Abstract
We generated a line of transgenic mice using a yeast artificial chromosome containing the Ret mutation responsible for the multiple endocrine neoplasia type 2B syndrome (MEN 2B). The resulting animals did not develop any of the expected neoplasms associated with MEN 2B. Transgenic animals were then bred with animals lacking murine Ret (RetM) to further evaluate the function of human mutated Ret (RetH2B) in the murine context. Whereas mice lacking RetM exhibit intestinal aganglionosis and the absence of kidneys with other genitourinary anomalies, expression of the RetH2B transgene in RetM-deficient mice allowed significant renal development with a partial rescue of the enteric nervous system. These RetH2B-positive/RetM-deficient mice exhibit normal Ret expression and survive longer than RetM-deficient mice, but still die at 3 to 5 days of age with evidence of enterocolitis. We conclude that the normal expression of a human Ret proto-oncogene with the MEN 2B mutation does not cause any features of MEN 2B in mice. Although the gene is normally expressed in the appropriate target tissues, there is incomplete phenotypic rescue in mice lacking murine Ret. These results suggest important interspecies differences between humans and mice in the function of the Ret oncogene.
The Ret proto-oncogene encodes a receptor tyrosine kinase that regulates the growth and development within the neurological and excretory systems.1–3 The gene was discovered using the NIH3T3 transfection assay and the name Ret derives from this discovery (rearranged during transfection).4 More recently, it was discovered that certain germ line mutations in the Ret proto-oncogene are associated with the multiple endocrine neoplasia (MEN) syndromes types 2A and 2B and familial medullary thyroid carcinoma (FMTC).5–9 These syndromes are inherited in an autosomal dominant manner. Patients with MEN 2A develop medullary thyroid carcinomas (MTCs), pheochromocytomas, and hyperparathyroidism; similarly, those with MEN 2B also develop MTCs and pheochromocytomas, but additionally develop mucosal neuromas of the lips, oral mucosa, and alimentary tract. Finally, FMTC is characterized only by the development of medullary thyroid carcinoma.10–12
To study the normal function of murine Ret (designated RetM), transgenic mice were created that lacked either Ret, the co-receptor GFR-α1, or glial cell line-derived neurotrophic factor (GDNF), the cognate ligand for the receptor complex.13–18 The Ret, GFR-α1, and GDNF knockout mice are all missing the enteric ganglion cells distal to the stomach and also exhibit varying degrees of renal agenesis or dysgenesis.18 The absence of intestinal ganglion cells is reminiscent of Hirschsprung’s disease in humans, in which there is the congenital absence of enteric ganglia in the distal gut. Indeed, inactivating Ret mutations are also the most common defined cause of Hirschsprung’s disease in humans. The importance of Ret in the development of the nervous system was further demonstrated when the other Ret ligands persephin, artemin, and neuturin were characterized and demonstrated to interact with Ret to support the growth of neurons in the peripheral, central, and enteric nervous systems (ENSs).19–23
Recently, to study the dominant transforming activity of the MEN 2B mutation of Ret, investigators created a transgenic mouse line containing the equivalent codon 918 Met to Thr mutation in the mouse Ret gene.24 Interestingly, these mice did not precisely model the human phenotype. Whereas they developed thyroid C-cell hyperplasia and pheochromocytomas, the mice did not develop MTC. Moreover, the animals did not possess the neuromas of the lips, oral mucosa, or gastrointestinal tract found in humans with MEN 2B. These results suggest that there are species differences between humans and mice that are responsible for the different phenotypes associated with the MEN 2B mutation of Ret. However, Ret with the MEN 2B mutation does support normal development of the kidney and ENS, even in mice homozygous for MEN 2B.24 In contrast to the above mouse model, two other studies25,26 found that overexpression of a human MEN 2B transgene causes developmental renal malformations.
In an attempt to create a mouse model of MEN 2B that recapitulates all of the sequelae of the human disease, we created a line of transgenic mice possessing the human Ret gene with the codon 918 Met to Thr mutation responsible for MEN 2B. To assure faithful expression of the gene through embryonic development and in the appropriate tissues, we used a yeast-associated chromosome (YAC) containing the human Ret gene with the MEN 2B mutation (designated RetH2B). This allows the inclusion of a considerable of amount of genetic material upstream and downstream from the coding elements of the gene, to ensure the inclusion of genetic elements responsible for the regulation of Ret expression. These transgenic animals were followed to see whether they developed the features of the human MEN 2B syndromes. To further evaluate the functionality of the human transgene, we bred these RetH2B transgenic mice to animals lacking one murine Ret allele (designated RetM), and then performed the genetic backcrosses to create mice that only express RetH2B. Our aims in this portion of the study were to determine whether the RetH2B gene corrects the phenotype associated with the RetM knockout and to investigate whether the human Ret gene with the MEN 2B mutation, expressed outside the context of the mouse Ret gene, impacts the development of tumors associated with MEN 2B.
Materials and Methods
Introduction of the MEN 2B Mutation into the Human Ret Proto-Oncogene
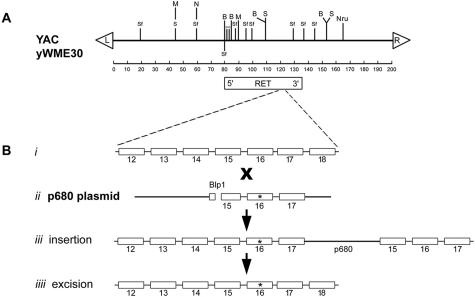
The Ret proto-oncogene measures ∼50 kb in length, and is contained within YAC yWME30, obtained from the Washington University Human Genome Sequencing Center. This YAC, measuring ∼200 kb in length, was transferred from the native AB1380 yeast strain into the YPH985 strain by kar-1 mating.27 The location and orientation of Ret within the YAC was ascertained by Southern analysis. The restriction map is depicted in Figure 1 and is consistent with other published results.28 To create the mutated gene that causes MEN 2B, the codon 918 T to C mutation responsible for the syndrome was introduced into the YAC by the two-step mutation method essentially as described.27 Briefly, the polymerase chain reaction (PCR) was used to isolate a ∼2-kb length of Ret from DNA obtained from a patient with MEN 2B. This genetic segment extended from the 5′ end of exon 15 to the 3′ end of exon 17, containing the intervening introns and the 16th exon, in which the point mutation resides. The PCR oligonucleotide names and sequences are 15F (5′TCATAAGCTTCATGGCCTGACGACTCGTCGTATT) and 17R (5′TCATCTCGAGCAAGTGAGGCTGGCCAAAGC). The exonic coding segments of the resulting PCR product were sequenced to ensure the absence of PCR fidelity errors, and the product was cloned into the yeast vector p680.27,29 This construct, containing the bp change of interest, was linearized with BlpI, and the mutated region of Ret was incorporated into the YAC using targeted homologous recombination as described.27 Again, exons 15 through 17 were sequenced in the YAC clone to ensure that the mutation of interest had been introduced into the gene, and that no other abnormalities were present. Finally, Southern analysis was performed to document that no gross rearrangements in Ret or in the YAC had occurred. The final YAC genetic construct with the mutation associated with MEN 2B was designated RetH2B.
Figure 1.
A: Restriction map of YAC yWME30 containing the Ret proto-oncogene. The arrowheads denote the left and right arms. Ret is ∼50 kb in length and the 5′ end is located ∼80 kb from the left arm of the YAC. The restriction endonucleases are denoted: S, SacI; B, BamHI; SF, SfiI; M, Mlu; N, NotI. B: Site-directed mutagenesis to introduce the 918Met→Thr mutation into Ret within the YAC. ii, The PCR was used to amplify the length of DNA containing Ret exons 15, 16, and 17, with the intervening introns, from DNA obtained from a patient with MEN 2B. The amplified DNA, containing the 918Met→Thr mutation in exon 16 (asterisk), was cloned into the p680 plasma containing the LEU2 gene (for positive selection) and CYH2s gene (for negative selection). iii, The restriction endonuclease BlpI was used to linearize the plasmid, which then allowed targeted transformation of yeast cells containing the YAC by selecting in Leu+ medium for colonies containing the LEU2 gene. iiii, The resulting strains were grown in medium containing cycloheximide to select for the occasional colony in which the p680 plasmid was deleted by homologous recombination, leaving the MEN 2B point mutation in yWME30.
Generation and Characterization of Transgenic Mice with the Mutated Ret Proto-Oncogene
To purify the YAC for pronuclear injection, the mutation-containing YAC was first separated from the other yeast chromosomes using pulsed-field gel electrophoresis.30 The edges of the gel were removed and ethidium-stained to determine where the YAC band had migrated. This band was cut from the 1% agarose gel, cut in half, and the two gel slices were placed side-by-side within a 4% agarose gel in a direction 90° to the pulsed-field gel electrophoretic run. The YAC DNA was concentrated by running the DNA through the 1% agarose into the 4% gel. One of the bands was stained with ethidium bromide to localize the DNA. The unstained gel slice containing the YAC DNA was dialyzed in digestion buffer, and subjected to GELase (Epicenter, Inc., Madison, WI) digestion. Purified YAC DNA (concentration 4 ng/μl) was injected into male pronuclei of fertilized eggs from F1 hybrid mice (C57BL/6 × B6C3H), and founder transgenic mice were generated using standard techniques.31,32 A total of 162 animals underwent tail-DNA screening for the human gene using PCR analysis to amplify the mutated 16th exon (oligonucleotides 16F 5′AGAGAGTAGAGTAACTTCAATGTC, 16R 5′CTACATGTATAAGGGTGTTT),33 followed by FokI digestion to document presence of the expected mutation.5 Three transgenic animals were generated, and in each of these lines, there was successful germline transmission of human Ret (RetH2B). To ensure that the transgene contained the entire Ret coding sequence, PCR was used to amplify the first and 20th exons. We found that a genetic rearrangement had occurred in one of the transgenic lines, resulting in the deletion of exon 1. The intact human Ret proto-oncogene was present in two lines of transgenic mice (line numbers 7 and 24) but there was a deletion in the inserted YAC from transgenic line 7 that resulted in the inclusion of only a very short length of intact DNA upstream to the first exon. The animals from line 24 were bred, and evaluated histologically for the development of the expected thyroid and adrenal neoplasms associated with Ret mutations. These animals were also bred with mice heterozygous for murine Ret (RetM+/−) to generate mice expressing RetH2B and heterozygous for murine Ret (RetH2B/RetM+/−). These animals were then backcrossed with murine Ret heterozygotes (RetM+/−) to generate mice with the genotype RetH2B/RetM−/− possessing only the mutated human YAC transgene. All of these experiments were performed with the prior approval of the Institutional Animal Care and Use Committees at Duke University and Washington University.
Histopathological Analysis
Anesthetized animals were sacrificed by transcardiac perfusion with phosphate-buffered saline, followed by 4% paraformaldehyde. In adult animals, tissues were dissected and embedded in paraffin before sectioning and staining with hematoxylin and eosin using standard procedures. Newborn pups were similarly perfused, and whole pups were paraffin-embedded and sectioned either longitudinally or transversely through the entire specimen before staining for histological analysis.
Evaluation of Ret Expression
RNA was purified from transgenic mouse tissues using Triazol (Life Technologies, Gaithersburg, MD) reagent, and reverse-transcribed into cDNA using a kit and protocol from Life Technologies. We assayed for expression of both the long (Ret51) and short (Ret9) transcripts of the human Ret proto-oncogene using species-specific oligonucleotides with the following sequences: upstreamhuman CCAGTTAAATGGACGGCAATTG (exon 16); downstream human RET9 AGACTTTGGTTTTGTTCAGAC (exon 19); downstream human RET51 CGGTAGACTTTCCATTCTCAG (exon 20); upstream mouse CCCGTCAAGTGGATGGCAATTG; downstream mouse RET9 AAGCTTTGGTGTCGGTGGGAT; downstream mouse RET51 CGGTGGACTTGATGCTCTTGG. The locations of the mouse primers correspond to those of the human primers. Ret was amplified from mouse cDNA for 40 cycles using the following parameters: 94°C for 30 seconds, 50°C for 30 seconds, 72°C for 45 seconds. The PCR products were then resolved on a 1.2% agarose gel.
Assays to quantitatively compare the transcription of the human and murine Ret transcripts were performed using the Clontech PCR MIMICS kit (B.D. Biosciences Clontech, Palo Alto, CA), essentially according to the manufacturer’s instructions. Species-specific oligonucleotides were designed to anneal within the coding region of the Ret gene (exon 15, forward; exon 17, reverse), as well as the v-erb internal standard mimic oligonucleotide (murine: forward 5′ TGGCACACCTCTGCTCTATGTGTTATACAGGGAGATGAAA; reverse 5′ TGTTCCCAGGAACTGTGGTCTCTGTCAATGCAGTTTGTAG; human: forward 5′ GATTTCGGCTTGTCCCGAGATGTTATACAGGGAGATGAAA; reverse 5′ TCAGGAGGAATCCCAGGATAGTCTGTCAATGCAGTTTGTAG).
Acetylcholinesterase Histochemistry
Acetylcholinesterase staining was performed on the alimentary tract of transgenic animals as described.14 Briefly, the alimentary tract was removed and fixed in 4% paraformaldehyde for 1 to 2 hours at 4°C. After overnight storage in saturated sodium sulfate, the gut was incubated in buffer [0.2 mmol/L ethopromazine HCl (Sigma Chemical Co., St. Louis, MO), 4 mmol/L acetylthiocholine iodide (Sigma), 10 mmol/L glycine, 2 mmol/L cupric sulfate, 65 mmol/L sodium acetate, pH 5.5] for 2 to 4 hours. Finally, staining for acetylcholinesterase was developed by incubating for 1.5 minutes in sodium sulfide (1.25%, pH 6.0), and the specimens were photographed under a dissecting microscope.
Genetic Mapping of the Integrated YAC
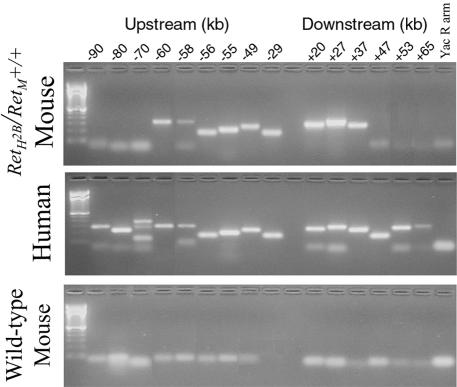
To verify the amount of human genetic material upstream and downstream from Ret, and to investigate whether genetic material was lost from the YAC during the manipulations required to create the transgenic mouse line, genetic mapping was used to investigate which portions of the YAC were incorporated into the transgenic mice. Oligonucleotides for PCR analysis were synthesized corresponding to human sequences adjacent to Ret at intervals of 3 to 5 kb along chromosome 10 upstream and downstream from the upstream and downstream ends of the gene. As demonstrated in Figure 2, we found that a total of ∼150 kb of the YAC were successfully transferred into the transgenic mouse. There were ∼60 kb 5′ to Ret and 37 kb 3′ to the gene. The oligonucleotide sequences are listed in Table 1.
Figure 2.
Genetic mapping to determine the regions of YAC yWME30 integrated into the transgenic mouse genome. Oligonucleotides were designed for use in PCR to selectively amplify human DNA in the region of the Ret proto-oncogene. Markers were located at variable distances 5′ and 3′ to Ret at the positions noted, and resolved by agarose gel electrophoresis. The top photograph demonstrates the results obtained from transgenic mouse DNA, the middle photograph demonstrates the results from normal human DNA, and the bottom photograph demonstrates the results from wild-type mouse DNA, to demonstrate the human specificity of the PCR reactions. The YAC was integrated from a region of 60 kbp upstream from Ret to 37 kbp downstream.
Table 1.
Oligonucleotides for YAC Mapping
| Position relative to Ret (kb) | Primer sequence |
|---|---|
| −90 | F-TCTCTCCATTTATGGCTGGG |
| R-TGCAAGGTGAAGCAAGAATG | |
| −80 | F-CAGACTGCTGACCTCCACAA |
| R-GGGCCTGCAGAGACACTAAG | |
| −70 | F-AGGAGAATGGCATGAACCTG |
| R-ACAAAACCAGTTCCTGGTGC | |
| −60 | F-AGACTCCGTCTTCTCTACAAAA |
| R-AAGAGAGGAAGAGCGATACGTCC | |
| −58 | F-TCTAATTCCTTCCAGAAATGCT |
| R-TGGACCGACAAAAATAACCAGT | |
| −56 | F-TTGCTCCATGGAGAACTGAG |
| R-GGCAATGAGGGAACTGGAAA | |
| −55 | F-GGGAGCTAATTTCTCCCTGG |
| R-GAGCCTCGACTAAGGGAATA | |
| −49 | F-GCCTGCTCATTAGAGGGATTAT |
| R-TTCTTCAAATTCAGAGTCACAG | |
| −29 | F-TTTAAGCCTCTGTTTCAGCT |
| R-AGAAAGCCAGAACTCCCATT | |
| +20 | F-TGAAAAGTCCAGGCCAAGAG |
| R-AGGTCGAGAGTTCAAGACCA | |
| +27 | F-AATGGGCAATGCCCATACT |
| R-GGGAGCTGTGCCAGTTTTAT | |
| +37 | F-AATTACTTTTTGGGGTGGGA |
| R-GTTGGATGAGAAGTCAGGTT | |
| +47 | F-TGAGTGAAAGAAGCCAGTCATA |
| R-TAGAGCATTTCACCCCAAAAAG | |
| +53 | F-CCATCCCTTTTGAAGCAGAA |
| R-GGAGTGAGAGGCCTCATTCA | |
| +65 | F-TTTCACGCATCAACCTTTGG |
| R-CCAAACCACAGAGCAGGAAG | |
| YAC right arm | F-ACGTCACTGTGAATGTTGCGG |
| R-TGCTTGGGAGTTGGGAATTGAAG |
Results
The RetH2B Transgene Does Not Cause MEN 2B Symptoms in Mice
In humans, the visible phenotypic features associated with the MEN 2B syndrome include facial pigmentation spots and submucosal neuromas of the tongue and lips, and a marfanoid appearance.34 Patients with MEN 2B also develop mucosal neuromas in the intestine and exhibit optic nerve hypertrophy. The first generation of transgenic mice with the human RetH2B proto-oncogene YAC construct also contained both normal mouse Ret alleles, and were designated genotypically RetH2B/RetM+/+. Standard anatomical and histological analysis of more than 25 transgenic animals revealed none of the phenotypic features seen in affected humans.34 Moreover, our breeding studies did not find any adverse effects of the transgene on fetal survival. The various genotypes were present in newborn mice at the expected frequency. We followed the animals longitudinally to determine whether they developed any of the expected neoplasms associated with MEN 2B. Anatomical analysis of more than 100 animals as old as 24 months revealed no neoplasms or other histological abnormalities in the thyroid or adrenal glands.
To exclude the possibility that our findings were related to the presence of three Ret alleles (the mutated human RetH2B and two normal mouse Ret genes), instead of the normal genetic complement of two alleles, we bred the F1 transgenic animal with mice lacking one RetM allele to generate animals possessing the RetH2B gene in the context of only one murine Ret allele, designated genotypically as RetH2B/RetM+/−.18 Again, there were no mucosal neuromas in the face or in the intestine, nor were there any obvious abnormalities in the central nervous system. Moreover, the animals did not develop any of the thyroid or adrenal neoplasms characteristic of the human MEN 2B syndrome throughout 24 months of follow up (more than 100 animals examined). Thus, we found no phenotypic effect in transgenic mice possessing the human Ret proto-oncogene with the mutation associated with MEN 2B, irrespective of whether the animals had the mutated gene in the context of either one or two wild-type murine Ret alleles.
Expression of RetH2B from the YAC Transgene Was Similar to that of Normal Murine Ret
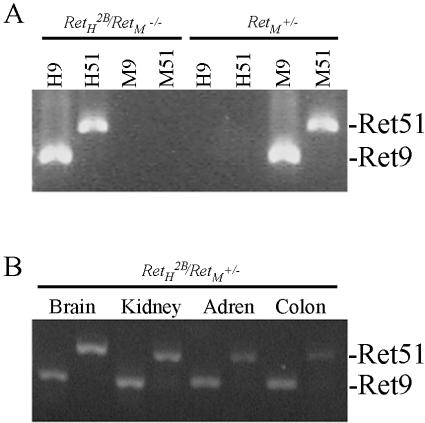
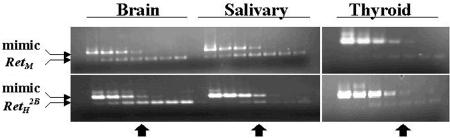
To determine whether the absence of MEN 2B-associated neoplasms was the result of improper expression of the RetH2B transgene in the target tissues, we used reverse transcriptase-PCR to evaluate expression of the human Ret transgene in RetH2B/RetM+/− mice (ie, in animals possessing one each of the human and mouse alleles). In humans, there are multiple Ret transcripts that encode two distinct Ret proteins that differ in the C-terminal region.35 These two moieties are termed Ret9 and Ret51 and demonstrate different downstream signaling characteristics.36–39 Using human-specific primers, we evaluated the expression of Ret9 and Ret51 by reverse transcriptase-PCR in transgenic mice in the brain, kidney, adrenal gland, and colon. The results are presented in Figure 3, and, although not quantitative, demonstrate that both isoforms of the human RetH2B oncogene are expressed in the transgenic mice. To determine whether the human YAC-transgene is expressed at physiological levels, we used the PCR mimics technique to quantitate Ret expression. As shown in Figure 4, the human gene was expressed at approximately the same level as the native murine gene in these animals in the brain, salivary gland, and thyroid gland. Also tested were the kidney and adrenal (not shown). Although we have not tested every tissue in which Ret might be expressed, in those tissues examined, the human Ret oncogene appears to be appropriately expressed at physiological levels.
Figure 3.
PCR products amplified from whole transgenic mice and selected mouse tissues. A: Using species-specific primer sets, cDNA was amplified from a whole RetH2B/RetM−/− mouse expressing only the human RetH2B transgene or the RetM+/− mouse expressing only the mouse Ret allele. Both primer sets from human and mouse correctly amplified the Ret splice variants from the corresponding cDNA. Importantly, the mouse primers did not amplify either splice variant of human Ret nor did the human primers amplify mouse Ret demonstrating species specificity of the primer sets. Each lane is labeled with the primer set used in the amplification: H9 and H51 amplify human Ret9 and Ret51; M9 and M51 amplify the corresponding murine products. The genotype of the source cDNA is labeled above the corresponding lanes. B: cDNA from mouse tissues with a RetH2B/RetM+/− genotype was amplified using the human primer sets for Ret9 and Ret51. The source tissue is labeled above the corresponding lanes and the PCR products are labeled on the right. In the transgenic mouse brain, kidney, adrenal gland, and colon, both human RetH2B splice variants are amplified.
Figure 4.
Comparison of expression levels for human and murine Ret from brain, salivary gland, and thyroid glands obtained from transgenic mice possessing the human RetH2B YAC transgene. Species-specific primers were used to amplify Ret from cDNA at varying dilutions, and compared to the mimic product by agarose gel electrophoresis. The arrowheads denote the dilution at which the human and murine transcripts are expressed at equivalent levels compared to the PCR mimic, demonstrating that human and murine Ret are expressed at the same levels in these tissues.
The RetH2B Transgene Partially Rescues the RetM−/− Kidney and ENS Phenotype
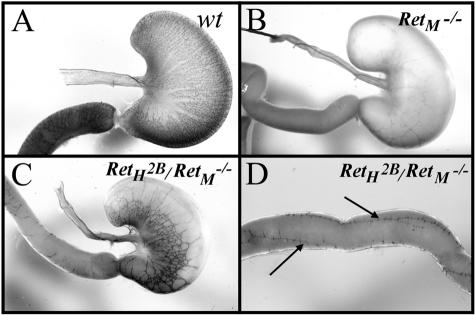
We further evaluated the function of the YAC transgene RetH2B by backcrossing the heterozygous RetH2B/RetM+/− transgenic mice with the Ret heterozygotes RetM+/− to generate mice with the genotype RetH2B/RetM−/− possessing only the mutated human YAC transgene. If the human gene were able to compensate for the absence of murine Ret, then these animals would be expected to exhibit the same phenotype as RetM+/− animals or as mice described by Smith-Hicks and colleagues24 in which normal murine Ret was replaced by two copies of Ret with the MEN 2B mutation (RetM2B+/+) (ie, normal kidneys and normal abundance of enteric ganglion cells). The genotypes of the litters were distributed as predicted, demonstrating no increased intrauterine mortality in any of the genotypes. Interestingly, the newborn pups generated from our backcross breeding experiments having the RetH2B/RetM−/− genotype were normal at birth, but all died within 3 to 5 days after birth. In evaluating these animals shortly after death, the stomachs were distended with milk, and there was a striking presence of air in the wall of the intestine as demonstrated in Figure 5. This pneumatosis intestinalis is similar to that which occurs in human newborns with necrotizing enterocolitis or enterocolitis related to congenital aganglionosis. The animals apparently succumbed to either dehydration or overwhelming infection resulting from enterocolitis.
Figure 5.
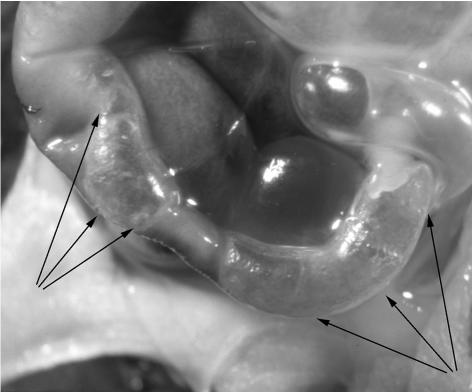
Pneumatosis intestinalis in transgenic mice possessing only the human Ret transgene with the MEN 2B mutation (genotype RetH2B/RetM−/−). Mouse pup was dissected shortly after death at 5 days of life. The arrowheads indicate the obvious bubbles of gas within the intestinal wall.
Histological analysis of cross sections of the bowel from the RetH2B/RetM−/− transgenic animals demonstrated the absence of enteric ganglion cells in the small bowel and colon of these mice. This phenotype is very similar to that found in mice lacking both Ret alleles. Because bowel cross-sections provide a limited view of the ENS and could miss a subtle rescue of ENS development, we next performed whole mount acetylcholinesterase staining to examine the ENS. The staining patterns were compared between wild-type animals, RetM−/− mice, and RetH2B/RetM−/− animals and are shown in Figure 6. Whereas the RetM−/− mice had no enteric neurons in the small bowel or colon and exhibited severe hypoganglionosis of the stomach as previously reported,18 the RetH2B/RetM−/− mice showed considerable ENS development in the stomach and a few chains of enteric ganglia extending for several centimeters down the proximal small bowel. The RetH2B/RetM−/− mice were still missing most of the neurons in the small bowel and all of the enteric neurons in the colon. These chains of enteric ganglia were strikingly different from the RetM−/− phenotype in which the ganglia are not observed in the small bowel except for a very few scattered nuclei in the first millimeter of the duodenum.18 These results suggest that the human YAC RetH2B transgene partially rescues the function of native mouse Ret in the ability to support the development of the ENS.
Figure 6.
Acetylcholinesterase staining of ENS in the stomach and duodenum. A: Wild-type stomach and duodenum (arrow) showing the fine network of nerves of the ENS. B: Stomach and duodenum (arrow) of mouse lacking Ret (genotype RetM−/−) showing aganglionosis of the ENS. C: Stomach and duodenum (arrow) of transgenic mouse with genotype RetH2B/RetM−/− showing partial rescue of the ENS by expression of human RetH2B. D: Distal to the stomach in the RetH2B/RetM−/− mouse, acetylcholinesterase staining is limited to a small number of longitudinal ENS bundles (arrows) in the proximal small intestine.
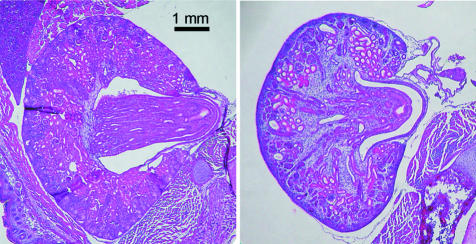
The other significant finding in RetH2B/RetM−/− mice is the frequent absence of kidneys, along with other anomalies of the urinary tract. We evaluated the excretory system in 25 animals with the RetH2B/RetM−/− genotype. In most cases, there were two kidneys, but in five (20%) of the transgenic mice, there was only one kidney present. The extra-renal excretory tracts were unremarkable. Histological analysis of the kidneys demonstrated some dysplasia in transgenic animals, histologically shown in Figure 7. Thus, whereas Ret knockout mice have renal aplasia and other abnormalities in the genitourinary tract, mice having the human RetH2B allele generally had grossly normal excretory systems with varying degrees of mild renal dysplasia.
Figure 7.
Photomicrographs of kidneys from newborn mice. Image on left is from a wild-type animal, and the right image is from a pup with genotype RetH2B/RetM−/−, demonstrating mild cortical dysplasia. Original magnifications, ×40.
Discussion
The Ret proto-oncogene is critical for the formation of the ENS and kidneys in mice. Ret activation also is essential for normal development of the parasympathetic and sympathetic nervous system.18 By activating both the MAPK and PI-3 kinase pathways, Ret tyrosine kinase signaling in many parts of the body regulates cell survival, migration, and proliferation during development.40–42 Inadequate Ret signaling causes intestinal aganglionosis in both humans and mice and renal hypoplasia/aplasia in mice.18,43 In fact, inactivating Ret mutations are the most frequent identifiable cause of Hirschsprung’s (intestinal aganglionosis) in humans.44,45
Increased Ret activation causes cancer in humans. There are three inherited cancer syndromes (FTMC, MEN 2A, and MEN 2B) that result from excessive and inappropriate Ret activation.5–9,46 Of these syndromes, MEN 2B is the most severe and results from a single point mutation in the Ret kinase domain changing Met918 to Thr918. This mutation increases Ret activation and may also change the intracellular signal transduction pathways activated by Ret.47 Humans with the MEN 2B mutation uniformly develop medullary carcinoma of the thyroid by age 20. They also have abnormalities of bone development leading to a marfanoid body habitus, and develop pheochromocytomas and mucosal neuromas.10–12
We generated a transgenic mouse expressing the human Ret gene with a Met918 to Thr918 mutation, expecting to develop a mouse model for MEN 2B. To ensure that appropriate regulatory signals were present in the transgene to recapitulate the normal Ret expression patterns, we elected to express Ret in the mouse from a human YAC clone with the M918T mutation. This 150-kb clone was expected to include the regulatory signals necessary for physiological Ret expression in the mouse. Indeed, human YAC transgenes have successfully rescued absent murine genes in a number of mouse knockout models of human disease.48–50 In other cases, however, human YACs have been unable to completely correct for the absence of a mouse gene.51,52 Our data demonstrate that this human YAC RetH2B clone apparently resulted in normal levels of both Ret splice isoforms in several target tissues including the kidney, brain, thyroid, and salivary and adrenal glands. Further, human RET protein exhibits an overall 83% identity with murine RET protein, and exhibits a 95% identity over the C-terminal 400 amino acids, which encompasses the intracellular signaling domain. Thus, we expected that the RetH2B transgene would create a good model for MEN 2B in mice.
To our surprise, none of the RetH2B mice developed cancer even when maintained for up to 2 years. Careful analysis of the thyroid and adrenal glands, tissues in which humans with MEN 2B typically develop cancer, did not reveal any evidence of neoplasia, despite normal expression pattern of the human transgene. This was true both in mice with two copies of wild-type murine Ret in addition to the transgene and in mice with a single wild-type murine Ret and a single human YAC RetH2B transgene. So why do these mice not develop MTC, the life-threatening neoplasm that occurs in virtually every human with MEN 2B34 or any of the other Ret-associated neoplasms for that matter? To answer this question, one must consider what previous studies have shown: 1) expression of physiological levels of human Ret with the MEN 2B mutation in mice will not induce tumor formation (MTC, pheochromocytoma, or intestinal ganglioneuromas); 2) expression of physiological levels of murine Ret with the MEN 2B mutation in mice will induce C-cell hyperplasia, pheochromocytomas, and certain ganglioneuromas, but not MTC;24 and 3) expression of supraphysiological levels of human Ret with the MEN 2B mutation (or the MEN 2A mutation) in mice will induce MTC and other associated tumors.53–57 It seems possible that intrinsic differences in the human and mouse RET proteins exist such that oncogenic signals from physiological levels of mutant human RET in a mouse are insufficient to drive tumor formation (and that this insufficiency can be overcome by overexpressing mutant human RET). Alternatively, modifier genes may exist that must also be expressed in addition to activated Ret for the development of MTC and that this requirement for modifier genes can be overcome by overexpressing mutant human RET. Further, these modifier genes may or may not be present in any particular strain of inbred mouse, which could explain the observed penetrance of MTC.54 Lastly, it is possible that subtle, undetected differences in Ret expression from the RetH2B transgene are ultimately responsible for the lack of tumor formation. This would take further detailed expression studies to confirm.
To test the biological activity of the human YAC RetH2B transgene, we bred the transgenic mice with RetM+/− heterozygous animals to generate RetH2B/RetM−/− mice. Whereas RetM−/− mice have no kidneys, the RetH2B/RetM−/− mice have essentially normal sized kidneys. Microscopic examination of the kidneys, however, demonstrates abnormalities in the kidney architecture. The cause of these developmental renal anomalies is not yet clear. Interestingly, Gestblom and colleagues26 observed renal dysgenesis in their RetMEN2B-transgenic mice. They found that sympathoadrenal hyperplasia caused the renal malformations by locally competing for ligand during renal development. However, we did not observe any gross sympathoadrenal hyperplasia in our RetH2B/RetM−/− mouse pups. In our mice, we expected that the RetH2B transgene would have increased activity compared to the native RetM gene. If, however, the RetH2B transgene had increased activity compared to the native mouse RetM gene, we would not have expected to see any mice with only a single kidney. The unilateral renal agenesis and dysplastic kidney phenotype in the RetH2B/RetM−/− mice is reminiscent of the renal abnormalities observed in GDNF+/− mice suggesting that despite the mutation, the RetH2B transgene exhibits less downstream signaling activity in the mouse than the wild-type murine Ret gene.16 This overall reduction in Ret activity of the RetH2B transgene compared to the native murine Ret, might also explain the absence of tumors in our mice. In fact, the extracellular portion of human Ret exhibits only a 77% identity with the same portion of murine Ret (versus a 95% identity within the intracellular portion). Thus, reduced Ret-related signaling during kidney development may be a function of reduced interaction with murine GDNF.
If the RetH2B transgene is really less active than wild-type RetM, defects might also be expected in the ENS of RetH2B/RetM−/−. Whereas RetM−/− mice have essentially no enteric neurons distal to the pylorus, and have a reduced density of neurons within the stomach, heterozygous RetM+/− mice have normal numbers of enteric neurons in the colon and small bowel.58 Thus, unlike in humans, haploinsufficiency in mice for RetM does not seem to cause Hirschsprung’s disease (intestinal aganglionosis) or hypoganglionosis. The ENS phenotype in the RetH2B/RetM−/− mice is unusual because single chains of enteric ganglia with thick interconnecting nerve fiber bundles extend down the proximal small bowel. Interestingly, although there are thick bundles of nerve fibers that connect ganglia along a chain that extends down the bowel, there are very few connections between individual chains of ganglia. An explanation for this finding would be that single ENS precursors actually give rise to linear arrays of interconnected ganglia as they migrate down the bowel. In this case, the chains of ganglia would represent the progeny of a single ENS precursor cell. This intestinal hypoganglionosis would be comparable to the piebaldism or coat spotting phenotype that occurs in endothelin receptor B or endothelin-3-deficient mice, in which inadequate proliferative signals for developing melanocytes result in regions of the mouse coat that are devoid of melanocytes, instead of a diffuse reduction in melanocytes spread over the surface of the skin.59,60 An alternative explanation is that the few surviving and proliferating ENS precursors are attracted to each other and stay bundled in ganglia rather than spreading out into the bowel. As the proliferating ENS precursors migrate down the bowel, they also stay connected by neuronal projections. The observation that there are few connections between the chains of ganglion cells present in the same region of the bowel suggests that extending neuronal fibers find it more difficult to reach distant chains of enteric ganglia, leading to limited interactions between the separate chains of enteric ganglia. Regardless of how these ganglia of the ENS develop, it is clear that the partial rescue of the ENS is mediated by human Ret encoded by the RetH2B transgene. However, some defect in development of the ENS still exists. Because murine Ret with the MEN 2B mutation has been shown to be sufficient to direct the normal development of the ENS,24 it seems that our phenotype could be explained by either a lack of proper RetH2B expression in the distal ENS or a lack of proper downstream signaling from mutant human Ret within the ENS. Thus, although the RetH2B transgene supports kidney development, it may lack those genetic elements required for the proper temporal expression of Ret in the ENS. Alternatively, there may exist intrinsic differences between human and murine Ret such that defects in Ret-related signaling results in only a partial rescue of the ENS.
One incompletely characterized aspect of Ret function is the contribution of the gene to the regulation of respiratory function. This connection is suggested by the coincidence of Hirschsprung’s disease with the congenital central hypoventilation syndrome in 22% of cases.61,62 Alterations in Ret have been described infrequently in patients having both Hirschsprung’s disease and central and congenital central hypoventilation syndrome.63,64 However, it is likely that mice lacking Ret have central hypoventilation, because they all die within 24 hours of birth, which is sooner than one might expect if such mice only had renal agenesis and total intestinal aganglionosis.18 To study this, investigators evaluated the effect of hypercapnea on mice lacking either one or both Ret alleles.65 Mice lacking Ret demonstrated depressed ventilatory response to inhaled CO2, leading to the conclusion that the Ret gene is an important factor in the development of the neurological regulation of ventilation. In contrast to mice lacking Ret, animals possessing one human Ret allele having the mutation associated with MEN 2B (genotypically RetH2B/RetM−/−) live until the age of 4 to 5 days. We believe this time of death is most consistent with the development of enterocolitis and/or dehydration related to the pseudo-obstruction of the intestine from their hypoganglionosis, rather than a hypoventilation syndrome. Whereas we did not formally measure the response of the transgenic animals to hypercapnea, these results suggest that the introduction of the human Ret proto-oncogene into mice lacking Ret is able to correct in part the neurological phenotype present in the knockout animals, despite the limited ability to support the development of the enteric nerves.
To summarize our findings, we introduced into mice a YAC transgene containing the human Ret oncogene with the mutation associated with MEN 2B. Surprisingly, the resulting transgenic mice did not develop any of the neoplasms associated with MEN 2B in humans. Moreover, introduction of the transgene into mice lacking Ret resulted in only partial correction of the murine knockout phenotype consisting of renal aplasia and partial intestinal aganglionosis. In particular, whereas the RetH2B/RetM−/− transgenic mice were able to develop kidneys, albeit slightly dysplastic ones, they were unable to develop a complete ENS. Moreover, whereas knockout mice possess dysregulation of their response to hypercapnea and ventilation, and presumably die of apnea within the first day of life, this phenotypic feature was corrected by the introduction of the human transgene. These results are in contrast to the case in which a normal murine Ret allele is present; such mice exhibit complete correction of the knockout phenotype.
Acknowledgments
We thank Frank Costantini, Columbia University, for the heterozygous Ret knockout mice.
Footnotes
Address reprint requests to Michael A. Skinner, M.D., Duke University Medical Center, Box 3815, Durham, NC 27710. E-mail: skinn009@mc.duke.edu.
Supported in part by the Children’s Miracle Network.
References
- Avantaggiato V, Dathan NA, Grieco M, Fabien N, Lazzaro D, Fusco A, Simeone A, Santoro M. Developmental expression of the RET protooncogene. Cell Growth Dev. 1994;5:305–311. [PubMed] [Google Scholar]
- Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F, Pachnis V. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122:349–358. doi: 10.1242/dev.122.1.349. [DOI] [PubMed] [Google Scholar]
- Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42:581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Carlson KM, Dou S, Chi D, Scavarda N, Toshima T, Jackson CE, Wells SA, Jr, Goodfellow PJ, Donis-Keller H. Single missense mutation in the tyrosine kinase domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci USA. 1994;91:1579–1583. doi: 10.1073/pnas.91.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, Howe JR, Moley JF, Goodfellow P, Wells SA., Jr Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet. 1993;2:851–856. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- Eng C, Smith DP, Mulligan LM, Nagal MA, Healey CS, Ponder MA, Gardner E, Scheumann GFW, Jackson CE, Tunacliffe A, Ponder BAJ. Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum Mol Genet. 1994;3:237–241. doi: 10.1093/hmg/3.2.237. [DOI] [PubMed] [Google Scholar]
- Hofstra RMW, Landsvater RM, Ceccherini I, Stulp RP, Stelwagon T, Luo Y, Pasini B, Hoppener JWM, van Amstel HKP, Romeo G, Lips CJM, Buys CHCM. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367:375–376. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- Mulligan LM, Kwok JBJ, Healey CS, Elsdon MJ, Eng C, Gardner E, Love DR, Mole SE, Moore JK, Papi L, Ponder MA, Telenius H, Tunnacliffe A, Ponder BAJ. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363:458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- Skinner MA, DeBenedetti MK, Moley JF, Norton JA, Wells SA., Jr Medullary thyroid carcinoma in children with multiple endocrine neoplasia types 2A and 2B. J Pediatr Surg. 1996;31:177–182. doi: 10.1016/s0022-3468(96)90343-7. [DOI] [PubMed] [Google Scholar]
- Wells SA, Jr, Chi DD, Toshima K, Dehner LP, Coffin CM, Dowton B, Ivanovich J, DeBenedetti MK, Dilley WG, Moley JF, Norton JA, Donis-Keller H. Predictive DNA testing and prophylactic thyroidectomy in patients at risk for multiple endocrine neoplasia type 2A. Ann Surg. 1994;220:237–250. doi: 10.1097/00000658-199409000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells SA, Jr, Skinner MA. Prophylactic thyroidectomy, based on direct genetic testing, in patients at risk for the multiple endocrine neoplasia type 2 syndromes. Exp Clin Endocrinol Diabetes. 1998;106:29–34. doi: 10.1055/s-0029-1211946. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang L-C, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A. GFRa1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Milbrandt J. GFRα1 deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- Skinner MA, Wells SA. Medullary thyroid carcinoma of the thyroid gland and the MEN 2 syndromes. Semin Pediatr Surg. 1997;6:134–140. [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Hui SZ, Granholm A-C, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of ganglion cells in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Constantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM, Milbrandt J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRα3-RET receptor complex. Neuron. 1998;21:1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM, Milbrandt J. Gene targeting reveals a critical role for Neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;2:253–263. doi: 10.1016/s0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- Kotzbauer PT, Lampe PA, Heukeroth RO, Golden JP, Creedon DJ, Johnson EM, Jr, Milbrandt J. Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature. 1996;384:467–470. doi: 10.1038/384467a0. [DOI] [PubMed] [Google Scholar]
- Milbrandt J, de Sauvage FJ, Fahrner TJ, Baloh RH, Leitner ML, Tansey MG, Lampe PA, Heuckeroth RO, Kotzbauer PT, Simburger KS, Golden JP, Davies JA, Vejsada R, Kato AC, Hynes M, Sherman D, Nishimura M, Wang L-C, Vandlen R, Moffat B, Klein RD, Poulsen K, Gray C, Garces A, Henderson CE, Phillips HS, Johnson EM. Persephin, a novel neurotrophic factor related to GDNF and neuturin. Neuron. 1998;20:245–253. doi: 10.1016/s0896-6273(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Smith-Hicks CL, Sizer KC, Powers JF, Tischler AS, Costantini F. C-cell hyperplasia, pheochromocytoma and sympathoadrenal malformation in a mouse model of multiple endocrine neoplasia type 2B. EMBO J. 2000;19:612–622. doi: 10.1093/emboj/19.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser DA, Froelick GJ, Matsumoto AM, Kafer KE, Marck B, Palmiter RD, Kapur RP. Ganglio neuromas and renal anomalies are induced by activated RETMEN2B in transgenic mice. Oncogene. 1999;18:877–886. doi: 10.1038/sj.onc.1202376. [DOI] [PubMed] [Google Scholar]
- Gestblom C, Sweetser DA, Doggett B, Kapur RP. Sympathoadrenal hyperplasia causes renal malformations in RETMEN2B-transgenic mice. Am J Pathol. 1999;155:2167–2179. doi: 10.1016/S0002-9440(10)65534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer F, Ketner G, Connelly C, Hieter P. Targeted recombination-based cloning and manipulation of large DNA segments in yeast. Methods. 1993;5:161–175. [Google Scholar]
- Pasini B, Hofstra RMW, Yin L, Bocciardi R, Santamaria G, Grootscholten PM, Ceccherini I, Patrone G, Priolo M, Buys CHCM, Romeo G. The physical map of the RET proto-oncogene. Oncogene. 1995;11:1737–1743. [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl A, Beerman F, Thies E, Monoliu L, Kelsey G, Schütz G. Transgenic mice generated by pronuclear injection of a yeast artificial chromosome. Nucleic Acids Res. 1992;20:3073–3077. doi: 10.1093/nar/20.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Cold Spring Harbor: Cold Spring Harbor Press; Manipulating the Mouse Embryo. (ed 2) 1994 [Google Scholar]
- Jaenisch R. Transgenic animals. Science. 1988;240:1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- Ceccherini I, Hofstra RMW, Luo Y, Stulp RP, Barone V, Stelwagon T, Bocciardi R, Nijveen H, Bolino A, Seri M, Roncheto P, Pasini B, Bozzano M, Buys CHCM, Romeo G. DNA polymorphisms and conditions for SSCP analysis of the 20 exons of the ret proto-oncogene. Oncogene. 1994;9:3025–3029. [PubMed] [Google Scholar]
- O’Riordan DS, O’Brien T, Crotty TB, Gharib H, Grant CS, van Heerden JA. Multiple endocrine neoplasia type 2B, More than an endocrine disorder. Surgery. 1995;118:936–942. doi: 10.1016/s0039-6060(05)80097-2. [DOI] [PubMed] [Google Scholar]
- Lorenzo MJ, Eng C, Mulligan LM, Stonehouse TJ, Healey CS, Ponder BA, Smith DP. Multiple mRNA isoforms of the human RET proto-oncogene generated by alternate splicing. Oncogene. 1995;10:1377–1383. [PubMed] [Google Scholar]
- de Graaff E, Srinivas S, Kilkenny C, D’Agati V, Mankoo BS, Costantini F, Pachnis V. Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 2001;15:2433–2444. doi: 10.1101/gad.205001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo MJ, Gish GD, Houghton C, Stonehouse TJ, Pawson T, Ponder BA, Smith DP. RET alternate splicing influences the interactions of the activated RET with SH2 and PTB domains of Shc, and the SH2 domain of Grb2. Oncogene. 1997;14:763–771. doi: 10.1038/sj.onc.1200894. [DOI] [PubMed] [Google Scholar]
- Pelet A, Geneste O, Edery P, Pasini A, Chappuis S, Atti T, Munnich A, Lenoir G, Lyonnet S, Billaud M. Various mechanisms cause RET-mediated signaling defects in Hirschsprung’s disease. J Clin Invest. 1998;101:1415–1423. doi: 10.1172/JCI375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Ahrens RC, Crowder RJ, Milbrandt J, Johnson EM., Jr The long and short isoforms of Ret function as independent signaling complexes. J Biol Chem. 2002;277:34618–34625. doi: 10.1074/jbc.M203580200. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Crawford PA, Gorodinsky A, Heuckeroth RO, Johnson EM, Jr, Milbrandt J. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development. 2001;128:3963–3974. doi: 10.1242/dev.128.20.3963. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Ichihara M, Iwashita T, Murakami H, Shimono Y, Kawai K, Kurokawa K, Murakumo Y, Imai T, Funahashi H, Nakao A, Takahashi M. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene. 2000;19:4469–4475. doi: 10.1038/sj.onc.1203799. [DOI] [PubMed] [Google Scholar]
- Mason I. The RET receptor tyrosine kinase, activation, signalling and significance in neural development and disease. Pharm Acta Helv. 2000;74:261–264. doi: 10.1016/s0031-6865(99)00048-5. [DOI] [PubMed] [Google Scholar]
- Parisi MA, Kapur RP. Genetics of Hirschsprung disease. Curr Opin Pediatr. 2000;12:610–617. doi: 10.1097/00008480-200012000-00017. [DOI] [PubMed] [Google Scholar]
- Edery P, Pelet A, Mulligan LM, Abel L, Attie T, Dow E, Bonneau D, David A, Flintoff W, Jan D, Journel H, Lacombe D, Le Merrer M, Meijers C, Parent P, Philip N, Plauchu H, Sarda P, Verloes A, Nihoul-Fekete C, Williamson R, Ponder BAJ, Munnich A, Lyonnet S. Long segment and short segment familial Hirschsprung’s disease, variable clinical expression at the RET locus. J Med Genet. 1994;31:602–606. doi: 10.1136/jmg.31.8.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo R, Ronchetto P, Luo Y, Barone V, Serl M, Ceccherini I, Pasini B, Bocclardl R, Lerone M, Kaalainen H, Marucciello G. Point mutations affecting the tyrosine domain of the RET proto-oncogene in Hirschsprung’s disease. Nature. 1994;367:377–378. doi: 10.1038/367377a0. [DOI] [PubMed] [Google Scholar]
- Jhiang SM. The RET proto-oncogene in human cancers. Oncogene. 2000;19:5590–5597. doi: 10.1038/sj.onc.1203857. [DOI] [PubMed] [Google Scholar]
- Pandit SD, Donis-Keller H, Iwamoto T, Tomich JM, Pike LJ. The multiple endocrine neoplasia type 2B point mutation alters long-term regulation and enhances the transforming capacity of the epidermal growth factor receptor. J Biol Chem. 1996;271:5850–5858. doi: 10.1074/jbc.271.10.5850. [DOI] [PubMed] [Google Scholar]
- Giraldo P, Montoliu L. Size matters, use of YACs, BACs, and PACs in transgenic animals. Transgenic Res. 2001;10:83–103. doi: 10.1023/a:1008918913249. [DOI] [PubMed] [Google Scholar]
- Hodgson JG, Smith DJ, McCutcheon K, Koide HB, Nishiyama K, Dinulos MB, Stevens ME, Bissada N, Nasir J, Kanazawa I, Disteche CM, Rubin EM, Hayden MR. Human Huntington derived from human YAC transgenes compensates for loss of murine Huntington by rescue of the lethal phenotype. Hum Mol Genet. 1993;5:1875–1885. doi: 10.1093/hmg/5.12.1875. [DOI] [PubMed] [Google Scholar]
- Majumder K, Shawlot W, Schuster G, Harrison W, Elder FFB, Overbeck PA. YAC rescue of downless locus mutations in mice. Mamm Genome. 1998;9:863–868. doi: 10.1007/s003359900884. [DOI] [PubMed] [Google Scholar]
- Lakshmanan G, Lieuw KH, Grosveld F, Engel JD. Partial rescue of GATA-3 by yeast artificial chromosome transgenes. Dev Biol. 1998;204:451–463. doi: 10.1006/dbio.1998.8991. [DOI] [PubMed] [Google Scholar]
- Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland, and throughout nephrogenesis. Development. 1999;126:1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- Acton DS, Velthuyzen D, Lips CJM, Höppener JWM. Multiple endocrine neoplasia type 2B mutation in human RET oncogene induces medullary thyroid carcinoma in transgenic mice. Oncogene. 2000;19:3121–3125. doi: 10.1038/sj.onc.1203648. [DOI] [PubMed] [Google Scholar]
- Cranston AN, Ponder BAJ. Modulation of medullary thyroid carcinoma penetrance suggests the presence of modifier genes in a RET transgenic mouse model. Cancer Res. 2003;63:4777–4780. [PubMed] [Google Scholar]
- Michiels FM, Chappuis S, Caillou B, Pasini A, Talbot M, Monier R, Lenoir GM, Feunteun J, Billaud M. Development of medullary thyroid carcinoma in transgenic mice expressing the RET protooncogene altered by a multiple endocrine neoplasia type 2A mutation. Proc Natl Acad Sci USA. 1997;94:3330–3335. doi: 10.1073/pnas.94.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan I, Gestblom C, Kapur RP. RETMen2B-transgene produces sympathoadrenal tumors but does not prevent intestinal aganglionosis in gdnf or gfrα-1−/− mice. Pediatr Dev Pathol. 2001;4:446–453. doi: 10.1007/s10024001-0039-9. [DOI] [PubMed] [Google Scholar]
- Reynolds L, Jones K, Winton DJ, Cranston A, Houghton C, Howard L, Ponder BAJ, Smith DP. C-Cell and thyroid epithelial tumours and altered follicular development in transgenic mice expressing the long isoform of MEN 2A RET. Oncogene. 2001;20:3986–3994. doi: 10.1038/sj.onc.1204434. [DOI] [PubMed] [Google Scholar]
- Gianino S, Grider JR, Cresswell J, Enomoto H, Heuckeroth RO. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development. 2003;130:2187–2198. doi: 10.1242/dev.00433. [DOI] [PubMed] [Google Scholar]
- Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Haddad GG, Mazza NM, Defendini R, Blanc WA, Driscoll JM, Epstein MA, Epstein RA, Mellins RB. Congenital failure of autonomic control of ventilation, gastrointestinal motility, and heart rate. Medicine. 1978;57:517–526. doi: 10.1097/00005792-197811000-00003. [DOI] [PubMed] [Google Scholar]
- Minutillo C, Pemberton PJ, Goldblatt J. Hirschsprung’s disease and Ondine’s curse, further evidence for a distinct syndrome. Clin Genet. 1989;36:200–203. doi: 10.1111/j.1399-0004.1989.tb03189.x. [DOI] [PubMed] [Google Scholar]
- Bolk S, Angrist M, Schwartz S, Silvestri JM, Weese-Mayer DE, Chakravarti A. Congenital central hypoventilation syndrome, mutation analysis of the receptor tyrosine kinase RET. Am J Med Genet. 1996;63:603–609. doi: 10.1002/(SICI)1096-8628(19960628)63:4<603::AID-AJMG14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Sakai T, Wakizaka A, Nirasawa Y. Congenital central hypoventilation syndrome associated with Hirschsprung’s disease, mutation analysis of the RET and endothelin-signaling pathways. Eur J Pediatr Surg. 2001;11:335–337. doi: 10.1055/s-2001-18552. [DOI] [PubMed] [Google Scholar]
- Burton MD, Kawashima A, Brayer JA, Kazemi H, Shannon DC, Schuchardt A, Costantini F, Pachnis V, Kinane TB. RET proto-oncogene is important for the development of respiratory CO2 sensitivity. J Auton Nerv Syst. 1997;63:137–143. doi: 10.1016/s0165-1838(97)00002-7. [DOI] [PubMed] [Google Scholar]