Abstract
We evaluated the significance of aberrant DNA methyltransferase 1 (DNMT1) protein expression during gastric carcinogenesis. The protein expression of DNMT1, Muc2, human gastric mucin, E-cadherin, and proliferating cell nuclear antigen was examined immunohistochemically in gastric cancers and corresponding noncancerous mucosae from 134 patients. The DNA methylation status of the CpG islands of the p16, human MutL homologue 1 (hMLH1), E-cadherin, and thrombospondin-1 (THBS-1) genes and the methylated in tumor (MINT)-1, -2, -12, and -31 clones was examined by methylation-specific polymerase chain reaction and combined bisulfite restriction enzyme analysis. Epstein-Barr virus (EBV) infection was detected by in situ hybridization. Nuclear immunoreactivity for DNMT1 was not detected in any of the noncancerous epithelia, except in proliferative zones (positive internal control), but was found in 97 (72%) of the gastric cancers. DNMT1 overexpression correlated significantly with poorer tumor differentiation (P < 0.001), but not with the phenotype (gastric type versus intestinal type) of the cancer cells. It also correlated significantly with DNA hypermethylation of the CpG islands of the hMLH1 (P = 0.024) and THBS-1 genes (P = 0.043), and with the CpG island methylator phenotype in the gastric cancers (P = 0.007). Reduced E-cadherin expression correlated significantly with poorer tumor differentiation (P = 0.002), DNA hypermethylation of the E-cadherin gene (P < 0.001) and DNMT1 overexpression (P = 0.014). DNMT1 overexpression was also associated with EBV infection (a potential etiological factor in gastric carcinogenesis) but not with the proliferative activity of the cancer cells as indicated by the proliferating cell nuclear antigen-labeling index. These results suggest that DNMT1 overexpression may not be just a secondary effect of increased cancer cell proliferative activity, but may be associated with EBV infection and other etiological factors during gastric carcinogenesis. Furthermore, DNMT1 may play a significant role in the development of poorly differentiated gastric cancers by inducing frequent DNA hypermethylation of multiple CpG islands.
DNA methylation plays an important role in transcriptional regulation and chromatin remodeling in mammalian cells.1 Both overall DNA hypomethylation and more regional DNA hypermethylation have been well documented in various cancers.1–8 Aberrant DNA methylation may be involved in carcinogenesis as a result of 1) increased gene mutagenicity because of deamination of 5-methylcytosine to thymine; 2) a possible association of aberrant DNA methylation with allelic loss; and 3) repression of gene transcription through methylation of CpG islands in regulatory regions of specific genes, including tumor-suppressor genes.1
To date, three enzymes, DNA methyltransferase 1 (DNMT1),9 DNMT3a, and DNMT3b,10 have been confirmed to possess DNMT activity. Of these, DNMT1 is the major and best known. As DNMT1 shows a preference for hemimethylated rather than unmethylated substrates in vitro,11 and targets replication foci by binding to proliferating cell nuclear antigen (PCNA),12 it seems to be a maintenance form of DNMT that copies methylation patterns after DNA replication. However, some workers have proposed that DNMT1 possesses both maintenance and de novo DNA methylation activity in vivo, regardless of its in vitro substrate preference.13
Overexpression of DNMT1 has been detected in several human cancers.14–16 With regard to gastric cancer, we have reported that DNMT1 mRNA expression levels were significantly higher in cancer tissues than in normal gastric mucosae.16 Moreover, during this previous study, we found that increased DNMT1 mRNA expression correlated significantly with the CpG island methylator phenotype (defined as frequent DNA hypermethylation of C-type CpG islands that are methylated in a cancer-specific but not an age-dependent manner17) in gastric and colorectal cancers.16 However, most previous analyses concerning DNMT1 expression in human cancers have been performed at the mRNA level. To our knowledge, DNMT1 protein expression in gastric cancers has never been reported. The aim of this study was therefore to evaluate the significance of aberrant DNMT1 protein expression during gastric carcinogenesis. Firstly, we searched for correlations between DNMT1 protein expression and the clinicopathological features of gastric cancers. Secondly, to determine the targets of aberrantly expressed DNMT1 during gastric carcinogenesis, we examined the correlations between DNMT1 protein expression on the one hand and the DNA methylation status of multiple C-type CpG islands and E-cadherin expression on the other. Thirdly, to clarify the background behind aberrant DNMT1 protein expression, we investigated correlations with the proliferative activity of cancer cells (as indicated by the PCNA-labeling index) and with etiological factors that are believed to be involved in gastric carcinogenesis, such as Helicobacter pylori18 and Epstein-Barr virus (EBV) infection.19
Materials and Methods
Patients and Tissue Samples
Cancerous tissues and corresponding noncancerous mucosae were obtained from 134 patients with primary gastric cancer. These patients underwent surgery at the National Cancer Center Hospital, Tokyo, Japan, between 1998 and 2002. They included 92 men and 42 women with a mean (± SD) age of 61 ± 7 years (range, 45 to 83 years). None of the patients received any preoperative treatment, such as radiation or chemotherapy. Based on histological examinations, the 134 tumors were classified as 23 well differentiated, 31 moderately differentiated, and 80 poorly differentiated (including signet ring cell and mucinous carcinomas) adenocarcinomas.
Immunohistochemistry
Five-μm-thick sections of formalin-fixed, paraffin-embedded tissue specimens from all 134 patients were deparaffinized and dehydrated. For antigen retrieval, the sections were heated for 10 minutes at 120°C in an autoclave. Nonspecific reactions were blocked with 2% normal swine serum. All sections were incubated with specific primary antibodies that recognized DNMT1 (goat polyclonal antibody, sc-10219, dilution 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), Muc2 (mouse monoclonal antibody, Ccp58, dilution 1:100; Novocastra, Newcastle-on-Tyne, UK), human gastric mucin (mouse monoclonal antibody, 45M1, dilution 1:50; Novocastra), E-cadherin (mouse monoclonal antibody, HECD-1;20 dilution 1:500), PCNA (mouse monoclonal antibody, p56720, dilution 1:200; Transduction Laboratories, Lexington, KY) and H. pylori (rabbit polyclonal antibody, B0471, dilution 1:20; DAKO, Glostrup, Denmark), respectively. We previously confirmed the specificity of the goat anti-human DNMT1 polyclonal antibody by Western blotting analysis: an immunoreactive band of ∼193.5 kd, corresponding to the molecular mass of DNMT1, was detected in human cancer cells, but no nonspecific bands were detected with this antibody.21 All primary antibody incubations were conducted at 4°C overnight and were followed by incubation with biotinylated secondary antibodies (anti-goat IgG, anti-mouse IgG, or anti-rabbit IgG, dilution 1:200; Vector Laboratories, Burlingame, CA) at room temperature for 30 minutes. The sections were then treated with Vectastain Elite ABC reagent (Vector Laboratories). 3.3′-Diaminobenzidine tetrahydrochloride was used as the chromogen. All sections were counterstained with hematoxylin.
The gastric cancers were classified into three phenotypes according to previously described criteria:22 the gastric type (positive for human gastric mucin), the intestinal type (positive for Muc2), and the mixed type (positive for both human gastric mucin and Muc2). For the evaluations of DNMT1 and PCNA expression, nuclear immunoreactivity in the proliferative zones of noncancerous foveolar epithelia was used as a positive internal control for all sections. Similarly, immunoreactivity in the cell membranes of noncancerous foveolar epithelia was used as a positive internal control for all sections during the evaluation of E-cadherin expression. As a negative control, the primary antibodies were omitted from the reaction sequence.
Methylation-Specific Polymerase Chain Reaction (MSP) and Combined Bisulfite Restriction Enzyme Analysis (COBRA)
High-molecular-weight DNA was extracted from 105 fresh paired samples of cancerous tissues and their corresponding noncancerous mucosae by phenol-chloroform extraction and dialysis. Bisulfite conversion was performed using 1 μg of genomic DNA and the reagents provided in the CpGenome DNA modification kit (Intergen, Purchase, NY). This process converts unmethylated cytosine residues to uracil, whereas methylated cytosine residues remain unchanged. The DNA methylation status of the CpG islands of the p16, MutL homologue 1 (hMLH1), and E-cadherin genes was determined by MSP. This technique is based on the principle that the DNA sequences of methylated and unmethylated genomic regions differ after bisulfite conversion and can thus be distinguished by sequence-specific polymerase chain reaction (PCR) primers.23 The bisulfite-modified DNA of the p16 gene was amplified using the primer sets provided in the CpG WIZ amplification kit (Intergen) and that of the hMLH124 and E-cadherin23 genes was amplified using the previously described primers. The DNA methylation status of the thrombospondin-1 (THBS-1) gene and the methylated in tumor (MINT)-1, -2, -12, and -31 clones was determined by COBRA.25 Bisulfite-modified DNA was amplified by PCR using previously described primers that were designed to amplify methylated and unmethylated genomic regions equally.17 The amplified fragments were digested with restriction enzymes that digest DNA only if the CpG sites in their recognition sequences are methylated: TaqI for the THBS-1 gene and the MINT-1 and -2 clones, MaeII for the MINT-12 clone and BstUI for the MINT-31 clone, respectively. The reaction products were separated electrophoretically on a 3% agarose gel and stained with ethidium bromide. Signal intensities were measured using an image analyzer (model FMBIO-2; Takara, Ohtsu, Japan).
In Situ Hybridization
Five-μm-thick sections of formalin-fixed, paraffin-embedded tissue specimens from all 134 patients were deparaffinized, dehydrated, and predigested with proteinase K. The sections were then hybridized with a digoxigenin-labeled EBV encoding RNA (EBER) 1 oligonucleotide probe (EBV detection kit; Nichirei, Tokyo, Japan) for 2 hours at 37°C. Anti-digoxigenin antibody-alkaline phosphatase was used with a nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate substrate to detect the EBER1 signal. Positive control specimens were provided by the manufacture. As a negative control, the EBER1 probe was omitted from the reaction sequence.
Statistics
Correlations between the incidence of DNMT1 immunoreactivity and variables including clinicopathological parameters, the DNA methylation status of CpG islands, E-cadherin expression, and H. pylori and EBV infection were analyzed using the chi-square test. Correlations between the PCNA-labeling index and clinicopathological parameters or DNMT1 immunoreactivity were analyzed using the Mann-Whitney U-test or the Kruskal-Wallis test. Differences with P values <0.05 were considered significant.
Results
Clinicopathological Significance of DNMT1 Protein Overexpression in Gastric Cancers
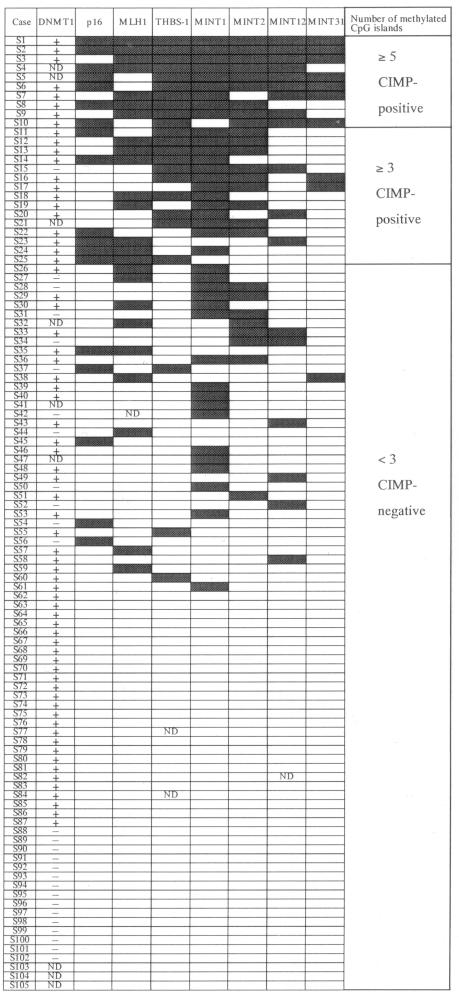
Immunoreactivity for DNMT1 was detected in the nuclei, but not in the cytoplasm or cell membranes, of cells in the proliferating zones of foveolar epithelia, lymphocytes, and cancer cells (Figure 1). To discriminate definitely positive cases from cases with leaky background level signal, if more than 30% of the cells in a tissue sample exhibited nuclear staining the sample was considered to show positive immunoreactivity. None (0%) of the 134 noncancerous epithelia exhibited DNMT1 immunoreactivity (except in the proliferative zones, which acted as the positive internal control for the analysis), whereas 97 (72%) of the 134 gastric cancers were DNMT1-positive.
Figure 1.
Immunohistochemical examination using anti-human DNMT1 goat polyclonal antibody. In the noncancerous mucosal sample from patient S121 (A), only the proliferative zones of the foveolar epithelia showed nuclear immunoreactivity for DNMT1, whereas the other epithelial cells did not. Although most cancer cells in a well-differentiated adenocarcinoma from patient S91 lacked nuclear immunoreactivity for DNMT1 (B), the moderately differentiated adenocarcinoma from patient S115 (C) and the poorly differentiated adenocarcinoma from patient S130 (D) showed strong nuclear immunoreactivity. Original magnifications: ×180 (A); ×360 (B–D).
Correlations between the incidence of nuclear immunoreactivity for DNMT1 and the clinicopathological features of the gastric cancers are shown in Table 1. DNMT1 protein overexpression was significantly associated with the degree of histological differentiation (P < 0.001).
Table 1.
DNMT1 Protein Expression and the PCNA-Labeling Index in the Gastric Cancers
| Variables | Analyzed | DNMT-1 positive [number of cases (%)] | P† | PCNA-labeling index [mean ± SD (%)] | P |
|---|---|---|---|---|---|
| Tumor differentiation | |||||
| Well differentiated | 23 | 7 (30%) | <.001 | 62 ± 25 | 0.165‡ |
| Moderately differentiated | 31 | 18 (58%) | 64 ± 17 | ||
| Poorly differentiated | 80 | 72 (90%) | 58 ± 27 | ||
| Phenotype* | |||||
| Gastric type | 50 | 38 (76%) | 0.137 | 60 ± 29 | 0.793‡ |
| Intestinal type | 34 | 21 (62%) | 57 ± 29 | ||
| Mixed type | 50 | 34 (68%) | 62 ± 23 | ||
| Depth of invasion | |||||
| Mucosa/submucosa | 38 | 28 (74%) | 0.423 | 56 ± 32 | 0.125‡ |
| Muscularis propria/subserosa | 11 | 6 (55%) | 70 ± 18 | ||
| Serosa | 85 | 63 (74%) | 60 ± 24 | ||
| Vascular involvement | |||||
| Negative | 55 | 41 (75%) | 0.494 | 56 ± 30 | 0.533§ |
| Positive | 79 | 56 (71%) | 63 ± 23 | ||
| Lymphnode metastasis | |||||
| Negative | 81 | 60 (74%) | 0.931 | 59 ± 27 | 0.465§ |
| Positive | 53 | 37 (70%) | 61 ± 26 |
Cellular phenotypes are defined as described in the Materials and Methods section.
Chi-square test,
Kruskal-Wallis test.
Mann-Whitney U-test.
Next, we evaluated cellular phenotypes based on immunohistochemistry for Muc2 and human gastric mucin, as shown in Figure 2. Fifty (37%) of the cancers showed a gastric phenotype, 34 (26%) showed an intestinal phenotype, and a further 50 (37%) showed a mixed cellular phenotype. There was no significant correlation between DNMT1 protein overexpression and the cellular phenotypes.
Figure 2.
Immunohistochemical examination using anti-human gastric mucin (A) and Muc2 (B) mouse monoclonal antibodies. A: An adenocarcinoma with a gastric phenotype and noncancerous foveolar epithelia (*) from patient S19. Both showed strong cytoplasmic immunoreactivity for human gastric mucin. B: An adenocarcinoma with an intestinal phenotype from patient S78, showing strong cytoplasmic immunoreactivity for Muc2. Original magnifications, ×360.
We then focused on the histological features of the noncancerous mucosae, as intestinal metaplasia is considered to be a precancerous lesion for adenocarcinomas with an intestinal phenotype. There was no significant correlation between DNMT1 protein overexpression in the gastric cancers and the presence or absence (or, if present, the degree) of intestinal metaplasia in the corresponding noncancerous mucosae (data not shown).
DNMT1 protein overexpression was not significantly associated with other parameters relating to cancer aggressiveness, such as the depth of invasion, vascular involvement, or lymph node metastasis.
Correlation between DNMT1 Protein Overexpression and the DNA Methylation Status of Multiple CpG Islands
Figure 3 shows examples of the PCR products from MSP and COBRA. The incidence of DNA methylation of the C-type CpG islands of each of the genes and MINT clones tested in the noncancerous mucosae and gastric cancers is summarized in Table 2. DNA methylation of at least one C-type CpG island was seen in 50 (48%) of the 105 noncancerous mucosae and 83 (80%) of the 105 gastric cancers examined. For all patients showing DNA methylation of a certain CpG island in both their noncancerous mucosa and cancer, the signal intensity of the reaction products reflecting the presence of methylated DNA was increased in the cancer compared with the corresponding noncancerous mucosa (Figure 3A). The DNA methylation status of each C-type CpG island in the gastric cancers is shown in Figure 4. When DNA hypermethylation was seen on three or more C-type CpG islands, we regarded the patient as being CpG island methylator phenotype (CIMP)-positive, based on previously described criteria.17 Twenty-five (24%) of the 105 gastric cancers were considered CIMP-positive. Furthermore, there were significant correlations between DNMT1 protein overexpression and DNA hypermethylation of each CpG island of the hMLH1 (P = 0.024) and THBS-1 (P = 0.043) genes in the gastric cancers (Table 3). There was also a significant correlation between DNMT1 protein overexpression and CIMP for the gastric cancer tissues (P = 0.007, Table 3).
Figure 3.
Examples of PCR products from DNA methylation analyses of multiple CpG islands in patients with gastric cancer (S). N, noncancerous mucosae; T, cancer tissue. The DNA methylation status of the CpG islands of the p16 (A), hMLH1 (B), and E-cadherin (C) genes was evaluated by MSP. In this analysis, the PCR products generated by primer sets M and U reflect the presence of methylated and unmethylated genes, respectively. Primer set W was used to confirm the completeness of the bisulfite modification. A: Methylated gene was detected in S24N, but the signal intensity of the M fragment was markedly increased in S24T compared with S24N. This indicates that a greater number of cells had undergone DNA hypermethylation in T than in N, although N can contain precursor cells for cancers and/or precancerous lesions. DNA methylation of the CpG islands of the THBS-1 (D) gene and the MINT-1, -2, -12, and -31 clones (E, F, G, and H, respectively) was evaluated by COBRA. In this analysis, only methylated genes (arrows) were digested by the restriction enzymes.
Table 2.
The DNA Methylation Status of the C-Type CpG Islands of Various Genes and Clones in Noncancerous Mucosae and Gastric Cancers
| CpG islands | Number of tissue samples (%)
|
|||
|---|---|---|---|---|
| Noncancerous mucosae
|
Gastric cancers
|
|||
| Analyzed | DNA hypermethylation detected | Analyzed | DNA hypermethylation detected | |
| p16 | 104 | 18 (17) | 103 | 23 (22) |
| hMLH1 | 105 | 15 (14) | 105 | 18 (17) |
| THBS-1 | 105 | 2 (2) | 104 | 25 (24) |
| MINT1 | 105 | 23 (22) | 105 | 39 (37) |
| MINT2 | 105 | 1 (1) | 105 | 26 (25) |
| MINT12 | 104 | 6 (6) | 104 | 18 (17) |
| MINT31 | 105 | 0 (0) | 105 | 10 (10) |
Figure 4.
Protein expression of DNMT1 and DNA methylation profiles for seven C-type CpG islands in 105 gastric cancers. The DNA methylation status was examined using MSP or COBRA (see Figure 3). Patient numbers are indicated on the vertical columns and the seven CpG islands are indicated on the top row. +, DNMT1 protein overexpression-positive; −, DNMT1 protein overexpression-negative; solid box, methylated; open box, unmethylated; ND, not done. When DNA hypermethylation was seen in three or more CpG islands, the patient was regarded as being CIMP-positive.
Table 3.
Correlations between DNMT1 Protein Overexpression and the DNA Methylation Status of Each C-Type CpG Island, CIMP and E-Cadherin Protein Expression in Gastric Cancers
| DNMT1 expression (number of tissue samples)
|
P* | ||
|---|---|---|---|
| Positive | Negative | ||
| CpG islands | |||
| p16 | |||
| Methylated | 13 | 3 | 0.361 |
| Unmethylated | 56 | 24 | |
| hMLH1 | |||
| Methylated | 20 | 2 | 0.024 |
| Unmethylated | 49 | 25 | |
| THBS-1 | |||
| Methylated | 18 | 2 | 0.043 |
| Unmethylated | 51 | 25 | |
| MINT1 | |||
| Methylated | 28 | 6 | 0.091 |
| Unmethylated | 41 | 21 | |
| MINT2 | |||
| Methylated | 18 | 4 | 0.237 |
| Unmethylated | 51 | 23 | |
| MINT12 | |||
| Methylated | 13 | 3 | 0.361 |
| Unmethylated | 56 | 24 | |
| MINT31 | |||
| Methylated | 10 | 0 | 0.503 |
| Unmethylated | 59 | 27 | |
| CIMP | |||
| Positive | 10 | 1 | 0.007 |
| Negative | 59 | 26 | |
| E-cadherin expression | |||
| Maintained | 48 | 27 | 0.014 |
| Reduced | 49 | 10 | |
Chi-square test. Reduced: over 50% of the cancer cells in a particular patient’s sample lacked or showed only slight E-cadherin immunoreactivity.
Correlation between DNMT1 Protein Overexpression and Reduced E-Cadherin Expression
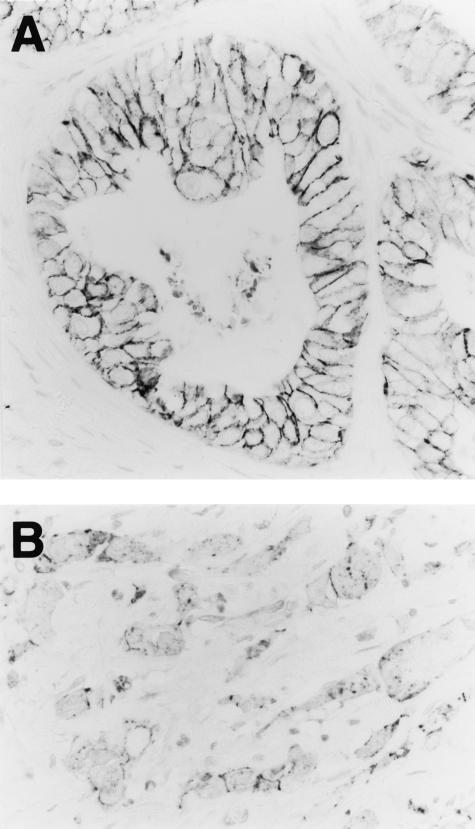
E-cadherin was detected in the cell membranes of epithelia from all (100%) of the 134 noncancerous mucosae. However, E-cadherin protein expression was considered to be reduced when more than 50% of the gastric cancer cells in a particular patient’s sample lacked or showed only slight membranous E-cadherin immunoreactivity. Reduced E-cadherin expression was observed in 59 (44%) of the 134 gastric cancers (Figure 5). The incidence of reduced E-cadherin expression was 22% in well, 23% in moderately, and 59% in poorly differentiated adenocarcinomas, respectively, and reduced E-cadherin expression was significantly associated with poorer tumor differentiation (P = 0.002). DNA methylation of CpG island of the E-cadherin gene was seen in 20 (19%) of the 105 gastric cancers examined (Figure 3C) and there was a significant correlation between DNA hypermethylation of CpG island of the E-cadherin gene and reduced E-cadherin expression in the gastric cancers (P < 0.001). Furthermore, there was a significant correlation between DNMT1 protein overexpression and reduced E-cadherin expression in gastric cancers (P = 0.014, Table 3). In fact, coincidence of nuclear immunoreactivity of DNMT1 and lack of membrane immunoreactivity of E-cadherin was frequently observed in individual cancer cells.
Figure 5.
Immunohistochemical examination using the anti-human E-cadherin mouse monoclonal antibody HECD-1. Although the well-differentiated adenocarcinoma from patient S31 maintained strong E-cadherin immunoreactivity at the cell-cell borders (A), E-cadherin expression was reduced in the poorly differentiated adenocarcinoma from patient S64 (B). Original magnifications, ×360.
DNMT1 Protein Expression and the PCNA-Labeling Index
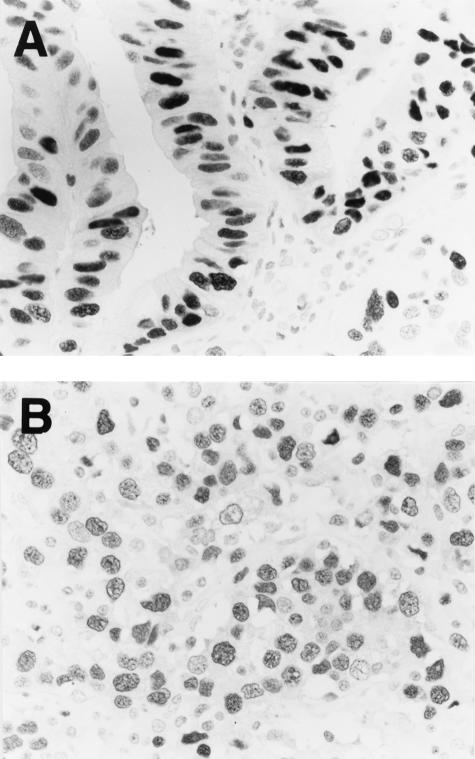
Examples of the results of immunohistochemistry for PCNA are shown in Figure 6. To evaluate the PCNA-labeling index, ∼300 cells per specimen were examined at a magnification of ×400 under a microscope and the cells that did and did not show nuclear immunoreactivity for PCNA were counted. The PCNA-labeling index was expressed as the percentage of the total cells that showed nuclear immunoreactivity. The PCNA-labeling index was increased even in well-differentiated adenocarcinomas, in which the incidence of overexpression of DNMT1 protein was still low (Table 1), and coincidence of nuclear immunoreactivity of PCNA and lack of nuclear immunoreactivity of DNMT1 was frequently observed in individual well-differentiated cancer cells. Thus, DNMT1 protein overexpression was not significantly associated with the PCNA-labeling index in the gastric cancers (P = 0.309, Figure 7).
Figure 6.
Immunohistochemical examination using anti-human PCNA mouse monoclonal antibody. A: A well-differentiated adenocarcinoma from patient S125. B: A poorly differentiated adenocarcinoma from patient S30. Original magnifications, ×360.
Figure 7.
Average PCNA-labeling indices in DNMT1 protein overexpression-positive (n = 97) and -negative (n = 37) gastric cancers. Error bar, SD DNMT1 protein overexpression was not significantly associated with the PCNA-labeling index in the gastric cancers (P = 0.309, Mann-Whitney U-test).
Correlation between DNMT1 Protein Overexpression and Etiological Factors
To understand the background behind DNMT1 protein overexpression, etiological factors considered to be involved in gastric carcinogenesis were examined. Although 44 (46%) of the 96 patients examined showed H. pylori infection in their noncancerous mucosae, there was no significant correlation between H. pylori infection and DNMT1 protein overexpression (P = 0.113). Similarly, there was no correlation between H. pylori infection and CIMP (P = 0.146). The incidence of EBV infection was examined by in situ hybridization in the same cohort (Figure 8). Four patients (4%) had EBV infection in their cancer cells, and all four of these cancers showed DNMT1 protein overexpression. Furthermore, EBV infection was significantly associated with DNA hypermethylation of five or more C-type CpG islands (P < 0.001).
Figure 8.
In situ hybridization investigation for EBV infection using the EBER1 oligonucleotide probe. A poorly differentiated adenocarcinoma from patient S45, showing diffuse positivity in the nuclei. Original magnification, ×180.
Discussion
We believe that this is the first report describing immunohistochemical examination for DNMT1 protein in gastric cancers. We have previously reported an increase in DNMT1 mRNA expression levels in gastric cancers compared with corresponding noncancerous mucosae.16 In the present study, we showed that DNMT1 expression was also increased at the protein level in gastric cancers, suggesting that DNMT1 overexpression has some significance during gastric carcinogenesis. DNMT1 protein overexpression showed no significant correlations with either the cellular phenotype (gastric type versus intestinal type) or the presence, absence, or degree of intestinal metaplasia (a precancerous lesion for adenocarcinomas with an intestinal phenotype) in corresponding noncancerous mucosae, suggesting that DNMT1 protein overexpression is associated with gastric carcinogenesis regardless of the cellular origin or phenotype. Our results also suggest that DNMT1 may particularly affect the stage of development at which cancers begin to show poorer differentiation. However, in a previous study, overexpression was detected even in precancerous conditions when we used quantitative reverse transcriptase-PCR analysis to examine DNMT1 mRNA expression levels in a cohort with hepatocellular carcinomas (HCCs).15 Although there appears to be a discrepancy between the previous and present findings, when the same cohort was examined by immunohistochemistry protein overexpression was not detected in precancerous conditions but only in moderately or poorly differentiated HCCs.21 This may be attributable to methodological differences: quantitative reverse transcriptase-PCR is so sensitive that it can detect small elevations in DNMT1 mRNA levels in precancerous conditions, whereas immunohistochemistry cannot detect such elevations until protein expression reaches a certain level in more malignant HCCs.21 By analogy with hepatocarcinogenesis, we cannot rule out the possibility that a small elevation in DNMT1 expression had already occurred in the earlier stages of gastric carcinogenesis, before the DNMT1 expression level reached the threshold of detection for the immunohistochemical methods used.
Regional DNA hypermethylation of CpG islands was detected even in noncancerous mucosae, which can contain precursor cells for cancers and/or precancerous lesions, such as intestinal metaplasia. However, the incidence and degree of DNA hypermethylation was increased in the gastric cancers compared with the noncancerous mucosae. These data are consistent with previous findings in precancerous conditions and cancers of various organs.3–8 Twenty-four percent of the gastric cancers were CIMP-positive, confirming that aberrant DNA methylation is associated with the multistage development of certain subgroups of gastric cancers. Targeting of the substrate DNA by DNMT1 may be disrupted by mechanisms such as dysfunction of p21WAF1,26 which competes with DNMT1 for binding to PCNA, in cancer cells.12 Moreover, it has recently been suggested that DNMT1 is capable of de novo methylating activity as well as having a maintenance function.13 Therefore, it is feasible that, in cancers, DNMT1 participates in the DNA hypermethylation of CpG islands that are not methylated in normal cells. We have previously reported that DNMT1 mRNA overexpression correlates significantly with CIMP in gastric and colorectal cancers.16 In the present study, we demonstrated a significant correlation between DNMT1 expression and CIMP in gastric cancers, even at the protein level. Moreover, among the C-type CpG islands examined, those of the hMLH1 and THBS-1 genes may be particularly targeted by overexpressed DNMT1. This is compatible with previous reports that silencing of the hMLH1 gene results in frequent microsatellite instability in gastric cancers.27,28
E-cadherin is one of the most important molecules involved in intracellular adhesion and cancer morphogenesis.29 In addition to the development of mutations,30–32 it has become apparent that the E-cadherin gene can be silenced by DNA hypermethylation around its promoter regions in gastric cancers.33,34 Indeed, reduced E-cadherin protein expression associated with DNA hypermethylation around the promoter region of the E-cadherin gene was significantly correlated with poorer glandular differentiation of the cancers examined in this study. We showed a significant correlation between DNMT1 protein overexpression and reduced E-cadherin protein expression in gastric cancers, indicating that the E-cadherin gene may also be a target of overexpressed DNMT1.
Although DNMT1 is a major DNMT in humans, so far two other enzymes, DNMT3a and DNMT3b, have also been shown to possess DNMT activity.1 Genomic methylation patterns may be established through cooperation among these three DNMTs, even in cancer cells.13 Moreover, it may be that unknown co-factors potently target these DNMTs to unmethylated substrates in a sequence-specific manner in cancers. Thus, DNMT1 overexpression alone may not explain regional DNA hypermethylation in cancers. Further studies on the cooperation between DNMT1 and other components of the DNA methylation machinery in tissue specimens may further our understanding of the basis of regional DNA hypermethylation during gastric carcinogenesis. Although the degree of DNA hypermethylation in noncancerous mucosae seemed slight, DNA methylation of CpG islands actually occurred in a considerable number of noncancerous mucosae that can contain precursor cells for cancer. However, DNMT1 overexpression was not detected immunohistochemically in noncancerous mucosae except for proliferative zones, in which DNMT1 performs maintenance methylation at the replication foci. The protein expression levels of DNMT1 in noncancerous mucosae may not reach the threshold that can be detected by the present immunohistochemical method, even though it promotes slight DNA hypermethylation. Otherwise, slight DNA hypermethylation in noncancerous mucosae may be attributable to alterations in components of the DNA methylation machinery other than DNMT1. Although frequent regional DNA hypermethylation has been reported in gastric type tumors,35 DNMT1 protein expression was not different between gastric and intestinal phenotypes in the present study. We assume again that unknown components of the DNA methylation machinery may potently target DNMT1 to substrate DNA, or that DNMTs other than DNMT1 may also participate in regional DNA hypermethylation in cancers showing gastric phenotype.
On the other hand, the overall 5-methylcytosine level is lower in cancer tissues than in normal tissues.1 In common with the previously reported immunohistochemical findings for DNMT1 in colorectal cancers36 and HCCs,21 some cancer cells exhibited very weak or no DNMT1 immunoreactivity, even in a gastric cancer that was considered DNMT1-positive according to the criteria described in the Materials and Methods section. Cancer cells with low DNMT1 levels may be prone to overall DNA hypomethylation.
An initial increase in DNMT1 mRNA expression in colon cancers became far more modest when the expression level was normalized according to that of a cell proliferation marker.37 DNMT1 mRNA is expressed mainly during the S-phase and, because tumor tissue is presumed to contain a greater proportion of dividing cells than normal tissue, some debate has arisen as to whether increased DNMT1 expression is because of an increase in the proportion of dividing cells or to an acute increase in DNMT1 expression per individual cell. This continuing discussion prompted us to compare DMNT1 immunoreactivity and the PCNA-labeling index in gastric cancers. In the present study, DNMT1 protein overexpression was not significantly associated with increased proliferative activity of gastric cancer cells; we have previously observed a similar discrepancy between DNMT1 expression and cell proliferative activity in precancerous conditions for urinary bladder carcinomas38 and in certain subgroups of HCCs.21 These findings suggest that DNMT1 protein overexpression does not result entirely from increased numbers of dividing cells during carcinogenesis.
Finally, we focused on etiological factors believed to be involved in gastric carcinogenesis to clarify the background behind DNMT1 protein overexpression. Although DNMT1 protein overexpression was not significantly associated with the incidence of H. pylori infection in corresponding noncancerous mucosae, all four of the EBV-positive cancers showed DNMT1 protein overexpression. EBV infection was significantly associated with the DNA hypermethylation of five or more C-type CpG islands, in accordance with a previous report that the average number of methylated CpG islands was higher in EBV-positive gastric cancers than in EBV-negative ones.39 These observations indicate that DNMT1 may play an important role in EBV-related gastric carcinogenesis via aberrant DNA methylation. Although it has previously been reported that transfection of latent membrane protein-1 of EBV induces the expression and activity of DNMT1,40 latent membrane protein-1 is not typically expressed in EBV-associated gastric cancers.41 Although the molecular mechanism governing how EBV infection results in overexpression of DNMT1 protein requires further elucidation, and our present findings must be interpreted with caution because of the small number of EBV-positive gastric cancers studied, EBV infection and other etiological factors may be associated with DNMT1 overexpression and contribute toward gastric carcinogenesis by inducing frequent DNA hypermethylation of multiple CpG islands.
Footnotes
Address reprint requests to Yae Kanai, Pathology Division, National Cancer Center Research Institute, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan. E-mail: ykanai@ncc.go.jp.
Supported by a Grant-in-Aid for the Second Term Comprehensive 10-Year Strategy for Cancer Control; a Grant-in-Aid for Cancer Research from the Ministry of Health, Labor, and Welfare of Japan; and a research resident fellowship from the Foundation for Promotion of Cancer Research in Japan (to T.N.).
References
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- Vachtenheim J, Horakova I, Novotna H. Hypomethylation of CCGG sites in the 3′ region of H-ras protooncogene is frequent and is associated with H-ras allele loss in non-small cell lung cancer. Cancer Res. 1994;54:1145–1148. [PubMed] [Google Scholar]
- Kanai Y, Ushijima S, Tsuda H, Sakamoto M, Sugimura T, Hirohashi S. Aberrant DNA methylation on chromosome 16 is an early event in hepatocarcinogenesis. Jpn J Cancer Res. 1996;87:1210–1217. doi: 10.1111/j.1349-7006.1996.tb03135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Ushijima S, Hui AM, Ochiai A, Tsuda H, Sakamoto M, Hirohashi S. The E-cadherin gene is silenced by CpG methylation in human hepatocellular carcinomas. Int J Cancer. 1997;71:355–359. doi: 10.1002/(sici)1097-0215(19970502)71:3<355::aid-ijc8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Eguchi K, Kanai Y, Kobayashi K, Hirohashi S. DNA hypermethylation at the D17S5 locus in non-small cell lung cancers: its association with smoking history. Cancer Res. 1997;57:4913–4915. [PubMed] [Google Scholar]
- Kanai Y, Ushijima S, Ochiai A, Eguchi K, Hui AM, Hirohashi S. DNA hypermethylation at the D17S5 locus is associated with gastric carcinogenesis. Cancer Lett. 1998;122:135–141. doi: 10.1016/s0304-3835(97)00380-7. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Kanai Y, Sakamoto M, Mizokami M, Ueda R, Hirohashi S. Genetic instability and aberrant DNA methylation in chronic hepatitis and cirrhosis—a comprehensive study of loss of heterozygosity and microsatellite instability at 39 loci and DNA hypermethylation on 8 CpG islands in microdissected specimens from patients with hepatocellular carcinoma. Hepatology. 2000;32:970–979. doi: 10.1053/jhep.2000.19797. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Ushijima S, Tsuda H, Sakamoto M, Hirohashi S. Aberrant DNA methylation precedes loss of heterozygosity on chromosome 16 in chronic hepatitis and liver cirrhosis. Cancer Lett. 2000;148:73–80. doi: 10.1016/s0304-3835(99)00316-x. [DOI] [PubMed] [Google Scholar]
- Bestor T, Laudano A, Mattaliano R, Ingram V. Cloning and sequencing of a cDNA encoding DNA methyltransferase of mouse cells. The carboxyl-terminal domain of the mammalian enzymes is related to bacterial restriction methyltransferases. J Mol Biol. 1988;203:971–983. doi: 10.1016/0022-2836(88)90122-2. [DOI] [PubMed] [Google Scholar]
- Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998;19:219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- Bestor TH. Activation of mammalian DNA methyltransferase by cleavage of a Zn binding regulatory domain. EMBO J. 1992;11:2611–2617. doi: 10.1002/j.1460-2075.1992.tb05326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang LS, Ian HI, Koh TW, Ng HH, Xu G, Li BF. Human DNA-(cytosine-5) methyltransferase-PCNA complex as a target for p21WAF1. Science. 1997;277:1996–2000. doi: 10.1126/science.277.5334.1996. [DOI] [PubMed] [Google Scholar]
- Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, Schuebel KE, Cui H, Feinberg AP, Lengauer C, Kinzler KW, Baylin SB, Vogelstein B. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–556. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- Sun L, Hui AM, Kanai Y, Sakamoto M, Hirohashi S. Increased DNA methyltransferase expression is associated with an early stage of human hepatocarcinogenesis. Jpn J Cancer Res. 1997;88:1165–1170. doi: 10.1111/j.1349-7006.1997.tb00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Kanai Y, Sakamoto M, Saito H, Ishii H, Hirohashi S. Expression of mRNA for DNA methyltransferases and methyl-CpG-binding proteins and DNA methylation status on CpG islands and pericentromeric satellite regions during human hepatocarcinogenesis. Hepatology. 2001;33:561–568. doi: 10.1053/jhep.2001.22507. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Ushijima S, Kondo Y, Nakanishi Y, Hirohashi S. DNA methyltransferase expression and DNA methylation of CpG islands and peri-centromeric satellite regions in human colorectal and stomach cancers. Int J Cancer. 2001;91:205–212. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1040>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipponen P. Helicobacter pylori: a cohort phenomenon. Am J Surg Pathol. 1995;19(Suppl 1):S30–S36. [PubMed] [Google Scholar]
- Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992;140:769–774. [PMC free article] [PubMed] [Google Scholar]
- Shimoyama Y, Hirohashi S, Hirano S, Noguchi M, Shimosato Y, Takeichi M, Abe O. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989;49:2128–2133. [PubMed] [Google Scholar]
- Saito Y, Kanai Y, Nakagawa T, Sakamoto M, Saito H, Ishii H, Hirohashi S. Increased protein expression of DNA methyltransferase (DNMT) 1 is significantly correlated with the malignant potential and poor prognosis of human hepatocellular carcinomas. Int J Cancer. 2003;105:527–532. doi: 10.1002/ijc.11127. [DOI] [PubMed] [Google Scholar]
- Egashira Y, Shimoda T, Ikegami M. Mucin histochemical analysis of minute gastric differentiated adenocarcinoma. Pathol Int. 1999;49:55–61. doi: 10.1046/j.1440-1827.1999.00824.x. [DOI] [PubMed] [Google Scholar]
- Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, Markowitz S, Willson JK, Hamilton SR, Kinzler KW, Kane MF, Kolodner RD, Vogelstein B, Kunkel TA, Baylin SB. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z, Laird PW. COBRA: a sensitive and quantitative DNA methylation assay. Nucleic Acids Res. 1997;25:2532–2534. doi: 10.1093/nar/25.12.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin SB. Tying it all together: epigenetics, genetics, cell cycle, and cancer. Science. 1997;277:1948–1949. doi: 10.1126/science.277.5334.1948. [DOI] [PubMed] [Google Scholar]
- Yanagisawa Y, Akiyama Y, Iida S, Ito E, Nomizu T, Sugihara K, Yuasa Y, Maruyama K. Methylation of the hMLH1 promoter in familial gastric cancer with microsatellite instability. Int J Cancer. 2000;85:50–53. doi: 10.1002/(sici)1097-0215(20000101)85:1<50::aid-ijc9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Esteller M, Tamura G, Rashid A, Stine OC, Yin J, Zou TT, Abraham JM, Kong D, Nishizuka S, James SP, Wilson KT, Herman JG, Meltzer SJ. Hypermethylation of the hMLH1 gene promoter is associated with microsatellite instability in early human gastric neoplasia. Oncogene. 2001;20:329–335. doi: 10.1038/sj.onc.1204104. [DOI] [PubMed] [Google Scholar]
- Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T, Kanai Y, Oyama T, Yoshiura K, Shimoyama Y, Birchmeier W, Sugimura T, Hirohashi S. E-cadherin gene mutations in human gastric carcinoma cell lines. Proc Natl Acad Sci USA. 1994;91:1858–1862. doi: 10.1073/pnas.91.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54:3845–3852. [PubMed] [Google Scholar]
- Muta H, Noguchi M, Kanai Y, Ochiai A, Nawata H, Hirohashi S. E-cadherin gene mutations in signet ring cell carcinoma of the stomach. Jpn J Cancer Res. 1996;87:843–848. doi: 10.1111/j.1349-7006.1996.tb02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci USA. 1995;92:7416–7419. doi: 10.1073/pnas.92.16.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura G, Yin J, Wang S, Fleisher AS, Zou T, Abraham JM, Kong D, Smolinski KN, Wilson KT, James SP, Silverberg SG, Nishizuka S, Terashima M, Motoyama T, Meltzer SJ. E-cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst. 2000;92:569–573. doi: 10.1093/jnci/92.7.569. [DOI] [PubMed] [Google Scholar]
- Endoh Y, Tamura G, Ajioka Y, Watanabe H, Motoyama T. Frequent hypermethylation of the hMLH1 gene promoter in differentiated-type tumors of the stomach with the gastric foveolar phenotype. Am J Pathol. 2000;157:717–722. doi: 10.1016/S0002-9440(10)64584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marzo AM, Marchi VL, Yang ES, Veeraswamy R, Lin X, Nelson WG. Abnormal regulation of DNA methyltransferase expression during colorectal carcinogenesis. Cancer Res. 1999;59:3855–3860. [PubMed] [Google Scholar]
- Lee PJ, Washer LL, Law DJ, Boland CR, Horon IL, Feinberg AP. Limited up-regulation of DNA methyltransferase in human colon cancer reflecting increased cell proliferation. Proc Natl Acad Sci USA. 1996;93:10366–10370. doi: 10.1073/pnas.93.19.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kanai Y, Saito Y, Kitamura T, Kakizoe T, Hirohashi S: Increased DNA methyltransferase (DNMT) 1 protein expression in human transitional cell carcinomas of the urinary bladder. J Urol (in press) [DOI] [PubMed] [Google Scholar]
- Kang GH, Lee S, Kim WH, Lee HW, Kim JC, Rhyu MG, Ro JY. Epstein-Barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol. 2002;160:787–794. doi: 10.1016/S0002-9440(10)64901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CN, Tsai CL, Tse KP, Chang HY, Chang YS. The Epstein-Barr virus oncogene product, latent membrane protein 1, induces the downregulation of E-cadherin gene expression via activation of DNA methyltransferases. Proc Natl Acad Sci USA. 2002;99:10084–10089. doi: 10.1073/pnas.152059399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands DC, Ito M, Mangham DC, Reynolds G, Herbst H, Hallissey MT, Fielding JW, Newbold KM, Jones EL, Young LS, Niedobitek G. Epstein-Barr virus and carcinomas: rare association of the virus with gastric adenocarcinomas. Br J Cancer. 1993;68:1014–1019. doi: 10.1038/bjc.1993.472. [DOI] [PMC free article] [PubMed] [Google Scholar]