Abstract
A progressive destruction of the myocardium occurs in ∼30% of Trypanosoma cruzi-infected individuals, causing chronic chagasic cardiomyopathy, a disease so far without effective treatment. Syngeneic bone marrow cell transplantation has been shown to cause repair and improvement of heart function in a number of studies in patients and animal models of ischemic cardiopathy. The effects of bone marrow transplant in a mouse model of chronic chagasic cardiomyopathy, in the presence of the disease causal agent, ie, the T. cruzi, are described herein. Bone marrow cells injected intravenously into chronic chagasic mice migrated to the heart and caused a significant reduction in the inflammatory infiltrates and in the interstitial fibrosis characteristics of chronic chagasic cardiomyopathy. The beneficial effects were observed up to 6 months after bone marrow cell transplantation. A massive apoptosis of myocardial inflammatory cells was observed after the therapy with bone marrow cells. Transplanted bone marrow cells obtained from chagasic mice and from normal mice had similar effects in terms of mediating chagasic heart repair. These results show that bone marrow cell transplantation is effective for treatment of chronic chagasic myocarditis and indicate that autologous bone marrow transplant may be used as an efficient therapy for patients with chronic chagasic cardiomyopathy.
The discovery of the pluripotency of adult bone marrow stem cells has opened new perspectives for the treatment of patients with chronic degenerative diseases for which there are no effective treatments available.1 This is the case of chronic chagasic cardiomyopathy (CChC), caused by the Trypanosoma cruzi protozoan and one of the leading causes of heart failure in Latin America. CChC is characterized by a diffuse inflammatory reaction composed mainly of mononuclear cells, leading to myocytolysis and to heart malfunctioning and enlargement.2 Most T. cruzi-infected individuals live throughout their lives without showing any signs or symptoms of the disease, in a subclinical state called the indeterminate form of Chagas’ disease.2 The mechanisms driving the development of CChC in ∼30% of T. cruzi-infected individuals are not clear. In the chronic phase of the disease, the finding of T. cruzi in blood or in the affected organs is a very rare event. In the past few years, several reports have provided strong evidence for an autoimmune component targeted to heart antigens in the pathogenesis of CChC.3–5 The disease progressively debilitates the patient, who finally evolves to a clinical state in which heart transplantation is the only option for treatment. However, heart transplantation in chagasic patients, in addition to its high financial costs and failure ratio, brings about the additional complication of relapse of the acute T. cruzi infection because of the transplantation-related immunosuppressive treatment.6,7
Adult bone marrow contains pluripotent stem cells with the ability to differentiate into diverse cell types, including neurons, adipocytes, hepatocytes, chondrocytes, and muscle cells.1,8 The regenerative potential of bone marrow stem cells has been tested in experimental models of ischemic cardiomyopathy, yielding very promising results. In these models, the proliferation of myofibers, angiogenesis, vasculogenesis, and improvement of cardiac function were observed after treatment of the ischemic myocardial lesions with bone marrow cells (BMCs).9–11 The potential use of BMC therapy was also tested in patients with ischemic cardiomyopathy.12–14 In the present report, we describe the effects of adult bone marrow transplant in a model of dilated cardiomyopathy of infectious etiology. Our results show the efficacy of BMC therapy in chagasic myocarditis and indicate that it may be efficient for treating this and similar dilated cardiomyopathies in humans.
Materials and Methods
Mice and Parasite
Specific pathogen-free, 8-week-old female or male BALB/c and C57BL/6 mice were used for T. cruzi infection and as noninfected controls. BALB/c, C57BL/6, C57BL/6 EGFP, and B6.129 Gtrosa26 transgenic mice were used as BMC donors. Gtrosa26 transgenic mice breeder pairs were purchased from the Jackson Laboratory, Bar Harbor, ME. All mice were raised and maintained at the animal facilities at the Gonçalo Moniz Research Center–FIOCRUZ/BA, and provided with rodent food and water ad libitum. Trypomastigotes of the myotropic Colombian T. cruzi strain15 were obtained from culture supernatants of infected LLC-MK2 cells.
Induction of Chagasic Myocarditis
Infection of BALB/c and C57BL/6 mice was performed by the inoculation of 100 and 1000 T. cruzi trypomastigotes in saline, respectively, by the intraperitoneal route. Infected mice were followed-up by parasitemia evaluation at various times after infection and after BMC transplantation, through counting the number of trypomastigotes in peripheral blood aliquots. All T. cruzi-infected mice used in the work had positive acute-phase parasitemia and anti-T. cruzi serology. All nontransplanted infected mice developed myocarditis, already present at the moment of bone-marrow transplantation.
BMC Transplantation
BMCs obtained from femurs of 4- to 6-week-old BALB/c, C57BL/6 EGFP, and B6.129 Gtrosa26 transgenic mice were used in transplantation experiments. BMCs were purified by centrifugation in Ficoll gradient at 1000 × g for 15 minutes (Histopaque 1119 and 1077, 1:1; Sigma, St. Louis, MO). After two washings in RPMI medium (Sigma), the cells were resuspended in saline, filtered over nylon wool, and injected intravenously in chagasic mice (3 × 106 cells/mouse). Total BMCs were washed twice with RPMI, resuspended in saline, and injected intravenously in chagasic mice (2 × 107 cells per mouse). In some experiments, groups of chagasic mice received transplant of BMCs obtained from chronically infected littermates. Nontransplanted control mice received intravenous injections of the same volume (200 μl) of saline. The percentage of Ficoll-purified c-kit+ cells was determined by flow cytometry using an anti-c-kit fluorescein isothiocyanate conjugate purchased from PharMingen (San Diego, CA). Analysis was done using a FACScalibur (Beckton-Dickinson, San Jose, CA).
Histopathology
Groups of chagasic and control BALB/c and C57BL/6 mice were sacrificed at various time points after bone marrow transplant. Hearts were removed and fixed in 4% buffered formalin. Sections were analyzed by light microscopy after paraffin-embedding and standard hematoxylin and eosin staining. Inflammatory cells infiltrating heart tissue were counted using a digital morphometric evaluation. Images were digitalized using a color digital video camera (CoolSnap cf, Media Cybernetics, Carlsbad, CA) adapted to an AX-70 microscope (Olympus, Tokyo, Japan), with motorized xyz stage (Media Cybernetics). The images were analyzed using the Image Pro Program (Media Cybernetics), so that the inflammatory cells were counted and integrated by area. The percentage of fibrosis was determined in Masson’s trichrome-stained heart sections using the Image Pro Program, to integrate the areas of fibrotic and nonfibrotic tissue in each field. Ten fields per section, in 5 to 10 sections per heart, were counted. The number of fibrosis foci was counted in 28 to 32 fields and expressed as number of foci per 6 mm2.
Detection of Donor Cells by Immunofluorescence
Hearts of BMC-transplanted mice and control chagasic mice were collected at various times after transplantation. Five-μm frozen sections were prepared and fixed in cold acetone. To detect the presence in the heart tissue of transplanted BMCs, obtained from EGFP or Gtrosa26 transgenic mice, sections were incubated with fluorescein isothiocyanate-conjugated anti-GFP antibody (Santa Cruz Biotechnologies, Santa Cruz, CA) and a biotin-conjugated anti-β-Gal antibody (Sigma), followed by a streptavidin-fluorescein isothiocyanate conjugate. Some sections were double-stained with the anti-β-Gal-biotin, followed by the fluorescein isothiocyanate conjugate, and with a biotin-conjugated anti-myosin (American Qualex, San Clemente, CA), revealed with streptavidin-cychrome 3 (PharMingen). A biotin blocking kit (Vector Laboratories, Burlingame, CA) was used before and after staining with anti-β-Gal in double-stained sections. Sections were counterstained with a 0.0001% Evans blue solution. In all cases, negative control reactions, lacking the primary antibodies, were performed, producing completely negative staining (no visible fluorescence). The presence of fluorescent cells was determined by observation in an AX-70 microscope with an epifluorescence system, using appropriate filters (Olympus).
Detection of Apoptotic Cells
To determine the presence of apoptotic cells in heart tissue, 5-μm paraffin-embedded sections of heart, from control or BMC-treated infected BALB/c mice, were examined, by means of a terminal dUTP nick-end labeling (TUNEL)-based in situ cell death detection, TACS TdT kit fluorescein (R&D Systems, Minneapolis, MN), according to the manufacturer’s instructions. In brief, cell membranes were permeabilized with a proteinase K solution to make DNA accessible to the labeling enzyme. Endogenous peroxidase activity was quenched using hydrogen peroxide. Biotinylated nucleotides were then incorporated into the 3′-OH ends of the DNA fragments by terminal deoxynucleotidyl transferase, and detected by using a streptavidin-fluorescein conjugate. Sections were incubated with TO-PRO-3 dye for nuclear staining (Molecular Probes, Eugene, OR) and analyzed in a FV-500 confocal system with IX 81 inverted microscope (Olympus).
Statistical Analyses
Data were analyzed using Student’s t-test, one-way analysis of variance, or Newman-Keuls multiple comparison test, as indicated in the text. Differences were considered significant when P was <0.05.
Results
Syngeneic BMCs from Normal Mice Cause the Healing of Damaged Myocardium in Chronic Chagasic Mice without Affecting Parasitism
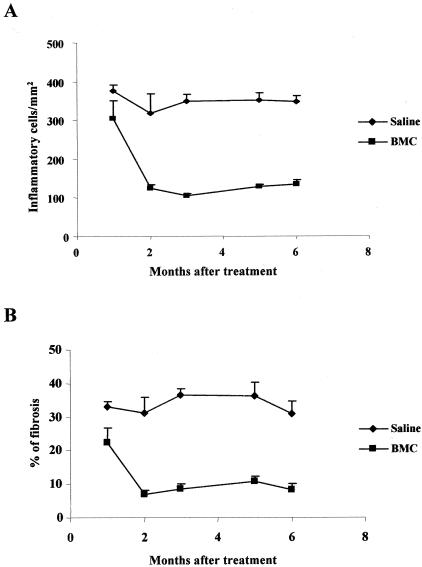
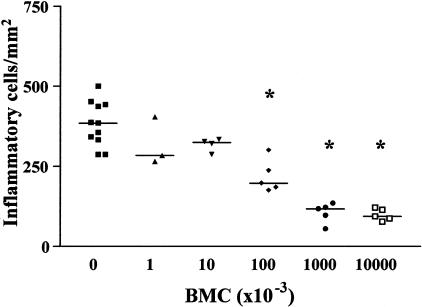
Hearts of chagasic BALB/c mice, treated with Ficoll gradient-purified BMCs 6 months after T. cruzi infection, had a small, but significant, decrease in the number of inflammatory cells and in the intensity of interstitial fibrosis 1 month after BMC therapy (Figure 1; P < 0.05, Newman-Keuls multiple comparison test). A marked decrease in number of inflammatory cells was observed 2 months after transplantation, when compared with those of control infected mice (Figure 1A and Figure 2, A and B; P < 0.001). There was a threefold reduction in area of fibrosis observed in the hearts of BMC-treated mice at that time (Figure 1B and Figure 2, C and D; P < 0.001, Newman-Keuls multiple comparison test). Fibrotic foci were reduced both in size and in numbers after BMC transplantation (saline-injected, 17 ± 5 foci/6 mm2; BMC-treated, 10 ± 4 foci/6 mm2). Hearts of BMC-treated mice remained with significantly less (threefold to fourfold) inflammation and fibrosis, 5 and 6 months after transplantation, compared to those of control chagasic mice (Figure 1). Transplantation of 107 or 106 purified BMCs caused a similar improvement in chronic chagasic myocarditis (71 to 75% reduction in the numbers of inflammatory cells), whereas treatment of chagasic mice with 105 BMCs caused a reduction of 43% in the number of inflammatory cells (Figure 3). No significant effects were observed in mice treated with 104 or less cells (Figure 3).
Figure 1.
Reduction of inflammation and fibrosis in established chronic chagasic myocarditis after BMC transplantation. BALB/c mice were infected with 100 trypomastigotes of T. cruzi and treated 6 months later with 3 × 106 per mouse of Ficoll-purified BMCs obtained from normal BALB/c mice by the intravenous route. Groups of control (saline-injected) or BMC-treated chagasic mice were sacrificed 1, 2, 3, 5, and 6 months after treatment. Heart sections were stained with conventional H&E stain for analysis of inflammatory infiltrate (A) or with Masson’s trichrome stain for quantification of interstitial fibrosis (B). Vertical bars represent the standard deviations of the means of the results obtained from four to six mice.
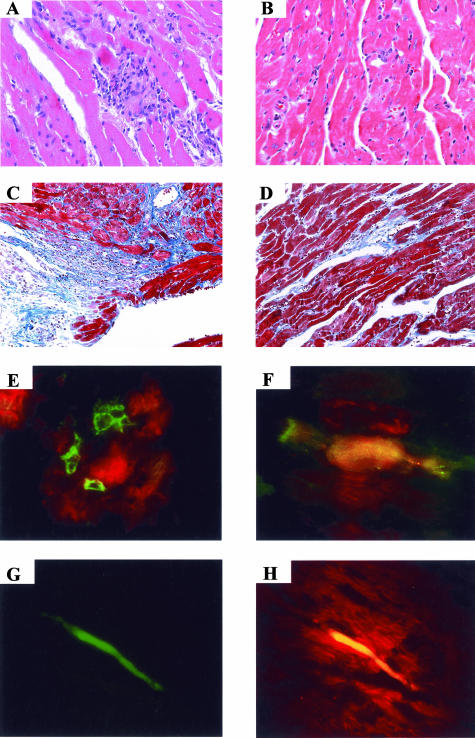
Figure 2.
Heart sections of saline-injected and BMC-treated chronic chagasic mice. BALB/c mice were transplanted intravenously with Ficoll-purified BMCs (3 × 106/mouse) from normal BALB/c donors 10 months after T. cruzi infection. Two months later control (saline-injected) mice (A and C) or BMC-treated mice (B and D) were sacrificed. Heart sections were prepared and stained with H&E (A and B) or Masson’s trichrome staining (C and D). E: Heart sections from C57BL/6 chagasic mice (8 months of infection) sacrificed 2 days after transplantation with Ficoll-purified BMCs obtained from Gtrosa26 transgenic mice. The section was double stained with anti-β-Gal (green) and anti-myosin (red). F–H: Heart sections of chagasic C57BL/6 mice (8 months of infection) sacrificed 4 days after transplantation with Ficoll-purified BMCs (3 × 106/mouse) obtained from EGFP transgenic mice. F and G: EGFP (green) in heart section (counterstained with Evans blue in F). H: Combination of EGFP (green) and myosin (red). Original magnifications: ×100 (C, D); ×200 (A, B, E, G, H); ×400 (F).
Figure 3.
Dose-response effect of transplanted BMCs in myocardial repair. Groups of BALB/c mice were transplanted with the numbers of Ficoll-purified BMCs, obtained from normal donors, indicated on the x axis. The number of inflammatory cells per mm2 was determined by analysis of H&E 2 months after transplantation. Each symbol represents data obtained from an individual mouse. The horizontal lines represent the arithmetic mean of each group of mice. *, P < 0.001, in relation to control (saline-injected, no cells) group; Newman-Keuls multiple comparison test.
BALB/c mice with well-established chronic chagasic lesions, 18 months after infection, also had their myocarditis greatly ameliorated by treatment with 2 × 107 syngeneic BMCs. Two months after transplantation, the hearts of these mice had ∼80% less inflammatory cells than those of control mice, which had an intense myocarditis (saline-injected control, 317.7 ± 49.9 cells/mm2; BMC-treated, 66.5 ± 22.4 cells/mm2; P < 0.001, Newman-Keuls multiple comparison test). Similarly, areas of fibrosis were rarely seen in heart sections of these BMC-treated chagasic mice, whereas the corresponding control chagasic mice had extensive areas of fibrosis (saline-injected control, 38.7 ± 3.0%; BMC-treated, 3.9 ± 1.0%; P < 0.001, Newman-Keuls multiple comparison test). No parasites could be observed either in the blood or in heart sections 1, 2 and 4 weeks and 2 to 6 months after BMC transplantation, by optical microscopy (not shown).
Syngeneic BMCs from Normal and Chagasic Donors Have Similar Effects in the Healing of Damaged Myocardium in Chronic Chagasic Mice
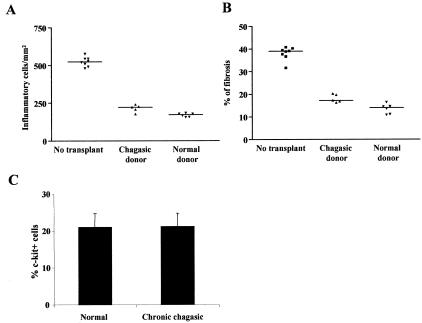
The efficacy of BMCs obtained from normal and from chronic chagasic mice were compared. Heart sections from C57BL/6 mice that received BMC transplantation from chronically infected littermates 4 months after T. cruzi infection had significantly less inflammatory cells and tissue fibrosis than sections of control mice 2 months after transplantation (Figure 4, A and B). The percentage of c-kit+ cells in BMC preparations obtained from normal and chagasic donors were similar (Figure 4C).
Figure 4.
BMCs obtained from chagasic donors and from normal donors have similar effects on myocarditis healing and percentages of c-kit+ cells. A and B: C57BL/6 chagasic mice with myocarditis (4 months after infection with 1000 T. cruzi trypomastigotes) were either injected with saline only (no transplant) or treated with Ficoll-purified BMCs (3 × 106/mouse) obtained from normal (normal donor) or from chagasic (chagasic donor) littermates. Nontransplanted chagasic mice and BMC-treated mice were sacrificed 6 months after infection. The numbers of inflammatory cells per mm2 (A) and the percentages of fibrosis (B) in the hearts were determined by analysis of H&E and of Masson’s trichrome-stained sections, respectively, 2 months after BMC treatment. Each symbol represents data obtained from an individual mouse. The horizontal lines represent the arithmetic mean of each group of mice. C: Percentage of c-kit+ cells in Ficoll-purified BMCs obtained from normal and T. cruzi-infected (6 months) (chronic chagasic) mice. Vertical bars represent the standard errors of the means of the results obtained from three mice per group.
Migration to Heart and Myosin Expression of Intravenously Injected BMCs in Chronic Chagasic Mice
Transplanted β-Gal+ cells were observed near cardiomyocytes 2 days after injection of Ficoll-purified BMCs from Gtrosa26 mice into chagasic mice (Figure 2E). When Ficoll-purified BMCs from EGFP-transgenic mice were injected into C57BL/6 chagasic mice, these cells could be observed in their hearts 4 days after BMC injection (Figure 2, F and G). A proportion (∼10%) of transplanted EGFP+ cells were also positive for cardiac myosin (Figure 2H). β-Gal+ and GFP+ cells were not observed in the hearts of normal mice transplanted with BMCs from Gtrosa26 or EGFP-transgenic mice (not shown).
Inflammatory Cells Undergo Apoptosis after BMC Transplant
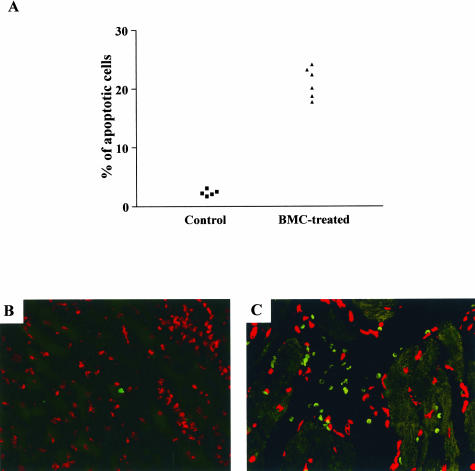
The number of apoptotic inflammatory cells was determined by TUNEL immunofluorescence assay. Chagasic mouse hearts had 10-fold higher numbers of apoptotic cells 1 month after BMC therapy than hearts of control chagasic mice, in which rare positive cells were observed per section (Figure 5; P < 0.01, Student’s t-test). Using the same methodology, no β-Gal+ cells undergoing apoptosis were observed in the hearts of chagasic mice transplanted with BMCs obtained from Gtrosa26 mice (data not shown).
Figure 5.
Inflammatory cells undergo apoptosis after BMC transplantation in chronic chagasic mice. BALB/c mice infected with T. cruzi (18 months of infection) were intravenously treated with BMCs (2 × 107cells/mouse) and sacrificed 1 month later. Heart sections of control (saline-injected) or BMC-treated chagasic mice were incubated with a TUNEL-based assay for apoptosis detection. A: The percentages of apoptotic inflammatory cells were determined. Each symbol represents data obtained from an individual mouse. B and C: Confocal microscopy images of heart sections stained of control (B) and BMC-treated (C) mice 1 month after transplantation after staining for apoptotic cells (green) followed by nuclear staining with TO-PRO-3 dye (red). Original magnifications, ×400.
Discussion
Research performed throughout the past few years has revealed the potential of BMC therapy in restoring hearts damaged by ischemic injuries.9–14 In this report we demonstrate the recovery, after bone marrow transplantation, of hearts with an ongoing inflammatory process because of infection by T. cruzi. The recovery involved the clearance of inflammatory cells and possibly the remodeling of damaged tissue through the regeneration of myocytes and resorption of interstitial fibrosis. This effect could be observed for at least 6 months after BMC transplantation, as demonstrated in our work. More importantly, BMCs obtained from age-matched chronic chagasic mice had similar recovery induction potential to that of BMCs obtained from uninfected mice. In this and in other disease models, further investigation is needed to fully understand the mechanisms involved in the remodeling processes triggered by cardiac or peripheral injection of BMCs.
The decrease in the heart inflammatory infiltrate of BMC-treated animals seems to correlate well with the increased apoptosis of inflammatory cells. Because the majority of cells injected during BMC transplantation are lymphocytes and monocytes, it could be proposed that most of the apoptotic cells in the transplanted animals’ hearts were of donor origin. However, this is clearly not the case, because in mice transplanted with BMCs from EGFP and Gtrosa26 transgenic donors, all of the apoptotic inflammatory cells could be shown to be of host origin. This may imply that the donor cells that migrate to the T. cruzi-infected hearts are really stem cells, which would not undergo apoptosis themselves but would be able to induce, through an as yet unknown, direct, or indirect mechanism, apoptosis in the resident inflammatory cells.
The fact that ∼70% of the T. cruzi-infected individuals remain throughout their lives in a subclinical state called the indeterminate form of the disease is a well-known feature of T. cruzi infection. However, careful observations of heart tissues in experimental animals during the indeterminate form of the disease indicate that a pathological process is in progress, but it is counterbalanced by a healing process: foci of inflammation are somehow modulated and damaged tissue is repaired, resulting in absence of disease.16 The finding in chronic chagasic hearts of only small numbers of apoptotic inflammatory cells is consistent with the hypothesis that apoptosis of inflammatory cells plays a role in keeping the development of disease in check.17 Thus, in those individuals in whom spontaneous repair would not be sufficient to prevent progressive damage, a cell-based therapy such as the one demonstrated herein would perhaps restore the equilibrium state found in asymptomatic individuals.
BMC therapy has been successfully used in patients with autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis.18–21 The mechanisms by which autologous BMC transplant regulates the immune-inflammatory process in these cases are unknown, but they might also come into operation in our model of Chagas’ disease, in which an important autoimmune component has been demonstrated.3–5 Thus, the benefits of BMC transplantation may result from tissue remodeling as well as from regulation of pathological immune responses. In fact, recent reports have demonstrated that BMCs inhibit T-cell responses.22,23 We are currently investigating whether BMC transplantation affects the immune response in chronic chagasic mice. The fact, described herein, that there was no evident increase in blood or heart parasitism in the BMC-transplanted animals indicates that the transplantation did not cause a marked nonspecific down-regulation of the immune system.
In the present work, the migration of transplanted BMCs to the myocardium of chagasic mice was demonstrated. The possibility that endothelial alterations in cardiac vasculature may signal to circulating stem cells so as to cause their tissue immigration, in this and in other pathological conditions, is open to investigation. A recent report showing that BMCs differentiate into cardiomyocytes and endothelial cells in a model of ischemic cardiomyopathy10 is consistent with the finding of a proportion of the transplanted cells in chagasic hearts expressing the muscle marker, myosin, reported herein. The presence of cardiomyocytes bearing the host’s phenotype in heart-transplanted patients24 also indicates that migrant stem cells can home-in and differentiate into the myocardium. The possibility of cell fusion between transplanted cells and host myocytes, however, cannot be excluded. In addition, it has still to be ascertained whether a differentiation of stem cells into myocytes, and/or a possible stem cell-mediated stimulation of the division of resident cells in the myocardium, play a role in the myocardium regeneration.
A recent study demonstrated that c-kit+ BMCs are responsible for tissue regeneration in a myocardial infarct model.10 In our study we found that BMC preparations obtained from normal and from chronic chagasic mice had similar numbers of c-kit+ cells. The role played by this BMC subpopulation in tissue regeneration in chagasic cardiomyopathy is still unknown.
BMC-treated mice had a marked reduction in the percentage of fibrotic areas. Myocardium damage leads to inappropriate collagen production and development of fibrosis, causing heart dysfunction.25 Several factors regulate the process of extracellular matrix degradation and collagen deposition, including cytokines, metalloproteinases, and peptides.25 The elevated production of the proinflammatory cytokine tumor necrosis factor-α in the heart causes the development of fibrosis and of dilated cardiomyopathy.26–29 Tumor necrosis factor-α-producing cells were detected in hearts of mice and of patients with chagasic cardiomyopathy.30–33 The reduction of fibrosis by BMC transplantation shown herein could be perhaps ascribed to its effect on inflammation, possibly decreasing the number of inflammatory, tumor necrosis factor-α-producing cells in the heart.
The potential use of bone marrow stem cells in therapies for chronic degenerative diseases has been recently extended with the identification of a bone marrow stem cell population bearing a plasticity compared to that of embryonic stem cells.34 The healing-promoting activity of BMCs on chagasic hearts described herein indicates that autologous BMC transplant should be considered as a possible treatment for CChC. More importantly, the study was performed using methodologies permitted for clinical use, such as transplantation of total and Ficoll-purified BMCs.
Acknowledgments
We thank Dr. Masaru Okabe who kindly provided breeder pairs of EGFP transgenic C57BL/6 mice and Fabíola N. Conceição for a careful review of this manuscript.
Footnotes
Address reprint requests to Ricardo Ribeiro dos Santos, Centro de Pesquisas Gonçalo Moniz, FIOCRUZ/BA, Rua Waldemar Falcão, 121—Brotas, Salvador, BA, CEP: 40295-001, Brazil. E-mail: rrsantos@cpqgm.fiocruz.br.
Supported by grants from the Brazilian Ministry of Science and Technology (Brazilian National Research Council, CNPq, Millenium Institute of Tissue Bioengineering) and NIH (HL073732-01).
References
- Krause DS. Plasticity of marrow-derived stem cells. Gene Ther. 2002;9:754–758. doi: 10.1038/sj.gt.3301760. [DOI] [PubMed] [Google Scholar]
- Köberle F. Chagas’ disease and Chagas’ syndromes: the pathology of American trypanosomiasis. Adv Parasitol. 1968;6:63–116. doi: 10.1016/s0065-308x(08)60472-8. [DOI] [PubMed] [Google Scholar]
- Pontes-de-Carvalho L, Santana CC, Soares MBP, Oliveira GG, Cunha-Neto E, Ribeiro-dos-Santos R. Experimental chronic Chagas’ disease myocarditis is an autoimmune disease preventable by induction of immunological tolerance to myocardial antigens. J Autoimmun. 2002;18:131–138. doi: 10.1006/jaut.2001.0574. [DOI] [PubMed] [Google Scholar]
- Soares MBP, Pontes-de-Carvalho L, Ribeiro-dos-Santos R. The pathogenesis of Chagas’ disease: when autoimmune and parasite-specific immune responses meet. An Acad Bras Cienc. 2001;73:547–559. doi: 10.1590/s0001-37652001000400008. [DOI] [PubMed] [Google Scholar]
- Leon JS, Godsel LM, Wang K, Engman DM. Cardiac myosin autoimmunity in acute Chagas’ heart disease. Infect Immun. 2001;69:5643–5649. doi: 10.1128/IAI.69.9.5643-5649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchi EA, Bellotti G, Mocelin AO, Uip D, Bacal F, Higuchi ML, Amato-Neto V, Fiorelli A, Stolf NA, Jatene AD, Pileggi F. Heart transplantation for chronic Chagas’ heart disease. Ann Thorac Surg. 1996;61:1727–1733. doi: 10.1016/0003-4975(96)00141-5. [DOI] [PubMed] [Google Scholar]
- Stolf NA, Higushi L, Bocchi E, Bellotti G, Auler JO, Uip D, Amato Neto V, Pileggi F, Jatene AD. Heart transplantation in patients with Chagas’ disease cardiomyopathy. J Heart Transplant. 1987;6:307–312. [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauer BE, Brehm M, Zeus T, Gattermann N, Hernandez A, Sorg RV, Kogler G, Wernet P. Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction. Dtsch Med Wochenschr. 2001;126:932–938. doi: 10.1055/s-2001-16579-2. [DOI] [PubMed] [Google Scholar]
- Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, Schumichen C, Nienaber CA, Freund M, Steinhoff G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, Silva GV, Belem L, Vivacqua R, Rangel FO, Esporcatte R, Geng YJ, Vaughn WK, Assad JA, Mesquita ET, Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- Federici EE, Abelmann WB, Neva FA. Chronic and progressive myocarditis in C3H mice infected with Trypanosoma cruzi. Am J Trop Med Hyg. 1964;13:272–280. doi: 10.4269/ajtmh.1964.13.272. [DOI] [PubMed] [Google Scholar]
- Andrade ZA. Immunopathology of Chagas disease. Mem Inst Oswaldo Cruz. 1999;94:71–80. doi: 10.1590/s0074-02761999000700007. [DOI] [PubMed] [Google Scholar]
- Rossi MA, Souza AC. Is apoptosis a mechanism of cell death of cardiomyocytes in chronic chagasic myocarditis?. Int J Cardiol. 1999;68:325–331. doi: 10.1016/s0167-5273(98)00375-1. [DOI] [PubMed] [Google Scholar]
- Moore J, Tyndall A, Brooks P. Stem cells in the aetiopathogenesis and therapy of rheumatic disease. Best Pract Res Clin Rheumatol. 2001;15:711–726. doi: 10.1053/berh.2001.0189. [DOI] [PubMed] [Google Scholar]
- Traynor AE, Barr WG, Rosa RM, Rodriguez J, Oyama Y, Baker S, Brush M, Burt RK. Hematopoietic stem cell transplantation for severe and refractory lupus. Analysis after five years and fifteen patients. Arthritis Rheum. 2002;46:2917–2923. doi: 10.1002/art.10594. [DOI] [PubMed] [Google Scholar]
- El-Badri NS, Wang BY, Steele A, Cherry, Marikar Y, Mizobe K, Good RA. Successful prevention of autoimmune disease by transplantation of adequate number of fully allogeneic hematopoietic stem cells. Transplantation. 2000;70:870–877. doi: 10.1097/00007890-200009270-00004. [DOI] [PubMed] [Google Scholar]
- Kim KC, Lee IH, Choi JH, Oh MR, Ahn MJ, Kim SY. Autologous stem cell transplantation in the treatment of refractory rheumatoid arthritis. J Korean Med Sci. 2002;17:129–132. doi: 10.3346/jkms.2002.17.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naïve and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- Weber KT, Brilla CG, Campbell SE, Zhou G, Matsubara L, Guarda E. Pathologic hypertrophy with fibrosis: the structural basis for myocardial failure. Blood Press. 1992;1:75–85. doi: 10.3109/08037059209077497. [DOI] [PubMed] [Google Scholar]
- Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- Matsumori A, Yamada T, Suzuki H, Matoba Y, Sasayama S. Increased circulating cytokines in patients with myocarditis and cardiomyopathy. Br Heart J. 1994;72:561–566. doi: 10.1136/hrt.72.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- Li YY, Feng YQ, Kadokami T, McTiernan CF, Draviam R, Watkins SC, Feldman AM. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc Natl Acad Sci USA. 2000;97:12746–12751. doi: 10.1073/pnas.97.23.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunnemark D, Ulfgren AK, Orn A, Harris RA. Cytokine production in hearts of Trypanosoma cruzi-infected CBA mice: do cytokine patterns in chronic stage reflect the establishment of myocardial pathology? Scand J Immunol. 1996;44:421–429. doi: 10.1046/j.1365-3083.1996.d01-328.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Tarleton RL. Persistent production of inflammatory and anti-inflammatory cytokines and associated MHC and adhesion molecule expression at the site of infection and disease in experimental Trypanosoma cruzi infections. Exp Parasitol. 1996;84:203–213. doi: 10.1006/expr.1996.0106. [DOI] [PubMed] [Google Scholar]
- Cunha-Neto E, Rizzo LV, Albuquerque F, Abel L, Guilherme L, Bocchi E, Bacal F, Carrara D, Ianni B, Mady C, Kalil J. Cytokine production profile of heart-infiltrating T cells in Chagas’ disease cardiomyopathy. Braz J Med Biol Res. 1998;31:133–137. doi: 10.1590/s0100-879x1998000100018. [DOI] [PubMed] [Google Scholar]
- Reis MM, Higuchi ML, Benvenuti LA, Aiello VD, Gutierrez PS, Bellotti G, Pileggi F. An in situ quantitative immunohistochemical study of cytokines and IL-2R+ in chronic human chagasic myocarditis: correlation with the presence of myocardial Trypanosoma cruzi antigens. Clin Immunol Immunopathol. 1997;83:165–172. doi: 10.1006/clin.1997.4335. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]