Abstract
Polycystic kidney (PCK) rats exhibit a multiorgan cyst pathology similar to human autosomal recessive polycystic kidney disease, and are proposed as an animal model of Caroli’s disease with congenital hepatic fibrosis (CHF). This study investigated the expression and function of selected components of the mitogen activated protein kinase (MAPK) pathway in cultured intrahepatic biliary epithelial cells (BECs) of PCK rats. Compared to the proliferative activity of cultured BECs of control rats, those of the PCK rats were hyperresponsive to epidermal growth factor (EGF). The increase in BEC proliferation was accompanied by overexpression of MAPK/extracellular signal-regulated protein kinase (ERK) kinase 5 (MEK5), and subsequent phosphorylation of ERK5 in vitro. The increased proliferative activity was significantly inhibited by the transfection of short interfering RNA against MEK5 mRNA. An EGF receptor tyrosine kinase inhibitor, gefitinib (“Iressa”, ZD1839), also significantly inhibited the abnormal growth of cultured BECs of PCK rats. By contrast, treatment with PD98059 and U0126, inhibitors for MEK1/2, was less effective. These results suggest that the activation of the MEK5-ERK5 cascade plays a pivotal role in the biliary dysgenesis of PCK rats, and also provide insights into the pathogenesis of Caroli’s disease with CHF. As the MEK5-ERK5 interaction is highly specific, it may represent a potential target of therapy.
Caroli’s disease is characterized by a congenital dilatation of the intrahepatic bile ducts, and is frequently associated with portal fibrosis corresponding to congenital hepatic fibrosis (CHF).1 Caroli’s disease with CHF is known as a hepatic manifestation of autosomal recessive polycystic kidney disease (ARPKD). The hepatobiliary lesions in Caroli’s disease are related to the persistence of or lack of remodeling of the embryonic ductal plate (so-called “ductal plate malformation”).2 The ductal changes are thought to be caused by a combination of uneven and disaproportionate overgrowth of biliary epithelial cells (BECs) and their supporting connective tissue,1 but the exact mechanism remains unexplored.
Renal pathologies found in polycystic kidney disease (PKD) have been well studied.3,4 Using spontaneous murine models that exhibit a multiorgan pathology similar to that seen in human ARPKD, a number of studies have identified misexpressed genes in the cystic kidneys, which are associated with increased cell proliferation, apoptosis, and matrix remodeling with an altered differentiation of the epithelial cells lining the renal cysts.5–16 At present, several murine and rat models of ARPKD are available, and these include BALB/c-bpk mice, BALB/c-cpk mice, C57BL/6J-cpk mice, and the polycystic kidney (PCK) rat.17 While none of the rodent models of ARPKD exhibit defects in the same genetic locus, the gene responsible for human ARPKD (the PKHD1 gene) was identified on human chromosome 6 as fibrocystin, and a mutation of PKHD1 was found in the PCK rat.18,19
The PCK rat is a mutant animal established from a Crj:CD/SD strain.17,20 This rat develops collecting duct-derived renal cysts, and multiple segmental and saccular dilatations of intrahepatic bile ducts due to ductal plate malformation, and also progressive portal fibrosis with increased numbers of small bile ducts and variable inflammatory cell infiltration. Recently, we reported that the PCK rat could be a useful and promising animal model of Caroli’s disease with CHF.21 We also demonstrated that an imbalance between cell proliferation and apoptosis might be responsible for the aberrant proliferation of BECs of PCK rats in vivo.
Epithelial cell proliferation and differentiation are modulated by various cytokines and growth factors. Epidermal growth factor (EGF) is a major participant in cellular events including proliferation and differentiation. By binding to a prototype transmembrane tyrosine kinase receptor (EGF receptor, EGFR), EGF activates the mitogen activated protein kinases (MAPKs), which relay signals from the cell membrane to the nucleus. The MAPK pathway consists of three protein kinases that act sequentially within a pathway: a MAPK kinase kinase, a MAPK/ERK (extracellular signal-regulated protein kinase) kinase (MEK), and a MAPK (ERK). The first and best studied is the MAPK pathway which consists of Raf-1 or B-Raf, MEK1/2, and ERK1/2. Recently, the novel MEK5-ERK5 pathway has been implicated in the regulation of cellular proliferation by acting with EGF. That is, EGF activates ERK5, and this activation requires the direct and specific upstream activator, MEK5.22
In murine ARPKD models, abnormal expression of EGF and EGFR has been implicated in the pathogenesis of cysts in the kidneys.16,23–25 ERK1/2 has been shown to be activated in renal epithelilal cells within cysts in a rat model of autosomal dominant PKD (ADPKD).26 In addition, Nauta et al27 demonstrated that BECs obtained from mice with ARPKD were hyperresponsive to EGF. These findings suggest that activation of the MAPK pathway is critically involved in the development of cystic changes in the liver of PCK rats. To date, however, few studies have examined the pathogenesis associated with the extrarenal cystic organs in PKD, including the liver.
Using the PCK rat as a model of Caroli’s disease with CHF, the involvement of the MAPK pathway in the development of biliary dysgenesis was examined in this study.
Materials and Methods
Animals
Crj:CD (control) and PCK rats were purchased from Charles River Japan (Sagamihara, Japan), and a colony of PCK rats was maintained at the Laboratory Animal Institute of Kanazawa University School of Medicine, Kanazawa, Japan. All animal experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals at Takara-machi Campus of Kanazawa University.
Antibodies
The antibodies used were anti-cytokeratin (CK)-7 (clone OV-TL 12/30, mouse monoclonal, Dako, Glostrup, Denmark), anti-γ-glutamyl transpeptidase (γ-GTP) (clone 5B9, mouse monoclonal, Cosmo-Bio, Tokyo, Japan), anti-vimentin (clone V9, mouse monoclonal, Dako), anti-EGF (sc-1343, goat polyclonal, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-EGFR (sc-03, rabbit polyclonal, Santa Cruz), anti-mast cell tryptase (AA1, mouse monoclonal, Dako), anti-MEK1/2 (sc-436, rabbit polyclonal, Santa Cruz), anti-phosphospecific (p)-MEK1/2 (sc-7995R, rabbit polyclonal, Santa Cruz), anti-MEK5 (KAP-MA003, rabbit polyclonal, Stressgen, San Diego, CA), anti-ERK1/2 (KAP-MA001, rabbit polyclonal, Stressgen), anti-p-ERK1/2 (44–680, rabbit polyclonal, Biosource International, Camarillo, CA), anti-ERK5 (KAS-MA002, rabbit polyclonal, Stressgen), anti-p-ERK5 (E7153, rabbit polyclonal, Sigma, St. Louis, MO), and anti-Ki-67 protein (MIB-5, mouse monoclonal, Immunotech, Marseille, France).
Cell Culture of Rat Intrahepatic BECs
Eight-week-old male rats were used. The isolation and passage culture of intrahepatic BECs were performed as described previously.28 Briefly, the livers were first perfused with Hanks’ balanced salt solution without (Ca2+) and (Mg2+) [HBSS(−)] via the portal vein trunk, and then with Dulbecco’s modified Eagle’s medium and Ham F-12 (DMEM/F-12) containing 0.04% collagenase (collagenase S-1, Nitta Zeratin, Osaka, Japan) and 0.22% dispase (Life Technologies, Grand Island, NE) for 20 minutes. After removal of the digested hepatic parenchyma, several tissue fragments were cut from parts of the intrahepatic large bile ducts, and then floated for 48 hours in DMEM/F-12 medium (standard medium) containing 10% Nu-Serum V culture supplement (Becton Dickinson, Bedford, UK), forskolin (2.1 μg/ml, Wako Pure Chemical Industries, Osaka, Japan), EGF (20 ng/ml, Upstate Biotechnology, New York, NY), and antibiotics in a 5% CO2 incubator. The tissue fragments were then placed as an explant on type I collagen gel (Cellmatrix Type I-A, Nitta Zeratin), and cultured in a 5% CO2 incubator.
BECs on collagen gel proliferated and spread from these explants. Areas of this sheet composed entirely of intrahepatic BECs were cut out and placed on other collagen gels for subculturing. Subcultured BECs on gels were fed with standard medium in a 5% CO2 incubator, and passaged every 3 weeks. After four passages, the cells were scraped off and placed in DMEM/F-2 medium containing 0.04% collagenase and 0.22% dispase. After centrifugation, the pellets were added to HBSS(−) and cells were set on collagen-1-coated plastic bottles for 3 weeks until confluent. Then, cultured cells were scraped off and stored for the following experiments.
Characterization of Cultured BECs
BECs cultured on collagen gel for 72 hours were fixed in 10% neutral-buffered formalin, and cut into several slices which were embedded in paraffin. Then, several sections, 3-μm thick, were cut and deparaffinized, and stained with HE and alcian blue at pH 2.5 for acid glycoprotein.
The BECs cultured on collagen-coated cover-glass (Sumitomo Bakelite, Tokyo, Japan) for 2 days were fixed in cold acetone for 10 minutes. After pretreatment with blocking reagent (Dako), primary antibodies against CK-7 (1:100), γ-GTP (1:50), and vimentin (1:50) were applied. Then, fluorescein isothiocyanate-labeled secondary polyclonal antibody against mouse IgG (Serotec, Raleigh, NC) was applied, and the sections were counterstained with propidium idodide (Vectashield, Vector Laboratories, Burlingame, CA). They were examined under a confocal laser microscope (LSM 410, Carl Zeiss, Gottingen, Germany). CK-7 and γ-GTP are known as biliary epithelial cell markers, while vimentin is a mesenchymal cell marker.
cDNA Microarray Analysis
BECs cultured in the presence of EGF (20 ng/ml) at the subconfluent stage were used, and a cDNA microarray assay was performed once. Total RNA was isolated from BECs using a RNA extraction kit (RNeasy Mini Kits, Qiagen, Tokyo, Japan). Digestion with DNase was performed using a RNase-Free DNase Set (Qiagen). The RNA was dissolved in RNase-free water. The purity and integrity of the RNA were examined spectrometrically, and the concentration was adjusted to 10 μg/μl.
Using 19 μl of RNA sample, cDNA synthesis was performed with the use of an Atlas Glass Fluorescent Labeling Kit (Clontech Company, Tokyo, Japan) according to the manufacturer’s instructions. The cDNA obtained was labeled with the Cy3 dye using the Atlas Glass Fluorescent Labeling Kit. Cy3 dye (Cy3 Mono-Reactive Dye Pack) was purchased from Amersham Biosciences (Tokyo, Japan). Cy3 fluorescent-labeled cDNA samples were then hybridized with the Atlas Glass Rat 1.0 Microarray (Clontech), which consisted of 1081 unique probes arrayed onto glass slides, according to the manufacturer’s instructions.
The fluorescence generated by Cy3 immobilized at the target sequence on the slides was measured using a scanning system, GenePix 4000B (InterMedical, Co., Nagoya, Japan). The fluorescent intensity of each plot was analyzed with image processing software (GenePix Pro 3.0.6, InterMedical). The intensity was corrected by the expression of reference genes (housekeeping genes) and by subtracting background signals. When the fluorescent signals of an individual gene were increased over 2.0-fold and decreased by more than half, the gene was determined to be overexpressed and underexpressed, respectively.
Quantitative and Semiquantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA (1 μg) extracted from the liver and cultured BECs was used to synthesize cDNA with reverse transcriptase (Reverse Transcriptase XL, Takara Biochemicals, Ohtsu, Japan). The sequences of the primers and conditions for PCR used are shown in Table 1. Amplification was performed in a total volume of 25 μl containing 1 μl of cDNA, 0.2 mmol/L dNTPs, 1 μmol/L each of 5′- and 3′-primers, and 2.5 U of TaqDNA polymerase (Takara EX Taq, Takara Biochemicals). For each reaction, an initial denaturation cycle of 94°C for 3 minutes and a final cycle of 72°C for 10 minutes were incorporated. The PCR products were subjected to 2% agarose gel electrophoresis and stained with ethidium bromide. Semiquantitative analysis of the gel images was performed using the public domain NIH image software in the exponential range of each PCR amplification. Fold difference compared with β-actin expression was calculated.
Table 1.
Sequences of the Primers and PCR Conditions Used in this Study
| Gene | Sequences (5′-3′) | Product size (bp) | Annealing temperature (°C) | PCR cycles |
|---|---|---|---|---|
| MEK5 | GCTTGTAAACACAAGCGGAC | 269 | 60 | 27 |
| CCTCATCAACAATGCACTGC | ||||
| TK | GCAGATCCAGGTGATTCTCG | 235 | 60 | 27 |
| GATCGATGCCAATGACAGCCA | ||||
| TGF-β3 | GCCATTAGGGGACAGATC | 594 | 55 | 40 |
| CAGTATGTCTCCATTGGG | ||||
| TGFR1 | GTTGCCCATCTTCACATGGAG | 276 | 60 | 27 |
| CATTGCATAGATGTCAGCACG | ||||
| EGF | CTACTGTCTCGACGTTGATG | 259 | 55 | 40 |
| GCTGACATCGTTCTCCAATA | ||||
| EGFR | CAACCCTGAGTATCTCAACA | 260 | 55 | 30 |
| CTGGAAAGTCCGGTTTGTAA | ||||
| bFGF | GCGTGGACGGCGTCCGGGAG | 280 | 65 | 40 |
| GGCCCCGTTTTGGATCCGAG | ||||
| GPx3 | CCTAGTCTCAAGTACGTTCG | 279 | 60 | 32 |
| CATCTTCACGTTGCTGACTG | ||||
| β-actin | ACCTTCAACACCCCAGCCATGTACG | 910 | 60 | 25 |
| CTGATCCACATCTGCTGGAAGGTGG |
Abbreviations: MEK5, mitogen-activated protein/extracellular signal-regulated protein kinase kinase 5; TK, thymidine kinase; TGF-β3, transforming growth factor-β3; TGFR1, TGF-β receptor type 1; EGF, epidermal growth factor; EGFR, EGF receptor; bFGF, basic fibroblast growth factor; GPx3, plasma glutathione peroxidase.
For the determination of MEK5 and EGFR mRNA expression, real-time quantitative RT-PCR was performed using pre-made MEK5 (FAM), EGFR (FAM), and β-actin (FAM)-specific primers and the ABI Prism 7700 Sequence Detection System (PE Applied Biosystems, Warrington, UK). RT-PCR was carried out with the TaqMan Universal RT-PCR Master Mix (PE Applied Biosystems) using 5 μl of cDNA in a 25-μl final reaction mixture. Cycling conditions were incubation at 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Fold difference compared with β-actin expression was calculated.
Western Blot Analysis
Proteins were extracted from cultured BECs using T-PER Tissue Protein Extraction Reagent (Pierce Chemical Company, Rockford, IL), and total protein was measured spectrometrically. First, 100 μg of the protein was subjected to 10% SDS-polyacrylamide electrophoresis, and then electrophoretically transferred onto a nitrocellulose membrane. The membrane was incubated with primary antibodies against MEK1/2 (1:1000), p-MEK1/2 (1:100), MEK5 (1:100), ERK1/2 (1:1000), p-ERK1/2 (1:200), ERK5 (1:500), and p-ERK5 (1:100). The protein expression was detected using secondary antibody conjugated to peroxidase-labeled polymer, EnVision+ system (Dako). 3,3′-Diaminobenzidine tetrahydrochloride was used as the chromogen.
WST1 Proliferation Assay and MAPK Pathway Inhibition Studies
The proliferative activity of BECs was assessed using a WST1 assay according to the manufacturer’s instructions (Roche, Mannhein, Germany). The WST-1 assay is a formazan-based colorimetric assay which has been frequently used for the determination of in vitro cell proliferative activity.29 In addition, to confirm that the proliferative activity assessed by the WST-1 assay can be regarded as an indicator of the actual cell number, an equal number of BECs obtained from control and PCK rats was seeded on collagen gels in 35-mm dishes. After a 48- and a 96-hour incubation with the standard medium containing 20 ng/ml of EGF, they were scraped off as a whole, and digested in the collagenase dissolution medium in test tubes. They were centrifuged, and the pellets were collected. After 0.05% crystal violet solution was added, the cells were lysed with a mixer, and the nuclei were counted using a hemocytometer. For comparison, the WST1 assay was performed as described below in a 96-well format.
A total of 1250 cells per well were used in a 96-well collagen-coated plate. After 24-hour preincubation with the standard medium, the medium was exchanged for that containing appropriate concentrations of the signaling inhibitors and/or EGF, and incubation continued for a further 24 hours. For MAPK cascade inhibitor experiments, PD98059 (Calbiochem, La Jolla, CA) and U0126 (Sigma) were used. Gefitinib (“Iressa”, ZD1839) was provided by AstraZeneca (Macclesfield, UK). The WST1 reagent was added and incubated for 2 hours before reading the plate. Each assay was conducted in six sets.
Inhibition of MEK5 was achieved using the RNA interference technique. The short interfering RNA (siRNA) target sequence for MEK5 mRNA was designed by B-Bridge International Inc. (San Jose, CA). Scramble II Duplex (B-Bridge International) was used as a negative control. The transfection of siRNA in BECs was performed using TransMessenger Transfection Reagent (Qiagen) according to the manufacturer’s instructions. The optimal amount and transfection time were determined in preliminary experiments, and 0.15 μg/well of siRNA was transfected with an incubation time of 2 hours. After 72 hours incubation with the standard medium, the proliferative activity of the cells was measured. Western blot analysis was used to confirm the inhibitory effects of siRNA on protein expression of MEK5.
Immunohistochemistry
Immunohistochemistry was performed with primary antibodies against EGF, EGFR, p-ERK1/2, p-ERK5, and Ki-67. Formalin-fixed, paraffin-embedded liver sections, 4-μm thick, were prepared from rats at 3 weeks, 2 months, and 10 months of age. After deparaffinization, antigen retrieval was performed for p-ERK1/2, p-ERK5, and Ki-67 protein by microwaving in 10 mmol/L citrate buffer, pH 6.0. After the blocking of the endogenous peroxidase, the sections were incubated overnight at 4°C with individual primary antibodies: anti-EGF (1:400), anti-EGFR (1:50), anti-p-ERK1/2 (1:200), anti-p-ERK5 (1:100), and anti-Ki-67 protein (1:50). Then, the sections were incubated with secondary antibodies conjugated to peroxidase-labeled polymer, EnVision+ system (Dako) or Histofine Simple Stain MAX PO(G) (Nichirei, Tokyo, Japan). Color development was performed using 3,3′-diaminobenzidine tetrahydrochloride, and the sections were counterstained with hematoxylin.
Ki-67-positive cells were quantitatively assessed. That is, more than 500 BECs were surveyed and the percentage of Ki-67-positive BECs was defined as the Ki-67 labeling index in each rat.
Double-immunostaining for EGF and mast cell tryptase was conducted as follows; the deparaffinized sections were incubated overnight at 4°C with anti-mast cell tryptase (1:50), and then incubated using the EnVision+ system (Dako). Color development was performed using the Vector Blue Alkaline Phosphatase Substrate Kit III (Vector Laboratories). Then, the detection of EGF was performed as above. Similarly, double-immonostaining for EGFR and Ki-67 protein was performed using primary antibodies against EGFR and Ki-67 protein, and color development was performed using the benzidine reaction for EGFR and Vector blue reaction for Ki-67 protein.
Control sections were evaluated by substitution of the primary antibodies with nonimmunized serum, which resulted in no signal detection. For the determination of EGF expression, additional control sections were incubated with the primary antibody absorbed with the respective peptide antigen.
Statistics
The means and SEM were calculated for all parameters determined. Statistical significance was evaluated by using analysis of variance or Mann-Whitney’s U-test. P < 0.05 was accepted as statistically significant.
Results
Characteristics of Cultured BECs
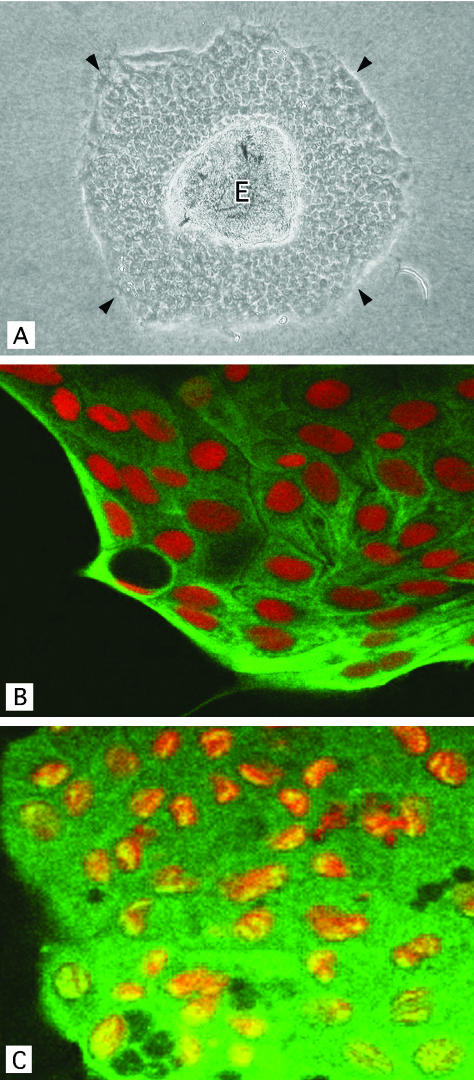
The dilatation of bile ducts spread throughout the liver of PCK rats, involving both intrahepatic large and small bile ducts. Since the dilatation of intrahepatic large bile ducts has been regarded as an essential feature of Caroli’s disease, BECs were isolated from intrahepatic large bile ducts, and cultured for the analysis. Isolated and cultured intrahepatic BECs from the livers of both control and PCK rats proliferated and spread as an epithelial cell sheet on the collagen gel (Figure 1A). Histologically, the cultured BECs after four passages, which were used for the subsequent experiments, showed a monolayer of cuboidal epithelial cells on the collagen gel. An oval or round nucleus was located in the middle of the cytoplasm of BECs, and acid glycoprotein was clearly detected on the apical cell surface (data not shown). CK-7 and γ-GTP were spread diffusely in the cytoplasm of cultured BECs (Figure 1, B and C), while vimentin was absent. These histological and phenotypic features were similar in the cultured BECs of control and PCK rats.
Figure 1.
Characteristics of cultured intrahepatic biliary epithelial cells (BECs) obtained from PCK rats. A: BECs formed an epithelial cell sheet (arrowheads) spreading from the isolated epithelial cell explant (E). There was no contamination of mesenchymal cells. Phase contrast microscope. B and C: Confocal immunocytochemistry for cultured BECs derived from a PCK rat. The cytoplasm of BECs was positive for cytokeratin-7 (green) (B) and γ-glutamyl transpeptidase (green) (C). The nucleus was counterstained with propidiumidodide. Original magnification: ×100 (A), ×1000 (B and C).
cDNA Microarray Analysis of Cultured BECs
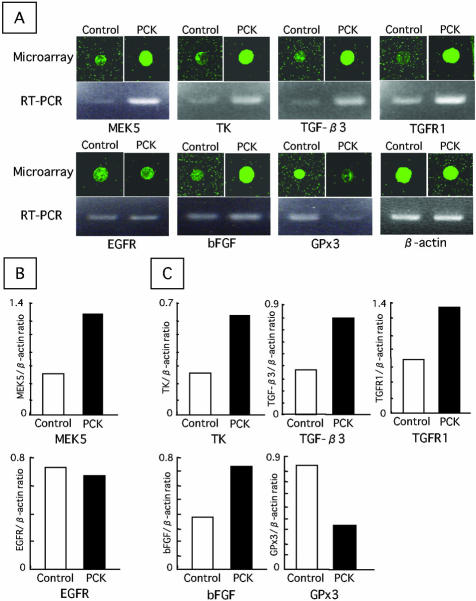
The overall differences in gene expression between cultured BECs of control and PCK rats were examined using a cDNA microarray analysis. The vast majority of the 1081 genes examined were expressed to a similar degree in both rats. In the PCK rat, the expression of 18 genes was enhanced by at least twofold, and the expression of three genes was reduced by half or less compared with the control. The 21 differentially expressed genes are listed in Table 2. Several of these genes were associated with cell proliferation, apoptosis, and/or fibrotic processes. These included the transcripts encoding MEK5, thymidine kinase (TK), junD, transforming growth factor (TGF)-β3, TGF-β receptor type 1 (TGFR1), basic fibroblast growth factor (bFGF), and plasma glutathione peroxidase (GPx3) (Figure 2A). Quantitative (Figure 2B) and semiquantitative (Figure 2C) RT-PCR analysis were further added to confirm the differentially expressed genes of interest.
Table 2.
Transcripts Expressed at >2.0-Fold Higher or <0.5-Fold Lower Levels in Biliary Epithelial Cells of PCK Rats
| Gene | Fold difference |
|---|---|
| Glycine transporter Glyt-1 | 12.0 |
| ADP-ribosylation factor 2 | 5.4 |
| Cationic amino acid transporter 3 | 5.2 |
| Potassium channel RCK4 | 4.4 |
| Voltage-gated dihydropyridine-sensitive L-type calcium channel beta 3 subunit | 4.0 |
| Basic fibroblast growth factor (bFGF) | 3.4 |
| junD proto-oncogene | 3.1 |
| Inhibitor of DNA binding 1 | 2.7 |
| Transforming growth factor-β3 (TGF-β3) | 2.7 |
| Thymidine kinase (TK) | 2.4 |
| Synaptobrevin 2 | 2.5 |
| Tripeptidylpeptidase II | 2.3 |
| Mitogen-activated protein/extracellular signal-regulated protein kinasekinase 5 (MEK5) | 2.2 |
| TGF-β receptor type 1 (TGFR1) | 2.2 |
| Adipocyte fatty acid-binding protein | 2.2 |
| G protein, gamma 5 subunit | 2.1 |
| ADP-ribosylation factor 3 | 2.1 |
| Acetylcholine receptor beta | 2.0 |
| Annexin 1 (ANX1) | 0.3 |
| Plasma glutathione peroxidase (GPx3) | 0.4 |
| Rab-11A | 0.4 |
Figure 2.
A: Microarray and RT-PCR analysis of the expression of eight genes in cultured BECs. B: Real time quantitative RT-PCR analysis for MEK5 and EGFR mRNA expression. C: Semiquantitative RT-PCR analysis for the mRNA expression. MEK5, mitogen-activated protein kinase/extracellular signal-regulated protein kinase kinase 5; TK, thymidine kinase; TGF-β3, transforming growth factor-β3; TGFR1, TGF-β receptor type 1; EGFR, epidermal growth factor receptor; bFGF, basic fibroblast growth factor; GPx3, plasma glutathione peroxidase. Results of RT-PCR analysis represent the mean of three independent experiments.
Among the products of the 21 differentially expressed genes, MEK5 is a component of the MAPK pathway, which is typically involved in epithelial cell proliferation by acting with EGF. In the following experiments, therefore, we focused on selected components of the MAPK pathway.
Expression of EGF and EGFR
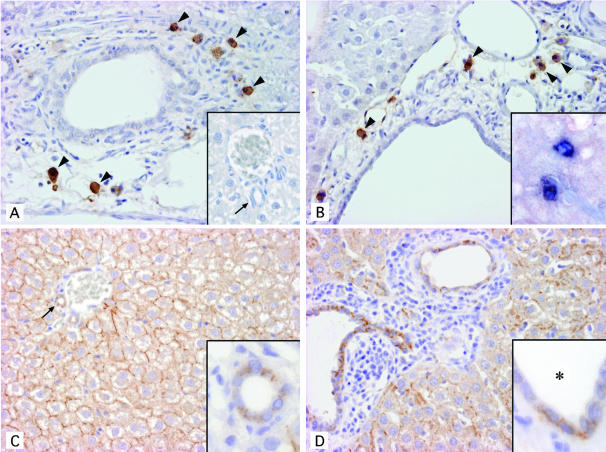
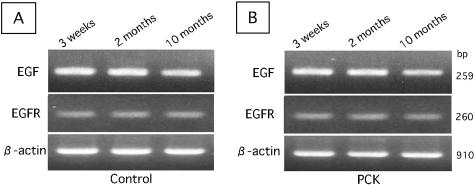
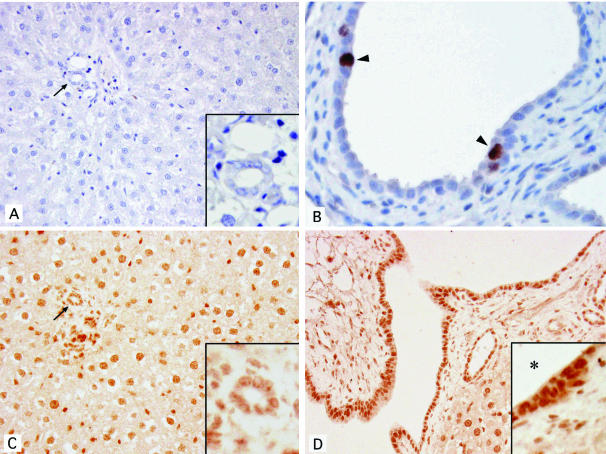
EGF was immunohistochemically localized in the mononuclear cells around the relatively large bile ducts in control rats and around dilated bile ducts of all sizes in PCK rats (Figure 3, A and B). There was a tendency for more EGF-positive cells to accumulate around dilated large bile ducts rather than small ones in the PCK rat. EGF-positive cells were not seen in the small portal tracts of the control rat. The signals were diminished when the sections were incubated with the primary antibody absorbed with the respective peptide antigen, suggesting the specificity of positive signals for EGF. Furthermore, RT-PCR disclosed that EGF mRNA was similarly detected in the liver at 3 weeks, 2 months, and 10 months of age and also similarly in the control and PCK rats (Figure 4, A and B). Double-immunostaining demonstrated that EGF-positive cells were also positive for mast cell tryptase, that is, mast cells, in both rats (Figure 3B, inset).
Figure 3.
Immunohistochemical analysis of the expression of EGF (A and B) and EGFR (C and D) in liver sections of 3-week-old control and PCK rats. EGF was detected in mononuclear cells around the large bile ducts in control rats (A) and around dilated bile ducts in PCK rats (B) (arrowheads). EGF-positive cells were not seen in the small portal tract of control rats (A, inset). Double immunostaining of EGF colored by the benzidine reaction and of mast cell tryptase colored by the Vector blue reaction showed that EGF-positive cells were also positive for typtase, that is, mast cells (B, inset). EGFR was expressed on the basolateral surface of almost all BECs and on the cell membrane of almost all hepatocytes showing a honeycomb appearance in both control (C) and PCK (D) rats. The subcellular localization of EGFR was evident at higher magnification (C and D, insets). Arrows indicate interlobular bile ducts. *, denotes bile duct lumen. Original magnification: ×200 (A to D); insets, ×200 (A), ×400 (B–D).
Figure 4.
RT-PCR analysis of the expression of EGF and EGFR mRNA in whole liver of control and PCK rats. EGF and EFGR mRNA were detected similarly at different ages (3 weeks, 2 months, and 10 months) in control (A) and PCK rats (B).
The expression of EGFR in the liver sections was seen on almost all BECs as well as hepatocytes in both rats (Figure 3, C and D). The subcellular localization of EGFR on BECs was mostly basolateral (Figure 3, C and D, insets), and positive EGFR signals were also focally seen on apical surfaces. Hepatocytes showed cell membrane expression presenting a honeycomb appearance in both rats. Consistent with the results of immunohistochemistry, EGFR mRNA was similarly detected in liver tissue of control and PCK rats at different ages by RT-PCR (Figure 4, A and B), and was also detected in cultured BECs of control and PCK rats to the same degree as in the quantitative RT-PCR analysis (Figure 2B). Thus, the degree and localization of immunohistochemical expression of EGF and EGFR in BECs and hepatocytes were similar at the different ages (3 weeks, 2 months, and 10 months) of both rats. Double immunostaining of EGFR and Ki-67 expression disclosed that while almost all BECs of both rats expressed EGFR, a few and a considerable number of these BECs were positive for Ki-67 in control and PCK rats, respectively (see below).
Proliferative Activity of Cultured BECs and Effects of EGF
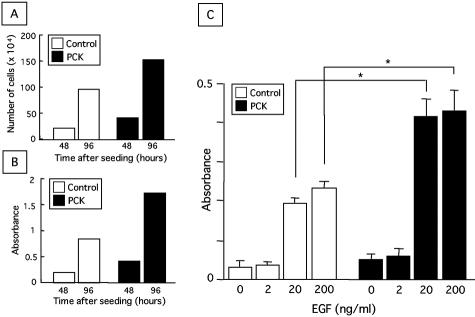
As for the relation between the WST-1 assay and cell proliferative activity, the counted cell number was identical to the value measured with the WST-1 assay in both control and PCK rats at 48 and 96 hours after the seeding of cultured BECs (Figure 5, A and B), confirming that the measured value obtained using WST-1 reagent is directly proportional to the cell number, and thus, the proliferative activity assessed by WST-1 assay can be regarded as an indicator of the actual cell number in this study.
Figure 5.
Effects of EGF on cell proliferative activity of cultured BECs. A and B: Demonstration of the positive correlation between counted cell number of cultured BECs (A) and cell proliferative activity assessed by the WST1 assay (B). A: Actual cell number was determined as described in the Materials and Methods. The data represent the mean of three sets per group. B: For comparison, the WST1 assay was performed in a 96-well format as described in the Materials and Methods. The data represent the mean of six sets per group. C: Effects of EGF on cell proliferative activity. After a 24-hour preincubation with the standard medium, BECs were cultured under different concentrations of EGF for an additional 24 hours, and the proliferative activity was measured using the WST1 assay. The data represent the mean ± SEM of six per group. *, P < 0.05.
The proliferative activity of the cultured BECs of both rats increased dose-dependently in response to EGF (Figure 5C). Compared to the proliferative activity of BECs of control rat, a significant increase in that of PCK rats was observed on the stimulation with EGF at concentrations of 20 and 200 ng/ml. In the subsequent experiments in vitro, the concentration of EGF in the culture medium was adjusted to 20 ng/ml.
Expression of Selected Components of the MAPK Pathway
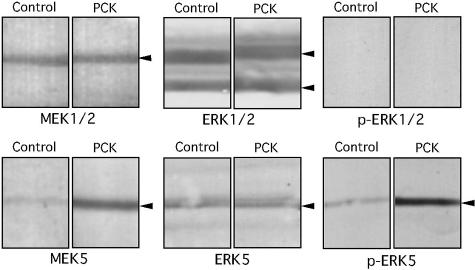
The expression of MAPK kinases (MEK1/2, p-MEK1/2, and MEK5), and MAPKs (ERK1/2, p-ERK1/2, ERK5, and p-ERK5) in the BECs was examined by Western blot analysis. Under the stimulation with EGF, overexpression of MEK5 was observed in cultured BECs of the PCK rat (Figure 6), which corresponded with its gene expression. Accordingly, the expression of p-ERK5 was increased in the PCK rat. Although the expression of MEK1/2, ERK1/2, and ERK5 was similarly detected in cultured BECs of both rats, the expression of p-MEK1/2 and p-ERK1/2 was faint or invisible (Figure 6, data not shown for p-MEK1/2).
Figure 6.
Western blot analysis of the selective components of the MAPK pathway in cultured BECs of control and PCK rats. BECs were cultured in the presence of EGF (20 ng/ml), and 100 μg of total protein extracted from BECs was used. Overexpression of MEK5 and of p-ERK 5 was observed in cultured BECs of PCK rats in comparison with control rats. There were no differences in the expression of MEK1/2, ERK1/2, and ERK5, and p-ERK1/2 was not detected in either of the rats.
Immunohistochemically, no positive signals for p-ERK1/2 were observed in interlobular bile ducts of control rats, while a few positive nuclear signals were seen in the bile duct epithelium of PCK rats (Figure 7, A and B). Positive signals for p-ERK5 were spread diffusely in the nuclei of BECs and hepatocytes in both control and PCK rats, and the signal intensity in BECs was remarkably higher in the PCK rat (Figure 7, C and D). This increased expression of p-ERK5 in BECs of the PCK rat was observed at the different ages of the rats. The Ki-67 labeling index of BECs was continuously higher in the PCK rat at the different ages, with a value of 10.5%, 8.2%, and 13.5% at 3 weeks, 2 months, and 10 months of age, respectively, when compared to the index of the control rats (2.1%, 0.5%, and 0.3% at 3 weeks, 2 months, and 10 months of age), suggesting that p-ERK5 overexpression was involved in cell proliferative activity in the PCK rat.
Figure 7.
Immunohistochemical analysis of the expression of p-ERK1/2 (A and B) and p-ERK5 (C and D) in liver sections of 3-week-old rats. No positive signals for p-ERK1/2 were observed in the portal area of control rats (A), while a few positive nuclear signals were seen in dilated bile duct epithelium of the PCK rat (B, arrowheads). Positive signals for p-ERK5 were diffusely seen in the nuclei of control (C) and PCK (D) rats, and the signal intensity in BECs was greater in PCK rats (D) than in control rats (C). Higher magnifications of bile ducts are given in the insets (A, C, and D). Arrows indicate interlobular bile ducts. *, denotes bile duct lumen. Original magnification: ×200 (A, C and D), ×400 (B); insets, ×400.
In Vitro MAPK Pathway Inhibitor Experiments
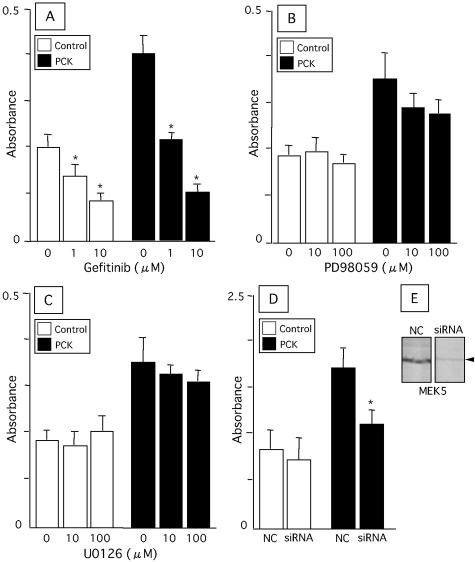
Gefitinib, an EGFR tyrosine kinase inhibitor, significantly inhibited the proliferative activity of BECs of both rats in a dose-dependent fashion (Figure 8A). Both PD98059 and U0126, inhibitors for MEK1/2, were less effective in the inhibition of BECs from both rats (Figure 8, B and C). The EGF-induced proliferative activity of BECs of the PCK rat was significantly inhibited by the transfection of siRNA against MEK5 mRNA (Figure 8D). Western blot analysis confirmed that siRNA against MEK5 mRNA blocked the protein expression in the cultured BECs of PCK rats (Figure 8E).
Figure 8.
Effects of inhibiting the MAPK pathway on proliferative activity of cultured BECs. BECs were treated with gefitinib (A), PD98059 (B), and U0126 (C) in the presence of EGF (20 ng/ml) for 24 hours. Inhibition of MEK5 was performed using short interfering RNA (siRNA) against MEK5 mRNA. After transfection of the siRNA, BECs were incubated with the standard medium containing EGF (20 ng/ml) for 72 hours, and the proliferative activity was measured (D). The proliferative activity was measured using the WST1 proliferation assay. Western blot analysis showed that siRNA against MEK5 mRNA blocked the protein expression in BECs of the PCK rat (E). The data represent the mean ± SEM of six per group. NC, negative control; *, P < 0.05 versus control.
Discussion
In this study, we identified the essential involvement of the MEK5-ERK5 pathway in the abnormal growth of BECs of the PCK rat. At first, we determined differentially expressed genes in the cultured BECs of PCK rats using a cDNA microarray analysis. Gene analyses using cultured BECs are preferable, because lymphocytes and neutrophils infiltrate to various degrees the portal tracts and the bile duct lumen of the PCK rat during the course of aging, and such secondary effects on BECs due to inflammatory or immunological reactions can be negligible. Although most of the 21 differentially expressed genes identified here have not been well studied with regard to function in BECs, seven genes (MEK5, TK, junD, TGF-β, TGFR1, bFGF, and GPx3) seemed to be related to cell proliferation, apoptosis, and/or fibrotic responses.
TGF-β3 is reported to trigger the apoptosis of epithelial cells.30,31 A recent study showed that the TGF-β signaling for apoptosis involved activated protein (AP)-1 and Smad proteins, and the JunD + FosB form of the AP-1 complex was markedly activated during TGF-β-dependent apoptosis.32 The overexpression of transcripts encoding TGF-β3, its receptor TGFR1 and JunD in BECs of the PCK rat indicates that TGF-β enhances the apoptosis of BECs by interacting with its receptor. In addition, TGF-β is known to induce fibrogenesis in the liver.33,34 The overexpression of the transcript encoding TGF-β3 as well as one of the other fibrogenic growth factors, bFGF, in the PCK rat indicates that these growth factors are involved in the progressive portal fibrogenesis.35
Maser et al36 reported that various organs including the kidney of mice with ARPKD expressed extracellular GPx, an antioxidant enzyme, and the enzymatic activity of GPx was reduced in kidneys and blood plasma of ARPKD mice. It is considered that GPx reduces excessive oxidative stress and protects tissues from the deleterious effects of reactive oxygen species. Recent findings suggest that intracellular oxidants are involved in the induction of apoptosis.37 The reduced expression of GPx3 mRNA in the BECs of the PCK rat may lead to excessive oxidative stress, promoting apoptosis. Although the discussion concerning the results of the cDNA microarray analysis remains speculative, the imbalanced cell kinetics including enhanced apoptosis in the BECs of the PCK rat may be related to the dilatation of intrahepatic bile ducts.21
There have been a number of studies on the role of EGF and EGFR in the development of the renal cystic pathology. According to previous reports, EGF expression is generally diminished in rodent models of PKD,16,23,38 while EGFR in the kidneys is overexpressed in murine5,16,25 and human39 PKD. EGF inhibits developmentally regulated apoptosis in the kidney,40 and neonatal injections of exogenous EGF have been shown to inhibit cystic enlargement and/or renal dysfunction in infantile forms of PKD,5,41 indicating the early protective effects of EGF and the involvement of apoptosis in these effects. However, continued administration of EGF during the postnatal period has been shown to accelerate the disease.42 Similarly, there have been studies showing that promotion of proliferation by members of the EGF family and/or excessive stimulation of EGFR also can increase cell proliferation and therefore exacerbate the progression of PKD.43,44 Diminished renal EGF may be an early contributor to PKD by inhibiting collecting duct differentiation, and leading to an up-regulation of EGFR that affects disease progression, but the precise role of EGF and EGFR in the renal cystic pathology and progression of PKD is still unclear.
This study showed that EGFR mRNA expression was observed to the same degree in the cultured BECs of both control and PCK rats by cDNA microarray and quantitative RT-PCR analysis. EGFR mRNA was also detectable in the liver tissue of control and PCK rats, and EGFR was similarly expressed immunohistochemically on the cell membrane of almost all BECs of both control and PCK rats. However, Ki-67 was detected in a few and a considerable number of these BECs in control and PCK rats, respectively, suggesting that another factor(s) in addition to EGFR expressed on BECs is important in the proliferation of BECs of PCK rats. In this study, EGF mRNA was detected in the liver of control and PCK rats by RT-PCR and EGF was shown to be located in mast cells around the bile ducts by double-immunostaining. These EGF-expressing mast cells were rather dense around small and large bile ducts of PCK rats in comparison with control rats in which EGF-positive mast cells were seen around the large bile ducts but not the small portal tracts, suggesting that these EGF-positive cells are involved in the proliferation of BECs and cystic dilatation of bile ducts in the PCK rat. Although mast cells are known for their role in allergic reactions, there are also nonimmune-related roles of mast cells that predominate in connective tissue. These nonimmune mast cells are a source of various growth factors, thereby participating in cell differentiation and the synthesis of extracellular matrix molecules.45 Recently, mast cells have been shown to express abundant EGF mRNA in the rat.46 The interaction of EGF and EFGR was shown in isolated BECs of rats,47 suggesting that mast cell-derived EGF and EGFR expressed on BECs is critically involved in the cystic bile duct enlargement in the PCK rat. In addition, EGF in the bile and plasma, which mainly originates from submandibular glands and has been implicated in liver pathophysiology,48,49 may relate to the stimulation of BEC growth.
Nauta et al27 demonstrated that BECs from BALB/c-bpk mice showed increased sensitivity to the proliferative effect of EGF. In other reports, renal cyst epithelial cells were hypersensitive to EGF-induced mitogenesis in some,50 but not all, cases.51 Nagao et al26 showed that activation of the MAPK pathway, including B-Raf and ERK1/2, in renal cyst epitheilal cells might be responsible for the development of cysts in ADPKD. In our experiments, BECs of the PCK rat were hyperresponsive to EGF, and this effect was mediated primarily by the overexpression of MEK5, which subsequently led to the increased phosphorylation of ERK5, rather than by the activation of the MAPK pathway consisting of MEK1/2 and ERK1/2. The findings obtained in this study that p-ERK5 was overexpressed and cell proliferative activities assessed by Ki-67 labeling were increased in a considerable number of BECs of PCK rats at different ages, when compared to control rats, support this scenario. We also demonstrated that the enhanced proliferative activity of BECs of the PCK rat was significantly inhibited by the transfection of siRNA against MEK5 mRNA. Kato et al22 demonstrated that the MEK5-ERK5 pathway is critically involved in the mitogenic activation by EGF, and also that ERK5 is required for EGF-induced cell proliferation and progression through the cell cycle from G1 to S phase. In addition, overexpression of the transcript encoding TK, a rate-limiting enzyme for DNA synthesis, observed in this study, may be associated with the overgrowth of BECs of the PCK rat. We consider that the activation of the MEK5-ERK5 cascade plays a pivotal role in the biliary dysgenesis of the PCK rat.
Gefitinib, an EGFR tyrosine kinase inhibitor, was originally developed as an anticancer agent.52 In this study, we used gefitinib to inhibit the overgrowth of BECs of the PCK rat. As expected, this agent had prominent inhibitory effects on the growth of BECs. It has been demonstrated that treatment of BALB/c-bpk mice with a tyrosine kinase inhibitor (EKI-785) inhibited renal enlargement, the development of renal dysfunction, and hepatic pathology,53 suggesting the possibility of using tyrosine kinase inhibitors as therapeutic agents for ARPKD.
In normal BECs, gefitinib had an inhibitory effect on cell proliferative activity, but inhibitors for MEK1/2 (PD98059 and U0126) and siRNA for MEK5 mRNA did not, suggesting that the proliferative effect of EGF on normal BECs is mediated neither by MEK1/2 nor by MEK5. In renal epithelial cells, it has been shown that activation of EGFR led to cell proliferation, and this proliferative effect was mediated by phosphoinositide-3-kinase (PI3K) and subsequent phosphorylation of Akt, rather than by phosphorylation of ERK1/2.54 One possible mechanism is that cell proliferation in response to EGF is mediated by the PI3K pathway in normal BECs.
Proliferating bile duct epithelial cells have been shown to be a major source of connective tissue growth factor, and thus proliferating BECs can be a contributor to liver fibrogenesis.55 Recently, Mehta et al56 demonstrated that overexpression of MEK5 led to the upregulation of the transcript encoding matrix metalloproteinase-9, an extracellular matrix degrading protease, in human prostatic cancer, and the overexpression was positively associated with tumor progression. Their data indicate the involvement of MEK5 in the kinetics of extracellular matrix molecules. Inhibition of the MEK5-ERK5 pathway by the administration of gefitinib may be effective for the inhibition of cyst development in the PCK rat, and this inhibition may also lead to an improvement in the progressive portal fibrosis. Therefore, further study in vivo is warranted.
Recently, Masyuk et al57 have showed that fibrocystin, a protein product of PKHD1, is expressed in cholangiocyte cilia and the disruption of PKHD1 results in abnormalities in ciliary morphology. Although the precise role of fibrocystin is yet to be determined and the causal relation between fibrocystin and overexpression of MEK5 remains unknown,18,19 the present study provided evidence to support an important role of activation of the MEK5-ERK5 pathway in the pathogenesis of cysts in the PCK rat, and identify key aspects of dysregulated BEC growth. The data obtained here also provide insights into the pathogenesis of biliary dysgenesis and hepatic fibrosis in Caroli’s disease with CHF. As the MEK5-ERK5 interaction is highly specific, it may represent a potential target of therapy for Caroli’s disease.
Acknowledgments
We thank AstraZeneca, Macclesfield, UK for kindly providing the Gefitinib (“Iressa”), a trademark of the AstraZeneca group of companies.
Footnotes
Address reprint requests to Yasuni Nakanuma, M.D., Ph.D., Department of Human Pathology, Kanazawa University, Graduate School of Medicine, Kanazawa 920-8640, Japan. E-mail: pbcpsc@kenroku.kanazawa-u.ac.jp.
References
- Nakanuma Y, Terada T, Ohta G, Kurachi M, Matsubara F. Caroli’s disease in congenital hepatic fibrosis and infantile polycystic disease. Liver. 1982;2:346–354. doi: 10.1111/j.1600-0676.1982.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Desmet VJ. Congenital diseases of intrahepatic bile ducts: variations on the theme “ductal plate malformation”. Hepatology. 1992;16:1069–1083. doi: 10.1002/hep.1840160434. [DOI] [PubMed] [Google Scholar]
- Murcia NS, Sweeney WE, Jr, Avner ED. New insights into the molecular pathophysiology of polycystic kidney disease. Kidney Int. 1999;55:1187–1197. doi: 10.1046/j.1523-1755.1999.00370.x. [DOI] [PubMed] [Google Scholar]
- Ramasubbu K, Gretz N, Bachmann S. Increased epithelial cell proliferation and abnormal extracellular matrix in rat polycystic kidney disease. J Am Soc Nephrol. 1998;9:937–945. doi: 10.1681/ASN.V96937. [DOI] [PubMed] [Google Scholar]
- Ricker JL, Gattone VH, II, Calvet JP, Rankin CA. Development of autosomal recessive polycystic kidney disease in BALB/c-cpk/cpk mice. J Am Soc Nephrol. 2000;11:1837–1847. doi: 10.1681/ASN.V11101837. [DOI] [PubMed] [Google Scholar]
- Harding MA, Gattone VH, II, Grantham JJ, Calvet JP. Localization of overexpressed c-myc mRNA in polycystic kidneys of the cpk mouse. Kidney Int. 1992;41:317–325. doi: 10.1038/ki.1992.44. [DOI] [PubMed] [Google Scholar]
- Cowley BD, Jr, Chadwick LJ, Grantham JJ, Calvet JP. Elevated proto-oncogene expression in polycystic kidneys of the C57BL/6J (cpk) mouse. J Am Soc Nephrol. 1991;1:1048–1053. doi: 10.1681/ASN.V181048. [DOI] [PubMed] [Google Scholar]
- Ali SM, Wong VY, Kikly K, Fredrickson TA, Keller PM, DeWolf WE, Jr, Lee D, Brooks DP. Apoptosis in polycystic kidney disease: involvement of caspases. Am J Physiol. 2000;278:R763–R769. doi: 10.1152/ajpregu.2000.278.3.R763. [DOI] [PubMed] [Google Scholar]
- Gattone VH, II, Calvet JP, Cowley BD, Jr, Evan AP, Shaver TS, Helmstadter K, Grantham JJ. Autosomal recessive polycystic kidney disease in a murine model: a gross and microscopic description. Lab Invest. 1988;59:231–238. [PubMed] [Google Scholar]
- Nakamura T, Ebihara I, Nagaoka I, Tomino Y, Nagao S, Takahashi H, Koide H. Growth factor gene expression in kidney of murine polycystic kidney disease. J Am Soc Nephrol. 1993;3:1378–1386. doi: 10.1681/ASN.V371378. [DOI] [PubMed] [Google Scholar]
- Rocco MV, Neilson EG, Hoyer JR, Ziyadeh FN. Attenuated expression of epithelial cell adhesion molecules in murine polycystic kidney disease. Am J Physiol. 1992;262:F679–F686. doi: 10.1152/ajprenal.1992.262.4.F679. [DOI] [PubMed] [Google Scholar]
- Ali SM, Nambi P, Fredrickson TA, Brooks DP. Epithelin mRNA expression in polycystic kidney disease. Peptides. 1999;20:1489–1495. doi: 10.1016/s0196-9781(99)00160-6. [DOI] [PubMed] [Google Scholar]
- Ebihara I, Killen PD, Laurie GW, Huang T, Yamada Y, Martin GR, Brown KS. Altered mRNA expression of basement membrane components in a murine model of polycystic kidney disease. Lab Invest. 1988;58:262–269. [PubMed] [Google Scholar]
- Rankin CA, Itoh Y, Tian C, Ziemer DM, Calvet JP, Gattone VH., II Matrix metalloproteinase-2 in a murine model of infantile-type polycystic kidney disease. J Am Soc Nephrol. 1999;10:210–217. doi: 10.1681/ASN.V102210. [DOI] [PubMed] [Google Scholar]
- Ebihara I, Nakamura T, Takahashi T, Yamamoto M, Tomino Y, Nagao S, Takahashi H, Koide H. Altered extracellular matrix component gene expression in murine polycystic kidney. Renal Physiol Biochem. 1995;18:73–80. doi: 10.1159/000173902. [DOI] [PubMed] [Google Scholar]
- Gattone VH, Ricker JL, Trambaugh CM, Klein RM. Multiorgan mRNA misexpression in murine autosomal recessive polycystic kidney disease. Kidney Int. 2002;62:1560–1569. doi: 10.1046/j.1523-1755.2002.00632.x. [DOI] [PubMed] [Google Scholar]
- Katsuyama M, Masuyama T, Komura I, Hibino T, Takahashi H. Characterization of a novel polycystic kidney rat model with accompanying polycystic liver. Exp Anim. 2000;49:51–55. doi: 10.1538/expanim.49.51. [DOI] [PubMed] [Google Scholar]
- Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schoneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70:1305–1317. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lager DJ, Qian Q, Bengal RJ, Ishibashi M, Torres VE. The pck rat: a new model that resembles human autosomal dominant polycystic kidney and liver disease. Kidney Int. 2001;59:126–136. doi: 10.1046/j.1523-1755.2001.00473.x. [DOI] [PubMed] [Google Scholar]
- Sanzen T, Harada K, Yasoshima M, Kawamura Y, Ishibashi M, Nakanuma Y. Polycystic kidney rat is a novel animal model of Caroli’s disease associated with congenital hepatic fibrosis. Am J Pathol. 2001;158:1605–1612. doi: 10.1016/S0002-9440(10)64116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395:713–716. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- Gattone VH, II, Andrews GK, Niu FW, Chadwick LJ, Klein RM, Calvet JP. Defective epidermal growth factor gene expression in mice with polycystic kidney disease. Dev Biol. 1990;138:225–230. doi: 10.1016/0012-1606(90)90192-l. [DOI] [PubMed] [Google Scholar]
- Horikoshi S, Kubota S, Martin GR, Yamada Y, Klotman PE. Epidermal growth factor (EGF) expression in the congenital polycystic mouse kidney. Kidney Int. 1991;39:57–62. doi: 10.1038/ki.1991.7. [DOI] [PubMed] [Google Scholar]
- Orellana SA, Sweeney WE, Neff CD, Avner ED. Epidermal growth factor receptor expression is abnormal in murine polycystic kidney. Kidney Int. 1995;47:490–499. doi: 10.1038/ki.1995.62. [DOI] [PubMed] [Google Scholar]
- Nagao S, Yamaguchi T, Kusaka M, Maser RL, Takahashi H, Cowley BD, Grantham JJ. Renal activation of extracellular signal-regulated kinase in rats with autosomal-dominant polycystic kidney disease. Kidney Int. 2003;63:427–437. doi: 10.1046/j.1523-1755.2003.00755.x. [DOI] [PubMed] [Google Scholar]
- Nauta J, Sweeney WE, Rutledge JC, Avner ED. Biliary epithelial cells from mice with congenital polycystic kidney disease are hyperresponsive to epidermal growth factor. Pediatr Res. 1995;37:755–763. doi: 10.1203/00006450-199506000-00014. [DOI] [PubMed] [Google Scholar]
- Katayanagi K, Kono N, Nakanuma Y. Isolation, culture, and characterization of biliary epithelial cells from different anatomical levels of the intrahepatic and extrahepatic biliary tree from a mouse. Liver. 1998;18:90–98. doi: 10.1111/j.1600-0676.1998.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Weinreich DM, Elaraj DM, Puhlmann M, Hewitt SM, Carroll NM, Feldman ED, Turner EM, Spiess PJ, Alexander HR. Effect of interleukin 1 receptor antagonist gene transduction on human melanoma xenografts in nude mice. Cancer Res. 2003;63:5957–5961. [PubMed] [Google Scholar]
- Rodriguez GC, Nagarsheth NP, Lee KL, Bentley RC, Walmer DK, Cline M, Whitaker RS, Isner P, Berchuck A, Dodge RK, Hughes CL. Progestin-induced apoptosis in the macaque ovarian epithelium: differential regulation of transforming growth factor-beta. J Natl Cancer Inst. 2002;94:50–60. doi: 10.1093/jnci/94.1.50. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Tumour-stromal interactions: transforming growth factor-beta isoforms and hepatocyte growth factor/scatter factor in mammary gland ductal morphogenesis. Breast Cancer Res. 2001;3:230–237. doi: 10.1186/bcr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura Y, Hua X, Bergelson S, Lodish HF. Critical role of Smads and AP-1 complex in transforming growth factor-beta-dependent apoptosis. J Biol Chem. 2000;275:36295–36302. doi: 10.1074/jbc.M006023200. [DOI] [PubMed] [Google Scholar]
- Roulot D, Sevcsik AM, Coste T, Strosberg AD, Marullo S. Role of transforming growth factor beta type II receptor in hepatic fibrosis: studies of human chronic hepatitis C and experimental fibrosis in rats. Hepatology. 1999;29:1730–1738. doi: 10.1002/hep.510290622. [DOI] [PubMed] [Google Scholar]
- Milani S, Herbst H, Schuppan D, Stein H, Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991;139:1221–1229. [PMC free article] [PubMed] [Google Scholar]
- Napoli J, Prentice D, Niinami C, Bishop GA, Desmond P, McCaughan GW. Sequential increases in the intrahepatic expression of epidermal growth factor, basic fibroblast growth factor, and transforming growth factor beta in a bile duct ligated rat model of cirrhosis. Hepatology. 1997;26:624–633. doi: 10.1002/hep.510260314. [DOI] [PubMed] [Google Scholar]
- Maser RL, Vassmer D, Magenheimer BS, Calvet JP. Oxidant stress and reduced antioxidant enzyme protection in polycystic kidney disease. J Am Soc Nephrol. 2002;13:991–999. doi: 10.1681/ASN.V134991. [DOI] [PubMed] [Google Scholar]
- Bauer G. Reactive oxygen and nitrogen species: efficient, selective, and interactive signals during intercellular induction of apoptosis. Anticancer Res. 2000;20:4115–4139. [PubMed] [Google Scholar]
- Cowley BD, Jr, Rupp JC. Abnormal expression of epidermal growth factor and sulfated glycoprotein SGP-2 messenger RNA in a rat model of autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;6:1679–1681. doi: 10.1681/ASN.V661679. [DOI] [PubMed] [Google Scholar]
- Klingel R, Dippold W, Storkel S, Meyer zum Buschenfelde KH, Kohler H. Expression of differentiation antigens and growth-related genes in normal kidney, autosomal dominant polycystic kidney disease, and renal cell carcinoma. Am J Kidney Dis. 1992;19:22–30. doi: 10.1016/s0272-6386(12)70198-1. [DOI] [PubMed] [Google Scholar]
- Coles HS, Burne JF, Raff MC. Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development. 1993;118:777–784. doi: 10.1242/dev.118.3.777. [DOI] [PubMed] [Google Scholar]
- Gattone VH, II, Lowden DA, Cowley BD., Jr Epidermal growth factor ameliorates autosomal recessive polycystic kidney disease in mice. Dev Biol. 1995;169:504–510. doi: 10.1006/dbio.1995.1164. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Gattone VH, II, Sweeney WE, Avner ED. Renal dysfunction but not cystic change is ameliorated by neonatal epidermal growth factor in bpk mice. Pediatr Nephrol. 2001;16:45–50. doi: 10.1007/s004670000495. [DOI] [PubMed] [Google Scholar]
- Gattone VH, II, Kuenstler KA, Lindemann GW, Lu X, Cowley BD, Jr, Rankin CA, Calvet JP. Renal expression of a transforming growth factor-alpha transgene accelerates the progression of inherited, slowly progressive polycystic kidney disease in the mouse. J Lab Clin Med. 1996;127:214–222. doi: 10.1016/s0022-2143(96)90081-5. [DOI] [PubMed] [Google Scholar]
- Richards WG, Sweeney WE, Yoder BK, Wilkinson JE, Woychik RP, Avner ED. Epidermal growth factor receptor activity mediates renal cyst formation in polycystic kidney disease. J Clin Invest. 1998;101:935–939. doi: 10.1172/JCI2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church MK, Levi-Schaffer F. The human mast cell. J Allergy Clin Immunol. 1997;99:155–160. doi: 10.1016/s0091-6749(97)70089-7. [DOI] [PubMed] [Google Scholar]
- Gilbert RE, Rumble JR, Cao Z, Cox AJ, van Eeden P, Allen TJ, Kelly DJ, Cooper ME. Endothelin receptor antagonism ameliorates mast cell infiltration, vascular hypertrophy, and epidermal growth factor expression in experimental diabetes. Circ Res. 2000;86:158–165. doi: 10.1161/01.res.86.2.158. [DOI] [PubMed] [Google Scholar]
- Ishii M, Vroman B, LaRusso NF. Morphologic demonstration of receptor-mediated endocytosis of epidermal growth factor by isolated bile duct epithelial cells. Gastroenterology. 1990;98:1284–1291. doi: 10.1016/0016-5085(90)90346-3. [DOI] [PubMed] [Google Scholar]
- Jones DE, Jr, Tran-Patterson R, Cui DM, Davin D, Estell KP, Miller DM. Epidermal growth factor secreted from the salivary gland is necessary for liver regeneration. Am J Physiol. 1995;268:G872–G878. doi: 10.1152/ajpgi.1995.268.5.G872. [DOI] [PubMed] [Google Scholar]
- Grau M, Rodriguez C, Soley M, Ramirez I. Relationship between epidermal growth factor in mouse submandibular glands, plasma, and bile: effects of catecholamines and fasting. Endocrinology. 1994;135:1854–1862. doi: 10.1210/endo.135.5.7956907. [DOI] [PubMed] [Google Scholar]
- Wilson PD, Du J, Norman JT. Autocrine, endocrine and paracrine regulation of growth abnormalities in autosomal dominant polycystic kidney disease. Eur J Cell Biol. 1993;61:131–138. [PubMed] [Google Scholar]
- Carone FA, Nakamura S, Schumacher BS, Punyarit P, Bauer KD. Cyst-derived cells do not exhibit accelerated growth or features of transformed cells in vitro. Kidney Int. 1989;35:1351–1357. doi: 10.1038/ki.1989.134. [DOI] [PubMed] [Google Scholar]
- Baselga J, Averbuch SD. ZD1839 (“Iressa”) as an anticancer agent. Drugs. 2000;60(Suppl 1):33–40. doi: 10.2165/00003495-200060001-00004. 41–32. [DOI] [PubMed] [Google Scholar]
- Sweeney WE, Chen Y, Nakanishi K, Frost P, Avner ED. Treatment of polycystic kidney disease with a novel tyrosine kinase inhibitor. Kidney Int. 2000;57:33–40. doi: 10.1046/j.1523-1755.2000.00829.x. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Dang Y, Schnellmann RG. Requirement of the epidermal growth factor receptor in renal epithelial cell proliferation and migration. Am J Physiol. 2004;287:F365–F372. doi: 10.1152/ajprenal.00035.2004. [DOI] [PubMed] [Google Scholar]
- Sedlaczek N, Jia JD, Bauer M, Herbst H, Ruehl M, Hahn EG, Schuppan D. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol. 2001;158:1239–1244. doi: 10.1016/S0002-9440(10)64074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PB, Jenkins BL, McCarthy L, Thilak L, Robson CN, Neal DE, Leung HY. MEK5 overexpression is associated with metastatic prostate cancer and stimulates proliferation, MMP-9 expression, and invasion. Oncogene. 2003;22:1381–1389. doi: 10.1038/sj.onc.1206154. [DOI] [PubMed] [Google Scholar]
- Masyuk TV, Huang BQ, Ward CJ, Masyuk AI, Yuan D, Splinter PL, Punyashthiti R, Ritman EL, Torres VE, Harris PC, LaRusso NF. Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology. 2003;125:1303–1310. doi: 10.1016/j.gastro.2003.09.001. [DOI] [PubMed] [Google Scholar]