Abstract
The placenta functions both as site for nutrition and protection of the fetus. Transport proteins, including members of the multidrug resistance protein (MRP)/ABCC subfamily, have been recognized to contribute to the latter function. MRP5 (ABCC5) was identified as transmembrane transport protein for cyclic nucleotides, especially 3′,5′-cyclic GMP (cGMP), indicating an additional role in signal transduction and a potential role in placenta development. We therefore studied expression, localization, and function of MRP5 in placenta of different gestational ages. Quantitative real-time polymerase chain reaction revealed expression of MRP5 in all 60 samples from pre-term and term placenta, with a decreasing mean expression with gestational age (MRP5/18S-ratio × 1000; < 32 weeks: 2.91 ± 0.73, n = 15; 32 to 37 weeks: 2.10 ± 0.87, n = 15; > 37 weeks: 0.46 ± 0.08, n = 30; P < 0.01). Immunofluorescence microscopy with an anti-MRP5 antibody indicated localization of MRP5 preferentially in the basal membrane of syncytiotrophoblasts and in and around fetal vessels. ATP-dependent [3H]cGMP transport as evidence for MRP5 function could be demonstrated in isolated basal membrane vesicles. Moreover, the influence of cellular differentiation on MRP5 expression was studied in isolated trophoblasts, revealing an increase of the MRP5 expression in parallel with the hCG production (MRP5/18S-ratio × 1000 was 2.4 ± 0.5 at day 5 of culture and 1.45 ± 0.5 at day 0 of culture, n = 3 preparations, significant difference with P < 0.05). In conclusion, MRP5 expression depends on gestational age and varies throughout the differentiation process. In view of the important role of cGMP for cellular differentiation, MRP5 may play a role in placental development in context with a specific need for cellular cGMP export.
Placenta, the fetomaternal interface, functions as site for nutrient supply as well as an effective barrier protecting the fetus against exogenous substances. It is assumed that syncytiotrophoblasts contol these somewhat controversial functions. Both, localization of these multinuclear cells, surrounding the villous tree free floating in maternal blood and their polarized development support this assumption. During placental development the formation of the multinuclear syncytiotrophoblast results from fusion of cytotrophoblasts. These latter cells loose their proliferative activity during the maturation process. Therefore, the fraction of cytotrophoblasts undergoing differentiation decreases with increasing gestational age.1 In vitro experiments using isolated cytotrophoblasts in cell culture showed spontaneous differentiation into syncytiotrophoblasts.2,3
The protective function of placenta against xenobiotics affecting fetal development is thought to be mediated in part by transport proteins which prohibit maternofetal transfer of potentially toxic compounds. Different transport proteins of the ATP Binding Cassette (ABC) superfamily were described to be expressed in placenta.4 These include proteins already known to be involved in drug resistance of tumor cells, like the MDR1 gene product P-glycoprotein (Pgp), the breast cancer resistance protein (BCRP), and several members of the MRP (ABCC) subfamily.5–9 One example is MRP2 (ABCC2), which is known to be involved in the hepatobiliary excretion of conjugated bilirubin and conjugated drugs.10 MRP2 is expressed in the apical membrane of syncytiotrophoblasts.8
Another member of the MRP (ABCC) subfamily, MRP5 (ABCC5), has been shown to be expressed in human placenta by RT-PCR and Western blot analyses.7,11 Aside from its potential role in drug disposition, eg, by transporting nucleoside-based antiviral drugs, MRP5 is of particular interest for signal transduction. MRP5 has been shown to mediate the cellular efflux of 3′,5′-cyclic nucleotides, cAMP, and cGMP.12,13 This export may serve as an alternative mechanism in the control of intracellular cyclic nucleotide levels, in addition to the well-established metabolic degradation by phosphodiesterases. Furthermore, this export may have a paracrine signaling function, since biological effects of extracellular cAMP and cGMP have been reported.14–16 It has been demonstrated, that cGMP enhances trophoblast differentiation.17 This latter effect may be influenced by the expression of MRP5.
We therefore investigated expression, localization, and function of MRP5 in human placenta and isolated cytotrophoblasts. Furthermore, we describe the influence of gestational age and differentiation on the MRP5 expression.
Materials and Methods
Human Samples
Chorionic villous tissues were obtained from women undergoing caesarian section and normal birth with no known medication. A total of 60 samples from pre-term and term placentas were used in the present study following written informed consent. The pre-terms with a termination under 37 weeks gestation were defined as pre-terms according to the World Health Organization definition. The pre-terms were divided into two groups as clinically significant pre-term delivery is before 32 weeks gestation. Placentas were collected within 15 minutes after normal vaginal deliveries and caesarian sections. Pathological samples such as gestational diabetes and pre-eclampsia were excluded. The clinical diagnosis of pre-term deliveries was placental insufficiency and cervical insufficiency. Demographic data of the three groups are summarized in Table 1. Samples for isolation of trophoblasts were taken from term placentas of normal deliveries. Samples for preparation of membrane vesicles were taken from two term and two pre-term (27 and 36 weeks of gestation) placentas of normal delivery.
Table 1.
Summary of Demographic Data on Terms, Late, and Early Pre-Terms of Secondary Trimester
| Terms | Late pre-terms | Early pre-terms | |
|---|---|---|---|
| Mean age of the pregnant | 29.3 ± 5 | 28.0 ± 7 | 27.2 ± 6 |
| Mean of gestational weeks | 39 ± 3 days | 34 ± 5 days | 28 ± 5 days |
| Number of gravidities | 2.0 | 2.1 | 2.4 |
| Number of births before | 1.8 | 1.7 | 2.0 |
| BMI at the beginning of gravidity | 24.5 | 23.2 | 22.1 |
| BMI at the end of gravidity | 39.7 | 27.2 | 24.6 |
| Caesarean sections | 63% | 73% | 78% |
| Number | 30 | 15 | 15 |
BMI, body mass index.
Cytotrophoblast Culture
Cytotrophoblasts were isolated as described previously by Kliman et al.18 Placental cotyledons of vaginal deliveries (n = 3) were prepared, minced, and washed with 0.9% saline. The tissue was then digested three times using trypsin (Sigma-Aldrich Corp., St. Louis, MO) and DNase I (Roche Diagnostics GmbH, Mannheim, Germany) solved in Hanks’ balanced salt solution without Ca2+ and Mg2+ (Gibco-Invitrogen Corporation, Carlsbad, CA) and 25 mmol/L N-2-hydroxyethyl piperazine-N-2-ethane sulfonic acid (HEPES) (pH 7.4). After 20 minutes of incubation in a shaking water bath at 37°C, 140 ml of the cell solution was decanted, filtered through gaze and four layers of mull. Enzymatic activity in the disaggregated supernatant was stopped by centrifugation through 5 ml 85%-fetal calf serum (FCS) (Biochrom AG, Berlin, Germany) for 10 minutes at 1000 × g. The pellet was resolved in 35 ml Dulbecco’s modified Eagle’s medium (DMEM)-25 mmol/L HEPES-DNase. After a further centrifugation at 500 × g for 10 minutes with 10 ml 90% Percoll, the pellet was resolved in cold DMEM-25 mmol/L HEPES. Cytotrophoblasts were separated using density gradient centrifugation at 1500 × g for 45 minutes on discontinuous Percoll (10 to 70%). Cells between the 40% and 50%-Percoll bands were collected, washed with DMEM, and plated onto 35-mm2 culture dishes at a density of 5 × 106 cells/dish. Cell culture experiments were performed at 37°C. The cells were grown in M199 medium (Biochrom AG), supplemented with 10% FCS and 100 units/ml penicillin/streptomycin and 5 ng/ml EGF, in 5% CO2 humidified atmosphere. The cells were maintained in culture for 5 days. Human chorionic gonadotropin (hCG) was determined in the supernatant using a clinical ELISA kit (Abbott Laboratories, Abbott Park, IL).
The purity of the isolated cell population was assessed by staining with antibodies against cytokeratin 18 (Sigma, specific for trophoblasts), vimentin (Alexis Corp., Gruenberg, Germany) and the endothelial marker PECAM-I (Santa Cruz Biotechnology Inc., Santa Cruz, CA). At least 95% of the isolated cells were cytokeratin 18-positive and vimentin- and PECAM-I-negative.
RNA Isolation and Analysis
RNA was isolated from 30 term and 30 premature placentas and three different cytotrophoblast preparations isolations using RNeasy Mini Extraction Kit (Qiagen, Frankfurt, Germany). Villous material of the placenta was separated and stored at −80°C. After mechanical homogenization with a microdismembranator (Braun, Melsungen, Germany) at 2500 rpm for 2 minutes, frozen samples were homogenized in guanidinium thiocyanate containing buffer. The isolation of total RNA was performed using the kit according to the manufacturer’s instructions. The integrity of the RNA was checked by ethidium bromide staining in formaldehyde containing 1% agarose gel.
The isolated RNA was reverse transcribed using random hexamer primer and the TaqMan reverse transcription (RT) kit (Applied Biosystems, Foster City, CA). The resulting cDNA was amplified by real-time PCR with intron-spanning primers and probes for human MRP5. Primer and probe oligonucleotides for PCR were designed based on the cDNA sequence published under GenBank Accession No. NM 005688. Real-time quantitative PCR was performed using forward primer 5′-CACCATCCACGCCTACAATAAA-3′, reverse primer 5′-CACCGCATCGGCACACGTA-3′ and the probe 5′-6FAM-GCTTGGTTGTCATCCAGCAGCTCCTG-XTp detecting both MRP5 and SMRP5, a splicing variant of MRP5.19 For detection of 18S rRNA in the placental and the cytotrophoblastic RNA samples, a pre-developed primer and probe mix was purchased from Applied Biosystems; the probe was VIC labeled. The measurement of MRP5 amount was performed using 15 ng cDNA; 0.15 ng cDNA were used for the detection of 18S rRNA. The universal PCR mastermix of Applied Biosystems was used for amplification of the PCR products and analyzed on a real-time PCR cycler (ABI Prism 7700 Sequence Detector, Applied Biosystems). For quantification of MRP5, a cloned PCR product of MRP5 in the vector pGEM-Teasy (Promega GmbH, Mannheim, Germany) was used as standard. Fluorescence intensities were plotted against PCR cycle numbers. The amplification cycle displaying the first significant increase of the fluorescence signal was defined as threshold cycle (CT). The CT value of each sample was compared to the CT values of the standardization series, which consisted of the cloned MRP5 PCR fragment resulting in a quantification of copy numbers mRNA.
Immunofluorescence Microscopy
The localization of MRP5 was investigated by confocal laser scanning immunofluorescence microscopy, using the polyclonal antibody AMF. This antibody was raised in rabbit against the deduced C-terminal sequence (AMFAAENKVAVKG) of human MRP5 and has been characterized previously.13,20
After delivery, placentas of 40 and 30 weeks of gestational age as samples of term and pre-term placenta (n = 3 each) were frozen in liquid nitrogen and 5-μm sections were cut using a cryotome at −20°C. After drying at room temperature for 2 hours, the sections were fixed in acetone at −20°C and then washed in phosphate-buffered saline (PBS) (pH 7.2). Blocking was performed in 5% FCS (Biochrom AG) in PBS for 45 minutes, followed by incubation with AMF-Serum (diluted 1:50) and the pre-immune serum (diluted 1:50) at room temperature overnight. After three washes with PBS, the sections were incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG or Alexa Fluor 568-conjugated anti-mouse IgG from Molecular Probes (Eugene, OR) for 2 hours.
The fluorescence was detected by confocal laser scanning microscopy. For staining of the basal membrane an antiserum against the organic anion transporting polypeptide B (OATP-B) was used in a concentration of 1:200. This antibody was raised in rabbits against the 15 amino acids at the carboxy terminus of the deduced OATP-B sequence (LLVSGPGKKPEDSRV) coupled to keyhole limpet hemocyanin. Moreover a peptide-competition of the immunfluorescent staining with the AMF-antibody was performed. Fifty μg of AMF-peptide (90 μmol/L) were pre-incubated with the diluted antiserum for 2 hours at room temperature after that the cryosections were processed as described above. For staining of the nuclei in cryosections, propidium iodide was added to the secondary antibody (1:800).
For double-labeling, formalin-fixed, paraffin-embedded samples were used. The slides were deparaffinized in xylene substitute for 10 minutes after which the paraffin sections were hydrated in alcohol at different dilutions for 5 minutes (100%, 98%, 75%, and 50%). After boiling the tissue sections in a citrate puffer (10 mmol/L citric acid, pH 6.0), the slides were rinsed in PBS and blocked with 5% FCS in PBS. The incubation with primary and secondary antibodies was performed as described above. For double-staining, the anti-CD-31 (anti-PECAM-I, Santa Cruz Biotechnology), anti- smooth muscle actin (clone 1A4, DAKO, Hamburg, Germany), and anti-MRP2 (MRP2 II-6, Alexis Corp.) antibodies as well as the monoclonal anti-cytokeratin 18 antibody (Sigma) were used. For staining of the nuclei, TOTO-3 iodide (Molecular Probes) was added to the anti-fading mounting medium in a dilution of 1:2000.
Immunohistochemistry
Isolated cytotrophoblasts were cultured on coverslips using supplemented medium as described above. The differentiating cells were fixed with paraformaldehyde and permeabilized with 0.1% Triton-X-100 after 6 hours, 72 hours, and 144 hours in culture. The cells were then rinsed with water three times. The slides were incubated with 0.3% H202 for quenching endogenous peroxidase activity and washed with PBS. For blocking of non-specific staining the Normal Serum of Vectastain ABC Kit (Vector Laboratories, Burlingame, CA) was used. After a 20-minute incubation, the excess of serum was decanted and the cultured cells were incubated overnight at 4°C with the diluted primary antibody against MRP5 (details above). After washing with PBS, the primary antibodies were labeled using the Vectastain ABC Kit as described by the producer. The peroxidase substrate diaminobenzidine tetrahydrochloride was used for developing the staining. The reaction was stopped after 10 minutes by rinsing the coverslips with tap water. The nuclei were stained using hemalaun. Staining was detected by microscopy.
Preparation of Trophoblastic Vesicles from Human Placental Tissue
Five placentas obtained after normal delivery at term and four placentas of pre-term deliveries were used for preparation of basal and apical vesicles according to methods described previously.21,22 In brief, the placental cotelydones were washed several times with cold PBS, 20 g of the placental tissue were minced and then washed again. After that, the tissue was homogenized in incubation buffer (250 mmol/L sucrose and 10 mmol/L Tris/HCl, pH 7.4) supplemented with protease inhibitors (0.1 mmol/L phenylmethylsulfonyl fluoride, 0.3 μmol/L aprotinin, and 1 μmol/L pepstatin) using a Potter-Elvehjem homogenizer (20 strokes, 1000 rpm). After incubation for 1 hour on ice, the homogenate was centrifuged at 9000 × g for 10 minutes. The supernatants were centrifuged (100,000 × g for 35 minutes) and the pellets were resuspended in incubation buffer and homogenized by 15 strokes with a loose-fitting Dounce B homogenizer. For separation of basal and apical membranes, MgCl2 was added to 10 mmol/L. After a 10-minute incubation on ice, a centrifugation (2200 × g for 12 minutes) was performed, separating the basal (pellet) and apical (supernatant) membranes. The pellets were resuspended in homogenization buffer and the supernatants with the apical membranes were homogenized using a tight-fitting Dounce B homogenizer (30 strokes), washed by centrifugation (100.000 × g for 35 minutes), and homogenized again using the tight-fitting Dounce B homogenizer (30 strokes). After centrifugation (100.000 × g for 35 minutes), the pellets of both membrane fractions were resuspended in 1 ml homogenization buffer and the membrane suspensions were passed 20 times through a 27-gauge needle for vesicle formation. Membrane vesicles were frozen and stored in liquid nitrogen.
Purity and Orientation of Trophoblastic Vesicles
Alkaline phosphatase activity as marker for the apical membrane was assayed according to Pekarthy et al23 and Na+/K+-ATPase activity according to Scharschmidt et al.24 To assess the orientation and integrity of the vesicles, the activity of the ectoenzyme nucleotide pyrophosphatase was measured in the presence and absence of detergent as described before.25
Immunoblot Analysis
Membrane vesicles were loaded onto a 7.5% sodium dodecylsulfate-polyacrylamid gel after incubation in sample buffer at 37°C for 30 minutes. Immunoblotting was performed using a tank blotting system (BioRad, Hercules, CA) and an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Freiburg, Germany). Primary antibodies were diluted in Tris-buffered saline containing 0.05% Tween 20 and 1% bovine serum albumin to the following final concentrations: AMF serum 1:1000, MRP2 II-6 monoclonal anti-MRP2 antibody (Alexis Corp.) 1:1000. Secondary horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG antibodies (BioRad) were used at a 1:2000 dilution. Band intensity was analyzed using KODAK 4.0 IMAGE Software.
Vesicle Transport Studies
To demonstrate MRP5 function, we used the prepared vesicles of basal and apical membrane fraction of placental tissue for measuring the ATP-dependent transport of [8-3H]cGMP (0.3 TBq/mmol; Hartmann Analytic, Braunschweig, Germany). The transport studies were performed using a rapid filtration through nitrocellulose filters as described elsewhere.13 One hundred-μg total vesicular protein were incubated in the presence of 4 mmol/L ATP, 10 mmol/L MgCl2, 10 mmol/L creatin phosphate, 250 mmol/L sucrose, and 4 μmol/L [3H]cGMP in incubation buffer containing 250 mmol/L sucrose and 10 mmol/L Tris/HCl, pH 7.4. At the end the incubation volume was 75 μl. In control incubations ATP was replaced by 5′-AMP. Aliquots of 20 μl were taken at the indicated time, diluted in 1 ml of ice-cold incubation buffer, and filtered immediately through nitrocellulose filters (0.2-μm pore size, pre-soaked in incubation buffer). Filters were rinsed with 5 ml of incubation buffer, dissolved in liquid scintillation fluid, and counted for radioactivity. Rates of net ATP-dependent transport were calculated by subtracting values obtained in the presence of 5′-AMP as a blank from those in the presence of ATP and are given in pmol[3H]cGMP × mg protein−1(1 pmol × mg protein−1 = 667 atomic disintegrations per minute (DPM)).
Statistical Analysis
Statistical analysis was performed using Student’s t-test for two independent groups or analysis of variance for the comparison of three independent groups. P < 0.05 was considered statistically significant.
Results
Detection of MRP5 mRNA in Placental Tissue Samples
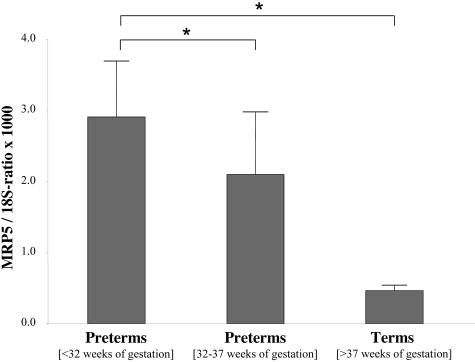
The expression of MRP5 was analyzed by quantitative real-time PCR in 60 samples from pre-term placentas, with a gestational age below 37 weeks, and term placentas. For quantification of MRP5 mRNA, a cloned MRP5 PCR fragment was used and the values were normalized to 18S rRNA. MRP5 mRNA was detectable in all samples with a decreasing mean expression with gestational age (Figure 1). Early pre-term placentas with a gestational age between 23 to 32 weeks showed about a sixfold higher mean amount of the transporter mRNA than term placentas. However, we observed a high inter-individual variability, especially in the pre-term placentas (Figure 1).
Figure 1.
Expression of MRP5 as a function of gestational age. Total RNA isolated from early pre-term (gestational age under 32 weeks of gestation, left column) late pre-term (gestational age from 32 until 37 weeks of gestation, middle column), or term placenta (right column) was reverse transcribed and amplified by real-time PCR using MRP5-specific primers. Data are expressed as MRP5/18S-rRNA ratio (×1000) (mean values ± SEM, early pre-term placentas, n = 15; late pre-term placentas, n = 15; term placentas, n = 30). *, Significant difference P < 0.01, using analysis of variance.
Immunolocalization of MRP5
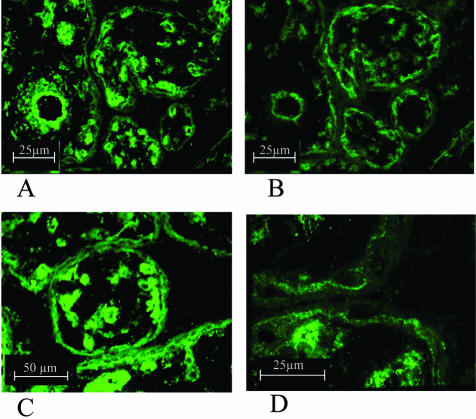
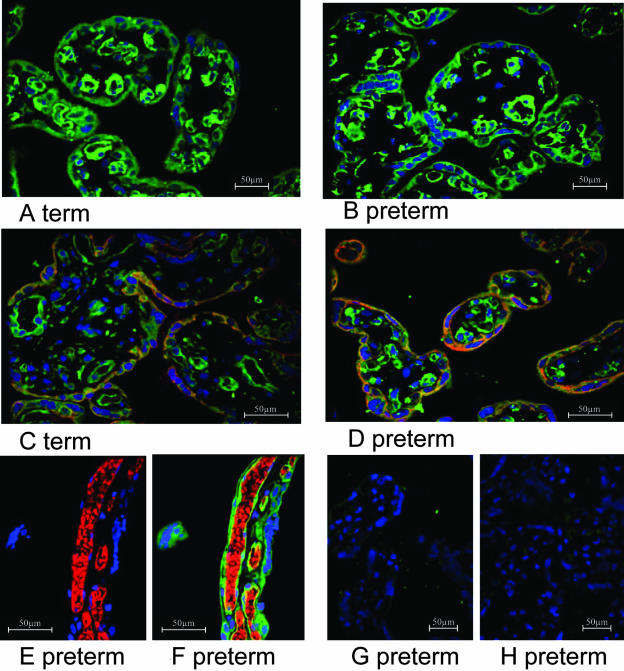
The localization of MRP5 in pre-term and term placentas was visualized by immunofluorescence microscopy using the anti-MRP5 polyclonal antibody AMF, which has been characterized before13,20 (Figures 2 and 3). In pre-term placentas the majority of MRP5 is located to the basal membrane of the syncytiotrophoblasts. Besides, a well-defined signal was detected in the vicinity of fetal blood vessels (Figure 2, A, C, and D). The basal staining of the syncytiotrophoblasts was confirmed by staining following sections of pre-term placenta with an OATP-B antiserum, a transport protein which has been demonstrated to be expressed in the basal membrane of the syncytium in human placenta (Figure 2, A and B).26,27 In term placentas some staining was observed, in the apical membrane of the syncytiotrophoblast in addition to the basal staining (Figure 3, A and C). The localization of MRP5 in the syncytiotrophoblast was confirmed by double-staining with a specific anti-cytokeratin 18 antibody in term (Figure 3C) and pre-term placentas (Figure 3D). The staining of endothelial cells of fetal blood vessels, confirmed by double-staining with the endothelial-specific anti-CD31 antibody (Figure 3, E and F) and an anti-smooth muscle actin antibody (not shown), was not affected by gestational age. Control staining of pre-term placentas with the pre-immune serum and the secondary anti-rabbit antibody only revealed the specificity of the reaction of the AMF antiserum with the above-described placental structures (Figure 3, G and H). Moreover, the fluorescent signal in the syncytiotrophoblast was abolished after pre-incubation of the anti-MRP5 serum with the AMF-peptide (data not shown).
Figure 2.
Immunofluorescence microscopy of cryosections of human pre-term placenta. A, C and D: Detection of MRP5 with the AMF anti-MRP5 antiserum (green fluorescence) in placenta. A–B: Staining of the basal membrane of syncytiotrophoblasts in following cryosections (5 μm) of pre-term placenta with AMF (A) and OATP-B (B) antiserum. C–D: Detection of MRP5 in pre-term placenta indicating the basal localization of this transport protein.
Figure 3.
Immunofluorescence microscopy of human placenta after formalin-fixation and paraffin-embedding. A to F: Detection of MRP5 with the AMF anti-MRP5 antiserum in term (A and C) and pre-term (B and D and E to H) placenta. Double-staining with the anti-cytokeratin 18 antibody (red fluorescence in C and D) indicates the localization of MRP5 in the syncytium. Staining with an endothelial-specific anti-CD31 antibody (red fluorescence in E and F) and the overlay with the MRP5 staining (green in F) show the localization of MRP5 in the vicinity of fetal vessels. G and H: Control staining of pre-term placentas with the pre-immune serum (G) and the secondary anti-rabbit antibody only (H). Counterstaining of the nuclei with TOTO-3 iodide (blue fluorescence in A to H).
Characterization of Trophoblastic Membrane Vesicles
To further investigate the localization and function of MRP5 in the basal or apical membrane of the syncytiotrophoblast, membrane vesicles enriched in basal or apical membranes were prepared from five term placentas and four early pre-term placentas. The vesicles were characterized and found to be enriched for marker enzymes as follows: mean enrichment factors (relative to the homogenate) of Na+/K+-ATPase activity as plasma membrane marker were 17.8 ± 1.1, 27.2 ± 3.1, 6.1 ± 1.2, and 7.2 ± 3.8 in pre-term basal, term basal, pre-term apical, and term apical membranes, respectively (mean values ± SD). Alkaline phosphatase activity was determined as marker for the apical trophoblast membrane, with enrichment factors of 6.0 ± 0.9, 8.4 ± 3.5, 19.4 ± 4.8, and 32.5 ± 8.1 in pre-term basal, term basal, pre-term apical, and term apical membranes, respectively. The sidedness of the membrane vesicles was determined by measurement of nucleotide pyrophosphatase activity in the presence or absence of detergent. The percentage of inside-out vesicles was 40.0 ± 20.1, 37.6 ± 15.1, 33.0 ± 15.2, and 30.8 ± 10.2% in pre-term basal, term basal, pre-term apical, and term apical membranes, respectively.
Immunoblot Analysis of Trophoblastic Membrane Vesicles
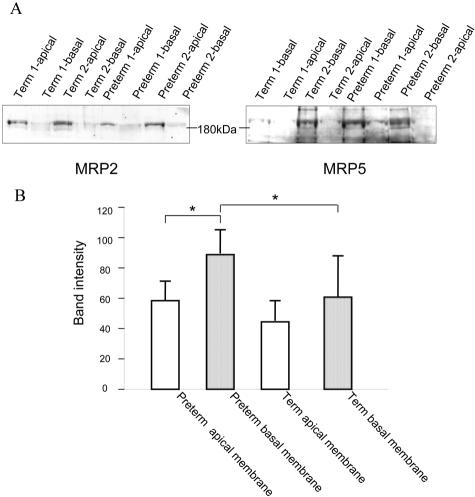
Immunoblots of the vesicle preparations were probed with the MRP5-specific antiserum AMF (Figure 4A, right panel). The 190-kd MRP5 glycoprotein was detected in vesicle preparations from term and pre-term placenta. In addition, a band of about 160 kd was observed in some MRP5-containing membranes including the membranes of MRP5-transfected cells (not shown), representing probably the only core-glycosylated MRP5 or a proteolytic degradation product. The 190-kd bands in the basal membranes from term and pre-term placenta were shifted to about 160 kd after treatment with peptide N-glycosidase F (not shown). The blots were also probed with a monoclonal antibody against MRP2 as marker protein known to be expressed in the apical membrane of the syncytiotrophoblasts.8 In contrast to MRP5, MRP2 was markedly enriched in the apical membrane preparations (Figure 4A, left panel).
Figure 4.
Immunodetection of MRP2 and MRP5 in basal and apical placenta membrane vesicles (75 μg of total protein). The monoclonal anti-MRP2 (MRP2 I-4) antibody (left) and the AMF anti-MRP5 serum (right) were used for staining. B: The quantification of band intensity of Western blot analysis of MRP5 in term and pre-term placentas (n = 3 individuals). The transport protein is expressed in all membrane fractions showing the highest expression in basal membrane fraction of pre-term placentas. *, Significant difference P < 0.05, using Student’s t-test.
In accordance with the immunfluorescence staining, MRP5 was detected in vesicles of the basal membranes and, to a minor extent, of the apical membranes. Quantification of band intensity showed a statistical significant higher expression of MRP5 in the basal membrane fraction of pre-term placentas compared to the apical fraction (t-test, P < 0.05). The combined detection of MRP5 in basal and apical membranes (sum of the band intensities) was also statistically significant higher in pre-term placentas than in term placentas (148.5 ± 29.8 and 106.3 ± 32.7, in pre-term and term placentas, P < 0.05). Furthermore, the ratio of the MRP5 detection in the apical to basal membranes was slightly higher in the term (0.77) compared to pre-term placenta (0.64). However, this difference was not statistically significant (Figure 4B).
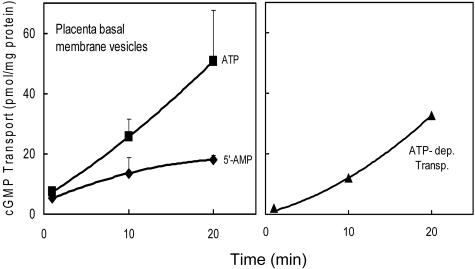
ATP-Dependent Transport of cGMP in Trophoblastic Vesicles
To investigate MRP5 function, ATP-dependent transport of [3H]cGMP, which proceeds into the fraction of inside-out oriented vesicles, was studied using the placenta vesicle preparations. As demonstrated in Figure 5, a significant ATP-dependent cGMP transport, calculated by subtracting the vesicle-associated radioactivity in the presence of 5′-AMP from the values obtained in the presence of ATP, could be detected in the basal membrane preparation. In accordance with the MRP5 immunodetection (Figure 4), the results of cGMP transport measurements in different membranes indicate significantly higher transport rates in the basal membrane preparations compared to the apical (P < 0.001, t-test) (Table 2). A low transport could be detected in the most apical membrane preparations but with no significant difference between term and pre-term placentas. However, we observed a high variation between the tissue samples.
Figure 5.
Basal membrane vesicles (100 μg of protein) from term placenta were incubated with [3H]cGMP (4 μmol/L) in the presence of 4 mmol/L ATP (▪) or 4 mmol/L 5′-AMP (left, ♦) and the vesicle-associated radioactivity was determined as described in the Materials and Methods section (mean values ± SD, from two separate experiments with duplicate determinations). The rate of net ATP-dependent transport (right, ▴) was calculated by subtracting transport in the presence of 5′-AMP as a blank from transport in the presence of ATP.
Table 2.
ATP-Dependent Transport of cGMP in Different Membrane Fractions from Human Placenta
| Membrane fraction | cGMP transport (pmol/mg protein/min) | |
|---|---|---|
| mean | ||
| Pre-term basal 1 | 1.41 ± 0.16* | 2.00 ± 0.80 |
| Pre-term basal 2 | 1.31 ± 0.33* | |
| Pre-term basal 3 | 3.01 ± 0.74* | |
| Pre-term basal 4 | 2.25 ± 0.51* | |
| Term basal 1 | 1.62 ± 0.42* | 1.76 ± 0.71 |
| Term basal 2 | 0.90 ± 0.28* | |
| Term basal 3 | 2.26 ± 0.68* | |
| Term basal 4 | 2.68 ± 0.94* | |
| Term basal 5 | 1.33 ± 0.28* | |
| Pre-term apical 1 | 0.18 ± 0.31 | 0.28 ± 0.17 |
| Pre-term apical 2 | 0.11 ± 0.30 | |
| Pre-term apical 3 | 0.50 ± 0.14** | |
| Pre-term apical 4 | 0.32 ± 0.14** | |
| Term apical 1 | 0.12 ± 0.13 | 0.28 ± 0.14 |
| Term apical 2 | 0.51 ± 0.20** | |
| Term apical 3 | 0.32 ± 0.07** | |
| Term apical 4 | 0.22 ± 0.07** | |
| Term apical 5 | 0.25 ± 0.08** | |
ATP-dependent cGMP transport was measured over 20 minutes as described in the legend to Figure 1. Data represent mean values ± SD from two separate experiments with duplicate determinations (n = 4).
, Significant difference between transport in the presence of 5′-AMP and ATP, P < 0.01 (*), P < 0.05 (**) Student’s t-test.
Expression of MRP5 in Differentiating Cytotrophoblasts
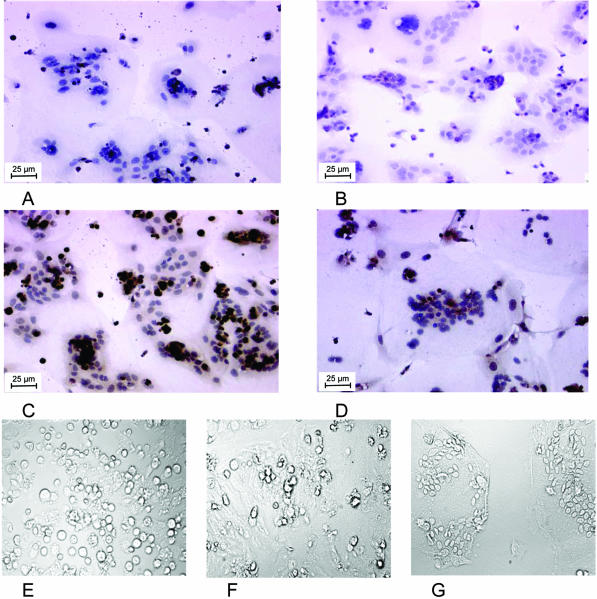
Cytotrophoblasts were isolated and cultured in EGF-containing medium. The cytotrophoblasts showed morphological and biochemical differentiation into a multinuclear syncytium in culture. Morphological differentiation with time in culture was monitored microscopically (Figure 6, F to G). After 1, 3, and 6 days in culture, the differentiated cells were immunohistochemically stained with the AMF antibody (Figure 6). A weak MRP5-staining of the cells was observed after 24 hours in culture, whereas the hemalaun-staining of the nucleus indicated the start of formation of the multinuclear syncytiotrophoblasts. With differentiation, the expression of MRP5 in the multinuclear syncytiotrophoblasts increased, indicated by an enhanced brown staining (Figure 6, B and C). However, with further spreading of the syncytiotrophoblast, the level of MRP5 seemed to decrease (Figure 6D). The AMF-specific staining of fixed cells is located to the cell membrane. A staining of perinuclear structures was also detected in control experiments without the AMF-antibody, indicating this represents a cross-reactivity of the secondary antibody used (Figure 6A).
Figure 6.
Detection of MRP5 in differentiating cytotrophoblasts in culture. B to D: Immunohistochemical detection of MRP5 with the AMF antibody (brown staining) in isolated cytotrophoblasts after one (B), three (C), and six days (D) in culture. The blue staining represents the nuclear staining with hemalaun. A: Control experiment without the AMF-antibody. E to G: Differentiation of the cytotrophoblasts to syncytiotrophoblasts after 1 (E), 3 (F), and 6 days (G) in culture as seen in the light microscopy.
MRP5 Expression in Relation to the hCG Production
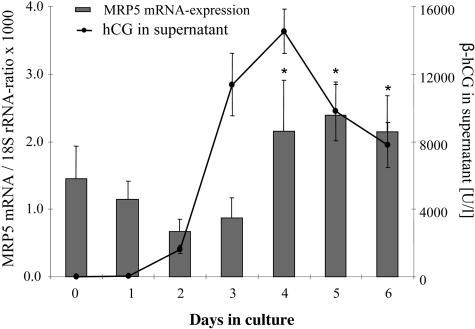
The hCG production of the cultured cytotrophoblasts was assessed in the supernatant, showing maximal concentration (above 15,000 U/L) at the fourth day of culture, decreasing to 50% of maximal concentration during the following days (Figure 7). Total RNA was isolated and reverse transcribed. The MRP5 mRNA amount detected by real-time RT-PCR (normalized to 18S rRNA) showed an increased expression of MRP5 in parallel with the hCG level in the supernatant.
Figure 7.
Correlation of hCG production and MRP5 expression in cytotrophoblasts in culture (means of three different preparations). Human chorionic gonadotropin (hCG) secretion (black line) of the cytotrophoblasts in culture for the period of time was determined as a marker for differentiation as described in the Materials and Methods section. MRP5 expression (columns) was measured by RT real-time PCR as described in the legend to Figure 1. The medium was replaced every 24 hours. *, Statistically significantly higher expression of MRP5 compared to day 0 (t-test; P < 0.05).
Discussion
In this study we demonstrate the expression pattern of MRP5 in human placenta on the mRNA and protein level. An increased amount of MRP5 mRNA was detected in second trimester placentas (divided in early 23 to 32 weeks gestation and late pre-terms 32 to 37 weeks gestation) in comparison with term placentas (Figure 1). Immunofluorescence microscopy with an anti-MRP5 antibody indicated localization of MRP5 preferentially in the basal membrane of syncytiotrophoblasts and in and around fetal vessels (Figure 2 and 3). In term placentas some signal was observed, in addition, in the apical membrane of the syncytiotrophoblast. In accordance, MRP5 was detected by immunoblotting in vesicles of the basal membranes and to a minor extent of the apical membranes (Figure 4). Furthermore, the combined detection of MRP5 protein in basal and apical membranes was also statistically significant higher in pre-term placentas than in term placentas. The ratio of the MRP5 detection in the apical to basal membranes was slightly higher in the term compared to pre-term placenta, however, this difference was not statistically significant. MRP5 function as a cGMP transport protein13 was shown in isolated vesicles of the membrane fractions (Figure 5 and Table 2). Moreover, MRP5 expression was detected during the in vitro differentiation of isolated cytotrophoboblasts, revealing an increase of the MRP5 expression in parallel with the hCG production and formation of the syncytiotrophoblasts (Figures 6 and 7). This increase with differentiation is in line with observations in the trophoblastic BeWo cell line recently published by Pascolo et al.9 In this report also a higher MRP5 mRNA expression was observed in placenta of the first trimester compared to term placenta.
MRP5 has been localized in smooth muscle cells and endothelial cells of blood vessels in the genitourinary system and in heart before.20,28 The expression and localization of this transporter in the membrane of the syncytiotrophoblasts is of particular interest in view of the function of MRP5 as an export pump for cyclic nucleotides, especially cGMP,13,29 thereby potentially either regulating the intracellular concentration of these second messengers or providing extracellular nucleotides for a paracrine action. During pregnancy, cAMP and cGMP excretion in urine rises, cGMP more rapidly and to a higher extent than cAMP.17 Both nucleotides have been shown to play a major role in cytotrophoboblast differentiation.17,30 Addition of 8-Br-cAMP and 8-Br-cGMP to cultured cytotrophoblasts has been shown to promote differentiation through independent pathways, with cGMP especially involved in membrane fusion.17
In addition to the role in cytotrophoblasts differentiation, cGMP plays an important role in the control of fetoplacental vascular tone by decreasing fetal blood pressure.31,32 Moreover, cGMP as the intracellular messenger of NO may regulate placental angiogenesis.33 The rapid fetal development and therefore the increasing metabolic demand in the later stages of pregnancy is managed by a strong increase in fetoplacental and uteroplacental blood flow. On the one hand, an increase of the maternal blood flow to the placenta has been described, beginning at approximately 12 weeks gestation.1 On the other hand, an increase of fetoplacental blood flow by vasodilatation and angioneogenesis has been shown.34,35 In view of the absence of neuronal regulation in placenta, locally produced vasoactive factors may play a critical role. It has been shown that with gestational age the expression of endothelial NO-synthetase in ovine fetoplacental vessels increases.36 In the same study an expression of the epithelial nitric oxide synthase (eNOs) has been described in human umbilical vein endothelial cells. Assuming that the increasing blood flow is mediated by NO-dependent vasodilatation, it may be associated with an increase of cGMP formation. This may trigger enhanced cGMP elimination, to which MRP5 could contribute, as indicated by the pronounced MRP5 staining in the surrounding of fetal vessels.
Aside from the potential role in cGMP signaling, MRP5 as an organic anion export pump may influence the distribution of potential toxic compounds and drugs. MRP5 was shown to confer resistance to nucleobase and nucleoside analogs used in anticancer and antiviral therapy, by cellular export of the intracellularly formed respective nucleoside monophosphate.29,37
In summary, our data indicate that MRP5 expression in placenta is regulated as a function of the organ development. Functional consequences remain to be elucidated.
Acknowledgments
We thank Prof. Dietrich Keppler (Division of Tumor Biochemistry, Deutsches Krebsforschungszentrum, Heidelberg, Germany) for generating the AMF antibody. We also thank Prof. W. Straube (Department of Obstetrics and Gynecology, University of Greifswald, Greifswald,Germany) for his collaboration. Moreover, we thank Dr. Christoph Ritter, Saskia Kuno, and Karen May for helpful discussions and support with the experiments. In addition, we thank Tina Brüggmann and Bärbel Uecker (Department of Pharmacology, University of Greifswald, Greifswald, Germany) for their excellent technical assistance.
Footnotes
Address reprint requests to Heyo K. Kroemer Ph.D., Institut für Pharmakologie, Ernst-Moritz-Arndt-Universität Greifswald, Friedrich-Loeffler-Str. 23d, D-17489 Greifswald, Germany. E-mail: kroemer@uni-greifswald.de.
Supported by the German Federal Ministry for Education and Research (NBL3 program, reference 01 ZZ 0103 to H.K.K.) and by the Karl und Lore Klein-Stiftung, Oy-Mittelberg, Germany (to H.MzS) and the Deutsche Forschungs-gemeinschaft (DFG. LI979/1-1 to K.L).
References
- Kliman HJ. Uteroplacental blood flow: the story of decidualization, menstruation, and trophoblast invasion. Am J Pathol. 2000;157:1759–1768. doi: 10.1016/S0002-9440(10)64813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloxam DL, Bax BE, Bax CM. Culture of syncytiotrophoblast for the study of human placental transfer. Part II: production, culture and use of syncytiotrophoblast. Placenta. 1997;18:99–108. doi: 10.1016/s0143-4004(97)90080-1. [DOI] [PubMed] [Google Scholar]
- Bloxam DL, Bax CM, Bax BE. Culture of syncytiotrophoblast for the study of human placental transfer. Part I: isolation and purification of cytotrophoblast. Placenta. 1997;18:93–98. doi: 10.1016/s0143-4004(97)90079-5. [DOI] [PubMed] [Google Scholar]
- Young AM, Allen CE, Audus KL. Efflux transporters of the human placenta. Adv Drug Deliv Rev. 2003;55:125–132. doi: 10.1016/s0169-409x(02)00174-6. [DOI] [PubMed] [Google Scholar]
- MacFarland A, Abramovich DR, Ewen SW, Pearson CK. Stage-specific distribution of P-glycoprotein in first-trimester and full-term human placenta. Histochem J. 1994;26:417–423. doi: 10.1007/BF00160054. [DOI] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- Pascolo L, Fernetti C, Pirulli D, Crovella S, Amoroso A, Tiribelli C. Effects of maturation on RNA transcription and protein expression of four MRP genes in human placenta and in BeWo cells. Biochem Biophys Res Commun. 2003;303:259–265. doi: 10.1016/s0006-291x(03)00327-9. [DOI] [PubMed] [Google Scholar]
- St. Pierre MV, Serrano MA, MacIas RI, Dubs U, Hoechli M, Lauper U, Meier PJ, Marin JJ. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol. 2000;279:R1495–R1503. doi: 10.1152/ajpregu.2000.279.4.R1495. [DOI] [PubMed] [Google Scholar]
- Pascolo L, Fernetti C, Garcia-Mediavilla MV, Ostrow JD, Tiribelli C. Mechanisms for the transport of unconjugated bilirubin in human trophoblastic BeWo cells. FEBS Lett. 2001;495:94–99. doi: 10.1016/s0014-5793(01)02357-2. [DOI] [PubMed] [Google Scholar]
- Koenig J, Nies A, Cui Y, Keppler D. MRP2, the apical pump for anionic conjugates. Holland IB, Cole SPC, Kuchler K, Higgins CF, editors. London: Academic Press,; ABC ProteinsFrom Bacteria to Man. 2003:pp 423–443. [Google Scholar]
- Belinsky MG, Bain LJ, Balsara BB, Testa JR, Kruh GD. Characterization of MOAT-C and MOAT-D, new members of the MRP/cMOAT subfamily of transporter proteins. J Natl Cancer Inst. 1998;90:1735–1741. doi: 10.1093/jnci/90.22.1735. [DOI] [PubMed] [Google Scholar]
- Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem. 2003;278:17664–17671. doi: 10.1074/jbc.M212723200. [DOI] [PubMed] [Google Scholar]
- Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275:30069–30074. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- Poulopoulou C, Nowak LM. Extracellular 3′,5′ cyclic guanosine monophosphate inhibits kainate-activated responses in cultured mouse cerebellar neurons. J Pharmacol Exp Ther. 1998;286:99–109. [PubMed] [Google Scholar]
- Touyz RM, Picard S, Schiffrin EL, Deschepper CF. Cyclic GMP inhibits a pharmacologically distinct Na+/H+ exchanger variant in cultured rat astrocytes via an extracellular site of action. J Neurochem. 1997;68:1451–1461. doi: 10.1046/j.1471-4159.1997.68041451.x. [DOI] [PubMed] [Google Scholar]
- Roelofsen H, Soroka CJ, Keppler D, Boyer JL. Cyclic AMP stimulates sorting of the canalicular organic anion transporter (Mrp2/cMoat) to the apical domain in hepatocyte couplets. J Cell Sci. 1998;111:1137–1145. doi: 10.1242/jcs.111.8.1137. [DOI] [PubMed] [Google Scholar]
- Sawai K, Azuma C, Koyama M, Hashimoto K, Kimura T, Samejima Y, Nobunaga T, Takemura M, Saji F. The novel role of 3′,5′-guanosine monophosphate (cGMP) on the differentiation of trophoblasts: comparison with the effects of 3′,5′-adenosine monophosphate (cAMP). Early Pregnancy. 1996;2:244–252. [PubMed] [Google Scholar]
- Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., III Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Nishio K, Sasaki H, Kurokawa H, Saito-Ohara F, Ikeuchi T, Tanabe S, Terada M, Saijo N. cDNA cloning of a short type of multidrug resistance protein homologue, SMRP, from a human lung cancer cell line. Biochem Biophys Res Commun. 1997;238:790–794. doi: 10.1006/bbrc.1997.7346. [DOI] [PubMed] [Google Scholar]
- Nies AT, Spring H, Thon WF, Keppler D, Jedlitschky G. Immunolocalization of multidrug resistance protein 5 in the human genitourinary system. J Urol. 2002;167:2271–2275. [PubMed] [Google Scholar]
- Kelley LK, Smith CH, King BF. Isolation and partial characterization of the basal cell membrane of human placental trophoblast. Biochim Biophys Acta. 1983;734:91–98. doi: 10.1016/0005-2736(83)90079-2. [DOI] [PubMed] [Google Scholar]
- Booth AG, Olaniyan RO, Vanderpuye OA. An improved method for the preparation of human placental syncytiotrophoblast microvilli. Placenta. 1980;1:327–336. doi: 10.1016/s0143-4004(80)80034-8. [DOI] [PubMed] [Google Scholar]
- Pekarthy JM, Short J, Lansing AI, Lieberman I. Function and control of liver alkaline phosphatase. J Biol Chem. 1972;247:1767–1774. [PubMed] [Google Scholar]
- Scharschmidt BF, Keeffe EB, Blankenship NM, Ockner RK. Validation of a recording spectrophotometric method for measurement of membrane-associated Mg- and NaK-ATPase activity. J Lab Clin Med. 1979;93:790–799. [PubMed] [Google Scholar]
- Bohme M, Muller M, Leier I, Jedlitschky G, Keppler D. Cholestasis caused by inhibition of the adenosine triphosphate-dependent bile salt transport in rat liver. Gastroenterology. 1994;107:255–265. doi: 10.1016/0016-5085(94)90084-1. [DOI] [PubMed] [Google Scholar]
- St Pierre MV, Hagenbuch B, Ugele B, Meier PJ, Stallmach T. Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J Clin Endocrinol Metab. 2002;87:1856–1863. doi: 10.1210/jcem.87.4.8431. [DOI] [PubMed] [Google Scholar]
- Ugele B, St Pierre MV, Pihusch M, Bahn A, Hantschmann P. Characterization and identification of steroid sulfate transporters of human placenta. Am J Physiol. 2003;284:E390–E398. doi: 10.1152/ajpendo.00257.2002. [DOI] [PubMed] [Google Scholar]
- Dazert P, Meissner K, Vogelgesang S, Heydrich B, Eckel L, Bohm M, Warzok R, Kerb R, Brinkmann U, Schaeffeler E, Schwab M, Cascorbi I, Jedlitschky G, Kroemer HK. Expression and localization of the multidrug resistance protein 5 (MRP5/ABCC5), a cellular export pump for cyclic nucleotides, in human heart. Am J Pathol. 2003;163:1567–1577. doi: 10.1016/S0002-9440(10)63513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, Beijnen JH, Scheper RJ, Hatse S, De Clercq E, Balzarini J, Borst P. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci USA. 2000;97:7476–7481. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinman MA, Kliman HJ, Caltabiano S, Strauss JF., III 8-Bromo-3′,5′-adenosine monophosphate stimulates the endocrine activity of human cytotrophoblasts in culture. J Clin Endocrinol Metab. 1986;63:1211–1217. doi: 10.1210/jcem-63-5-1211. [DOI] [PubMed] [Google Scholar]
- Clifton VL, Read MA, Leitch IM, Giles WB, Boura AL, Robinson PJ, Smith R. Corticotropin-releasing hormone-induced vasodilatation in the human fetal-placental circulation: involvement of the nitric oxide-cyclic guanosine 3′,5′-monophosphate-mediated pathway. J Clin Endocrinol Metab. 1995;80:2888–2893. doi: 10.1210/jcem.80.10.7559870. [DOI] [PubMed] [Google Scholar]
- Bainbridge SA, Farley AE, McLaughlin BE, Graham CH, Marks GS, Nakatsu K, Brien JF, Smith GN. Carbon monoxide decreases perfusion pressure in isolated human placenta. Placenta. 2002;23:563–569. doi: 10.1053/plac.2002.0845. [DOI] [PubMed] [Google Scholar]
- Yamahara K, Itoh H, Chun TH, Ogawa Y, Yamashita J, Sawada N, Fukunaga Y, Sone M, Yurugi-Kobayashi T, Miyashita K, Tsujimoto H, Kook H, Feil R, Garbers DL, Hofmann F, Nakao K. Significance and therapeutic potential of the natriuretic peptides/cGMP/cGMP-dependent protein kinase pathway in vascular regeneration. Proc Natl Acad Sci USA. 2003;100:3404–3409. doi: 10.1073/pnas.0538059100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Vagnoni KE, Bird IM, Magness RR. Expression of basic fibroblast growth factor, endothelial mitogenic activity, and angiotensin II type-1 receptors in the ovine placenta during the third trimester of pregnancy. Biol Reprod. 1997;56:1189–1197. doi: 10.1095/biolreprod56.5.1189. [DOI] [PubMed] [Google Scholar]
- Myatt L. Control of vascular resistance in the human placenta. Placenta. 1992;13:329–341. doi: 10.1016/0143-4004(92)90057-z. [DOI] [PubMed] [Google Scholar]
- Zheng J, Li Y, Weiss AR, Bird IM, Magness RR. Expression of endothelial and inducible nitric oxide synthases and nitric oxide production in ovine placental and uterine tissues during late pregnancy. Placenta. 2000;21:516–524. doi: 10.1053/plac.1999.0504. [DOI] [PubMed] [Google Scholar]
- Wielinga PR, Reid G, Challa EE, van dH I, van Deemter L, de Haas M, Mol C, Kuil AJ, Groeneveld E, Schuetz JD, Brouwer C, De Abreu RA, Wijnholds J, Beijnen JH, Borst P. Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Mol Pharmacol. 2002;62:1321–1331. doi: 10.1124/mol.62.6.1321. [DOI] [PubMed] [Google Scholar]