Abstract
The collapse of major histocompatiblity complex (MHC) class-I-dependent immune privilege can lead to autoimmune disease or fetal rejection. Pragmatic and instructive models are needed to clarify the as yet obscure controls of MHC class I down-regulation in situ, to dissect the principles of immune privilege generation, maintenance, and collapse as well as to develop more effective strategies for immune privilege restoration. Here, we propose that human scalp hair follicles, which are abundantly available and easily studied, are ideally suited for this purpose: interferon-γ induces ectopic MHC class I expression in the constitutively MHC class-I-negative hair matrix epithelium of organ-cultured anagen hair bulbs, likely via interferon regulatory factor-1, along with up-regulation of the MHC class I pathway molecules β2microglobulin and transporter associated with antigen processing (TAP-2). In the first report to identify natural immunomodulators capable of down-regulating MHC class I expression in situ in a normal, neuroectoderm-derived human tissue, we show that ectopic MHC class I expression in human anagen hair bulbs can be normalized by treatment with α-MSH, IGF-1, or TGF-β1, all of which are locally generated, as well as by FK506. These agents are promising candidates for immune privilege restoration and for suppressing MHC class I expression where this is clinically desired (eg, in alopecia areata, multiple sclerosis, autoimmune uveitis, mumps orchitis, and fetal or allograft rejection).
A select number of mammalian tissue sites, namely the brain, cornea, anterior chamber of the eye, testis, liver, fetotrophoblast, and the hamster cheek pouch, display a fascinating phenomenon called “immune privilege.”1–5 This name reflects that these tissue environments can award allotransplants relative protection from rejection by the host immune system.1,2
Immune privilege is generated and maintained by a number of mechanisms.1–7 These mechanisms include down-regulation or absence of major histocompatibility complex (MHC) class Ia expression, which serves to sequester (auto-) antigens in these sites so as to prevent their presentation to autoreactive CD8+ T cells, local production of potent immunosuppressants such as transforming growth factor (TGF)-β1, interleukin (IL)-10 and α-melanocyte stimulating hormone (MSH), functional impairment of antigen presenting cells, absence of lymphatics, construction of extracellular matrix barriers to hinder immune cell trafficking,2,5 and expression of non-classical MHC class Ib molecules.5,6,8 Immune privilege collapse may bring about (auto-) immune disease, such as multiple sclerosis,9 autoimmune uveitis,3,10 mumps orchitis,11 alopecia areata,7,12 autoimmune chronic active hepatitis,13,14 and fetal rejection.4,5,15
More than three decades ago, Billingham et al16 recognized that actively growing (anagen) hair follicles in guinea pigs provide a special milieu which allows transplanted allogeneic epidermal melanocytes to escape elimination by the host immune system. Only much later, it became recognized that the proximal epithelium of anagen hair follicles in humans, mice and rats indeed displays one of the key features of immune privilege, ie, absent or very low levels of MHC class I expression.17–20 The hair follicle immune privilege appears to be largely restricted to the lower (proximal) hair follicle epithelium, since the follicular connective tissue sheath and the dermal papilla are clearly MHC class I+ in murine and human anagen VI hair follicles.18,20–22 Compared to the epidermis and the distal follicle epithelium, the proximal anagen hair follicle (especially the hair matrix) also displays substantially fewer antigen-presenting cells (ie, CD1a+ or ultrastructurally identified Langerhans cells), which are functionally impaired since they do not express MHC class II antigens.18 The anagen hair bulb also lacks a lymphatic system and almost devoid of intraepithelial T cells.18,23,24 And finally, anagen hair bulbs locally generate potent immunosuppressants, such as TGF-β1,25,26 insulin growth factor (IGF)-127 and α-MSH.28,29 Taken together, this makes the hair follicle an excellent, easily accessible, and experimentally easily manipulated immune privilege model.7,18,21
To optimally exploit the human hair follicle for studying general principles of how immune privilege is generated, maintained, lost and restored in situ, a pragmatic and reductionist approach was chosen. Justified by the central role these parameters play in immune privilege generation and maintenance,1,3,7 we focused on the constitutive expression and the experimentally induced up- and down-regulation of MHC class I mRNA and antigen in situ, and on the MHC class I-pathway-related molecules transporter-associated with antigen processing (TAP)2 and β2microglobulin, which are critical for proper presentation of (self-) peptide via MHC class I.30–33 Previously, we had found that, compared to IL-1 and tumor necrosis factor (TNF)-α, IFN-γ is the most potent inducer of ectopic MHC class I expression in murine anagen hair bulbs in vivo.22 Also in tumor immunology studies, IFN-γ is frequently employed to up-regulate MHC class I and II expression.34,35 Therefore, immune privilege collapse in human scalp hair follicles was simulated by treating microdissected, organ-cultured anagen hair bulbs with IFN-γ. Organ culture of normal human scalp hair follicle, as pioneered by Philpott et al,36 was chosen since this is readily available surplus material from plastic surgery and allows facile and well-controlled experimental manipulations in vitro. We then studied whether or not selected candidate molecules reported in the in vitro literature as down-regulators of MHC class I expression in selected cultured cell types, namely, TGF-β1,37 IGF-1,38 α-MSH,39 or IL-1040 could down-regulate ectopic, IFN-γ-induced human leukocyte antigen (HLA)-A/B/C expression and/or the expression of TAP2 and β2microglobulin in organ-cultured human scalp hair follicles in situ.
Materials and Methods
Specimen
Skin biopsies were obtained after informed consent from normal human scalp skin as samples during routine plastic or hair transplantation surgery. The specimen used for cryosections were snap-frozen in liquid nitrogen, and stored at −80°C until use. Before staining, specimens were embedded and processed for longitudinal cryosections (8 μm) as described before.18 Skin samples, used for hair follicle microdissection and organ culture, were collected and stored in refrigerator at 4°C.
Hair Follicle Organ Culture
The hair follicles microdissected from human scalp skin samples36 were cultured in William’s E medium (Biochrom AG, Berlin, Germany), which was supplemented with insulin (10 μg/ml) (Sigma, Taufkirchen, Germany), hydrocortisone (10 ng/ml) (Sigma), penicillin (Sigma), streptomycin (Sigma) and L-glutamine (2 mmol/L) (Sigma) using 24-well culture plate according to the well-established Philpott model.41 Control hair follicles were cultured with vehicle for 4 days. Test groups were treated with 75 IU/ml IFN-γ (Peprotech, Rocky Hill, NJ) for 4 days. In addition, 25, 50, 75 and 100 ng/ml IGF-1 (Sigma),42,43 0.2 μg/ml or 0.4 μg/ml α-MSH (Peprotech),44 100 ng/ml IL-10 (Peprotech),45 15 ng/ml or 30 ng/ml TGF-β1 (R&D Systems, Wiesbaden-Nordenstadt, Germany)46 and 10−8 M FK506 (LC Laboratories, Woburn, MA)47 were added on day 2 in each group. These doses were chosen on the basis of their use for keratinocytes in vitro.42–47 Four days after microdissection, these follicles were embedded in cryogel and frozen in liquid nitrogen and were processed for cryosectioning.
Immunohistology
Cryosections of normal human scalp skin and of cultured human hair follicles were immunostained, using a monoclonal mouse antibody against human HLA-A/B/C (DAKO, Hamburg, Germany), a monoclonal mouse antibody against human β2microglobulin (BD Biosciences, San Jose, CA), a polyclonal rabbit antibody against human TAP2 (Santa Cruz Biotechnology, Santa Cruz, CA), and calnexin (Santa Cruz), using the extrasensitive DAKO EnVision ADAAP technique (DAKO). Staining intensity of MHC class I-immunoreactivity was scored as: −, absent; +, weak; ++, moderate; +++, strong. The quantification of immunoreactivity (Figure 2, C to E) was performed in a blinded manner (ie, in ignorance of the group assignment of the analyzed section) by a different observer than the one who had generated and photodocumented the immunostains. This light microscopic technique was complemented with a highly sensitive immunofluorescent tyramide signal amplification (TSA) technique (PerkinElmer Life Sciences, Boston, MA).48 Briefly, sections of normal human scalp skin and cultured human hair follicles were immunostained using the monoclonal mouse antibody against human HLA-A/B/C (DAKO), the monoclonal mouse antibody against human HLA-DP/DQ/DR (DAKO), monoclonal mouse antibody against human β2microglobulin (BD Biosciences, Heidelberg, Germany), the monoclonal rabbit antibody against human interferon regulatory factor (IRF)-1 (Santa Cruz) and the polyclonal goat antibody against human TAP2 (Santa Cruz), as the primary antibodies diluted in TNB blocking buffer for 60 minutes at room temperature, followed by incubation with the biotinylated antibody against mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) (1:200 in TNB, 30 minutes at room temperature). Next, streptavidin horseradish peroxidase was administrated (1:50 in TNB, 30 minutes at room temperature). Finally, the reaction was amplified by tetramethylrhodamine- or fluorescein isothiocyanate-tyramide amplification reagent at room temperature for 3 minutes (1:50 in amplification diluent provided with the kit). The TSA signals were visualized under a fluorescent microscope (Zeiss, Jena, Germany) with CCD camera (Hamamatsu Photonics, Hamamatsu, Japan). The mean fluorescence intensity was measured at four previously defined reference areas in the hair matrix of anagen hair bulbs (Figure 1) by NIH image, and the average mean fluorescence intensity was calculated (n = 60 hair follicles/each group, derived from more than 3 patients).
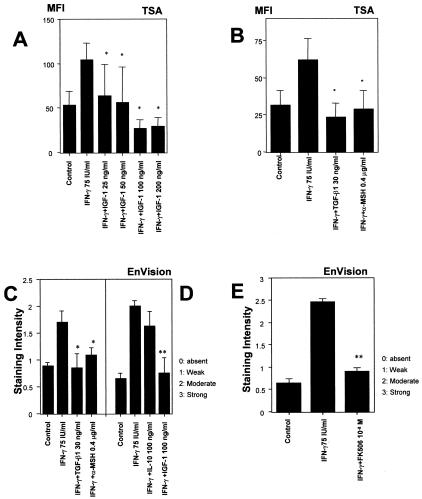
Figure 2.
IGF-1, TGF-β, α-MSH, and FK506 can down-regulate IFN-γ-induced ectopic MHC class I expression in hair matrix. A: IGF-1 (25 to 200 ng/ml) down-regulates the fluorescent mean intensity of MHC class I in hair matrix by dose dependent manner. B: TSA, which is high-sensitive immunohistochemical technique, revealed that 30 ng/ml TGF-β1 and 0.4 μg/ml α-MSH significantly down-regulate the mean intensity of MHC class I in the hair matrix. C and D: EnVision also shows that 100 ng/ml IGF-1, 30 ng/ml TGF-β1, 0.4 μg/ml α-MSH significantly down-regulate the staining intensity of MHC class I in the hair matrix. D: IL-10 fails to down-regulate the IFN-γ-induced ectopic MHC class I expression. E: The expression of MHC class I was significantly down-regulated in hair matrix treated by 10−8 M FK506. The MFI of MHC class I expression was measured at four previously defined reference areas in the hair matrix of anagen hair bulbs by NIH image, and the average MFI was calculated (n = 60 HFs from 3 patients/group). Staining intensity of MHC class I-immunoreactivity was scored as follows: −, absent; +, weak; ++, moderate; +++, strong (n = 60 HFs from 3 patients/each group), indicated as mean values ± SEM (n = 60 HF/group from 3 patients). *, P < 0.05 versus IFN-γ-treated hair follicles; **, P < 0.01 versus IFN-γ-treated hair follicles.
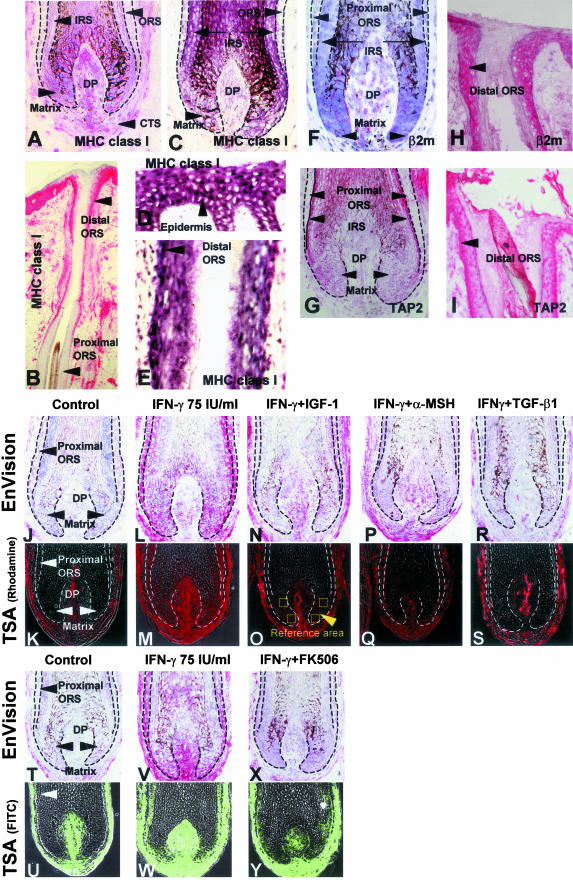
Figure 1.
A to I: Anagen hair bulbs express very low epithelial MHC class I and its pathway molecules in normal human scalp skin sections. A: Extra-sensitive APAAP-based immunohistology techniques (EnVision) show very low or absent MHC class-I-like immunoreactivity in the hair matrix, inner root sheath (IRS), and proximal ORS. Dermal papilla cells show marked variation in MHC class I immunoreactivity between individuals. Some dermal papilla cells have strong immunoreactivity for MHC class I and others have only weak immunoreactivity. B: The epidermis, distal ORS, and the connective tissue sheath of anagen VI scalp hair follicle have high MHC class-I-like immunoreactivity. In the ORS, MHC class I immunoreactivity declines from distal to proximal. C: On in situ hybridization technique, the proximal ORS and matrix keratinocytes show very little detectable HLA-B gene expression, supporting the MHC class I protein expression data (A). D and E: Note the strongly positive HLA-B mRNA signals in the epidermis (human scalp skin) and the distal ORS. F: Proximal ORS and hair matrix keratinocytes show low or absent expression level of β2microglobulin immunoreactivity similar to the expression of MHC class I. In comparison with proximal ORS and hair matrix keratinocytes, dermal papilla cells express moderate expression of β2microglobulin. G: Proximal ORS, hair matrix, and dermal papilla cells show low expression of TAP2 immunoreactivity. H: Distal ORS and epidermis show high levels of β2microglobulin immunoreactivity. I: Distal ORS shows high level of TAP2 immunoreactivity. J to Y: IGF-1, α-MSH, TGF-β1, and FK506 down-regulate IFN-γ-induced ectopic MHC class I protein expression in vitro. J and K: EnVision (J) and Tyramide signal amplification (TSA)46 (K) show the low expression of MHC class I in the vehicle-treated control follicle matrix and proximal ORS in cultured human hair follicles. L and M: 75 IU/m IFN-γ induces the ectopic MHC class I expression in human hair matrix and proximal ORS. N to S: 100 ng/ml IGF-1 (N and O), 0.4 μg/ml α-MSH (P and Q), and 30 ng/ml TGF-β1 (R and S) down-regulate the IFN-γ-induced ectopic MHC class I expression in human hair matrix and proximal ORS. T to Y: In comparison with vehicle-treated control hair follicles (T and U), 75 IU/ml IFN-γ-treated human hair matrix has a strong MHC class I immunoreactivity (V and W), which is down-regulated by 1 × 10−8 FK506 (X and Y) in vitro. The mean fluorescence intensity (MFI) was measured at four previously defined reference areas in the hair matrix of anagen hair bulbs (N and O) by NIH image, and the average MFI was calculated [n = 60 hair follicles (HFs) from 3 patients/group]. β2m, β2microglobulin; CTS, connective tissue sheath; DP, dermal papilla.
HLA-B Probe
A HLA-B cDNA plasmid (450 bp) cloned in pSPT 19 was kindly provided by Dr. Elisabeth H. Weiss (Munich, Germany).49 This plasmid recognizes all MHC class Ia transcripts. The plasmid was transformed into Epicurean coli XL1-Blue (Stratagene, La Jolla, CA). Plasmid DNA was isolated by QIAGEN Plasmid Mini Kit (QIAGEN, Hilden, Germany). The sense probe was prepared using the PstI cut fragment (Roche, Mannheim, Germany) and the SP 6 promoter. Antisense probe was prepared with the PuvII cut fragment (Roche) and T7 promoter. Sense and antisense HLA-B probes were labeled with biotin dUTP by Biotin Labeling Mix (Roche).
In Situ Hybridization
In situ hybridization was performed by a kit for the detection of biotinylated probes (DAKO), following the manufacturer’s instructions. Briefly, biotinylated DNA probes were hybridized with acetone-fixed cryosections of human scalp or cultured hair follicles for 90 minutes at 56°C. The slide was immersed for 30 minutes at room temperature in a stringent washing buffer (provided in the kit), followed by incubation with streptavidin-alkaline phosphatase for 20 minutes. Finally, hybridization products were visualized by BCIP/NBT substrate solution for 60 minutes.
RT-PCR Analysis of HLA-B mRNA in Cultured Human Hair Follicles
The expression of mRNA for the HLA-B in cultured hair follicles was determined by semiquantitative reverse transcription-polymerase chain reaction (RT-PCR). 15 hair follicles were obtained from each group from more than 3 patients. (Vehicle, IFN-γ 75 IU/ml, IFN-γ 75 U/ml + IGF-I 100 ng/ml). The total RNA was extracted from proximal hair bulbs using the RN easy kit (QIAGEN). The extracted RNA was reverse-transcribed cDNA by first-strand cDNA Synthesis Kit for RT-PCR (Roche) and was amplified in the UNO-Thermoblock (Biometra, Gottingen, Germany). The primers used in this study were as follows: HLA-B, sense: 5′-CCG GAC TCA GAA TCT CCT CAG-3′, antisense: 5′-AAA CAC AGG TCA GCA TGG GAA C-3′; β-actin, sense: 5′-CGA CAA CGG CTC CGG CAT GTG C-3′, anti-sense: 5′-CGT CAC CGG AGT CCA TCA CGA TGC-3′ (Sigma ARK, Germany).50 Hot start (5 minutes at 94°C), and 30 cycles of amplification at 94°C for 30 seconds, 55°C for 1 minute, and 72°C for 1 minute, followed by a final extension at 72°C for 10 minutes. The obtained PCR products were analyzed by electrophoresis on a 2% agarose gel. The gel was stained with 20 ng/ml ethidium bromide visualized by the ultraviolet transilluminator. The photographed bands were quantified by NIH image. The intensity of mRNA expression was measured by calculating the relative density of PCR bands against β-actin in the same sample.
Results
Normal Anagen Hair Bulbs Express Very Low MHC Class I Protein and mRNA
Low or absent expression of MHC class I protein in the proximal hair follicle epithelium, namely in the keratinocytes of the hair matrix, is the hallmark of hair follicle immune privilege (Figure 1A), compared with the MHC class I expression of the distal outer root sheath (ORS) and epidermal keratinocytes in normal human scalp skin (Figure 1B).7,17,18 However, it had been unknown whether this reflects an unusually low rate of MHC class I transcription. Here, we show by in situ hybridization that HLA-B-related transcript signals in the anagen hair matrix and the proximal ORS (Figure 1C) are substantially weaker than in the epidermis (Figure 1D) or the distal hair follicle epithelium (distal ORS) (Figure 1E) in normal human scalp skin sections.
β2microglobulin and TAP2 Mirror the Follicular Expression of MHC Class I
The stable assembly and the cell surface expression of functional MHC class I molecules not only require intracellular uptake of a self peptide (eg, autoantigen, viral proteins in virus infected cells, mutated cells in tumor cells) but also a complex, tightly regulated sequence of interactions with MHC class I pathway molecules, including β2microglobulin, TAP1, and TAP2.31–33 Therefore, we also studied the follicular expression of these MHC class I pathway molecules by immunohistology. β2microglobulin and TAP2 immunoreactivity mirrored the low or absent expression level of HLA-A/B/C molecules in the proximal ORS and hair matrix of anagen hair bulbs (Figure 1, F and G), compared with distal ORS and epidermis (Figure 1, H and I) in the normal human scalp skin sections. Therefore, this immunoprivileged region of the skin epithelium is characterized by a down-regulation not only of HLA-A/B/C, but also of major MHC class I pathway molecules. This mirrors the situation in murine hair follicles in vivo22 and underscores the relative defect of these follicular tissue compartments in the presentation of self-peptides to CD8+ T cells: the few MHC class I molecules that may be expressed here are likely to form an unstable, functionally impaired antigen-presentation MHC class I complex.51–53
Experimental Imitation of Immune Privilege Collapse: IFN-γ Up-Regulates Ectopic MHC Class I Gene and Protein Expression in Normal Human Hair Follicles
To imitate one key event of immune privilege collapse, ie, the ectopic expression of MHC class I molecules in previously MHC class I-negative tissue sites, normal human scalp hair follicles in the active/growth stage of the hair cycle (anagen VI)54,55 were microdissected and organ-cultured, and treated with low-dose IFN-γ (75 IU/ml). IFN-γ was chosen because we had previously shown in murine back skin that, compared to other cytokines that reportedly act as potent up-regulators of MHC class I expression (eg, IL-1β, TNF-α), only IFN-γ served as strong MHC class I inducer in anagen hair bulbs in vivo.22
By careful titration studies, 75 IU/ml IFN-γ were identified as the minimally effective dose of IFN-γ that sufficed to induce the ectopic MHC class I expression in organ-cultured human anagen hair bulbs, while higher doses (100 to 1000 IU/ml IFN-γ) also caused significant, premature hair follicle regression (catagen) in vitro. Since hair follicles physiologically lose their immune privilege during each catagen phase,7,19,23 because the hair follicles involute during catagen primarily by apoptotic deletion of hair bulb keratinocytes that were those previously MHC class I negative,23,55,56 catagen induction was undesired and had to be avoided for the purpose of this study. Therefore, 75 IU/ml IFN-γ were used in all subsequent experiments.
We evaluated the MHC class I expression in cultured hair follicles using two complementary techniques, TSA (Figure 1, K, M, U, and W, Figure 2, A and B) and DAKO EnVision (Figure J, L, T, and V, Figure 2, C to E). Both techniques revealed that low-dose IFN-γ (75 IU/ml) strongly up-regulated not only MHC class I immunoreactivity in areas of the hair follicle epithelium with previously low or absent MHC class I expression (in particular in the proximal hair matrix), and further enhanced MHC class I in constitutively positive hair follicle compartments (eg, tissue sheath, dermal papilla), but also up-regulated HLA-B mRNA expression in situ in these regions (Figure 3, A and B) in vitro. Given the central importance of MHC class I-mediated autoantigen presentation in immune disorders characterized by immune privilege collapse,7,13,14 this ectopic, IFN-γ-induced MHC class I expression in the proximal epithelium of organ-cultured human anagen hair follicles was exploited as a highly reductionist, yet very pragmatic, easily reproducible, and well-standardized general model for experimentally imitating and manipulating one key event during immune privilege collapse: the ectopic MHC class I expression on the gene and protein level.
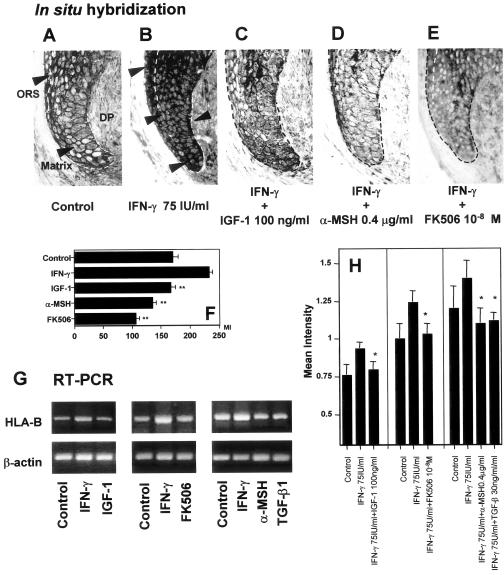
Figure 3.
HLA-B gene expression is down-regulated by IGF-1, α-MSH, TGF-β, and FK506. A: In situ hybridization revealed that HLA-B mRNA expression was very low in control hair matrix and proximal hair ORS which is same as the result shown in Figure 1. B: 75 U/ml IFN-γ strongly up-regulated HLA-B mRNA expression in matrix. C: 100 ng/ml IGF-1 repressed the number of ectopic HLA-B mRNA-like signals. D: 0.4 μg/ml α-MSH repressed the number of ectopic HLA-B mRNA-like signals. E: 1 × 10−8 M FK506 repressed the number of ectopic HLA-B mRNA-like signals. F: The mean intensity of mRNA expression by in situ hybridization was evaluated by NIH image in matrix and distal ORS. G and H: Semiquantitative RT-PCR supports the result by immunohistochemistry that shows IGF-1, FK506, α-MSH, and TGF-β1 inhibits 75 IU/ml IFN-γ-induced ectopic HLA-B mRNA transcription. The mean intensity of each amplified signals were measured and compared with that of β-actin using NIH image software. The level of mean intensity ratio was down-modulated in HF treated with 100 ng/ml IGF-1, 1 × 10−8M FK506, 0.4 μg/ml, and 30 ng/ml TGF-β1. *, P < 0.05 versus IFN-γ-treated hair follicles; ** P < 0.01 versus IFN-γ-treated HFs.
IFN-γ Likely Up-Regulates MHC Class I in the Anagen Hair Bulb via IRF-1
In vitro studies, eg, in neural cells, have established that IFN-γ-induced MHC class I expression is primarily mediated via interferon regulatory factor-1 (IRF-1).57,58 Interestingly, by immunohistology, IRF-1-like immunoreactivity was very weak in the proximal hair matrix and the proximal ORS of anagen hair bulbs in normal human scalp skin sections (Figure 4A), but very prominent in the distal ORS (Figure 4B), the basal layer epidermis (Figure 4C) and throughout the epithelium of catagen follicles (Figure 4D). Instead, compared to vehicle controls (Figure 4E), the hair matrix and proximal ORS of IFN-γ-treated hair follicles show strong expression of IRF-1 (Figure 4F) in vitro. This suggests that IFN-γ likely up-regulates MHC class I in the anagen hair bulb via IRF-1, and that IRF-1 is an important element in the generation, collapse, and restoration of the immune privilege of anagen hair follicles.
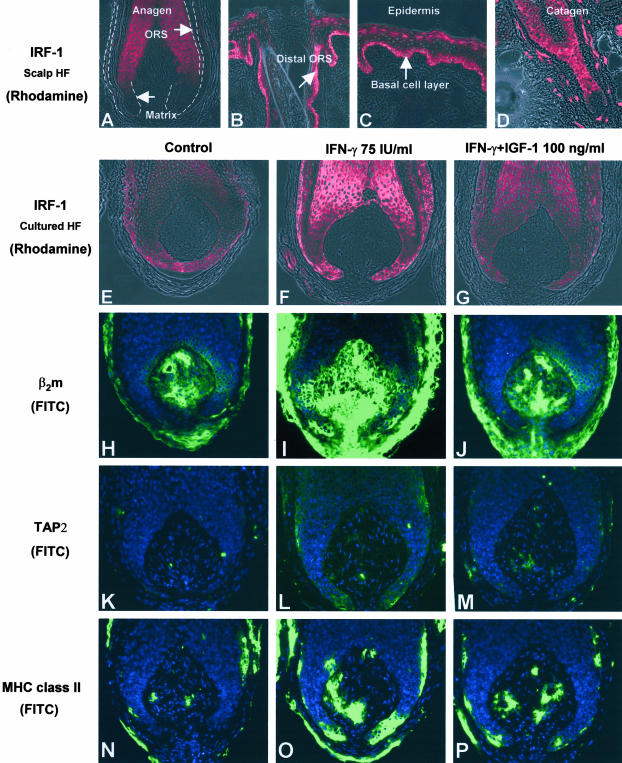
Figure 4.
A: HF matrix has absent or low IRF-1-like-immunoreactivity in anagen hair bulb. B: In anagen hair follicle, IRF-1 immunoreactivity is declined from epidermis to distal ORS. C: Basal layer epidermis shows IRF-1 strong immunoreactivity in human scalp skin. D: Very prominent immunoreactivity throughout the epithelium of catagen follicles. E: Control HF shows low immunoreactivity of IRF-1. F and G: 75 IU/ml IFN-γ up-regulated IRF-1 immunoreactivity in matrix and proximal ORS (F), which is restored by 100 ng/ml IGF-1 (G). H to P: IFN-γ-induced ectopic MHC class I pathway molecules and MHC class II immunoreactivity was down-regulated by IGF-1. H: TSA revealed that control HF has very low immunoreactivity of β2microglobulin like MHC class I expression. I and J: 75 IU/ml IFN-γ up-regulate β2microglobulin immunoreactivity in hair follicle matrix, and down-regulated by 100 ng/ml IGF-1. K to M: IFN-γ-induced ectopic TAP2 immunoreactivity was also down-regulated by 100 ng/ml IGF-1. IGF-1 also down-regulates IFN-γ- induced MHC class II expression. N: The expression of MHC class II in control HFs were very low in matrix and proximal ORS. O to P: Control HF shows a few MHC class-II-positive cells in the connective tissue sheath; 75 IU/ml IFN-γ prominently induced MHC class II expression not only in the connective tissue sheath but also in the proximal ORS (keratinocytes), which could be down-regulated again by 100 ng/ml IGF-1 (P).
TGF-β1, IGF-1, α-MSH, and FK506 Restore Hair Follicle Immune Privilege
Next, we attempted to restore the hair follicle IP by applying a number of agents to the medium that had been reported to down-regulate MHC class I expression in cell culture such as TGF-β1,37 IGF-1,38 α-MSH,39 IL-10,40 and FK506.59 Among the surprisingly few recognized MHC class I suppressors, we selected natural bioregulatory compounds that we and others had shown before to be locally generated in normal murine or human anagen hair bulbs, ie, TGF-β1,25,26 IGF-1,27 and α-MSH,28,29 and also chose IL-10, which was originally characterized as an immunosuppressive cytokine,60,61 hypothesizing that one or several of these factors may be involved in generation and/or maintenance of the normal immune privilege of the anagen hair bulb. If so, we reasoned that they should be capable of suppressing ectopic MHC class I expression. For greater simplicity, IFN-γ-induced ectopic expression of MHC class I in the proximal hair matrix was taken as an indicator of “immune privilege collapse”, while its down-regulation was interpreted as a sign of (at least partial) “immune privilege restoration”.
As shown in Figure 1, N (EnVision) and O (TSA), IGF-1 substantially down-regulated IFN-γ-induced the ectopic MHC class I expression in the proximal hair matrix of normal, organ-cultured human anagen hair bulbs in a dose-dependent manner in vitro (Figure 2A). Both of EnVision and TSA technique confirmed that a similar down-regulation was seen with 0.2 μg/ml or 0.4 μg/ml α-MSH, 15 ng/ml or 30 ng/ml TGF-β1, and 10−8 M FK506 (Figure 1, P to S, X, and Y, and Figure 2, B, C, and E) (0.2 μg/ml α-MSH, 15 ng/ml TGF-β1; data not shown) but not with 100 ng/ml IL-10 (Figure 2D) in vitro. In addition, a variable degree of MHC class I down-regulation was seen also in other constitutively MHC class-I-positive-hair follicle compartments, and in (non-immunoprivileged) areas of the follicle mesenchyme where IFN-γ treatment had strongly up-regulated MHC class I expression (this phenomenon was particularly prominent with α-MSH and TGF-β1; see Figure 1, P to S). This is the first evidence that two growth factors (IGF-1, TGF-β1) and a proopiomelanocortin-derived neuropeptide (α-MSH) can down-regulate MHC class I expression in a normal human tissue in situ. It is intriguing to note that the same agents are also constitutively expressed in the proximal hair follicle epithelium.25–29
IGF-1, α-MSH, and FK506 Inhibit Ectopic MHC Class I Transcription
To clarify whether IGF-1, α-MSH and FK506 also inhibit MHC class I mRNA transcription, in situ hybridization and RT-PCR were used. In situ hybridization revealed that 75 U/ml IFN-γ induced ectopic MHC class I mRNA signals in the hair matrix compared with control (Figure 3, A and B). Corresponding to our immunohistological findings on the protein level (Figure 1, J to Q and T to Y), 100 ng/ml IGF-1, 0.4 μg/ml α-MSH, and 10−8 M FK506 all down-modulated ectopic MHC class I mRNA-related signals (Figure 3, C to E) in cultured hair follicles. The mean intensity of the expression was measured by NIH image (Figure 3F). Semiquantitative RT-PCR on mRNA extracted from microdissected, cultured hair bulbs supported that IGF-1, α-MSH, TGF-β1, and FK506 indeed inhibit IFN-γ induced MHC class I mRNA transcription (Figure 3, G and H).
IGF-1 Possibly Down-Regulates MHC Class I Expression via IRF-1
To probe the mechanism of MHC class I down-regulation, we studied how IGF-1 affected IRF-1 expression. As shown by immunohistology, the immunoreactivity for IRF-1 was down-regulated in IGF-1-treated hair matrix and proximal ORS in vitro (Figure 4G), compared to the hair follicles that had been treated only with IFN-γ (Figure 4F). Even though conclusive evidence for this remains to be provided, this is in line with the concept that MHC class I protein expression is suppressed by IGF-1 via direct or indirect IRF-1 down-regulation, since IRF-1 has recently been identified as the most important enhancer of MHC class I gene promoter activity.57,58
IGF-1 Down-Regulates IFN-γ-Induced Ectopic β2microglobulin and TAP2 Expression in the Hair Matrix
Furthermore, we checked whether IGF-1 also modulates the expression of the important MHC class I pathway molecules, β2microglobulin and TAP2, since this would further impair potential (auto)antigen presentation via MHC class I molecules. Because IGF-1 had given the strongest and best-reproducible MHC class I down-regulatory effects, we studied hair follicles that had first been treated by IFN-γ, followed by IGF-1. β2microglobulin immunoreactivity, which is very low in the matrix of control hair follicles, was up-regulated by 75 U/ml IFN-γ compared to controls (Figure 4, H and I). This ectopic expression in the hair matrix was down-regulated again by 100 ng/ml IGF-1 in vitro (Figure 4J).
In vitro, TAP is generally stimulated by IFN-γ.62,63 Here we show that IFN-γ indeed up-regulates TAP2 also in immunoprivileged areas of the human hair follicle epithelium, ie, in the hair matrix and the proximal ORS (Figure 4, K and L), exactly as we had previously seen in murine back skin hair follicles in vivo.22 After IGF-1 treatment, TAP2 immunoreactivity declined in the hair matrix and proximal ORS (Figure 4M). This suggests that IGF-1 can also suppress effective self-antigen presentation via inhibiting the TAP-mediated transport of proteasome-digested autoantigenic peptides into the endoplasmic reticulum, where they are taken up by MHC class I.30–33
Interestingly, constitutively MHC class I+ fibroblasts of the follicular dermal papilla (Figure 1A), whose level of MHC class I expression is further augmented by IFN-γ treatment (Figure 1, L, M, V, and W), became virtually TAP2-negative after IGF-1 treatment in vitro (Figure 4M). IGF-1 treatment may, therefore, also impair antigen presentation to CD8+ T cells in this crucial mesenchymal “command center” of the hair follicle54,55 since it would be expected that (auto)antigens cannot be transported into the endoplasmic reticulum because of insufficient availability of TAP2.51–53 This finding is particularly intriguing in the context of the most frequent autoimmune disease of human hair follicles alopecia areata,7,12 where an autoimmune attack on the follicle mesenchyme (ie, the dermal papilla) has also been implicated in the pathogenesis.64
IGF-1 Also Down-Regulates IFN-γ-Induced Ectopic MHC Class II Expression
In normal skin epithelium, MHC class II is only expressed on professional antigen presenting cells (Langerhans cells).65 However, MHC class II is not expressed on the very few Langerhans cells that can occasionally be detected in human anagen hair bulbs by electromicroscopy or CD1a staining, which suggests that these are functionally impaired in this immunoprivileged region of the hair follicle.7,18 Under pro-inflammatory conditions, namely after IFN-γ stimulation, many skin cell types (including keratinocytes) can become non-professional antigen presenting cells via up-regulation of MHC class II.66,67 If this happens in an area of relative immune privilege, this must be expected to further accelerate and aggravate immune privilege collapse.7,12,67
Therefore, it is interesting to note that control hair follicles, as expected, show MHC class-II-positive cells only on a few cells in connective tissue sheath in vitro (Figure 4N). Then, 75 U/ml IFN-γ, as expected, prominently induces MHC class II expression in the proximal ORS (Figure 4O), which can be down-regulated again by 100 ng/ml IGF-1 in cultured hair follicles (Figure 4P).
Discussion
Here, we propose that human scalp hair follicles offer an ideal, far-too-long ignored model for the dissection of general principles of immune privilege generation, maintenance, and collapse. We show that this model also helps to identify novel, and hopefully more effective strategies for immune privilege restoration via MHC class I-down-modulation wherever this would be clinically beneficial (eg, multiple sclerosis,9 autoimmune uveitis,3,10 mumps orchitis,11 alopecia areata,7,12 autoimmune chronic acute hepatitis,13,14 and fetal rejection4,5,15). Furthermore, this new immune privilege model allows one to dissect the essentially unknown controls of MHC class I down-regulation in neuroectodermally derived human tissue compartments in situ.
Our data indicate that hair matrix and proximal ORS are largely negative for MHC class I not only on the protein, but also on the mRNA level (Figure 1, A and C) and that this is accompanied by a relatively low expression of important MHC class I pathway molecules (TAP2 and β2microglobulin) (Figure 1, F and G). Since TAP subunits are critical for MHC class I assembly and thereby for antigen translocation into the endoplasmic reticulum, while β2microglobulin is needed for the stabilization of MHC class I and endogenous antigen complex, this coordinated down-regulation of MHC class I, TAP2 and β2microglobulin molecules in the anagen hair matrix can be expected to severely hinder (self-)antigen presentation in the human anagen hair bulb. Since all keratinocytes in the anagen hair bulb arise from MHC class I+ stem cells in the bulge region of the ORS or the secondary hair germ,55,68,69 spontaneous anagen development55 actually offers a most intriguing natural model that only awaits exploitation in molecular and ultrastructural studies how exactly MHC class I transcription/translation and surface expression as well as the expression of associated MHC class I-pathway molecules are cyclically shut-down in selected “transit amplifying” cells that arise from this epithelial stem cell pool.68,69 Due to the virtual impossibility of studying early stages of anagen development in the human hair follicle, we are currently exploring this in depilation-induced anagen during the murine hair cycle. Since the data reported here designate α-MSH, TGF-β1, and IGF-1 reasonable candidates as major constitutive, anagen-associated MHC class I suppressors, we are focusing on expression switches of the corresponding genes in the bulge and secondary hair germ during anagen I-III.
We show that the prototypic pro-inflammatory cytokine IFN-γ is a key inducer of ectopic MHC class I expression (Figure 1, L, M, V, and W), likely via IRF-1 (Figure 4F), the most important enhancer of MHC class I gene promoter activity.57,58 That IFN-γ also up-regulates the MHC class I pathway molecules β2microglobulin and TAP2 in the anagen hair bulb (Figure 4, I and L) underscores the potency of this cytokine as an instigator of immune privilege collapse and designates IFN-γ-induced signaling a prime molecular target for immune intervention in MHC class I-dependent autoimmune disease and transplant rejection. Recently, IFN-κ, a novel type I interferon, was identified in human keratinocytes, which is significantly enhanced by IFN-γ.70 Therefore, IFN-κ may also play a role in our current model.
While the relative immune privilege of the anagen hair bulb had been noted before,17,19 it has remained elusive how the low or absent MHC class I expression that appears to be at the basis of hair follicle immune privilege is controlled.7 Intriguingly, the current study suggests that locally generated, secreted molecules, which have previously been recognized as potent modulators of hair follicle growth cycling and/or pigmentation,55,71,72 ie, TGF-β125,26 IGF-1,27 α- MSH,28,29 are likely candidates as constitutive down-modulators of MHC class I expression in the proximal hair follicle epithelium (Figure 1, N to S). This is the first report to identify natural immunomodulators capable of down-regulating MHC class I expression in situ in a normal, neuroectoderm-derived human tissue. IGF-1 even suppresses the MHC class I pathway molecules TAP2 and β2microglobulin (Figure 4, J and M) as well as ectopic MHC class II expression (Figure 4P), thus underscoring its potency as an “immune privilege restorer”.
This finding is exciting since, to the best of our knowledge, this is not only the first report implicating this well-known growth factor for fibroblasts and keratinocytes73 in immunosuppressive activities, but also since IGF-1 is locally generated in the hair follicle, where it is recognized as a key hair growth modulator: IGF-1 mRNA and protein are abundantly expressed in various hair follicle compartments, including the human hair bulb,27 which also expresses IGF-1 receptors.25,42 Physiological concentrations of IGF-1 are potent stimulators of hair follicle growth, and suppress hair follicle regression (catagen).74 Though it remains to be dissected how IGF-1 down-regulates IFN-γ-induced ectopic MHC class I expression, in the current study, IRF-1 expression in situ was inhibited by IGF-1 (Figure 4G), suggesting a key role for IRF-1.
TGF-β1 is also locally expressed in murine and human hair follicles,25,26 and is a potent immunosuppressor.3,75,76 That TGF-β1 can down-regulate IFN-γ-induced follicular MHC class I expression in situ (Figure 1, R and S) fits well to the available in vitro data.37 The proopiomelanocortin-derived neuropeptide α-MSH is expressed prominently in the hair matrix,71,72 and is also appreciated for its potent immunosuppressive activity.77,78,79 For example, α-MSH down-regulates the activity or production of many cytokines, including IL-1β, IL-6, TNF-α, and IFN-γ, and may as a natural cytokine antagonist.77,80 Here, we provide the first available evidence that this melanocortin also is an effective suppressor of ectopic MHC class I expression in situ (Figure 1, P and Q, Figure 2, B and C, and Figure 3, D, F, G, and H), which strongly supports an isolated preliminary report of MHC class I inhibition by α-MSH in an epithelial cell line (A431) in vitro.39
In contrast, the potent natural immunosuppressant IL-10,81,82 which reportedly can down-regulate MHC class I expression in human melanoma cells in vitro40 and thus may aid in tumor escape from immunosurveillance, failed to suppress IFN-γ-induced ectopic MHC class I expression in the human hair bulb in situ (Figure 2D). Interestingly, IL-10-deficient mice are actually less susceptible to developing alopecia areata than wild-type controls,83 while transgenic expression of IL-10 in pancreatic islet cells of non-obese diabetic mice accelerates autoimmune insulinitis and diabetes.84 Together with these reports, the failure of IL-10 to inhibit MHC class I expression in the human hair bulb further questions the conventional wisdom of IL-10 as an “immunosuppressant.”81,82
That the potently immunosuppressive immunophilin ligand, FK506, markedly down-modulates epithelial MHC class I expression in organ-cultured human hair follicles (Figure 1, X and Y, and Figure 2E) is a clinically most encouraging finding: on the one hand, FK506 is already widely used in clinical medicine, eg, for the suppression of liver transplant rejection,85 on the other, FK506 has already been shown to reverse hair loss in murine and rat models of alopecia areata.86 Therefore, the current study identifies with FK506 a widely available, registered drug that could, in principle, be tested immediately for its MHC class I down-regulating capacity in a wide range of clinical conditions where this is therapeutically desirable (eg, multiple sclerosis,9 autoimmune uveitis,10 mumps orchitis,11 autoimmune chronic active hepatitis,13,14 and alopecia areata7,12). In addition, the natural, locally generated MHC class I suppressors IGF-1, TGF-β1, and α-MSH, also are promising candidates for immune privilege restoration and for MHC class I suppression whenever this is clinically desired. In particular, α-MSH carries a very low risk of toxicity, since the systemic administration of α-MSH in man is very well tolerated.87,88
Although the role of NK and NK-T cells in MHC class-I-negative hair follicle immune privilege remains to be elucidated,7 the current study already opens new therapeutic perspectives not only in alopecia areata, where MHC class I suppression may well be the key to achieving the long-awaited, yet still elusive therapeutic breakthrough,12 but also in other clinical conditions where an efficient down-regulation of MHC class-presented self- or non-self-antigens would be of great therapeutic value.
Acknowledgments
We thank Dr. Elisabeth H. Weiss (Munich, Germany) for providing HLA-B cDNA plasmid for in situ hybridization, and S. Wegerich, G. Pilnitz-Stolze, and M. Dietrich for excellent technical assistance.
Footnotes
Address reprint requests to Ralf Paus, M.D., Department of Dermatology, University Hospital Hamburg-Eppendorf, Martinistr. 52 D-20246 Hamburg, Germany. E-mail: paus@uke.uni-hamburg.de.
Supported in part by a grant from Cutech Srl, Padova (to R. P.).
References
- Head JR, Billingham RE. Immunologically privileged sites in transplantation immunology and oncology. Perspect Biol Med. 1985;29:115–131. doi: 10.1353/pbm.1985.0038. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Immune privilege as the result of local tissue barriers and immunosuppressive microenvironments. Curr Opin Immunol. 1993;5:428–432. doi: 10.1016/0952-7915(93)90064-y. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY. Immunology and immunomodulation of corneal transplantation. Int Rev Immunol. 2002;21:173–196. doi: 10.1080/08830180212064. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. Immunology at the maternal-fetal interface: lessons for T cell tolerance and suppression. Annu Rev Immunol. 2000;18:367–391. doi: 10.1146/annurev.immunol.18.1.367. [DOI] [PubMed] [Google Scholar]
- Erlebacher A. Why isn’t the fetus rejected? Curr Opin Immunol. 2001;13:590–593. doi: 10.1016/s0952-7915(00)00264-8. [DOI] [PubMed] [Google Scholar]
- Fuzzi B, Rizzo R, Criscuoli L, Noci I, Melchiorri L, Scarselli B, Bencini E, Menicucci A, Baricordi OR. HLA-G expression in early embryos is a fundamental prerequisite for the obtainment of pregnancy. Eur J Immunol. 2002;32:311–315. doi: 10.1002/1521-4141(200202)32:2<311::AID-IMMU311>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Paus R, Ito N, Takigawa M, Ito T. The hair follicle and immune privilege. J Invest Dermatol Symp Proc. 2003;8:188–194. doi: 10.1046/j.1087-0024.2003.00807.x. [DOI] [PubMed] [Google Scholar]
- Menier C, Riteau B, Dausset J, Carosella ED, Rouas-Freiss N. HLA-G truncated isoforms can substitute for HLA-G1 in fetal survival. Hum Immunol. 2000;61:1118–1125. doi: 10.1016/s0198-8859(00)00194-4. [DOI] [PubMed] [Google Scholar]
- Bruno R, Sabater L, Sospedra M, Ferrer-Francesch X, Escudero D, Martinez-Caceres E, Pujol-Borrell R. Multiple sclerosis candidate autoantigens except myelin oligodendrocyt glycoprotein are transcribed in human thymus. Eur J Immunol. 2002;32:2737–2747. doi: 10.1002/1521-4141(2002010)32:10<2737::AID-IMMU2737>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Streilein JW, Ohta K, Mo JS, Taylor AW. Ocular immune privilege and the impact of intraocular inflammation. DNA Cell Biol. 2002;21:453–459. doi: 10.1089/10445490260099746. [DOI] [PubMed] [Google Scholar]
- Filippini A, Riccioli A, Padula F, Lauretti P, D’Alessio A, De Cesaris P, Gandini L, Lenzi A, Ziparo E. Control and impairment of immune privilege in the testis and in semen. Hum Reprod Update. 2001;7:444–449. doi: 10.1093/humupd/7.5.444. [DOI] [PubMed] [Google Scholar]
- Paus R, Slominski A, Czarnetzki BM. Is alopecia areata an autoimmune-response against melanogenesis-related proteins, exposed by abnormal MHC class I-expression in the anagen hair bulb? Yale J Biol Med. 1994;66:541–554. [PMC free article] [PubMed] [Google Scholar]
- Lobo-yeo A, Senaldi G, Portmann B, Mowat AP, Mieli-Vergani G, Vergani D. Class I and class II major histocompatibility complex antigen expression on hepatocytes: a study in children with liver disease. Hepatology. 1990;12:224–232. doi: 10.1002/hep.1840120208. [DOI] [PubMed] [Google Scholar]
- Franco A, Barnaba V, Natali P, Balsano C, Musca A, Balsano F. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology. 1988;8:449–454. doi: 10.1002/hep.1840080302. [DOI] [PubMed] [Google Scholar]
- Weetman AP. The immunology of pregnancy. Thyroid. 1999;9:643–646. doi: 10.1089/thy.1999.9.643. [DOI] [PubMed] [Google Scholar]
- Billingham RE, Silvers WK. A biologist’s reflections on dermatology. J Invest Dermatol. 1971;57:227–240. doi: 10.1111/1523-1747.ep12261543. [DOI] [PubMed] [Google Scholar]
- Harrist TJ, Ruiter D, Mihm MC, Jr, Bhan AK. Distribution of major histocompatibility antigen in normal skin. Br J Dermatol. 1983;109:623–633. doi: 10.1111/j.1365-2133.1983.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Christoph T, Müller-Rover S, Audring H, Tobin DJ, Hermes B, Cotsarelis G, Rückert R, Paus R. The human hair immune system: cellular composition and immune privilege. Br J Dermatol. 2000;142:862–873. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- Westgate GE, Craggs RI, Gibson WT. Immune privilege in hair growth. J Invest Dermatol. 1991;97:417–420. doi: 10.1111/1523-1747.ep12481002. [DOI] [PubMed] [Google Scholar]
- Paus R, Eichmüller S, Hofmann U, Czarnetzki BM, Robinson P. Expression of classical and non-classical MHC class I antigens in murine hair follicles. Br J Dermatol. 1994;131:171–183. doi: 10.1111/j.1365-2133.1994.tb08488.x. [DOI] [PubMed] [Google Scholar]
- Paus R, Christoph T, Müller-Röver S. Immunology of the hair follicle: a short journey into terra incognita. J Invest Dermatol Symp Proc. 1999;4:226–234. doi: 10.1038/sj.jidsp.5640217. [DOI] [PubMed] [Google Scholar]
- Rückert R, Hofmann U, van der Veen C, Bulfone-Paus S, Paus R. MHC class I expression in murine skin: developmentally controlled and strikingly restricted intraepithelial expression during hair follicle morphogenesis and cycling, and response to cytokine treatment in vivo. J Invest Dermatol. 1998;111:25–30. doi: 10.1046/j.1523-1747.1998.00228.x. [DOI] [PubMed] [Google Scholar]
- Paus R, Hofmann U, Eichmüller S, Czarnetzki BM. Distribution and changing density of gamma-delta T cells in murine skin during the induced hair cycle. Br J Dermatol. 1994;130:281–289. doi: 10.1111/j.1365-2133.1994.tb02922.x. [DOI] [PubMed] [Google Scholar]
- Paus R, van der Veen C, Eichmüller S, Kopp T, Hagen E, Müller-Röver S, Hofmann U. Generation and cyclic remodeling of the hair follicle immune system in mice. J Invest Dermatol. 1998;111:7–18. doi: 10.1046/j.1523-1747.1998.00243.x. [DOI] [PubMed] [Google Scholar]
- Welker P, Foitzik K, Bulfone-Paus S, Henz BM, Paus R. Hair cycle-dependent changes in the gene expression and protein content of transforming factor beta 1 and beta 3 in murine skin. Arch Dermatol Res. 1997;289:554–557. doi: 10.1007/s004030050239. [DOI] [PubMed] [Google Scholar]
- Foitzik K, Lindner G, Müller-Röver S, Maurer M, Botchkareva N, Botchkarev V, Handjiski B, Metz M, Hibino T, Soma T, Dotto GP, Paus R. Control of murine hair follicle regression (catagen) by TGF-β1 in vivo. FASEB J. 2000;14:752–760. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- Rudman SM, Philpott MP, Thomas GA, Kealey T. The role of IGF-I in human skin and its appendages: morphogen as well as mitogen? J Invest Dermatol. 1997;109:770–777. doi: 10.1111/1523-1747.ep12340934. [DOI] [PubMed] [Google Scholar]
- Slominski A, Heasley D, Mazurkiewicz JE, Ermak G, Baker J, Carlson JA. Expression of proopiomelanocortin (POMC)-derived melanocyte-stimulating hormone (MSH) and adrenocorticotropic hormone (ACTH) peptides in skin of basal cell carcinoma patients. Hum Pathol. 1999;30:208–215. doi: 10.1016/s0046-8177(99)90278-2. [DOI] [PubMed] [Google Scholar]
- Kono M, Nagata H, Umemura S, Kawana S, Osamura Y. In situ expression of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skin. EMBO J. 2001;15:2297–2299. doi: 10.1096/fj.01-0254fje. [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr How the immune system protects the host from infection. Microbes Infect. 2001;13:1167–1171. doi: 10.1016/s1286-4579(01)01477-0. [DOI] [PubMed] [Google Scholar]
- Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- Momburg F, Tan P. Tapasin: the keystone of the loading complex optimizing peptide binding by MHC class I molecules in the endoplasmic reticulum. Mol Immunol. 2002;39:217–233. doi: 10.1016/s0161-5890(02)00103-7. [DOI] [PubMed] [Google Scholar]
- Bouvier M. Accessory proteins and the assembly of human class I MHC molecules: a molecular and structural perspective. Mol Immunol. 2003;39:697–706. doi: 10.1016/s0161-5890(02)00261-4. [DOI] [PubMed] [Google Scholar]
- Kooy AJ, Prens EP, Van Heukelum A, Vuzevski VD, Van Joost T, Tank B. Interferon-γ-induced ICAM-1 and CD40 expression, complete lack of HLA-DR and CD80 (B7.1), and inconsistent HLA-ABC expression in basal cell carcinoma: a possible role for interleukin-10? J Pathol. 1999;187:351–357. doi: 10.1002/(SICI)1096-9896(199902)187:3<351::AID-PATH227>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, Bonish B, Bacon P, Qin JZ, Denning MF, Foreman K, Diaz MO, Robinson J, Nickoloff BJ. Role for Id-1 in immunobiology of normal keratinocytes and in basal cell carcinoma. Exp Dermatol. 2003;12:255–260. doi: 10.1034/j.1600-0625.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- Philpott MP, Green MR, Kealey T. Human hair growth in vitro. J Cell Sci. 1990;97:463–471. doi: 10.1242/jcs.97.3.463. [DOI] [PubMed] [Google Scholar]
- Napolitano G, Montani V, Giuliani C, Di Vincenzo S, Bucci I, Todisco V, Laglia G, Coppa A, Singer DS, Nakazato M, Kohn LD, Colletta G, Monaco F. Transforming growth factor-β I down-regulation of major histocompatibility complex class I in thyrocytes: coordinate regulation of two separate element by thyroid-specific as well as ubiquitous transcription factors. Mol Endocrinol. 2000;14:486–505. doi: 10.1210/mend.14.4.0454. [DOI] [PubMed] [Google Scholar]
- Saji M, Moriarty J, Ban Singer DS, Kohn LD, Singer D. Hormonal regulation of major histocompatibility complex class I genes in rat thyroid FRTL-5 cells: thyroid-stimulating hormone induces a cAMP-mediated decrease in class I expression. Proc Natl Acad Sci USA. 1992;89:1944–1948. doi: 10.1073/pnas.89.5.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer E, Köck A, Schwarz T, Lugar TA: Regulation of MHC class I antigen expression by keratinocyte alpha-MSH. Arch Dermatol Res 1993, 258:56 (Abstract) [Google Scholar]
- Yue FY, Dummer R, Geertsen R, Hofbauer G, Laine E, Manolio S, Burg G. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class-I, HLA class-II and ICAM-1 molecules. Int J Cancer. 1997;16:630–637. doi: 10.1002/(sici)1097-0215(19970516)71:4<630::aid-ijc20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Philpott MP, Sanders D, Westgate GE, Kealey T. Human hair growth in vitro: a model for the study of hair follicle biology. J Dermatol Sci. 1994;7:S55–S72. doi: 10.1016/0923-1811(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Hodak E, Gottlieb AB, Anzilotti M, Krueger JG. The insulin-like growth factor 1 receptor is expressed by epithelial cells with proliferative potential in human epidermis and skin appendages: correlation of increased expression with epidermal hyperplasia. J Invest Dermatol. 1996;106:564–570. doi: 10.1111/1523-1747.ep12344044. [DOI] [PubMed] [Google Scholar]
- Wraight CJ, Werther A. Insulin-like growth factor-I and epidermal growth factor regulate insulin-like growth factor binding protein-3 (IGFBP-3) in the human keratinocyte cell line HaCaT. J Invest Dermatol. 1995;105:602–607. doi: 10.1111/1523-1747.ep12323716. [DOI] [PubMed] [Google Scholar]
- Redondo P, García-Foncillas J, Okroujnov I, Bandrés E. α-MSH regulates interleukin-10 expression by human keratinocytes. Arch Dermatol Res. 1998;290:425–428. doi: 10.1007/s004030050330. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Eicheler W, Huth A, Wenzel E, Happle R. Cytokines and growth factors influence hair growth in vitro. Possible implications for the pathogenesis and treatment of alopecia areata. Arch Dermatol Res. 1996;288:153–156. doi: 10.1007/BF02505825. [DOI] [PubMed] [Google Scholar]
- Paterson IC, Patel V, Sandy JR, Prime SS, Yeudall WA. Effects of transforming growth factor β-1 on growth-regulatory genes in tumour-derived human oral keratinocytes. Br J Cancer. 1995;72:922–927. doi: 10.1038/bjc.1995.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A, Matsue H, Shibaki A, Kawashima T, Kobayashi H, Ohkawara A. The effects of cyclosporin A and FK506 on proliferation and IL-8 production of cultured human keratinocytes. J Dermatol Sci. 1995;10:130–138. doi: 10.1016/0923-1811(95)00395-9. [DOI] [PubMed] [Google Scholar]
- Roth KA, Adler K, Bobrow MN. Enhanced tyramide signal amplification immunohistochemical detection. J Histochem Cytochem. 1999;47:1644–1645. [PubMed] [Google Scholar]
- Pohla H, Kuon W, Tabaczewski P, Doerner C, Weiss EH. Allelic variation in HLA-B and HLA-C sequences and the evolution of the HLA-B alleles. Immunogenetics. 1998;29:297–307. doi: 10.1007/BF00352839. [DOI] [PubMed] [Google Scholar]
- Willers J, Urosevic M, Laine E, Geertsen R, Kundig T, Burg G, Dummer R. Decreased intraindividual HLA-class I expression is due to reduced transcription in advanced melanoma and dose not correlate with HLA-G expression. J Invest Dermatol. 2001;117:1498–1504. doi: 10.1046/j.0022-202x.2001.01586.x. [DOI] [PubMed] [Google Scholar]
- Song R, Porgador A, Harding CV. Peptide-receptive class I major histocompatibility complex molecules on TAP-deficient and wild-type cells and their roles in the processing of exogenous antigens. Immunology. 1999;97:316–324. doi: 10.1046/j.1365-2567.1999.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankat-Buttgereit B, Tampe R. The transporter associated with antigen processing: function and implications in human diseases. Physiol Rev. 2002;82:187–204. doi: 10.1152/physrev.00025.2001. [DOI] [PubMed] [Google Scholar]
- Ritz U, Seliger B. The transporter associated with antigen processing (TAP): structural integrity, expression, function, and its clinical relevance. Mol Med. 2001;7:149–158. [PMC free article] [PubMed] [Google Scholar]
- Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R. Analysis of apoptosis during hair follicle regression (catagen). Am J Pathol. 1997;151:1601–1719. [PMC free article] [PubMed] [Google Scholar]
- Jarosinski KW, Massa PT. Interferon regulatory factor-1 is required for interferon-γ-induced MHC class I genes in astrocytes. J Neuroimmunol. 2002;122:74–84. doi: 10.1016/s0165-5728(01)00467-2. [DOI] [PubMed] [Google Scholar]
- Massa PT, Hsiaoling WU. Interferon regulatory factor element and interferon regulatory factor 1 in the induction of major histocompatibility complex I gene in neural cells. J Interferon Cytokine Res. 1995;15:799–810. doi: 10.1089/jir.1995.15.799. [DOI] [PubMed] [Google Scholar]
- Panhans-Gross A, Novak N, Kraft S, Bieber T. Human epidermal Langerhans cells are targets for the immunosuppressive macrolide tacrolimus (FK506). J Allergy Clin Immunol. 2001;107:345–352. doi: 10.1067/mai.2001.112600. [DOI] [PubMed] [Google Scholar]
- Fiorentina DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th2 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatelia MA, Meda L, Gasperini S, Calzetti F, Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994;179:1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer R, Baron J, Dagtekin G, Jahnen-Dechent W, Zwadlo-Klarwasser G. Differential regulation of the expression of transporters associated with antigen processing, TAP1 and TAP2, by cytokines and lipopolysaccharide in primary human macrophages. Inflamm Res. 2002;51:403–408. doi: 10.1007/pl00000321. [DOI] [PubMed] [Google Scholar]
- Elbe-Burger A, Mommaas AM, Prieschl EE, Fiebiger E, Baumruker T, Stingl G. Major histocompatibility complex class II-fetal skin dendritic cells are potent accessory cells of polyclonal T-cell responses. Immunology. 2000;101:242–253. doi: 10.1046/j.1365-2567.2000.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin DJ, Fenton DA. Kendall MD, Cell degeneration in alopecia areata. An ultrastructural study. Am J Dermatopathol. 1991;13:248–256. doi: 10.1097/00000372-199106000-00006. [DOI] [PubMed] [Google Scholar]
- Soos JM, Krieger JI, Stuve O, King CL, Patarroyo JC, Aldape K, Wosik K, Slavin AJ, Nelson PA, Antel JP, Zamvil SS. Malignant glioma cells use MHC class II transactivator (CIITA) promoters III and IV to direct IFN-gamma-inducible CIITA expression and can function as nonprofessional antigen presenting cells in endocytic processing and CD4(+) T-cell activation. Glia. 2001;36:391–405. doi: 10.1002/glia.1125. [DOI] [PubMed] [Google Scholar]
- Sundstrom JB, Ansari AA. Comparative study of the role of professional versus semiprofessional or nonprofessional antigen presenting cells in the rejection of vascularized organ allografts. Transpl Immunol. 1995;3:273–289. doi: 10.1016/0966-3274(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Landau M, Assy B, Shalaginov R, Serafimovich S, Kalish RS. Mediation of alopecia areata by cooperation between CD4+ and CD8+ T lymphocytes: transfer to human scalp explants on Prkdc (scid) mice. Arch Dermatol. 2002;138:916–922. doi: 10.1001/archderm.138.7.916. [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Panteleyev AA, Jahoda CA, Christiano AM. Hair follicle predetermination. J Cell Sci. 2001;114:3419–3431. doi: 10.1242/jcs.114.19.3419. [DOI] [PubMed] [Google Scholar]
- LaFleur DW, Nardelli B, Tsareva T, Mather D, Feng P, Semenuk M, Taylor K, Buergin M, Chinchilla D, Roshke V, Chen G, Ruben SM, Pitha PM, Coleman TA, Moore PA. Interferon-κ, a novel type I interferon expressed in human keratinocytes. J Biol Chem. 2001;276:39765–39771. doi: 10.1074/jbc.M102502200. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Wortsman J. On the potential role of proopiomelanocortin in skin physiology and pathology. Mol Cell Endocrinol. 1993;93:C1–C6. doi: 10.1016/0303-7207(93)90131-3. [DOI] [PubMed] [Google Scholar]
- Paus R, Botchkarev VA, Botchkareva NV, Mecklenburg L, Luger T, Slominski A. The skin POMC system (SPS). Leads and lessons from the hair follicle. Ann NY Acad Sci. 1999;885:350–363. doi: 10.1111/j.1749-6632.1999.tb08690.x. [DOI] [PubMed] [Google Scholar]
- Tavakkol A, Varani J, Elder JT, Zoubolis CZ. Maintenance of human skin in organ culture: role for insulin-like growth factor-1 receptor and epidermal growth factor receptor. Arch Dermatol Res. 1999;291:643–651. doi: 10.1007/s004030050469. [DOI] [PubMed] [Google Scholar]
- Philpott MP, Sanders DA, Kealey T. Effects of insulin and insulin-like growth factors on cultured human hair follicles: IGF-I at physiologic concentrations is an important regulator of hair follicle growth in vitro. J Invest Dermatol. 1994;102:857–861. doi: 10.1111/1523-1747.ep12382494. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85–91. doi: 10.1016/s0165-2478(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Taylor AW. Aqueous humor induces transforming growth factor-β (TGF-β)-producing regulatory T-cells. Curr Eye Res. 1997;16:900–908. doi: 10.1076/ceyr.16.9.900.5043. [DOI] [PubMed] [Google Scholar]
- Luger TA, Scholzen T, Brzoska T, Becher E, Slominski A, Paus R. Cutaneous immunomodulation and coordination of skin stress responses by α-melanocyte-stimulating hormone. Ann NY Acad Sci. 1998;840:381–394. doi: 10.1111/j.1749-6632.1998.tb09577.x. [DOI] [PubMed] [Google Scholar]
- Gatti S, Colombo G, Buffa R, Turcatti F, Garofalo L, Carboni N, Ferla L, Fassati LR, Lipton JM, Catania A. α-Melanocyte-stimulating hormone protects the allograft in experimental heart transplantation. Transplantation. 2002;74:1678–1684. doi: 10.1097/00007890-200212270-00005. [DOI] [PubMed] [Google Scholar]
- Lipton JM, Catania A. Anti-inflammatory actions of the neuroimmunomodulator α-MSH. Immunol Today. 1997;18:140–145. doi: 10.1016/s0167-5699(97)01009-8. [DOI] [PubMed] [Google Scholar]
- Oktar BK, Alican I. Modulation of the peripheral and central inflammatory responses by α-melanocyte stimulating hormone. Curr Pro Pep Sci. 2002;3:623–628. doi: 10.2174/1389203023380396. [DOI] [PubMed] [Google Scholar]
- Bellinghausen I, Knop J, Saloga J. The role of interleukin 10 in the regulation of allergic immune responses. Int Arch Allergy Immunol. 2001;126:97–101. doi: 10.1159/000049499. [DOI] [PubMed] [Google Scholar]
- Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103:131–136. doi: 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyschmidt-Paul P, McElwee KJ, Happle R, Kissling S, Wenzel E, Sundberg JP, Zoller M, Hoffmann R. Interleukin-10-deficient mice are less susceptible to the induction of alopecia areata. J Invest Dermatol. 2002;119:980–982. doi: 10.1046/j.1523-1747.2002.00230.x. [DOI] [PubMed] [Google Scholar]
- Moritani M, Yoshimoto K, Tashiro F, Hashimoto C, Miyazaki J, Ii S, Kudo E, Iwahana H, Hayashi Y, Sano T, Itakura M. Transgenic expression of IL-10 in pancreatic islet A cells accelerates autoimmune insulitis and diabetes in non-obese diabetic mice. Int Immunol. 1994;6:1929–1936. doi: 10.1093/intimm/6.12.1927. [DOI] [PubMed] [Google Scholar]
- Cohen SM. Current immunosuppression in liver transplantation. Am J Ther. 2002;9:119–125. doi: 10.1097/00045391-200203000-00006. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Rushton DH, Trachy R, Oliver RF. Topical FK506: a potent immunotherapy for alopecia areata? Studies using the Dundee experimental bald rat model. Br J Dermatol. 1997;137:491–497. doi: 10.1111/j.1365-2133.1997.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Lerner AB, McGuire JS. Effect of α- and β-melanocyte stimulating hormones on the skin colour of man. Nature. 1961;189:176–179. doi: 10.1038/189176a0. [DOI] [PubMed] [Google Scholar]
- Bernasconi S, Torresani T, Illig R. The effect of a-MSH on plasma growth hormone, cortisol and TSH in children. J Clin Endocrinol Metab. 1974;40:759–763. doi: 10.1210/jcem-40-5-759. [DOI] [PubMed] [Google Scholar]