Abstract
Since Hodgkin and Reed-Sternberg (HRS) cells of Hodgkin lymphoma (HL) generally have immunoglobulin gene rearrangements, they are considered to be of B-cell origin. One of the characteristics of HRS cells is a prominent production of various cytokines and chemokines. Cytokine production is generally driven by expression of T-cell transcription factors (TFs). Only limited information is available on the expression of T-cell TFs in HL. Expression of four T-cell TFs and the target cytokine spectrum of these TFs were analyzed in six HL and three large B-cell lymphoma (LBCL) cell lines using quantitative PCR. ERM expression was observed in all HL and LBCL cell lines. Out of HL cell lines, T-bet was expressed in five, GATA-3 in four, and c-Maf in two cell lines. Immunohistochemistry in HL tissues revealed that in 11 of 12 (92%) of the classical HL cases HRS cells were GATA-3 and/or T-bet positive. In three of six cases of nodular lymphocyte predominance type of HL, the neoplastic cells were T-bet positive. Overall, the T-cell TF and cytokine profiles of the HL cell lines showed a considerable degree of consistency. The expression of T-cell TFs may explain the production of various cytokines by HL cell lines and HRS cells.
Although the origin of the neoplastic cells of HL was a contentious issue for a long time, results of several studies in recent years have confirmed that the neoplastic cells of classical HL (CHL) as well as of nodular lymphocyte predominance type of HL (NLPHL) are derived from germinal center B cells in the vast majority of the cases.1–4 Immunohistochemical analysis of the neoplastic cells of CHL has revealed an immunophenotype that is very specific, but highly unusual for a B-cell-derived tumor. The so-called Hodgkin and Reed-Sternberg (HRS) cells typically express CD30 and CD15, have inconsistent expression of CD20 and CD79a, and typically lack J-chain and immunoglobulins.5,6 The neoplastic cells of NLPHL, the so-called lymphocytic and histiocytic (L&H)-type HRS cells, do express classical B-cell markers like CD20, CD79a, and J-chain7 but lack CD30 and CD15.8
More recently, the expression of lineage-specific nuclear proteins, including TFs associated with lymphoid cell maturation and activation was studied. PAX5/BSAP, a TF essential for B-cell commitment in early B cells and maintenance of B-cell identity in mature B cells, was reported to be expressed in the majority of NLPHL cases9–11 while in CHL cases the reported percentages of positive cases vary from 17%10 to all cases.9,11,12 Other B-cell TFs like BOB-1/OBF.1 and OCT-2 that are important for proper B-cell function were reported to be absent9,13 or expressed in less than half of CHL cases.10,14,15 In most of these studies, the majority of NLPHL cases showed consistent expression of these TFs. Expression of another octamer binding TF Oct-1 was more consistent and present in almost all CHL and NLPHL cases.14 Thus, although the various reports are in agreement regarding the down-regulation of B-cell-associated TFs, the intensity and number of cases showing reactivity is quite variable, in contrast to findings in other B lymphomas.14 Only a few cases of CHL of T-cell origin have been reported16,17 and only limited information is available on the expression of T-cell TFs in HRS cells.18–20 The transcriptional activator Notch1 is strongly expressed in the majority of HRS cells.21
HRS cells are known to produce cytokines, creating their own reactive cell microenvironment, which may contribute to stimulation of their growth and survival.19,22 Generally, the production of cytokines is driven by activation of T-cell TFs.23–25 In this study, we examined the expression of T-cell TFs, GATA-3, c-Maf, T-bet, and ERM in HL using immunohistochemistry and quantitative PCR (qPCR). In addition, we studied the cytokine gene expression patterns of HL cell lines to determine the possible association with the target cytokine spectrum of the T-cell TFs.
Materials and Methods
Cell Lines
Five of the HL cell lines used were of B-cell origin and one (HDLM-2) of T-cell origin. L428, L591, and HDLM-2 originated from patients with HL of the nodular sclerosis (NS) type, L1236 and KM-H2 from mixed cellularity (MC) type, and DEV from NLPHL type. L428, L1236, and L591 cell lines were provided kindly by Dr. Volker Diehl (University of Cologne, Cologne, Germany); HDLM-2 and KM-H2 cell lines were kindly provided by Dr. Ralf Kuppers (University of Essen Medical School, Essen, Germany). The t(14;18)-positive DLBL cell line SU-DHL-6 cell line was kindly provided by Dr. Alan Epstein (University of Southern California, Los Angeles, CA, USA). The other two DLBL cell lines, VER, t(8;14) positive and Rose, t(14;18) positive, were established in our laboratory (unpublished data).
All cell lines were cultured in RPMI-1640 with ultraglutamine-1 (BioWhittaker Europe, Verviers, Belgium), supplemented with FCS (fetal calf serum) (5% for L428, 10% for L591, HDLM-2, KM-H2, L1236, SU-DHL-6, Rose and VER, 20% for DEV) and 50 U/ml penicillin/streptomycin at 37°C in an atmosphere containing 5% CO2.
Tissue Samples
Formalin-fixed, paraffin-embedded and frozen samples of a series of previously diagnosed HL tissues, comprising 7 NLPHL and 16 CHL cases were obtained from the files of the Department of Pathology and Laboratory Medicine, Groningen University Medical Center, The Netherlands. The CHL group was comprised of 10 NS, 3 MC, 1 lymphocyte depleted (LD), and 2 unclassifiable HL cases (see Table 3). Reactive tonsil and normal adult thymus were used as control tissues.
Table 3.
Immunohistochemical Expression of GATA-3 and T-bet in the Neoplastic Cells of HLs
| Case no.
|
GATA-3
|
T-bet
|
||
|---|---|---|---|---|
| NLPHL | % positive cells | Intensity | % positive cells | Intensity |
| 1 | 0 | − | 25–50 | +++ |
| 2 | 0 | − | 10–25 | ++ |
| 3 | 0 | − | 25–50 | ++ |
| 4 | 0 | − | 0 | − |
| 5 | 0 | − | 0 | − |
| 6 | 0 | − | 0 | − |
| 7 | 0 | − | nd | na |
| Total no. of positive cases | 0/7 | 3/6 | ||
| CHL* | % positive cells | Intensity | % positive cells | Intensity |
| 8 | 25–50 | ++ | >75 | +++ |
| 9 | 50–75 | ++ | >75 | +++ |
| 10 | 0 | − | >75 | +++ |
| 11 | 0 | − | >75 | +++ |
| 12 | Nd | na | >75 | +++ |
| 13 | >75 | +++ | >75 | +++ |
| 14 | 50–75 | +++ | 50–75 | +++ |
| 15 | 0 | − | <10 | ++ |
| 16 | >75 | +++ | 0 | − |
| 17 | 50–75 | +++ | 0 | − |
| 18 | 0 | − | >75 | +++ |
| 19 | Nd | na | >75 | +++ |
| 20 | Nd | na | >75 | +++ |
| 21 | >75 | +++ | 0 | − |
| 22 | 25–50 | ++ | 0 | − |
| 23 | ND | na | 0 | − |
| Total no. of positive cases | 8/12 | 11/16 | ||
na, not applicable; nd, not done (material not available); −, no signal; +, weak; ++, moderate; +++, strong.
, Among CHL cases 8–17 are NS type, 18–20 are MC type, 21 are LD type, 22 and 23 are unclassifiable HL cases.
Immunohistochemistry
Four-μm thick paraffin-embedded tissue sections were cut from routine blocks; coated on aminopropyltriethoxysilane (APES)-coated slides (Lo-Laboroptik GMBH, Friedrichsdaf, Germany), dried, deparaffinized in xylene, and rehydrated in graded alcohols. Antigen retrieval was carried out by treating the sections in 1 mmol/L Tris-EDTA (Tris hydroxymethyl, Merck, Darmstadt, Germany) aminomethane-ethylenediaminetetraacetic acid) buffer (pH 8.0) for c-Maf and in 10 mmol/L citrate buffer (Merck) (pH 6.0) for T-bet in a microwave oven (Amana RS591SS, Salm & Kipp, Breukolen, The Netherlands) (three times for 5 minutes at 700W). After antigen retrieval, the slides were incubated for 1 hour at room temperature with the primary antibodies (Table 1), followed by a 30-minute incubation with the secondary antibody, and for an additional 30 minutes with the tertiary antibody. Antibody binding was detected by DAB (3,3′- diaminobenzidine (Sigma, St. Louis, MO)) staining. For GATA-3 staining, 4-μm thick frozen sections from tonsils and lymph nodes were air-dried, fixed in acetone, and incubated for 1 hour with primary antibody, followed by application of horseradish peroxidase-labeled second and tertiary antibody incubation steps for 30 minutes each. AEC (3-amino-9-ethylcarbazole (Sigma)) was used to visualize the positive cells. Cytospins prepared from cell cultures were air-dried and stained by the same procedure as the frozen sections. Positive-control tissues as well as negative controls, sections stained in parallel without primary antibody, were used in all instances. The percentage of positive-neoplastic cells expressing the TFs was assigned to one of the following categories: 0, <10%, 10 to 25%, 25% to 50%, 50% to 75%, and >75%. Only definite staining on unambiguous malignant cells was accepted as positive. The intensity (+ weak, ++ moderate, or +++ strong) of the staining was also recorded. We also tried to confirm protein expression of ERM on frozen and paraffin-embedded tissues, but none of the antigen retrieval methods yielded good results in paraffin-embedded tissues and no staining was obtained in frozen sections with the available antibody.
Table 1.
Antibodies Used in the Present Study
| Transcription factor | Antibody | Source | Dilution |
|---|---|---|---|
| T-bet | Anti-T-bet (monoclonal) | Prof. L.H. Glimcher, Harvard Medical School, USA | 1/50 |
| PolyFast Rabbit anti T-bet | Zymed Laboratories | 1/50 | |
| (polyclonal) | |||
| GATA-3 | HG3–31(monoclonal) | Santa Cruz Biotechnology | 1/50 |
| ERM | sc-1955 (polyclonal) | Santa Cruz Biotechnology | 1/50 |
| c-Maf | sc-7866 (polyclonal) | Santa Cruz Biotechnology | 1/50 |
Quantitative PCR
qPCR analysis based on the TaqMan methodology was performed using an ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA). Primers and probes for five of the cytokines and the housekeeping gene have been designed using Primer Express Software (Applied Biosystems). As a precaution, to prevent amplification of genomic DNA, primer sequences were chosen to span exon junctions or to lie in distant exons separated by long introns. The Taqman probes were labeled at the 5′-end with a reporter fluorochrome (FAM = 6-carboxy-fluorescein) and at the 3′-end with a quencher fluorochrome (TAMRA = 6-carboxy-tetramethylrhodamine). The sequences of the primers and the probes for these cytokine mRNAs and housekeeping gene mRNAs are shown in Table 2. T-cell-TFs, IL-13, and TGFβ-1 gene-specific Taqman probe and primer sets were obtained from Applied Biosystems as Assays-on-Demand (AOD) gene expression products. The AOD ID’s were ERM (NM-004454), Hs00231790-m1; GATA-3 (NM-002051), Hs00231122-m1; T-bet (NM-013351), Hs00203436-m1; c-Maf (NM-005360), Hs00193519-m1; IL-13 (NM-002188), Hs00174379-m1; TGFβ-1 (NM-000660), Hs00171257-m1.
Table 2.
Primer and Probe Sequences of the Cytokines and the Housekeeping Gene Designed Using Primer Express Software
| Gene | Primer and probe |
|---|---|
| IL-2 | S: 5′- AAG AAT CCC AAA CTC ACC AGG AT -3′ |
| A: 5′- TGC TGA TTA AGT CCC TGG GTC TTA -3′ | |
| P: 5′- (FAM)- CAT GCC CAA GAA GGC CAC AGA ACT G -(TAMRA) 3′ | |
| IL-4 | S: 5′- ACA GGC ACA AGC AGC TGA TC -3′ |
| A: 5′- CCT TCA CAG GAC AGG AAT TCA A -3′ | |
| P: 5′- (FAM)- TGA AAC GGC TCG ACA GGA ACC TCT G -(TAMRA) -3′ | |
| IL-5 | S: 5′-CTT GGA GCT GCC TAC GTG TAT G -3′ |
| A: 5′- ATG TAC AGG AAC AGG AAT CCT CAG A -3′ | |
| P: 5′- (FAM)- CCC CAC AGA AAT TCC CAC AAG TGC A -(TAMRA) -3′ | |
| IL-10 | S: 5′- ATG AAG GAT CAG CTG GAC AAC TT -3′ |
| A: 5′- CCT TGA TGT CTG GGT CTT GGT -3′ | |
| P: 5′- (FAM)- ACC TGG GTT GCC AAG CCT TGT CTG -(TAMRA) -3′ | |
| IFN-γ | S: 5′- GAA ACG AGA TGA CTT CGA AAA GC -3′ |
| A: 5′- CGA CCT CGA AAC AGC ATC TG -3′ | |
| P: 5′- (FAM)- CCA AGT GAT GGC TGA ACT GTC GCC-(TAMRA) -3′ | |
| HPRT | S: 5′- GGC AGT ATA ATC CAA AGA TGG TCA A -3′ |
| A: 5′- GTC TGG CTT ATA TCC AAC ACT TCG T -3′ | |
| P: 5′-(FAM)-CAA GCT TGC TGG TGA AAA GGA CCC C -(TAMRA) -3′ |
S, sense; A, antisense; P, probe.
Total RNA was extracted using Absolutely RNA Miniprep Kit (Stratagene, La Jolla, CA). Complementary DNA (cDNA) was synthesized from 10 μg of total cellular RNA by First Strand cDNA Synthesis System using Superscript II RT (Invitrogen, Carlsbad, CA) using random hexamers in a volume of 20 μl and further diluted to 2 ng/μL concentration. The qPCR reaction mixture contained 5 μl cDNA, 10 μl 2X TaqMan Universal PCR Master Mix (Eurogentec, Seraing, Belgium), 900 nmol/L of each primer, and 200 nmol/L probe in a total reaction volume of 20 μl. All assays were performed in triplicate. cDNA samples from the peripheral blood of healthy donors obtained by centrifugation on a Ficoll-Paque gradient were used as a positive control. Reaction tubes without template cDNA served as negative controls. The PCR plate was incubated for 2 minutes at 50°C to optimize the uracil-N-glycosylase enzyme activity and at 95°C for 10 minutes to activate the Taq polymerase. This was followed by 45 cycles of denaturation at 95°C for 15 seconds, annealing and extension at 60°C for 1 minute. The CT (threshold cycle) parameter was defined as the fractional cycle number at which the fluorescence generated by cleavage of the probe passed a preset threshold. The samples with CT values >37 were considered as not expressing the given cytokine mRNA. The CT is inversely proportional to the logarithmic scale of the starting quantity of template cDNA. Consequently, the gene dosage was deduced by calculating the difference in CT from the CT of the reference gene HPRT. The average CT values for target genes were subtracted from the average housekeeping gene CT values to yield the ΔCT. Results were finally expressed as 2−ΔCT which is an index of the relative amount of cytokine or T-cell TF expressed in each cell line.
Results
Analysis of HL and LBCL Cell Lines for T-Cell TFs and Various Cytokines
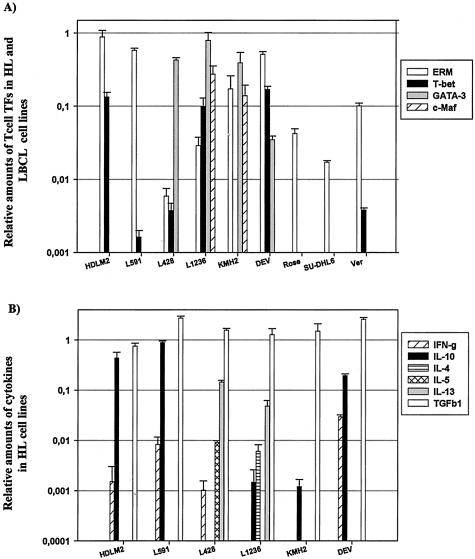
All HL cell lines expressed mRNA for at least two T-cell TFs at the mRNA level (Figure 1A). The T-cell-derived HL cell line HDLM-2 expressed only the Th1-associated TFs T-bet and ERM. ERM expression was observed in all HL cell lines, albeit at different levels. T-bet was expressed in five, GATA-3 in four, and c-Maf in two of the HL cell lines. The three LBCL cell lines expressed ERM. Only VER had additionally expression of T-bet at a very low level. GATA-3 protein was detected in L1236, L428, and KM-H2 but not in DEV. L428 and L1236 cells had a strong homogenous nuclear staining for GATA-3 with clearly negative nucleoli whereas KM-H2 showed weaker GATA-3 protein expression (Figure 2A). c-Maf and T-bet antibodies worked only in paraffin sections and we did not get reliable results with ERM antibody in cytospins and tissue sections. None of the HL cell lines had IL-2 mRNA expression, whereas all showed significant TGFβ-1 expression. L591, DEV, and HDLM-2 had a comparable expression profile, including IFN-γ, IL-10, and TGFβ-1. Although the expression levels were different for each cell line, relative expression levels were concordant; a low level of IFN-γ, and 10 to 100 times more IL-10 and TGFβ-1 than IFN-γ. IL-13 was expressed by two of the six cell lines, whereas IL-4 was only detected in L1236 and IL-5 only in L428 (Figure 1B).
Figure 1.
Results of qPCR showing relative quantities of T-cell TFs in HL and LBCL cell lines and cytokine expression in HL cell lines. The vertical axis indicates relative amount of T-cell TFs (A) or cytokine (B) expressed in each cell line. Results are expressed as 2−ΔCT values normalized to HPRT. All results represent the mean of a triplicate experiment.
Figure 2.
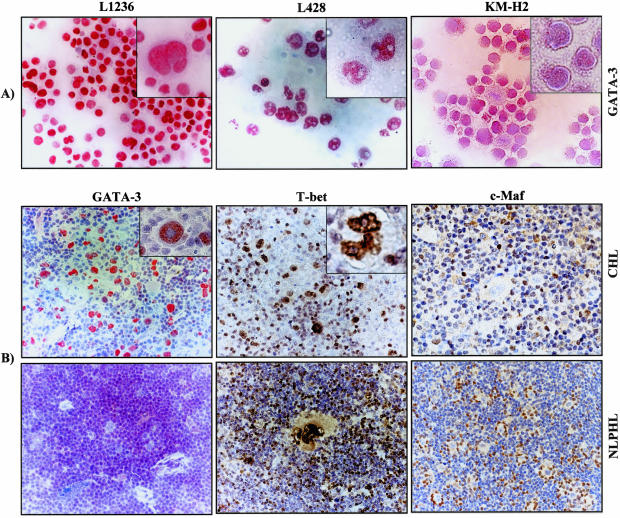
Immunostaining of HL cell lines and tissues involved by HL for T-cell TFs. A: GATA-3 staining in HL cell lines. B: GATA-3, T-bet, c-Maf immunostaining of CHL and NLPHL cases. For GATA-3, frozen sections were used and for c-Maf and T-bet staining, paraffin sections were used. Original magnification ×100 for A–B, original magnification ×157.5 for insets.
T-Cell TF Protein Expression in CHL Cases
GATA-3 was detected in HRS cells in 8 of 12 CHL (67%) cases (Table 3). In all instances the positive staining was confined to the nuclei and the nucleoli stood out as negative. Although some variation was observed, the staining intensity was stronger in neoplastic cells than in nonmalignant infiltrating cells (Figure 2B). In three cases more than 75% of the HRS cells and in another three cases between 50 to 75% of the neoplastic cells showed GATA-3 protein expression.
The most striking reactivity of anti-T-bet antibody was with HRS cells that showed stronger nuclear staining than the non-neoplastic background lymphocytes (Figure 2B). Nine of 16 (56%) cases expressed T-bet protein in more than 75% of the neoplastic cells. Considering the cases in which GATA-3 and T-bet staining were both performed, 92% of the cases (11 of 12 cases) showed GATA-3 and/or T-bet staining in 25 to 90% of the neoplastic cells (Table 3).
Staining for c-Maf was not observed in HRS cells, despite good staining of the background lymphocytes. Immunohistochemical stains for ERM did not work.
T-Cell TF Protein Expression in NLPHL Cases
In two NLPHL cases, T-bet expression was present in approximately to 25 to 50% of the L&H cells (Figure 2B), whereas in another NLPHL case 10% to 25% of L&H cells showed weak to moderate T-bet expression and in three cases the L&H cells were completely negative (Table 3). The lymph node with the strongest expression came from a 26-year-old male with inguinal lymphadenopathy; the L&H cells in this case were relatively strongly CD30 positive, but otherwise the histology and immunohistology was consistent with the diagnosis of NLPHL. No GATA-3 or c-Maf expression was observed in L&H cells. Notably, strong c-Maf staining was detected in the lymphocytes forming rosettes around L&H cells (Figure 2B).
Discussion
Defective lymphocyte transcriptional machinery is one of the mechanisms proposed for the lack of Ig expression in the neoplastic cells of CHL.15,26 A global B-cell transcriptional deregulation was demonstrated by immunohistochemistry on primary HRS cells.13,15,27 Gene expression profiling of HL cell lines in comparison to normal B-cell subsets demonstrated decreased mRNA levels for nearly all B-lineage-specific genes.28 On the other hand, HRS cells produce many cytokines and chemokines that are normally produced by T cells and dendritic cells.19,29
In this study, mRNA and protein expression of four T-cell TFs that are considered to be important in Th lineage commitment were analyzed. In contrast to the previously reported deficiency of B-cell-specific TF expression in HL, we found frequent expression of T-cell TFs in HRS cells and HL cell lines, which may indicate a transcriptional switch in HL.
All HL and LBCL cell lines had ERM mRNA expression. Unfortunately, the anti-ERM antibody was not effective in immunohistochemical stains. ERM or Ets variant gene-5 is induced by IL-12 in a STAT-4-dependent manner and has been reported to be Th1-specific although the target genes for ERM have not been identified yet.30 ERM is also involved in tumorigenesis and developmental processes and interact with promoters of several genes via the ETS domain.31 Finding of significant high ERM expression in all HL and LBCL cell lines is not surprising. Overexpression of ERM was also reported in B-CLL and in mantle cell lymphoma.32
Three of the HL cell lines showed relatively strong and two showed relatively weak T-bet mRNA expression, whereas in 69% of the CHL cases and 50% of the NLPHL cases, the tumor cells showed T-bet protein expression. GATA-3 mRNA was expressed in HL cell lines L428, L1236, and KM-H2, and also with low levels in DEV, whereas GATA-3 protein expression could only be demonstrated in L428, L1236, and KM-H2. Although HL cell lines and primary HL cases were previously reported to lack expression of GATA-3,20 GATA-3 protein expression was present in 67% of the CHL cases and in four HL cell lines. Kuppers et al18 reported that GATA-3 was upregulated in the L1236 cell line and detected GATA-3 protein expression by Western blot analysis in L1236, L428, and KM-H2 but not in HDLM-2. Our results are in concordance with this report. In cases where frozen and paraffin tissue could be analyzed, GATA-3 and/or T-bet expression was observed in 11 of 12 (92%) of the CHL cases and in 25 to 90% of HRS cells.
c-Maf mRNA was expressed in L1236 and KM-H2, but no c-Maf protein was detected in HRS or L&H cells in tissue sections. Overexpression of c-Maf is reported to occur at an incidence of about 25% in multiple myeloma characterized by t(14;16)(q32;q23) translocation involving the c-Maf locus at chromosome 16.33 Recently Hurt et al34 observed c-Maf expression also in myeloma cell lines lacking c-Maf translocations. Cytogenetic analyses of L1236 and KM-H2 revealed several composite translocated chromosomes including −der 1635,36 but it is not clear whether or not the c-Maf locus is involved.
During murine Th1/Th2 commitment, the TFs T-bet and ERM are selectively expressed in Th1 cells, and GATA-3 and c-Maf in Th2 cells.37 In human lymphocytes these TFs are expressed in a broadly analogous manner, but with some important differences.38 T-bet, also known as T-box 2139 is an important TF for maintenance of the Th1 phenotype.24 Following in vitro differentiation of murine naive CD4+ T cells, T-bet is selectively expressed in Th1 cells but not in Th2 cells. T-bet is critical for IFN-γ expression and suppresses IL-2 in T-bet overexpression studies in murine T cells.24 In human T cells, concomitant expression of IFN-γ and IL-2 was found during in vitro differentiation of human Th1 cells and high levels of human T-bet mRNA were detected in naive T cells and Th1 cells.38 In human lymphocytes, ERM mRNA is preferentially expressed in activated Th1 cells, but is also weakly present in resting and activated T cells and Th2 cells.38 In committed and developing murine Th1 cells, overexpression of GATA-3 caused induction of the Th2 cytokines.40 In human, GATA-3 mRNA levels increased only under Th2-inducing conditions.41 GATA-3 has been identified as being critical in the regulation of the IL-4/IL-5/IL-13 locus in mice.25,42 However, IL-5 is the only cytokine which appears to be regulated by GATA-3 in human.43 In mice, c-Maf has a critical and selective function in IL-4 gene transcription.44 c-Maf mRNA has predominantly been observed in human and murine Th2 cells.37,38
Expression of IL-10 and TGF-β was detected in five HL cell lines. IL-10 is a pleiotropic cytokine with a crucial role in immunoregulation.45 The ubiquitous presence of IL-10 in HL cell lines may result from the fact that its expression can be regulated by the constitutively and ubiquitously expressed Sp1 and Sp3 TFs.46 Overall, the cytokine expression profile of most of the HL cell lines is highly reminiscent of a CD4+ T-cell subset, designated as the T regulatory subset, characterized by the ability to produce high levels of IL-10 and TGFβ.47,48
Cytokine expression in the HL cell lines was found to be heterogeneous. Two cell lines, L1236 and L428, have a strong expression of IL-13 combined with lower levels of IL-4 and IL-5, respectively. Cousins et al38 examined the interrelations among expression of Th1 and Th2 cytokines in individual human T cells using intracellular cytokine staining at various times during the differentiation process. Concomitant expression of IL-10 and other cytokines was rare, and IL-10 and IL-5 were found to be mutually exclusive. IL-4-, IL-5-, and IL-13-positive cells were only present at high frequency once IL-10 cells had completely disappeared.
Our cytokine data for L428 and L1236 including IL-13, is in agreement with the previous data.49–51 However, Kapp et al50 also found strong IL-13 expression in KM-H2 and weak expression in HDLM-2. The discrepancy with our data may be caused by the use of different techniques. In their study, microarray and Northern blot analysis were used, whereas we have measured IL-13 gene expression with the qPCR Taqman gene expression assay. Although it is not clear which probe was used by Kapp et al50 for the aforementioned techniques, the difference in results could originate from the potential presence of the short splice variant of IL-13 that we cannot detect with qPCR.
Overall, the T-cell TF and cytokine profiles of the cell lines show a considerable degree of correlation (Figure 1; Table 4). GATA-3 was expressed in four of six cell lines and in L428 and L1236 the downstream targets of GATA-3, being IL-4, IL-5 and IL-13 were indeed present. T-bet was observed in five cell lines, and IFN-γ was expressed in four of these. c-Maf was detected in two cell lines and IL-4 was indeed expressed in one of these. All HL cell lines showed prominent mRNA expression of the Th1-specific TF ERM, however its function is not yet known.30
Table 4.
T-Cell Transcription Factors and Their Downstream Cytokine Targets
| T-cell TF | Downstream cytokine target | Reference no. |
|---|---|---|
| ERM | Not known | 30 |
| T-bet | ↑IFN- γ (m and h) | 24, 38 |
| ↓ IL-2 (m) | 24 | |
| ↑ IL-2 (h) | 38 | |
| GATA-3 | ↑ IL-4 (m) | 25, 40, 42 |
| ↑IL-5 (m,h) | 40, 42, 43 | |
| ↑ IL-13 (m) | 25, 42 | |
| c-Maf | ↑ IL-4 (m) | 44 |
m, mice; h, human; ↑, induces; ↓, suppresses.
Is there an explanation for the T-cell TF expression in HRS cells, considering that these represent a clonal progeny of post-germinal center B cells?1–4 Recently, two populations of mouse effector B cells were identified (Be1 and Be2) that produce Th1- and Th2-like cytokine patterns, respectively IFN-γ and IL-4, depending on the cytokine environment in which the cells are activated during their primary antigen encounter.52 Polarized B-cell subsets may subsequently regulate T-cell differentiation and function. The role of T-bet in B-lymphocyte development is not well understood. T-bet is shown to promote IgG2a class switching in response to IFNγ signaling in murine B cells.53 Splenic B cells do produce IFN-γ and express T-bet on treatment with anti-CD40 mAb, rIL-12, and rIL-18 for 72 hours.24 Recently Durali et al54 showed constitutive T-bet mRNA expression as well as IL-12-induced IFN-γ expression leading to strong expression of T-bet in B cells. Ongoing external stimulation of the neoplastic cells via surface receptors or the cytokine milieu can thus be an explanation of T cell TFs accumulation in HRS cells.
It has been speculated that, in CHL, the transforming events may interfere with the expression and/or the function of TFs that are involved in cell fate or commitment decisions in the B-cell lineage.55,56 Constitutive activation of the Notch1 receptor and a high expression of Notch1 have been demonstrated in HRS cells and HL cell lines.21 In normal lymphopoiesis, activation of the Notch pathway results in a block of early B-cell lymphopoiesis.57 The balance between Notch1 and Pax5 may dictate developmental decisions in lymphoid ontogeny; forced Pax5 expression in hematopoietic progenitors results in a skewing toward B-cell development at the expense of T-cell development by specifically repressing Notch1.58 Outcome of Notch1 activation in HRS cells may thus be a complete block of the B-cell developmental pathway with absence of Ig and B-cell-specific antigens and aberrant expression of other hematopoietic lineage markers.58,59 However, there are no data in the literature about the effect of Notch-1 activation on the expression levels of T-cell TFs and the related cytokines in committed B cells. The presence of nuclear expression of T-cell TFs in HRS cells might explain the production of cytokines and chemokines in HL tissues and HL cell lines.
Footnotes
Address reprint requests to Dr. Sibrand Poppema, Department of Pathology and Laboratory Medicine, Groningen University Medical Center, Hanzeplein 1, P.O. Box 30.001 9700 RB Groningen, The Netherlands. E-mail: s.poppema@med.rug.nl.
Supported by Groningen University Institute for Drug Exploration.
References
- Muschen M, Kuppers R, Spieker T, Brauninger A, Rajewsky K, Hans-mann ML. Molecular single-cell analysis of Hodgkin- and Reed-Sternberg cells harboring unmutated immunoglobulin variable region genes. Lab Invest. 2001;81:289–295. doi: 10.1038/labinvest.3780237. [DOI] [PubMed] [Google Scholar]
- Kuppers R, Klein U, Hansmann ML, Rajewsky K. Cellular origin of human B-cell lymphomas. N Engl J Med. 1999;341:1520–1529. doi: 10.1056/NEJM199911113412007. [DOI] [PubMed] [Google Scholar]
- Kanzler H, Kuppers R, Hansmann ML, Rajewsky K. Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J Exp Med. 1996;184:1495–1505. doi: 10.1084/jem.184.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marafioti T, Hummel M, Foss HD, Laumen H, Korbjuhn P, Anagnostopoulos I, Lammert H, Demel G, Theil J, Wirth T, Stein H. Hodgkin and Reed-Sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood. 2000;95:1443–1450. [PubMed] [Google Scholar]
- Zukerberg LR, Collins AB, Ferry JA, Harris NL. Coexpression of CD15 and CD20 by Reed-Sternberg cells in Hodgkin’s disease. Am J Pathol. 1991;139:475–483. [PMC free article] [PubMed] [Google Scholar]
- Schmid C, Pan L, Diss T, Isaacson PG. Expression of B-cell antigens by Hodgkin’s and Reed-Sternberg cells. Am J Pathol. 1991;139:701–707. [PMC free article] [PubMed] [Google Scholar]
- Poppema S. The diversity of the immunohistological staining pattern of Sternberg-Reed cells. J Histochem Cytochem. 1980;28:788–791. doi: 10.1177/28.8.6777426. [DOI] [PubMed] [Google Scholar]
- von Wasielewski R, Werner M, Fischer R, Hansmann ML, Hubner K, Hasenclever D, Franklin J, Sextro M, Diehl V, Georgii A. Lymphocyte-predominant Hodgkin’s disease: an immunohistochemical analysis of 208 reviewed Hodgkin’s disease cases from the German Hodgkin Study Group. Am J Pathol. 1997;150:793–803. [PMC free article] [PubMed] [Google Scholar]
- Torlakovic E, Tierens A, Dang HD, Delabie J. The transcription factor PU. 1, necessary for B-cell development is expressed in lymphocyte predominance, but not classical Hodgkin’s disease. Am J Pathol. 2001;159:1807–1814. doi: 10.1016/S0002-9440(10)63027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimle-Grauer SA, Tinguely M, Seada L, Fellbaum C, Hansmann ML. Expression patterns of transcription factors in progressively transformed germinal centers and Hodgkin lymphoma. Virchows Arch. 2003;442:284–293. doi: 10.1007/s00428-002-0735-5. [DOI] [PubMed] [Google Scholar]
- Foss HD, Reusch R, Demel G, Lenz G, Anagnostopoulos I, Hummel M, Stein H. Frequent expression of the B-cell-specific activator protein in Reed-Sternberg cells of classical Hodgkin’s disease provides further evidence for its B-cell origin. Blood. 1999;94:3108–3113. [PubMed] [Google Scholar]
- Krenacs L, Himmelmann AW, Quintanilla-Martinez L, Fest T, Riva A, Wellmann A, Bagdi E, Kehrl JH, Jaffe ES, Raffeld M. Transcription factor B-cell-specific activator protein (BSAP) is differentially expressed in B cells and in subsets of B-cell lymphomas. Blood. 1998;92:1308–1316. [PubMed] [Google Scholar]
- Re D, Muschen M, Ahmadi T, Wickenhauser C, Staratschek-Jox A, Holtick U, Diehl V, Wolf J. Oct-2 and Bob-1 deficiency in Hodgkin and Reed Sternberg cells. Cancer Res. 2001;61:2080–2084. [PubMed] [Google Scholar]
- Saez AI, Artiga MJ, Sanchez-Beato M, Sanchez-Verde L, Garcia JF, Camacho FI, Franco R, Piris MA. Analysis of octamer-binding transcription factors Oct2 and Oct1 and their coactivator BOB.1/OBF.1 in lymphomas. Mod Pathol. 2002;15:211–220. doi: 10.1038/modpathol.3880518. [DOI] [PubMed] [Google Scholar]
- Stein H, Marafioti T, Foss HD, Laumen H, Hummel M, Anagnostopoulos I, Wirth T, Demel G, Falini B. Down-regulation of BOB.1/OBF.1 and Oct2 in classical Hodgkin disease but not in lymphocyte predominant Hodgkin disease correlates with immunoglobulin transcription. Blood. 2001;97:496–501. doi: 10.1182/blood.v97.2.496. [DOI] [PubMed] [Google Scholar]
- Seitz V, Hummel M, Marafioti T, Anagnostopoulos I, Assaf C, Stein H. Detection of clonal T-cell receptor gamma-chain gene rearrangements in Reed-Sternberg cells of classic Hodgkin disease. Blood. 2000;95:3020–3024. [PubMed] [Google Scholar]
- Muschen M, Rajewsky K, Brauninger A, Baur AS, Oudejans JJ, Roers A, Hansmann ML, Kuppers R. Rare occurrence of classical Hodgkin’s disease as a T cell lymphoma. J Exp Med. 2000;191:387–394. doi: 10.1084/jem.191.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppers R, Klein U, Schwering I, Distler V, Brauninger A, Cattoretti G, Tu Y, Stolovitzky GA, Califano A, Hansmann ML, Dalla-Favera R. Identification of Hodgkin and Reed-Sternberg cell-specific genes by gene expression profiling. J Clin Invest. 2003;111:529–537. doi: 10.1172/JCI16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss HJ, Pinto A, Duyster J, Poppema S, Herrmann F. Hodgkin’s disease: a tumor with disturbed immunological pathways. Immunol Today. 1997;18:156–163. doi: 10.1016/s0167-5699(97)84661-0. [DOI] [PubMed] [Google Scholar]
- Hsu PL, Xie SS, Hsu SM. Absence of T-cell- and B-cell-specific transcription factors TCF-1, GATA-3, and BSAP in Hodgkin’s Reed-Sternberg cells. Lab Invest. 1996;74:395–405. [PubMed] [Google Scholar]
- Jundt F, Anagnostopoulos I, Forster R, Mathas S, Stein H, Dorken B. Activated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphoma. Blood. 2002;99:3398–3403. doi: 10.1182/blood.v99.9.3398. [DOI] [PubMed] [Google Scholar]
- Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99:4283–4297. doi: 10.1182/blood-2002-01-0099. [DOI] [PubMed] [Google Scholar]
- Ho IC, Glimcher LH. Transcription: tantalizing times for T cells. Cell. 2002;109(Suppl):S109–S120. doi: 10.1016/s0092-8674(02)00705-5. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- Theil J, Laumen H, Marafioti T, Hummel M, Lenz G, Wirth T, Stein H. Defective octamer-dependent transcription is responsible for silenced immunoglobulin transcription in Reed-Sternberg cells. Blood. 2001;97:3191–3196. doi: 10.1182/blood.v97.10.3191. [DOI] [PubMed] [Google Scholar]
- Hertel CB, Zhou XG, Hamilton-Dutoit SJ, Junker S. Loss of B cell identity correlates with loss of B cell-specific transcription factors in Hodgkin/Reed-Sternberg cells of classical Hodgkin lymphoma. Oncogene. 2002;21:4908–4920. doi: 10.1038/sj.onc.1205629. [DOI] [PubMed] [Google Scholar]
- Schwering I, Brauninger A, Klein U, Jungnickel B, Tinguely M, Diehl V, Hansmann ML, Dalla-Favera R, Rajewsky K, Kuppers R. Loss of the B-lineage-specific gene expression program in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2003;101:1505–1512. doi: 10.1182/blood-2002-03-0839. [DOI] [PubMed] [Google Scholar]
- Maggio E, van den Berg A, Diepstra A, Kluiver J, Visser L, Poppema S. Chemokines, cytokines, and their receptors in Hodgkin’s lymphoma cell lines and tissues. Ann Oncol. 2002;13(Suppl 1):52–56. doi: 10.1093/annonc/13.s1.52. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Jacobson NG, Bhattacharya D, Gorham JD, Fenoglio D, Sha WC, Murphy TL, Murphy KM. The Ets transcription factor ERM is Th1-specific and induced by IL-12 through a Stat4-dependent pathway. Proc Natl Acad Sci USA. 1999;96:3888–3893. doi: 10.1073/pnas.96.7.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte D, Baert JL, Defossez PA, de Launoit Y, Stehelin D. Molecular cloning and characterization of human ERM, a new member of the Ets family closely related to mouse PEA3 and ER81 transcription factors. Oncogene. 1994;9:1397–1406. [PubMed] [Google Scholar]
- Korz C, Pscherer A, Benner A, Mertens D, Schaffner C, Leupolt E, Dohner H, Stilgenbauer S, Lichter P. Evidence for distinct pathomechanisms in B-cell chronic lymphocytic leukemia and mantle cell lymphoma by quantitative expression analysis of cell cycle and apoptosis-associated genes. Blood. 2002;99:4554–4561. doi: 10.1182/blood.v99.12.4554. [DOI] [PubMed] [Google Scholar]
- Chesi M, Bergsagel PL, Shonukan OO, Martelli ML, Brents LA, Chen T, Schrock E, Ried T, Kuehl WM. Frequent dysregulation of the c-maf proto-oncogene at 16q23 by translocation to an Ig locus in multiple myeloma. Blood. 1998;91:4457–4463. [PubMed] [Google Scholar]
- Hurt EM, Wiestner A, Rosenwald A, Shaffer AL, Campo E, Grogan T, Bergsagel PL, Kuehl WM, Staudt LM. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell. 2004;5:191–199. doi: 10.1016/s1535-6108(04)00019-4. [DOI] [PubMed] [Google Scholar]
- Wolf J, Kapp U, Bohlen H, Kornacker M, Schoch C, Stahl B, Mucke S, von Kalle C, Fonatsch C, Schaefer HE, Hansmann ML, Diehl V. Peripheral blood mononuclear cells of a patient with advanced Hodgkin’s lymphoma give rise to permanently growing Hodgkin-Reed Sternberg cells. Blood. 1996;87:3418–3428. [PubMed] [Google Scholar]
- Joos S, Granzow M, Holtgreve-Grez H, Siebert R, Harder L, Martin-Subero JI, Wolf J, Adamowicz M, Barth TF, Lichter P, Jauch A. Hodgkin’s lymphoma cell lines are characterized by frequent aberrations on chromosomes 2p and 9p including REL and JAK2. Int J Cancer. 2003;103:489–495. doi: 10.1002/ijc.10845. [DOI] [PubMed] [Google Scholar]
- Glimcher LH, Murphy KM. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 2000;14:1693–1711. [PubMed] [Google Scholar]
- Cousins DJ, Lee TH, Staynov DZ. Cytokine coexpression during human Th1/Th2 cell differentiation: direct evidence for coordinated expression of Th2 cytokines. J Immunol. 2002;169:2498–2506. doi: 10.4049/jimmunol.169.5.2498. [DOI] [PubMed] [Google Scholar]
- Zhang WX, Yang SY. Cloning and characterization of a new member of the T-box gene family. Genomics. 2000;70:41–48. doi: 10.1006/geno.2000.6361. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Takemoto N, Kurata H, Kamogawa Y, Miyatake S, O’Garra A, Arai N. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J Exp Med. 2000;192:105–115. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapenko A, Leipe J, Niesner U, Devriendt K, Beetz R, Radbruch A, Kalden JR, Lipsky PE, Schulze-Koops H. GATA-3 in human T cell helper type 2 development. J Exp Med. 2004;199:423–428. doi: 10.1084/jem.20031323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DH, Yang L, Cohn L, Parkyn L, Homer R, Ray P, Ray A. Inhibition of allergic inflammation in a murine model of asthma by expression of a dominant-negative mutant of GATA-3. Immunity. 1999;11:473–482. doi: 10.1016/s1074-7613(00)80122-3. [DOI] [PubMed] [Google Scholar]
- Schwenger GT, Fournier R, Kok CC, Mordvinov VA, Yeoman D, Sanderson CJ. GATA-3 has dual regulatory functions in human interleukin-5 transcription. J Biol Chem. 2001;276:48502–48509. doi: 10.1074/jbc.M107836200. [DOI] [PubMed] [Google Scholar]
- Kim JI, Ho IC, Grusby MJ, Glimcher LH. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity. 1999;10:745–751. doi: 10.1016/s1074-7613(00)80073-4. [DOI] [PubMed] [Google Scholar]
- Mocellin S, Marincola F, Riccardo Rossi C, Nitti D, Lise M. The multifaceted relationship between IL-10 and adaptive immunity: putting together the pieces of a puzzle. Cytokine Growth Factor Rev. 2004;15:61–76. doi: 10.1016/j.cytogfr.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Tone M, Powell MJ, Tone Y, Thompson SAJ, Waldmann H. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J Immunol. 2000;165:286–291. doi: 10.4049/jimmunol.165.1.286. [DOI] [PubMed] [Google Scholar]
- Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Mason D, Powrie F. Control of immune pathology by regulatory T cells. Curr Opin Immunol. 1998;10:649–655. doi: 10.1016/s0952-7915(98)80084-8. [DOI] [PubMed] [Google Scholar]
- Malec M, Soderqvist M, Sirsjo A, MacNamara B, Lewin N, Sjoberg J, Bjorkholm M, Porwit-MacDonald A. Real-time polymerase chain reaction determination of cytokine mRNA expression profiles in Hodgkin’s lymphoma. Haematologica. 2004;89:679–685. [PubMed] [Google Scholar]
- Kapp U, Yeh WC, Patterson B, Elia AJ, Kagi D, Ho A, Hessel A, Tipsword M, Williams A, Mirtsos C, Itie A, Moyle M, Mak TW. Interleukin 13 is secreted by and stimulates the growth of Hodgkin and Reed-Sternberg cells. J Exp Med. 1999;189:1939–1946. doi: 10.1084/jem.189.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinnider BF, Elia AJ, Gascoyne RD, Patterson B, Trumper L, Kapp U, Mak TW. Signal transducer and activator of transcription 6 is frequently activated in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2002;99:618–626. doi: 10.1182/blood.v99.2.618. [DOI] [PubMed] [Google Scholar]
- Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci USA. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durali D, de Goer de Herve MG, Giron-Michel J, Azzarone B, Delfraissy JF, Taoufik Y. In human B cells, IL-12 triggers a cascade of molecular events similar to Th1 commitment. Blood. 2003;102:4084–4089. doi: 10.1182/blood-2003-02-0518. [DOI] [PubMed] [Google Scholar]
- Rolink AG, Schaniel C, Andersson J, Melchers F. Selection events operating at various stages in B cell development. Curr Opin Immunol. 2001;13:202–207. doi: 10.1016/s0952-7915(00)00205-3. [DOI] [PubMed] [Google Scholar]
- Schebesta M, Heavey B, Busslinger M. Transcriptional control of B-cell development. Curr Opin Immunol. 2002;14:216–223. doi: 10.1016/s0952-7915(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC, Pear WS. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- Souabni A, Cobaleda C, Schebesta M, Busslinger M. Pax5 promotes B lymphopoiesis and blocks T cell development by repressing Notch1. Immunity. 2002;17:781–793. doi: 10.1016/s1074-7613(02)00472-7. [DOI] [PubMed] [Google Scholar]
- Mikkola I, Heavey B, Horcher M, Busslinger M. Reversion of B cell commitment upon loss of Pax5 expression. Science. 2002;297:110–113. doi: 10.1126/science.1067518. [DOI] [PubMed] [Google Scholar]