Abstract
JC virus (JCV), a member of the polyomavirus family, causes a demyelinating disease of the central nervous system (CNS) in humans known as progressive multifocal leukoencephalopathy. Although glial cells are the principal target of JCV productive infection in progressive multifocal leukoencephalopathy patients, little is known regarding the site of JCV persistence and the mechanisms by which the virus spreads to the CNS to cause disease. Previous work has demonstrated the presence of replicating JCV DNA in B lymphocytes from peripheral blood, tonsil, and spleen and it has been hypothesized that lymphocytes may be one site of JCV persistence. Detection of viral gene products in renal tubules and excretion of JC virions in the urine suggests JCV persistence in the kidney. A respiratory route of viral transmission has also been hypothesized implicating the lung as another possible site of persistent JCV infection. Earlier studies from our laboratory have shown that terminal α2,6-linked sialic acid is a critical component of the JCV receptor. In this report we examined the tissue distribution of this JCV receptor-type sialic acid in a panel of normal human tissues. Our results demonstrate that in normal brain JCV receptor-type sialic acids are expressed on oligodendrocytes and astrocytes, but not on cortical neurons. The receptor-type sialic acid is also more highly expressed on B lymphocytes than on T lymphocytes in normal human spleen and tonsil. In addition, both the kidney and lung express abundant levels of α2-6-linked sialic acids. Our data show a striking correlation between the expression of the JCV receptor-type sialic acid on cells and their susceptibility to infection by the virus. These findings also support the hypothesis of JCV persistence in lymphoid tissue and B-cell-facilitated viral dissemination to the CNS.
The polyomavirus JC (JCV) is a common human pathogen, infecting 80% of the population worldwide.1 After initial asymptomatic infection of the host, the virus establishes a life-long persistent infection in the peripheral tissues of healthy individuals. Severe immunosuppression provides suitable conditions for increased JCV replication and spread to the central nervous system (CNS) where the virus selectively targets and destroys the myelin-producing glial cells, the oligodendrocytes.2,3 The loss of these cells leads to a patchy and subsequently confluent demyelination of the CNS referred to as progressive multifocal leukoencephalopathy (PML).4,5 Histopathologically the PML lesions are poorly outlined and are characterized by a discoloration of the white matter, presence of bizarre astrocytes with giant atypical nuclei, and loss of oligodendrocytes.4–8 The nuclei of the remaining oligodendrocytes are enlarged and contain viral particles.2,9 The clinical manifestations of this neurological disorder vary and may include motor weakness, dementia, visual and cognitive impairment, and cranial nerve palsies.4,10 The acquired immune deficiency syndrome pandemic has led to a concomitant rise in the incidence of PML as human immunodeficiency virus-1-associated immunosuppression is the underlying disease in 85% of the PML cases.11,12 In addition, involvement of human immunodeficiency virus-1 secreted products has been proposed to stimulate JCV replication in immunocompromised patients and ultimately aid JCV dissemination to the CNS.13–16
Epidemiological observations reveal that more than 50% of the healthy adults harbor JCV DNA in their kidneys.17–19 The occasional excretion of JC virions in the urine of immunocompetent individuals suggests that the kidney is one site of JCV persistent infection.20,21 Although JCV renal infection is common in PML patients, no clinical abnormalities of the kidney tissue have been reported.18,20,22 The mode of viral dissemination from the periphery where primary infection occurs to the CNS where the disease develops remains undefined. Microscopic examination of early-stage PML brain reveals multiple perivascular areas of white matter deterioration suggestive of viral spread to the CNS facilitated by a hematogenous route.5,6 Subsequent investigations of JCV association with circulating lymphocytes revealed that JCV DNA is indeed present in the blood of healthy individuals and at a higher rate in acquired immune deficiency syndrome patients.23–28 The B-cell population appears to be the main target of JCV in peripheral blood.29–31 The presence of viral DNA and proteins was detected by polymerase chain reaction and immunohistochemistry in B-cell-rich lymphoid organs such as tonsil, spleen, and lymph nodes.32,33 In addition, fluorescence-activated cell sorting analysis demonstrated that labeled JC virions preferentially bound to primary human glial cells and primary human B cells, but did not bind to primary human T cells.34 The putative lymphotropic nature of JCV outside the CNS strongly suggests that in addition to the kidney, the lymphoid organs could be another site of viral persistence and may facilitate viral spread to the CNS by infected B cells.35,36
The primary interaction between a virus and its host is achieved by the attachment of the viral particle to specific cell-surface molecules. The nature and stability of this initial contact has a strong impact on the subsequent progression through the viral life cycle as well as on the virus tropism and pathogenesis resulting from the infection.37 It has been established that JCV utilizes an N-linked glycoprotein containing terminal α2,6-linked sialic acid (SA) to infect human glial cells but the identity of the proteinaceous moiety has not yet been determined. Crude neuraminidase from Vibrio cholerae, but not recombinant α2,3-specific neuraminidase inhibits infection by JCV, and JCV inhibits the binding of labeled Sambucus nigra lectin (SNA), α2,6-specific lectin, but not of Maackia amurensis (MAA), α2,3-specific lectin, to glial cells.38 SAs are negatively charged molecules abundantly expressed on the cell surface as parts of glycoconjugates, and as such, they are easily accessible and likely to interact with viral particles that are trying to enter a target cell. These molecules have been implemented as low-affinity adhesion co-receptors;37,39 regulators of viral pathogenicity, tissue tropism, and spread;37,39–42 and determinants in host range specificity.39,43,44 Virus binding to SAs as a primary mode of low-avidity attachment has been shown to concentrate viral particles on the cell surface and thus, increase the probability of interaction with a high-avidity receptor.37 The most commonly expressed SA in human cells is N-acetyl neuraminic acid, which occurs in three main configurations: α2,3, α2,6, and α2,8. Because JCV binding and infection of glial cells is α2,6 SA-dependent and α2,3 SA-independent, it is suitable to name the former the receptor-type SA and the latter, the nonreceptor-type SA. Viral spread and pathogenicity are governed not only by the ability to bind SA but also by the type and linkage of SA that the virus recognizes. Bauer and colleagues41 clearly demonstrated that alterations in the SA-binding preference of the mouse polyomavirus capsid protein, VP1, parallel variations in virus disease outcome. Large plaque mouse polyomavirus strains are highly pathogenic. They recognize and bind to straight chain sialyloligosaccharides terminating in α2,3-linked SA.41 In contrast, small-plaque strains are much less pathogenic than large plaque because of their interaction with both branched and straight chain sialyloligosaccharides. Recognition of multiple SA linkages may result in entrapment of the virus on cells that do not express the high-avidity receptor thereby leading to a nonproductive infection. This emphasizes the importance of proper recognition of the both the proteinaceous part of the receptor and the SA linkage by the virus.41
We have examined and compared the expression of the JCV receptor-type SA, α2,6, and the nonreceptor-type SA, α2,3, in normal human spleen, tonsil, brain, kidney, and lung by immunofluorescence analysis. We report that the JCV receptor-type SA is expressed on oligodendrocytes, astrocytes, and B lymphocytes, to a lesser extent on T lymphocytes, and not on cortical neurons. Abundant distribution of this SA linkage is also seen in human kidney and lung. The presence of the receptor-type SA on cells closely parallels their susceptibility to JCV infection thus underlining the significant role of α2,6-linked SA in JCV interactions with its host.
Materials and Methods
Tissue Collection and Preparation
Normal human lung, kidney, spleen, and tonsil specimens were obtained from the Cooperative Human Tissue Network (Philadelphia, PA). The tissues were snap-frozen in O.C.T. compound (n-octane model of mega-8 detergent) and stored at −80°C. The specimens were sectioned at a thickness of 5.0 μm on Leica CM 3050 S cryostat and fixed in acetone at 4°C for 20 minutes. The samples were then stored at −20°C or used immediately for immunofluorescence analysis.
Immunofluorescence
Tonsil and spleen sections were rinsed in Tris-buffered saline (TBS), endogenous biotin, biotin receptors, and avidin binding sites were saturated using the avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA). The slides were then blocked for several hours in 10% normal goat serum to reduce nonspecific antibody binding. The tissue sections were then incubated with anti-CD19 10 μg/ml (clone HD37, Chemicon, Temecula, CA) or with anti-CD3 10 μg/ml (clone UCHT1, Novocastra Laboratories, Newcastle, UK) at 4[degree]C overnight. The sections were rinsed extensively in Tris-buffered saline and the primary antibodies were detected using 30 μg/ml of fluorescein isothiocyanate (FITC)- or Texas Red-conjugated goat anti-mouse IgG (Jackson Immunoresearch, West Grove, PA) for 1 hour at room temperature). Biotinylated MAA (α2,6 linkage) and SNA (α2,6-linkage) purchased from Vector Laboratories were applied to the tissue sections at a dilution of 5.0 μg/ml for 1 hour at room temperature. Lectin binding was detected using 2 μg/ml of fluorescein dichlorotriazine-conjugated streptavidin (Jackson Immunoresearch) for 1 hour at room temperature. The slides were mounted with Vectashield medium and analyzed using a Nikon Eclipse E 800 epifluorescent microscope (Nikon Inc., Mellville, NY) equipped with Hamamatsu ORCA-ER digital camera (Hamamatsu Inc., Bridgewater, NJ) and Openlab software (Improvision Inc., Lexington, MA). Lung and kidney samples were only probed with biotinylated lectins for the expression of the SA molecules as described above. Frontal cortex sections were stained free-floating. Briefly, sections were rinsed in Tris-buffered saline, blocked in 5% normal goat serum-0.1% bovine serum albumin in HEPES buffer (10 mmol/L HEPES, 150 mmol/L NaCl, 0.1 mmol/L MgCl2, pH 7.2) overnight. Slides were incubated with monoclonal antibodies (mAbs) directed against glial fibrillary acidic protein (GFAP) (Sigma, St. Louis, MO) 1:50 or myelin/oligodendrocyte-specific protein (MOSP) (Chemicon) 1:500, or neurofilament (NF) (Novocastra Laboratories) 1:10 and either biotinylated MAA or SNA for 72 hours at 4°C. Binding of the primary antibodies and lectins was visualized using goat anti-mouse IgG (GFAP and NF) or IgM (MOSP) Alexa Flour 594 conjugates (Molecular Probes, Eugene OR), 4 μg/ml, and streptavidin conjugated to Alexa Fluor 488 (Molecular Probes) at 1 μg/ml. The slides were mounted with Vectashield mounting medium and analyzed by confocal microscopy.
Confocal Microscopy
Confocal images were acquired with a Nikon PCM 2000 (Nikon Inc.) using the argon488 and the green helium-neon543 lasers. Serial optical sections were performed with Simple 32, C-imaging computer software (Compix Inc., Cranberry Township, PA). Z-series sections were collected at 0.5 μmol/L with a ×60 PlanApo lens and a scan zoom of ×2. Images were processed and reconstructed in NIH Image shareware (National Institutes of Health, Springfield, VA). Adobe Photoshop was used to convert the images to CMYK and in the assembly of figures.
Isolation of Tonsillar Lymphocytes and Flow Cytometric Analysis
Tonsillar lymphocytes were prepared by mincing and passage through a 70-μm nylon cell strainer (Falcon, Franklin Lakes, NJ). After washing three times in Hanks’ balanced salt solution, cell suspensions were layered on a Histopaque-1077 gradient (Sigma-Aldrich, St. Louis, MO) for density separation. Tonsillar lymphocytes were collected after centrifugation for 30 minutes at 900 × g. Cells were suspended in buffer comprised of HEPES containing 5% bovine serum albumin. Cells were then stained with biotinylated-MAA or biotinylated-SNA for 30 minutes at 4°C. After two washes, anti-CD19-R-phycoerthryn (R-PE) (clone HD37, Chemicon), anti-CD3-allophycocyanin (APC) (clone UCHT1; eBioscience, San Diego, CA), or isotype controls, and streptavidin-peridinin chlorophyll A protein (PerCP) (BD PharMingen, San Diego, CA) were added for another 30 minutes. Cells were then washed and fixed in 2% paraformaldehyde in phosphate-buffered saline and 2 × 105 events were collected on a FACScalibur. The data were acquired and analyzed using CellQuest software (Becton Dickinson, Franklin Lakes, NJ).
Results
Expression of the JCV Receptor-Type SA on B Cells in Human Tonsil
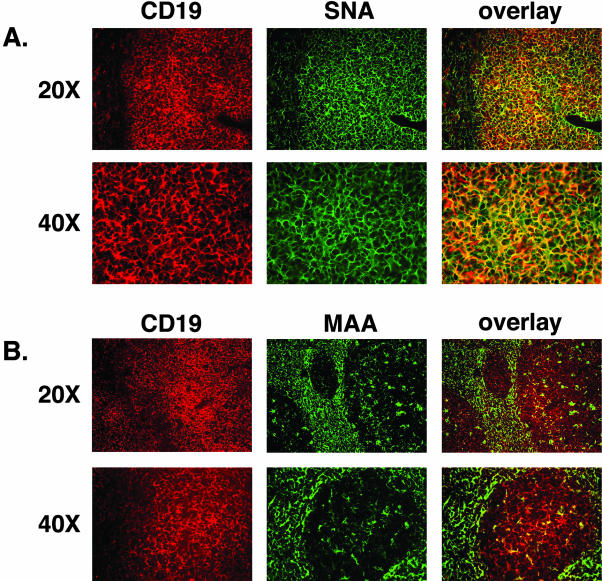
JCV DNA has previously been detected in tonsil tissue isolated from both children and adults.32 The oropharyngeal anatomical location of the tonsils and their function in detecting and responding to pathogens in the oral cavity implicates these secondary lymphoid organs as a potential site of primary JCV infection via a respiratory route of transmission.30,32 We sought to determine whether the JCV receptor-type SA was expressed in tonsillar tissue. After tonsillectomy, palatine tonsils were snap-frozen and sectioned at 5.0 μm for further immunofluorescence analysis. The specimens were stained with a mAb to the B-cell-specific surface marker CD19, which in turn was visualized by a Texas Red-conjugated secondary antibody (Figure 1, A and B). The receptor-type SA, α2,6, and the nonreceptor-type SA, α2,3, were detected by the biotinylated lectins SNA and MAA, respectively. The bound lectins were recognized by FITC-conjugated streptavidin (Figure 1, A and B). The overlaid image of the two markers reveals strong co-localization of the receptor-type SA on CD19-positive B cells. In contrast, the control, or nonreceptor-type SA, did not co-localize with CD19 on B cells.
Figure 1.
The JCV receptor-type SA is expressed on B lymphocytes in the tonsil. B cells were labeled with anti-C19 mAb and visualized with goat anti-mouse Texas Red-conjugated secondary Ab (A and B, first column). After the sections were incubated with biotinylated SNA (A) or MAA (B) lectin followed by fluorescein dichlorotriazine-streptavidin. CD19-positive cells co-stained with the SNA lectin as shown by the overlay image (A, third column). MAA did not bind CD19-positive cells (B, third column).
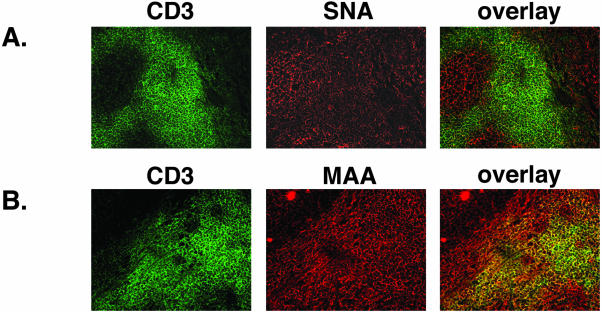
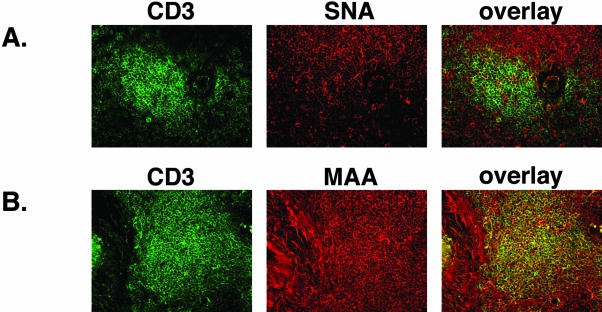
Using a similar staining procedure and a mAb to the CD3 antigen of T cells, we identified the T-cell population in the interfollicular regions of the tonsil. The binding of the cellular marker CD3 was visualized with FITC-conjugated secondary antibody. The tonsillar sections were then incubated with the biotinylated lectins SNA and MAA, which were detected by a Texas Red-conjugated streptavidin (Figure 2, A and B). The CD3-labeled T cells did not co-stain for the expression of the receptor-type SA, α2,6 (Figure 2A, third column). In contrast, the T cells were positive for the presence of the nonreceptor-type SA, α2,3, as indicated by the overlap of anti-CD3 antibody and MAA lectin binding (Figure 2B, third column).
Figure 2.
The JCV receptor-type SA is not expressed on CD3-positive cells in the tonsil. Frozen tonsil sections were double-labeled with anti-CD3 mAb and SNA or MAA lectin. Binding to CD3 was visualized by FITC-conjugated anti-mouse Ab (A and B, first column). The overlay images reveal lack of expression of the α2,6-linked SA on CD-3-positive cells (A, third column). In contrast, the α2, 3-linked SA is present on CD3-expressing cells (B, third column).
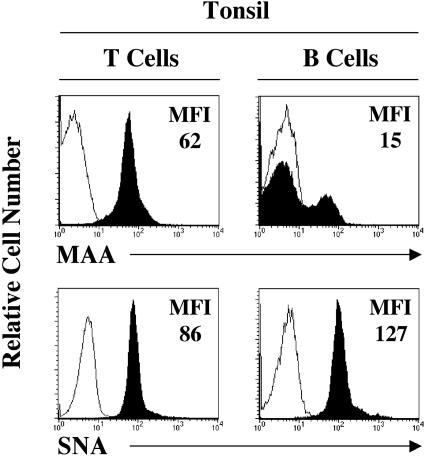
To confirm our histological observations, we analyzed the expression of the receptor-type and the nonreceptor-type SA on primary lymphocytes by flow cytometry. Fresh tonsils were obtained from patients undergoing routine tonsillectomy. The mononuclear cells were isolated by mechanical disruption of the tissue followed by Histopaque-1077 gradient centrifugation. The lymphocyte population was labeled with fluorophore-conjugated B- and T-cell-specific markers CD19-R-PE and CD3-APC, respectively, and with biotinylated SNA and MAA lectins followed by streptavidin-PerCP. Our results show that all of the CD19-positive cells expressed high levels of the receptor-type SA, and only a small subset (∼20%) of them stained positive for the presence of the nonreceptor-type SA (Figure 3, second column). The pattern of receptor-type SA distribution determined by flow cytometry closely resembled our immunofluorescence findings in the tissue. The nonreceptor-type SA was present on the CD3-positive T lymphocytes (Figure 3, first column). The receptor-type SA was also detected on the surface of the T cells, but it was significantly reduced when compared with the binding of the SNA lectin to the B lymphocytes (Figure 3). Identical results were obtained on human peripheral blood lymphocytes (data not shown).
Figure 3.
Expression of JCV receptor-type and nonreceptor-type SA on T and B tonsillar lymphocytes. Lymphocytes were isolated from the tonsil and analyzed for the expression of CD3, CD19, and MAA or SNA by flow cytometry. The expression of MAA or SNA on stained cells (shaded histograms) versus unstained cells (solid lines) is shown on T cells (defined as CD3+ and CD19−) and B cells (defined as CD3− and CD19+). MFI, mean fluorescence intensity. One experiment representative of three is shown.
Expression of the JCV Receptor-Type SA on B Cells in Human Spleen
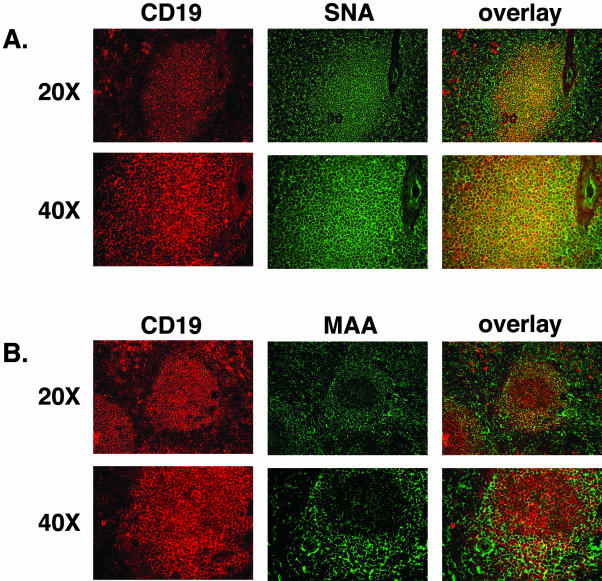
The spleen represents a major accumulation of lymphoid tissues in the body. One of the important functions of the spleen is the production of activated lymphocytes, which are released into the blood stream. JCV infection of B lymphocytes in the spleen has been observed by in situ hybridization and immunocytochemistry to viral capsid antigens.33 The detection of JCV in the spleen suggests availability of viral receptors in this secondary lymphoid organ. We obtained frozen spleen specimens from the Cooperative Human Tissue Network. Analogous to the staining experiment performed on tonsillar tissue, we evaluated the expression pattern of the receptor and nonreceptor-type SA in the B- and T-cell-rich regions in the spleen. Figure 4A shows staining of a B-cell lymphoid nodule with CD19 that entirely co-localizes with the expression of α2,6-linked SA. Binding of the MAA lectin to the α2,3-linked SA is excluded from the majority of the B-cell-populated lymphoid follicle (Figure 4B). The receptor-type SA is not found on the CD3-positive T cells in the spleen as demonstrated by the lack of overlap between the cellular marker and the SNA lectin (Figure 5A, overlay). As seen in the tonsil, the splenic T cells stained positive for the presence of the nonreceptor-type SA, α2,3 by binding the MAA lectin (Figure 5B, third column).
Figure 4.
The JCV receptor-type SA but not the nonreceptor-type SA is present on B lymphocytes in the spleen. Spleen sections were double-labeled with monoclonal anti-CD19 antibody and biotinylated SNA (A) or MAA (B) lectin overnight at 4°C. CD19 binding was visualized with Texas Red-conjugated goat anti-mouse Ab, and lectin binding was detected with fluorescein dichlorotriazine-conjugated streptavidin. Both panels in A demonstrate co-localization in the binding reactivity of anti-CD19 Ab and the SNA lectin, whereas no co-staining is seen with the MAA lectin in CD-19-positive regions in the spleen.
Figure 5.
The JCV receptor-type SA is not present on T lymphocytes in the spleen. Frozen spleen specimens were sectioned at a thickness of 5 μm and incubated with anti-CD3 mAb detected with goat anti-mouse FITC-conjugated antibody. Then the sections were probed with biotinylated SNA (A) or biotinylated MAA (B) lectin and visualized with Texas Red-conjugated streptavidin. The overlay of CD3-positve cells and SNA binding reveals absence of co-localization (A, right). In contrast, there is complete overlap in staining of CD3 T cells and MAA lectin (B).
Expression of the JCV Receptor-Type SA on Glial Cells but Not on Cortical Neurons in Human Brain
Several different techniques such as in situ hybridization, immunocytochemistry, and light and electron microscopy have shown that the presence of JCV DNA, proteins, and virions in the CNS is confined to the nuclei and cytoplasm of glial cells.45–48 The oligodendrocytes are the primary target of JCV lytic infection.2,3,49 The astrocytes are also susceptible to infection by JCV although replication of viral late protein and progeny production remains at low levels.46,49–52 Except for a few isolated reports JC virions, protein, and DNA cannot be detected in neurons.45,53
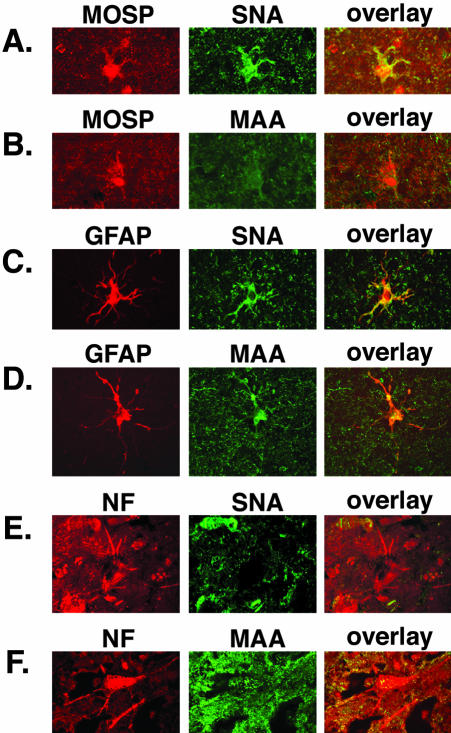
Our next step was to determine the pattern of SA expression in the normal human brain. Frozen frontal cortex specimens were obtained from Rhode Island Hospital brain bank and sectioned at a thickness of 40 μm. The oligodendrocytes were detected by incubating the sections with a mAb to the myelin/oligodendrocyte-specific protein (MOSP), followed by an AlexaFlour 594-conjugated secondary antibody (Figure 6B). The receptor-type SA, α2,6, is abundantly expressed on the surface of the oligodendrocytes as revealed by the binding of the SNA lectin. The overlay image shows strong co-localization of the cellular marker MOSP and the SNA lectin (Figure 6A). The presence of the nonreceptor-type SA is less prominent in oligodendrocytes. The MAA lectin stained the oligodendrocytes with a similar intensity as the background.
Figure 6.
Expression of α2,6-, and α2,3-linked SA in human frontal cortex. Double labeling with cellular markers and lectins was used to determine the pattern of the JCV receptor-type SA. Oligodendrocytes were labeled with anti-MOSP Ab and visualized with Alexa Fluor 594-conjugated secondary Ab (A and B, first column). As shown on the overlays of A and B, the oligoglial cells stain positive for expression of α2,6-linked SA (A). The MAA binding is weak (B). Astrocytes labeled with GFAP (B and C, first column) express both SA linkages. Complete overlap of the cellular marker and the SNA (C) or MAA (D) lectins is seen (C and D, third column). Neurons were labeled with anti-NF Ab (E and F, first column). They do not co-stain with SNA (E, third column) but stain with MAA (F, overlay). Original magnifications, ×120.
Unlike the results with the oligodendrocytes, the astrocytes were found to express both the receptor-type and the nonreceptor-type SA as revealed by the complete overlap in GFAP and SNA binding (Figure 6C) or GFAP and MAA binding (Figure 6D).
In contrast the cortical neurons did not stain positive for the presence of the receptor-type SA (Figure 6E). A mAb directed against the neurofilament triplet (NF) was used to identify neurons. The SNA lectin did not bind the NF-labeled neurons (Figure 6E). The MAA lectin recognized the nonreceptor-type SA, α2,3 on the surface of some but not all cortical neurons (Figure 6F).
Expression of the JCV Receptor-Type SA in Human Kidney and Lung
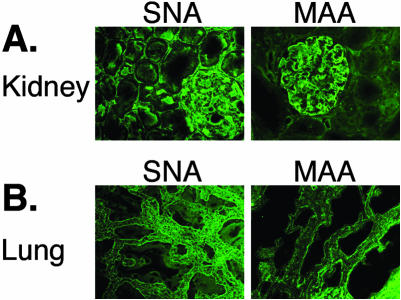
Even though JCV infection has never revealed any histopathological changes in the kidney, viral DNA is commonly detected in the renal cortex and medulla of more than 50% of individuals.20,54 We used the SNA and MAA lectins to probe 5.0-μm-thick kidney sections for the presence of α2,6- and α2,3-linked SA, respectively. Figure 6A shows that the receptor-type SA expression is high in the kidney demonstrated by staining of the renal glomerulus and the surrounding tubules. The nonreceptor-type SA is less prevalent in the kidney, confined mostly to the renal glomerulus (Figure 7A).
Figure 7.
Expression of JCV receptor- and nonreceptor-type SA in the kidney and lung. Frozen kidney and lung specimens were sectioned and probed for the presence of α2,6- and α2,3-linked SA with SNA and MAA lectin, respectively. The SNA lectin bound more strongly to both tissues (A and B, first column) compared to the binding affinity of the MAA lectin (A and B, second column).
Detection of JCV in tonsillar tissue32 and the suggested hypothesis that the respiratory tract may be the site of initial viral infection prompted us to look at the expression of the receptor-type SA in the lung. The lung parenchyma displays stronger reactivity with the SNA lectin than with the MAA lectin, nevertheless, α2,6 and α2,3 linkages are present abundantly in this tissue (Figure 7B).
Discussion
Recognizing and binding to α2,6-linked SA is indispensable for initiation of JCV productive infection.38 In this study we have demonstrated that the JCV receptor-type SA, α2,6, is present on B lymphocytes in the tonsil and spleen, on oligodendrocytes and astrocytes in the brain, and in kidney and lung. All of these tissues and cell types have been implicated to play a role in JCV infection and spread in the human host.4,32,33,55–57 Although several reports link JCV abortive infection to tumorigenesis in glial cells58 PML is the only recognized adverse outcome of JCV lytic infection in the brain.4 Development of the disease occurs in the context of profound immunosuppression and active JCV replication in the brain. The high prevalence of JC asymptomatic infection in the human population contrasts the low incidence of PML and brings up the inevitable questions of sites of JCV persistence. Availability of proper cell surface receptor(s) is the first important determinant of viral tissue tropism and mechanism of spread in the host. SAs are most commonly found as terminal monosaccharides of many membrane-anchored glycoproteins and glycosphingolipids.59 Their biological role is yet to be fully elucidated. The negative charge displayed by biological membranes has been partially attributed to SAs; they are known to participate in cell-cell, cell-matrix interactions and allow survival of erythrocytes in circulation.59 A number of viruses successfully use certain types of SA linkages as attachment and entry receptors.42,44,60,61 It is reasonable to expect that a virus using a given type of SA linkage in its entry process will target a tissue or a cell type possessing high levels of that SA. The incorporation of SA structures into glycoconjugates is achieved by five linkage-specific sialyltransferases.62 The expression level of these enzymes has been shown to be tissue and temporally regulated, thus accounting for the differential and cell-specific pattern in SA linkage production.62 The α2,6-linkage is the SA moiety that mediates JCV infection. The virus infects independently of the presence of other SA molecules because the enzymatic removal of α2,3-linked SA does not inhibit infection.38 Therefore, we examined the proposed sites of JCV persistent and lytic infection for the presence of the receptor-type SA, α2,6. We performed dual-labeling experiments on frozen tonsil and spleen sections. Our findings demonstrate that B lymphocytes express the receptor-type SA as indicated by the co-localization of the B-cell-specific marker CD19 and the α2,6 linkage-specific lectin SNA. The nonreceptor-type SA was not detected on the B-cell population in the tonsil and spleen because the α2,3-specific lectin MAA did not co-localize with the CD19 marker. The reciprocal pattern of SA linkage distribution is observed on the T lymphocytes in the tonsil and spleen. Double-staining experiments reveal that antibody to the T-cell-specific marker CD3 overlaps in binding with the MAA lectin, but does not co-localize with the SNA lectin, which binds to the receptor-type SA. To confirm these findings we analyzed single cell suspensions of lymphocytes isolated from fresh tonsils by flow cytometry for expression of the relevant ligands. Consistent with the histological data we found that B lymphocytes express higher levels of the receptor-type SA than T lymphocytes. Previous work in our laboratory has shown that JCV binds to the primary B lymphocytes, and does not bind to T lymphocytes suggesting the lack of JC interaction moieties on the T cells.34 Moreover, neuraminidase pretreatment of the B lymphocytes to remove the cell surface-associated SAs, abrogated their ability to transmit JCV infection to the highly susceptible glial cells.34 Experimental evidence demonstrates that human B cells not only bind JC virions but also support low level of productive viral infection30,34 indicating the need for and availability of a functional JCV receptor on these cells. In concordance with these observations, we show that the B-lymphocyte population in tonsil and spleen stain positive for the presence of the JCV receptor-type SA.
Because the brain is the site of JCV-induced pathogenesis and the glial cells are the main targets of JCV infection, we next examined the presence of the receptor-type SA in the permissive oligodendrocytes and the semipermissive astrocytes. The oligodendrocytes are the myelin-producing cells in the CNS. Their lytic destruction by JCV with secondary demyelination constitutes PML.2 Our double-labeling experiment clearly showed the co-localization of the oligodendrocytes-specific marker, MOSP and the SNA lectin indicating the presence of the receptor-type SA on the surface of mature oligodendrocytes. The SNA lectin also bound to the GFAP-positive astrocytes, the other common target of JCV in the brain. These observations are in agreement with previous findings in our laboratory demonstrating that JCV binds to primary human fetal glial cells.34 Probing of the oligodendrocytes with the MAA lectin did not produce the robust signal seen in MAA lectin binding to the astrocytes.
The demyelination process in PML results from the metabolic and eventually cytocidal damage by JCV on glial cells.2,9 There are no definitive reports establishing the involvement of neurons as host cells for JCV. The neuronal cells do not appear to support either viral replication or subsequent spread of JCV throughout the brain.45,53 Our staining experiments revealed that the JCV receptor-type SA, α2,6-linked SA is not expressed on the NF-labeled cortical neurons as indicated by the lack of SNA binding to this cell type. There are a number of cellular factors required for the successful execution of the viral life cycle. The absence of the receptor-type SA acid from the surface of cortical neurons does not definitively account for the inability of these cells to support productive JCV infection. Nevertheless, it is important to note the correlation between the absence of receptor-type SA from the neuronal cells’ surface and their low susceptibility to JCV infection.
The search for sites of JCV primary infection and latency prompt us to examine the distribution of the receptor-type SA in the lung and the kidney. There is an abundant expression of this SA linked in both tissues. A respiratory route of transmission has been proposed for the closely related human polyomavirus BKV.63,64 Although no respiratory symptoms have ever been associated with seroconversion to JCV as seen with BKV, the presence of JCV in pediatric tonsil specimens suggests that initial infection by the virus can use the respiratory route.32 Others have detected JCV DNA in lung tissue collected from a pediatric PML patient.57,65
In healthy immunocompetent individuals the rate of renal JCV excretion increases with age.66,67 The intermittent JC viruria is believed to result from the activation of already established infection in the urinary tract.68 In the kidney JCV DNA can be detected throughout the renal parenchyma.21,69,70 As mentioned earlier JCV persistence in the kidney remains primarily subclinical without altering the renal morphology and physiology.20 The JCV receptor-type SA, α2,6 linkage is abundantly present in the kidney indicating the availability of JCV binding sites on renal epithelial cells.
The results from the staining experiments presented here describe a correlation between JCV susceptibility and distribution of the receptor-type SA. Our observations maintain the established necessity for α2,6-linked SA in JCV infection and support the previously suggested mechanism of JCV hematogenous spread from the periphery to the CNS.36
Acknowledgments
We thank all members of the Atwood laboratory for critical discussion during the course of this work, Judith Nathanson for critical help with computer graphics, Virginia Hovanesian for confocal microscopy, and Amanda Robinson and Lorie St. Pierre for administrative assistance.
Footnotes
Address reprint requests to Dr. Walter J. Atwood, Department of Molecular Microbiology and Immunology, Brown University, Box G-B616, 171 Meeting St., Providence, RI 02912. E-mail: walter_atwood@brown.edu.
Supported by the National Cancer Institute (R01 CA71878), the National Institute of Neurological Disorders and Stroke (R01 NS43097), and the Department of Education (G.A.A.N.N. training grant P200A030100 to S.H.R.).
References
- Major EO, Amemiya K, Tornatore CS, Houff SA, Berger JR. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992;5:49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Rhein G, Chou S. Particles resembling papovaviruses in human cerebral demyelinating disease. Science. 1965;148:1477–1479. doi: 10.1126/science.148.3676.1477. [DOI] [PubMed] [Google Scholar]
- Padgett B, ZuRhein G, Walker D, Echroade R, Dessel B. Cultivation of papova-like virus from human brain with progressive multifocal leukoencephalopathy. Lancet. 1971;I (7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Astrom K, Mancall E, Richardson EP., Jr Progressive multifocal leukoencephalopathy. Brain. 1958;81:93–127. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- Cavanagh J, Lond M. Cerebral demyelination associated with disorders of the reticuloendothelial system. Lancet. 1959;33:524–529. doi: 10.1016/s0140-6736(59)91774-x. [DOI] [PubMed] [Google Scholar]
- Richardson EP. Progressive multifocal leukoencephalopathy. N Engl J Med. 1961;265:815–823. doi: 10.1056/NEJM196110262651701. [DOI] [PubMed] [Google Scholar]
- Richardson EP, Johnson PC. Atypical progressive multifocal leukoencephalopathy with plasma cell infiltrates. Acta Neuropathol Suppl. 1975;6:247–250. doi: 10.1007/978-3-662-08456-4_43. [DOI] [PubMed] [Google Scholar]
- Silverman L, Rubinstein L. Electron microscopic observations on a case of progressive multifocal leukoencephalopathy. Acta Neuropathol (Berl) 1965;5:215–224. doi: 10.1007/BF00686519. [DOI] [PubMed] [Google Scholar]
- Zu Rhein G. Polyoma-like virions in a human demyelinating disease. Acta Neuropathol (Berl) 1967;8:57–68. doi: 10.1007/BF00686650. [DOI] [PubMed] [Google Scholar]
- Weber F, Goldmann C, Kramer M, Kaup FJ, Piclhardt M, Young P, Petry H, Weber T, Luke W. Cellular and humoral immune response in progressive multifocal leukoencephalopathy. Ann Neurol. 2001;49:636–642. [PubMed] [Google Scholar]
- Berger JR, Concha M. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J Neurovirol. 1995;1:5–18. doi: 10.3109/13550289509111006. [DOI] [PubMed] [Google Scholar]
- Major EO, Ault GS. Progressive multifocal leukoencephalopathy: clinical and laboratory observations on a viral induced demyelinating disease in the immunodeficient patient. Curr Opin Neurol. 1995;8:184–190. [PubMed] [Google Scholar]
- Chowdhury M, Taylor JP, Tada H, Rappaport J, Wong-Staal F, Amini S, Khalili K. Regulation of the human neurotropic virus promoter by JCV-T antigen and HIV-1 tat protein. Oncogene. 1990;5:1737–1742. [PubMed] [Google Scholar]
- Chowdhury M, Taylor JP, Chang CF, Rappaport J, Khalili K. Evidence that a sequence similar to TAR is important for induction of the JC virus late promoter by human immunodeficiency virus type 1 Tat. J Virol. 1992;66:7355–7361. doi: 10.1128/jvi.66.12.7355-7361.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury M, Kundu M, Khalili K. GA/GC-rich sequence confers Tat responsiveness to human neurotropic virus promoter, JCVL, in cells derived from central nervous system. Oncogene. 1993;8:887–892. [PubMed] [Google Scholar]
- Cullen B. The HIV-1 Tat protein: an RNA sequence-specific processivity factor? Cell. 1990;63:655–657. doi: 10.1016/0092-8674(90)90129-3. [DOI] [PubMed] [Google Scholar]
- Gardner SD, Mackenzie EF, Smith C, Porter AA. Prospective study of the human polyomaviruses BK and JC and cytomegalovirus in renal transplant recipients. J Clin Pathol. 1984;37:578–586. doi: 10.1136/jcp.37.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesters PM, Heritage J, McCance DJ. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 1983;147:676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- Hogan TF, Borden EC, McBain JA, Padgett BL, Walker DL. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med. 1980;92:373–378. doi: 10.7326/0003-4819-92-3-373. [DOI] [PubMed] [Google Scholar]
- Dorries K, Elsner C. Persistent polyomavirus infection in kidney tissue of patients with disease other then progressive multifocal leucoencephalopathy. Virol Adv. 1991;10:51–61. [Google Scholar]
- Dorries K, ter Meulen V. Progressive multifocal leucoencephalopathy: detection of papovavirus JC in kidney tissue. J Med Virol. 1983;11:307–317. doi: 10.1002/jmv.1890110406. [DOI] [PubMed] [Google Scholar]
- Lafon ME, Dutronc H, Dubois V, Pellegrin I, Barbeau P, Ragnaud JM, Pellegrin JL, Fleury HJA. JC virus remains latent in peripheral blood B lymphocytes but replicates actively in urine from AIDS patients. J Infect Dis. 1998;177:1502–1505. doi: 10.1086/515305. [DOI] [PubMed] [Google Scholar]
- Dubois V, Lafon ME, Ragnaud JM, Pellegrin JL, Damasio F, Baudouin C, Michaud V, Fleury HJA. Detection of JC virus DNA in the peripheral blood leukocytes of HIV-infected patients. AIDS. 1996;10:353–358. doi: 10.1097/00002030-199604000-00001. [DOI] [PubMed] [Google Scholar]
- Dubois VL, Lafon M, Ragnaud JM, Pellegrin JL, Fleury HJA: JC virus mRNA in the peripheral blood of JC-positive HIV-infected patients. X1 International Conference on AIDS/Vancouver 1996, Abstract no. Th. A. 4054 [Google Scholar]
- Dubois V, Dutronc H, Lafon ME, Poinsot V, Pellegrin JL, Ragnaud JM, Ferrer AM, Fleury HJA. Latency and reactivation of JC virus in peripheral blood of human immunodeficiency virus type-1 infected patients. J Clin Microbiol. 1997;35:2288–2292. doi: 10.1128/jcm.35.9.2288-2292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois V, Moret H, Lafon ME, Janvresse CB, Dussaix E, Icart J, Karaterki A, Ruffault A, Taoufik Y, Vignoli C, Ingrand D. Prevalence of JC virus viraemia in HIV-infected patients with or without neurological disorders: a prospective study. J Neurovirol. 1998;4:539–544. doi: 10.3109/13550289809113498. [DOI] [PubMed] [Google Scholar]
- Andreoletti L, Lescieux A, Lambert V, Si-Mohamed A, Matta M, Wattre P, Belec L. Semiquantitative detection of JCV-DNA in peripheral blood leukocytes from HIV-1-infected patients with or without progressive multifocal leukoencephalopathy. J Med Virol. 2002;66:1–7. doi: 10.1002/jmv.2103. [DOI] [PubMed] [Google Scholar]
- Koralnik IJ, Schmitz JE, Lifton MA, Forman MA, Letvin NL. Detection of JC virus DNA in peripheral blood cell subpopulations of HIV-1-infected individuals. J Neurovirol. 1999;5:430–435. doi: 10.3109/13550289909029484. [DOI] [PubMed] [Google Scholar]
- Atwood WJ, Amemiya K, Traub R, Harms J, Major EO. Interaction of the human polyomavirus JCV, with human B-lymphocytes. Virology. 1992;190:716–723. doi: 10.1016/0042-6822(92)90909-9. [DOI] [PubMed] [Google Scholar]
- Monaco MGC, Atwood WJ, Gravell M, Tornatore CS, Major EO. JCV infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implication for viral latency. J Virol. 1996;70:7004–7012. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco MC, Shin J, Major EO. JC virus infection in cells from lymphoid tissue. Dev Biol Stand. 1998;94:115–122. [PubMed] [Google Scholar]
- Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol. 1998;72:9918–9923. doi: 10.1128/jvi.72.12.9918-9923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houff SA, Major EO, Katz DA, Kufta CV, Sever JL, Pittaluga S, Roberts JR, Gitt J, Saini N, Lux W. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. N Engl J Med. 1988;318:301–305. doi: 10.1056/NEJM198802043180507. [DOI] [PubMed] [Google Scholar]
- Wei G, Liu CK, Atwood W. JC virus binds to primary human glial cells, tonsillar stromal cells, and B-lymphocytes, but not to T-lymphocytes. J Neurovirol. 2000;6:127–136. doi: 10.3109/13550280009013156. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Berger JR, Houff SA, Curfman B, Meyers K, Winfield D, Major EO. Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann Neurol. 1992;31:454–462. doi: 10.1002/ana.410310426. [DOI] [PubMed] [Google Scholar]
- Sabath BF, Major EO. Traffic of JC virus from sites of initial infection to the brain: the path to progressive multifocal leukoencephalopathy. J Infect Dis. 2002;186:S180–S186. doi: 10.1086/344280. [DOI] [PubMed] [Google Scholar]
- Barton ES, Youree BE, Ebert DH, Forrest JC, Connolly JL, Valyi-Nagy T, Washington K, Wetzel JD, Dermody TS. Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease. J Clin Invest. 2003;111:1823–1833. doi: 10.1172/JCI16303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CK, Wei G, Atwood WJ. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal alpha 2-6 linked sialic acids. J Virol. 1998;72:4643–4649. doi: 10.1128/jvi.72.6.4643-4649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnberg N, Pring-Akerblom P, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor on Chang C cells. J Virol. 2002;76:8834–8841. doi: 10.1128/JVI.76.17.8834-8841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwegmann-Wessels CZG, Laude H, Enjuanes L, Herrler G. Binding of transmissible gastroenteritis coronavirus to cell surface sialoglycoproteins. J Virol. 2002;76:6037–6043. doi: 10.1128/JVI.76.12.6037-6043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PH, Cui C, Stehle T, Harrison SC, DeCaprio JA, Benjamin TL. Discrimination between sialic acid containing receptors and pseudoreceptors regulates polyomavirus spread in the mouse. J Virol. 1999;73:5826–5832. doi: 10.1128/jvi.73.7.5826-5832.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi H, Lipton H. Heparan sulfate mediates infection of high-neurovirulence Theiler’s viruses. J Virol. 2002;76:8400–8407. doi: 10.1128/JVI.76.16.8400-8407.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Suzuki Y, Mitnaul L, Vines A, Kida H, Kawaoka Y. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology. 1997;227:493–499. doi: 10.1006/viro.1996.8323. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ito T, Suzuki T, Holland RE, Chambers TM, Kiso M, Ishida H, Kawaoka Y. Sialic acid species as a determinant of the host range of influenza A viruses. J Virol. 2000;74:11825–11831. doi: 10.1128/jvi.74.24.11825-11831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budka H, Shah KV. Papovavirus antigens in paraffin sections of PML brains. Prog Clin Biol Res. 1983;105:299–309. [PubMed] [Google Scholar]
- Itoyama YH, Webster deF, Sternberger NH, Richardson EP, Walker DL, Quarles RH, Padgett BL. Distribution of papovavirus, myelin-associated glycoprotein, and myelin basic protein in progressive multifocal leukoencephalopathy lesions. Ann Neurol. 1982;11:369–407. doi: 10.1002/ana.410110414. [DOI] [PubMed] [Google Scholar]
- Mazlo M, Tariska I. Morphological demonstration of the first phase of polyomavirus replication in oligodendroglia cells of human brain in progressive multifocal leukoencephalopathy (PML). Neuropathology. 1980;49:133–143. doi: 10.1007/BF00690753. [DOI] [PubMed] [Google Scholar]
- Mazlo M, Herndon R. Progressive multifocal leukoencephalopathy: ultrastructural findings in two brain biopsies. Neuropathol Appl Neurol. 1977;3:323–339. [Google Scholar]
- Watanabe I, Preskorn S. Virus-cell interaction in oligodendroglia, astroglia and phagocyte in progressive multifocal leukoencephalopathy. An electron microscopic study. Acta Neuropathol (Berl) 1976;36:101–115. doi: 10.1007/BF00685273. [DOI] [PubMed] [Google Scholar]
- Boldorini R, Cristina S, Vago L, Tosoni A, Guzzetti S, Costanzi G. Ultrastructural studies in the lytic phase of progressive multifocal leukoencephalopathy in AIDS patients. Ultrastruct Pathol. 1993;17:599–609. doi: 10.3109/01913129309027796. [DOI] [PubMed] [Google Scholar]
- Mazlo M, Tariska I. Are astrocytes infected in progressive multifocal leukoencephalopathy (PML)? Acta Neuropathol (Berl) 1982;56:45–51. doi: 10.1007/BF00691181. [DOI] [PubMed] [Google Scholar]
- Stoner G, Ryschkewitsch C, Walker D, Webster H. JC papovavirus large tumor (T)-antigen expression in brain tissue of acquired immune deficiency syndrome (AIDS) and non-AIDS patients with progressive multifocal leukoencephalopathy. Proc Natl Acad Sci USA. 1986;83:2271–2275. doi: 10.1073/pnas.83.7.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintaku MMR, Sawa H, Nagashima K. Infection with JC virus and possible dysplastic ganglion-like transformation of the cerebral cortical neurons in a case of progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2000;59:921–929. doi: 10.1093/jnen/59.10.921. [DOI] [PubMed] [Google Scholar]
- Aoki N, Kitamura T, Tominaga T, Fukomori N, Sakamoto Y, Kato K, Mori M. Immunohistochemical detection of JC virus in nontumorous renal tissue of a patient with renal cancer but without progressive multifocal leukoencephalopathy. J Clin Microbiol. 1999;37:1165–1167. doi: 10.1128/jcm.37.4.1165-1167.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault GS, Ryschkewitsch CF, Stoner GL. Type-specific amplification of viral DNA using touchdown and hot start PCR. J Virol Methods. 1994;46:145–156. doi: 10.1016/0166-0934(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Richardson EP, Webster H deF. Progressive multifocal leukoencephalopathy: its pathological features. Sever JL, Madden D, editors. New York: Alan R. Liss, Inc.; Polyomaviruses and Human Neurological Disease. 1983:pp 191–203. [PubMed] [Google Scholar]
- Newman JT, Frisque RJ. Detection of archetype and rearranged variants of JC virus in multiple tissues from a pediatric PML patient. J Med Virol. 1997;52:243–252. doi: 10.1002/(sici)1096-9071(199707)52:3<243::aid-jmv2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Del Valle L, Gordon J, Ferrante P, Khalili K. JC virus in experimental and clinical brain tumorigenesis. Khalili K, Stoner GL, editors. New York: Wiley-Liss; Human PolyomavirusesMolecular and Clinical Perspectives. 2001:pp 409–430. [Google Scholar]
- Schauer R. Achievements and challenges of sialic acid research. Glycoconj J. 2000;17:485–499. doi: 10.1023/A:1011062223612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GN, Paulson JC. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Chen MH, Benjamin T. Roles of N-glycans with alpha 2,6 as well as alpha 2,3 linked sialic acid in infection by polyoma virus. Virology. 1997;233:440–442. doi: 10.1006/viro.1997.8596. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Paulson JC. Differential expression of five sialyltransferase genes in human tissues. J Biol Chem. 1994;269:17872–17878. [PubMed] [Google Scholar]
- Mantyjarvi R, Karjalainen H, Laaksonen M. Cell-mediated immune reaction against BK virus-transformed cells. Cancer Immunol Immunother. 1984;17:28–32. doi: 10.1007/BF00205493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit, Wertheim-van Dillen P, van Strien A, van der Noordaa J. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10:91–99. doi: 10.1002/jmv.1890100203. [DOI] [PubMed] [Google Scholar]
- Newman JT, Frisque RJ. Identification of JC virus variants in multiple tissues of pediatric and adult PML patients. J Med Virol. 1999;58:79–86. [PubMed] [Google Scholar]
- Agostini HT, Ryschkewitsch CF, Stoner GS. Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. J Clin Microbiol. 1996;34:159–164. doi: 10.1128/jcm.34.1.159-164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini HT, Ryschkewitsch CF, Mory R, Singer EJ, Stoner GL. JC virus (JCV) genotypes in brain tissue from patients with progressive multifocal leukoencephalopathy (PML) and in urine from controls without PML: increased frequency of JCV type 2 in PML. J Infect Dis. 1997;176:1–8. doi: 10.1086/514010. [DOI] [PubMed] [Google Scholar]
- Dorries K. Latent and persistent polyomavirus infection. Khalili K, Stoner GL, editors. New York: Wiley-Liss, Inc.; Human PolyomavirusesMolecular and Clinical Perspectives. 2001:pp 97–235. [Google Scholar]
- Grinnell B, Padgett B, Walker D. Distribution of non-integrated DNA from JC papovavirus in organs of patients with progressive multifocal leukoencephalopathy. J Infect Dis. 1983;147:669–675. doi: 10.1093/infdis/147.4.669. [DOI] [PubMed] [Google Scholar]
- McCance D. Persistence of animal and human papovaviruses in renal and nervous tissues. Prog Clin Biol Res. 1983;105:343–357. [PubMed] [Google Scholar]