Abstract
We recently identified mutations in the fukutin related protein (FKRP) gene in patients with congenital muscular dystrophy type 1C (MDC1C) and limb girdle muscular dystrophy type 2I (LGMD2I). The sarcolemma of these patients typically displays an immunocytochemical reduction of α-dystroglycan. In this report we extend these observations and report a clear correlation between the residual expression of α-dystroglycan and the phenotype. Three broad categories were identified. Patients at the severe end of the clinical spectrum (MDC1C) were compound heterozygote between a null allele and a missense mutation or carried two missense mutations and displayed a profound depletion of α-dystroglycan. Patients with LGMD with a Duchenne-like severity typically had a moderate reduction in α-dystroglycan and were compound heterozygotes between a common C826A (Leu276Ileu) FKRP mutation and either a missense or a nonsense mutation. Individuals with the milder form of LGMD2I were almost invariably homozygous for the Leu276Ile FKRP mutation and showed a variable but subtle alteration in α-dystroglycan immunolabeling. Our data therefore suggest a correlation between a reduction in α-dystroglycan, the mutation and the clinical phenotype in MDC1C and LGMD2I which supports the hypothesis that dystroglycan plays a central role in the pathogenesis of these disorders.
The dystroglycan complex is widely distributed and has been implicated in development, cell adhesion, and signaling in both muscle and various non-muscle tissues.1–3 A single gene DAG1 encodes for a polypeptide that is posttranslationally modified to yield the two glycoproteins referred to as α- and β-dystroglycan.4 α-dystroglycan is a membrane-associated extracellular glycoprotein and binds to the laminin α2 chain, perlecan, biglycan, neurexin, and agrin within the extracellular matrix,5–7 whereas β-dystroglycan is transmembrane and links α-dystroglycan to the actin cytoskeleton via either dystrophin or utrophin.8 The disruption of this linkage underlies several forms of muscular dystrophy,9 underscoring its importance in striated muscle and contribution to the structural integrity of the sarcolemma.10
α-dystroglycan together with integrin α7β1D form the two main laminin receptors in skeletal muscle. Work in ES cells shows that both contribute to the preferential polymerization of laminin on the cell surface during the early stages of basement membrane formation11–14; however, rather than being uniquely required for basement membrane assembly recent evidence suggests that these receptors may instead act to control the turnover of major basement membrane components.15 Consistent with this, chimeric mice, in which the deficiency in dystroglycan is restricted to skeletal muscle, show an apparently normal deposition of laminin but do develop a progressive muscle pathology together with defects at the neuromuscular junction.16 While genetic defects of dystroglycan have not as yet been identified, it is well recognized that both α- and β-dystroglycan are secondarily but markedly reduced at the sarcolemma of both patients with Duchenne Muscular Dystrophy (DMD) and dystrophin deficient mdx mice.17 A reduction of α- and β-dystroglycan has also been demonstrated in the various sarcoglycan-deficient limb girdle muscular dystrophies and their respective animal models,18,19 although others have reported that β-dystroglycan is not perturbed in these conditions.18,20 None of these disorders has been associated with any alteration in the expression of laminin α2 chain within the extracellular matrix.
More recently a number of muscular dystrophies caused by mutations in putative glycosyltransferases have been described. These disorders are associated with marked alterations in the glycosylation of α-dystroglycan and have been shown to be due to genetic defects in fukutin, (Fukuyama congenital muscular dystrophy, FCMD), POMGnT1 (Muscle-Eye-Brain disease, MEB), POMT1 (Walker-Warburg syndrome, WWS),21 and fukutin related protein (FKRP) (congenital muscular dystrophy 1C, MDC1C and limb girdle muscular dystrophy 2I, LGMD2I). Our group first reported a series of mutations in the FKRP gene which are associated with an unusually wide clinical spectrum. These include children who present at birth and never acquire the ability to walk, patients with a severe, Duchenne-like LGMD phenotype (severe end of the LGMD2I spectrum) and patients whose symptoms only start in the third or fourth decade of life and follow a significantly milder clinical course (mild LGMD2I). We also demonstrated a reduction in the immunolabeling of α- but not β-dystroglycan,22,23 and a variable reduction in the laminin α2 chain. These features are similar to those previously reported for FCMD, WWS and MEB.
To determine whether the observed alterations in α-dystroglycan are likely to be central to the pathogenesis of MDC1C and LGMD2I, we characterized in detail the pattern of expression of this and other proteins in a number of patients in whom the FKRP gene mutation has been identified. Using four different antibodies, two of which were directed toward the core protein, we demonstrated a severe depletion of α-dystroglycan in all patients with MDC1C phenotype, accompanied by a moderate reduction of the laminin α2 chain. A significant depletion of α-dystroglycan and a variable reduction of the laminin α2 chain was also identified in patients at the severe end of the LGMD2I spectrum. Patients with milder LGMD2I disease course, however, displayed normal immunolabeling of the laminin α2 chain with a variable but only subtle alteration in α-dystroglycan. The results of the molecular genetic studies were correlated with the clinical and pathological findings and allowed us to recognize three groups of patients: 1) MDC1C, which were either compound heterozygotes for 1 nonsense and 1 missense or homozygotes for missense mutations, 2) LGMD21 severe (DMD-like) which had the leu276Ile mutation plus one null mutation (but not exclusively null), and 3) LGMD2I mild, which almost invariably were homozygotes for the common Leu276Ileu mutation. This latter mutation appears to be a mild mutation as the patients homozygous for it had the milder phenotype and better preserved α-dystroglycan staining while it was never observed in patients with MDC1C. These results suggest that the perturbation of the dystroglycan axis plays a central role in the pathogenesis of MDC1C and LGMD2I.
Materials and Methods
Clinical Description of FKRP Families
The clinical features of children with MDC1C have previously been reported.23 Briefly, they presented soon after birth with hypotonia and feeding difficulties, never acquired the ability to walk because of severe weakness, which also affected the facial muscles. Calf hypertrophy was common together with a markedly elevated serum creatine kinase (CK). Cognitive development, intelligence, and vision were normal, as was brain MRI, while cardiac studies showed evidence of left ventricular impairment in several children. The clinical features of patients with LGMD2I have also been previously described.22,24 This ranged from one patient presenting in the first few years of life and following a Duchenne-like course, with loss of independent ambulation in the teens, to a later presentation and slow progression with patients remaining ambulant well into adult life. Mild facial weakness and neck flexion weakness was present in most patients. Calf and, to a lesser extent, brachioradialis hypertrophy were common features and proximal muscles were weaker than distal muscles. A dilated cardiomyopathy was documented in several patients. Serum CK was significantly elevated in all patients. Laminin α2 chain expression was immunohistochemically normal in the muscle biopsy of the mild LGMD2I patients; however, Western blotting using an antibody that recognizes the 80-kd laminin α2 fragment revealed an absence or near-absence of labeling in all patients.24
Immunocytochemistry
Unfixed frozen 8-μm sections were incubated with monoclonal antibodies to: β-spectrin (Novocastra, Newcastle, UK), laminin α2, α5, β1, γ1 (Chemicon International Inc., Temecula, CA), 11H6 (gift of Kevin Campbell, Upstate Biotechnology, Lake Placid, NY) is an anti α-dystroglycan directed against a carbohydrate epitope25 and has been previously shown to functionally block laminin binding in in vitro assays.26,27 V1A4–1 (Upstate Biotechnology) is a monoclonal antibody directed against α-dystroglycan. The sheep polyclonal antibody was raised against a core peptide of α-dystroglycan.28 A second core peptide antibody IB7 (monoclonal) was a kind gift from S. Carbonetto (McGill University, Quebec, Canada).29 Additional antibodies included β-dystroglycan 43DAG/8D5 (Novocastra) a monoclonal antibody directed against the C terminus of the cytoplasmic domain, perlecan (Chemicon), a mouse monoclonal antibody M1 directed against a 180-kd extracellular matrix protein shown to be deficient in FCMD muscle (P180, a gift of Yoshihide Sunada, Kawasaki Medical School, Kurashika, Japan),30 integrin β1D (ab 8991 AbCam Ltd, Cambridge, UK), Collagen VI (Chemicon clones 1944 and 3303), collagen IV (Southern Biotechnology) and integrin α7B (gift of Dr. Ulrike Mayer, University of Manchester, Manchester, UK).
All primary antibodies were applied for 1 hour and revealed with an appropriate biotinylated secondary antibody (Amersham 1:200) for 30 minutes, followed by streptavidin conjugated to Alexa 594 (Molecular Probes, Eugene, OR) for 20 minutes. Double labeling used an appropriate secondary antibody conjugated with Alexa 488 (Molecular Probes). All dilutions and washings were made in phosphate-buffered saline. Control sections were labeled without primary antibodies, and all sections were compared with control samples from other neuromuscular disorders, and with normal muscle.
All sections were mounted in aqueous mountant and viewed with epifluorescence using a Leica DMR microscope linked to Metamorph (Universal Imaging Inc., Downington, PA).
Western Blotting
Human fetal muscle was obtained from fetuses at gestational ages ranging from 10 to 21 weeks. All samples were obtained with approval from the ethics committee. Muscle from MDC1C and LGMD2I patients was obtained by needle biopsy. Proteins were extracted in sample buffer consisting of 1 mol/L Tris HCL, 1% sodium dodecyl sulfate, 2-mercaptoethanol plus a cocktail of protease inhibitors (antipain, aprotinin, and leupeptin). Soluble protein was resolved using a NuPage Pre-cast gel (4 to 12% Bis-Tris) (Invitrogen, Carlsbad, CA) and then electrophoretically transferred to nitrocellulose membrane (Sartorius). Nitrocellulose strips were blocked in 3% bovine serum albumin in Tris-buffered saline Tween and probed with antibodies to α-dystroglycan (V1A4–1) and β-dystroglycan (Novocastra), washed and incubated with horseradish peroxidase anti-mouse (Jackson Laboratories). Membranes were visualized using chemiluminescence (ECL+Plus, Amersham).
Genetic Analysis
Genomic DNA was extracted from white blood cells by standard methods after obtaining informed consent. A 1.6-kb fragment containing the FKRP coding sequence was amplified using Advantage-GC Genomic Polymerase Mix (BD Biosciences Clontech, Palo Alto, CA) and primers FKRP-1F (AAAGGGAATTGAGAAAGAGC) and FKRP-5 (GCTCACACAGAGCTTCTCC). PCR products were separated by agarose gel electrophoresis, purified (Qiagen) and used for direct sequencing. Sequencing reactions were carried out using ABI Prism BigDye Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA) and primers FKRP-1R (GCAGGAAGGAGTCTACCAG), FKRP-2R(CCGAGAGGTTGAAGAGGT),FKRP- 3R(CTCCTCGTAGAGGTAGGC), FKRP-4R (CCTTCTCCCATACGAAGC), and FKRP-5R (GCTCACACAGAGCTTCTCC). Sequencing products were separated on an ABI377 automated sequencer (Applied Biosytems) and analyzed using SeqEd (Applied Biosytems).
Results
In normal muscle all four antibodies directed against α-dystroglycan clearly delineated individual muscle fibers with no apparent variation between fiber types (see Figure 1).
Figure 1.
A: Immunolabeling of α-dystroglycan in MDC1C patient 2 (B, D, F) and age-matched control muscle (A, C, E) using IIH6 (A and B), V1A4–1 (C and D) and the sheep antibody to the core protein (E and F). B: Immunolabeling of α-dystroglycan using the sheep polyclonal to the core peptide (A) and IB7 (monoclonal antibody directed against the core peptide; a gift of S. Carbonetto) (B) in MDC1C patient 1 with an age-matched control labeled with IB7 (C). C: Western blot of α-dystroglycan in normal control muscle (lane 1) 16-week fetal muscle (lane 2) and C2C12 cells (lane 3) with IIH6. Lane 4 shows labeling for laminin α2 in human muscle, molecular weight markers from Invitrogen are shown on the left of the blot, and those from Bio-Rad (Hercules, CA) on the right hand side, thus defining the dynamic range of the gel system used. D: Western blot of C2C12 cells (lanes 1 and 3), and normal human muscle (lanes 2 and 4) labeled with IIH6 (lanes 1 and 2) and V1A4–1 (lanes 3 and 4). IIH6 can be seen to identify a slightly higher molecular weight product than V1A4–1.
MDC1C Immunocytochemistry
As previously reported, the muscle from patients with MDC1C showed a marked depletion of α-dystroglycan as determined by immunocytochemistry. This was evident using three antibodies (IIH6, V1A4–1, and sheep polyclonal against the core peptide) against α-dystroglycan (Figure 1). Serial sections of the same patient (patient 2; see Table 1) showed that IIH6 immunolabeled fewer fibers than either V1A4–1 or the core antibody (Figure 1, B, D, and F). Such variation may be a reflection of the relative affinities of each of the antibodies or related to the expression/availability of the specific epitope that each recognizes. In relation to the latter possibility, it is perhaps relevant that IIH6 identifies a slightly higher molecular weight isoform than V1A4–1 (Figure 1, C and D) in both extracts of human muscle and C2C12 cells.
Table 1.
Patient Mutations
| Patient | FKRP mutation | Protein change | Clinical |
|---|---|---|---|
| 1* | A926G, C1154A | Tyr309Cys, Ser384Stop | MDC1C |
| 2* | C1378T homozygous | Pro448Leu | MDC1C |
| 3* | 165InsGGAG, G1016A | Asp60Stop, Arg339His | MDC1C |
| 4† | C826A, C1384T | Leu276Ile, Pro462Ser | LGMD2I (severe) |
| 5‡ | C826A homozygous | Leu276Ile | LGMD2I (mild) |
| 6† | C826A, 158InsCT GGT | Leu276Ile, Val51fs | LGMD2I (severe) |
| 7† | C826A, G1078A | Leu276Ile, Asp360Asn | LGMD2I (severe) |
| 8† | C826A, C1154A | Leu276Ile, Ser385Stop | LGMD2I (mild) |
| 9‡ | C826A homozygous | Leu276Ile | LGMD2I (mild) |
| 10‡ | C826A homozygous | Leu276Ile | LGMD2I (mild) |
| 11† | C826A homozygous | Leu276Ile | LGMD2I (mild) |
| 12† | C826A homozygous | Leu276Ile | LGMD2I (mild) |
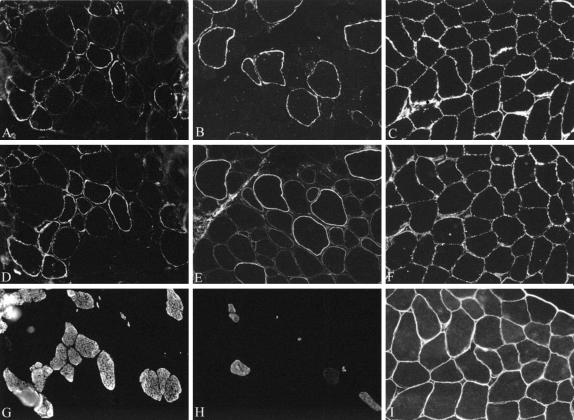
Comparisons between patients showed some significant variation in the number of fibers depleted of α-dystroglycan (Figure 2) but near normal labeling for β-dystroglycan relative to controls. For example, while patient 1 displayed an almost total absence of α-dystroglycan (using V1A4–1) the muscle of patient 3 contained a small proportion of fibers that expressed clear labeling at the sarcolemma. These fibers also showed reduced or absent α-dystroglycan using the two antibodies to the core protein (Figure 1). Assuming that there has been no epitope masking these observations suggest that there is a reduction in both glycosylated epitopes and the core protein in MDC1C. The nerve visible in Figure 2C shows clear labeling for α-dystroglycan as does the muscle spindle, the former of which is consistent with the reported absence of peripheral nerve involvement in these patients.23
Figure 2.
Immunolabeling of β-dystroglycan (A and B) α-dystroglycan (epitope recognized by V1A4–1) (C and D) and neonatal myosin (E and F) in patients 1 (A, C, E) and 2 (B, D, F) with MDC1C. β-dystroglycan immunolabeling is similar in both patients, although some of the smaller fibers show a reduction relative to control muscle. These fibers also expressed neonatal myosin, indicating that they were immature. Patient 1 shows a more marked depletion of sarcolemmal associated α-dystroglycan than patient 2. Comparisons of serial areas show that a proportion of the fibers in patient 2 that retain some α-dystroglycan also express neonatal forms of myosin and are therefore immature. Note the apparent preservation of α-dystroglycan on the muscle spindle and nerve in patient 1, the former of which is consistent with the absence of nervous system involvement in this patient.
The expression of a variety of proteins is affected by muscle regeneration. Muscle biopsies taken from MDC1C patients typically contain a large number of regenerating fibers (Figure 2, E and F). Analyses of serial sections immunostained for α- and β-dystroglycan and neonatal myosin showed that the number of fibers with an immunocytochemical depletion of α-dystroglycan was far greater than the number expressing neonatal myosin (Figure 2). Interestingly in one patient2 a proportion of the fibers that tended to retain α-dystroglycan at the membrane also expressed neonatal myosin. Some of the immature fibers in these patients are therefore capable of producing or retaining a glycoform of α-dystroglycan that is lost from the sarcolemma of more mature fibers. While immunostaining for β-dystroglycan was relatively well preserved in all patients there was a reduction in the labeling of some smaller fibers that were also positive for neonatal myosin indicating that they were relatively immature (Figure 2). Immunostaining for α, β, δ, and γ sarcoglycan also showed a mild reduction on some of the smaller fibers relative to control (Figure 3). These observations suggest that in MDC1C there are only subtle changes in other components of the dystrophin-associated complex that appear to relate to the high number of regenerating fibers.
Figure 3.
Immunolabeling of α, β, δ, and γ sarcoglycan in MDC1C patient 2 (A, C, E, G) and an age-matched control (B, D, F, H). Only a mild reduction in all sarcoglycans can be seen in the smaller relative to the larger fibers, suggesting that this feature relates to maturity rather than the absence of α-dystroglycan.
P180 is an extracellular matrix protein (180 kd) that has been shown to be specifically deficient in muscle from patients with FCMD.30 Antibodies directed toward P180 stain the sarcolemma of normal muscle (Figure 4E). In all MDC1C patients immunolabeling at the sarcolemma was greatly reduced (Figure 4, A and F).
Figure 4.
Immunolabeling for P180 in MDC1C patient 1 (A), LGMD2I at the severe end of the clinical spectrum (B and C) patients 6 and 7, LGMD2I patient homozygous for common Leu276Ile change (mild clinical phenotype) patient 5 (D), control muscle (E), and MDC1C patient 3 (F).
Integrin α7β1D is a major receptor for laminin α2 in muscle and has been reported to be up-regulated in both DMD patients and dystrophin deficient mdx mice but reduced in the muscle of patients with laminin α2 deficiency.31 In MDC1C immunolabeling for integrin α7B was variable between fibers and markedly reduced on a proportion relative to controls (Figure 5, A and B). In addition, the capillary staining clearly seen in control muscle appeared less evident. β1D was also variable between fibers in MDC1C with strong immunolabeling in a proportion of fibers yet an apparent reduction in others relative to control muscle (Figure 5, D and E). These observations suggest that integrin mediated adhesion may be altered in this disease, however, as to whether this is a consequence of the regeneration or the reduced expression of α-dystroglycan at the sarcolemma remains to be determined.
Figure 5.
Immunolabeling for integrin α7 (A, B, C) and β1D (D, E, F) in control (A and D), MDC1C patient 3 (B and E), and LGMD2I patient 7 (C and F). Immunolabeling for both integrin α7 and β1D was reduced on a proportion of fibers. However, on others labeling appeared to be increased relative to controls, this was particularly evident with the β1D antibody. A similar increase in immunolabeling was observed with both integrin α7 and β1D antibodies in most fibers of LGMD2I muscle. Capillary staining seen with integrin α7 is less evident in MDC1C and LGMD2I.
A variable secondary reduction in laminin α2 has been previously reported in patients with mutations in FKRP and in one patient (patient 1; see Table 1) reported to be associated with a reduction in laminin β1 and γ1. However, changes in laminin α2 chain labeling were variable and much less obvious in another patient (patient 2) (data not shown). Given the role of α-dystroglycan in basement membrane assembly we also examined the expression of laminin α5, perlecan, collagen IV, and collagen VI (data not shown). Immunocytochemically perlecan and collagen IV were indistinguishable from controls. Collagen VI expression was examined using two commercially available antibodies (1944 and 3303) but showed only minimal changes in one of the two patients examined.
LGMD2I: Immunocytochemistry
Overall the adult cases showed an immunocytochemical reduction of α-dystroglycan that was milder than that seen in MDC1C. This was evident using either an antibody to a glycosylated epitope (V1A4–1) or to the core protein (sheep polyclonal); however, patients at the severe end of the clinical spectrum tended to show the greatest reduction in immunolabeling of α-dystroglycan (Figure 6, B and E) while those at the milder end of the LGMD2I clinical spectrum, with the Leu276Ile mutation, generally displayed relatively well preserved α-dystroglycan (Figure 6, C and F). Patients with LGMD2I display a variable number of regenerating fibers which sometimes immunostained more strongly with antibodies to both the glycosylated epitopes or the core peptide (Figure 7, A, D, and G). However, this pattern of staining was not evident in all regenerating fibers of all patients as is shown in Figure 7, B, E, and H). There were no discernable alterations in the immunocytochemical labeling for laminin α2 in this group of patients (data not shown).
Figure 6.
Immunolabeling for α-dystroglycan using V1A4–1 (A, B, C) and the core antibody (D, E, F) in control (A and D), patient 4 (B and E, severe end of the LGMD2I spectrum), and patient 5 (C and F, mild end of the LGMD2I clinical spectrum). V1A4–1 shows a greater reduction in patient 4 relative to 5; this difference is less obvious with the core antibody, although both patients show a reduction relative to control muscle.
Figure 7.
Immunolabeling for α-dystroglycan in two patients (11 and 12) with LGMD2I (A, D, G and B, E, H, respectively), together with an age-matched control (C, F, I). Patient 11 shows variable immunolabeling with both IIH6 (A) and V1A4–1 (D). The fibers labeled most strongly also label with neonatal myosin (G). Patient 12 also shows variable immunolabeling for α-dystroglycan with both IIH6 (B) and the antibody to the core protein (E). However, in this patient the most intensely stained fibers are not positive for neonatal myosin. Control muscle labeled for IIH6, V1A4–1, and the core protein (sheep polyclonal) is shown in C, F, and I, respectively.
With respect to P180 there was some significant variation between patients with patients at the severe end of the clinical spectrum showing a more marked reduction than those at the milder end although this was variable as shown by a comparison of Figure 4, B and C. The one patient examined which was homozygous for the Leu276Ileu mutation showed levels comparable to that of control muscle (Figure 4D).
MDC1C: Western Blot Analysis
The lower molecular weight and hence less glycosylated forms of α-dystroglycan are characteristically expressed by immature muscle as shown by Figure 8A which shows the Western blot analysis of human fetal muscle between the ages of 10 and 21 weeks of gestation. As can be seen from the molecular weight markers α-dystroglycan apparently runs at an approximate molecular weight of 100 kd. This seems to be due to an apparent compression of high molecular weight proteins in the minigel system making accurate estimations of molecular weight unreliable. This was confirmed by running muscle extracts on a full-length system using the same markers. Under these conditions the molecular weight of α-dystroglycan was approximately 156 kd, ie, the same as that previously published for this protein (data not shown). All of the gels presented here were run using the minigel system and controls were always included to make comparative analyses between samples.
Figure 8.
A: Western blot analysis of human fetal muscle at 9.4 weeks (lane 1), 10.9 weeks (lane 2), 13.6 weeks (lane 3), and 16.6 weeks (lane 4) of gestation, neonatal muscle (lane 5), adult muscle 9 (lane 6), DMD muscle (lane 7), and normal adult muscle (lane 8) are included for comparison. The average molecular weight of α-dystroglycan can be seen to increase with gestational age. B: Western blot shown in A probed for β-dystroglycan. C: Western blot analysis using V1A4–1 of muscle from control (lane 1) and LGMD2I (lane 2) at the severe end of the clinical spectrum. D: Western blot analysis using V1A4–1 of muscle from control (lane 1), LGMD2I homozygous for the Leu276Ile change (lane 2), MDC1C (lane 3), and human fetal muscle at 9.4 weeks gestation (lane 4).
Western blot analysis of the muscle from patient 2 showed a marked reduction in the average molecular weight of α-dystroglycan relative to control muscle (Figure 8C). These results and those previously published22,23 suggest that the apparently preserved immunocytochemical expression of α-dystroglycan on immature fibers in MDC1C is due to a residual lower molecular weight form of α-dystroglycan produced by the immature fibers and that the higher molecular weight product characteristic of mature muscle is absent. No significant alterations in the molecular weight of other components of the dystrophin associated complex such as dystrophin and α and γ sarcoglycans were noted (data not shown).
None of the MDC1C samples blotted for β-dystroglycan showed any alteration in molecular weight (data not shown). Previous reports have shown the presence of an additional 30-kd fragment of β-dystroglycan in some cases of sarcoglycanopathy.32 This has now be shown to be due to proteolytic processing of the extracellular domain of β-dystroglycan by membrane-associated matrix metalloproteinase (MMP) activity.33 We identified this fragment in some of the human fetal muscle samples but failed to detect it in any of our MDC1C patient samples thus confirming the integrity of β-dystroglycan in these patients (data not shown).
LGMD2I: Western Blot Analysis
Western blot analysis reflected the immunolabeling results with patient 5, at the mild end of the LGMD2I spectrum, showing no clear alteration in molecular weight relative to controls (Figure 8D). Muscle obtained from a MDC1C patient and a human fetus at 9.4 weeks is also included for comparison. By contrast patient 4, affected by a DMD-like severity showed a marked loss of the high molecular weight isoforms (Figure 8D) and total absence of laminin α2 (data not shown).
Mutational Analysis
The mutations detected in the patients included in this study are shown in Table 1. In the three MDC1C families, affected individuals from families 1 and 2 were compound heterozygotes of one missense and one nonsense mutation, while the affected individual from the consanguineous family 3 had a homozygous missense change. In contrast, all seven affected individuals with the LGMD phenotype shared the C826A/Leu279Ile mutation. Patients at the severe end of the LGMD2I spectrum (DMD-like) were compound heterozygotes between the common C826A/Leu279Ile mutation and either nonsense or missense mutations, while families at the milder end of the spectrum were either heterozygous for this change or homozygous. These latter families had the mildest phenotype.
Discussion
Mutations in the genes encoding for a number of components of the dystroglycan complex result in various forms of muscular dystrophy including Duchenne Muscular Dystrophy (DMD), the sarcoglycanopathies and laminin α2-deficient CMD (MDC1A).34 However, more recently mutations have been reported in proteins that appear to be involved in the processing of α-dystroglycan. These include Fukuyama muscular dystrophy which is due to a deficiency in fukutin and MEB disease due to mutations in the gene encoding for the enzyme POMGnT1,35 and WWS due to mutations in POMT1.21 While it has yet to be proven that fukutin and POMGnT1 act directly on α-dystroglycan it has nonetheless been shown that the hypoglycosylation of α-dystroglycan is characteristic of these two conditions and plays a central role in their pathogenesis by disrupting neurexin, agrin, and laminin binding.36 We have previously demonstrated that mutations in FKRP, a putative glycosyltransferase, also leads to an apparent alteration in the glycosylation of α-dystroglycan. Mutations in FKRP underlie both MDC1C and LGMD2I, and are thus associated with a wide spectrum of clinical severity. The reasons for this are at the present time incompletely understood. In the present study we have therefore sought to characterize the spectrum of α-dystroglycan abnormalities in patients with both MDC1C and LGMD2I to determine whether there is any correlation between α-dystroglycan expression, FKRP mutation, and clinical phenotype.
We found that patients with MDC1C displayed a profound reduction in α-dystroglycan that was evident using antibodies directed to either the glycosylated regions or different epitopes in the core protein. Since IIH6 (one of the antibodies directed toward a glycosylated epitope) is known to interact specifically with the laminin binding region of α-dystroglycan, the marked reduction in staining obtained with this antibody strongly suggest that, as has been previously demonstrated in MEB and FCMD, laminin binding is perturbed in these patients. Interestingly, these patients show no central nor peripheral nervous system involvement, despite the expression of FKRP in brain.23 Immunolabeling for α-dystroglycan appeared normal on the nerves that were present in one biopsy. The reasons for this are unclear but may relate to tissue-specific processing (the molecular weights of α-dystroglycan in nerve and muscle are 120 kd and 160 kd, respectively) and/or different binding partners in the two locations. All of the MDC1C patients examined had either missense or nonsense mutations in the FKRP gene. FKRP is a putative glycosyltransferase that has been shown to localize to the Golgi.37 Furthermore, one of the missense mutations reported in the present cohort of MDC1C patients is associated with mis-localization of FKRP in the cell and altered α-dystroglycan processing.37 These results provide further evidence in support of the hypothesis that α-dystroglycan is one of the main targets (direct or indirect) for FKRP.
A significant number of regenerating fibers are present in both MDC1C and LGMD2I patient muscle. Interestingly, we noted that some but not all of these fibers were immunolabeled more strongly for α-dystroglycan relative to the surrounding fibers. Similar observations have been recently reported for a group of patients with a mild form of LGMD, the genetic cause of which was not determined.38 This was suggested by the authors to contribute to their milder phenotype; however, our observations suggest that this is a feature that varies between different LGMD2I patients and is also seen in MDC1C.
POMGnT1, the enzyme defective in MEB participates in the synthesis O-mannosyl glycan, a modification that is rare in mammals but which is nevertheless known to be present in α-dystroglycan.39 Fukutin, the protein deficient in FCMD, has been less well-defined but is thought to be an enzyme involved in the modification of cell surface molecules. Recent work shows that antibodies to glycosylated epitopes fail to detect α-dystroglycan in either of these disorders, whereas an antibody to the core protein shows levels of expression similar to that of control.36 The protein detected in these cases with this antibody shows a marked drop in molecular weight relative to control muscle, strongly suggesting that both diseases are associated with a similar loss of carbohydrate groups.
These observations differ from those made in the present study since in patients with FKRP mutations α-dystroglycan appears reduced using all antibodies, ie, those to the glycosylated epitopes and also the core protein. This may be due to either an aberrantly glycosylated form of α-dystroglycan that results in masking of some of the epitopes identified by the sheep polyclonal antibody, thus giving rise to an apparent immunocytochemical reduction, or alternatively that there is a true reduction in α-dystroglycan protein. The fact that two different antibodies against the core protein showed similar results might be a finding in favor of this second hypothesis. In regard to this it is interesting that both we and others have observed immunolabeling, albeit reduced, using the antibody to the core protein in a MEB patient with a confirmed mutation in POMGnT1 (Brown and Muntoni, unpublished observations) and WWS with a confirmed mutation in POMT1,21 thus confirming that this antibody is able to recognize a hypoglycosylated form of α-dystroglycan. Unfortunately since the sheep polyclonal antibody does not work reliably on Western blot it was not possible to determine whether there was an additional lower molecular weight product in our patients as has been previously reported for MEB and FCMD.36 Nonetheless, Western blotting does show that the average molecular weight of α-dystroglycan detected in our patients with the antibody to α-glycosylated epitope is similar to that at 10 weeks gestation in the human fetus.
We typically found a severe depletion of α- but not β-dystroglycan in all patients with the MDC1C phenotype, which was accompanied by a variable reduction of the laminin α2 chain. Similar changes were also identified in those patients with LGMD2I who followed a severe, DMD-like course. A significant number of these patients were compound heterozygote between a missense mutation and a null allele; the type of the missense mutation was crucial in determining if patients followed a MDC1C or LGMD2I course as the common Leu276lle mutation was never observed in MDC1C patients, thus underscoring the finding that this mutation is invariably associated with a milder phenotype. Patients homozygous for the common Leu276Ile mutations clearly had the mildest form of muscular dystrophy. In these patients α-dystroglycan abnormalities were much more subtle on immunocytochemistry and Western blot analysis. These results suggest a significant perturbation of the dystroglycan axis in patients with FKRP mutations and a correlation between the clinical phenotype and the amount of detectable α-dystroglycan.
The predominant form of laminin in skeletal muscle is merosin, which is composed of α2, β1, and γ1.40 MDC1C patients show a variable (sometimes only subtle) alteration in laminin α2 chain immunolabeling. A partial deficiency of laminin α2 chain has also been reported in other neuromuscular disorders and is a feature of both FCMD, MEB,39 and MDC1B.41,42 Both FCMD and MEB, produce a hypoglycosylated form of muscle α-dystroglycan that is less able to bind laminin α2.36 Laminin α2 also interacts with α7β1 integrin and it is interesting to note that some fibers showed an apparent increase in immunolabeling for integrin α7 and β1D. suggesting that integrin up-regulation may be a response to the marked reduction in α-dystroglycan. However, the integrin α7 subunit is developmentally regulated and, as such, alterations in the laminin α2 binding network may not have been the only factor contributing to these observations. Further indirect proof of similarities of muscle pathology observed in FCMD and MDC1C comes from the observation that P180, an as yet uncharacterized protein which has been reported as being specifically deficient in muscle from FCMD patients,30 was also absent or greatly reduced in MDC1C but not LGMD2I. It is possible that P180 is another glycoprotein affected by the deficiency of fukutin and FKRP, in addition to α-dystroglycan.
α-dystroglycan has been attributed with a role in regulating protein turnover rather than initiating basement membrane assembly, as has previously been suggested.13 In regard to this it is interesting to note the apparent absence of laminin α2 on Western blot in patients with LGMD2I, despite apparently normal immunolabeling on muscle sections.24 We also observed this apparent absence of laminin α2 (80-kd fragment) in one of the severe cases of LGMD2I. The reasons for this are at present unclear but could possibly be due to altered solubility of this protein or the unmasking of a proteolytic cleavage site normally masked by α-dystroglycan binding. These possibilities would both support the idea of an alteration at the molecular level within the basement membrane of LGMD2I.
The dystroglycan and sarcoglycan complexes are known to be tightly associated and the presence of an intact sarcoglycan complex is thought to be required for the stable presence of α-dystroglycan at the sarcolemma. There was no evidence of any alteration in the immunolabeling of any of the sarcoglycans in either MDC1C or LGMD2I, while an abnormally low expression of dystrophin-associated proteins has previously been reported in FCMD.43,44 In addition there was no 30-kd cleavage product of β-dystroglycan as has been occasionally seen in muscle biopsies from patients with sarcoglycanopathy. This cleavage product, unlike the 43-kd protein, does not associate with α-dystroglycan and has been found to be prevalent in certain carcinoma cell lines.45 We have now noted the presence of this product in selected samples of fetal muscle which we suggest may be due to the increased susceptibility of fetal muscle to metalloproteinase attack. Interestingly, the selective absence of α-dystroglycan was not associated with any such cleavage.
In conclusion, the loss of high molecular weight observed in α-dystroglycan in MDC1C and, to a lesser extent, LGMD2I, supports the prediction that FKRP is a glycosyltransferase. There is now substantial evidence showing that the abnormal glycosylation of α-dystroglycan has a number of functional consequences, including the binding with laminin α2 chain. However, the absence of brain involvement in MDC1C and LGMD2I suggests that unlike FCMD and MEB the binding of other partners more relevant to neural migration and synaptic function is either not perturbed or is compensated for by other components. The abnormal posttranslational modification of α-dystroglycan identifies a novel pathway responsible for muscle degeneration, abnormalities of which are now observed in a growing number of human conditions.
Footnotes
Address reprint requests to Dr. Susan C. Brown, Dubowitz Neuromuscular Unit, Department of Paediatrics, Imperial College London, Hammersmith Hospital, Du Cane Road, London W12 ONN, UK. E-mail: s.brown@ic.ac.uk.
Supported by The Muscular Dystrophy Campaign (F.M.), the European Community (E.C. project Myo-Cluster, contract number GLG1-CT-1999–00870 to F.M.), the Alexander Krupp von Bohlen und Halbach Foundation, and by the Deutsche Forschungsgemeinschaft (Str 498/2–2 to T.V.).
References
- Moll J, Barzaghi P, Lin S, Bezakova G, Lochmuller H, Engvall E, Muller U, Ruegg MA. An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature. 2001;413:302–307. doi: 10.1038/35095054. [DOI] [PubMed] [Google Scholar]
- Winder SJ. The complexities of dystroglycan. Trends Biochem Sci. 2001;26:118–124. doi: 10.1016/s0968-0004(00)01731-x. [DOI] [PubMed] [Google Scholar]
- Chamberlain JS, Corrado K, Rafael JA, Cox GA, Hauser M, Lumeng C. Interactions between dystrophin and the sarcolemma membrane. Soc Gen Physiol Ser. 1997;52:19–29. [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Milatovich A, Ozcelik T, Yang B, Koepnick K, Francke U, Campbell KP. Human dystroglycan: skeletal muscle cDNA, genomic structure, origin of tissue specific isoforms and chromosomal localization. Hum Mol Genet. 1993;2:1651–1657. doi: 10.1093/hmg/2.10.1651. [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP. Dystroglycan inside and out. Curr Opin Cell Biol. 1999;11:602–607. doi: 10.1016/s0955-0674(99)00024-1. [DOI] [PubMed] [Google Scholar]
- Holt KH, Crosbie RH, Venzke DP, Campbell KP. Biosynthesis of dystroglycan: processing of a precursor propeptide. FEBS Lett. 2000;468:79–83. doi: 10.1016/s0014-5793(00)01195-9. [DOI] [PubMed] [Google Scholar]
- Bowe MA, Mendis DB, Fallon JR. The small leucine-rich repeat proteoglycan biglycan binds to α-dystroglycan and is upregulated in dystrophic muscle. J Cell Biol. 2000;148:801–810. doi: 10.1083/jcb.148.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Ohlendieck K, Ionasescu VV, Tome FM, Nonaka I, Burghes AH, Mora M, Kaplan JC, Fardeau M, Campbell KP. The role of the dystrophin-glycoprotein complex in the molecular pathogenesis of muscular dystrophies. Neuromuscul Disord. 1993;3:533–535. doi: 10.1016/0960-8966(93)90110-6. [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP. Dystroglycan: an extracellular matrix receptor linked to the cytoskeleton. Curr Opin Cell Biol. 1996;8:625–631. doi: 10.1016/s0955-0674(96)80103-7. [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP. A role for dystroglycan in basement membrane assembly. Cell. 1998;95:859–870. doi: 10.1016/s0092-8674(00)81708-0. [DOI] [PubMed] [Google Scholar]
- Colognato H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol. 1999;145:619–631. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MD, Satz JS, Brakebusch C, Costell M, Gustafsson E, Fassler R, Campbell KP. Distinct roles for dystroglycan, β1 integrin and perlecan in cell surface laminin organization. J Cell Sci. 2001;114:1137–1144. doi: 10.1242/jcs.114.6.1137. [DOI] [PubMed] [Google Scholar]
- Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP. Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum Mol Genet. 1997;6:831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, Yurchenco PD. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J Cell Biol. 2002;157:1279–1290. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote PD, Moukhles H, Lindenbaum M, Carbonetto S. Chimaeric mice deficient in dystroglycans develop muscular dystrophy and have disrupted myoneural synapses. Nat Genet. 1999;23:338–342. doi: 10.1038/15519. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Campbell KP. Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve. 1994;17:2–15. doi: 10.1002/mus.880170103. [DOI] [PubMed] [Google Scholar]
- Araishi K, Sasaoka T, Imamura M, Noguchi S, Hama H, Wakabayashi E, Yoshida M, Hori T, Ozawa E. Loss of the sarcoglycan complex and sarcospan leads to muscular dystrophy in β-sarcoglycan-deficient mice. Hum Mol Genet. 1999;8:1589–1598. doi: 10.1093/hmg/8.9.1589. [DOI] [PubMed] [Google Scholar]
- Hack AA, Groh ME, McNally EM. Sarcoglycans in muscular dystrophy. Microsc Res Tech. 2000;48:167–180. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<167::AID-JEMT5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Vainzof M, Passos-Bueno MR, Canovas M, Moreira ES, Pavanello RC, Marie SK, Anderson LV, Bonnemann CG, McNally EM, Nigro V, Kunkel LM, Zatz M. The sarcoglycan complex in the six autosomal recessive limb-girdle muscular dystrophies. Hum Mol Genet. 1996;5:1963–1969. doi: 10.1093/hmg/5.12.1963. [DOI] [PubMed] [Google Scholar]
- Beltran-Valero DB, Currier S, Steinbrecher A, Celli J, Van Beusekom E, Van Der ZB, Kayserili H, Merlini L, Chitayat D, Dobyns WB, Cormand B, Lehesjoki AE, Cruces J, Voit T, Walsh CA, Van Bokhoven H, Brunner HG. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet. 2002;71:1033–1043. doi: 10.1086/342975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington M, Yuva Y, Prandini P, Brown SC, Torelli S, Benson MA, Herrmann R, Anderson LV, Bashir R, Burgunder JM, Fallet S, Romero N, Fardeau M, Straub V, Storey G, Pollitt C, Richard I, Sewry CA, Bushby K, Voit T, Blake DJ, Muntoni F. Mutations in the fukutin-related protein gene (FKRP) identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum Mol Genet. 2001;10:2851–2859. doi: 10.1093/hmg/10.25.2851. [DOI] [PubMed] [Google Scholar]
- Brockington M, Blake DJ, Prandini P, Brown SC, Torelli S, Benson MA, Ponting CP, Estournet B, Romero NB, Mercuri E, Voit T, Sewry CA, Guicheney P, Muntoni F. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin α2 deficiency and abnormal glycosylation of α-dystroglycan. Am J Hum Genet. 2001;69:1198–1209. doi: 10.1086/324412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushby K, Anderson LV, Pollitt C, Naom I, Muntoni F, Bindoff L. Abnormal merosin in adults. A new form of late onset muscular dystrophy not linked to chromosome 6q2. Brain. 1998;121:581–588. doi: 10.1093/brain/121.4.581. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Dystrophin-associated glycoproteins: their possible roles in the pathogenesis of Duchenne muscular dystrophy. Mol Cell Biol Hum Dis Ser. 1993;3:139–166. doi: 10.1007/978-94-011-1528-5_6. [DOI] [PubMed] [Google Scholar]
- Durbeej M, Larsson E, Ibraghimov-Beskrovnaya O, Roberds SL, Campbell KP, Ekblom P. Non-muscle α-dystroglycan is involved in epithelial development. J Cell Biol. 1995;130:79–91. doi: 10.1083/jcb.130.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SC, Fassati A, Popplewell L, Page AM, Henry MD, Campbell KP, Dickson G. Dystrophic phenotype induced in vitro by antibody blockade of muscle α-dystroglycan-laminin interaction. J Cell Sci. 1999;112:209–216. doi: 10.1242/jcs.112.2.209. [DOI] [PubMed] [Google Scholar]
- Herrmann R, Straub V, Blank M, Kutzick C, Franke N, Jacob EN, Lenard HG, Kroger S, Voit T. Dissociation of the dystroglycan complex in caveolin-3-deficient limb girdle muscular dystrophy. Hum Mol Genet. 2000;9:2335–2340. doi: 10.1093/oxfordjournals.hmg.a018926. [DOI] [PubMed] [Google Scholar]
- Moukhles H, Roque R, Carbonetto S. α-dystroglycan isoforms are differentially distributed in adult rat retina. J Comp Neurol. 2000;420:182–194. doi: 10.1002/(sici)1096-9861(20000501)420:2<182::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Sunada Y, Saito F, Higuchi I, Matsumura K, Shimizu T. Deficiency of a 180-kDa extracellular matrix protein in Fukuyama type congenital muscular dystrophy skeletal muscle. Neuromuscul Disord. 2002;12:117–120. doi: 10.1016/s0960-8966(01)00251-6. [DOI] [PubMed] [Google Scholar]
- Hodges BL, Hayashi YK, Nonaka I, Wang W, Arahata K, Kaufman SJ. Altered expression of the α7β1 integrin in human and murine muscular dystrophies. J Cell Sci. 1997;110:2873–2881. doi: 10.1242/jcs.110.22.2873. [DOI] [PubMed] [Google Scholar]
- Anderson LV, Davison K. Multiplex Western blotting system for the analysis of muscular dystrophy proteins. Am J Pathol. 1999;154:1017–1022. doi: 10.1016/S0002-9440(10)65354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Saito F, Fukuta-Ohi H, Zhong D, Hase A, Arai K, Okuyama A, Maekawa R, Shimizu T, Matsumura K. Processing of β-dystroglycan by matrix metalloproteinase disrupts the link between the extracellular matrix and cell membrane via the dystroglycan complex. Hum Mol Genet. 2001;10:1563–1569. doi: 10.1093/hmg/10.15.1563. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M, Herrmann R, Straub V, Talim B, Voit T, Topaloglu H, Toda T, Endo T. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell. 2001;1:717–724. doi: 10.1016/s1534-5807(01)00070-3. [DOI] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Esapa CT, Benson MA, Schroder JE, Martin-Rendon E, Brockington M, Brown SC, Muntoni F, Kroger S, Blake DJ. Functional requirements for fukutin-related protein in the Golgi apparatus. Hum Mol Genet. 2002;11:3319–3331. doi: 10.1093/hmg/11.26.3319. [DOI] [PubMed] [Google Scholar]
- Cohn RD, Henry MD, Michele DE, Barresi R, Saito F, Moore SA, Flanagan JD, Skwarchuk MW, Robbins ME, Mendell JR, Williamson RA, Campbell KP. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell. 2002;110:639–648. doi: 10.1016/s0092-8674(02)00907-8. [DOI] [PubMed] [Google Scholar]
- Kano H, Kobayashi K, Herrmann R, Tachikawa M, Manya H, Nishino I, Nonaka I, Straub V, Talim B, Voit T, Topaloglu H, Endo T, Yoshikawa H, Toda T. Deficiency of α-dystroglycan in muscle-eye-brain disease. Biochem Biophys Res Commun. 2002;291:1283–1286. doi: 10.1006/bbrc.2002.6608. [DOI] [PubMed] [Google Scholar]
- Sewry CA, Muntoni F. Inherited disorders of the extracellular matrix. Curr Opin Neurol. 1999;12:519–526. doi: 10.1097/00019052-199910000-00005. [DOI] [PubMed] [Google Scholar]
- Muntoni F, Taylor J, Sewry CA, Naom I, Dubowitz V. An early onset muscular dystrophy with diaphragmatic involvement, early respiratory failure and secondary α2 laminin deficiency unlinked to the LAMA2 locus on 6q22. Eur J Paediatr Neurol. 1998;2:19–26. doi: 10.1016/1090-3798(98)01001-9. [DOI] [PubMed] [Google Scholar]
- Brockington M, Sewry CA, Herrmann R, Naom I, Dearlove A, Rhodes M, Topaloglu H, Dubowitz V, Voit T, Muntoni F. Assignment of a form of congenital muscular dystrophy with secondary merosin deficiency to chromosome 1q42. Am J Hum Genet. 2000;66:428–435. doi: 10.1086/302775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Nonaka I, Campbell KP. Abnormal expression of dystrophin-associated proteins in Fukuyama-type congenital muscular dystrophy. Lancet. 1993;341:521–522. doi: 10.1016/0140-6736(93)90279-p. [DOI] [PubMed] [Google Scholar]
- Arahata K, Hayashi YK, Mizuno Y, Yoshida M, Ozawa M. Dystrophin-associated glycoprotein and dystrophin co-localisation at sarcolemma in Fukuyama congenital muscular dystrophy. Lancet. 1993;342:623–624. doi: 10.1016/0140-6736(93)91454-t. [DOI] [PubMed] [Google Scholar]
- Losasso C, Di Tommaso F, Sgambato A, Ardito R, Cittadini A, Giardina B, Petrucci TC, Brancaccio A. Anomalous dystroglycan in carcinoma cell lines. FEBS Lett. 2000;484:194–198. doi: 10.1016/s0014-5793(00)02157-8. [DOI] [PubMed] [Google Scholar]