Abstract
Altered corneal epithelial barrier function is the cause for ocular irritation and visual morbidity in dry eye disease. Increased matrix metalloproteinase (MMP)-9 activity has been observed in the tear fluid of dry eye patients. To determine the pathogenic role of MMP-9 in the corneal epithelial disease of dry eye, the effects of experimentally induced dry eye on corneal epithelial morphology and barrier function were compared in MMP-9 knockout mice and their wild-type littermates. Dry eye was created through cholinergic blockade and exposure to a desiccating environment. The tear fluid MMP-9 concentration increased in response to dryness in wild-type mice. Corneal epithelial permeability to three different-sized molecules increased in dry eye wild-type mice, but not in MMP-9 knockout mice. Topical administration of active MMP-9 to dry eye MMP-9 knockout mice significantly increased corneal epithelial permeability. Compared to MMP-9 knockout mice, wild-type mice showed greater desquamation of differentiated apical corneal epithelial cells that expressed the tight junction protein occludin in response to dryness. This was accompanied by an increase in lower sized (50 kd) occludin in the corneal epithelia of wild-type mice. These findings could be replicated in cultured human corneal epithelial cells that were treated with active MMP-9. These studies indicate that increased MMP-9 activity on the ocular surface in response to dryness disrupts corneal epithelial barrier function. This appears to be because of accelerated loss of tight junction bearing superficial corneal epithelial cells, perhaps by proteolytic cleavage of occludin.
Corneal epithelial disease, termed keratitis sicca, is a severe and sight-threatening complication of dry eye.1 A key clinical feature of keratitis sicca is disruption of corneal epithelial barrier function.2–4 This results in eye irritation, corneal surface irregularity, blurred and fluctuating vision, and increased risk for corneal ulceration.4–8 Ocular surface inflammation has been implicated in the pathogenesis of keratitis sicca. Elevated levels of proinflammatory cytokines, such as interleukin (IL)-1, have been detected in the tear fluid of patients with this condition.9–11 Furthermore, the concentration and activity of matrix metalloproteinase (MMP)-9 in the tear fluid was found to be significantly increased in these eyes, with the highest concentrations observed in eyes with the severe corneal epithelial disease or sterile corneal ulcers.10–12
We hypothesize that MMP-9 plays an important role in the disruption of corneal epithelial barrier function in dry eye. We previously reported an experimental murine model of dry eye that disrupts corneal epithelial barrier function similar to human dry eye disease.13 The purpose of this study was to compare the effects of experimentally induced dry eye (EIDE) on corneal epithelial morphology and barrier function in MMP-9 knockout mice and their wild-type (WT) littermates.
Materials and Methods
Mice
This research protocol was approved by the Baylor College of Medicine Center for Comparative Medicine and it conformed to the standards in the Association for Research in Vision and Ophthalmology (ARVO) Statement for the use of animals in ophthalmic and vision research. MMP-9 (gelatinase B) knockout mice (referred to as BKO mice) were created on a 129SvEv/CD-1 mixed background as previously reported.14 WT (GelB +/+) littermates were used as controls.
Mouse genotypes were verified throughout the study by polymerase chain reaction (PCR) performed on tail genomic DNA. Genomic DNA was isolated from tails of 1-month-old mice using a Genomic DNA Isolation kit (Sigma, St. Louis, MO) according to the manufacturer’s instructions. A specific primer pair including a sense primer (GCATACTTGTACCGCTATGGT) and an anti-sense primer (TGTGATGTTATGATGGTCCC) was designed from the sequence of MMP-9 gene exon 2 (accession no. X72794), which was knocked out and replaced in BKO mice by a cassette containing the neomycin phosphotransferase cDNA (neo′) driven by the phosphoglycerate kinase (PGK) promoter.15 PCR amplification was performed in a 96-well GeneAmp PCR System 9700 using a GeneAmp PCR kit (Applied Biosystems, Foster City, CA) in a 50-μl volume containing 1 μg of genomic DNA, dNTPs, Taq polymerase, and the specific primers.
Creation of Dry Eye
EIDE was created by subcutaneous injection of 0.5 mg/0.2 ml scopolamine hydrobromide four times a day (8:00 a.m., 12:00 p.m., 2:00 p.m., and 5:00 p.m.), alternating between the left and right flanks of 4- to 6-week-old WT and BKO mice as previously described.16 Up to five mice were placed in a cage with a perforated plastic screen on one side to allow airflow from a fan (Cafrano, Wiarton, Ontario, Canada) placed 6 inches in front of it for 16 hours per day. Tear production was measured with phenol-red impregnated cotton threads (Zone-Quick; Oasis, Glendora, CA) placed into the tear meniscus of the lateral canthus for 30 seconds. Tear fluorescein clearance (the residual concentration of fluorescein dye in the tear fluid 15 minutes after instillation) was measured as previously reported.13
Gelatin Zymography
The relative amount of MMP-9 in tear washings was measured by gelatin zymography. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis gelatin zymography was performed using a previously reported method.17 Tear fluid was collected using 1.0-μl polished glass capillary pipettes (Drummond, Broomhall, PA) immediately after a 1.0-μl drop of Brij buffer was placed on the ocular surface. Pooled tear samples from treated or untreated WT and BKO mouse were fractionated in an 8% polyacrylamide gel containing gelatin (0.5 mg/ml). The gels were soaked in 0.25% Triton X-100 for 30 minutes at room temperature, and incubated in a digestion buffer containing 5 mmol/L phenylmethyl sulfonyl fluoride at 37°C overnight. They were stained with 0.25% Coomassie brilliant blue R-250 in 40% isopropanol for 2 hours, and destained overnight in 7% acetic acid. Gelatinolytic activities appeared as clear bands of digested gelatin against a dark blue background of stained gelatin.
RNA Isolation and Semiquantitative Reverse Transcriptase (RT)-PCR
MMP-9 RNA expression in the cornea epithelium was measured by RT-PCR. Total RNA was isolated from corneal epithelia (six eyes per group) of WT or BKO mice with or without EIDE by acid guanidium thiocyanate-phenol-chloroform extraction method. The 371 bp of the mouse MMP-9 gene was amplified by semiquantitative RT-PCR using the sense primer TGTACCGCTATGGTTACACCCG and anti-sense primer CGCGACACCAAACTGGATGAC, which was specifically designed to amplify a segment from exon 2 to exon 3 of the mouse MMP-9 sequence (accession no. NM_013599 and X72794), of which the part of exon 2 and all of intron 2 were replaced in BKO mouse. The housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was amplified as an internal control using the primer pair, GCCAAGGTCATCCATGACAAC and GTCCACCACCCTGTTGCTGTA, which was specifically designed from exon 7 and exon 9 of the mouse GAPDH sequence (accession no. M32599).
Corneal Epithelial Permeability
Corneal epithelial permeability to three different molecules [carboxyfluorescein, Alexa-Fluor-dextran (AFD), and horseradish peroxidase (HRP)] was assessed in six eyes of control mice and mice with EIDE for 2 weeks. One μl of 0.3% carboxyfluorescein (Holles Laboratory, Cohasset, MA) or AFD (Molecular Probes, Eugene, OR) (10 kd molecular weight) was placed on the ocular surface 15 minutes before euthanasia. Corneas were excised, rinsed four times in phosphate-buffered saline (PBS), and placed in wells of a 96-well plate (Costar-Corning, Corning, NY) containing 100 μl of PBS, covered, wrapped in aluminum foil, and placed on a shaker for 60 minutes. Carboxyfluorescein and AFD concentrations were measured with a Cytofluor II fluorometer (Perseptive Biosystems, Framingham, MA) as previously reported.13 HRP uptake by the cornea was measured by placing 1 μl of a 1 U/ml HRP solution on the ocular surface 15 minutes before euthanasia. HRP was detected in excised corneas using an Amplex Red detection system (Molecular Probes) and measuring absorbance using a 530-nm absorbance and 590-nm emission filters at 10, 20, and 30 minutes.
To assess the affects of topical application of MMP-9 on corneal epithelial permeability in BKO mice, 1 μl of a 1 μg/ml solution of active MMP-9 (Oncogene Research Products, Boston, MA) prepared in PBS was administered topically every 2 hours for 10 hours a day for 2 days to mice with and without EIDE. Control mice received 1 μl of PBS every 2 hours or no drops. Three eyes of three animals were evaluated in each treatment group.
Histology
Eyes from WT and BKO mice with and without EIDE were surgically excised, fixed in 10% formalin, and embedded in paraffin. Six-μm sections were stained with hematoxylin and eosin or periodic acid-Schiff (PAS) reagent. Sections from three different eyes in each group were examined and photographed with a Nikon Eclipse E400 (Garden City, NY) microscope equipped with a Nikon DXM 1200 digital camera.
Transmission Electron Microscopy
After fixation, the corneal samples from WT and BKO mice with and without EIDE (n = 2) were rinsed in buffer and postfixed in PIPES-buffered osmium tetroxide (pH 7.2) for 1 hour at room temperature, then rinsed in several changes of distilled water, and dehydrated through a graded series of ethanol. The dehydrated tissues were incubated in two 45-minute changes of propylene oxide followed by a 1:1 mixture of propylene oxide and Spurr’s resin for 1 hour and 30 minutes. The tissue pieces were then incubated in pure resin for 1 hour and 30 minutes, after which they were transferred to fresh resin in block molds and allowed to cure at 60°C overnight. Thick sections (1 μm) cut from the hardened blocks were mounted on glass slides, stained with an alcoholic solution of toluidine blue and basic fuchsin, and examined under the light microscope. Areas of interest were trimmed and 60-nm sections were cut and mounted on copper grids (300 mesh). The grids were stained with uranyl acetate and lead citrate and photographed with a Zeiss EM-900 transmission electron microscope (Peabody, MA). Photographs were taken with Kodak 4489 electron microscope film (Rochester, NY).
Confocal Microscopy
Whole freshly harvested murine corneas from WT and BKO mice with and without EIDE (n = 3) were fixed in cold methanol for 10 minutes at −20°C. After fixation, they were permeabilized with PBS containing 0.1% Triton X for 10 minutes. The tissues were blocked with 20% goat serum in PBS for 1 hour at room temperature to reduce nonspecific labeling. The tissues were then incubated with polyclonal rabbit anti-occludin (1:25 dilution; Zymed, San Francisco, CA) diluted in 5% goat serum and PBS, overnight at 4°C. Tissues without primary antibody were used as negative controls. After extensive washing with PBS, Alexa Fluor-488-conjugated goat anti-rabbit antibody (1:300, Molecular Probes) was applied for 1 hour at room temperature. Tissues were rinsed and counterstained with propidium iodide (1 μg/ml in PBS) for 30 minutes. After washing with PBS, corneal tissues were flattened on microscope slides, mounted with anti-fade Gel/Mount (Fisher, Atlanta, GA) and coverslips were applied.
Digital confocal images (512 × 512 pixels) were captured with a laser-scanning confocal microscope (LSM 510, Zeiss with krypton-argon and He-Ne laser; Zeiss, Thornwood, NY) with 488-excitation and 543-nm emission filters, LP505 and LP560, respectively. They were acquired with a 40/1.3× oil-immersion objective. Samples from treated and untreated animals were captured by using identical photomultiplier tube gain settings and processed using Zeiss LSM-PC software and Adobe Photoshop 6.0. The number of desquamating superficial epithelial cells was counted in three microscopic fields per cornea in paraffin-embedded histological sections from three corneas obtained from each of four groups of mice: control WT, control BKO, WT with EIDE, and BKO with EIDE.
Corneal Epithelial Explant Cultures
Primary human corneal epithelial cells were grown from limbal explants taken from corneoscleral tissues provided by the Lions Eye Bank of Texas (Houston, TX) using a previously described method.18 Explants were grown in six-well culture plates for ∼20 days until they were near confluence. Groups of three wells were exposed to MMP-9 (0.5 and 1 μg/ml) for 8 and 24 hours, and another group of three wells served as a media control. The morphology of these cells was observed and photographed before and after MMP-9 treatment by phase and fluorescent microscopy after staining with calcein AM dye (10 μg/ml, Molecular Probes) for 30 minutes. Immunostaining for occludin was performed as described above.
Western Blot
To make soluble and insoluble pools, mouse corneal epithelial cells obtained by scraping were lysed in a buffer containing 1% Triton X-100, 100 mmol/L NaCl, 10 mmol/L HEPES, 2 mmol/L ethylenediaminetetraacetic acid (EDTA) and a EDTA-free protease inhibitor cocktail tablet (Roche Applied Science, Indianapolis, IN), then centrifuged at 15,000 × g for 30 minutes at 4°C. This supernatant was considered the Triton-soluble pool. The pellet was solubilized in 1% SDS and referred to as the Triton-insoluble pool. The total protein concentrations of the cell extracts were measured by a Micro BCA protein assay kit (Pierce, Rockford, IL). The protein samples (50 μg) were mixed with 6× SDS-reducing sample buffer and boiled for 5 minutes before loading. Proteins were separated by SDS-polyacrylamide gel electrophoresis (4 to 15% Tris-HCl, gradient gels; Bio-Rad, Hercules, CA), and transferred electronically to polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were blocked with 5% nonfat milk in TTBS (50 mmol/L Tris, pH 7.5, 0.9% NaCl, and 0.1% Tween-20) for 1 hour at room temperature, and then incubated 2 hours at room temperature with a 1:160 dilution (1.56 μg/ml) of rabbit anti-occludin antibody (Zymed) with 5% nonfat milk in TTBS. After three washings with TTBS, the membranes were incubated for 1 hour at room temperature with HRP-conjugated secondary antibody donkey anti-rabbit IgG (1:10,000 dilution; Promega, Madison, WI), or goat anti-rat IgG (1:5000 dilution; Pierce, Rockford, IL). After washing the membranes for four times, the signals were detected with an ECL advance chemiluminescence reagent (Amersham, Piscataway, NJ) and the images were acquired and analyzed by a Kodak image station 2000R (Eastman-Kodak, New Haven, CT). Band intensities from three different experiments were averaged. Lysates from control and MMP-9-treated human corneal epithelial cell cultures were also analyzed in a similar manner.
Immunprecipitation
The corneal epithelia of untreated WT mice were lysed in 150 μl of RIPA buffer containing 50 mmol/L Tris-HCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.2% SDS, 150 mmol/L NaCl, 10 mmol/L HEPES, pH 7.3, 2 mmol/L EDTA, 20 mmol/L sodium fluoride, and a EDTA-free protease inhibitor cocktail tablet (Roche Applied Science, Indianapolis, IN). The cell extracts were centrifuged at 15,000 × g for 30 minutes at 4°C and the supernatants were incubated with 20 μl of polyclonal anti-occludin antibody (Zymed) followed by rotation at 4°C overnight. Subsequently, 15 μL of protein A agarose (Sigma, St. Louis, MO) and 100 μl of ImmunoPure IgG-binding buffer (Pierce) were added with further incubation for 2 hours at 4°C. The immunoprecipitates were collected by centrifugation at 3000 × g, washed three times in wash buffer I (20 mmol/L Tris-HCl, pH 8.0, 400 mmol/L KCl, 0.5 mmol/L EDTA, 10% glycerol, and 0.25% Nonidet P-40) and one time in wash buffer II (20 mmol/L Tris-HCl, pH 8.0, 100 mmol/L KCl, 0.5 mmol/L EDTA, 10% glycerol, and 0.25% Nonidet P-40). Bound protein was eluted in 6× SDS-reducing sample buffer and boiled for 5 minutes for immunoblot analysis. Immunoblot were probed with 1:625 dilution (0.8 μg/ml) of monoclonal anti-occludin antibody (Zymed). Cell lysates from control and MMP-9-treated human corneal epithelial cell cultures were immunoprecipitated in a similar manner.
Statistics
The Student’s t-test or the Mann-Whitney test were used where appropriate for between group statistical comparisons. Analysis of variance with Turkey posthoc analysis was used for the MMP-9 reconstitution experiment and comparison of corneal epithelial desquamation.
Results
Genotype
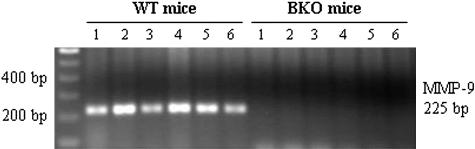
Genotyping was performed with PCR to confirm that BKO mice lacked a full-length MMP-9 gene. Using a primer pair spanning a sequence of MMP-9 gene exon 2 that was knocked out in BKO mice, a 225-bp fragment was generated from WT animals, but not from BKO mice (Figure 1).
Figure 1.
Genotype determination by PCR using mouse tail genomic DNA and a specific primer pair designed from the MMP-9 gene exon 2 sequence, which was knocked out in BKO mice. The PCR products were analyzed by 1.5% agarose gel electrophoresis. A 225-bp band was generated from all six WT mice (left, lanes 1 to 6) tested but not from six BKO mice (right, lanes 1 to 6).
Induction of Dry Eye
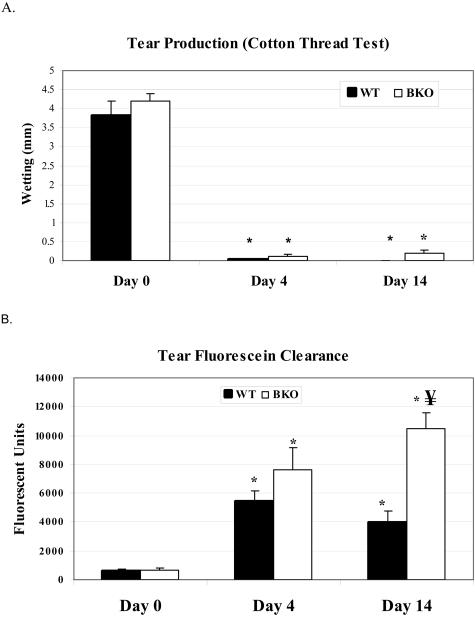
EIDE with scopolamine injections and a fan resulted in a significant decrease in tear production (Figure 2A) and worsening of tear fluorescein clearance (Figure 2B) within 4 days that was sustained throughout the treatment period in both groups. There was no difference in tear production between groups at any time point; however, clearance of fluorescein from the tear film was significantly more delayed in the BKO group at day 14 (P < 0.0001, Figure 2B).
Figure 2.
A: Aqueous tear production measured in mm with a cotton thread in mice before (day 0) and after 4 and 14 days of EIDE. *, P < 0.001 compared to baseline. B: Measurement of clearance of fluorescein dye from the tear fluid 15 minutes after instillation of 1 μl of 2% sodium fluorescein in mice before (day 0) and 4 and 14 days after EIDE. *, P < 0.001 compared to baseline; ¥, P < 0.001 compared to WT at day 14. Values are mean ± SD from 14 eyes of seven mice.
Tear Fluid MMP-9 Increases with Experimental Dryness in WT Mice
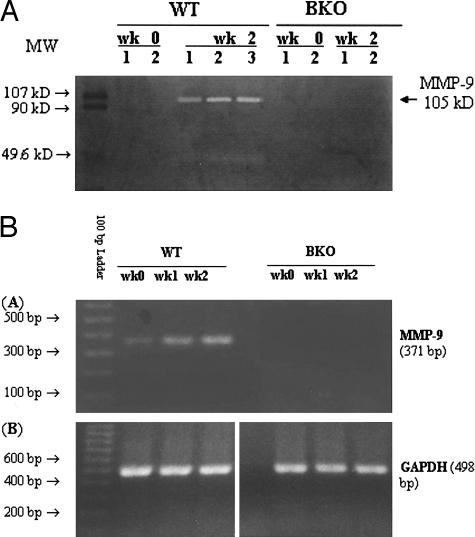
MMP-9 could not be detected in pooled tear fluid washings of untreated (week 0) WT or BKO mice by gelatin zymography (Figure 3A). Distinct MMP-9 bands were noted in the tear fluid of WT mice after 2 weeks of EIDE, but not in BKO mice with EIDE. Mouse MMP-9 has been reported to have a higher molecular weight (107 kd) than human MMP-9 (92 kd).15 Western blot of tear MMP-9 could not be performed because of the low protein concentration in the pooled tear washings.
Figure 3.
A: Gelatin zymography of pooled tear fluid washings obtained from WT and BKO mice before (wk 0) and after 2 weeks (wk 2) of EIDE. Mouse MMP-9 has a molecular weight of 107 kd.15 B: Expression of MMP-9 and GAPDH RNA in the corneal epithelium of WT and BKO mice by RT-PCR before (wk 0) and after 1 (wk 1) and 2 (wk 2) weeks of EIDE.
To confirm that BKO mice do not produce a functional MMP-9 gene product, MMP-9 RNA expression in the corneal epithelium was evaluated by RT-PCR using primers that span the deleted region of the MMP-9 gene in BKO mice. A PCR product of the appropriate size (371 bp) was obtained in WT mice and there was a progressive increase in intensity throughout a 2-week period in response to EIDE. In contrast, no MMP-9 RNA expression was observed in BKO mice at any time point (Figure 3B).
Disruption of the Corneal Epithelial Permeability Barrier Is Significantly Less in Dry Eye BKO Mice
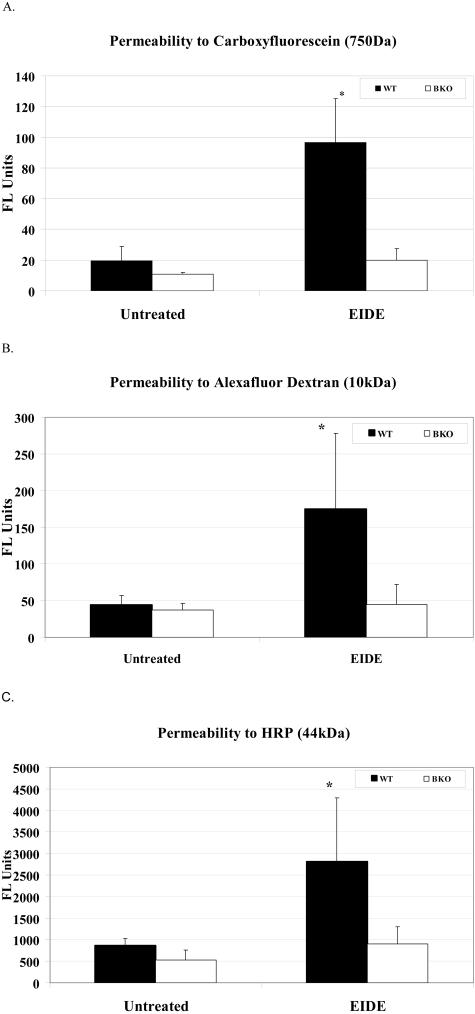
A hallmark of human keratoconjunctivitis sicca is increased corneal epithelial permeability to the diagnostic dye sodium fluorescein. Corneal epithelial permeability to molecules of three different sized molecules (carboxyfluorescein, molecular weight 750 kd; AFD, molecular weight, 10 kd; and HRP, molecular weight, 44 kd) was assessed in control and EIDE mice. Compared to baseline, permeability to all three molecules was significantly increased in WT mice, but not in BKO mice after 2 weeks of EIDE (Figure 4).
Figure 4.
Corneal permeability to carboxyfluorescein (A, FL units = units of fluorescein emission at 530 nm), AFD (B, FL units = units of fluorescein emission at 530 nm), and HRP (C, FL units = units of fluorescein emission from activated peroxidase substrate Amplex Red at 590 nm) in untreated control and after 2 weeks of EIDE in WT and BKO mice Values are mean ± SD for four eyes. A: *, P = 0.002 WT with EIDE versus untreated control WT and BKO with EIDE; B: *, P = 0.048 WT with EIDE versus untreated control WT and BKO with EIDE; C: *, P < 0.03 WT with EIDE versus untreated control WT and treated BKO.
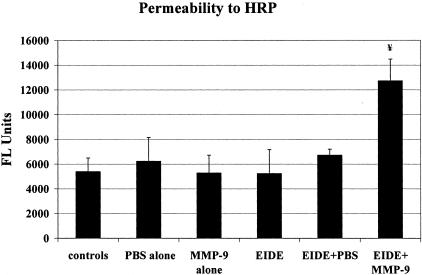
To determine whether the addition of MMP-9 to the ocular surface of BKO mice would worsen corneal epithelial barrier function in response to EIDE, control BKO mice and BKO mice with EIDE received topical application of activated MMP-9 to their ocular surface every 2 hours for 10 hours per day for 2 days, then corneal permeability to HRP was measured. There was no difference in corneal epithelial barrier function in BKO mice treated with topical MMP-9 or saline compared to the untreated control group. In contrast, a significant increase (P < 0.05) in corneal epithelial permeability to HRP was noted in BKO mice with EIDE that received topical MMP-9 compared to mice receiving saline or nothing (Figure 5). Treatment with MMP-9 was not continued beyond 2 days because MMP-9-treated mice with EIDE began to develop frank corneal epithelial sloughing.
Figure 5.
Corneal permeability to HRP in untreated controls and mice treated 10 hours per day for 2 days with PBS, MMP-9 (1 μl of 1 μg/ml), EIDE, EIDE plus PBS, EIDE plus MMP-9 (1 μl of 1 μg/ml). Drops were administered every 2 hours. Values are mean ± SD for 3 eyes. ¥, Between group difference was 0.002 ANOVA. Post-hoc analysis showed significant differences between EIDE + MMP-9 and controls (P < 0.05), EIDE (P < 0.01), and EIDE + PBS (P < 0.05). FL, fluorescent.
Corneal Histology and Ultrastructure
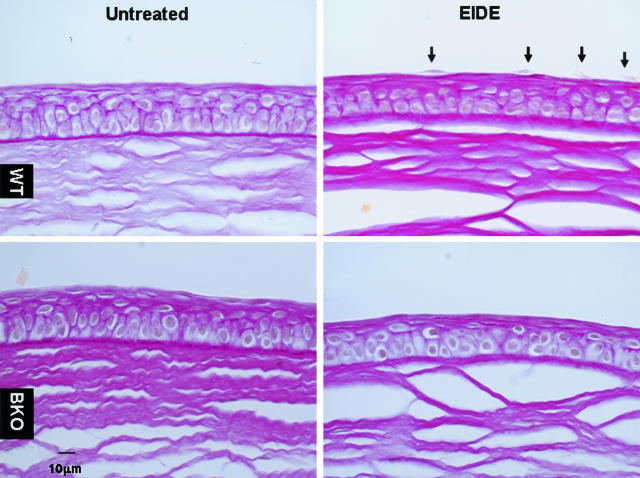
To determine the mechanism by which MMP-9 increases corneal epithelial permeability in dry eye, corneal histology and ultrastructure were compared in WT and BKO mice. Compared to corneas from untreated control animals without EIDE, detaching superficial corneal epithelial cells were observed only in WT mice (mean, 4.2 ± 0.8 per 100-μm segments) in paraffin sections stained with PAS, but not in the corneas of BKO mice (n = 3, Figure 6).
Figure 6.
PAS-stained sections of representative central corneas from untreated WT and BKO mice before (left, top, and bottom, respectively) and after EIDE for 14 days (right, top, and bottom, respectively). Detaching apical cells (arrows) were in the corneas of WT mice with EIDE (top, right), but not in BKO mice with EIDE (bottom, right).
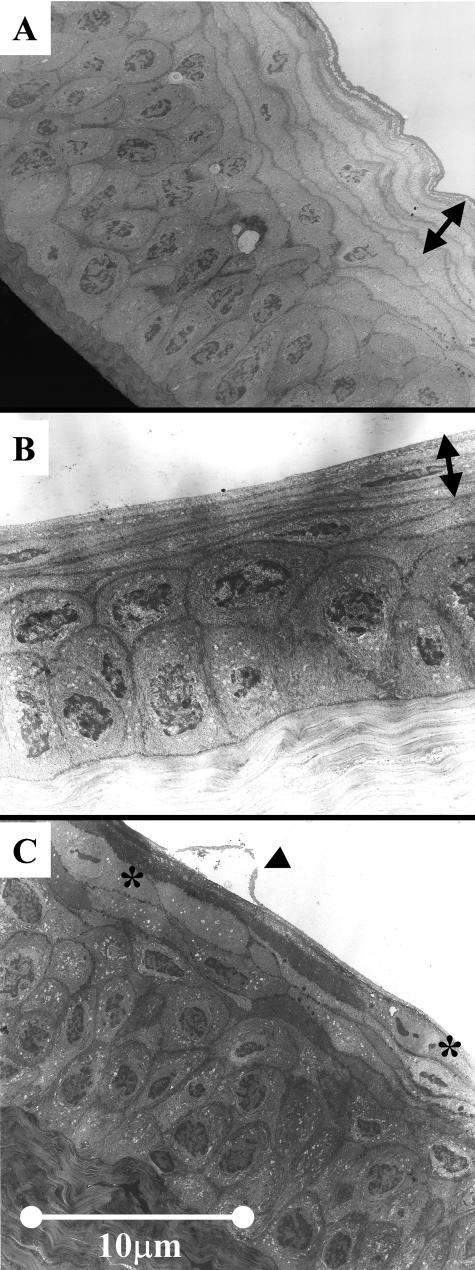
Transmission electron microscopy showed a similar appearance of the corneal epithelium in control WT and BKO mice with multiple layers of thin superficial epithelial cells with barely detectable nuclei (Figure 7C, arrow). The superficial corneas of BKO mice with EIDE had a similar appearance to untreated eyes (Figure 7B, arrow). In contrast, detaching apical epithelial cells were observed in response to EIDE in WT mice (Figure 7C, arrow). Taken together, these results indicate there is greater desquamation of apical epithelial cells in response to EIDE in WT mice than in BKO mice.
Figure 7.
Transmission electron micrograph of untreated WT mouse cornea (A), cornea of BKO mouse with EIDE for 14 days (B) (arrows in A and B indicate layers of thinned apical epithelium with barely detectable cell nuclei), and cornea of a WT mouse with EIDE for 14 days (C). Arrow indicates detaching apical epithelial cell. Superficial cells with cleaved nuclei are marked with asterisks.
Expression of the Tight Junction Protein Occludin
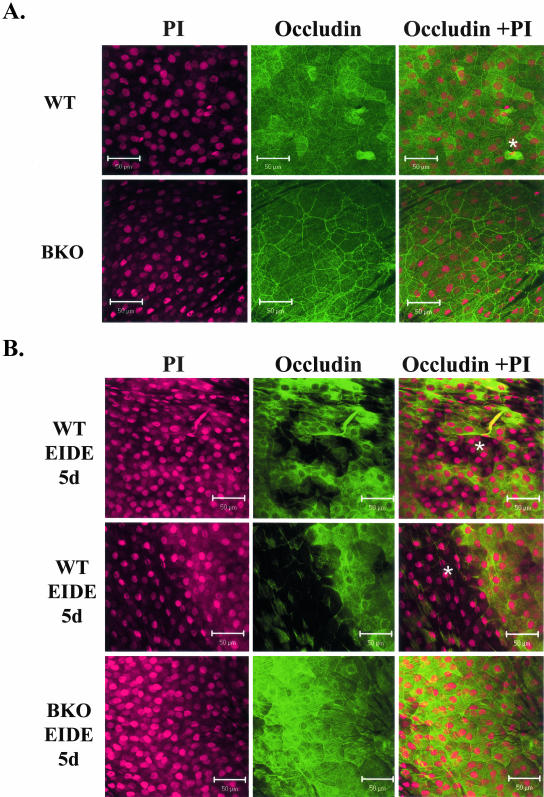
Barrier function is maintained in the normal cornea by tight junctions (zonula occludins) in the differentiated apical corneal epithelial cells. Certain protein constituents of the tight junction complex, such as occludin are substrates of MMPs, including MMP-9.19,20 To determine whether disruption of corneal epithelial tight junctions may be a cause for the accelerated desquamation and altered barrier function in dry eye, the expression of the tight junction protein occludin was evaluated by laser-scanning confocal microscopy and Western blot. In control WT and BKO mice, strong occludin staining in the intercellular junctions and faint staining in the cytoplasm was observed in the apical corneal epithelial layers (Figure 8A). Detaching apical epithelial cells were occasionally observed in the central corneas of both WT and BKO mice, although with a greater frequency in the WT (means, 5.6 ± 1.5 and 2.2 ± 1.3 in nine microscopic fields, respectively; P < 0.01). After 1 week of EIDE, numerous detached apical epithelial cells (11.6 ± 1.9) were observed in every field examined in WT corneas (Figure 8B, top; P < 0.001 versus control). A large area of the central corneal epithelial sloughing was noted in one of the three WT EIDE corneas examined (Figure 8B, middle). The corneas of BKO mice had fewer detached apical corneal epithelial cells in response to EIDE (3.0 ± 0.7; Figure 8B, bottom) than the WT corneas (P < 0.001).
Figure 8.
Confocal microscopy of whole mount corneas stained with polyclonal occludin antisera. A: Apical corneal epithelium of control WT (top) and BKO (bottom) mice. Detached epithelium is noted with asterisk. B: Apical corneal epithelium in WT mice with EIDE for 5 days (top and middle). Areas of epithelial detachment are noted with asterisk. The middle figure shows the border of a large central circular area of detachment (∼1 mm diameter). A representative cornea from a BKO mouse with EIDE for 5 days is shown on the bottom. Cells were counterstained with propidium iodide (PI) (left), and polyclonal occludin antisera (middle). The merged image is on the right.
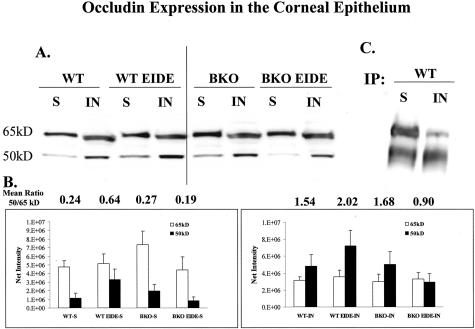
Increased expression of MMPs by cultured human umbilical vein endothelial cells has been reported to induce a shift in the molecular size of occludin from 65 kd to 50 kd.19 The ratio of 50-kd to 65-kd occludin bands was evaluated in Triton-soluble and -insoluble protein fractions obtained from the corneal epithelia of WT and BKO mice with and without EIDE (Figure 9). There was a predominance of the 65-kd occludin species in the Triton-soluble fraction of control untreated WT and BKO corneas (Figure 9, A and B). An increase in the 50-kd occludin band was observed in both Triton-soluble and -insoluble fractions in the corneal epithelium of WT mice in response to EIDE (Figure 9, A and B). In contrast, the ratio between the 50-kd and 65-kd bands decreased in the corneal epithelia of BKO mice with EIDE (Figure 9, A and B). The identity of these two occludin bands was confirmed by immunoprecipitation of mouse corneal epithelial lysates using a polyclonal occludin antisera, followed by immunoblotting with a monoclonal occludin antibody (Figure 9C).
Figure 9.
A: Occludin Western blot of triton soluble (S) and insoluble (IN) fractions of corneal epithelial lysates from control WT and BKO mice and WT and BKO mice with EIDE for 5 days (WT 5 day and BKO 5 day, respectively). B: Graphs of mean 65- and 50-kd band densities from triton soluble (S) and insoluble (IN) corneal epithelial fractions from three blots. The mean 50:65-kd ratio for each sample is provided above the bar graphs. C: Immunoprecipitation of triton soluble (S) and insoluble (IN) fractions of corneal epithelial lysates from control WT mice using polyclonal occludin antisera followed by immunoblotting with monoclonal occludin antibody.
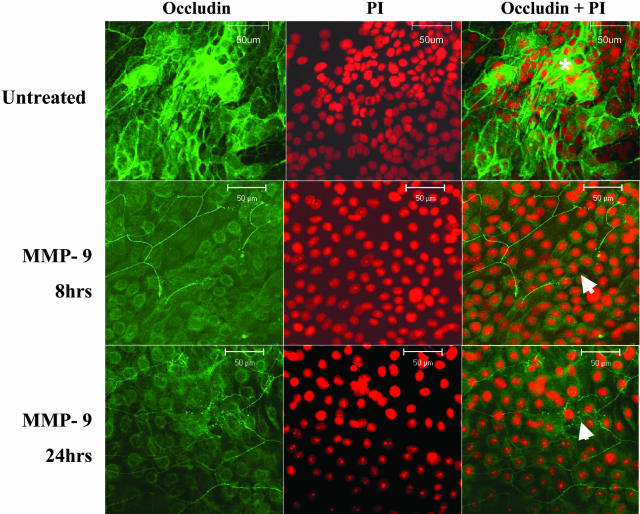
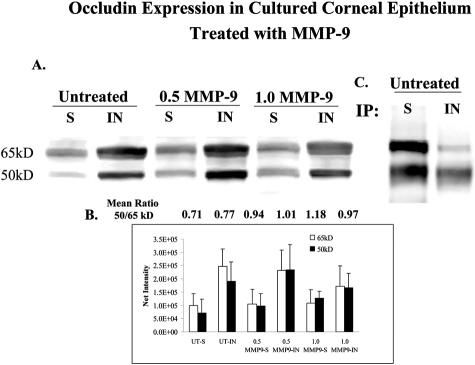
To determine whether exposure to MMP-9 promotes detachment of corneal epithelial cells, confluent primary human corneal epithelial cultured cells were treated for 8 and 24 hours with active MMP-9 and their cellular morphology and occludin expression were evaluated. The MMP-9-treated cultures showed rounding and detachment of superficial epithelial cells in areas of stratification. This was accompanied by a loss or discontinuity of the honeycomb membrane-staining pattern that was seen in differentiated stratified epithelia in control cultures (Figure 10). These MMP-9-induced changes were observed in all three sets of treated cultures. These changes in immunoreactivity were accompanied by a decrease in the density of the 65-kd occludin band and an increase in the 50-kd band in MMP-9-treated cells, compared to media controls (Figure 11). The identity of these two occludin bands was confirmed by immunoprecipitation of human corneal epithelial lysates (Figure 11C).
Figure 10.
Confocal microscopy of control untreated primary cultured human corneal epithelium in media (top), primary cultured human corneal epithelium treated with MMP-9 (1 μg/ml) for 8 hours (MMP-9 8 hours, middle), and primary cultured human corneal epithelium treated with MMP-9 (1 μg/ml) for 24 hours (MMP-9 24 hours, bottom). In each group, cells were stained with polyclonal occludin antisera (left), counterstained with propidium iodide (PI, middle), and the merged image is on the right. A honeycomb pattern of occludin staining was observed in areas of stratification (asterisk) in control cultures; areas of discontinuity of occludin staining in MMP-9-treated cultures are indicated by arrows.
Figure 11.
A: Occludin Western blot of triton soluble (S) and insoluble (IN) fractions of lysates from untreated confluent primary human corneal epithelial cultures and cultures treated with 0.5 and 1.0 μg/ml of activated MMP-9 (0.5 MMP-9 and 1.0 MMP-9, respectively) for 24 hours. B: Graph of mean 65- and 50-kd band densities from triton soluble (S) and insoluble (IN) corneal epithelial fractions from three blots. The mean 50:65-kd ratio for each sample is provided above the bar graphs. C: Immunoprecipitation of triton soluble (S) and insoluble (IN) fractions of corneal epithelial lysates from control human corneal epithelial cultures using polyclonal occludin antisera followed by immunoblotting with monoclonal occludin antibody.
Discussion
Similar to human keratitis sicca, EIDE was found to disrupt corneal epithelial barrier function in WT mice. The concentration of MMP-9 protein in the tear fluid of these mice was below the level of detection before the induction of dry eye and increased to clearly detectable levels after 2 weeks of EIDE. Increased tear fluid MMP-9 was accompanied by a significant increase in corneal epithelial permeability to three different sized molecules, ranging from 0.750 to 44 kd. In contrast, MMP-9 knockout (BKO) mice developed only a minimal nonsignificant increase in corneal permeability to these molecules in response to dry eye. To determine whether this difference in corneal epithelial permeability could be attributed directly to MMP-9, or to other changes in these mice secondary to their lack of MMP-9 production, we performed a reconstitution experiment in which active MMP-9 was administered topically to the ocular surface of normal BKO mice and BKO mice with EIDE. After 2 days of MMP-9 treatment, corneal epithelial permeability was significantly increased in the reconstituted BKO mice with EIDE, but not in the control BKO mice with normal tear production. This suggests that changes in the ocular surface environment of dry eye mice renders them more susceptible to the barrier-disrupting effects of MMP-9, perhaps because of decreased concentrations of MMP-9 inhibitors (eg, TIMPs) that are secreted into the tear film by the lacrimal glands.17 It is possible that topical treatment with MMP-9 for a longer period of time, or at a higher concentration would disrupt corneal epithelial barrier function even in the control BKO mice with normal tear production.
Increased concentration and activity of MMP-9 has been observed in the tear fluid of human dry eye patients.10–12 The tear fluid concentration of MMP-9 was observed to increase as tear clearance decreased.11 Consistent with this finding is the observation that tear MMP-9 concentration increases in the nocturnal closed eye.21 We previously observed that among dry eye patients, the highest tear MMP-9 concentrations were in patients with Sjögren’s syndrome, the most severe dry eye condition in which the ability to reflex tear is lost, similar to the scopolamine-treated mice in this study.10 Among these eyes, the highest MMP-9 concentrations were observed in patients who developed corneal epithelial defects and sterile stromal ulceration.10 The source of the increased MMP-9 in dry eye tear fluid has not been established. Possible sources include activated ocular surface epithelial cells and leukocytes that infiltrate the ocular surface.22,23 The current study indicates that increased expression of MMP-9 by the corneal epithelium is certainly one source. In experimental corneal epithelial wounds, increased MMP-9 production has been observed in the basal corneal epithelial cells.24,25 In contrast, increased MMP-9 has been observed in all layers of the corneal epithelium, including the superficial cells in inflammatory conditions, such as herpetic keratitis.26 Therefore, MMP-9 may affect the superficial corneal epithelium either by direct production by these cells, or by paracellular diffusion from deeper epithelial layers, a process that can be enhanced by the epithelial stress of dry eye.
Our studies indicate that MMP-9 disrupts tight junctions in the superficial layers of the corneal epithelium, promoting cell detachment and exposure of the less differentiated underlying wing epithelial cells. MMP-9 has numerous substrates, including the tight junction protein occludin.19,20,27 The increase in lower sized (50 kd) occludin in the corneal epithelia of WT mice with EIDE may be because of proteolytic cleavage of occludin by MMP-9, or it may represent incompletely processed occludin in the less differentiated wing epithelial cells that represent a larger percentage of the corneal epithelial population after the well-differentiated apical epithelia detach. Stimulation of protease production by cultured human umbilical vein endothelial cells with protein tyrosine phosphate inhibitors was previously found to increase 50-kd occludin, which was reported to be an occludin degradation product because this shift was blocked by the MMP inhibitor 1,10-phenanthroline.19
These findings indicate that MMP-9 may play a physiological role in normal corneal desquamation. Vitamin A deficiency has been reported to decrease MMP-9 production by the corneal epithelium and it is associated with reduced desquamation of the apical corneal epithelium, leading to increased corneal epithelial thickness.28,29 The effects of MMP-9 on cultured human corneal epithelial cells in the current study further support this concept. MMP-9 treatment was observed to promote epithelial detachment and loss of the honeycomb occludin staining pattern found in stratified differentiated cells. This was accompanied by an increase in 50-kd occludin in MMP-9-treated cultures.
The findings of our study suggest that targeting MMP-9 in human keratitis sicca may lessen the severity of clinical disease. There is currently no approved therapy that inhibits MMP-9 production, release, or activation. Corticosteroids and doxycycline, two agents that have been observed to decrease the severity of human keratitis sicca30–32 have been reported to decrease the level of MMP-9 in conditioned media from primary human cornea epithelial cultures.22,33 It is likely that other more specific inhibitors of MMP-9 will become available in the future.
Footnotes
Address reprint requests to Stephen C. Pflugfelder, M.D., Cullen Eye Institute, 6565 Fannin, NC 201, Houston, TX 77030. E-mail: stevenp@bcm.tmc.edu.
Supported by the National Eye Institute Grant EY11915, an unrestricted grant from Research to Prevent Blindness, The Oshman Foundation, and the William Stamps Farish Fund (to S.C.P.) and R01 EY1265, P30 EY14801, and the Walter G. Ross Foundation (E.M.F.).
References
- Murillo-Lopez F, Pflugfelder SC. Dry eye. Krachmer J, Mannis M, Holland E, editors. St. Louis: Mosby; The Cornea. 1996:pp 663–686. [Google Scholar]
- Yokoi N, Kinoshita S. Clinical evaluation of corneal epithelial barrier function with the slit-lamp fluorophotometer. Cornea. 1995;14:485–489. [PubMed] [Google Scholar]
- Gobbels M, Spitznas M. Corneal epithelial permeability of dry eyes before and after treatment with artificial tears. Ophthalmology. 1992;99:873–878. doi: 10.1016/s0161-6420(92)31879-2. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Tseng SC, Sanabria O, Kell H, Garcia CG, Felix C, Feuer W, Reis BL. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17:38–56. doi: 10.1097/00003226-199801000-00007. [DOI] [PubMed] [Google Scholar]
- Rolando M, Iester M, Macri A, Calabria G. Low spatial-contrast sensitivity in dry eyes. Cornea. 1998;17:376–379. doi: 10.1097/00003226-199807000-00006. [DOI] [PubMed] [Google Scholar]
- de Paiva CS, Lindsey JL, Pflugfelder SC. Assessing the severity of keratitis sicca with videokeratoscopic indices. Ophthalmology. 2003;110:1102–1109. doi: 10.1016/s0161-6420(03)00245-8. [DOI] [PubMed] [Google Scholar]
- de Paiva SC, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004;137:109–115. doi: 10.1016/s0002-9394(03)00897-3. [DOI] [PubMed] [Google Scholar]
- Ormerod LD, Fong LP, Foster CS. Corneal infection in mucosal scarring disorders and Sjogren’s syndrome. Am J Ophthalmol. 1988;105:512–518. doi: 10.1016/0002-9394(88)90243-7. [DOI] [PubMed] [Google Scholar]
- Barton K, Monroy DC, Nava A, Pflugfelder SC. Inflammatory cytokines in the tears of patients with ocular rosacea. Ophthalmology. 1997;104:1868–1874. doi: 10.1016/s0161-6420(97)30014-1. [DOI] [PubMed] [Google Scholar]
- Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- Afonso A, Sobrin L, Monroy DC, Selzer M, Lokeshwar B, Pflugfelder SC. Tear fluid gelatinase B activity correlates with IL-1β concentration and fluorescein tear clearance. Invest Ophthalmol Vis Sci. 1999;40:2506–2512. [PubMed] [Google Scholar]
- Smith VA, Rishmawi H, Hussein H, Easty DL. Tear film MMP accumulation and corneal disease. Br J Ophthalmol. 2001;85:147–153. doi: 10.1136/bjo.85.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun D, Wang M, Monroy D, Li DQ, Lokeshwar BL, Stern ME, Pflugfelder SC. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43:632–638. [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, Lee Y, McCarthy BE, Wollenberg KR, Jester JV, Wang M, Welgus HG, Shipley JM, Senior RM, Fini ME. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem. 2002;277:2065–2072. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- Yeh S, Song XJ, Farley W, Li DQ, Stern ME, Pflugfelder SC. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol Vis Sci. 2003;44:124–129. doi: 10.1167/iovs.02-0581. [DOI] [PubMed] [Google Scholar]
- Sobrin L, Selzer MG, Lokeshwar BL, Pflugfelder SC. Stromelysin (MMP-3) activates pro-MMP-9 secreted by corneal epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:1703–1709. [PubMed] [Google Scholar]
- Li DQ, Shang TY, Kim HS, Solomon A, Lokeshwar BL, Pflugfelder SC. Regulated expression of collagenases MMP-1, -8, and -13 and stromelysins MMP-3, -10, and -11 by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:2928–2936. doi: 10.1167/iovs.02-0874. [DOI] [PubMed] [Google Scholar]
- Wachtel M, Frei K, Ehler E, Fontana A, Winterhalter K, Gloor SM. Occludin proteolysis and increased permeability in endothelial cells through tyrosine phosphatase inhibition. J Cell Sci. 1999;112:4347–4356. doi: 10.1242/jcs.112.23.4347. [DOI] [PubMed] [Google Scholar]
- Behzadian MA, Wang XL, Windsor LJ, Ghaly N, Caldwell RB. TGF-beta increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest Ophthalmol Vis Sci. 2001;42:853–859. [PubMed] [Google Scholar]
- Sack RA, Beaton A, Sathe S, Morris C, Willcox M, Bogart B. Towards a closed eye model of the pre-ocular tear layer. Prog Retin Eye Res. 2000;19:649–668. doi: 10.1016/s1350-9462(00)00006-9. [DOI] [PubMed] [Google Scholar]
- Li DQ, Lokeshwar BL, Solomon A, Monroy D, Ji Z, Pflugfelder SC. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res. 2001;73:449–459. doi: 10.1006/exer.2001.1054. [DOI] [PubMed] [Google Scholar]
- Stern ME, Gao J, Schwalb TA, Ngo M, Tieu DD, Chan CC, Reis BL, Whitcup SM, Thompson D, Smith JA. Conjunctival T-cell subpopulations in Sjogren’s and non-Sjogren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–2614. [PubMed] [Google Scholar]
- Daniels JT, Geerling G, Alexander RA, Murphy G, Khaw PT, Saarialho-Kere U. Temporal and spatial expression of matrix metalloproteinases during wound healing of human corneal tissue. Exp Eye Res. 2003;77:653–664. doi: 10.1016/j.exer.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Sivak JM, West-Mays JA, Yee A, Williams T, Fini ME. Transcription factors Pax6 and AP-2alpha interact to coordinate corneal epithelial repair by controlling expression of matrix metalloproteinase gelatinase B. Mol Cell Biol. 2004;24:245–257. doi: 10.1128/MCB.24.1.245-257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YN, Bauer D, Wasmuth S, Steuhl KP, Heiligenhaus A. Matrix metalloproteinases (MMP-2 and 9) and tissue inhibitors of matrix metalloproteinases (TIMP-1 and 2) during the course of experimental necrotizing herpetic keratitis. Exp Eye Res. 2003;77:227–237. doi: 10.1016/s0014-4835(03)00112-x. [DOI] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining SS, Schulte DP, Zhou X, Wilson PM, Fish BL, Moulder JE. Changes in rat corneal matrix metalloproteinases and serine proteinases under vitamin A deficiency. Curr Eye Res. 1997;16:158–165. doi: 10.1076/ceyr.16.2.158.5085. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson L, Tanaka M, Kuwabara T, Bieri JG. Early corneal changes in vitamin A deficient rats. Exp Eye Res. 1980;30:261–269. doi: 10.1016/0014-4835(80)90006-8. [DOI] [PubMed] [Google Scholar]
- Akpek EK, Merchant A, Pinar V, Foster CS. Ocular rosacea: patient characteristics and follow-up. Ophthalmology. 1997;104:1863–1867. [PubMed] [Google Scholar]
- Sainz de la Maza Serra SM, Simon Castellvi C, Kabbani O. Nonpreserved topical steroids and punctual occlusion for severe keratoconjunctivitis sicca. Arch Soc Esp Oftalmol. 2000;75:751–756. [PubMed] [Google Scholar]
- Marsh P, Pflugfelder SC. Topical non-preserved methylprednisolone therapy of keratoconjunctivitis sicca. Ophthalmology. 1999;106:811–816. doi: 10.1016/S0161-6420(99)90171-9. [DOI] [PubMed] [Google Scholar]
- Dursun D, Kim MC, Solomon A, Pflugfelder SC. Treatment of recalcitrant recurrent corneal epithelial erosions with inhibitors of matrix metalloproteinases-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001;132:8–13. doi: 10.1016/s0002-9394(01)00913-8. [DOI] [PubMed] [Google Scholar]