recovery was a result of increased proliferation of basal cells in response to Clara cell ablation. Furthermore, the observation that increased proliferation of basal cells preceded restoration of the CE cell population suggests that basal cells act as the major progenitor cell in the Clara-cell-depleted bronchial epithelium.
K14-IR cells were first detected on recovery day 1 (Figure 2M) and were among the most abundant BrdU-labeled cell populations on days 1 to 6 of recovery (Figure 3, L to N and Figure 4B). At the day 2 and 3 time points approximately 50% of K14-IR cells were labeled with BrdU (Figure 3L and Figure 4B). Proliferation of K14-IR cells decreased between recovery days 3 and 6 (Figure 3, M and N and Figure 4B), with the labeling index decreasing to 0.4 ± 0.4% by day 9 (Figure 3O and Figure 4B). The contribution of K14 IR cells to the total proliferative population was low and not significantly different from control levels on days 6 and 9 of recovery (Figure 4C). Collectively, these data indicate that a subpopulation of basal cells phenotypically defined by expression of K14 contributes to renewal of the bronchial epithelium following Clara cell depletion.
Phenotypic Plasticity among Progenitor Cells of the Regenerating Bronchial Epithelium
In addition to changes in the molecular phenotype of basal cells morphological alterations to these cells were observed during the postnaphthalene recovery period. Three days after naphthalene treatment, GSI-B4-reacitve and K14-IR cells exhibited an atypical cuboidal morphology suggestive of secretory cell differentiation. Adjacent section analysis of tissue from animals recovered for days 6 or 9 demonstrated that these morphologically distinct K14-IR cells were localized to regions of the bronchial epithelium harboring nascent CCSP-IR cells (Figure 2, C, D, K, and L). Dual-immunofluorescence analysis of CCSP and K14 was used to determine whether these morphologically distinct cells represented a potential intermediate in secretory cell differentiation. A small number of CCSP/K14 dual-positive cells were observed on day 3 of recovery (Figure 5D). Representation of these dual-positive cells reached a maximum on day 6 (Figure 5, A to D) and a similar number of these cells were observed within the bronchial epithelium on day 9 of recovery (Figure 5D). Cells with molecular characteristics of both CE and basal cells and the dynamic regulation of lineage-specific markers within these cells during epithelial regeneration suggests that basal cells of the bronchial epithelium may be capable of differentiating into CE cells in response to severe Clara cell injury. Regenerative CCSP-IR cells within the neuroepithelial body (NEB) or terminal bronchiole-associated regenerative microenvironments of the bronchiolar airway epithelium did not express detectable GSI-B4 reactivity or K14 immunoreactivity at any recovery time point (data not shown). Thus, CCSP/K14 dual-positive cells were limited to the bronchial epithelium of intrapulmonary conducting airways, suggesting that the CE cell lineage within bronchial epithelium may represent a distinct lineage from those of the bronchiolar epithelium.
Figure 5.
CCSP/K14 dual-positive cells. The occurrence of bronchial cells that co-express CCSP (A, green) and K14 (B, red) was determined by dual immunofluorescence and confocal microscopy. The single-color images were merged (C) and used to determine the number of dual-positive cells per millimeter of BM in control (Ctrl) and naphthalene-treated mice following 1.5 to 9 days (d) of recovery (D). The images presented in A to C are from an animal that had been recovered 6 days. Original magnification, ×600. The histograms represent the mean ± SEM (n = 4 to 5). *, P < 0.05.
Basal Cells Function as a Multipotent Progenitor Cell for Renewal of the Bronchial Epithelium
The differentiation potential of basal cells was determined through introduction of a genetic tag into K14-expressing cells of the bronchial epithelium. This model used the previously described K14-CreERt transgene29 in combination with the ROSA26-floxed-neo-LacZ RS31 allele to generate tagged K14 expressing cells in a temporally regulated fashion. The K14-CreERt transgene utilizes the human cytokeratin 14 promoter to direct basal cell-specific expression of a fusion protein composed of the bacteriophage P1 Cre recombinase and a tamoxifen-regulated estrogen receptor ligand binding region (CreERt).39,40 In the presence of ligand (Tamoxifen, t) the Cre-ERt fusion protein translocates to the nucleus and acts in a site-specific fashion to recombine DNA segments demarcated by LoxP sites. In this study, excision of a LoxP-neo-LoxP resulted in juxtaposition of the ubiquitous ROSA 26 promoter and the LacZ coding sequence. Regulation of the recombined allele by the ubiquitous ROSA26 promoter allowed expression of the lineage tag in all cells derived from a K14-expressing progenitor cell. Bitransgenic K14/RS mice derived from each of three K14 lines were screened for ligand-dependent and cell-type-specific recombination in the epidermis following topical administration of vehicle or Tamoxifen29 and in the bronchial epithelium following systemic administration of vehicle or Tamoxifen using histochemical detection of LacZ on whole mounted tissue. Ligand-dependent, cell-type-specific recombination was observed in the bronchial epithelium of all 3 lines of K14 transgenic mice. Recombination was not detected in the bronchial epithelium of K14/RS bitransgenic mice exposed to carrier or in RS mice exposed to Tamoxifen (data not shown). The number 3 line, which demonstrated the highest recombination frequency in steady-state animals, was chosen for the present study.
To assess the differentiation of basal cells in the regenerating bronchial epithelium, adult K14/RS bitransgenic mice were treated with 275 mg/kg naphthalene (i.p.) to deplete the Clara cell population and with 4 mg Tamoxifen dissolved in corn oil (i.p.) on recovery days 2 to 4 to induce recombination. Groups of 4 to 6 K14/RS mice were sacrificed 4-, 12-, or 20 days after naphthalene treatment, and the lungs fixed and whole-mount stained for identification of cells expressing E. coli β-galactosidase (β-Gal). The kinetics and pattern of Clara cell injury and restoration of the epithelium in K14/RS mice treated with naphthalene and Tamoxifen was similar to that observed in wild-type FVB/n mice (data not shown). Rare β-gal-expressing cells (on average 2 to 10 per lobe) were detected in the bronchial epithelium of naphthalene/Tamoxifen-treated mice on recovery day 4 and these cells were usually present as isolated cells (Figure 6A). A marked increase in the number of β-gal-positive epithelial cells within the bronchial epithelium was noted on days 12 (data not shown) and 20, and these tagged cells were usually clustered in groups of variable size (Figure 6D). These data suggest that proliferation and differentiation of isolated β-gal-expressing cells detected at the 4-day time point resulted in generation of the clusters of tagged cells identified at the later time points.
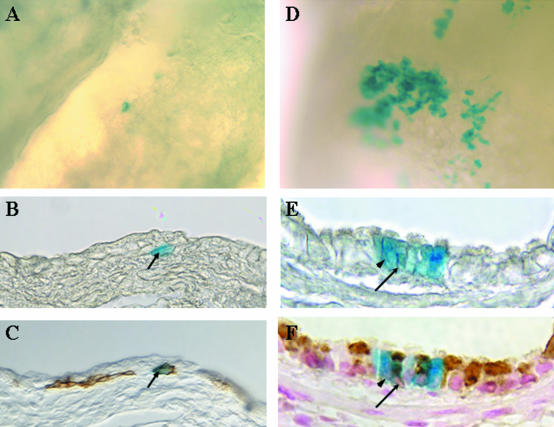
Figure 6.
Lineage of cytokeratin-14 expressing cells of the bronchial epithelium. K14-cre-ERt/RS bitransgenic mice were treated with naphthalene on day 0 and with Tamoxifen on recovery days 2 to 4. β-gal-expressing cells within the bronchial epithelium (blue-stained cells) were detected by wholemount histochemistry on recovery days 4 (A to C) and 20 (D to F). Tissue was initially imaged as a wholemount (A and D) and subsequently embedded, sectioned, and re-imaged (B and E). Sections containing β-gal-expressing cells were analyzed by immunohistochemistry for expression of K14 (C) or CCSP (F). Arrows in B and C indicate a β-gal/K14 dual-positive cell. Arrowheads in E and F indicate a β-gal-positive ciliated cell and the arrows indicate a β-gal/CCSP dual-positive cell.
To determine the phenotype of the tagged cells, X-gal-stained bronchial tissue was embedded in paraffin and sections containing β-gal-expressing cells were immunostained for cell-type specific markers. On recovery day 4, all X-gal-stained cells exhibited typical basal cell morphology (Figure 6B) and expressed K14 antigen (Figure 6C) but not CCSP (data not shown). In contrast, the majority of β-gal-positive cells detected on days 12 and 20 were cuboidal in morphology (Figure 6E) and no longer expressed K14 (data not shown). However, both ciliated and non-ciliated cells were detected within the tagged population at this time point (Figure 6E) and a subset of non-ciliated cells expressed CCSP (Figure 6F). Interestingly, at day 20 of recovery when the number of basal cells returned to the steady-state level, most of the β-gal-positive clusters lacked GSI-B4-reactive basal cells (data not shown). These observations demonstrate that the β-gal-positive ciliated and non-ciliated bronchial epithelial cells are derived from K14-expressing basal cells that proliferate and differentiate early in the repair process. As such, we conclude that basal cells are a multipotent progenitor population that gives rise to basal, Clara and ciliated cells, and therefore, plays an important role in renewal of the bronchial epithelium following depletion of the secretory cell population.
Discussion
The present study is the first to demonstrate that cytokeratin-14-expressing basal cells represent a multipotent cell type capable of restoring a normal bronchial epithelium following in vivo ablation of an abundant progenitor cell population (CE cells). These findings are in contrast to our previous studies showing that renewal of bronchiolar airways is dependent on CE cells.14,16,18 This disparity between bronchial and bronchiolar airways is consistent with a mechanism in which the activity of distinct progenitor cell pools accounts for regional differences in both lineage specification during lung development and in the cellular composition of tracheobronchial and bronchiolar airways. The tracheobronchial epithelium of the mouse forms a pseudostratified epithelium composed of ciliated, nonciliated secretory (CE, serous, and goblet) and basal cells.3,41 In contrast, bronchiolar airways are lined by a simple cuboidal to columnar epithelium which includes nonciliated secretory (Clara) and ciliated cells with sparsely distributed neuroepithelial cells that are either solitary or organized into clusters.3,42 The notion of regional differences in the type and differentiation potential of progenitor cells is supported by studies using purified cell populations to reconstitute denuded rat tracheal xenografts.43,44 In these studies, bronchiolar Clara cells isolated from the rabbit lung reconstituted an epithelium resembling bronchiolar epithelium, yet a mixed population of rabbit tracheal epithelial cells re-established a pseudostratified epithelium similar to that found in the trachea.
We have previously used naphthalene exposure to induce selective depletion of naphthalene-sensitive CE (Clara) cells or a transgenic mouse model to ablate all CE cells to reveal the identity and location of candidate stem cell populations within airways.14–18 In these studies naphthalene-resistant CE cells were found to exhibit properties of a multipotent tissue-specific stem cell, including infrequent cycling in the steady-state and a relatively undifferentiated phenotype. Findings of the present study indicate that K14-expressing basal cells contribute to renewal of the bronchial epithelium and are consistent with our previous demonstration that GCV-mediated depletion of CE cells in CCtk transgenic mice results in the proliferation of another cell type within the bronchial epithelium (Figure 1).16 These data also implicate the basal cell as either a secondary progenitor or a multipotent stem cell for this airway location. Interestingly, intrapulmonary bronchi represent a transitional regenerative zone in which both the widespread basal-cell-dependent (present study) and focal NEB-associated regenerative processes14,18 contribute to epithelial maintenance following naphthalene-induced ablation of the Clara cell progenitor pool. The bronchial versus bronchiolar regenerative patterns observed within this transitional zone could be distinguished not only by the distribution of regenerative cells but also the kinetics with which regenerating CE cells were observed; basal cell-derived CE cells observed in the present study appeared approximately 6 days after naphthalene-induced airway injury, whereas focal regeneration of bronchiolar epithelium re-establishes depleted CE cells as early as 48 hours post naphthalene-exposure.18 Data from the present study also suggest a complex relationship among progenitor cell populations in bronchial airways involving multiple distinct cell types capable of functioning as a multipotent progenitor cell.
Dynamic changes in the molecular phenotype of basal cells as they repopulate surface epithelial cell types of the airway indicate their multipotent differentiation potential, a conclusion that was confirmed through introduction of permanent lineage tags into the K14-expressing basal cell population. These findings are consistent with results of others in which basal-cell-like intermediates have been observed either following mechanical injury to the tracheal epithelium or during reconstitution of denuded tracheas by isolated tracheal epithelial cells.7,33,45 However, the identity of precursor cells contributing to renewal of scrape wounds introduced into the rat trachea7 or among isolated cells exhibiting multipotential differentiation potential when seeded into tracheal xenografts23,43 could not be determined. Our findings characterizing cell types contributing to regeneration of the bronchial epithelium demonstrate that K14-expressing basal cells have multipotential differentiation potential and, in the context of Clara cell depletion, contribute to restoration of CE progenitor cell populations. These findings are consistent with the demonstration of a subpopulation of K14-expressing cells of the tracheal epithelium that we have shown to exhibit multipotent differentiation capacity in vivo (Hong et al, in press).
The existence of multiple progenitor cell types within the bronchial epithelium and a hierarchy with which they participate in epithelial maintenance is of fundamental importance to the understanding of mechanisms that may lead to epithelial remodeling in the setting of chronic lung disease. We have demonstrated that infrequent proliferation of epithelial cells in the steady-state bronchial epithelium can be accounted for principally by CE progenitor cells, with little participation of GSI-B4-reactive or K14-IR basal cell populations. However, basal cell proliferation was dramatically increased following CE cell depletion, a mode of injury that reveals the multipotent differentiation potential of the bronchial basal cell population. The dynamic nature of progenitor cell utilization observed in the present study is similar to that observed by Evans and co-workers12 within the bronchial epithelium and by Lane and Gordon13 within the tracheal epithelium of rats. In studies by Evans et al, 12 both basal and nonciliated secretory cell populations contributed to epithelial maintenance in the steady-state; however, only nonciliated columnar epithelial cells contributed to the proliferative pool following oxidant-induced injury to ciliated cells. In contrast, mechanical injury to the rat tracheal epithelium resulted in proliferation of basal cells leading to restoration of a normal epithelium.13 Collectively, findings by others and those of this study argue for a hierarchy of progenitor cell utilization within the bronchial epithelium that may be influenced by a variety of factors, including the identity of target cells injured by environmental or physical agents. Even though activation of basal cell progenitor populations following naphthalene-induced Clara cell depletion results in restoration of an epithelium that includes all major cell types, it has yet to be determined whether appropriate functionality is conferred on a basal-cell-derived epithelium compared to that of the steady-state epithelium. It is possible that preferential activation of a non-preferred progenitor cell population within the chronically injured airway may contribute to functional alterations within the epithelium that may be transient or persistent. This notion is supported by the recent findings of Bucchieri and colleagues,46 who demonstrated that functional differences between bronchial epithelia from asthmatic and normal subjects are maintained in culture, indicating irreversible alterations in lineage specification. The ability to introduce lineage tags within selective progenitor cell populations in vivo provides tools to unambiguously define the contribution that differential progenitor cell utilization makes toward altered epithelial cell function in airway disease.
Acknowledgments
We thank Jennifer LaRuffa for husbandry of animals used in these studies.
Footnotes
Address reprint requests to Dr. Barry R. Stripp, Department of Environmental and Occupational Health, University of Pittsburgh, FORBL Room 314, 3343 Forbes Avenue, Pittsburgh, PA 15260. E-mail: brs2@pitt.edu.
Supported by National Institutes of Health grants HL64888 and ES08964.
References
- Shannon JM. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev Biol. 1994;166:600–614. doi: 10.1006/dbio.1994.1340. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212:482–494. doi: 10.1002/(SICI)1097-0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Jeffery PK, Reid L. New observations of rat airway epithelium: a quantitative and electron microscopic study. J Anat. 1975;120:295–320. [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Moller PC. Biology of airway basal cells. Exp Lung Res. 1991;17:513–531. doi: 10.3109/01902149109062862. [DOI] [PubMed] [Google Scholar]
- Inayama Y, Hook GER, Brody AR, Cameron GS, Jetten AM, Gilmore LB, Gray T, Nettesheim P. The differentiation potential of tracheal basal cells. Lab Invest. 1988;58:706–717. [PubMed] [Google Scholar]
- Inayama Y, Hook GER, Brody AR, Jetten AM, Gray T, Mahler J, Nettesheim P. In vitro and in vivo growth and differentiation of clones of tracheal basal cells. Am J Pathol. 1989;134:539–549. [PMC free article] [PubMed] [Google Scholar]
- Randell SH, Comment CE, Ramaekers FCS, Nettesheim P. Properties of rat tracheal epithelial cells separated based on expression of cell surface α-galactosyl end groups. Am J Respir Cell Mol Biol. 1991;4:544–554. doi: 10.1165/ajrcmb/4.6.544. [DOI] [PubMed] [Google Scholar]
- Boers JE, Amberge AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med. 1998;157:2000–2006. doi: 10.1164/ajrccm.157.6.9707011. [DOI] [PubMed] [Google Scholar]
- Breuer R, Zajicek G, Christensen TG, Lucey EC, Snider GL. Cell kinetics of normal adult hamster bronchial epithelium in the steady state. Am J Respir Cell Mol Biol. 1990;2:51–58. doi: 10.1165/ajrcmb/2.1.51. [DOI] [PubMed] [Google Scholar]
- Donnelly GM, Haack DG, Heird CS. Tracheal epithelium: cell kinetics and differentiation in normal rat tissue. Cell Tissue Kinet. 1982;15:119–130. doi: 10.1111/j.1365-2184.1982.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Cabral-Anderson LJ, Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest. 1978;38:648–655. [PubMed] [Google Scholar]
- Evans MJ, Shami SG, Cabral-Anderson LJ, Dekker NP. Role of nonciliated cells in renewal of the bronchial epithelium of rats exposed to NO2. Am J Pathol. 1986;123:126–133. [PMC free article] [PubMed] [Google Scholar]
- Lane BR, Gordon R. Regeneration of rat tracheal epithelium after mechanical injury. I. The relationship between mitotic activity and cellular differentiation. Proc Soc Exp Biol Med. 1974;145:1139–1144. doi: 10.3181/00379727-145-37968. [DOI] [PubMed] [Google Scholar]
- Reynolds SD, Giangreco A, Power JHT, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaingreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- Stevens TP, McBride JT, Peake JL, Pinkerton KE, Stripp BR. Cell proliferation contributes to PNEC hyperplasia after acute airway injury. Am J Physiol. 1997;272:L486–L493. doi: 10.1152/ajplung.1997.272.3.L486. [DOI] [PubMed] [Google Scholar]
- Stripp BR, Maxson K, Mera R, Singh G. Plasticity of airway cell proliferation and gene expression following acute naphthalene injury. Am J Physiol. 1995;269:L791–L799. doi: 10.1152/ajplung.1995.269.6.L791. [DOI] [PubMed] [Google Scholar]
- Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, Stripp BR. Conditional ablation of Clara cells in transgenic mice reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol. 2000;278:L1256–L1263. doi: 10.1152/ajplung.2000.278.6.L1256. [DOI] [PubMed] [Google Scholar]
- Keenan KP, Wilson TS, McDowell EM. Regeneration of hamster tracheal epithelium after mechanical injury. IV. Histochemical, immunocytochemical and ultrastructural studies. Virchows Arch B Cell Pathol. 1983;43:213–240. doi: 10.1007/BF02932958. [DOI] [PubMed] [Google Scholar]
- McDowell EM, Keenan KP, Huang M. Restoration of mucociliary tracheal epithelium following deprivation of vitamin A. A quantitative morphologic study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45:221–240. doi: 10.1007/BF02889866. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Allen ED, Wilson JM. Reconstitution of tracheal grafts with a genetically modified epithelium. Proc Natl Acad Sci USA. 1991;88:11192–11196. doi: 10.1073/pnas.88.24.11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JF, Schlossber H, Yankaskas JR, Dudus L. Progenitor cells of the adult human airway involved in submucosal gland development. Development. 1995;121:2031–2046. doi: 10.1242/dev.121.7.2031. [DOI] [PubMed] [Google Scholar]
- Liu JY, Nettesheim P, Randell SH. Growth and differentiation of tracheal epithelial progenitor cells. Am J Physiol. 1994;266:L296–L307. doi: 10.1152/ajplung.1994.266.3.L296. [DOI] [PubMed] [Google Scholar]
- Davies DE. The bronchial epithelium in chronic and severe asthma. Curr Allergy Asthma Rep. 2001;1:127–133. doi: 10.1007/s11882-001-0080-9. [DOI] [PubMed] [Google Scholar]
- Maestrelli P, Saetta M, Mapp CE, Fabbri LM. Remodeling in response to infection and injury. Airway inflammation and hypersecretion of mucus in smoking subjects with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:S76–S80. doi: 10.1164/ajrccm.164.supplement_2.2106067. [DOI] [PubMed] [Google Scholar]
- Dunnill MS. Carcinoma of the bronchus and lung. New York: Churchill Livingstone; Pulmonary Pathology. 1987:pp 333–401. [Google Scholar]
- Spencer H. Carcinoma of the lung. New York: Pergamon Press; Pathology of the Lung. 1985:pp 837–927. [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BR, Beddington R, Constantini F, Lacy E. Production of transgenic mice. Plainview, New York: Cold Spring Harbor Press; Manipulating the Mouse Embryoa Laboratory Manual. 1994:pp 226–250. [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Flint FF, Schulte BA, Spicer SS. Glycoconjugate with terminal α galactose. Histochemistry. 1986;84:387–395. doi: 10.1007/BF00482968. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Nishihara M, Kwaguchi S, Sakakura Y. Expression of phenotypic markers during regeneration of rat tracheal epithelium following mechanical injury. Am J Respir Cell Mol Biol. 1994;11:85–94. doi: 10.1165/ajrcmb.11.1.7517145. [DOI] [PubMed] [Google Scholar]
- Reynolds SD, Mango GW, Gelein R, Bøe IM, Lund J, Stripp BR. Normal function and lack of fibronectin accumulation in kidneys of Clara cell secretory protein/uteroglobin deficient mice. Am J Kidney Dis. 1999;33:541–551. doi: 10.1016/s0272-6386(99)70192-7. [DOI] [PubMed] [Google Scholar]
- Purkis PE, Steel JB, Mackenzie IC, Nathrath WBJ, Leigh IM, Lane EB. Antibody markers of basal cells in complex epithelia. J Cell Sci. 1990;97:39–50. doi: 10.1242/jcs.97.1.39. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I, Fuchs E. The cDNA sequence of a human epidermal keratin: divergence of sequence but conservation of structure among intermediate filament proteins. Cell. 1982;31:243–252. doi: 10.1016/0092-8674(82)90424-x. [DOI] [PubMed] [Google Scholar]
- Knapp AC, Rentrop M, Schweizer J, Winter H. Three cDNA sequences of mouse type I keratins. Cellular localization of the mRNAs in normal and hyperproliferative tissues. J Biol Chem. 1987;262:938–945. [PubMed] [Google Scholar]
- Van Winkle LS, Gunderson AD, Shimizu JA, Baker GL, Brown CD. Gender differences in naphthalene metabolism and naphthalene-induced acute lung injury. Am J Physiol. 2002;282:L1122–L1134. doi: 10.1152/ajplung.00309.2001. [DOI] [PubMed] [Google Scholar]
- Brocard J, Warot X, Wendling O, Messaddeq N, Vonesch JL, Chambon P, Metzger D. Spatio-temporally controlled site-specific somatic mutagenesis in the mouse. Proc Natl Acad Sci USA. 1997;94:14559–14563. doi: 10.1073/pnas.94.26.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood TD, Hancock DC, Danielian PS, Parker MG, Evan GI. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack RJ, Al-Ugaily LH, Morris G, Widdicombe JG. The distribution and structure of cells in the tracheal epithelium of the mouse. Cell Tissue Res. 1980;208:65–84. doi: 10.1007/BF00234174. [DOI] [PubMed] [Google Scholar]
- Peake JL, Reynolds SD, Stripp BR, Stephens KE, Pinkerton KE. Alteration of pulmonary neuroendocrine cells during epithelial repair of naphthalene-induced airway injury. Am J Pathol. 2000;156:1–8. doi: 10.1016/S0002-9440(10)64728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AR, Hook GER, Cameron GS, Jetten AM, Butterick CJ, Nettensheim P. The differentiation capacity of Clara cells isolated from the lungs of rabbits. Lab Invest. 1987;57:219–229. [PubMed] [Google Scholar]
- Hook GER, Brody AR, Cameron GS, Jetten AM, Gilmore LB, Nettensheim P. Repopulation of denuded tracheas by Clara cells isolated from the lungs of rabbits. Exp Lung Res. 1987;12:311–329. doi: 10.3109/01902148709062843. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Nettesheim P, Ramaekers FCS, Randell SH. Expression of “cell-type-specific” markers during rat tracheal epithelial regeneration. Am J Respir Cell Mol Biol. 1992;7:30–41. doi: 10.1165/ajrcmb/7.1.30. [DOI] [PubMed] [Google Scholar]
- Bucchieri F, Puddicombe SM, Lordan JL, Richter A, Buchanan D, Wilson SJ, Ward J, Zummo G, Howarth PH, Djukanovic R, Holgate ST, Davies DE. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Biol. 2002;27:179–185. doi: 10.1165/ajrcmb.27.2.4699. [DOI] [PubMed] [Google Scholar]
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- Hutton E, Paladini RD, Yu QC, Yen M, Coulombe PA, Fuchs E. Functional differences between keratins of stratified and simple epithelia. J Cell Biol. 1998;143:487–499. doi: 10.1083/jcb.143.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]