Abstract
Age-related macular degeneration (AMD) is a leading cause of blindness in the elderly. In its severest form, choroidal neovessels breach the macular Bruch’s membrane, an extracellular matrix compartment comprised of elastin and collagen laminae, and grow into the retina. We sought to determine whether structural properties of the elastic lamina (EL) correspond to the region of the macula that is predilected toward degeneration in AMD. Morphometric assessment of the macular and extramacular regions of 121 human donor eyes, with and without AMD, revealed a statistically significant difference in both the integrity (P < 0.0001) and thickness (P < 0.0001) of the EL between the macular and extramacular regions in donors of all ages. The EL was three to six times thinner and two to five times less abundant in the macula than in the periphery. The integrity of the macular EL was significantly lower in donors with early-stage AMD (P = 0.028), active choroidal neovascularization (P = 0.020), and disciform scars (P = 0.003), as compared to unaffected, age-matched controls. EL thickness was significantly lower only in individuals with disciform scars (P = 0.008). The largest gaps in macular EL integrity were significantly larger in all categories of AMD (each P < 0.0001), as compared to controls. EL integrity, thickness, and gap length in donors with geographic atrophy did not differ from those of controls. These structural properties of the macular EL correspond spatially to the distribution of macular lesions associated with AMD and may help to explain why the macula is more susceptible to degenerative events that occur in this disease.
Bruch’s membrane is a stratified extracellular matrix complex that lies between the retinal pigment epithelium (RPE) and the choroidal capillary bed, or choriocapillaris. It is comprised of two collagen-rich layers, referred to as the inner and outer collagenous layers, that flank a central domain of elastin and elastin-associated proteins.1,2 A number of age-related changes have been described in Bruch’s membrane,3–23 the most prominent of which are drusen and basal laminar deposits.24–30 In addition, increases in thickness, enhanced basophilia and Sudanophilia, accumulation of membranous debris, decreases in hydraulic conductivity, and fragmentation and calcification of Bruch’s membrane, have been described.
These age-related alterations in Bruch’s membrane could lead to a loss of the normal function of Bruch’s membrane and promote degenerative changes in the aging eye. Various lines of evidence suggest that Bruch’s membrane functions as a physical barrier to the egress of cells and vessels from the choroid into the sub-RPE and subretinal spaces. Disruption of, or damage to, this barrier is associated with loss of vision in a variety of ocular diseases, often resulting from the growth of new blood vessels—termed choroidal neovascular membranes (CNVM)—from the choroid into the sub-RPE and/or subretinal spaces. CNVM formation occurs in a number of macular degenerative diseases, including age-related macular degeneration (AMD), the leading cause of irreversible blindness in the developed world.31 Moreover, CNVM formation can be induced experimentally by thermal damage to Bruch’s membrane in normal monkey eyes after laser photocoagulation32 and potentially, after laser treatment of human CNVM.33 Interestingly, extramacular treatment is relatively ineffective, suggesting that the macula is inherently susceptible to choroidal neovascularization (CNV).34
AMD-associated CNVM formation occurs in ∼4% of the population older than the age of 7535 and is primarily restricted to the macula. It accounts for 90% of severe visual loss associated with AMD, even though only ∼10 to 15% of individuals with AMD develop CNV. The macula is a unique 6-mm-diameter region of the posterior pole that lies directly in the visual axis of human and nonhuman primates (Figure 1A). This region of the retina develops postnatally and subserves fine acuity vision. The reason for the increased susceptibility of the macula to degeneration and CNVM formation in primates has not been elucidated.
Figure 1.
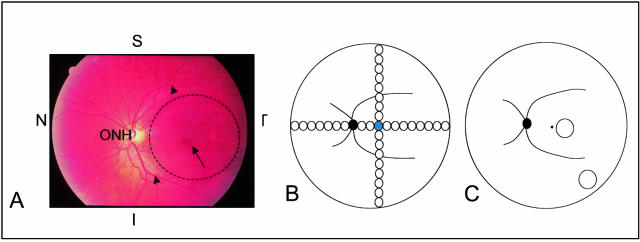
Ocular regions used in this study. A: Color funduscopic photograph of the left eye of a middle-aged individual without any clinical signs of AMD. The macula (dashed circle) is centered on the fovea (arrow) and lies within the major retinal vessels (arrowheads) that emanate from the optic nerve head (ONH). The superior (S), inferior (I), temporal (T), and nasal (N) axes are marked for orientation. B: Diagrammatic representation of the distribution of the 2-mm-diameter retinal-choroidal-scleral punches collected from a 14-year-old donor. The solid black circle depicts the location of the optic nerve head, the curved black lines emanating from the optic disk represent the major retinal vessels, and the blue circle denotes the punch that was centered on the fovea. The remaining circles represent serial 2-mm-diameter punches taken along the superior, inferior, temporal, and nasal axes. C: Illustration showing the macular and extramacular (mid-peripheral) sites (circles) from which 4-mm-diameter punches were collected for EL thickness and integrity measurements in the large set of AMD and unaffected donors. Landmarks are the same as those described in B.
We have speculated that one possible explanation for the tendency of new vessels to breach the macular Bruch’s membrane, as opposed to the extramacular regions, might be that there are regional differences in the structure and/or composition of Bruch’s membrane. It is likely that the integrity and nature of its collagen and elastin components primarily dictate the basic structural and functional properties of Bruch’s membrane. Hence, one might predict that the disruption of one or more layers of Bruch’s membrane might precede CNV in AMD. Accordingly, we recently observed what we interpreted to be robust differences in the integrity and thickness of the elastic lamina (EL) of Bruch’s membrane between the macular and extramacular regions in a series of human eyes. To evaluate further the topographical variation in the thickness and continuity of the EL, we assessed these parameters in a larger series of normal, unaffected donor eyes and compared them to those obtained from donors with early and late stages of AMD.
The data collected herein show that the macular EL is substantially thinner and more porous than that in the extramacular region. This information has lead to the development of a working hypothesis that the structural attributes of the macular EL of Bruch’s membrane may be related to, at least in part, the predilection of this region to degeneration in AMD, other macular dystrophies, and other conditions characterized by CNVM formation.
Materials and Methods
Human Donor Eyes and Morphological Procedures
The 121 human eyes used in this study were obtained from MidAmerica Transplant Services (St. Louis, MO), the Iowa Lions Eye Bank (Iowa City, IA), the Heartland Eye Bank (Columbia, MO), the Central Florida Lions Eye and Tissue Bank (Tampa, FL), and the Virginia Eye Bank (Norfolk, VA) after informed consent. Institutional Review Board committee approval for the use of human donor tissues was obtained from Human Subjects Committees at St. Louis University and the University of Iowa. All eyes were processed within 4 hours of death. The gross pathological features, as well as the corresponding fundus photographs and angiograms when available, of all eyes in this repository were read and classified by retinal specialists. Fundi were classified according to a modified version of the International AMD grading system.36 For this investigation, donors were placed into one of four categories: young unaffected (<62 years), age-matched unaffected (≥62 years), early-stage AMD (the youngest was 62 years old), and late-stage AMD. Late-stage AMD donors were subdivided into those with 1) geographic atrophy (GA), 2) active CNVMs, and 3) disciform scars preceded clinically by CNVM (CNV/DS). Note that some individuals refer to early-stage AMD as age-related maculopathy, or ARM; this term will not be used herein. Donors were classified as unaffected if they had no macroscopic or funduscopic signs of macular pathology or any ophthalmic history of AMD. Early AMD donors were defined based on an ophthalmic history of AMD and the presence of significant numbers of macular drusen, pigment disruption, and/or other clinical signs of early AMD.
Polyclonal antisera directed against tropoelastin (PCAB 94, gift from Dr. Robert Mecham, Washington University, St. Louis, MO; PR398, Elastin Products, Owensville, MO), as well as monoclonal antibodies directed against bovine elastin (clone BA-4, Sigma, St. Louis, MO; MM436, Elastin Products) and human aortic α-elastin (PR533, Elastin Products) were used to examine the EL of Bruch’s membrane. For experiments using Elastin Products’ antibodies, antigens were retrieved according to the manufacturer’s instructions. Reactivity of antibodies with the EL of Bruch’s membrane was analyzed in a series of young (<10 years), middle-aged (20 to 60 years), and aged (>60 years) donors. Posterior poles, or wedges of posterior poles spanning between the ora serrata and the macula, were fixed in 4% (para)formaldehyde in 100 mmol/L sodium cacodylate, pH 7.4, as described previously.29 After 2 to 4 hours of fixation, eyes were transferred to 100 mmol/L sodium cacodylate and rinsed (3 × 10 minutes), infiltrated, and embedded in acrylamide. These tissues were subsequently embedded in OCT, snap-frozen in liquid nitrogen, and stored at −80°C. In addition, unfixed posterior poles, or wedges thereof, were embedded directly in OCT, without acrylamide infiltration or embedment. Both fixed and unfixed tissues were sectioned to a thickness of 6 to 8 μm on a cryostat. Immunolabeling was performed as described previously,29,30 using Alexa 488-conjugated secondary antibodies (Molecular Probes, Eugene, OR). Adjacent sections were incubated with secondary antibody alone, to serve as negative controls. Some immunolabeled specimens were viewed by confocal laser-scanning microscopy.37 These cellular nuclei in these sections were counterstained with TO-PRO-3 (Molecular Probes).
Ocular tissues used for transmission electron microscopical studies were fixed by immersion fixation in one-half strength Karnovsky’s fixative, within 4 hours of death, for a minimum of 24 hours. Trephine-punched specimens (see below) were fixed, transferred to 100 mmol/L sodium cacodylate buffer, pH 7.4, and subsequently dehydrated, embedded in epoxy resin, sectioned, and photographed, as described previously.38,39
Morphometric Analyses
To establish the baseline structural and topographical characteristics of the EL, irrespective of aging or AMD, three separate analyses were performed. In the first analysis, baseline topographic data were collected from a series of oriented, 2-mm-diameter, full-thickness punchesof RPE-choroid-sclera that were collected using a trephine punch. These punches were taken at 2-mm intervals from the fovea to the ora serrata in the temporal, nasal, inferior, and superior quadrants in an eye from a 14-year-old donor and subsequently prepared for electron microscopy (Figure 1B). The average thickness and integrity of the EL in each of these punches were measured. A second series of 2-mm-diameter punches derived from two additional donors aged 7 and 25 were measured (these data were similar to those of the 14-year-old and are not depicted in this article).
In the second analysis, measurements of EL thickness and integrity were made at two defined locations, 1 to 2 mm and 12 to 13 mm, from the foveal center, in the infero-temporal quadrant, in a series of donors (Figure 1C). Fifty-six eyes from 56 unaffected (non-AMD) donors, ranging in age from 6 hours after birth to 96 years of age (mean age, 51.4 years), were used in this analysis. At least five donor eyes from each decade of life were included in this analysis. Oriented, 4-mm-diameter, full-thickness punches of RPE-choroid-sclera were taken using a trephine punch and prepared for electron microscopy, as described above.
In the third analysis, similar measurements of macular EL thickness and integrity were made from punches taken from 64 eyes derived from 64 human AMD donors, ranging in age from 62 to 99 years of age (Figure 1C). These included 24 donors with macular drusen, pigment disruption, and other signs of early-stage AMD; 15 donors with GA; 9 donors with active, patent CNVMs; and 16 donors with disciform scars (DS) that were preceded by documented CNVMs or in which a CNVM was present in the second eye. The mean ages of donors in these four categories were 79.0, 82.8, 83.7, and 88.2 years, respectively.
Outcome Measures
Four random photographic images were taken from each punched specimen using a JEOL JEM 1220 microscope (JEOL USA Inc., Peabody, MA), as described previously.38 Images were collected at ×5000 actual magnification for the first analysis (see above) and ×2500 actual magnification for the second and third analyses (see above). The thickness of the EL of Bruch’s membrane was measured at 20 points (five equal-distance points from each of the four images per specimen) using a micrometer. The average value of the 20 points was used in the statistical analyses. The integrity of the EL was defined as the total length of visually detectable elastin divided by the overall length of visible Bruch’s membrane within each of the four images. Thus, 100% integrity represented a fully intact (nonporous) EL and 0% integrity represented complete absence of elastin. The average integrity value derived from the four images of each punch was used for statistical analyses. The largest, or maximum, gap length (ie, discontinuity in the EL) present within the elastin lamina from the four micrographs used for each donor was identified and measured. The largest gap length from all donors in any given category was averaged (Figure 2F).
Figure 2.
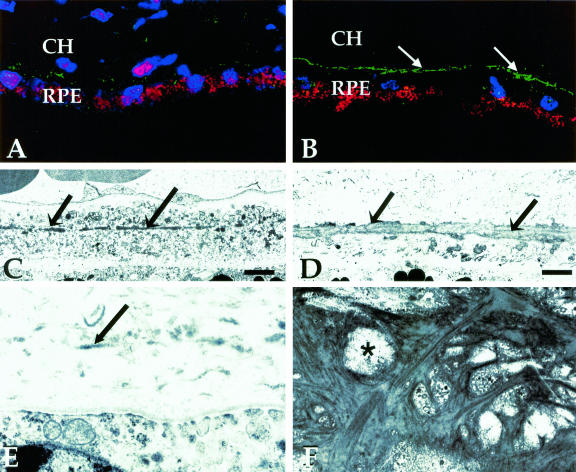
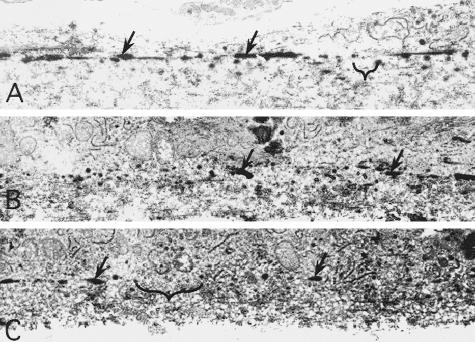
Confocal (A, B) and transmission electron microscopical (C–F) images of the elastic layer of Bruch’s membrane. Images were collected from macular (A, C, E) and extramacular (B, D, F) regions of Bruch’s membrane, as indicated in Figure 1. A and B: Labeling with an antibody directed against tropoelastin in a 78-year-old donor without macular disease. Tissues prepared for confocal microscopy were counterstained with TO-PRO-3 to visualize cellular nuclei (blue); RPE-associated lipofuscin is autofluorescent (red). Note that the elastin immunoreactivity of the elastic layer (green) is far more robust in the extramacular region (B) that it is in the macular region (A). The EL (arrows) in the macular region of an 82-year-old donor, depicted in C and D, is thinner and contains more numerous discontinuities than in the extramacular region. E: A higher magnification image of Bruch’s membrane directly adjacent to the foveal pit; the elastin fibers in this region are very sparse and thin (arrow). When the extramacular EL is viewed en face (F), its porosity (asterisk) is evident. These discontinuities contribute to the integrity and gap length values measured in this study. Original magnifications, ×400 (A, B). Scale bar, 2 μm (C, D).
Statistical analyses were performed using Microsoft Excel (Microsoft Inc., Redmond, WA) and S-Plus Statistical Packages (Insightful Corp., Seattle, WA). The Mann-Whitney U-test (Wilcoxon rank-sum test) was used to compare data series and linear regression analysis was used to explore age-related changes. To compare normal, unaffected donors to donors with AMD, statistical analyses were performed using only the normal, unaffected donors >61 years of age (23 unaffected, 23 early-stage AMD, and 40 late-stage AMD total). The mean age of normal donors >61 was 79.8 years; this mean age was not significantly different from that of each of the three groups of donors with AMD. Some data are represented graphically in the form of box and whiskers plots. These graphs show values for the median (bolded line within box), the first and third quartiles as a solid box, and the minimum and maximum observation range as whiskers. Any extreme values, defined as those further than 1.5× the interquartile range from the median, are represented as solitary, horizontal lines.
Results
Immunohistochemistry
The reactivity of anti-elastin antibodies with the macular EL of Bruch’s membrane differed markedly from that of extramacular regions in all donor eyes examined (Figure 2, A and B). Immunoreactive elastin was attenuated and highly discontinuous in the maculas of most donors, as compared to more peripheral regions where it was continuous and thick. This same pattern was observed with all elastin antibodies tested. The differences between these same structural attributes in the macular and extramacular regions are even more striking when viewed by transmission electron microscopy (Figure 2, C and D). In the center of the fovea, elastin was particularly sparse and thin (Figure 2E). These data provided the impetus for a more robust evaluation of these structural parameters in a larger set of samples derived from donors with and without a history of AMD.
EL Integrity and Thickness Maps Derived from a Young Donor
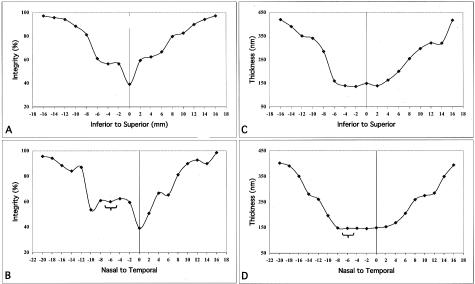
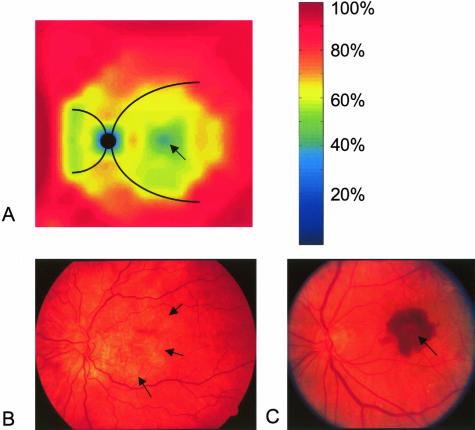
The mean integrity of the EL measured in the series of punches derived from a 14-year-old donor (Figure 1B) is depicted in Figure 3, A and B. The mean integrity was lowest (∼40%) in the central macula (fovea) and increased rapidly, approaching ∼80% at the main retinal vascular arcades (+8 mm and −8 mm) in the temporal, superior, and inferior regions. In the nasal quadrant, the integrity was lowest around the optic disk, especially on the nasal aspect, and rapidly increased in regions approaching 8 to 10 mm from the fovea, just outside the major vascular arcades. The mean thickness values exhibited a similar distribution, except there was not a significant fovea-associated dip (Figure 3, C and D). A calculated color-coded schematic map of the mean EL integrity of this eye is depicted in Figure 4A. In general, the area of the fundus with the thinnest and most porous EL corresponds spatially to the same region where the majority of lesions associated with AMD are manifest (Figure 4, B and C).
Figure 3.
Values for mean integrity (percent; A, B) and thickness (nm; C, D) of the elastic layer derived from the 14-year-old donor described in Figure 1B. Plots A and C depict measurements taken from the inferior ora serrata to the superior ora serrata, passing through the fovea (0), whereas plots B and D show measurements spanning between the nasal and temporal ora serrata, passing through the fovea (0) and optic nerve head (brackets; −5 mm to −7 mm). Note the extremely low integrity of the EL in the fovea (A and B; spanning between −2 mm and +2 mm) and adjacent to the optic nerve head on the nasal side (−8 mm to −10 mm in B). A step increase in integrity occurs in the vicinity of the major retinal vessels (∼−6 mm and +6 mm in A and B). The EL is thinnest in the macula area and increases abruptly in the region of the vascular arcades (∼−6 mm to +6 mm in C and D).
Figure 4.
A: A derived, color-coded schematic of the integrity of the elastic layer (relative percent scale shown to right) of Bruch’s membrane based on the data shown in Figure 3, A and B. The black circle represents the optic nerve head and the curved black lines emanating from it are the approximate positions of the major retinal vessels. Note that the integrity is lowest in the foveal region (arrow), central macula, and on the nasal aspect of the optic nerve head. These regions of decreased integrity correspond spatially to the distribution of the majority of macular lesions, such as GA (B; arrows depict the margin of macular atrophy) and CNVMs (C; arrow) that occur in individuals with AMD.
EL Integrity and Thickness in the Macular and Extramacular Regions of Donors without AMD
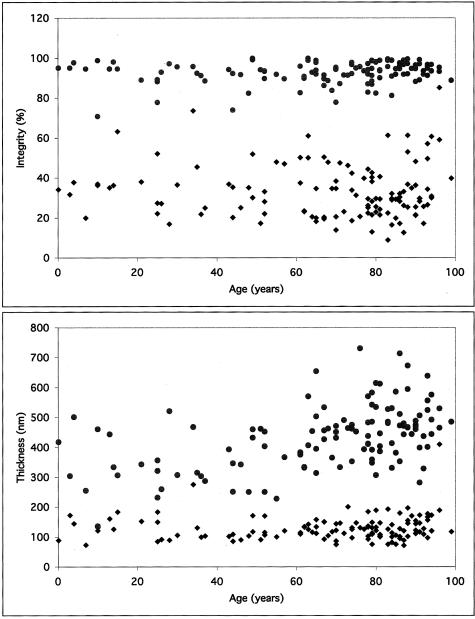
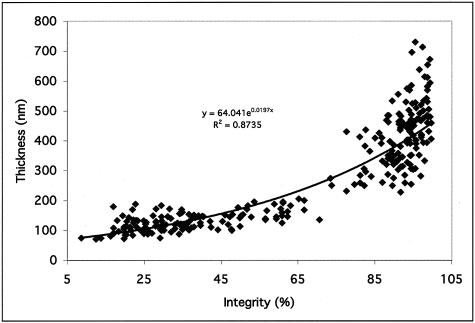
Scatter plots for the mean integrity and thickness of the EL of Bruch’s membrane in a series of normal, unaffected human donors are depicted in Figure 5. The mean integrity value of the EL in this series of normal human donors was 37.2% (SD = 7.9%) in the macula and 92.3% (SD = 4.7%) in the extramacula (Figure 5A). The mean thickness of the EL from the same series of donors was 134.2 nm (SD = 32.0 nm) in the macula and 391.7 nm (SD = 120.1 nm) in the extramacula (Figure 5B). This represents a statistically significant difference in both the integrity (P < 0.00001) and thickness (P < 0.00001) of the EL between the macular and extramacular regions. When the unaffected donors were split into young and age-matched groups, aging effects in both the macular and extramacular regions were observed. The thickness, but not the integrity, of the EL was significantly higher in the age-matched group, as compared to the young group, in both the macular and peripheral regions (P = 0.033 and P < 0.001, respectively). Scatter plots comparing the mean integrity and mean thickness values for each donor (Figure 6) suggest that a strong relationship exists between integrity and thickness of the EL in all regions in which these parameters were measured (exponential fit R2 = 0.8735).
Figure 5.
Scatter plots showing values of EL integrity (top; percent) and thickness (bottom; nm) derived from 121 individual donors, aged 1 day to 99 years. Note that the integrity of the EL in the macular region is approximately three times lower, on average, than that of the extramacular regions in all donors. The thickness of the EL in the macular regions is approximately four to six times lower, on average, than that of the extramacular region in all donors.
Figure 6.
Scatter plot showing the relationship between EL integrity and thickness for all donors examined in this investigation. Note that there is a strong relationship between these two parameters in all regions measured (exponential fit R2 = 0.8735).
EL Integrity and Thickness in the Macular Regions of Donors with Early and Advanced Forms of AMD
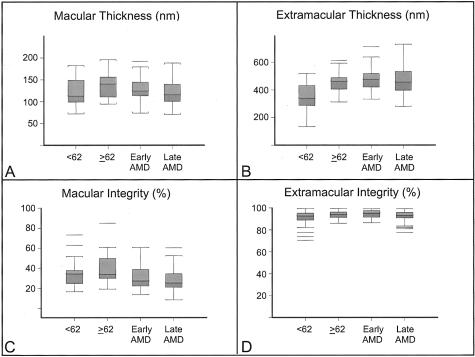
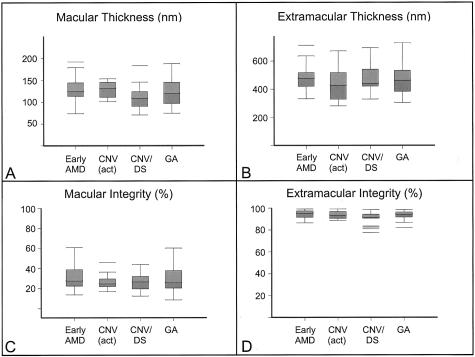
In the macular area, there was an apparent thinning of EL thickness and a loss of EL integrity from elderly normal individuals, through early-stage AMD to late-stage AMD (Table 1; Figures 7, 8, 9, and 10). For macular integrity, significant differences were observed between unaffected, age-matched controls and the following: all AMD (P = 0.003), early-stage AMD (P = 0.028), late-stage AMD (P = 0.003), active CNVM (P = 0.02), and CNV/DS (P = 0.003). For macular thickness, significant differences were observed between age-matched controls and donors with CNV/DS (P = 0.008), the latter which accounted for the apparent significant differences observed in the AMD (P = 0.035) and late-stage AMD (P = 0.024) groups (Table 1). These changes in EL thickness were in the opposite direction to those seen in normal aging. In contrast to the macula, no significant differences were found in the extramacular region between age-matched donors and those with early- or late-stage AMD. Importantly, no significant differences in EL parameters were observed between unaffected, age-matched controls and individuals with GA. Further analyses were made between early-stage AMD and late-stage AMD, between active CNVM and CNV/DS, between all CNV and GA, between active CNVM and GA, and between CNV/DS and GA (Table 1). No significant differences in macular or extramacular EL thickness or integrity were revealed in these comparisons.
Table 1.
Comparative Analyses of Elastic Lamina Parameters Using the Mann-Whitney U-Test
| Macular thickness | Macular integrity | Peripheral thickness | Peripheral integrity | |
|---|---|---|---|---|
| Aging comparisons | ||||
| <62 Young versus ≥62 age-matched | 0.033 | 0.34 | 0.001 | 0.28 |
| Age-matched to AMD comparisons | ||||
| ≥62 Age-matched versus all AMD | 0.035 | 0.003 | 0.77 | 0.94 |
| ≥62 Age-matched versus early-stage AMD | 0.19 | 0.028 | 0.42 | 0.49 |
| ≥62 Age-matched versus late-stage AMD | 0.024 | 0.003 | 0.92 | 0.55 |
| ≥62 Age-matched versus GA | 0.15 | 0.10 | 0.99 | 0.95 |
| ≥62 Age-matched versus active CNVM | 0.44 | 0.02 | 0.42 | 0.94 |
| ≥62 Age-matched versus CNV/DS | 0.008 | 0.003 | 0.79 | 0.24 |
| AMD subgroup comparisons | ||||
| Early-stage AMD versus late-stage AMD | 0.25 | 0.45 | 0.40 | 0.12 |
| Active CNVM versus CNV/DS | 0.089 | 0.64 | 0.35 | 0.51 |
| CNVM and CNV/DS versus GA | 0.61 | 0.41 | 0.72 | 0.35 |
| Active CNVM versus GA | 0.59 | 0.64 | 0.46 | 0.92 |
| CNV/DS versus GA | 0.28 | 0.40 | 0.98 | 0.23 |
Significant differences are bolded.
Figure 7.
Top: Box and whiskers plots showing data for EL thickness (A, B) and integrity (C, D) derived from the macular (A, C) and extramacular (B, D) regions of unaffected donors <62 years, age-matched donors ≥62 years, donors with early-stage AMD, and donors with late-stage AMD (includes donors with GA, active CNVMs, and DSs). Median values are shown as bold red lines within each box), first and third quartile values are represented as blue and magenta, respectively, within the solid box, the minimum and maximum observation ranges are shown as whiskers and extreme values are represented as solitary, horizontal lines. One young donor with an extreme macular elastic layer thickness of 275 nm and one age-matched control donor with an extreme macular elastic layer thickness of 409 nm were omitted from graph A for scaling purposes.
Figure 8.
Bottom: Box and whiskers plots showing data for EL thickness (A, B) and integrity (C, D) derived from the macular (A, C) and extramacular (B, D) regions of donors with early-stage AMD and subgroups of donors with late-stage AMD, including active CNVMs [CNV (act)], DSs (CNV/DS), and GA.
Figure 9.
Transmission electron micrographs depicting the EL of Bruch’s membrane in eyes from a 82-year-old, age-matched control donor (A), an 84-year-old donor with early AMD (B), and an 83-year-old AMD donor with active CNV (C). The elastic layers are depicted with arrows and longest gaps in macular EL integrity (maximum gap lengths) for each donor are bracketed.
Figure 10.
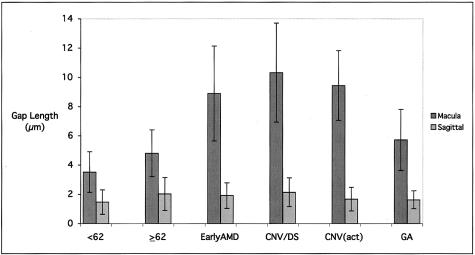
Bar graph depicting the average values for the largest gap length, or discontinuity, in the EL of donors with early-stage AMD and subgroups of donors with late-stage AMD (active CNVM, CNVM/DS, and GA). Significant differences in maximum gap length are noted between the both control groups (young and age-matched) and the early AMD, active CNVM, and CNVM/DS groups (all P < 0.0001), but not between the age-matched control group and the GA group (P = 0.11).
EL Gap Length in the Macular and Peripheral Regions of Donors with and without AMD
The largest gap length, or discontinuity, in the EL was assessed for each donor used in this study (Figures 9 and 10). There were no significant differences in largest elastin layer gap in the extramacular region between the various control and affected groups. In the macular area, the largest gaps in the elastin layer were significantly larger in the early AMD (P < 0.0001), active CNV (P < 0.0001), and CNV/DS (P < 0.0001) groups (in which the average gap length was ∼9 to 10 μm), as compared to both the age-matched and young unaffected control groups (in which the average gap width was 4 to 5 μm). No significant differences were observed between donors in the unaffected, age-matched control group and those in the classic GA group (P = 0.11).
Discussion
Bruch’s membrane likely provides a scaffold for RPE adhesion, regulates the diffusion of molecules between the choroid and retina, and serves as a physical barrier to cell movement, restricting the passage of cells between the choroid and retina.40 Numerous changes have been described at this interface that have the potential to disrupt the normal physiology of Bruch’s membrane and its surrounding tissues 3–13,16–19,21,22,25,28,41,42, however few of them help to explain the predilection of the macular Bruch’s membrane to degradation and dysfunction that occurs in individuals afflicted with AMD. To pursue preliminary findings suggesting the presence of topographical variation in the EL between the macular and extramacular regions we rigorously examined the morphology of this extracellular structure in a large series of human donor eyes.
The Macular EL Is Thinner and More Porous in All Individuals
In this study, we have found that the EL of Bruch’s membrane is three to six times thinner and two to five times more porous in the macular region than it is in the peripheral region at all ages. This profound difference was not anticipated based on previous investigations.4,14,17,27,43–47 It is intriguing to speculate about the mechanisms that might give rise to the observed topographical variation in the EL. One explanation is that the majority of the elastin in Bruch’s membrane is synthesized at a time before that of the final differentiation of the macula, which occurs during the first few years of postnatal life.48 This concept is supported by the fact that the majority of elastin is deposited in most tissues and organs during the latter stages of gestation.49 Elastin gene reactivation and neosynthesis does occur postnatally, typically in response to injury and inflammation, however its deposition rarely results in the reformation of ordered elastin fibers.50,51
It is also conceivable that differential thinning of Bruch’s membrane-associated elastin occurs during postnatal growth of the eye and this event is more pronounced in the macula because of differential rates of growth and/or stretching in this region. This process may be exacerbated in myopia, a condition characterized by an increased axial length of the eye and oftentimes by the secondary formation of macular atrophy and CNVMs.52
Our data also suggest that an interesting regional relationship between the porosity and thickness of the EL exists (Figure 6). Increasing integrity is associated with increasing thickness in a nearly exponential manner from the macula to the extramacular region. This relationship, which might prove to be predictive of AMD, will be examined in more detail in future studies.
The Macular EL in Individuals with AMD
This study also shows that the integrity of the macular EL is significantly lower in individuals with early-stage AMD, as compared to age-matched controls, whereas both the thickness and integrity of the EL are significantly lower in individuals with late-stage AMD, including those with active CNVMs and DSs (except for those with classic GA, as discussed below). It will be valuable to understand better the mechanisms that lead to even greater porosity and thinning of the macular EL in individuals with AMD. One explanation is that the overall integrity and thickness of the macular EL do not decrease with advancing age in individuals who develop AMD, but rather, that these parameters are lower in affected individuals from the time of birth. This could occur, for example, if ocular elastin synthesis was impaired during gestation, if specific isoforms of elastin were synthesized that predispose individuals to disease, or if one or more components of the elastin synthetic pathway were dysfunctional because of mutations in the genes encoding elastin or elastin-associated proteins. It has been established experimentally, for example, that exposure to increased amounts of vitamin D during gestation results in a reduction of aortic elastin content.53 Similarly, it has been proposed that the synthesis of aortic elastin is deficient in fetuses with impaired growth.54 Moreover, the expression of tissue-specific isoforms of tropoelastin,55 or the enhanced expression of the wrong isoform might predispose one to AMD.
Yet another explanation for the observed decrease in macular EL integrity in individuals with AMD is that the elastin layer is degraded in these patients more rapidly than it is in unaffected individuals. Along these lines, we20,37 and others47,56–60 have provided evidence that inflammatory and immune-mediated processes, particularly complement activation,30,61 are associated with the development of AMD. These processes are prominent at the RPE-Bruch’s membrane interface, and are typically more robust in the macular region than in the extramacular region. A potential consequence of these chronic inflammatory processes, if analogous to those that occur in a variety other age-related disease processes,62,63 would be an overall disruption, degradation, and/or remodeling of Bruch’s membrane-associated elastin and collagen. In cutus laxa, for example, a robust and dramatic loss of cutaneous elastic fibers is caused by local inflammation.64
If local, chronic inflammation participates in the destruction of the Bruch’s membrane, one would predict that macular CNVM formation would be associated with other chronic, systemic inflammatory diseases. Indeed, the development of neovascular membranes is not limited to AMD.65,66 On review, there are numerous immune-mediated and inflammation-based diseases including presumed ocular histoplasmosis syndrome, membranoproliferative glomerulonephritis, rubella, toxoplasma retinochoroiditis, sarcoidosis, Vogt-Koyanagi-Harada disease, birdshot choroidopathy, Bechet’s disease, chronic uveitis, and bacterial endocarditis,67–69 in which CNVMs can form. Importantly, the majority of these CNVMs form within the macula, an issue that is addressed in more detail below.
The Macular Elastic Layer in Classic GA
These data also show that the integrity and thickness of the macular EL in individuals with GA does not vary significantly from those of unaffected, age-matched controls. These data imply that differences or changes in the EL of individuals with AMD may be restricted to a specific phenotype(s) or genotype(s) of the disease. Importantly, they may also help to explain the distinct pattern of macular degeneration that is observed in individuals afflicted with GA.
Consequences of Decreased Macular EL Integrity in AMD
Whatever the derivation of the unique macula-associated features of Bruch’s membrane described herein, one would predict that any degeneration-inducing processes and/or modifications of the structural components of Bruch’s membrane (eg, collagen and elastin), whether genetic or acquired, might preferentially affect the overall function of the macular region. One consequence of an EL that is more porous in the macula might be that there is less substrate for RPE cell adhesion. If proven to be the case, this would help to explain the propensity for pigment epithelial cell detachments, basal laminar deposit accumulation, and soft drusen formation—all strong risk factors for the development of AMD—to form within the macula.7,16 Compromised adhesion of macular RPE cells might also help to explain the dysfunction of these cells that occurs in AMD.
Another consequence of a more porous macular EL might be the predilection of CNVMs to form within the macula. CNV—a pathological process in which neovessels emanate from the choroidal vasculature, breach the barrier imposed by Bruch’s membrane, and grow into the sub-RPE and subretinal spaces—is associated with a variety of disease processes,65,66 including AMD. CNVM formation can occur secondary to genetic alteration and degeneration of Bruch’s membrane, such as occur in pseudoxanthoma elasticum, Ehlers Danlos syndrome, Sorsby’s fundus dystrophy, and Menke’s disease,70–74 suggesting that genetic conditions resulting in elastin breakdown can predispose one to CNVM formation. CNVM formation may also be acquired in certain situations. For example, disruption of Bruch’s membrane that occurs as a consequence of mechanical stress in choroidal rupture, myopia, cryoinjury, ocular massage, and manipulation of the eye during cataract extraction is often associated with the subsequent formation of CNVM.23,52,75,76 Similarly, CNVM formation can be induced iatrogenically in human eyes77 and experimentally in monkey eyes by damage to Bruch’s membrane after laser photocoagulation.32 One recent study shows that there is a predilection of the macula to a higher incidence of CNVM formation after intense laser photocoagulation in monkeys.78 Moreover, in mouse models of CNV79,80 the majority of data provide evidence that neither choroidal nor retinal neovessels are capable of breaching Bruch’s membrane unless it is first disrupted,79–81 consistent with the notion that a robust Bruch’s membrane may safeguard against CNV, although one investigator has suggested that mechanical disruption is not prerequisite.82
Thus, it seems clear that the structural components of Bruch’s membrane must be compromised for CNV to occur and it is reasonable to postulate that a neovascular breach of Bruch’s membrane is primarily opportunistic, after a failure of its barrier function. However, a satisfactory explanation for this topographical predilection of CNVM formation to occur in the macula has not been made previously.
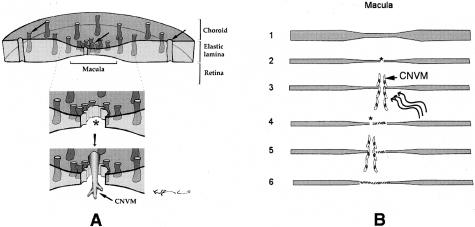
We propose that the data related to macular EL integrity may help to explain this predilection. In the proposed model of inflammation-induced destruction of Bruch’s membrane, as an example, the macular region might likely be compromised before that of the extramacular region, based on the morphological characteristics of Bruch’s membrane described herein. In other words, pan-choroidal inflammation would be expected to have a more rapid and dramatic effect in the macula, as compared to other regions, because of the more porous nature of its elastin layer (Figure 11A). Ironically, the elastin peptides that might be generated as a consequence of elastin fiber destruction in the macula could play a key role in subsequent neovascular events because of their highly angiogenic properties.83,84
Figure 11.
Models depicting possible scenarios that might lead to the enhanced susceptibility of the macula to CNVM formation (A) and CNVM recurrence (B). We have proposed that AMD-associated pathways, such as inflammation, that degrade or disrupt elastin might affect the barrier function of Bruch’s membrane in the macular region before that of the extramacular region because the elastic layer is the thinnest and most porous in this region (A and B, lane 1). The resulting breaches of Bruch’s membrane in the macula (asterisks; A and B, lane 2) might allow CNVMs to penetrate Bruch’s membrane and to grow into the subretinal and/or sub-RPE spaces. In treatments intended to destroy these neovessels, such as laser photocoagulation and photodynamic therapy (wavy lines; B, lane 3), the breached region of Bruch’s membrane might be replaced by fibrotic scar tissue (hatches; B, lane 4). Subsequently, the process of elastin degradation and neovascularization would continue at thinned, porous regions of the EL lying adjacent to that of the original vascular penetration (B, lanes 4 and 5). Ultimately, this process might continue until a scar formed over that portion of the macula (hatches; B, lane 6) where the EL was initially the thinnest and most porous.
In addition, the observation that the largest gap lengths (discontinuities) within the macular EL are significantly longer in individuals with AMD (with the exception of eyes with GA) than in age-matched controls may also help to explain the predilection toward CNVM formation in some eyes. For a neovessel to grow from the choroid into the sub-RPE space, its cells must be able to pass physically through Bruch’s membrane. Although the smallest theoretical pore through which choroidal neovessel-associated endothelial cells can migrate has not been established to our knowledge, it is intuitive that the larger gaps observed in some AMD donors would more readily allow penetration by developing neovascular fronds. Along these lines, it is interesting to note that the majority of images in the literature that depict early choroidal neovessels penetrating Bruch’s membrane show gaps greater than 5 μm in diameter. Our data show that many donors with AMD, except for those with GA, have EL gap widths greater than 5 μm. These data also imply that the decreased integrity observed in many of our AMD donors can be accounted for pores that are larger in diameter than in the unaffected controls.
Summary
In summary, the results of this study have documented the existence of unique structural features associated with the macular EL of Bruch’s membrane. These attributes correspond spatially to the distribution of the majority of macular lesions, including GA, CNV, and DS formation that are associated with AMD, a leading cause of blindness throughout the world. Our current working hypothesis is that insults to the structural integrity of Bruch’s membrane, whether mechanical, inflammatory, or compositional in nature, are prerequisite for invasion of choroidal neovessels into the sub-RPE and/or subretinal spaces, even in conditions in which blood vessel growth is abnormally enhanced. By extension, we suggest that once the macular EL is compromised, CNVM formation is likely to reoccur after mechanical or pharmaceutical destruction of an initial neovascular membrane (Figure 11B). Indeed, this phenomenon of macular neovessel recurrence is frequently observed after CNVM treatment modalities.85,86 We conclude that the quantitatively distinct features of the EL may help to explain the enhanced susceptibility of the macula to degeneration in AMD and other diseases characterized by neovascularization in the macular region.
Acknowledgments
We thank Dr. Stephen Russell for stimulating discussions and for evaluation of human donor eyes; Dr. Robert Mecham for his kind gift of anti-elastin polyclonal antibodies; James Borchardt, Ryan Lee, Amber Bain, Sheri McCormick, and Aleks Rozek for logistical assistance; Sepidah Amin and Karen Gehrs for helpful discussions; the staffs of the Iowa Lions Eye Bank, MidAmerica Transplant Services, the Heartland Eye Bank, the Central Florida Lions Eye and Tissue Bank, and the Virginia Eye Bank for their dedication in procuring the eyes used in these studies; and all of the families who unselfishly donated eyes of loved ones to this study.
Footnotes
Address reprint requests to Gregory S. Hageman, Ph.D., Department of Ophthalmology and Visual Sciences, Center for Macular Degeneration, The University of Iowa, 11190E PFP, 200 Hawkins Dr., Iowa City, IA 52240. E-mail: gregory-hageman@uiowa.edu.
Supported in part by the National Institutes of Health (grants EY11515 to G.S.H. and EY014563 to R.F.M.); The Ruth and Milton Steinbach Fund, Inc. (to G.S.H.); a Research to Prevent Blindness Lew R. Wasserman Merit Award (to G.S.H.); and The University of Iowa Center on Aging (grant AG00214 to R.F.M.).
References
- Marshall J, Hussain A, Starita C, Moore D, Patmore A. Aging and Bruch’s membrane. Marmor M, Wolfensberger T, editors. New York: Oxford University Press,; The Retinal Pigment Epithelium. 1988:pp 669–692. [Google Scholar]
- Guymer R, Bird A. Bruch’s membrane, drusen, and age-related macular degeneration. Marmor M, Wolfensberger T, editors. New York: Oxford University Press,; The Retinal Pigment Epithelium. 1988:pp 693–705. [Google Scholar]
- Hogan M. Bruch’s membrane and disease of the macula. Trans Ophthalmol Soc UK. 1967;87:113–161. [PubMed] [Google Scholar]
- Hogan M, Alvarado J. Studies on the human macula. Aging changes in Bruch’s membrane. Arch Ophthalmol. 1967;77:410–420. doi: 10.1001/archopht.1967.00980020412022. [DOI] [PubMed] [Google Scholar]
- Guymer R, Luthert P, Bird A. Changes in Bruch’s membrane and related structures with age. Prog Retinal Eye Res. 1999;18:59–90. doi: 10.1016/s1350-9462(98)00012-3. [DOI] [PubMed] [Google Scholar]
- Sarks S. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green W, McDonnell P, Yeo J. Pathologic features of senile macular degeneration. Ophthalmology. 1985;92:615–627. [PubMed] [Google Scholar]
- Grindle C, Marshall J. Ageing changes in Bruch’s membrane and their functional implications. Trans Ophthalmol Soc UK. 1978;98:172–175. [PubMed] [Google Scholar]
- Feeney-Burns L, Ellersieck M. Age-related changes in the ultrastructure of Bruch’s membrane. Am J Ophthalmol. 1985;100:686–697. doi: 10.1016/0002-9394(85)90625-7. [DOI] [PubMed] [Google Scholar]
- Pauleikhoff D, Sheraidah G, Marshall J, Bird A, Wessing A. Biochemical and histochemical analysis of age related lipid deposits in Bruch’s membrane. Ophthalmologe. 1994;91:730–734. [PubMed] [Google Scholar]
- Ramrattan R, van der Schaft T, Mooy C, de Bruijn W, Mulder P, de Jong P. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- Karwatowski W, Jeffries T, Duance V, Albon J, Bailey A, Easty D. Preparation of Bruch’s membrane and analysis of the age-related changes in the structural collagens. Br J Ophthalmol. 1995;79:944–952. doi: 10.1136/bjo.79.10.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D, Hussain A, Marshall J. Age-related variation in the hydraulic conductivity of Bruch’s membrane. Invest Ophthalmol Vis Sci. 1995;36:1290–1297. [PubMed] [Google Scholar]
- Kliffen M, van der Schaft TL, Mooy CM, de Jong PT. Morphologic changes in age-related maculopathy. Microsc Res Tech. 1997;36:106–122. doi: 10.1002/(SICI)1097-0029(19970115)36:2<106::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Kamei M, Apte SS, Rayborn ME, Lewis H, Hollyfield JG. TIMP-3 accumulation in Bruch’s membrane and drusen in eyes from normal and age-related macular degeneration donors. LaVail MM, Hollyfield JG, Anderson RE, editors. New York: Plenum Press,; Degenerative Retinal Diseases. 1997:pp 11–15. [Google Scholar]
- Zarbin M. Age related macular degeneration: a review of pathogenesis. Eur J Ophthalmol. 1998;8:199–206. doi: 10.1177/112067219800800401. [DOI] [PubMed] [Google Scholar]
- Spraul C, Lang G, Grossniklaus H, Lang G. Histologic and morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol. 1999;44:S10–S32. doi: 10.1016/s0039-6257(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Okubo A, Rosa R, Bunce C, Alexander R, Fan J, Bird A, Luthert P. Relationship of age changes in retinal pigment epithelium and Bruch’s membrane. IOVS. 1999;40:443–449. [PubMed] [Google Scholar]
- Curcio C, Millican C, Bailey T, Kruth H. Accumulation of cholesterol with age in human Bruch’s membrane. Invest Ophthalmol Vis Sci. 2001;42:265–274. [PubMed] [Google Scholar]
- Hageman G, Luthert P, Chong N, Johnson L, Anderson D, Mullins R. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Ret Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Hussain A, Rowe L, Marshall J. Age-related alterations in the diffusional transport of amino acids across the human Bruch’s-choroid complex. J Opt Soc Am. 2002;19:166–172. doi: 10.1364/josaa.19.000166. [DOI] [PubMed] [Google Scholar]
- Handa J, Verzijl N, Matsunaga H, Aotaki-Keen A, Lutty G, te Koppele J, Miyata T, Hjelmeland L. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Invest Ophthalmol Vis Sci. 1999;40:775–779. [PubMed] [Google Scholar]
- Yamamoto T, Yamashita H. Pseudopodia of choriocapillary endothelium. Jpn J Ophthalmol. 1989;33:327–336. [PubMed] [Google Scholar]
- Sarks J, Sarks S, Killingsworth M. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2:552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- Green W, Enger C. Age-related macular degeneration histopathologic studies. Ophthalmology. 1993;100:1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- Bressler N, Silva J, Bressler S, Fine S, Green W. Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina. 1994;14:130–142. [PubMed] [Google Scholar]
- Spraul C, Grossniklaus H. Characteristics of drusen and Bruch’s membrane in postmortem eyes with age-related macular degeneration. Arch Ophthalmol. 1997;115:267–273. doi: 10.1001/archopht.1997.01100150269022. [DOI] [PubMed] [Google Scholar]
- Curcio C, Millican C. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117:329–339. doi: 10.1001/archopht.117.3.329. [DOI] [PubMed] [Google Scholar]
- Hageman G, Mullins R, Russell S, Johnson L, Anderson D. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB J. 1999;13:477–484. doi: 10.1096/fasebj.13.3.477. [DOI] [PubMed] [Google Scholar]
- Mullins R, Anderson D, Russell S, Hageman G. Ocular drusen contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- Bhagat N, Flaxel C. Nonexudative macular degeneration. Lim J, editor. New York: Marcel Dekker,; Age-Related Macular Degeneration. 2002:pp 67–82. [Google Scholar]
- Ryan S. Subretinal neovascularization. Natural history of an experimental model. Arch Ophthalmol. 1982;100:1804–1809. doi: 10.1001/archopht.1982.01030040784015. [DOI] [PubMed] [Google Scholar]
- Malthieu D, Turut P, Francois P. Subretinal neovessels caused by photocoagulation. Bull Soc Ophthalmol Fr. 1988;88:645–646. [PubMed] [Google Scholar]
- Lee S, Shen W, Yeo I, Lai C, Mathur R, Tan D, Constable I, Rakoczy P. Predilection of the macular region to high incidence of choroidal neovascularization after intense laser photocoagulation in the monkey. Invest Ophthalmol Vis Sci. 2003;44 doi: 10.1001/archopht.122.3.353. E-abstract 3945. [DOI] [PubMed] [Google Scholar]
- Smith W, Assink J, Klein R, Mitchell P, Klaver C, Klein B, Hofman A, Jensen S, Wang J, de Jong P. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- Bird A, Bressler N, Bressler S, Chisholm I, Coscas G, Davis M, de Jong P, Klaver C, Klein B, Klein R, Mitchell P, Sarks J, Sarks S, Soubrane G, Taylor H, Vingerling J. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- Anderson D, Mullins R, Hageman G, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- Hageman G, Mullins R. Molecular composition of drusen as related to substructural phenotype. Mol Vis. 1999;5:28. [PubMed] [Google Scholar]
- Russell S, Mullins R, Schneider B, Hageman G. Basal laminar drusen are indistinguishable in location, substructure, and composition from drusen associated with aging and age-related macular degeneration. Am J Ophthalmol. 2002;129:205–214. doi: 10.1016/s0002-9394(99)00345-1. [DOI] [PubMed] [Google Scholar]
- Goldberg MF. Bruch’s membrane and vascular growth. Invest Ophthalmol. 1976;15:443–446. [PubMed] [Google Scholar]
- Yamomoto T, Yamashita H. Scanning electron microscopic observation of Bruch’s membrane with the osmium tetroxide treatment. Br J Ophthalmol. 1989;73:162–167. doi: 10.1136/bjo.73.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei M, Hollyfield J. TIMP-3 in Bruch’s membrane: changes during aging and in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1999;40:2367–2375. [PubMed] [Google Scholar]
- Tarkkanen A, Vannas S. Ultrastructure of Bruch’s membrane in senile macular degeneration. Acta Ophthalmol. 1967;45:694–698. doi: 10.1111/j.1755-3768.1967.tb06540.x. [DOI] [PubMed] [Google Scholar]
- Hollenberg M, Burt W. The fine structure of Bruch’s membrane in the human eye. Can J Ophthalmol. 1969;4:296–306. [PubMed] [Google Scholar]
- Bonnet M, Schiffer H. Modifications du tissu elastique de la membrane de Bruch dans la region maculaire en fonction de l’age. Bull Mem Soc Fr Ophthalmol. 1973;86:353–366. [PubMed] [Google Scholar]
- Newsome D, Huh W, Green W. Bruch’s membrane age-related changes vary by region. Curr Eye Res. 1987;6:1211–1221. doi: 10.3109/02713688709025231. [DOI] [PubMed] [Google Scholar]
- Killingsworth M, Sarks J, Sarks S. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye. 1990;4:613–621. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Yuodelis C. The morphological development of the human fovea. Ophthalmology. 1984;91:603–612. doi: 10.1016/s0161-6420(84)34247-6. [DOI] [PubMed] [Google Scholar]
- Davidson JM, Quaglino JD, Giro MG. Elastin repair. Cohen IK, Diegelman RE, Lindblad WJ, editors. Philadelphia: W.B. Saunders,; Wound HealingBiochemical and Clinical Aspects. 1992 [Google Scholar]
- Quaglino D, Jr, Nanney LB, Kennedy R, Davidson JM. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin. I. Excisional wound model. Lab Invest. 1990;63:307–319. [PubMed] [Google Scholar]
- Pasquali Ronchetti I, Quaglino D, Jr, Dyne KM, Zanaboni G, Cetta G. Ultrastructural studies on dermis from prolidase deficient subjects. J Submicrosc Cytol Pathol. 1991;23:439–445. [PubMed] [Google Scholar]
- Yoshida T, Ohno-Matsui K, Ohtake Y, Takashima T, Futagami S, Baba T, Yasuzumi K, Tokoro T, Mochizuki M. Long-term visual prognosis of choroidal neovascularization in high myopia: a comparison between age groups. Ophthalmology. 2002;109:712–719. doi: 10.1016/s0161-6420(01)01007-7. [DOI] [PubMed] [Google Scholar]
- Norman P, Moss I, Sian M, Gosling M, Powell J. Maternal and postnatal vitamin D ingestion influences rat aortic structure, function and elastin content. Cardiovasc Res. 2002;55:369–374. doi: 10.1016/s0008-6363(02)00444-3. [DOI] [PubMed] [Google Scholar]
- Martyn C, Greenwald S. Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet. 1997;350:953–955. doi: 10.1016/s0140-6736(96)10508-0. [DOI] [PubMed] [Google Scholar]
- Parks W, Roby J, Wu L, Grosso L. Cellular expression of tropoelastin mRNA splice variants. Matrix. 1992;55:156–162. doi: 10.1016/s0934-8832(11)80057-0. [DOI] [PubMed] [Google Scholar]
- Penfold P, Madigan M, Gillies M, Provis J. Immunological and aetiological aspects of macular degeneration. Prog Ret Eye Res. 2001;20:385–414. doi: 10.1016/s1350-9462(00)00025-2. [DOI] [PubMed] [Google Scholar]
- Heriot W, Henkind P, Bellhorn R, Burns M. Choroidal neovascularization can digest Bruch’s membrane. A prior break is not essential. Ophthalmology. 1984;91:1603–1608. doi: 10.1016/s0161-6420(84)34112-4. [DOI] [PubMed] [Google Scholar]
- Penfold P, Killingsworth M, Sarks S. An ultrastructural study of the role of leucocytes and fibroblasts in the breakdown of Bruch’s membrane. Aust J Ophthalmol. 1984;12:23–31. [PubMed] [Google Scholar]
- Penfold P, Killingsworth M, Sarks S. Senile macular degeneration. The involvement of giant cells in atrophy of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1986;27:364–371. [PubMed] [Google Scholar]
- Dastgheib K, Green W. Granulomatous reaction to Bruch’s membrane in age-related macular degeneration. Arch Ophthalmol. 1994;112:813–818. doi: 10.1001/archopht.1994.01090180111045. [DOI] [PubMed] [Google Scholar]
- Johnson L, Ozaki S, Staples M, Erickson P, Anderson D. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000;70:441–449. doi: 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole G, Cooper N, Eikelenboom P, Emmerling M, Fiebich B, Finch C, Frautschy S, Griffin W, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie I, McGeer P, O’Banion M, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel F, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps R. Atherosclerosis: the emerging role of inflammation and the CD40-CD40 ligand system. Proc Natl Acad Sci USA. 2000;97:6930–6932. doi: 10.1073/pnas.97.13.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J, Fazio M, Christiano A. Cutis laxa and premature aging syndromes. Royce P, Steinmann B, editors. New York: Wiley-Liss,; Connective Tissue and Its Heritable DisordersMolecular, Genetic, and Medical Aspects. 1993:pp 409–423. [Google Scholar]
- Green W, Wilson D. Choroidal neovascularization. Ophthalmology. 1986;93:1169–1176. doi: 10.1016/s0161-6420(86)33609-1. [DOI] [PubMed] [Google Scholar]
- Dolan B. Choroidal neovascularization not associated with age-related macular degeneration. Optom Clin. 1996;5:55–76. [PubMed] [Google Scholar]
- Meredith T, Green W, Key S, Dolin G, Maumenee A. Ocular histoplasmosis: clinicopathologic correlation of 3 cases. Surv Ophthalmol. 1977;22:189–205. doi: 10.1016/0039-6257(77)90055-8. [DOI] [PubMed] [Google Scholar]
- Olk R, Burgess D, McCormick P. Subfoveal and juxtafoveal subretinal neovascularization in the presumed ocular histoplasmosis syndrome. Visual prognosis. Ophthalmology. 1984;91:1592–1602. doi: 10.1016/s0161-6420(84)34113-6. [DOI] [PubMed] [Google Scholar]
- Ryan SJ. St. Louis: C.V. Mosby,; The Retina. 1989 [Google Scholar]
- Erkkila H, Raitta C, Niemi K. Ocular findings in four siblings with pseudoxanthoma elasticum. Acta Ophthalmol (Copenh) 1983;61:589–599. doi: 10.1111/j.1755-3768.1983.tb04349.x. [DOI] [PubMed] [Google Scholar]
- Weber B, Vogt G, Pruett R, Stohr H, Felbor U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby’s fundus dystrophy. Nat Genet. 1994;8:352–356. doi: 10.1038/ng1294-352. [DOI] [PubMed] [Google Scholar]
- Chong N, Alexander R, Gin T, Bird A, Luthert P. TIMP-3, collagen, and elastin immunohistochemistry and histopathology of Sorsby’s fundus dystrophy. Invest Ophthalmol Vis Sci. 2000;41:898–902. [PubMed] [Google Scholar]
- Wong S, Fong K, Lee N, Gregory-Evans K, Gregory-Evans C. Successful photodynamic therapy for subretinal neovascularization due to Sorsby’s fundus dystrophy: 1 year follow up. Br J Ophthalmol. 2003;87:796–797. doi: 10.1136/bjo.87.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S, Kuwabara T, Sanderson P. Menkes’ kinky hair disease: a light and electron microscopic study of the eye. Invest Ophthalmol. 1976;15:128–138. [PubMed] [Google Scholar]
- Cohen S. Etiology of choroidal neovascularization in young patients. Ophthalmology. 1996;103:1241–1244. doi: 10.1016/s0161-6420(96)30515-0. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein B, Wong T, Tomany S, Cruickshanks K. The association of cataract and cataract surgery with the long-term incidence of age-related maculopathy: the Beaver Dam eye study. Arch Ophthalmol. 2002;120:1551–1558. doi: 10.1001/archopht.120.11.1551. [DOI] [PubMed] [Google Scholar]
- Lim J. Iatrogenic choroidal neovascularization. Surv Ophthalmol. 1999;44:95–111. doi: 10.1016/s0039-6257(99)00077-6. [DOI] [PubMed] [Google Scholar]
- Shen W, Lee S, Yeo I, Lai C, Mathur R, Tan D, Constable I, Rakoczy P. Predilection of the macular region to high incidence of choroidal neovascularization after intense laser photocoagulation in the monkey. Arch Ophthalmol. 2004;122:353–360. doi: 10.1001/archopht.122.3.353. [DOI] [PubMed] [Google Scholar]
- Schwesinger C, Yee C, Rohan R. Intrachoroidal neovascularization in transgenic mice overexpressing vascular endothelial growth factor in the retinal pigment epithelium. Am J Pathol. 2001;158:1161–1172. doi: 10.1016/S0002-9440(10)64063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Matsui K, Hirose A, Yamamoto S, Saikia J, Okamoto N, Gehlbach P, Duh E, Hacket S, Chang M, Bok D, Zack D, Campochiaro P. Inducible expression of vascular endothelial growth factor in adult mice causes severe proliferative vitreoretinopathy and retinal detachment. Am J Pathol. 2002;160:711–719. doi: 10.1016/S0002-9440(10)64891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak N, Okamoto N, Wood J, Campochiaro P. VEGF is major stimulator in model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:3158–3164. [PubMed] [Google Scholar]
- Spilsbury K, Garrett K, Shen W, Constable I, Rakoczy P. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol. 2000;157:135–144. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nackman G, Karkowski F, Halpern V, Gaetz H, Tilson M. Elastin degradation products induce adventitial angiogenesis in the Anidjar/Dobrin rat aneurysm model. Surgery. 1997;122:39–44. doi: 10.1016/s0039-6060(97)90262-2. [DOI] [PubMed] [Google Scholar]
- Tummalapalli C, Tyagi S. Responses of vascular smooth muscle cell to extracellular matrix degradation. J Cell Biochem. 1999;75:515–527. [PubMed] [Google Scholar]
- Pollack A, Korte G, Weitzner A, Henkind P. Ultrastructure of Bruch’s membrane after krypton laser photocoagulation. I. Breakdown of Bruch’s membrane. Arch Ophthalmol. 1986;104:1372–1376. doi: 10.1001/archopht.1986.01050210126039. [DOI] [PubMed] [Google Scholar]
- Lafaut B, Aisenbrey S, Vanden Broecke C, Di Tizio F, Bartz-Schmidt K. Clinicopathologic correlation in exudative age-related macular degeneration: recurrent choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2001;239:5–11. doi: 10.1007/s004170000224. [DOI] [PubMed] [Google Scholar]