Abstract
Cardiac ankyrin repeat protein (CARP) was identified by subtractive hybridization as one of a group of genes that are rapidly modulated by acute wounding of mouse skin. Quantitative RT-PCR showed that CARP was strongly induced during the first day after wounding (157.1-fold), and the high level persisted for up to 14 days. Immunohistochemistry and in situ hybridization revealed that CARP was expressed in skeletal muscle, vessel wall, hair follicle, inflammatory cells, and epidermis in the wound area. To examine the effects of CARP on wound healing, we developed an adenoviral CARP vector to treat subcutaneously implanted sponges in either rats or Flk-1LacZ knock-in mice. Four days after infection, CARP-infected sponges in rats showed a remarkable increase in the vascular component in granulation tissue as compared to Ad-LacZ controls. This result was confirmed by CD34 immunostaining. By 7 days post-infection of sponge implants in Flk-1LacZ knock-in mice, granulation tissue showed many more LacZ-positive cells in Ad-CARP-infected sponges than in virus controls. Ad-CARP treatment also induced neovascularization and increased blood perfusion in rabbit excisional wounds in and ischemic rat wounds. These findings indicate that CARP could play a unique role in therapeutic angiogenesis during wound healing.
Angiogenesis is a critical component of several processes after birth, including endometrial proliferation, wound healing, and tumor growth. After wounding, the formation of a rich vascular bed is essential for optimal repair.1 Pre-clinical studies document that angiogenic growth factors, such as VEGF, FGF-1, FGF-2, TGF-β, hepatocyte growth factor (HGF), angiopoietin-related growth factor (AGF), and placenta growth factor (PIGF) can promote vessel growth in vivo and microvascular endothelial cell morphogenesis in vitro.2 Neovascularization is a highly organized sequence of events that requires the correct spatial and temporal expression of specific sets of genes leading to the development of a vascular network. Transcription factors have been shown to serve as master switches for regulating a number of developmental processes,3 and they undoubtedly are involved in angiogenesis.
Cardiac ankyrin repeat protein (CARP) was originally identified in 1995 as a cytokine-inducible transciptional regulator in cultured human endothelial cells.4 Subsequently, three laboratories independently isolated rat CARP in 1997.5–7 Northern blot analyses indicated that CARP was highly enriched in the adult heart and was detectable in skeletal muscle, lung, and placenta.6 The expression of CARP mRNA was also evident in several cell types including primary myocytes, rabbit aortic smooth muscle cells (C2/2), human umbilical vein endothelial cells (HUVEC), and bovine pulmonary artery endothelial cells (PAE).4,7,8 CARP promoter-LacZ transgenic mice displayed cardiac-specific expression in the early embryo.9 CARP was found to be a cardiac-restricted target for doxorubicin5 and a marker of cardiac hypertrophy.10 These data strongly suggest that CARP could play a role in cardiogenesis.5,8 It was recently reported that CARP is a TGF-β target gene in vascular smooth muscle cells,8 and it is involved in arteriogenesis induced in rabbits and mice.11 While these reports imply that CARP may also be involved in angiogenesis, there has been no direct evidence that CARP could induce or promote neovascularization.
Using subtractive hybridization between intact and wounded skin in mice, our preliminary data revealed that CARP was up-regulated during the skin repair process. To define the effect of CARP in wound healing, we developed and applied CARP adenovirus in several wound-healing animal models. Our data consistently showed that overexpression of CARP can induce neovascularization during wound repair.
Materials and Methods
Studies were carried out in the American Association for the Accreditation of Laboratory Animal Care approved facilities of Vanderbilt University, Nashville, Tennessee, under approvals from the local Subcommittee on Animal Care. All protocols met the approval of both the Institutional Animal Care and Use Committee at Vanderbilt University School of Medicine. In additional studies, mice were housed and fed according to German Federal guidelines under procedures approved by the local government of Bavaria.
Animal Models
Excisional Wound Models
Normal male BALB/c mice (8 to 10 weeks; Charles River Wiga, Sulzfeld, Germany) were used to generate four 5-mm full-thickness excisional wounds on the back of each animal. At different time points (from 1 hour to 14 days), mice were sacrificed and the complete wounds, including 2 mm of the wound margins, were harvested.
Gene Therapy Animal Models for Wound Healing
Male rats (400 g, Harlan Sprague-Dawley, Indianapolis, IN) and Flk-1LacZ knock-in mice (Jackson Laboratories, Bar Harbor, ME) were implanted subcutaneously with PVA (polyvinyl alcohol) sponges (Unipoint Laboratories, High Point, NC; 1 cm diameter, 2 mm thick). Three days after implantation of sponges in rats, 1 × 109 plaque-forming unit (PFU) of Ad-CARP or Ad-LacZ in 50 μl of HBS vehicle (20 mmol/L HEPES, 150 mmol/L NaCl, pH 7.8) were injected into sponges using a 0.33-ml insulin syringe with 28G#1/2“ needle (Becton Dickinson, Franklin Lakes, NJ). For Flk-1LacZ transgenic mice, 1 × 108 PFU of Ad-CARP or Ad-Luc-IRES-GFP in 50 μl HBS were percutaneously injected into the sponges 7 days post-implantation. At 7 days (rat) or 14 days (mouse) after implantation, the animals were sacrificed, and sponge samples were removed for histological analyses.
New Zealand White rabbits (10 to 12 weeks, Harlan Sprague-Dawley) were used to create four 6-mm wounds on the ventral surface of each ear with vascular punches (Boss Instruments, Ltd, Nashville, TN), which can prevent cartilage from damage by cutting upwards through the skin. Briefly, using 2% lidocaine with epinephrine (Astra USA, Inc., Westborough, MA) in a tuberculin syringe (Becton Dickinson, Franklin Lakes, NJ) with a small bore needle, a wheal on the inside surface of the rabbit ear was created. A spear-pointed scalpel with #11 blade (Feather Safety Razor Co. Ltd., Japan) was inserted into the wheal with the blade facing upward to create an incision across the diameter of the wheal of sufficient length to accommodate the 6-mm vascular punch. The thin side of a Freer elevator (Roboz Surgical Instrument, Rockville, MA) was inserted into the incision and swept into one-half of the wheal to undermine the skin and make enough space to accommodate one-half of the discoid punch blade. The half excision was made by cutting upwards with the vascular punch (see Figure 6H). The second half excision was made by the same procedure. Fifty μl of adenovirus suspension (1 × 108 PFU) was intradermally injected into the wound margins. OpSite dressing (Smith & Nephew, Largo, FL) was applied to each wound site, and the dressing was changed every 3 days. Laser Doppler perfusion imaging (Perimed Inc., North Royalton, OH) and digital photography were performed every 3 days. At 14 days post-surgery, the rabbits were sacrificed, and the wound samples were collected and subjected to histological analysis.
Figure 6.
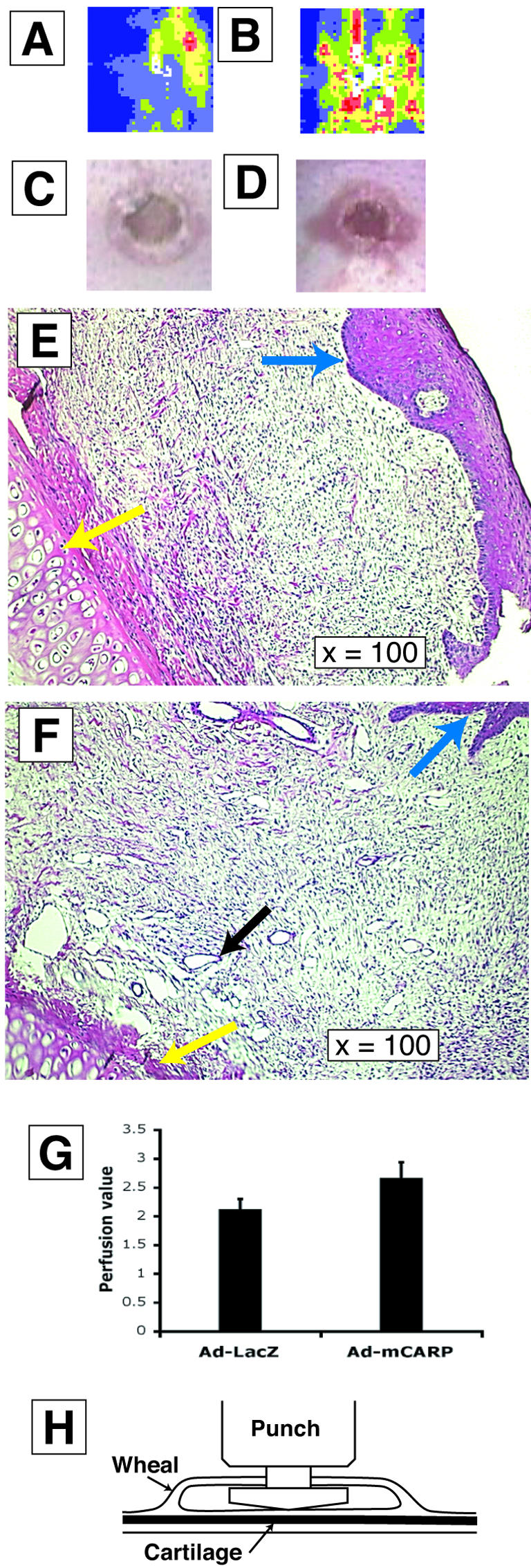
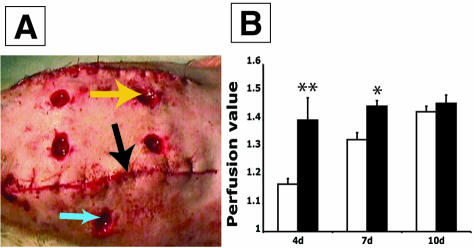
CARP-induced neovascularization in rabbit ear excisional wounds. Comparison of laser Doppler perfusion imaging (A–B), photography (C–D), and histology (E–F) is illustrated. A, C, and E represent wounds treated with Ad-LacZ and B, D, and F represent Ad-CARP-injected wounds. Yellow arrowheads indicate ear cartilage and blue arrowheads indicate epidermis (E). Black arrowheads indicate vessels (F). G: Compares the blood perfusion values of Ad-CARP- and Ad-LacZ-infected excisional wounds in rabbit ears (paired t-test, *, P < 0.05, n = 8). H: Schematic illustration of rabbit ear excisional wound model. The vascular punch was inserted through an incision made in a raised wheal formed by subdermal injection of a lidocaine:epinephirine solution, and the wound was made by cutting upward to prevent cartilage damage.
Male rats (400 g, Harlan Sprague-Dawley) were used to generate an ischemic wound model. Briefly, two 10-cm long, full-thickness, parallel incisions (4 cm apart) were made on the back to reduce the blood supply of the area bounded by the two incisions. The anchored flap was freed from underlying connective tissue by blunt dissection. Four full-thickness excisions were made in the central area with a 6-mm biopsy punch as well as two non-ischemic excisions in the area outside of the ischemic zone as control (see Figure 7A). Either 50 μl of Ad-CARP or Ad-Luc-GFP (1 × 109 PFU) were injected around the wound margins at the completion of surgery. Laser Doppler perfusion imaging and digital photography were performed on anesthetized animals at days 4, 7, and 10 post-surgery.
Figure 7.
CARP response in ischemic rat skin wounds. A: Illustration of surgical model. Yellow arrow indicates an excisional wound in an ischemic zone. Black arrow indicates incision dividing the ischemic and normal areas. Blue arrow marks a wound in non-ischemic area. B: Comparison of blood perfusion values between Ad-CARP- and Ad-Luc-GFP- infected ischemic wounds in rats. (paired t-test, **, P < 0.005 at d4 and *, P < 0.05 at d7, n = 20).
RNA Preparation and Real Time PCR Analysis
For preparation of RNA, the tissue from four mice was combined, immediately frozen in liquid N2, and stored at −80°C until used for RNA isolation. After homogenization of biopsies, total RNA was prepared by a modification of the method of Chomczynski and Sacchi12 to include a DNase I digestion step.
For cDNA synthesis, MultiScribe reverse transcriptase and random hexamers (Applied Biosystems, Foster City, CA) were used according to the manufacturer’s instructions. Specific cDNA transcripts were subsequently measured by means of quantitative RT-PCR using a SYBR green PCR master mix (Applied Biosystems). The quantification was carried out relative to GAPDH. Murine CARP specific primers were: forward: 5′-ACA GCG CGT GTC CTT TTG AT-3′; reverse: 5′-GCC AAG GAA AAC TGA GTT CGA-3′. GAPDH specific primers were: forward: 5′-ATC AAC GGG AAG CCC ATC A-3′ and reverse: 5′-GAC ATA CTC AGC ACC GGC CT-3′. Each PCR reaction was measured in triplicate.
Cloning
The full-length cDNA of mCARP was PCR amplified from a murine d1 wound first-strand cDNA, using the forward primer 5′-ATGATGGTACTGAGAGTAGAG-3′ and the reverse primer 5′-GAATGTAGCTATGCGAGAG-3′. The PCR reaction product was subsequently cloned into KpnI and PmlI sites of pMHint plasmid (derivative of the pMH plasmid, Boehringer Mannheim), generating the pMHint-CARP construct.
In Situ Hybridization
Tissue samples were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS), embedded in RNase-free paraffin, and sectioned. Deparaffinized tissue sections were successively treated with 0.2 mol/L HCl, 20 μg/ml proteinase K in TE buffer (50 mmol/L Tris-HCl, pH 8.5, 5 mmol/L EDTA) and 0.5% acetic anhydride for 10 to 20 minutes. Murine CARP cDNA was subcloned into pBluscript II-SK− in both sense and anti-sense orientations, and hybridization probes were prepared by labeling with digoxigenin-UTP using an in vitro transcription kit with T7 RNA polymerase (DIG RNA labeling kit, Roche, Germany) according to the manufacturer’s protocol. Incubation was performed with labeled probes in hybridization buffer (2X SSC, 2% blocking solution in kit, 0.02% SDS, 10% dextran sulfate, and 50% formamide) in a humid chamber for 4 to 6 hours at 55°C. After washing, the hybridization signal was detected with the DIG nucleic acid detection kit (Roche, Germany) using an anti-digoxigenin alkaline phosphatase conjugated antibody and NBT/BCIP color development system according to the manufacturer’s protocol. Tissue sections were counterstained with 0.02% fast green (Sigma, St. Louis, MO) for 1 to 2 minutes and mounted under coverslips with glycerol.
Adenovirus Construction
The KpnI/PmlI DNA fragment containing full-length murine CARP cDNA was cut from pMHint-CARP and subcloned into the pShuttle-CMV vector (Quantum Biotechnologies, Montreal, Canada). The resulting plasmid was co-transformed into BJ5381 cells with an Ad-Easy-1 adenoviral backbone DNA that is E1- and E3-deleted and is replication-deficient. The recombinant adenoviral construct was transfected into 293A cells to produce viral particles. To generate a control/reporter virus, an XbaI/SmaI DNA fragment containing an internal ribosome entry site (IRES) and GFP was isolated from pIRES-EGFP (Clontech, Palo Alto, CA). An XbaI/XhoI luciferase DNA fragment was cut from pGL-Basic (Promega, Madison, WI) and separately subcloned into the pShuttle-CMV vector. An Ad-Luc-IRES-GFP viral particle was generated by the recombination procedure described above. Adenovirus containing the β-galactosidase transgene (Ad-LacZ) was purchased from Quantum Biotechnologies, using the same adenoviral backbone DNA as above. After amplification and purification of Ad-CARP, Ad-Luc-IRES-GFP, and Ad-LacZ, the viral particle numbers were determined by absorption at 260 nm, and the PFU titers were detected by overlaying infected 293A cells with 1.25% SeaPlaque agarose (FMC Bioproducts, Rockland, ME) following the protocol from Quantum Biotechnologies. The final particle/PFU ratio ranged from 200:1 to 500:1.
Histology
Samples were harvested from wounds at indicated time points and fixed in 4% paraformaldehyde in PBS at least overnight, dehydrated, and embedded in paraffin. Serial 5-μm sections were obtained from the paraffin-embedded wounds and subjected to routine hematoxylin and eosin staining.
For LacZ staining, sponge samples from Flk-1lacZ mice were fixed in 2% formaldehyde/0.2% glutaraldehyde for 2 hours on ice, washed with PBS and permeabilized with 0.02% NP-40 in PBS for 15 minutes at room temperature. The samples were incubated in staining solution (2 mmol/L MgCl2, 5 mmol/L potassium ferricyanide, 5 mmol/L potassium ferrocyanide, 0.01% deoxycholic acid, 0.02% NP-40, and 1 mg/ml X-gal/DMSO in PBS) at 37°C overnight. After staining, the samples were washed with PBS and post-fixed in above fixing solution for 1 to 3 hours at 4°C. These samples were then dehydrated and embedded in paraffin. Five-μm sections were deparaffinized with xylene and counterstained with eosin.
Generation of CARP Antibody, Western Blot, and Immunostaining
An N-terminal 300-bp fragment of CARP cDNA (bases 31–349) was amplified by PCR and subcloned into pGEX-3X vector (Amersham Biosciences, Arlington Heights, IL) at the BamH1 restriction site. A GST-CARP fusion protein was generated according to the manufacturer’s instructions. To produce CARP polyclonal rabbit antisera, 2.0 mg of purified GST-CARP in PBS was injected intradermally into rabbits after emulsification in an equal volume of complete Freund’s adjuvant (Sigma) emulsion, followed 2 weeks later by a GST-CARP emulsion in incomplete Freund’s adjuvant (Sigma). Anti-CARP antisera were harvested 10 to 20 days after the second injection. To purify CARP antibody by affinity chromatography, either affinity-purified GST or GST-CARP protein was conjugated to CNBr-activated Sepharose 4B beads (Amersham Biosciences). CARP antisera were applied to the beads. After washing the beads with sodium acetate buffer (0.1 mol/L HOAc, 0.5 mol/L NaCl), anti-CARP IgG was eluted from the GST-CARP column with 0.15 mol/L glycine (pH 2.5). Anti-GST antibodies were removed by further passage of the eluate over a GST-Sepharose column. The antibody specificity and titer was confirmed by Western blot analysis using a protein extract from mouse heart (data not shown).
For Western blot analysis, whole cell extraction was lysated with RIPA buffer (10 mmol/L Tris-HCl, pH 8.0; 0.14 mol/L NaCl; 0.025% NaN3; 0.5% Triton X-100; 0.1% SDS and 1% sodium deoxycholate). Fifty μg of sample was separated in 10% SDS-PAGE and blotted with anti-CARP antibody (1:1000) at 4°C overnight, and anti-rabbit IgG (1:5000, Vector Laboratories, Burlingame, CA) at room temperature (RT) for 30 minutes.
Normal skin and excisional wounds from mice were fixed in 4% paraformaldehyde in PBS and embedded in paraffin. Tissue sections were blocked with 5% goat serum (Santa Cruz Biotechnology, Santa Cruz, CA) for 60 minutes at RT and incubated with anti-CARP antibody (1:100) at 4°C overnight. After washing with PBS, slides were incubated with peroxidase-conjugated anti-rabbit IgG (1:250) for 30 minutes at room temperature. Finally, the tissue sections were developed with a DAB kit (Vector Laboratories) and counterstained with hematoxylin. CD34 immunostaining was performed on deparaffinized tissue sections of sponge implants. Slides were pretreated in 0.1 mol/L citrate buffer (pH 6.0) in a microwave oven for 4 × 5 minutes. After blocking with 5% donkey serum, CD34 antibody (1: 100, Santa Cruz Biotechnology) was applied to slides at 4°C overnight. The same detection procedure for anti-CD34 was performed.
Laser Doppler Perfusion Imaging
The blood flow in excisional wounds on rabbits’ ears and rats’ dorsum was mapped with a high resolution PIM-2 Laser Doppler perfusion imager (Perimed Inc., North Royalton, OH). The parameter settings during the measurement were: scanning area, 20 × 20 mm; high resolution; distance between the scanner head and wound, 17.8 cm. The measurement of the image and perfusion value was carried out by the LDISOFT software package.
Electric Cell-Substrate Impedance Sensing (ECIS) Assay and Cell Survival Assay
ECIS assay was performed according to published procedures.13 To study endothelial cell behavior during wound healing in vitro, HUVEC were infected with either Ad-CARP or Ad-LacZ (MOI = 10:1) for 2 days. 4 × 104 cells were grown in the single electrode array (Applied BioPhysics, Troy, NY) containing gold film surface electrodes and were serum-starved in M199 medium (Gibco BRL, Gaithersburg, MD) containing 0.5% fetal bovine serum (FBS) overnight. The impedance in normal mode was recorded in the ECIS Model 100 (Applied BioPhysics) with an approximate constant current of 1μA at 4 kHz for 2 hours between the small measuring electrode (250 μm diameter) and a large counter electrode. To kill the cells that covered in the measuring electrode, a pulse of 3 volts at 40kHz was applied for 8 seconds. ECIS was returned to the normal mode for 15 hours to allow the cells re-migrate to the measuring electrode. To perform the survival study, 1 × 104 HUVEC after adenovirus infection were grown in 96-well plates in M199 medium containing 0.5% FBS for 1 to 3 days. At different time points, culture medium was aspirated from each well and cells were washed twice with 100 μl of PBS. One hundred μl of 0.02% SDS diluted in 1X SSC was added and incubated at 37°C for 1 hour. One hundred μl of PicoGreen reagent (Molecular Probes, Eugene, OR) diluted in 1X TE (10 mmol/L Tris-HCl, 1 mmol/L EDTA, pH 7.5) was applied to each well, and the fluorescence was read with CytoFluor II spectrofluorometer (excitation 480 nm; emission 520 nm; PerSeptive Biosystems, Framingham, MA). The total DNA was calculated from a DNA standard curve.
Statistical Analysis
Response of control and treated wounds in each animal were compared using the Student’s paired t-test.
Results
CARP mRNA and Protein Are Sharply Up-Regulated in the Excisional Wound
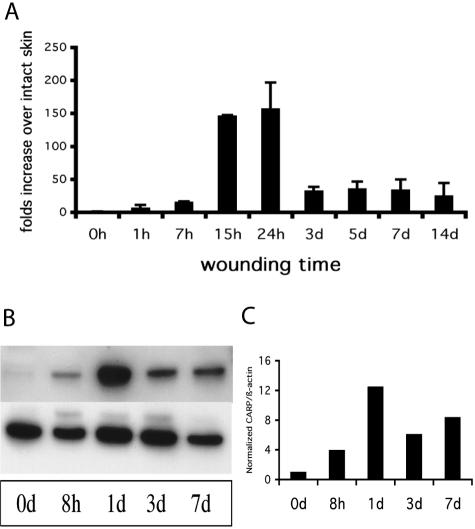
Using a subtractive hybridization approach, gene expression differences between murine wounds and intact skin were identified with cDNA libraries that had been constructed from intact and wounded murine skin to identify the genes that were either up- or down-regulated during wounding. This strategy identified CARP among a number of other genes to be strongly up-regulated within 1 day after wounding (data not shown). A time-course study using real time quantitative RT-PCR showed that CARP transcription began increasing within 1 hour after wounding, and it reached a peak at 24 hours after wounding (157.1-fold, Figure 1A). From 3 days to 14 days after wounding, the expression of CARP remained elevated at 24- to 35-fold over levels in intact skin. To confirm this result at the protein level, mouse excisional wound tissue was isolated at several time points. Whole tissue lysates were subjected to Western blot analysis to detect CARP protein levels using a polyclonal antibody we generated as described. Figure 1B shows that the abundance of CARP protein was consistent with the CARP transcript abundance pattern during the healing of mouse excisional wounds, although the relative CARP content in d1 wounds was not as dramatically different from intact skin as the mRNA data (Figure 1C).
Figure 1.
Comparison of CARP transcription and translation levels between intact skin and excisional wounds in mice by real-time RT-PCR and Western blot. A: Time course of CARP mRNA levels in normal mice after wounding. Real-time, quantitative RT-PCR data showed that CARP transcripts were dramatically up-regulated during the first day after wounding and maintained increased expression by 25- to 35-fold from 3 days to 14 days. Each PCR reaction was measured in triplicate. B: Western blot analysis of CARP protein abundance during wounding. Fifty μg of lysate protein from mouse skin was taken at the indicated time points after excisional wounding and fractionated by SDS-PAGE. Top, signal detected with an anti-mouse CARP antibody; bottom, signal from an anti-β-actin antibody. C: Band intensities of the CARP signal assessed by densitometry after normalization for loading with β-actin values.
Localization of CARP Expression in Mouse Skin Wounds
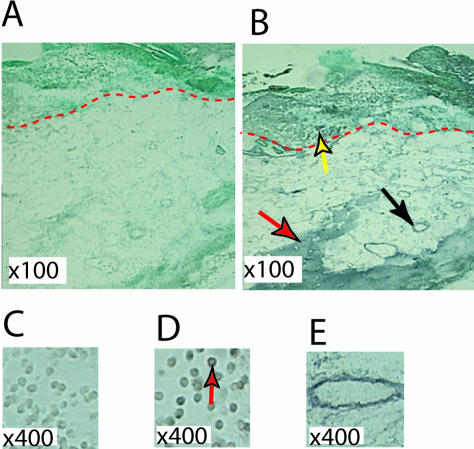
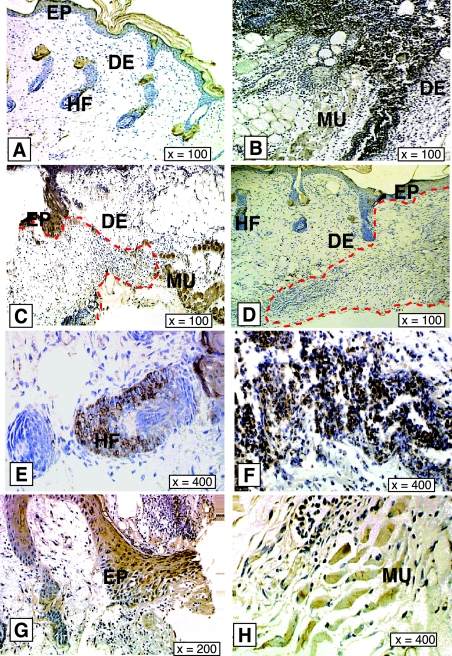
One-day mouse wounds were harvested and subjected to in situ hybridization as described. A strong CARP mRNA signal appeared in subcutaneous skeletal muscle and around vessel walls in the dermis (Figure 2, B and E). We also observed some CARP mRNA signal both in keratinocytes and inflammatory cells close to the wound area (Figure 2, B and D). To confirm these findings at the protein level, we performed immunostaining on wound sections at several time points. In intact skin, CARP immunoreactivity was only detected in the panniculus carnosus and in hair follicles (Figure 3, A, E, and H). One day after wounding CARP was expressed mainly in inflammatory cells in the wound field (Figure 3, B and F). By day 3, CARP protein was found in epidermis at the wound margin (Figure 3, C and G). By 7 days after wounding the CARP expression pattern was similar to intact skin. These data showed that after injury CARP seems not only to be expressed in vascular tissue and muscle, but also in inflammatory and epithelial cells.
Figure 2.
In situ hybridization of murine CARP in 1 day mouse skin wounds. Dark color represents the CARP mRNA signal. A and C: Sense probe hybridization. B, D, and E: Anti-sense hybridization. Skeletal muscle, vessel and epidermis signals are indicated by red, black and yellow arrowheads, respectively in B. Red dashed line indicates the basement membrane. CARP mRNA-positive inflammatory cells in the wound area are indicated by red arrowheads in D. High magnification of vessel signal is shown in (E).
Figure 3.
Immunostaining of CARP in mouse skin wounds. Expression patterns of CARP at 0 days, 1 day, 3 days, and 7 days in mouse skin excisional wounds are shown in A–D, respectively. E–H: High magnification images of CARP-positive cells in hair follicles and inflammatory cells in 1-day wounds, epidermis, and skeletal muscle cells in 3-day wounds respectively. Red dashed line indicates the boundary of wound and normal area in C and D. Only the wound bed is shown in B. EP, epidermis; DE, dermis; HF, hair follicle; MU, muscle.
Ad-CARP Infection Promotes Neovascularization in Experimental Granulation Tissue
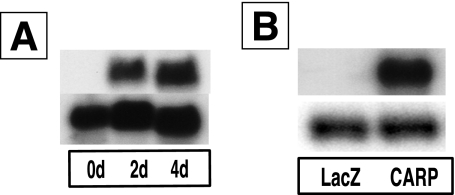
To determine the potential influence of CARP overexpression on wound healing, we constructed an adenovirus that constitutively overexpressed murine CARP mRNA under control of the CMV promoter. We confirmed the primary structure of the construct by restriction enzyme digestion and PCR analysis (data not shown). Northern blot analysis showed that a high level of CARP mRNA expression was present at both 2 days and 4 days after infection of 293A cells (Figure 4A). Viral particle concentration was determined spectrophotometrically (4.5 × 1012 particles/ml), and biological titration was performed using routine plaque assay (2 × 1010 PFU/ml). The ratio of particle concentration to PFU titer was always less than 500. The high quality virus was necessary to detect CARP biological function in wound healing.
Figure 4.
Adenovirus-mediated CARP transcription both in vitro and in vivo. A: Northern blot of RNA from 293A cells infected with Ad-CARP for 0, 2, and 4 days. Top, CARP coding region probe; bottom, GAPDH probe. B: Northern blot of RNA from implanted sponges infected with either Ad-LacZ or Ad-CARP. Top, CARP probe; bottom, GAPDH probe.
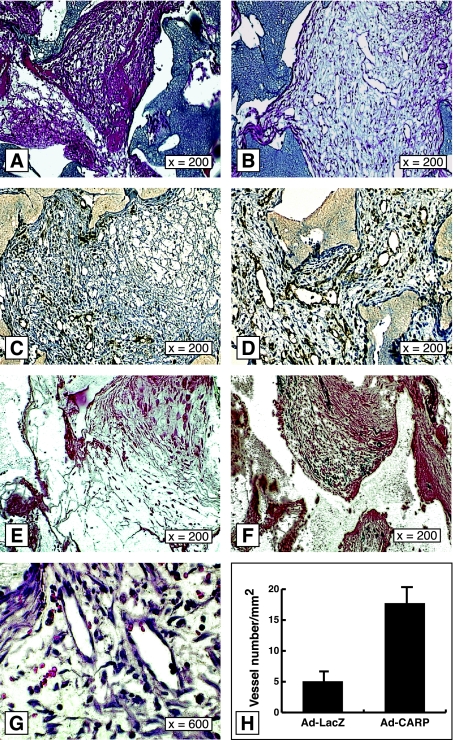
The formation of granulation tissue is a critical stage in wound healing. This process includes accumulation of macrophages, ingrowth of fibroblasts, deposition of loose connective tissue, and neovascularization.1 To determine the effect of CARP overexpression in granulation tissue, we implanted PVA sponges in rats subcutaneously and injected 1 × 109 PFU of Ad-CARP or Ad-LacZ into sponges 3 days after implantation. The control virus provided connection for the unspecific effects of viral infection and foreign protein expression in the target tissue. Ad-CARP-infected sponges showed high transcript levels of CARP by Northern blot analysis 3 days after infection (Figure 4B). One day later, Ad-CARP-infected granulation tissue displayed more numerous and larger vessels than did Ad-LacZ-infected tissue, in the absence of macroscopic hemorrhage (Figure 5, A and B). High magnification confirmed these structures to be dilated capillaries comprised of endothelial monolayers (Figure 5G). The neovascularization response was very impressive, with blood vessels being the dominant histological feature, and there appeared to be an excess of vascular tissue over connective tissue (supported by trichrome staining; data not shown), at least with a dose of 109 PFU of virus. We used the cell surface marker CD34 to confirm the identity of the endothelial cell lineage.14 Most of the immunoreactive signal was localized to endothelial cells of vessels in granulation tissue. Ad-CARP-infected granulation tissue showed three times more CD34-positive microvascular cells than Ad-LacZ-infected sponge granulation tissue (Figure 5, C, D, and H). While CD34 is a useful marker for endothelial cells, it is also expressed in endothelial progenitors and some hematopoietic cells.14,15 Since Flk-1 is a more specific marker for vessel wall endothelium, the Flk-1LacZ knock-in mouse was used as a model to reveal the expression of VEGF receptor 2 (Flk-1) in wound healing.16 PVA sponges were implanted subcutaneously in Flk-1LacZ mice, and Ad-CARP or Ad-Luc-IRES-GFP was injected into sponges 7 days after implantation. X-gal staining showed that CARP-infected granulation tissue displayed many more LacZ-positive cells than Ad-Luc-IRES-GFP-infected sponges (Figure 5, E and F). Thus, CARP overexpression was associated with a sharp rise in endothelial cell abundance and high levels of VEGF receptor-2 expression.
Figure 5.
Overexpression of CARP induces angiogenesis in experimental granulation tissue. H&E staining of subcutaneously implanted sponges in rat after the injection of either Ad-LacZ (A) or Ad-CARP (B). At 4 days post-injection, Ad-CARP-infected sponges showed more numerous and larger blood vessels. High magnification of the vessels is shown in G. CD34 immunostaining for endothelial precursors in subcutaneously implanted sponges in rats 4 days after injection with either Ad-LacZ (C) or Ad-CARP (D). X-gal staining for subcutaneously implanted sponges in the Flk-1LacZ knock-in mouse at 7-days after injection with either Ad-Luc-IRES-GFP (E) or Ad-CARP (F). Flk-1-positive cells, indicative of the endothelial lineage, displayed a dark blue color. H: Vessel number was counted in 1-mm2 grids in two complete non-consecutive cross-sections from CD34-stained sponges. Results are presented as mean number ± SEM, P < 0.005.
CARP Induces Neovascularization and Increased Blood Perfusion in Full-Thickness Wounds
The mouse and rat wound models showed that overexpression of CARP induced neovascularization in experimental granulation tissue. To address whether CARP increases blood perfusion in wound healing, we used a rabbit excisional wound model. Observation of wounds treated with Ad-CARP showed that the wound margin was more erythematous than the Ad-LacZ control (Figure 6, C and D). Histological examination showed again that CARP induced more and larger vessels in the wound site without increased inflammation (Figure 6, E and F). Laser Doppler perfusion imaging (LPDI) was performed 14 days after wounding, and the LDPI mean blood perfusion value in the Ad-CARP-infected wound was significantly higher than the Ad-LacZ control (Figure 6, A, B and G; P < 0.05). The macroscopic and LDPI data were very consistent with the histological data. Thus, overexpression of CARP not only induced neovascularization but also increased blood perfusion in a rabbit excisional wound model.
To address whether CARP is a potential gene therapy candidate in ischemic wounds, an ischemic wound model in rats was used (Figure 7A). LDPI data showed that overexpression of CARP increased blood perfusion by 20% at day 4 and 10% at day 7 after wounding. By day 10 there was no statistically significant difference between a single Ad-CARP treatment and the Ad-Luc-GFP control (Figure 7B).
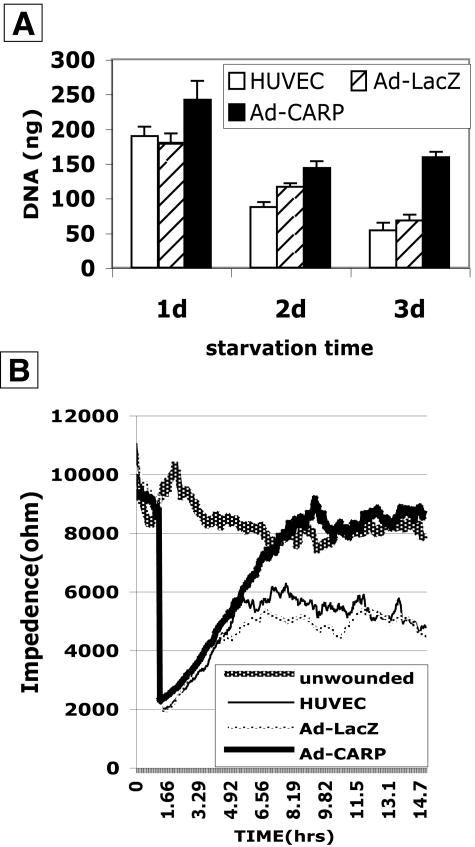
Overexpression of CARP Enhances HUVEC Survival and Promotes Migration
To explore the mechanism of CARP in neovascularization, CARP was overexpressed in HUVEC and subjected to assays for cell survival and cell migration. Figure 8A shows that Ad-CARP enhanced cell survival 1.34-, 1.23-, and 2.33-fold at 1 day, 2 days, and 3 days of serum starvation as compared to Ad-LacZ-infected HUVEC cells. Figure 8B shows the recovery of HUVEC monolayer impedance following injury. Following 2 hours of baseline data collection, six wells received an elevated current pulse of 3 volts at 40 kHz for 8 seconds to produce an injury. The impedance then dropped to a low level due to disruption of the monolayer. During the next 5 hours, the impedance of the CARP-infected HUVEC monolayer returned to the baseline level due to migration of cells into damaged zone. On the other hand, the impedance of Ad-LacZ-infected HUVEC rose only 56% after 14 hours. The same results were obtained with Ad-GFP as control virus (data not shown).
Figure 8.
Overexpression of CARP enhances HUVEC survival and improves endothelial wound healing in vitro. A: HUVEC were serum starved for 1 to 3 days after infection with nothing (HUVEC), Ad-LacZ, or Ad-CARP. DNA values are the means of three determinations ± SEM. B: ECIS was performed in HUVEC cell infected with Ad-CARP, Ad-LacZ. Unwounded cell layers show a relatively constant impedance over 16 hours, with treatment groups showing varying rates of impedance recovery reflective of cell layer integrity. Impedance tracings are the average of duplicate cultures.
Discussion
Having observed dynamic and persistent induction of CARP in wounds, we hypothesized that overexpression would reveal biological activity. The sponge implantation model of experimental granulation tissue was tested, since it emphasizes the fibroplasia and neovascularization aspects of tissue repair,17 and wound healing is a post-embryonic phenomenon that involves both vasculogenesis and angiogenesis.1 We reasoned that effective administration of a nuclear factor would require an efficient means of gene delivery to a large proportion of the target cells. Using an adenoviral luciferase reporter, we confirmed by in vivo observation of bioluminescence that adenoviral gene expression was confined to the injection site and persisted for several days (data not shown). The localized activity was probably enhanced by the tissue encapsulation of the implanted sponges. Ad-LacZ infection within the sponges confirmed that adenovirus infection stimulated transgene expression in a variety of cell types (data not shown). Our findings showed that CARP, a protein with nuclear localization and presumed transcriptional activity, was capable of stimulating neovascularization in a number of wound-healing models.
Regulation and Sources of CARP Expression during Wound Healing
CARP expression was remarkably up-regulated during excisional wound healing in normal mice. A less pronounced induction of CARP was observed in a animal model that exhibits a compromised wound-healing response, genetically diabetic db/db mice (data not shown), that have defects in granulation tissue formation.18 More detailed studies may show a correlation between CARP expression and impaired wound healing.
The endogenous stimulus for rapid CARP induction following injury is uncertain. Neovascularization is thought to be regulated by a group of soluble peptides including VEGF, Ang-1, and Ang-2, members of the FGF family and PIGF, among others.19 Vasculogenesis may involve mediators that mobilize and recruit circulating endothelial progenitor cells.20 CARP expression is rapidly increased when human dermal microvascular endothelial cells (HDMEC) are treated with TNF-α for 16 hours4 or when C2/2 rat vascular smooth muscle cells (VSMC) are stimulated with TGF-β for 24 hours.8 Thus, cytokines known to be mobilized in early wounds are capable of activating this transcriptional co-factor in vascular tissues, consistent with the hypothesis that CARP induction may have a bearing on neovascularization. However, most investigations have focused on the expression of CARP during cardiogenesis or experimental cardiomyopathy. Our in situ hybridization data showed that CARP was highly expressed in wounded skeletal muscle in the subdermis and vessel walls in the dermis, which is consistent with previous localization studies, and the CARP protein signal showed a similar distribution. We also detected CARP mRNA in keratinocytes and inflammatory cells in the wound vicinity. CARP has recently been identified as a biomarker of human skin keratinocytes by microarray analysis.21 It appears that CARP is expressed at a low level in keratinocytes under physiological conditions, and factors such as inflammatory cytokines or growth factors could amplify CARP expression in keratinocytes and other cell types during wound healing. The CARP-expressing cells in the inflammatory infiltrate had the morphology of leukocytes, but this population may also contain circulating endothelial cell progenitors as part of the vasculogenic response.
Kanai et al reported CARP was up-regulated at transcriptional level in response to exogenous TGF-β in cultured VSMCs.8,22 Genetic studies have revealed that TGF-β plays an important role in embryonic vascular assembly.23 In vivo, TGF-β induces angiogenesis after injection into rat sponge implants24 or the skin of newborn mice.25 In direct contrast, TGF-β has been identified as a potential negative regulator of endothelial and VSMC cell growth in vitro.26 The relation between CARP and other angiogenic regulators is not well defined, although it is not strongly induced in vitro by traditional angiogenic factors such as FGF-2 or VEGF (unpublished observations).
The Spatial and Temporal Expression Features of CARP Correlate with Effects on Vessel Morphology
Despite the initial discovery of CARP in vascular endothelium, studies of embryonic CARP expression turned the focus to embryonic cardiomyogenesis and myogenesis.5,7 Denervation rapidly induces CARP mRNA in the vicinity of vacated neuromuscular synapses.7 Perhaps more relevant to effects in tissue repair, CARP is strongly induced in cardiac muscle by cardiac hypertrophy,10 and it is up-regulated in experimental arterial injury.11 Transient transfection data showed that CARP was a transcriptional inhibitor in a VSMC cell line.5 Our data showed that overexpression of CARP could enhance cell survival and improve migration of endothelial cells.
The Mechanism of CARP-Induced Neovascularization
The relationship of CARP expression to either myogenesis or arteriogenesis is unclear. CARP has been identified as a nuclear protein. DNA binding experiments have indicated that CARP is probably a transcriptional co-factor, since its DNA binding activity was very low.4 The 319aa CARP polypeptide contains a number of protein motifs, including a bipartite nuclear localization signal, (71–80,94–103) a PEST-like sequence, (108–126) four ankyrin repeats (152–283) with an additional, less conserved ankyrin half-repeat, and several phosphorylation sites.4 Ankyrin repeat proteins are present in a large number of protein families, including transcription factors, developmental regulators, and CDK inhibitors.27 The function of the ankyrin repeat is to bind other proteins. Yeast two-hybrid studies have revealed that CARP interacts with YB-1, suggesting that CARP could be a co-activator or co-repressor in transcriptional process.6 The isolation of CARP-interactive proteins will be one way to suggest the actual mechanism of action.
If CARP is part of the innate mechanism of neovascularization, it could be part of the signal transduction pathway leading to or from known angiogenic factors. The mechanism of CARP-induced neovascularization may be due to its transcriptional properties in progenitor cells, possibly by inhibiting repressors of angiogenic activation or by activating classic angiogenic mediators, such as VEGF, Flk-1, or Ang-1. CARP overexpression in the sponge model leads to greater vascularization and relatively less fibrogenesis and collagen. This phenomenon is consistent with the action of several angiogenic factors; however, the absence of hemorrhage points toward FGF-like rather than VEGF-like activity. CARP may signal through other intermediates.
Recently, Boengler et al11 reported that overexpression of CARP in COS-1 cells lead to a 3.7-fold increase of the early growth response transcription factor 1 (Egr-1). We confirmed this result by transient transfection of hCARP into COS-1 cells (data not shown). Egr-1 is one member of an early response gene family that is expressed in both endothelial and vascular smooth muscle cells,28 and this transcription factor is a major mediator of the activation of tissue factor by VEGF in endothelial cells.29 The RNA-cleaving phosphodiester-linked DNA-based enzyme technique implicated Egr-1 signaling during FGF-dependent angiogenesis in subcutaneous Matrigel plugs and tumor growth in mice.30 Moreover, Egr-1 has been shown to mediate MT1-MMP transcription in endothelium, a enzymatic system by which endothelial cells can initiate an invasive response to tissue injury or mechanical stress.31 As a gene therapy candidate, Egr-1 can promote angiogenesis both in vivo and in vitro as well as accelerate wound healing in mice.32 Since these previous studies suggest possible mechanisms of CARP-mediated angiogenesis, a more detailed relation between CARP and Egr-1 is underway. Other cellular targets may be relevant because the adenovirus infection of cells is non-selective. Overexpression of CARP may directly affect vascular cells, or it may be acting indirectly to stimulate the release of paracrine factors from non-vascular cell types such as fibroblasts, leukocytes, or keratinocytes.
In summary, our study provides evidence suggesting that CARP could play a role not only in cardiogenesis but also in neovascularization during tissue repair. Further findings to be emphasized include the ability of functional genomics to identify new factors that have wound-healing activity and the potential of gene transfer approaches to reveal the activity of intracellular molecules involved in gene regulation. CARP therapy may represent a new strategy for neovascularization or a novel target for limiting local vessel growth.
Acknowledgments
We thank Dr. Steve Hanks (Vanderbilt University) for generously providing the ECIS equipment, and we thank other staff members of the laboratory for assisting us in this project, especially Jin-Hua Liu for technical support and Dr. Mariagabriella Giro for critical comments.
Footnotes
Address reprint requests to Jeffrey M. Davidson, Ph.D., Department of Pathology C3321 MCN, Vanderbilt University School of Medicine, Nashville, TN 37232-2562. E-mail: jeff.davidson@vanderbilt.edu.
Supported by grant FKZ 0312225 from Bundesministerium fur Bildung und Forschung (to A.G., S.W., E.W.), the NIDDK (to J.M.D.), the Department of Veterans Affairs (to J.M.D.), and Switch Biotech AG.
References
- Madri JA, Pratt BM. Angiogenesis. Clark RAF, Henson PM, editors. New York: Plenum; The Molecular and Cellular Biology of Wound Repair. 1996:pp 337–353. [Google Scholar]
- Emanueli C, Madeddu P. Angiogenesis gene therapy to rescue ischaemic tissues: achievements and future directions. Br J Pharmacol. 2001;133:951–958. doi: 10.1038/sj.bjp.0704155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettgen P. Transcriptional regulation of vascular development. Circ Res. 2001;89:380–388. doi: 10.1161/hh1701.095958. [DOI] [PubMed] [Google Scholar]
- Chu W, Burns DK, Swerlick RA, Presky DH. Identification and characterization of a novel cytokine-inducible nuclear protein from human endothelial cells. J Biol Chem. 1995;270:10236–10245. doi: 10.1074/jbc.270.17.10236. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan R, Poizat C, Baker RK, Abdishoo S, Isterabadi LB, Lyons GE, Kedes L. A novel cardiac-restricted target for doxorubicin: CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes. J Biol Chem. 1997;272:22800–22808. doi: 10.1074/jbc.272.36.22800. [DOI] [PubMed] [Google Scholar]
- Zou Y, Evans S, Chen J, Kuo HC, Harvey RP, Chien KR. CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2–5 homeobox gene pathway. Development. 1997;124:793–804. doi: 10.1242/dev.124.4.793. [DOI] [PubMed] [Google Scholar]
- Baumeister A, Arber S, Caroni P. Accumulation of muscle ankyrin repeat protein transcript reveals local activation of primary myotube endcompartments during muscle morphogenesis. J Cell Biol. 1997;139:1231–1242. doi: 10.1083/jcb.139.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai H, Tanaka T, Aihara Y, Takeda S, Kawabata M, Miyazono K, Nagai R, Kurabayashi M. Transforming growth factor-beta/Smads signaling induces transcription of the cell type-restricted ankyrin repeat protein CARP gene through CAGA motif in vascular smooth muscle cells. Circ Res. 2001;88:30–36. doi: 10.1161/01.res.88.1.30. [DOI] [PubMed] [Google Scholar]
- Kuo H, Chen J, Ruiz-Lozano P, Zou Y, Nemer M, Chien KR. Control of segmental expression of the cardiac-restricted ankyrin repeat protein gene by distinct regulatory pathways in murine cardiogenesis. Development. 1999;126:4223–4234. doi: 10.1242/dev.126.19.4223. [DOI] [PubMed] [Google Scholar]
- Aihara Y, Kurabayashi M, Saito Y, Ohyama Y, Tanaka T, Takeda S, Tomaru K, Sekiguchi K, Arai M, Nakamura T, Nagai R. Cardiac ankyrin repeat protein is a novel marker of cardiac hypertrophy: role of M-CAT element within the promoter. Hypertension. 2000;36:48–53. doi: 10.1161/01.hyp.36.1.48. [DOI] [PubMed] [Google Scholar]
- Boengler K, Pipp F, Fernandez B, Ziegelhoeffer T, Schaper W, Deindl E. Arteriogenesis is associated with an induction of the cardiac ankyrin repeat protein (carp). Cardiovasc Res. 2003;59:573–581. doi: 10.1016/s0008-6363(03)00511-x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Keese CR, Wegener J, Walker SR, Giaever I. Electrical wound-healing assay for cells in vitro. Proc Natl Acad Sci USA. 2004;101:1554–1559. doi: 10.1073/pnas.0307588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- Kappel A, Ronicke V, Damert A, Flamme I, Risau W, Breier G. Identification of vascular endothelial growth factor (VEGF) receptor-2 (Flk-1) promoter/enhancer sequences sufficient for angioblast and endothelial cell-specific transcription in transgenic mice. Blood. 1999;93:4284–4292. [PubMed] [Google Scholar]
- Davidson JM. Animal models for wound repair. Arch Dermatol Res. 1998;290(Suppl):S1–S11. doi: 10.1007/pl00007448. [DOI] [PubMed] [Google Scholar]
- Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, Nowygrod S, Wolf BM, Caliste X, Yan SF, Ster DM, Schmidt AM. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159:513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JM, Whitsitt JS, Pennington B, Ballas CB, Eming S, Benn SI. Gene therapy of wounds with growth factors. Curr Top Pathol. 1999;93:111–121. doi: 10.1007/978-3-642-58456-5_12. [DOI] [PubMed] [Google Scholar]
- Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Invest Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- Curto EV, Lambert GW, Davis RL, Wilborn TW, Dooley TP. Biomarkers of human skin cells identified using DermArray DNA arrays and new bioinformatics methods. Biochem Biophys Res Commun. 2002;291:1052–1064. doi: 10.1006/bbrc.2002.6542. [DOI] [PubMed] [Google Scholar]
- Topper JN. Transforming growth factor-beta (TGF-beta) and vascular disease: cARP as a putative TGF-beta target gene in the vessel wall. Circ Res. 2001;88:5–6. doi: 10.1161/01.res.88.1.5. [DOI] [PubMed] [Google Scholar]
- Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- Davidson MJ, Broadley K. Reversal of the wound healing deficit in diabetic rats by combined basic fibroblast growth factor and transforming growth factor-beta-1 therapy. Wound Repair Regen. 1997;5:77–88. doi: 10.1046/j.1524-475X.1997.50115.x. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, Fauci AS. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8:21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Sedgwick SG, Smerdon SJ. The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem Sci. 1999;24:311–316. doi: 10.1016/s0968-0004(99)01426-7. [DOI] [PubMed] [Google Scholar]
- Tsai JC, Liu L, Cooley BC, DiChiara MR, Topper JN, Aird WC. The Egr-1 promoter contains information for constitutive and inducible expression in transgenic mice. FASEB J. 2000;14:1870–1872. doi: 10.1096/fj.99-1072fje. [DOI] [PubMed] [Google Scholar]
- Mechtcheriakova D, Wlachos A, Holzmuller H, Binder BR, Hofer E. Vascular endothelial cell growth factor-induced tissue factor expression in endothelial cells is mediated by EGR-1. Blood. 1999;93:3811–3823. [PubMed] [Google Scholar]
- Fahmy RG, Dass CR, Sun LQ, Chesterman CN, Khachigian LM. Transcription factor Egr-1 supports FGF-dependent angiogenesis during neovascularization and tumor growth. Nat Med. 2003;9:1026–1032. doi: 10.1038/nm905. [DOI] [PubMed] [Google Scholar]
- Haas TL, Stitelman D, Davis SJ, Apte SS, Madri JA. Egr-1 mediate extracellular matrix-driven transcription of membrane type 1 matrix metalloproteinase in endothelium. J Biol Chem. 1999;274:22679–22685. doi: 10.1074/jbc.274.32.22679. [DOI] [PubMed] [Google Scholar]
- Bryant M, Drew GM, Houston P, Hissey P, Campbell CJ, Braddock M. Tissue repair with a therapeutic transcription factor. Hum Gene Ther. 2000;11:2143–2158. doi: 10.1089/104303400750001444. [DOI] [PubMed] [Google Scholar]