Abstract
Extracellular matrix metalloproteinase inducer (EMMPRIN) was originally identified on the tumor cell surface as an inducer of matrix metalloproteinase (MMP) production in neighboring fibroblasts. Here we demonstrate a role for EMMPRIN in MMP induction during corneal wound healing. MMP and EMMPRIN expression was analyzed in normal and ulcerated human corneas, as well as in corneal epithelial and stromal cells in culture using confocal microscopy, zymography, immunoblots, and real-time polymerase chain reaction. In normal cornea EMMPRIN was predominantly expressed in the epithelium but was markedly induced in the anterior stroma of ulcerated corneas. This coincided with MMP-2 induction that co-localized with EMMPRIN at the epithelio-stromal boundary. The role of epithelial-stromal interaction in MMP induction was investigated in an in vitro co-culture system and demonstrated an induction and co-localization of EMMPRIN and MMP-2 in the fibroblasts at the interface with epithelial cells. Direct contact of fibroblasts with EMMPRIN-containing purified epithelial cell membranes also induced MMP-1, MMP-2, and EMMPRIN and this was inhibited by a blocking anti-EMMPRIN antibody, suggesting that EMMPRIN was primarily responsible for this induction. These findings, and the up-regulation of EMMPRIN by epidermal growth factor and transforming growth factor-β, demonstrate a role for EMMPRIN in wound healing and suggest that sustained local up-regulation of EMMPRIN and MMPs in chronic situations in which healing is delayed may lead to excessive matrix degradation and corneal melts.
The cornea, first diopter of the eye, is a transparent anterior ocular tissue that has been extensively used as a model for studying wound healing because of its remarkably organized structure. It is composed of three distinct layers, an epithelium, a stroma, and an endothelium. The epithelium and the endothelium are separated from the stroma by three different structures, the epithelial basement membrane, Bowman layer, and the Descemet’s membrane. The separation of these tissue compartments is necessary for the normal homeostasis of the cornea and the disruption of the basement membrane was shown to be associated with activation of the wound healing process that comprise fibroblast activation and stromal remodeling in which synthesis and degradation of the extracellular matrix are tightly regulated to restore tissue homeostasis.1,2
Matrix metalloproteinases (MMPs), a family of proteolytic enzymes able, collectively, to degrade all of the molecules of the extracellular matrix, are central to this process. Their induction during wound healing is thought to play a role in extracellular matrix remodeling, cell-matrix interactions, inflammatory cell recruitment, cytokine activation, and regulation of angiogenesis.3–5 This family currently includes more than 25 members that can be divided into collagenases (MMP-1, -8, and -13), gelatinases (MMP-2 and -9), stromelysins (MMP-3 and -10), matrilysins (MMP-7 and -26), and the membrane-type MMPs (MMP-14 to -17 and -24), according to their structure and substrate specificity.
Barely detected in unwounded cornea, MMPs are strongly induced during wound healing after excimer laser treatment, epithelial scrape, or alkali burn injury.3,6,7 They have a complex pattern of expression and can be induced in both the migrating epithelium and in the anterior stroma of the healing cornea. Some MMPs, such as MMP-1 or MMP-9, are induced in the basal layer of the regenerating epithelium. Although MMP-1 is detected in the epithelium and the anterior stroma only when the basement membrane is disrupted, MMP-9 induction in regenerating epithelial cells appears to be independent of the integrity of the basement membrane.8–12 Other MMPs, such as MMP-2, MMP-3, and MT1-MMP were shown to be induced in the stroma of the healing cornea and are often detected in the subepithelial fibroblasts of the wounded area, even though MT1-MMP is normally expressed in the basal epithelial cells.13–15 The precise regulatory mechanisms involved in MMP induction in wound healing have not yet been elucidated despite extensive investigations, but are thought to involve cytokines, cell-matrix, and cell-cell interactions.2,4,16
Although in physiological wound healing MMPs become rapidly undetectable after wound closure, their deregulation and prolonged accumulation in the anterior stroma may lead to ulcerations and perforations in chronic wounds.6,17,18 In such situations, the induction of MMPs in the subepithelial layer of the corneal stroma is reminiscent of their regulation in cancer whereby MMPs have often been seen localized in the stromal tissues directly in contact with the tumor.19 It was initially thought that tumor cells themselves were responsible for the production of MMPs, but it then became apparent that the surrounding stromal fibroblasts represent the major source of MMP activity, suggesting that the interaction between tumor cells and stromal fibroblasts is responsible for the elevated MMP levels detected in the tumors.20 These observations led to the identification of EMMPRIN (extracellular matrix metalloproteinase inducer), or CD147, a 58-kd transmembrane glycoprotein enriched on the surface of most tumor cells that can stimulate stromal cells to produce elevated levels of several MMPs, namely MMP-1, MMP-2, and MMP-3.4,21–23 EMMPRIN has later been identified in several diseased human tissues such as in rheumatoid arthritis24 and venous leg ulcers25 as well as in normal keratinocytes and other epithelia.26,27 The fact that, during corneal wound healing, these same enzymes, MMP-1, MMP-2, and MMP-3 were shown to be up-regulated in the anterior stroma underneath the injured epithelium incited us to look for EMMPRIN as the corneal inducer of MMP production through direct epithelial cell-fibroblast interaction.
In this study we used noninfectious ulcerated corneas as a model to study MMP regulation because they are associated with excessive extracellular matrix degradation that may lead to corneal perforation and where prolonged increase in MMP expression has been demonstrated.18,28 We report the differential expression and localization of EMMPRIN in normal and ulcerated corneas, its regulation by cytokines, and its biological function in vitro.
Materials and Methods
Tissue, Cells, and Materials
Corneal tissues (n = 5) were obtained from the French Eye Bank (Dr. P. Sabatier, Banque Française des Yeux, Paris, France) and Belgian Eye Banque (Dr. Lejeune, Lieges, Belgium). Chronic ulcerated or perforated corneal specimens (n = 5) were obtained from patients undergoing penetrative keratoplasty and use of specimen was performed in accordance with protocols approved by the institutional committee. Tissues were flash-frozen in optimal cutting temperature (OCT) compound and kept at −80°C for immunohistochemistry.
Human corneal reconstructed epithelia, stratified by air lifting in vitro, were obtained in collaboration with SkinEthic Inc. (Nice, France). The corneal epithelial cell line, 1384 HCE-T (Dr. Araki-Sasaki, Riken Cell Bank, Saitama, Japan) was maintained in supplementary hormonal epithelial medium (SHEM) [50% HAMS-F12 and 50% Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10 ng/ml epidermal growth factor (EGF), 0.1 nmol/L cholera toxin, and 5 μg/ml insulin] containing 10% fetal bovine serum. Corneal fibroblasts cell line were cultured in DMEM supplemented with 10% fetal bovine serum.29 For experiments with cytokine treatment, cells were used at 60 to 80% confluence in 50% HAMS-F12 and 50% DMEM without supplements. Cells were incubated in serum-free medium 18 hours before cytokine addition.
The antibodies used were anti-EMMPRIN (anti-CD147) HIM6 monoclonal antibody (mAb), used for immunoblots and immunohistochemistry (Becton-Dickinson, San Diego, CA), and anti-EMMPRIN blocking antibody, UM-8D6, for inhibition studies (Research Diagnostics, Inc., Flanders, NJ). The neutralizing activity of this antibody has been confirmed in different assays and was shown to inhibit dose dependently and specifically both MMP-1 and MMP-2 production induced by recombinant EMMPRIN in fibroblasts, both of lung and dermal origin (L.Yan, unpublished data).
Anti-MMP-1 mAb was from Calbiochem (La Jolla, CA); anti-MMP-2 mAb CA 801 and polyclonal antibody (pAb)30 were gifts from Dr. Raphael Fridman (Wayne State University, Detroit, MI); horseradish peroxidase-conjugated anti-mouse antibody was from Jackson ImmunoResearch Laboratories Immunotech (Marseille, France); and anti-mouse IgG Alexa 488 and anti-rabbit IgG Alexa 594were from Molecular Probes (Invitrogen, Cergy Pontoise, France). Other materials were gelatin from Sigma (St. Louis, MO), ECL+ reagent from Amersham Bioscience (Buckinghamshire, UK), and protease inhibitor cocktail Set V ethylenediaminetetraacetic acid-free from Calbiochem. Recombinant EMMPRIN used in the study was an affinity-purified Flag-tag protein consisting of the extracellular domain of human EMMPRIN expressed in mammalian cells.
Cell Treatment
Corneal epithelial cells 1384 HCE-T were maintained in SHEM medium supplemented with 10 ng/ml EGF, 0.1 nmol/L cholera toxin, 5 μg/ml insulin, and 10% fetal bovine serum until they reached 70 to 80% confluence. The medium was then replaced with serum- and growth factor-free SHEM and cells were incubated for 18 hours before cytokine addition. Cells were then treated with either transforming growth factor (TGF)-β1 (10 ng/ml), EGF (10 ng/ml), or tumor necrosis factor-α (10 ng/ml) in the serum-free medium and incubated for either 6 hours, after which total RNA was extracted with the RNeasy kit (Qiagen, Hilden, Germany) for real-time quantitative reverse transcriptase (RT)-polymerase chain reaction (PCR) measurements, or for 24 hours, after which the cells were lysed in the Tris-buffered saline-Nonidet P-40 solution containing protease inhibitors and subjected to immunoblot analysis of EMMPRIN, as described below. The incubation times were chosen on the basis of several time-course experiments.
To obtain epithelial cells at different densities, 5 × 105 cells suspended in complete SHEM media were seeded in either 25-, 75-, or 175-cm2 dishes. Cells were allow to adhere and grow for ∼24 hours until a confluent monolayer was obtained in the 25-cm2 dish and the cells in the larger flasks were at correspondingly lower densities. The different density cultures were then washed with serum-free SHEM and lysed for either quantitative RT-PCR or immunoblots analysis. In some experiments different density cultures were obtained by seeding 5 × 104 cells in 25-cm2 dishes and allowing them to grow in complete media, collecting the cells each day up to 3 days when cells reached confluence.
Epithelial Fibroblast Co-Culture
Epithelial cell suspension containing 105 in 200 μl (1:1 SHEM:DMEM) was applied on a sterile microscope slide that was placed in a culture dish. After cells within the drop became confluent (48 hours) forming an epithelial sheet of ∼1 cm2, fibroblasts (105 in 200 μl) were added around the epithelial sheet and allowed to attach before the whole slide was completely submerged in medium. After 24 to 48 hours, a clear interface between the two cell types formed. The slides were then washed in phosphate-buffered saline (PBS); fixed in 4% paraformaldehyde; stained with 4,6-diamidino-2-phenylindole (DAPI), anti-EMMPRIN, anti-MMP-2 mAb; and analyzed by confocal microscopy.
Epithelial Cell Membrane Preparation
Cell membranes were prepared according to Lindenmeyer and colleagues.31 Briefly, HCE-T cells were scrapped with rubber policemen in serum-free SHEM medium containing 1/100 protease inhibitor cocktail Set V (AEBSF, aprotinin, E-64, leupeptin, and 1 mmol/L ethylenediaminetetraacetic acid), disrupted on ice by controlled sonication: three cycles of 10 seconds at 40 W (Vibra cell Sonifier; Bioblock Scientific, France) and centrifuged at 1000 × g for 10 minutes at 4°C to remove unbroken cells. The sonicate supernatant was centrifuged at 19,000 × g for 30 minutes to sediment the granules and the resulting supernatant was further centrifuged at 100,000 × g for 1 hour. After one wash in serum-free DMEM, the pellet containing the membrane fraction was resuspended in the same medium. Aliquots containing the membrane fraction diluted with serum-free DMEM (20 μg/ml) were added to the fibroblasts cultures (106 cells per 25-cm2 dish) and incubated for 24 hours.
Confocal Immunohistochemistry and Cytochemistry
Cryostat sections (8 μm) were prepared from frozen cornea or stratified epithelium and were immunostained as previously described.32 Briefly, the sections were fixed in chilled acetone for 10 minutes, then rehydrated in PBS, and incubated in blocking solution (3% bovine serum albumin) for 30 minutes. Sections were incubated for 1 hour with anti-EMMPRIN/CD-147 HIM6 mAb at 1/200 dilution, then for 30 minutes with conjugated affinity-purified donkey anti-mouse IgG (Alexa 488). For immunocytochemistry, cells were seeded on Lab-Tek glass slides, cultured for 2 days, fixed in 4% paraformaldehyde, and then processed as above using either CD-147 HIM6 or anti-MMP-2 pAb, and followed by conjugated affinity-purified donkey anti-mouse and/or anti-rabbit IgG (Alexa 488 and 594, respectively). The slides were mounted and examined with a laser-scanning confocal microscope (Leica Lasertechnik, Heidelberg, Germany). Negative controls were prepared using the same procedure, but phosphate-buffered saline was substituted for the primary antibody.
Immunoblot Analysis
Cells were lysed in Tris-buffered saline-Nonidet P-40 solution comprising 50 mmol/L Tris buffer, pH 7.5, 150 mmol/L NaCl, 1% Nonidet P-40, 0.5% Triton X-100, 2 mmol/L ethylenediaminetetraacetic acid, and 1/100 protease inhibitor cocktail Set V. After incubation on ice for 20 minutes and scrapping, the lysates were centrifuged for 3 minutes at 15,000 × g at 4°C. Protein concentration of the resulting supernatants was quantitatively determined using the Bradford Bio-Rad protein assay. Twenty μg of protein in Laemmli sample buffer were loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel for electrophoresis and then transferred to nitrocellulose filters. EMMPRIN or MMP-1 were revealed by incubation with 0.2 μg/ml HIM6 mAb or anti-MMP1 mAb overnight at 4°C followed by a 1-hour incubation with horseradish peroxidase-conjugated anti-mouse antibody and visualized with ECL+ reagent. Protein loading was verified by comparing the intensity of actin bands. Band intensities were quantified using Image Quant 5.2 (Molecular Dynamics, Piscataway, NJ).
Gelatin Zymography
The presence of the gelatinases, MMP-2 and MMP-9, in the serum-free conditioned media (10 μl) was determined by zymography in 10% polyacrylamide gels containing 1 mg/ml gelatin, as previously described.33 Band intensities were quantified using Image Quant 5.2 (Molecular Dynamics).
Real-Time Quantitative PCR
To evaluate the expression levels of MMP-1, MMP-2, and EMMPRIN normalized to the housekeeping β2-microglobulin gene in cultured corneal stromal cells, real-time quantitative PCR was performed using a LightCycler (Roche Diagnostics, France) according to described techniques.18,34 Selected sets of primers and labeled probes (Eurogentec, Biosense, Italy) are shown in Table 1. Standards for MMP-1, MMP-2, EMMPRIN, and β2-microglobulin were prepared from total normal RNA, amplified by RT-PCR, and cloned using the TOPO II TA cloning kit (Invitrogen, France) following the manufacturer’s recommendations.
Table 1.
List of the Primers and Probes Used
| Gene and oligonucleotide | Location | Sequence | PCR product size (bp) |
|---|---|---|---|
| EMMPRIN | |||
| Upper primer | 766U | 5′-GCAGCGGGCAGCACC-3′ | |
| Lower primer | 833L | 5′-CCACCTGCCTCAGGAAGAGTT-3′ | 68 |
| Probe | 788U | 5′-CAAAGGCAAGAACGTCCGCCAGAG-3′ | |
| MMP2 | |||
| Upper primer | 380U | 5′-CCGTCGCCCATCATCAA-3′ | |
| Lower primer | 450L | 5′-AGGTATTGCACTGCCAACTCTTT-3′ | 71 |
| Probe | 406U | 5′-CGATGTCGCCCCCAAAACGGA-3′ | |
| MMP9 | |||
| Upper primer | 610U | 5′-CATTCAGGGAGACGCCCA-3′ | |
| Lower primer | 673L | 5′-AACCACGACGCCCTTGC-3′ | 64 |
| Probe | 629U | 5′-TTCGACGATGACGAGTTGTGGTCCCT-3′ | |
| β2 Microglobulin | |||
| Upper primer | 20U | 5′-CGCTCCGTGGCCTTAGC-3′ | |
| Lower primer | 86L | 5′-GAGTACGCTGGATAGCCTCCA-3′ | 67 |
| Probe | 39U | 5′-TGCTCGCGCTACTCTCTCTTTCTG-3′ |
Results
Distribution of EMMPRIN in the Normal Cornea
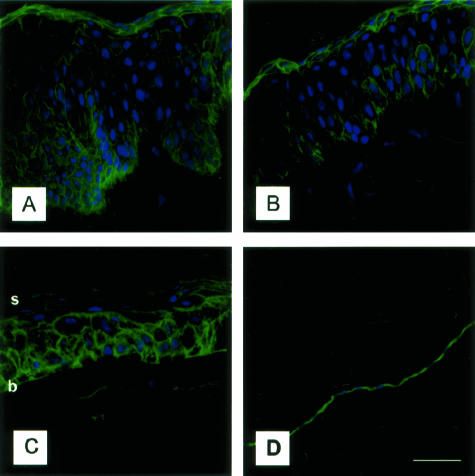
The presence and localization of EMMPRIN in the cornea was studied by confocal immunohistochemistry on 8-μm-thick frozen human corneal sections (limbus, peripheral, and central cornea). Figure 1 shows the presence of EMMPRIN in the different regions of the cornea, with the strongest staining seen on the surface of epithelial cells and endothelial cells. Some staining can also be noted on the cell membrane of keratocytes in the corneal stroma. A gradient of EMMPRIN staining was observed in the central epithelium (Figure 1C), strongest in the basal cells, and decreased toward superficial layers. In the limbal epithelium, EMMPRIN staining (Figure 1A) appeared to follow columns of cells originating from the basal layer, presenting a staining pattern different from that in the central cornea. In addition, the most superficial layers of the limbal and mid-peripheral epithelium (Figure 1, A and B) were more intensely stained compared to the central epithelium (Figure 1C).
Figure 1.
Immunodetection of EMMPRIN in the human cornea. A–C: Confocal EMMPRIN immunostaining was observed in the peripheral (A) and mid-peripheral (B) limbal corneal epithelium and in the central human corneal epithelium (C). EMMPRIN staining was intense in the epithelial cells whereas the stroma was weakly stained. D: Corneal endothelial cells were also intensely stained. Note the gradient in EMMPRIN staining from superficial to basal cells in the central corneal epithelium, and the increased staining in the superficial peripheral corneal epithelium (b and s denote basal and superficial layers, respectively. Green, EMMPRIN staining; blue, DAPI nuclear staining. Scale bar, 25 μm.
Distribution of EMMPRIN in Corneal Epithelial Cells in Culture
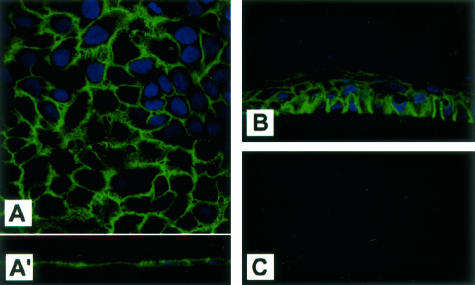
Corneal epithelial cell line in culture examined by confocal immunohistochemistry demonstrated intense immunoreactivity of EMMPRIN that was concentrated at the cell surface, forming small processes that projected from the cells (Figure 2). Confocal vertical section of the monolayer culture revealed that staining was homogeneously distributed around the cell surface (Figure 2, A and A′). Examination of reconstructed epithelium in culture, in which cells were allowed to stratify by air lifting, shows that stratification in vitro was associated with the formation of a gradient of EMMPRIN staining (Figure 2B) that contrasts with the homogenous staining of the monolayer cell culture, but resembles the one observed in the central epithelium of the cornea (Figure 1C), suggesting a regulation of EMMPRIN according to the differentiation state of the cells.
Figure 2.
Cell surface distribution of EMMPRIN in corneal epithelial cells in vitro. A: Confocal imaging of EMMPRIN staining in monolayer cultures of epithelial cells. Staining in A and vertical section in inset A′ show that EMMPRIN staining is distributed along the whole cell membrane and associated with small processes extending from the cells. B: EMMPRIN staining in stratified reconstituted epithelium in vitro shows a similar gradient to that seen in the central cornea. C: No signal was detected in control epithelium cultures when only the secondary antibody was used. Original magnifications, ×60.
Regulation of EMMPRIN by Cytokines and Cell Density
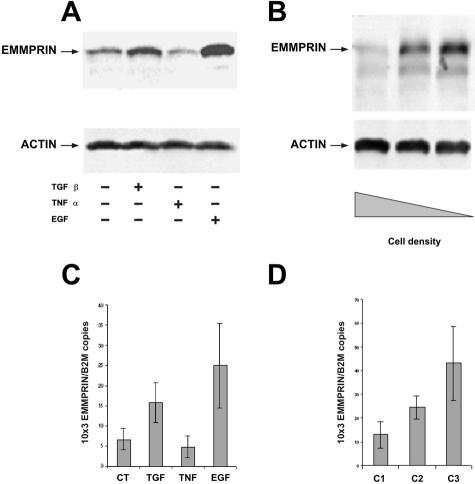
The modulatory role played by cytokines on the regulation of EMMPRIN in corneal epithelial cells was assessed by immunoblot (Figure 3, A and B) and real-time PCR (Figure 3, C and D) after treating these cells with TGF-β1, EGF, and tumor necrosis factor-α, cytokines implicated in wound healing. Immunoblot analysis of cell lysates revealed two bands at ∼40 and 60 kd corresponding to differently glycosylated forms of EMMPRIN.20 An up-regulation of EMMPRIN by TGF-β1 and EGF was obtained at both the mRNA and protein level (Figure 3, A and C). Tumor necrosis factor-α had a slight but not significant inhibition on EMMPRIN expression. To investigate the regulation of EMMPRIN as a function of the degree of confluence, cells were plated at different densities and cultured for 24 hours before immunoblot and PCR analysis. Figure 3, B and D, demonstrates an inverse regulation of EMMPRIN with cell density, with the highest level of EMMPRIN associated with sparse cultures. Similar results were obtained when cells were seeded at the same cell density and allowed to grow, EMMPRIN being analyzed each day up to confluence (not shown). Interestingly, the immunoblot analysis revealed in the sparse culture a greater increase in the higher Mr band at 60 kd (Figure 3B), suggesting a preferential up-regulation of the glycosylated form that was reported to be the most active.35
Figure 3.
Regulation of EMMPRIN in corneal epithelial cells by cytokines and cell density. Immunoblots (A, B) and real-time PCR (C, D) analysis of EMMPRIN regulation by cytokines (A, C) and cell density (B, D) in corneal epithelial cells. A and C: Cells were treated with TGF-β1 (10 ng/ml), EGF (10 ng/ml), or tumor necrosis factor-α (10 ng/ml) in serum-free medium for 24 hours (A) or 6 hours (C). B and D: Different cell density cultures were obtained by seeding 106 of cells in 25-cm2 (C1), 75-cm2 (C2), and 175-cm2 (C3) dishes. Cells were allowed to adhere for ∼24 hours to obtain confluent monolayer in the 25 cm2 (C1). For immunoblots, 20 μg of cell extracts were analyzed with either anti-EMMPRIN or anti-actin antibodies. C and D: Results are expressed as the mean of four separate experiments; bars, ±SD.
Up-Regulation and Co-Localization of EMMPRIN and MMP-2 in Chronic Ulcerated Corneas
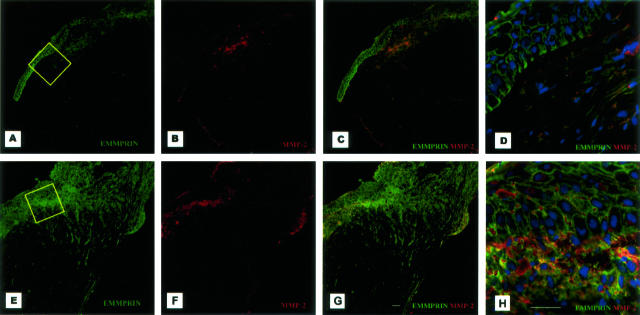
To assess the implication of EMMPRIN in wound healing we analyzed its expression and distribution, as well as that of MMP-2, in ulcerated corneas. To this end, tissue sections taken either from the center or at a distance of the ulcerated area were subjected to immunohistochemical and zymography analysis. Immunohistochemical analysis revealed a striking increase in EMMPRIN staining in the ulcerated area in all five corneas examined. This increase was predominantly observed in the wounded stroma, particularly at the epithelio-stromal boundary (Figure 4). Inaddition, the gradient of EMMPRIN staining clearly seen in the epithelium at a distance from the wounded area that resembled closely that of normal cornea (Figure 1) was lost at the edge of the ulceration. Although only faint staining of MMP-2 could be observed in the unwounded stoma, it was markedly increased in the wounded stroma, in particular at the epithelio-stromal boundary, where MMP-2 and EMMPRIN were shown to be co-localized by double-labeled confocal immunohistochemistry. The same MMP-2 staining pattern was observed with either the monoclonal or the polyclonal MMP-2 antibody used. Some areas, which appear to correspond to the intercellular space, displayed only MMP-2 staining and may represent extracellularly located enzyme. The increased MMP-2 level in the ulcerated areas compared to nonulcerated areas was also observed by zymography (results not shown).
Figure 4.
Induction and co-localization of EMMPRIN and MMP-2 in the stroma at the epithelio-stromal boundary in chronic corneal ulcers. Tissue sections at a distance from the ulcerations (A–D) and in the ulcerated area (E–H) were subjected to double-labeled confocal immunohistochemistry using mouse anti-human CD147 (A, C–E, G, H; green) and rabbit anti-human MMP-2 pAb antibodies (B–D, F–H; red) and counterstained with DAPI (D and H, blue). Merged staining of EMMPRIN and MMP-2 (C, D, G, H) revealed induction of EMMPRIN and MMP-2 in the ulcerated area in the stroma at the epithelio-stromal boundary (G, H) whereas MMP-2 staining was weak without co-localization with EMMPRIN in the nonulcerated cornea (D). D and H: Higher magnification of the areas in squares in A and E. Note the absence of staining in the acellular layer of Bowman (D) and the intense staining when continuity exists between epithelium and stroma in the ulcerated area (H). Scale bar, 25 μm.
Corneal Epithelial Cells Up-Regulate MMP Expression in Corneal Fibroblasts through Direct Cell Contact
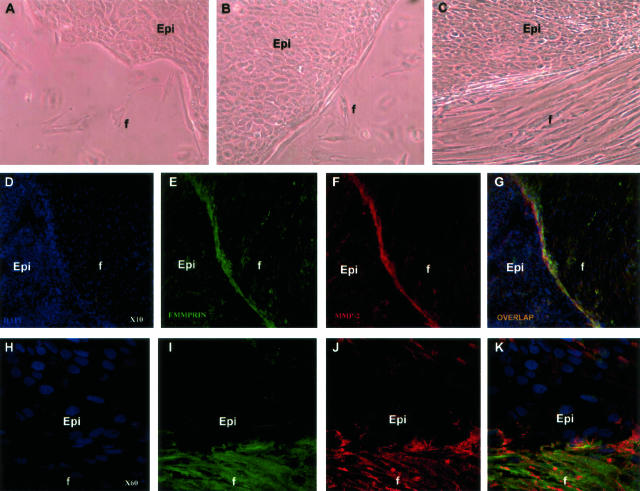
Because epithelial EMMPRIN is known to stimulate MMP expression in neighboring fibroblasts, we used a monolayer co-culture model of corneal epithelial cells and fibroblasts to evaluate MMP regulation in fibroblasts. For that, epithelial cell suspension was applied to one side of a sterile microscope slide and allowed to form a confluent epithelial sheet after which fibroblasts were added and left to grow until a clear interface between the two types of cells was formed (Figure 5; A to C). Immuno-confocal analysis demonstrated a strong staining of MMP-2 on the surface of fibroblasts limited to the interface between the two cell types (Figure 5, F and J), suggesting a regulation of MMP-2 by direct interaction. This induction of MMP-2 was associated with a similar increase in EMMPRIN expression (Figure 5, E and I) and the two were co-localized in the fibroblasts at the interface with epithelial cells, as shown by the overlapping staining (Figure 5, G and K). Thus, the MMP-2 and EMMPRIN induction and co-localization at the interface between the two cell types resembles that observed at the subepithelial wounded stroma in vivo and advocate for an involvement of a direct cellular interaction as a mechanism of MMP-2 induction via EMMPRIN.
Figure 5.
Induction and co-localization of EMMPRIN and MMP-2 in corneal fibroblasts at the interface with corneal epithelial cells in an in vitro co-culture system. Epithelial cell (epi) suspension containing 105 in 200 μl was applied, forming a large drop, on a sterile microscope slide that was placed in a culture dish. After cells within the drop became confluent (48 hours) forming an epithelial sheet, fibroblasts (f) were added and allowed to attach (A), proliferate, and migrate around the epithelial cells (B, C), forming after 24 to 48 hours a clear interface between the two types of cells (C). After further incubation for 24 hours, the slides were washed, fixed, and stained with DAPI (D and H, blue), anti-EMMPRIN/CD147, HIM6 mAb (E and I, green), and anti-MMP-2 pAb (F and J, red) and analyzed by confocal microscopy. G and K: Overlapping staining of EMMPRIN and MMP-2 in yellow. H–K: Higher magnifications of the epithelial/fibroblast interface shown in D–G, respectively. Original magnifications: ×10 (D–G); ×60 (H–K).
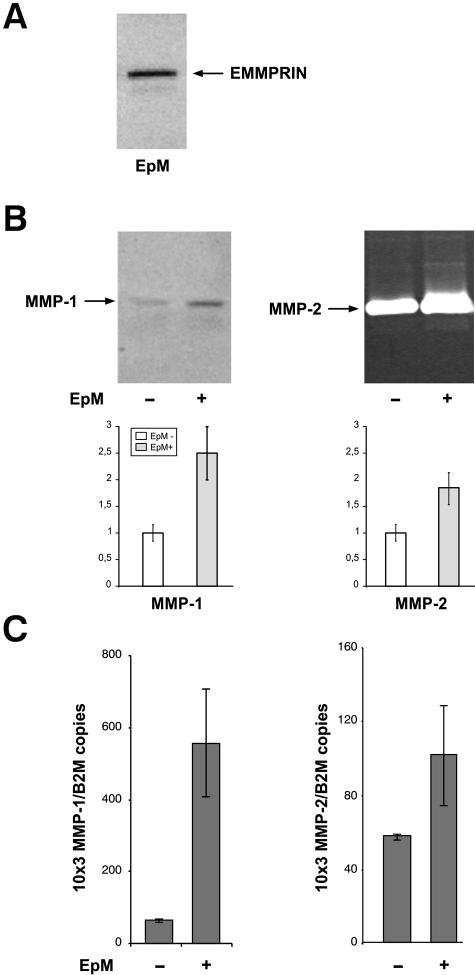
To further define the implication of direct cell contact in this induction, fibroblasts were incubated with purified epithelial cell membranes. Immunoblot analysis confirmed the presence of EMMPRIN in the epithelial membrane preparation (EpM) (Figure 6A) and these membranes stimulated protein secretion (Figure 6B) and mRNA expression (Figure 6C) of both MMP-1 and MMP-2 by fibroblasts. MMP-9 was not regulated by the epithelial cell membrane. No activity was detected in control experiments with isolated membranes incubated in the absence of fibroblasts (not shown). These experiments demonstrated that direct cellular contact mediated by the cell membrane can regulate MMP expression independently of secreted soluble factors. These results, together with the restricted localization of the induced MMP-2 to fibroblasts alongside epithelial cells, suggested that direct epithelio-stromal interaction (ESI) may represent an additional mechanism to that of cytokines in the regulation of MMP expression in the cornea.
Figure 6.
EMMPRIN-containing corneal epithelial cell membranes stimulate MMP production in corneal fibroblasts in culture. A: Corneal epithelial cell membranes (EpM) were isolated by differential centrifugation and 10 μg of membrane extract were analyzed for EMMPRIN content by immunoblotting. One major band at 60 kd and a lower Mr faint band at ∼40 kd, corresponding to the high and low glycosylated forms of EMMPRIN, can be observed. B: Fibroblast cultures (80% confluent) were incubated with 20 μg/ml of EpM for 24 hours in serum-free medium after which the conditioned medium, 5× concentrated, was analyzed by immunoblot for MMP-1 and gelatin zymography for MMP-2. The amount of EpM added was derived from the same number of epithelial cells to that of fibroblasts. The graphs represent densitometry quantification of MMP-1 protein and MMP-2 gelatinolytic activity. C: Real-time PCR analysis of the same cultures as in B incubated with the membranes for 18 hours before RNA extraction. Results are expressed as the mean of triplicate experiments; bars, ±SD.
MMP and EMMPRIN Induction in Corneal Fibroblasts by Epithelial Cell Membranes Is Mediated by EMMPRIN
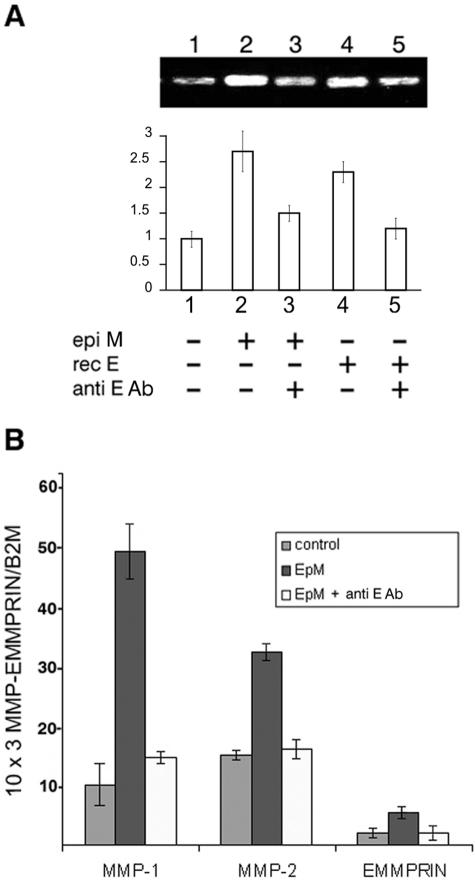
To define the role of EMMPRIN in the induction of MMP-2 observed after fibroblasts were incubated with epithelial cell membranes, we used an EMMPRIN blocking antibody in this system. Zymography analysis of the conditioned medium demonstrated increased MMP-2 after treatment with corneal epithelial membranes (Figure 7A, lane 2) or recombinant EMMPRIN (Figure 7A, lane 4). Both inductions were markedly suppressed when anti-EMMPRIN antibody was present (Figure 7A, lanes 3 and 5). This inhibition by the antibody was confirmed using real-time RT-PCR for both MMP-1 and MMP-2 (Figure 7B). Interestingly, we demonstrated that EMMPRIN mRNA was also induced by epithelial cell membrane and that this induction was inhibited by blocking the EMMPRIN antibody. Hence, EMMPRIN present at the surface of epithelial membrane is responsible for the induction of both MMP-2 and EMMPRIN in fibroblasts and may account for their induction at the interface with epithelial cells in vitro and represent a possible mechanism for MMP-2 and EMMPRIN induction in vivo.
Figure 7.
Anti-EMMPRIN-blocking antibody inhibits induction of fibroblasts MMPs and EMMPRIN by epithelial cell membrane. A: Corneal fibroblast cultures were incubated for 24 hours with 20 μg/ml of corneal epithelial cell membranes (EpM) or with 5 μg/ml of recombinant EMMPRIN (rec E), in the absence or presence of 20 μg/ml of anti-EMMPRIN blocking antibody (anti E Ab) in serum-free medium, after which the conditioned medium was analyzed by gelatin zymography. The fibroblasts were preincubated with the antibody for 15 minutes before the addition of the membranes. The graph represents the densitometry quantification of MMP-2 gelatinolytic activity. B: Real-time PCR analysis of MMP-1, MMP-2, and EMMPRIN mRNA expression in fibroblasts incubated with EpM for 18 hours in the absence or presence of 20 μg/ml of anti-EMMPRIN blocking antibody. Results are expressed as the mean of triplicate separate experiments; bars, ±SD.
Discussion
Although barely detected in normal cornea, MMPs are induced during wound healing processes. This induction may be mediated by different mechanisms because MMPs are known to be regulated by growth factors and cytokines, by integrin-mediated cell-matrix interactions, or by direct cell-cell interactions.5,16 EMMPRIN, a cell membrane glycoprotein enriched on epithelial cells and in particular on tumor cells, has been suggested to be responsible for MMP induction resulting from direct ESIs during carcinogenesis.20,22 In this investigation, we identified EMMPRIN in the cornea and demonstrated its implication in the localized MMP induction resulting from direct corneal ESI that may take place during corneal wound healing.
Direct ESI in the cornea is normally prevented by the presence of both the basement membrane and the Bowman layer.36 During pathological wound healing processes, such as corneal ulcerations, breakdown of these barriers may facilitate direct ESI and favor MMP-mediated complications including corneal melts or perforations.6,18,37 EMMPRIN was shown, in studies using either tumor cells, EMMPRIN transfected cells, or directly the recombinant molecule, to induce MMP-1, MMP-2, and MMP-3 in skin fibroblasts.19 These same proteinases have been detected in the subepithelial layer in corneal ulcerations/perforations of multiple origin,17,38 such as those associated with rheumatoid arthritis, Sjögren syndrome,28 alkali burn injury,6 or iatrogenic corneal melts,18 suggesting that they may be induced by direct ESI in the cornea, mediated by epithelial cell EMMPRIN. Our immunohistochemical analysis identified EMMPRIN in the healthy cornea and demonstrated a marked increase in its expression in ulcerated corneas, in particular in the area of stromal thinning. MMP-2 was also induced in the wounded stroma, and the increase in both EMMPRIN and MMP-2 was concentrated at the epithelio-stromal boundary. The increase in EMMPRIN staining in the anterior stroma was concomitant with the loss of its normal polarized distribution seen in the epithelium at a distance from the wounded area, as well as in the normal corneas, with the highest expression in the basal cells, and corresponded to the disruption of the epithelial organization in this region. Furthermore, double-labeled confocal immunohistochemistry revealed a close co-localization of MMP-2 and EMMPRIN in the subepithelial area of the wounded stroma, except in areas that appear to represent the extracellular matrix, where MMP-2 predominated. This may indicate a localized induction of MMP-2 by the accumulated EMMPRIN at this region. MMP-9, although also induced in corneal ulceration, exhibited different localization and appeared to be expressed predominantly by regenerating epithelial cells and by the inflammatory cells recruited to the wounded area, as was previously shown.11,18,39 MMP-9, which is more implicated in the delay of epithelial wound closure,8 was not co-localized with EMMPRIN (results not shown), in accordance with the general consensus that EMMPRIN does not induce MMP-9 in fibroblasts.40
The induction of both EMMPRIN and MMP-2 at the ESI interface observed in vivo was reproduced by our in vitro co-culture model, in which corneal epithelial cells and fibroblasts were allowed to come into direct contact, forming an epithelio-stromal border. The very strong staining of MMP-2 and EMMPRIN concentrated in the fibroblasts along the interface with the epithelial cells and their restricted localization is suggestive of direct cell-cell contact as a mechanism of induction. Although a paracrine regulation of MMPs involving secreted cytokines has been suggested3,41,42 and may also be involved to some extent in our co-culture system, it would be expected to result in a more generalized and homogenous staining. The experiments in which purified membrane preparations of epithelial cells induced both MMP-1 and MMP-2 in cultured fibroblasts confirms that EMMPRIN-containing epithelial cells are able to activate fibroblasts to produce more MMPs by direct cell-cell interaction. Furthermore, the induction of both MMP-1 and MMP-2 was greatly inhibited by a blocking antibody to EMMPRIN, indicating that the MMP induction observed in this system is mostly because of the effect of EMMPRIN. The increase in fibroblast EMMPRIN levels by the epithelial cell membrane is consistent with the increased EMMPRIN observed in the fibroblasts at the epithelio-stromal interface, both in the ulcerated corneas and in the in vitro co-culture model. The inhibition of EMMPRIN induction by the blocking antibody to EMMPRIN suggests that EMMPRIN itself was primarily responsible for its own induction in the fibroblasts. This is in agreement with the previously reported demonstration of a positive feedback regulation of EMMPRIN.43 However the importance of this new MMP-induction mechanism during corneal wound healing remains to be demonstrated in animal models in vivo.
In the normal cornea, EMMPRIN was detected mainly in the epithelium and endothelium. In the central epithelium, it was particularly concentrated in the basal cells with a gradual decrease toward the superficial cells. When isolated epithelial cells in culture were immunostained for EMMPRIN, a homogenous membrane staining was observed. However, the basoapical gradient observed in stratified epithelial cells in vitro, as in the central cornea in vivo, suggests that EMMPRIN expression may depend on the differentiation state of the epithelial cells, known to implicate cytokines and MMPs.8,44 Indeed, the growing list of MMP substrates, including cytokines, cell adhesion molecules, and signaling molecules, suggests that in addition to degrading the extracellular matrix, these proteases also contribute to communication between cells and their microenvironment and influence cell behavior such as cell growth and differentiation. However, despite the known role of EMMPRIN in regulating MMPs, an MMP-independent function in these cells cannot be excluded.
Although the cell surface localization of EMMPRIN both in cultured cells and tissue sections has been clearly demonstrated by our immunofluorescence staining as well as by numerous other studies, the release of a small fraction of soluble active EMMPRIN, either by proteolytic cleavage or by vesicular shedding has been previously described in tumor cell culture medium.43,45 The fact that soluble EMMPRIN retains its activity suggests that if EMMPRIN shedding can indeed occur in vivo, it may also be involved in mediating and modulating ESI in a diffusible paracrine manner, in a similar way to cytokines during corneal wound healing.
Although much attention was paid into the regulation of MMPs by EMMPRIN, little is known about how EMMPRIN itself is regulated and the nature of the factors that may induce its expression. During our investigations in vitro, we first observed an enhanced expression of EMMPRIN when corneal epithelial cells in vitro were incubated in the presence of serum (data not shown). We were able to demonstrate that at least two cytokines, TGF-β1 and EGF, positively regulate EMMPRIN in these cells. The effect of EGF is in agreement with our previous results identifying EGFR signaling as critical for EMMPRIN regulation in mammary tumor cells.34 Interestingly, the receptors for these two cytokines, TβR and EGFR, were also shown to concentrate in the basal layers of the corneal epithelium and were shown to be up-regulated during epithelial cell migration during wound closure.46,47 Taken together with the present results, it suggests that a complex regulation existing between EGFR signaling, cell-cell interaction, and EMMPRIN to regulate the proliferative and migratory state of the basal epithelial cell. In addition, cell density, which has previously been shown to regulate the functional expression of TGF-β receptors in corneal epithelial cells,48 also appears to be an important regulatory mechanism controlling EMMPRIN levels.
On the basis of these results the following scenario may be postulated on the possible events that follow deep wounding involving direct ESI. The decrease in epithelial cell density and the accumulation of cytokines known to be secreted during wound healing, such as TGF-β and EGF, would increase EMMPRIN levels in the epithelial cells in the wounded area. This can in turn induce fibroblasts, by direct interaction, to increase their own level of EMMPRIN. The resultant MMP induction would trigger either stromal remodeling to restore tissue homeostasis, or excessive MMP release and stromal destruction in chronic situations in which healing is delayed and direct ESI persists. The implication of EMMPRIN in stromal cell activation in response to injury, by increasing both MMPs and EMMPRIN, suggests that inhibition of EMMPRIN may represent a promising future therapeutic strategy in situations of excess extracellular matrix degradation associated with chronic wound healing.
Acknowledgments
We thank Bernard Coulomb for suggestions and discussions; Marie-Pierre Podgorniak for technical assistance with the RT-PCR measurements; F. Brau, C. Doliger, and M. Schmid for confocal microscopy imaging; and Elisabeth Savariau for the help with figure preparation.
Footnotes
Address reprint requests to Eric E. Gabison, Hôpital St. Louis, INSERM U 532, 1 Avenue Claude Vellefaux, 75010 Paris, France. E-mail: egabison@wanadoo.fr.
Supported by the Association Française des Amblyopes Unilatéraux (to E.E.G.) and the Fondation de France (to E.S.).
References
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 1999;18:293–309. doi: 10.1016/s1350-9462(98)00017-2. [DOI] [PubMed] [Google Scholar]
- Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res. 2002;21:1–14. doi: 10.1016/s1350-9462(01)00015-5. [DOI] [PubMed] [Google Scholar]
- Kahari VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997;6:199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Fini ME, Cook JR, Mohan R. Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res. 1998;290:S12–S23. doi: 10.1007/pl00007449. [DOI] [PubMed] [Google Scholar]
- Azar DT, Hahn TW, Jain S, Yeh YC, Stetler-Stevensen WG. Matrix metalloproteinases are expressed during wound healing after excimer laser keratectomy. Cornea. 1996;15:18–24. [PubMed] [Google Scholar]
- Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, Lee Y, McCarthy BE, Wollenberg KR, Jester JV, Wang M, Welgus HG, Shipley JM, Senior RM, Fini ME. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem. 2002;277:2065–2072. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- Li de Q, Shang TY, Kim HS, Solomon A, Lokeshwar BL, Pflugfelder SC. Regulated expression of collagenases MMP-1, -8, and -13 and stromelysins MMP-3, -10, and -11 by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:2928–2936. doi: 10.1167/iovs.02-0874. [DOI] [PubMed] [Google Scholar]
- Sudbeck BD, Pilcher BK, Welgus HG, Parks WC. Induction and repression of collagenase-1 by keratinocytes is controlled by distinct components of different extracellular matrix compartments. J Biol Chem. 1997;272:22103–22110. doi: 10.1074/jbc.272.35.22103. [DOI] [PubMed] [Google Scholar]
- Daniels JT, Geerling G, Alexander RA, Murphy G, Khaw PT, Saarialho-Kere U. Temporal and spatial expression of matrix metalloproteinases during wound healing of human corneal tissue. Exp Eye Res. 2003;77:653–664. doi: 10.1016/j.exer.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Daniels JT, Limb GA, Saarialho-Kere U, Murphy G, Khaw PT. Human corneal epithelial cells require MMP-1 for HGF-mediated migration on collagen I. Invest Ophthalmol Vis Sci. 2003;44:1048–1055. doi: 10.1167/iovs.02-0442. [DOI] [PubMed] [Google Scholar]
- Girard MT, Matsubara M, Kublin C, Tessier MJ, Cintron C, Fini ME. Stromal fibroblasts synthesize collagenase and stromelysin during long-term tissue remodeling. J Cell Sci. 1993;104:1001–1011. doi: 10.1242/jcs.104.4.1001. [DOI] [PubMed] [Google Scholar]
- Ye HQ, Azar DT. Expression of gelatinases A and B, and TIMPs 1 and 2 during corneal wound healing. Invest Ophthalmol Vis Sci. 1998;39:913–921. [PubMed] [Google Scholar]
- Ye HQ, Maeda M, Yu FS, Azar DT. Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal and wounded rat corneas. Invest Ophthalmol Vis Sci. 2000;41:2894–2899. [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Ambrosio R, Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20:625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- Gordon JM, Bauer EA, Eisen AZ. Collagenase in human cornea: immunologic localization. Arch Ophthalmol. 1980;98:341–345. doi: 10.1001/archopht.1980.01020030337022. [DOI] [PubMed] [Google Scholar]
- Gabison EE, Chastang P, Menashi S, Mourah S, Doan S, Oster M, Mauviel A, Hoang-Xuan T. Late corneal perforation after photorefractive keratectomy associated with topical diclofenac: involvement of matrix metalloproteinases. Ophthalmology. 2003;110:1626–1631. doi: 10.1016/S0161-6420(03)00486-X. [DOI] [PubMed] [Google Scholar]
- Li R, Huang L, Guo H, Toole BP. Basigin (murine EMMPRIN) stimulates matrix metalloproteinase production by fibroblasts. J Cell Physiol. 2001;186:371–379. doi: 10.1002/1097-4652(2000)9999:999<000::AID-JCP1042>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Toole BP. Emmprin (CD147), a cell surface regulator of matrix metalloproteinase production and function. Curr Top Dev Biol. 2003;54:371–389. doi: 10.1016/s0070-2153(03)54015-7. [DOI] [PubMed] [Google Scholar]
- Kataoka H, DeCastro R, Zucker S, Biswas C. Tumor cell-derived collagenase-stimulatory factor increases expression of interstitial collagenase, stromelysin, and 72-kDa gelatinase. Cancer Res. 1993;53:3154–3158. [PubMed] [Google Scholar]
- Kataoka H, Tanaka H, Nagaike K, Uchiyama S, Itoh H. Role of cancer cell-stroma interaction in invasive growth of cancer cells. Hum Cell. 2003;16:1–14. doi: 10.1111/j.1749-0774.2003.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Klein CA, Seidl S, Petat-Dutter K, Offner S, Geigl JB, Schmidt-Kittler O, Wendler N, Passlick B, Huber RM, Schlimok G, Baeuerle PA, Riethmuller G. Combined transcriptome and genome analysis of single micrometastatic cells. Nat Biotechnol. 2002;20:387–392. doi: 10.1038/nbt0402-387. [DOI] [PubMed] [Google Scholar]
- Konttinen YT, Li TF, Mandelin J, Liljestrom M, Sorsa T, Santavirta S, Virtanen I. Increased expression of extracellular matrix metalloproteinase inducer in rheumatoid synovium. Arthritis Rheum. 2000;43:275–280. doi: 10.1002/1529-0131(200002)43:2<275::AID-ANR6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Norgauer J, Hildenbrand T, Idzko M, Panther E, Bandemir E, Hartmann M, Vanscheidt W, Herouy Y. Elevated expression of extracellular matrix metalloproteinase inducer (CD147) and membrane-type matrix metalloproteinases in venous leg ulcers. Br J Dermatol. 2002;147:1180–1186. doi: 10.1046/j.1365-2133.2002.05025.x. [DOI] [PubMed] [Google Scholar]
- Gabison EE, Hoang-Xuan T, Mauviel A, Menashi S: EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie (in press) [DOI] [PubMed] [Google Scholar]
- DeCastro R, Zhang Y, Guo H, Kataoka H, Gordon MK, Toole B, Biswas G. Human keratinocytes express EMMPRIN, an extracellular matrix metalloproteinase inducer. J Invest Dermatol. 1996;106:1260–1265. doi: 10.1111/1523-1747.ep12348959. [DOI] [PubMed] [Google Scholar]
- Riley GP, Harrall RL, Watson PG, Cawston TE, Hazleman BL. Collagenase (MMP-1) and TIMP-1 in destructive corneal disease associated with rheumatoid arthritis. Eye. 1995;9:703–718. doi: 10.1038/eye.1995.182. [DOI] [PubMed] [Google Scholar]
- Griffith M, Osborne R, Munger R, Xiong X, Doillon CJ, Laycock NL, Hakim M, Song Y, Watsky MA. Functional human corneal equivalents constructed from cell lines. Science. 1999;286:2169–2172. doi: 10.1126/science.286.5447.2169. [DOI] [PubMed] [Google Scholar]
- Hoyhtya M, Fridman R, Komarek D, Porter-Jordan K, Stetler-Stevenson WG, Liotta LA, Liang CM. Immunohistochemical localization of matrix metalloproteinase 2 and its specific inhibitor TIMP-2 in neoplastic tissues with monoclonal antibodies. Int J Cancer. 1994;56:500–505. doi: 10.1002/ijc.2910560408. [DOI] [PubMed] [Google Scholar]
- Lindenmeyer F, Legrand Y, Menashi S. Upregulation of MMP-9 expression in MDA-MB231 tumor cells by platelet granular membrane. FEBS Lett. 1997;418:19–22. doi: 10.1016/s0014-5793(97)01336-7. [DOI] [PubMed] [Google Scholar]
- Lin HC, Chang JH, Jain S, Gabison EE, Kure T, Kato T, Fukai N, Azar DT. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest Ophthalmol Vis Sci. 2001;42:2517–2524. [PubMed] [Google Scholar]
- Fridman R, Toth M, Pena D, Mobashery S. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2). Cancer Res. 1995;55:2548–2555. [PubMed] [Google Scholar]
- Menashi S, Serova M, Ma L, Vignot S, Mourah S, Calvo F. Regulation of extracellular matrix metalloproteinase inducer and matrix metalloproteinase expression by amphiregulin in transformed human breast epithelial cells. Cancer Res. 2003;63:7575–7580. [PubMed] [Google Scholar]
- Tang W, Chang SB, Hemler ME. Links between CD147 function, glycosylation, and caveolin-1. Mol Biol Cell. 2004;15:4043–4050. doi: 10.1091/mbc.E04-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Hong JW. Bowman’s layer structure and function: critical or dispensable to corneal function? A hypothesis. Cornea. 2000;19:417–420. doi: 10.1097/00003226-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Fini ME, Girard MT, Matsubara M. Collagenolytic/gelatinolytic enzymes in corneal wound healing. Acta Ophthalmol Suppl. 1992;202:26–33. doi: 10.1111/j.1755-3768.1992.tb02165.x. [DOI] [PubMed] [Google Scholar]
- Wagoner MD, Kenyon KR. Distribution of collagenase and cell types in sterile ulceration of human corneal grafts. Acta Ophthalmol Suppl. 1989;192:65–71. doi: 10.1111/j.1755-3768.1989.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Girard MT, Kublin CL, Cintron C, Fini ME. Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodelling cornea. Dev Biol. 1991;147:425–439. doi: 10.1016/0012-1606(91)90300-r. [DOI] [PubMed] [Google Scholar]
- Caudroy S, Polette M, Nawrocki-Raby B, Cao J, Toole BP, Zucker S, Birembaut P. EMMPRIN-mediated MMP regulation in tumor and endothelial cells. Clin Exp Metastasis. 2002;19:697–702. doi: 10.1023/a:1021350718226. [DOI] [PubMed] [Google Scholar]
- Girard MT, Matsubara M, Fini ME. Transforming growth factor-beta and interleukin-1 modulate metalloproteinase expression by corneal stromal cells. Invest Ophthalmol Vis Sci. 1991;32:2441–2454. [PubMed] [Google Scholar]
- Matsubara M, Zieske JD, Fini ME. Mechanism of basement membrane dissolution preceding corneal ulceration. Invest Ophthalmol Vis Sci. 1991;32:3221–3237. [PubMed] [Google Scholar]
- Tang Y, Kesavan P, Nakada MT, Yan L. Tumor-stroma interaction: positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res. 2004;2:73–80. [PubMed] [Google Scholar]
- Yam HF, Pang CP, Fan DS, Fan BJ, Yu EY, Lam DS. Growth factor changes in ex vivo expansion of human limbal epithelial cells on human amniotic membrane. Cornea. 2002;21:101–105. doi: 10.1097/00003226-200201000-00021. [DOI] [PubMed] [Google Scholar]
- Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene. 2004;23:956–963. doi: 10.1038/sj.onc.1207070. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Hutcheon AE, Guo X, Chung EH, Joyce NC. TGF-beta receptor types I and II are differentially expressed during corneal epithelial wound repair. Invest Ophthalmol Vis Sci. 2001;42:1465–1471. [PubMed] [Google Scholar]
- Zieske JD, Takahashi H, Hutcheon AE, Dalbone AC. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest Ophthalmol Vis Sci. 2000;41:1346–1355. [PubMed] [Google Scholar]
- Wang M, Munier F, Araki-Saski K, Schorderet D. TGFBI gene transcript is transforming growth factor-beta1-responsive and cell density-dependent in a human corneal epithelial cell line. Ophthalmic Genet. 2002;23:237–245. doi: 10.1076/opge.23.4.237.13884. [DOI] [PubMed] [Google Scholar]