Abstract
The breakdown of the blood-retina barrier (BRB) is a common feature of diabetic retinopathy. The purpose of the present study is to determine whether there are genetic differences in susceptibility to the breakdown of the BRB in diabetic retinopathy using two rat models. In streptozotocin (STZ)-induced diabetes, Brown Norway (BN) rats developed sustained vascular hyperpermeability in the retina during the entire experimental period (16 weeks of diabetes), while diabetic Sprague Dawley (SD) rats only showed retinal hyperpermeability from 3 to 10 days after the onset of diabetes. The strain difference in permeability was not correlated with the blood glucose levels in these two strains. In oxygen-induced retinopathy (OIR), BN rats developed retinal vascular hyperpermeability from postnatal day 12 (P12) to P22 with a peak at P16, which was 8.7-fold higher than that in the age-matched normal controls. In OIR-SD rats, however, hyperpermeability was observed from P14 to P18, with a peak only 2.2-fold higher than that in the controls. The strain difference in vascular hyperpermeability was correlated with the different overexpression of vascular endothelial growth factor (VEGF) in the retina of these two models. This finding suggests that genetic backgrounds contribute to the susceptibility to diabetic retinopathy.
Macular edema is a major cause of vision loss in diabetic patients, which may occur at any stage of diabetic retinopathy, even before the clinical appearance of the disease.1,2 A large number of asymptomatic patients with preclinical macular edema fail to seek treatment until some degree of vision loss has already occurred. A timely and appropriate treatment can prevent 50% of moderate vision loss resulting from diabetic macular edema, while it may prevent up to 90% of severe vision loss in cases of proliferative diabetic retinopathy.2,3 Although laser treatment does deliver some results in reducing the severity of macular edema, its therapeutic application is limited because the laser causes scar formation, which is threatening to the central vision.4
Breakdown of the blood-retina barrier (BRB) is one of the most important pathophysiological changes in the early stages of diabetic retinopathy as well as of other ischemic or inflammatory retinal diseases, such as central retinal vein occlusion and uveitis.1,5–7 The enhancement of retinal vascular permeability (RVP) and vascular leakage resulting from BRB dysfunction has been observed both in patients with diabetes and streptozotocin (STZ)-induced diabetic animal models.8–11 Increased microvascular permeability is also hypothesized to be a crucial step in angiogenesis associated with tumors.12 The exact mechanisms underlying the breakdown of BRB and the increased RVP are largely unclear.
Vascular endothelial growth factor (VEGF) is a major angiogenic and mitogenic factor, which plays a crucial role in both normal and pathological angiogenesis.13 VEGF is also referred to as vascular permeability factor (VPF) based on its ability to induce vascular hyperpermeability.14 It has been shown that VEGF is one of the main mediators of increased RVP in ischemic and non-ischemic retinal diseases.15,16 The up-regulated expression of VEGF and its receptors are associated with increased RVP in rats with STZ-induced diabetes.17
STZ-induced diabetes is a commonly used model of type-1 diabetes.18 Rats are the animal species most often used as subjects of this model.19 The retinas in STZ-diabetic rats exhibit most of the pathological features of background diabetic retinopathy seen in humans, including blood vessel dilation, BRB breakdown, microaneurysm formation, and intraretinal microvascular abnormalities, except for the development of typical retinal neovascularization (NV).1,20 Therefore, the STZ-induced diabetic rat model is widely used in studies of the early stages of diabetic retinopathy, especially in those examining vascular hyperpermeability in the retina.21–25
The oxygen-induced retinopathy (OIR) model is widely accepted for studies of retinal ischemic neovascular diseases such as diabetic retinopathy and retinopathy of prematurity (ROP).26–29 Exposure to hyperoxia followed by normoxia results in retinal ischemia and subsequently induces retinal NV. Previously, we have shown that in the OIR model, pigmented Brown Norway (BN) rats are more susceptible to ischemia-induced retinal NV than Sprague Dawley (SD) rats, correlating with the differences in the up-regulation of VEGF and the down-regulation of the pigment epithelium-derived factor (PEDF), an angiogenic inhibitor in the retina.30 In the present study, we examined the strain-related differences of vascular permeability in the retina and iris in BN and SD rats with STZ-induced diabetes and in those with OIR, and the differences in VEGF expression in the diabetic retina.
Materials and Methods
Animals
BN and SD rats were purchased from Harlan Sprague-Dawley (Indianapolis, IN). Care, use, and treatment of all animals in this study were in strict agreement with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research as well as the guidelines set forth by The University of Oklahoma Health Science Center.
Induction of Experimental Diabetes
Eight-week-old BN and SD rats were given a single intravenous injection of STZ (50 mg/kg in 10 mmol/L of citrate buffer, pH 4.5) after an overnight fasting. Control rats received injections of citrate buffer alone. Serum glucose levels were checked 24 hours after STZ injection and every 2 days thereafter, and only the animals with glucose levels higher than 350 mg/dl were considered diabetic. At 1, 3, 7, and 10 days as well as 2, 4, 8, and 16 weeks after the STZ injection, vascular permeability in the retina and iris was measured using the Evans blue method.31
Oxygen-Induced Retinopathy
OIR was induced as described by Smith et al26 with some modifications.29 Briefly, newborn rats were randomly assigned to experimental and control groups. At postnatal day 7 (P7), rats in the experimental group were exposed to hyperoxia (75% O2) for 5 days (P7 to P12) and then returned to normoxia (room air). Control rats were kept at constant normoxia. At each time point of P12, P14, P16, P18, P22, P30, and P36, vascular permeability in the retina and iris was measured.
Measurement of Vascular Permeability
Vascular permeability was quantified by measuring albumin leakage from blood vessels into the retina and iris using the Evans blue method following a documented protocol31 with minor modifications. Evans blue dye (Sigma, St. Louis, MO) was dissolved in normal saline (30 mg/ml). The rats were anesthetized and Evans blue (30 mg/kg) was injected over 10 seconds through the femoral vein under microscopic inspection. Evans blue non-covalently binds to plasma albumin in the blood stream.32 Immediately after Evans blue infusion, the rats turned visibly blue, confirming their uptake and distribution of the dye. The rats were kept on a warm pad for 2 hours to ensure the complete circulation of the dye. Then the chest cavity was opened, and the rats were perfused via the left ventricle with 1% paraformaldehyde in citrate buffer (pH = 4.2), which was pre-warmed to 37°C to prevent vasoconstriction. The perfusion lasted 2 minutes under a pressure of 120 mmHg to clear the dye from the vessel. Immediately after perfusion, the eyes were enucleated, and the retina and iris were carefully dissected under an operating microscope. Evans blue dye was extracted by incubating each sample in 150 μl formamide for 18 hours at 70°C. The extract was centrifuged at 70,000 rpm (Rotor type: TLA-100.3, Beckman Coulter, Inc., Fullerton, CA) for 20 minutes at 4°C. Absorbance was measured using 100 μl of the supernatant at 620 nm. The concentration of Evans blue in the extracts was calculated from a standard curve and was normalized by the total protein concentration in each sample. Results were expressed in μg of Evans blue per milligrams of total protein content.
Induction of Vascular Hyperpermeability by Injection of VEGF and Insulin-Like Growth Factor-1
Purified VEGF was commercially purchased from Pepro Tech, Inc. (Rocky Hill, NJ), diluted to a concentration of 0.03 μg/μl (1.3 μmol/L) and sterilized. Purified insulin-like growth factor-1 (IGF-1) was a generous gift from Dr. Steve Rosenzweig at the Medical University of South Carolina. It was diluted to a concentration of 1.6 ng/μl (0.2 μmol/L) in phosphate-buffered saline (PBS) and sterilized. Eight-week-old BN and SD rats were given 3 μl/eye VEGF or IGF-1 intravitreal injection into the right eye and the same amount of sterilized PBS into the left eye. Six hours after the injection, the vascular permeability in the retina and iris was measured using the Evans blue method described above.
Western Blot Analysis
Western blot analysis was performed as described previously.27 As the VEGF level in a single retina of rat is too low to be detected by Western blot or ELISA (data not shown), the retinas from three animals of same group were pooled and homogenized in 1X sodium dodecyl-sulphate (SDS) gel loading buffer by sonication on ice. The insoluble pellet was removed by centrifugation. The protein concentration in the cytosolic fraction was measured with BioRad protein assay. One hundred μg of cytosolic protein was subjected to Western blot analysis using a polyclonal anti-VEGF antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA).
Immunohistochemistry
Frozen eye sections (5 μm) were cut and mounted onto super frost plus slides (VWR, West Chester, PA). After three washes with PBS, the sections were treated with 0.3% H2O2 in 10% methanol for 10 minutes to diminish the possible background. Then the slides were washed three times in a washing buffer (0.05 mol/L Tris-HCl, 0.15 mol/L NaCl, pH 7.6), for 5 minutes each. The sections were blocked with a blocking buffer (1% blocking reagent in washing buffer, Perkin Elmer Life Sciences, Boston, MA) for 1 hour, and then a sheep anti-rat albumin antibody (1:600 dilution, Bethyl, Montgomery, TX) was added and incubated with the slides for another 1 hour. After three washes with the washing buffer, the slides were incubated with TSA conjugated with FITC (1:500 dilution, Perkin Elmer Life Sciences, Boston, MA) for 5 minutes. Following washes with the washing buffer, the sections were mounted and covered with a coverslip. The immunosignals were examined under a fluorescent microscope (Axioplan2 Imaging, Carl Zeiss, Jena, Germany).
Visualization of Retinal Vessel Leakage by Evans Blue in Retinal Sections
Retinal vascular leakage was visualized by Evans blue dye, which irreversibly binds to albumin.33–35 Under deep anesthesia, the rats were given Evans blue (30 mg/kg) injection through the femoral vein, and kept on a warm pad for 20 minutes. The eyes were rapidly enucleated and fixed with 4% paraformaldehyde in PBS for 3 hours. The retina was then isolated and flat-mounted, and immediately examined under a microscope (Axioplan2 Imaging, Carl Zeiss).
Statistical Analysis
All results were expressed as mean ± SD unless otherwise indicated. The paired Student’s t-test was used for comparing the eyes from the same animal, while the unpaired t-test was used for inter-animal comparisons. Statistical difference was considered significant at a P value of less than 0.05.
Results
Blood Glucose Levels in STZ-Induced Diabetic BN and SD Rats
Diabetes was induced in 8-week-old BN and SD rats. The animals with blood glucose levels higher than 350 mg/dl were deemed diabetic. The incidences of diabetes induced by a single dose of STZ were 86% and 89% in BN and SD rats, respectively. After the same dose of STZ treatment, the average blood glucose levels in the BN rats were similar or lower than in SD rats at the time points measured (Table 1).
Table 1.
Blood Glucose Levels in Diabetic Rats
| Duration of diabetes | Blood glucose (mg/dl)
|
P value (n = 6) | |
|---|---|---|---|
| Brown Norway | Sprague Dawley | ||
| Normal | 138.57 ± 29.61 | 126.00 ± 21.17 | 0.182 |
| 3 days | 424.43 ± 85.15 | 514.29 ± 62.69 | 0.062 |
| 7 days | 413.00 ± 33.91 | 457.00 ± 39.68 | 0.082 |
| 10 days | 373.71 ± 33.37 | 424.86 ± 30.63 | 0.019 |
| 2 weeks | 423.57 ± 36.13 | 477.14 ± 40.57 | 0.006 |
| 3 weeks | 412.57 ± 54.99 | 479.71 ± 38.95 | 0.003 |
| 4 weeks | 429.50 ± 70.18 | 520.71 ± 59.44 | 0.014 |
| 8 weeks | 435.29 ± 96.31 | 476.57 ± 51.67 | 0.071 |
| 16 weeks | 467.67 ± 94.95 | 501.67 ± 62.04 | 0.239 |
, Values are fast blood glucose levels expressed as mean ± SD.
Time Course of Vascular Hyperpermeability in STZ-Diabetic BN and SD Rats
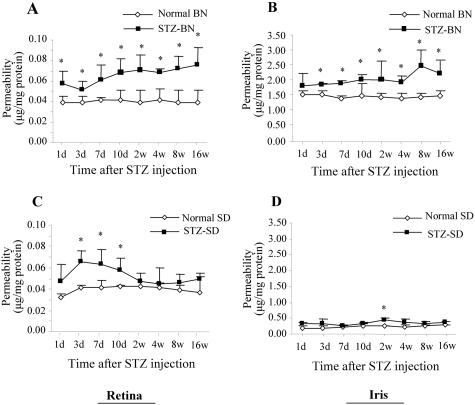
In normal adult BN and SD rats, there was no significant strain difference in the basal level of vascular permeability in the retina with an average of 0.04 μg of Evans blue per mg of protein in both normal BN and and SD rats (P > 0.1, n = 6) (Figure 1, A and C). On the other hand, in the normal iris, the basal level of vascular permeability was significantly higher in the BN than in the SD rats (Figure 1, B and D).
Figure 1.
Time courses of vascular hyperpermeability in BN and SD rats with STZ-induced diabetes. After intravenous injection of STZ (50 mg/kg), vascular permeability in the retina (A and C) and iris (B and D) was measured using the Evans blue method. Vascular permeability was measured at 1, 3, 7, and 10 days, and at 2, 4, 8, and 16 weeks after the STZ injection. Age-matched normal rats of each strain were used as controls. Evans blue was normalized by total protein concentration in the tissue. Permeability is expressed as μg of dye per mg of protein in the tissue (mean ± SD, n = 4 to 7). Values statistically different from the control are indicated by * (P < 0.05).
To compare vascular permeability changes in STZ-diabetic rats, RVP was measured 1, 3, 7, 10 days and 2, 4, 8, and 16 weeks after the onset of diabetes as well as in the age-matched normal controls. In diabetic BN rats, RVP increased early, with a 1.44-fold elevation over the control level (P = 0.0292, n = 4) on the first day after the onset of diabetes. RVP reached the plateau at 2 weeks with a level 1.8-fold higher than that found in the controls (P = 0.0074, n = 4). The hyperpermeability remained at higher levels during the entire experimental period of 16 weeks (1.92-fold, P = 0.0059, n = 4) (Figure 1A). In diabetic SD rats, RVP started to increase 3 days after the STZ-injection (1.59-fold over the control value, P = 0.0013, n = 4), remaining at elevated levels at 7 and 10 days (1.54- and 1.38-fold above those in the controls, respectively, P < 0.01, n = 4 to 6). By 2 weeks of diabetes, the permeability returned to the control level (Figure 1C).
In the iris of diabetic BN rats, the permeability increased at 3 days after the injection of STZ with an elevation of 1.21-fold as compared to the control value and remained at levels significantly higher than the normal level during the remaining experimental period (1.35-fold to 1.76-fold, P < 0.05, n = 4 or 6) (Figure 1B). In the iris of diabetic SD rats, the vascular permeability only showed a significant increase at 2 weeks after the induction of diabetes (P < 0.01) (Figure 1D).
Time Course of Vascular Hyperpermeability in OIR-BN and OIR-SD Rats
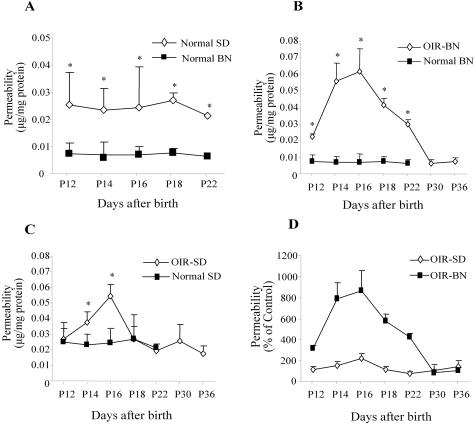
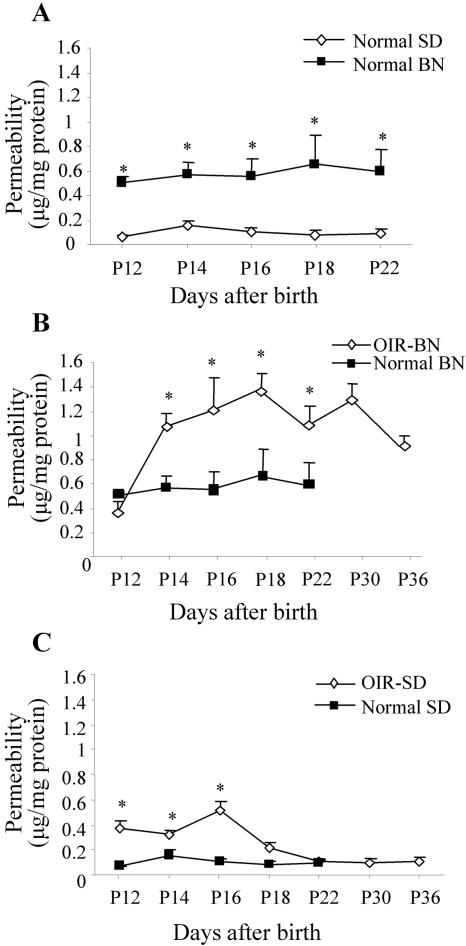
Normal neonatal BN and SD rats were kept under constant normoxia. The average basal level of RVP in normal BN rats was significantly lower than that in normal SD rats (0.0071 μg/mg in BN versus 0.0242 μg/mg in SD rats, P = 0.001 to 0.0158 for age-matched comparison, n = 4 to 7) (Figure 2A), suggesting that there may be some differences between these two strains with regard to the physiological status of BRB and regulation of vascular function at this developmental stage. The normal levels of vascular permeability in the retina were significantly lower than that in the iris of the same strain (Figure 2A and Figure 3A), suggesting that normal BRB has been developed at these ages.
Figure 2.
Time courses of vascular hyperpermeability in the retina of BN and SD rats with OIR. Rats were exposed to 75% O2 from age P7 to P12 and then returned to normoxia. Vascular permeability was measured at P12, P14, P16, P18, P22, P30, and P36 in OIR rats and in normal controls. A: Comparison of basal levels of RVP between normal BN and SD rats, RVP in OIR and normal BN rats at different ages (B), and RVP in OIR and normal SD rats at different ages (C). The Evans blue in the retina was normalized by total protein concentration. The result is expressed as μg of dye per mg of protein in the retina (mean ± SD, n = 4 to 7). Values statistically different from the respective controls are indicated by * (P < 0.05). D: Comparison of the increases of RVP in BN and SD rats with OIR. Values are expressed as percent increases of permeability over the respective controls (mean ± SD, n = 4 to 7).
Figure 3.
Time courses of vascular hyperpermeability in the iris of BN and SD rats with OIR. Rats were exposed to 75% O2 from age P7 to P12 and then returned to normoxia. Vascular permeability in the iris was measured at P12, P14, P16, P18, P22, P30, and P36 in OIR rats and normal controls. The Evans blue in the iris was normalized by total protein concentration. Permeability is expressed as μg of dye per mg of protein in the tissue (mean ± SD, n = 4 to 7). A: Basal levels of vascular permeability in normal BN and SD rats, vascular permeability in the iris of normal and OIR-BN rats (B), and vascular permeability in the iris of normal and OIR-SD rats (C). Values statistically different from those of the controls are indicated by * (P < 0.05).
In OIR-BN rats exposed to hyperoxia from P7 to P12, RVP was significantly higher than in the age-matched controls between P12 and P22 (P < 0.01, n = 4 to 6) with a peak at P16, which was 8.7-fold higher than that in the age-matched control (P < 0.001, n = 7). RVP in the retina returned to the normal value by P30 (Figure 2B).
In OIR-SD rats, following the same hyperoxia exposure, significantly increased retinal vascular permeability was observed from P14 to P16 with a peak 2.2-fold higher than that in the controls at P16. Vascular permeability declined to the control level by P18 (Figure 2C). The comparison of the two strains showed that retinal vascular hyperpermeability in the OIR-SD rats is lower at its peak and lasts for a shorter period of time, when compared to OIR-BN rats (Figure 2D).
In the iris, the basal level of vascular permeability in BN rats was significantly higher compared to that in SD rats at this developmental stage (Figure 3A). In the iris of the OIR-BN rats, vascular hyperpermeability started at P14 (1.87-fold over the control, P < 0.001) and lasted for the entire experimental period (P < 0.001, n = 4) (Figure 3B). In OIR-SD rats, the significant increase in vascular permeability in the iris was only observed from P12 to P16 (P < 0.001, n = 4) and returned to the control level by P18 (Figure 3C).
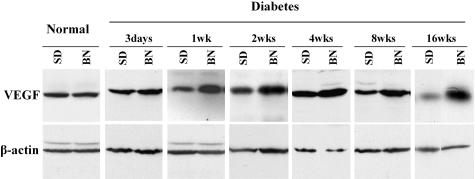
Differences in Retinal VEGF Levels in STZ-Diabetic BN and SD Rats
Previously, we demonstrated that in the OIR model, BN rats have significantly higher VEGF up-regulation than do SD rats, correlating with their different retinal hyperpermeability levels.30 In this study, we compared the VEGF levels of these two strains with the STZ-diabetes. Retinal VEGF levels were measured and semiquantified by Western blot analysis in BN and SD rats with STZ-induced diabetes and compared to respective age-matched non-diabetic controls at different time points after the onset of diabetes. The results showed that the basal level of retinal VEGF expression was similar in normal adult BN and in SD rats (Figure 4). Following the induction of diabetes by STZ, however, the retinal VEGF levels in diabetic BN rats were higher than those of diabetic SD rats during the time period of diabetes that lasted 3 days to 16 weeks (Figure 4). The retinal VEGF levels did not change with age in normal adult rats (data not shown). The expression levels of VEGF receptor-2 (VEGFR2, or KDR) in the retina were also compared in the diabetic BN and diabetic SD rats using a commercial ELISA kit (R&D Systems Inc., MN). No strain difference was found in VEGFR2 levels under diabetes (data not shown).
Figure 4.
Retinal VEGF levels in BN and SD rats with STZ-induced diabetes. The retinas were dissected from diabetic BN and SD rats at 3 days, and 1, 2, 4, 8, and 16 weeks following the STZ injection. The same amounts of soluble proteins were blotted with an antibody specific to VEGF. The same filter was stripped and re-blotted with the anti-β-actin antibody to normalize VEGF levels. The results are from pooled retinas of three animals at each time point.
Different Up-Regulations of Endogenous VEGF and Different Responses to Exogenous VEGF in BN and SD Rats
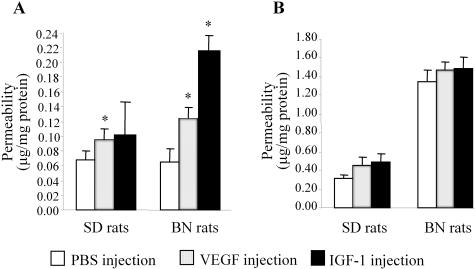
To determine whether the difference in RVP in diabetic BN and SD rats is caused only by the different up-regulations of VEGF expression in the retina or also by different responses of the retinal blood vessels to VEGF, we compared the vascular hyperpermeability in normal adult BN and SD rats induced by an intravitreal injection of exogenous VEGF or an injection of IGF-1, which is known to increase vascular permeability through the induction of endogenous VEGF expression.36 RVP was measured 6 hours after the injection. In BN rats, IGF-1 increased RVP more than threefold over that in the PBS-injected contralateral eyes (P < 0.05), while enhancing the permeability in SD rats only by 1.4-fold compared to its controls (Figure 5A). The hyperpermeability induced by IGF-1 in the BN rat retina was significantly higher than that observed in SD rats (P = 0.0035), suggesting that the endogenous VEGF up-regulation induced by IGF-1 injection is higher in BN than in SD rats. Moreover, the intravitreal injection of exogenous VEGF increased the RVP in BN rats by 1.8-fold as compared to contralateral eyes with PBS injection (P < 0.01, n = 4), while increasing the permeability only by 1.4-fold over the control value in SD rats (P < 0.05, n = 4) (Figure 5). The results demonstrate that the injection of exogenous VEGF induced a significantly higher RVP in BN rats than in SD rats (Figure 5A) (P = 0.038). In contrast, the injection of VEGF and IGF-1 did not affect permeability in the iris of these strains.
Figure 5.
Comparison of vascular permeability induced by the intravitreal injection of VEGF and IGF-1 in normal BN and SD rats. Normal 8-week-old adult BN and SD rats were given VEGF or IGF-1 intravitreal injections into the right eye and PBS into the left one as control. Six hours after the injection, vascular permeability in the retina (A) and iris (B) was measured using the Evans blue method. The results were normalized by total protein concentrations and expressed as μg of dye per mg of protein in the tissue (mean ± SD, n = 4). Values statistically different from the PBS-injected contralateral controls are indicated by * (P < 0.05).
Increased RVP in Diabetic Rats Confirmed by Direct Immunostaining of Albumin and by Visualization of Evans Blue-Albumin Complex in Retina Sections
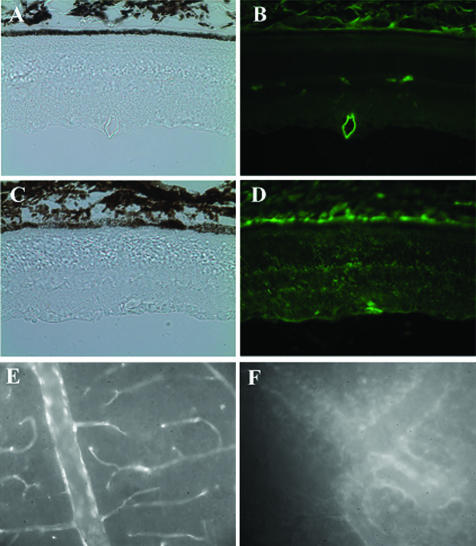
To confirm the increase of RVP in diabetic retina, immunohistochemistry of albumin was performed in BN rats at 4 weeks of diabetes and compared with that in age-matched normal controls. In the control (Figure 6, A and B), the albumin staining was only observed inside the blood vessels in the inner retina while no albumin was detected in the retina tissue. In diabetic retina (Figure 6, C and D), intensive and diffuse albumin staining was observed in the inner retina. Moreover, diffused albumin staining was also detected in the outer retina. This result provides the direct evidence showing that significant amount of albumin was leaked into retina tissue in BN rats at 4 weeks of diabetes.
Figure 6.
Immunostaining of albumin and visualization of Evans blue-albumin complex in retina sections. Immunohistochemistry using an anti-albumin antibody was performed in BN rats of 4 weeks of diabetes (C and D) and age-matched normal controls (A and B). The figure shows diffused albumin staining in the retina of diabetic rats, while no staining in the retina of normal control. A and C: Phase contrast images of the same fields of images (B) and (D), respectively. E and F: Retinal vascular leakage in OIR BN rat was revealed by visualization of Evans Blue-albumin complex in the retina. The figure shows that in the control retina (E) Evans blue-albumin complex was limited inside the blood vessels, while the dye leaked into the retina tissue in the OIR retina (F). Each image is a representative from three rats.
The retinal blood vessel leakage was also visualized by Evans blue-albumin complex in retinal flat mount of OIR BN rats. In the age-matched normal control retinas, Evans blue dye was limited inside the blood vessels (Figure 6E). In OIR BN retina at age P16, focal leakage of the dye from capillaries and larger vessels was noted, corroborating the RVP changes obtained by the quantitative Evans blue assay (Figure 6F).
Discussion
The BRB breakdown and the consequent retinal vascular hyperpermeability is one of the early features of diabetic retinopathy.5,6,9 Increased RVP results in the leakage of fluid, lipids, and plasma proteins from blood vessels to retinal tissue, which can further lead to macular edema, a major cause of vision loss in diabetic patients.1,2 The mechanisms underlying retinal vascular hyperpermeability are largely unclear. In our study described here, we compared the susceptibility to the BRB breakdown of BN and SD rats with STZ-induced diabetes or with OIR. The finding that BN rats develop more severe vascular leakage than do SD rats after receiving the same insults suggests that genetic background determines the susceptibility to vascular leakage and also its severity.
STZ-induced diabetic rats is commonly used as a model for the studies of diabetic complications.21–25 Several rat strains have been used for the STZ-induced diabetes model and retinal vascular permeability studies, including albino SD, Wistar, Lewis, and pigmented Long-Evans rats.6,22,37–39 It has been controversial as regard to whether the STZ-diabetic rats develop vascular hyperpermeability in the retina. The controversy may be explained by that different strains of rats were used and permeability was measured at different time points following the onset of diabetes in previous reports. Xu et al31 found that RVP increased by 1.7-fold in STZ-SD rats 1 week following the onset of diabetes, compared to normal controls. However, Lightman et al40 showed that there was no increase in RVP in STZ-SD at 3 weeks and 6 months following onset of diabetes. In addition, Granstam and Granstam41 found that there was no difference in nitric oxide (NO)-tone and retinal blood flow between STZ-SD rats at 3 weeks of diabetes and normal controls. These observations are quite consistent with the time course of RVP changes in STZ-SD rats, as shown in the present study, ie, elevated RVP only occur at the early stages (3 to 10 days) after the onset of diabetes, and RVP returns to the control level by 2 weeks following the onset of diabetes. Previous studies also showed that there is no significant change in the blood-nerve barrier in STZ-SD rats at 8 and 16 weeks after the onset of diabetes, suggesting that the changes of permeability in the nerve are consistent with that in the retina.42 There were no previous studies of RVP in STZ-BN rats. In STZ-diabetic Long-Evans rats, however, the changes of RVP are also controversial. Ennis and Betz43 showed that the BRB was unaffected at 2 and 6 weeks of diabetes, and there was only a small increase in permeability at 20 weeks. In another paper, Ennis44 showed that the passive permeability of the BRB was not increased at 2-, 4-, and 12-week diabetic Long-Evans rats. However, permeability studies using 125I-labeled albumin and the Evans blue methods both showed that the vascular permeability increased by 2.9-fold and 10.7-fold after 1 and 4 weeks of diabetes.31,45–48 Therefore, when we compare the vascular leakage in diabetic rats, rat strain, duration of diabetes, and the tracer methods used in the permeability assay should be taken into consideration.
In the present study, we performed a systematic comparison of the time courses of vascular permeability in pigmented BN and albino SD rats with STZ-induced diabetes. The results show that vascular leakage occurs earlier and lasts longer in diabetic BN rats than in diabetic SD rats. Moreover, the increases of RVP in STZ-BN and STZ-SD rats are not parallel with their blood glucose levels, suggesting that the strain differences in RVP cannot be ascribed to the different hyperglycemia levels. Instead, the BRB in BN rats may be more susceptible to hyperglycemia-induced vascular damage. The histological and genetic bases for the lower susceptibility to the BRB breakdown or the higher capacity of BRB repair in diabetic SD rats remain to be investigated in the future.
OIR is a widely used model for studies of ischemia-induced retinal NV.26–29 Previous studies have shown increased protein concentrations in the vitreous of OIR rats, before retinal neovascularization.49 In our recent studies, we compared retinal NV in BN and SD rats with OIR. Our results showed that in the OIR model, BN rats are more prone to develop ischemia-induced retinal NV than are SD rats. Moreover, OIR-BN rats have higher VEGF up-regulation in the retina.30 Since the increase in vascular permeability is considered a crucial step in the development of NV, and vascular leakage is a common feature in many ischemic retinal diseases such as central retinal vein occlusion, we have measured vascular permeability changes in the retina and iris of BN and SD rats with OIR at different stages of OIR. In OIR-BN rats, a significant increase in RVP was observed from P12 to P22, while it only occurred between P14 and P18 in the OIR-SD retina. In addition, the peak of the RVP change in OIR-BN rats was approximately four times higher than that in the OIR-SD ones. In the iris, OIR-SD rats showed a transient increase in vascular permeability, while OIR-BN rats demonstrated sustained hyperpermeability during the entire experimental period. These results reveal that OIR also result in vascular leakage in the retina, which is more severe and lasts longer in BN as compared to SD rats, indicating a difference in susceptibility to hypoxia-induced BRB dysfunction.
VEGF is believed to be an important mediator of increased vascular permeability by either decreasing the expression of junctional proteins, such as occludin or inducing their redistribution.7,21,50–52 Intravitreal injection of VEGF caused 26.7% ultrastructural opening of endothelial cell tight junctions in the retinas as opposed to the 11.4% opening detected in the saline-injected control retinas.53 VEGF-targeted strategies, such as anti-VEGF antibodies and antagonists of the VEGF receptors have shown beneficial effects on reducing vascular permeability.1,13,45,54 In the present study, we have compared the retinal VEGF levels in BN and SD rats with STZ-diabetes at different time points following the onset of diabetes. From 1 to 16 weeks of diabetes, the retinal VEGF levels were apparently higher in diabetic BN rats than it was in diabetic SD rats, while the basal levels of retinal VEGF in normal BN and SD rats were similar. In contrast, there was no strain difference in VEGFR2 levels. This result suggests that the differential up-regulation of VEGF by diabetes in these two strains may be responsible, at least in part, for the different vascular leakages in the STZ-diabetes model.
Our previous studies have demonstrated that VEGF levels in the OIR-BN retina are significantly higher than those in the OIR-SD ones, while the basal VEGF levels are similar in the neonatal rats of these two strains.30 Moreover, the peaks of VEGF overexpression appeared at P16, coinciding with that of hyperpermeability, further supporting that overexpression of VEGF plays a major role in the vascular leakage in OIR.27 The results derived from the OIR model also support the assumption that the difference between the above two strains in vascular hyperpermeability differences can be ascribed to the different up-regulations of VEGF by ischemia.30
To confirm the different VEGF regulations in the two rat strains, we have determined the VEGF expression induced by IGF-1 using vascular permeability as an indicator in normal adult BN and SD rats, as IGF-1 is known to induce hyperpermeability through the up-regulation of VEGF.36 The same amount of IGF-1 induced more severe hyperpermeability in BN rats when compared to that found in SD rats, reflecting higher induction of endogenous VEGF. This observation further confirms that the induction of VEGF expression is different in these two strains. It is noteworthy that injection of the same amount of an IGF-1 analogue, R3, which does not bind to its binding proteins, did not induce vascular leakage in either BN or SD rats (data not shown). This result suggests that the interactions with IGF-1-binding proteins may be essential for the activity of IGF-1 in induction of vascular leakage. As hypoxia and IGF-1 both induce VEGF expression through the hypoxia-inducible factor-1 (HIF-1), the difference in VEGF up-regulation in these two strains may be ascribed to different HIF-1 pathways or the HIF-1-binding sites in the VEGF promoter of the two strains.
In conclusion, the present study suggests that the strain difference in vascular leakage between BN and SD STZ-diabetic rats may be partially ascribed to different VEGF expression and VEGF signaling in these strains. Our results suggest that the BN rat is more susceptible to hypoxia-induced hyperpermeability in the retina of STZ-diabetes model and OIR model. This study also supports the conclusion that genetic background is a determinant of susceptibility to retinal NV and the breakdown of the BRB in diabetic retinopathy.
Acknowledgments
We thank Dr. Bruce Berkowitz for critical review and discussion of this manuscript.
Footnotes
Address reprint requests to Jian-xing Ma, M.D., Ph.D., The Oklahoma University Health Science Center, 941 Stanton L. Young Blvd., BSEB 328B, Oklahoma City, OK 73104-5043. E-mail: jian-xing-ma@ouhsc.edu.
Supported by NIH grants EY12231 and EY015650, Research Awards from the American Diabetes Association and the Juvenile Diabetic Foundation as well, as by a research grant from Novartis Pharma AG, Ophthalmology, Basel, Switzerland.
References
- Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86:363–365. doi: 10.1136/bjo.86.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105:998–1003. doi: 10.1016/S0161-6420(98)96025-0. [DOI] [PubMed] [Google Scholar]
- Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47:S253–262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- Bandello F, Lanzetta P, Menchini U. When and how to do a grid laser for diabetic macular edema. Doc Ophthalmol. 1999;97:415–419. doi: 10.1023/a:1002499920673. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz JG. The blood-ocular barriers: past, present, and future. Doc Ophthalmol. 1997;93:149–157. doi: 10.1007/BF02569055. [DOI] [PubMed] [Google Scholar]
- Do carmo A, Ramos P, Reis A, Proenca R, Cunha-vaz JG. Breakdown of the inner and outer blood retinal barrier in streptozotocin-induced diabetes. Exp Eye Res. 1998;67:569–575. doi: 10.1006/exer.1998.0546. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Mori F, Hikichi T, Takahashi J, Nagaoka T, Yoshida A. Dysfunction of active transport of blood-retinal barrier in patients with clinically significant macular edema in type 2 diabetes. Diabetes Care. 1248;25:1248–1249. doi: 10.2337/diacare.25.7.1248. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Gadegbeku C, Campochiaro PA, Green WR. Immunohistochemical localization of blood-retinal barrier breakdown in human diabetics. Am J Pathol. 1989;134:231–235. [PMC free article] [PubMed] [Google Scholar]
- Vinores SA, Derevjanik NL, Mahlow J, Berkowitz BA, Wilson CA. Electron microscopic evidence for the mechanism of blood-retinal barrier breakdown in diabetic rabbits: comparison with magnetic resonance imaging. Pathol Res Pract. 1998;194:497–505. doi: 10.1016/s0344-0338(98)80118-0. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Ishiko S, Kojima M, Ogasawara H. Permeability of the blood-ocular barrier in adolescent and adult diabetic patients. Br J Ophthalmol. 1993;77:158–161. doi: 10.1136/bjo.77.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal: similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Wong JS. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int Suppl. 2000;77:S113–S119. doi: 10.1046/j.1523-1755.2000.07718.x. [DOI] [PubMed] [Google Scholar]
- Senger DR, Connolly DT, Van de Water L, Feder J, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990;50:1774–1778. [PubMed] [Google Scholar]
- Murata T, Nakagawa K, Khalil A, Ishibashi T, Inomata H, Sueishi K. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab Invest. 1996;74:819–825. [PubMed] [Google Scholar]
- Hammes HP, Lin J, Bretzel RG, Brownlee M, Breier G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes. 1998;47:401–406. doi: 10.2337/diabetes.47.3.401. [DOI] [PubMed] [Google Scholar]
- Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408–2413. [PubMed] [Google Scholar]
- Balamurugan AN, Gu Y, Miyamoto M, Wang W, Inoue K, Tabata Y. Streptozotocin (STZ) is commonly used to induce diabetes in animal models. Pancreas. 2003;26:102–103. [Google Scholar]
- Kowluru RA. Retinal metabolic abnormalities in diabetic mouse: comparison with diabetic rat. Curr Eye Res. 2002;24:123–128. doi: 10.1076/ceyr.24.2.123.8158. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Kowluru RA, Frank RN, Kern TS, Hohman TC, Prakash M. Subnormal retinal oxygenation response precedes diabetic-like retinopathy. Invest Ophthalmol Vis Sci. 1999;40:2100–2105. [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells: Penn State Retina Research Group. Diabetes. 1998;47:1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- Carroll WJ, Hollis TM, Gardner TW. Retinal histamine systhesis is increased in experimental diabetes. Invest Ophthalmol Vis Sci. 1988;29:1201–1204. [PubMed] [Google Scholar]
- Clermont AC, Brittis M, Shiba T, McGovern T, King GL, Bursell SE. Normalization of retinal blood flow in diabetic rats with primary intervention using insulin pumps. Invest Ophthalmol Vis Sci. 1994;35:981–990. [PubMed] [Google Scholar]
- Ishibashi T, Tanaka K, Taniguchi Y. Disruption of iridial blood-aqueous barrier in experimental diabetic rats. Graefes Arch Clin Exp Ophthalmol. 1982;219:159–164. doi: 10.1007/BF02156840. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16:438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Visual Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Gao G, Li Y, Zhang D, Gee S, Crosson C, Ma J. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 2001;489:270–276. doi: 10.1016/s0014-5793(01)02110-x. [DOI] [PubMed] [Google Scholar]
- Gao G, Li Y, Gee S, Dudley A, Fant J, Crosson C, Ma JX. Down-regulation of vascular endothelial growth factor and up-regulation of pigment epithelium-derived factor: a possible mechanism for the anti-angiogenic activity of plasminogen kringle 5. J Biol Chem. 2002;277:9492–9497. doi: 10.1074/jbc.M108004200. [DOI] [PubMed] [Google Scholar]
- Zhang D, Kaufman PL, Gao G, Saunders RA, Ma JX. Intravitreal injection of plasminogen kringle 5, an endogenous angiogenic inhibitor, arrests retinal neovascularization in rats. Diabetologia. 2001;44:757–765. doi: 10.1007/s001250051685. [DOI] [PubMed] [Google Scholar]
- Gao G, Li Y, Fant J, Crosson CE, Becerra SP, Ma JX. Difference in ischemic regulation of vascular endothelial growth factor and pigment epithelium-derived factor in Brown Norway and Sprague Dawley rats contributing to different susceptibilities to retinal neovascularization. Diabetes. 2002;51:1218–1225. doi: 10.2337/diabetes.51.4.1218. [DOI] [PubMed] [Google Scholar]
- Xu Q, Qaum T, Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci. 2001;42:789–794. [PubMed] [Google Scholar]
- Radius RL, Anderson DR. Distribution of albumin in the normal monkey eye as revealed by Evans blue fluorescence microscopy. Invest Ophthalmol Vis Sci. 1980;19:238–243. [PubMed] [Google Scholar]
- Caspers-Velu LE, Wadhwani KC, Rapoport SI, Kador PF. Permeability of the blood-retinal and blood-aqueous barriers in galactose-fed rats. J Ocular Pharmacol Ther. 1995;11:469–487. doi: 10.1089/jop.1995.11.469. [DOI] [PubMed] [Google Scholar]
- Rungger-Brandle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41:1971–1980. [PubMed] [Google Scholar]
- Vinores SA. Assessment of blood-retinal barrier integrity. Histol Histopathol. 1995;10:141–154. [PubMed] [Google Scholar]
- Punglia RS, Lu M, Hsu J, Kuroki M, Tolentino MJ, Keough K, Levy AP, Levy NS, Goldberg MA, D’Amato RJ, Adamis AP. Regulation of vascular endothelial growth factor expression by insulin-like growth factor I. Diabetes. 1997;46:1619–1626. doi: 10.2337/diacare.46.10.1619. [DOI] [PubMed] [Google Scholar]
- Barouch FC, Miyamoto K, Allport JR, Fujita K, Bursell SE, Aiello LP, Luscinskas FW, Adamis AP. Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci. 2000;41:1153–1158. [PubMed] [Google Scholar]
- Kinoshita N, Kakehashi A, Inoda S, Itou Y, Kuroki M, Yasu T, Kawakami M, Kanazawa Y. Effective and selective prevention of retinal leukostasis in streptozotocin-induced diabetic rats using gliclazide. Diabetologia. 2002;45:735–739. doi: 10.1007/s00125-002-0820-y. [DOI] [PubMed] [Google Scholar]
- Kojima M, Sasaki K. Reinvestigation of streptozotocin-induced diabetic cataract as a standard experimental model. Nippon Ganka Gakkai Zasshi. 1993;97:324–332. [PubMed] [Google Scholar]
- Lightman S, Pinter G, Yuan L, Bradbury M. Permeability changes at blood-retinal barrier in diabetes and effect of aldose reductase inhibition. Am J Physiol. 1990;259:R601–605. doi: 10.1152/ajpregu.1990.259.3.R601. [DOI] [PubMed] [Google Scholar]
- Granstam E, Granstam SO. Regulation of uveal and retinal blood flow in STZ-diabetic and non-diabetic rats: involvement of nitric oxide. Curr Eye Res. 1999;19:330–337. doi: 10.1076/ceyr.19.4.330.5300. [DOI] [PubMed] [Google Scholar]
- Kihara M, Schmelzer JD, Poduslo JF, Curran GL, Nickander KK, Low PA. Aminoguanidine effects on nerve blood flow, vascular permeability, eletrophysiology, and oxygen free radicals. Proc Natl Acad Sci USA. 1991;88:6107–6111. doi: 10.1073/pnas.88.14.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis SR, Betz AL. Sucrose permeability of the blood-retinal and blood-brain barriers. Invest Ophthalmol Vis Sci. 1986;27:1095–1102. [PubMed] [Google Scholar]
- Ennis SR. Permeability of the blood-ocular barrier to mannitol and PAH during experimental diabetes. Curr Eye Res. 1990;9:827–838. doi: 10.3109/02713689008999555. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol. 2002;160:501–509. doi: 10.1016/S0002-9440(10)64869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Tsujikawa A, Qin W, Qaum T, Xu Q, Moromizato Y, Bursell SE, Wiegand SJ, Rudge J, Ioffe E, Yancopoulos GD, Adamis AP. Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol. 2002;160:1683–1693. doi: 10.1016/S0002-9440(10)61115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, Aiello LP, Ogura Y, Adamis AP. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci USA. 1999;96:10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulaki V, Qin W, Joussen AM, Hurlbut P, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest. 2002;109:805–815. doi: 10.1172/JCI13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Roberto KA, Penn JS. The vitreous protein concentration is increased prior to neovascularization in experimental ROP. Curr Eye Res. 1998;17:218–221. doi: 10.1076/ceyr.17.2.218.5604. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, Smith LE, King GL. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol. 1999;14:240–248. doi: 10.3109/08820539909069543. [DOI] [PubMed] [Google Scholar]
- Luna JD, Chan CC, Derevjanik NL, Mahlow J, Chiu C, Peng B, Tobe T, Campochiaro PA, Vinores SA. Blood-retinal barrier (BRB) breakdown in experimental autoimmune uveoretinitis: comparison with vascular endothelial growth factor, tumor necrosis factor alpha, and interleukin-1beta-mediated breakdown. J Neurosci Res. 1997;49:268–280. doi: 10.1002/(sici)1097-4547(19970801)49:3<268::aid-jnr2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Lu M, Adamis AP. Vascular endothelial growth factor gene regulation and action in diabetic retinopathy. Ophthalmol Clin North Am. 2002;15:69–79. doi: 10.1016/s0896-1549(01)00010-4. [DOI] [PubMed] [Google Scholar]