Abstract
Protein kinase C (PKC)-ε, a Ca2+-independent, phospholipid-dependent serine/threonine kinase, is among the PKC isoforms expressed in mouse epidermis. We reported that FVB/N transgenic mouse lines that overexpress (8- or 18-fold) PKC-ε protein in basal epidermal cells and cells of the hair follicle develop papilloma-independent squamous cell carcinoma (SCC) elicited by 7,12-dimethylbenz(a)anthracene initiation and 12-O-tetradecanoylphorbol-13-acetate-promotion or by repeated ultraviolet radiation exposures. The susceptibility to the development of SCC was proportional to the level of expression of the PKC-ε transgene. We now report that PKC-ε FVB/N transgenic mice (line 215) that overexpress in epidermis ∼18-fold PKC-ε protein more than their wild-type littermates spontaneously develop a myeloproliferative-like disease (MPD) in 100% of PKC-ε transgenic mice. The MPD was characterized by an excess of neutrophils and eosinophils, resulting in invasion of almost all vital organs of the mouse by 6 months of age. On gross examination these mice present with splenomegaly, hepatomegaly, and severe lymphadenopathy. Examination of the bone marrow revealed almost complete effacement by neutrophils, eosinophils, and their precursors. Furthermore, the spleen and lymph nodes were enlarged and exhibited marked extramedullary hematopoiesis. Complete pathological analysis of the second PKC-ε transgenic mouse (line 224) that expresses approximately eightfold PKC-ε protein more than their wild-type littermates revealed no remarkable findings in any of the affected organs as seen in line 215. However, peripheral blood analyses of PKC-ε transgenic mice indicated significant increases of neutrophils in the circulating blood in both PKC-ε transgenic lines. To determine whether there was an imbalance of cytokines in PKC-ε transgenic mice (line 215), resulting in aberrant myelopoiesis, we analyzed 17 cytokines in the peripheral blood. This analysis indicated that interleukin-5, interleukin-6, and granulocyte-colony stimulating factor were up-regulated as a function of age. The transgene PKC-ε was not detected in any of the affected organs (bone marrow, liver, spleen, lung) We suggest that overexpression of PKC-ε in the epidermis may lead to the induction of specific cytokines that may, in a paracrine mechanism, perturb normal hematopoiesis in bone marrow resulting in a granulocytic skew toward that of neutrophils and eosinophils. The susceptibility of PKC-ε transgenic mice to the induction of SCC and the spontaneous development of MPD are unrelated.
Hematopoiesis is a highly regulated process in the adult bone marrow that gives rise to all elements of the blood including erythrocytes, granulocytes, monocytes, lymphocytes, and platelets.1 All of these blood cells are believed to derive from one common precursor cell, the pluripotent hematopoietic stem cell. This common precursor gives rise to a lymphoid stem cell and myeloid stem cell, which are committed to producing lymphocytes and the cells of the myeloid lineage. The progenitor myeloid stem cell, a tri-lineage multipotent stem cell, gives rise to three types of committed stem cells, which differentiate along the erythroid/megakaryocytic, eosinophilic, or the polymorphonuclear-monocyte pathway. This process, called myelopoiesis, is tightly regulated to balance and coordinate proliferation, survival, and differentiation, and is regulated by growth factors and cytokines.
To date, four proteins have been identified that have the ability to induce the proliferation of myeloid stem cells. These proteins are called colony-stimulating factors (CSFs). One of these molecules induces macrophages (M-CSF), the second induces granulocytes (G-CSF), the third induces both macrophages and granulocytes (GM-CSF), and finally the fourth induces macrophages, granulocytes, mast cells, and erythroid cells [interleukin (IL)-3]. For a myeloid stem cell to give rise to a mature, functional, nondividing, differentiated cell requires a balance of cell survival, division, and differentiation associated with the ability to withdraw from the cell cycle at the time of maturity. Little evidence exists that the CSFs have the ability to induce both proliferation and differentiation. Rather it is thought that a complex cytokine network exists in which CSFs are responsible for triggering multiplication and non-CSF cytokines are responsible for triggering differentiation.2
Chronic myeloproliferative syndromes are a group of interrelated neoplastic disorders of the multipotent myeloid stem cell. As a result, this syndrome can present with imbalances of all lineages including erythroid, granulocytic, monocytic, as well as megakaryocytic cells. Clinically these diseases are broken into four subtypes each of which features a predominant cell lineage: essential thrombocythemia (megakaryocytes), polycythemia vera (erythrocytes), myeloid metaplasia (all myelopoietic lineages), and chronic myelogenous leukemia (granulocytes).
To study the in vivo function of individual CSFs, transplantation studies have been performed. Transgenic mice carrying the murine GM-CSF under the control of a retroviral promoter had accumulation of macrophages in eyes, striated muscle, and peritoneal and pleural cavities.3 Similar results were seen when murine bone marrow cells were transduced with a retrovirus containing the mouse GM-CSF cDNA and transplanted into lethally irradiated mice. The results were the development of nonneoplastic myeloproliferative disease with infiltration of neutrophils and macrophages into the spleen, lung, and liver.4 Similar retroviral approaches have shown that IL-3 can lead to chronic myeloid proliferative diseases.5 When murine bone marrow cells, transduced with a retrovirus containing G-CSF6 or IL-6,7 were introduced into recipient mice, a severe neutrophilic granulocytosis in all hematopoietic compartments was reported. In addition, neutrophil infiltration occurring in the lung, liver, and lymph nodes was accompanied by splenomegaly resulting from enhanced extramedullary hematopoiesis. These experimental approaches have defined the roles of individual CSFs, indicating that imbalances of such factors can lead to severe disturbances in myelopoiesis and subsequent formation of chronic myeloproliferative-like disease (MPD) and leukemias.
Protein kinase C (PKC) represents a large family of phosphatidylserine (PS)-dependent serine/threonine kinases.8–10 Based on structural similarities and co-factor dependence, 11 PKC isoforms have been classified into three subfamilies: the classical (cPKC), the novel (nPKC), and the atypical (aPKC). The cPKCs (α, βI, βII, γ) are dependent on PS, diacylglycerol, and calcium for their activation. The nPKCs (δ, ε, η, and θ) retain responsiveness to diacylglycerol and PS, but do not require calcium for full activation. The aPKCs (λ and σ) only require PS for their activation. The members of the PKC family exhibit functional diversity in their roles in the regulation of gene expression, cell growth, differentiation, and apoptosis.11–17 PKC-ε has been well documented as an oncogene.18,19 PKC-ε is a calcium-independent, 12-O-tetradecanoylphorbol-13-acetate (TPA)/diacylglycerol-activated serine/threonine kinase that participates in the regulation of diverse cellular functions, including gene expression,20–22 neoplastic transformation,18,19 cell adhesion,23 mitogenicity,24,25 and cellular motility.26 However, the role of PKC-ε in the production and release of cytokines remains unclear.
PKC-ε is among six isoforms (α, δ, ε, η, μ, ζ) expressed in the mouse skin. To determine the in vivo functional specificity of PKC-ε in mouse skin carcinogenesis, we generated PKC-ε transgenic mouse (FVB/N) lines 224 and 215 that overexpress ∼8- and 18-fold, respectively, PKC-ε protein over endogenous levels in basal epidermal cells. PKC-ε transgenic mice were observed to be highly sensitive to the development of squamous cell carcinoma (SCC) elicited by the 7,12-dimethylbenz(a)anthracene (DMBA) (100 nmol)-TPA (5 nmol) tumor promotion protocol or by repeated ultraviolet radiation exposures. In this communication, we report that PKC-ε transgenic line 215 exhibits a MPD accompanied by increased serum levels of IL-5, IL-6, and G-CSF with age. These mice show extreme neutrophil/eosinophil proliferation in the bone marrow, with increased numbers of these cells in the blood and marked infiltration of the lungs, liver, and kidneys. In addition, PKC-ε transgenic mice presented with massive splenomegaly resulting from increased extramedullary hematopoiesis. It appears PKC-ε mediated induction and release of specific cytokines may affect the proliferation of a putative myeloid stem cell with resultant development of a spontaneous MPD. These mice will be a useful tool to study the pathogenesis and treatment of chronic myeloid diseases.
Materials and Methods
PKC-ε Transgenic Mice
PKC-ε transgenic mice were generated as previously described.27 All animal care protocols were approved by an institutional review board. Transgenic mice were maintained by mating hemizygous transgenic mice with wild-type FVB/N mice. The mice were housed in groups of two to three in plastic bottom cages in light-, humidity-, and temperature-controlled rooms; food and water were available ad libitum. The animals were kept in a normal rhythm of 12-hour light and 12-hour dark periods. The transgene was detected by polymerase chain reaction (PCR) analysis using genomic DNA isolated from 1-cm tail clips.
Peripheral Blood Analysis
The mice were anesthetized with halothane. An incision along the axillary blood vessels was made and 500 μl of blood was withdrawn with a 22-gauge needle. The syringes were coated with 0.5 mol/L of ethylenediaminetetraacetic acid to prevent coagulation. The blood was placed immediately into ethylenediaminetetraacetic acid-coated tubes and mixed thoroughly. Cell analysis was performed by the Clinical Pathology Department at the University of Wisconsin College of Veterinary Medicine using the Advia 120 Chemistry Analyzer (Bayer Corp., Tarrytown, NY).
Histological Methods
Soft tissues were fixed in 10% neutral-buffered formalin for 24 hours, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E). Bones were surgically removed, cut longitudinally, and placed in 10% neutral-buffered formalin overnight. Decalcification was performed overnight using Fisher’s Cal-EX solution followed by paraffin-embedding and staining with H&E.
Routine Electron Microscopy
Liver tissue was cut into 1 mm cubes, fixed in glutaraldehyde, and postfixed in osmium tetroxide. Tissues were then dehydrated in an ethanol series and embedded in Epon 812. Thin sections for electron microscopy were cut with an LKB (Bromma, Sweden) ultramicrotome. Copper grids were stained with lead citrate and uranyl acetate and photographed in a Hitachi (Tokyo, Japan) electron microscope.
Cytokine Analysis
G-CSF levels in the serum and epidermis were quantified by enzyme-linked immunosorbent assay (ELISA) using a mouse G-CSF Quantikine ELISA kit (R&D Systems, Minneapolis, MN). Serum was collected by drawing blood from mice, incubating at room temperature for 30 minutes, followed by centrifugation for 10 minutes at 5000 × g. Fresh serum was used for ELISA analysis. Epidermis was collected by scrapping the epidermis, followed by homogenization of the sample in immunoprecipitation lysis buffer (50 mmol/L HEPES, pH 7.5, 150 mmol/L NaCl, 10% glycerol, 1% Triton X-100, 1.5 mmol/L MgCl2, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mmol/L phenylmethyl sulfonyl fluoride, 200 μmol/L Na3VO4, 200 μmol/L NaF, and 1 mmol/L ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid). The samples were spun for 30 minutes at maximum speed and supernatants were collected for analysis. For analysis of G-CSF in the media, media from treated and untreated cells were collected, centrifuged, and analyzed using the mouse G-CSF Quantikine ELISA kit (R&D Systems). G-CSF release to the media was normalized to the number of cells present at the onset of the experiment. Analysis of macrophage inflammatory protein1-α, GM-CSF, monocyte chemoattractant protein, keratinocyte-derived cytokine, regulated on activation normal T cell expressed and secreted (RANTES), interferon-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, IL-13, and tumor necrosis factor (TNF)-α at the indicated times were performed by Linco Diagnostics (St. Charles, MO) using the Luminex Multi-Analyte detection assay.
Western Analysis
Mice were euthanized by cervical dislocation and organs collected. The tissues were homogenized in immunoprecipitation lysis buffer (50 mmol/L HEPES, pH 7.5, 150 mmol/L NaCl, 10% glycerol, 1% Triton X-100, 1.5 mmol/L MgCl2, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mmol/L phenylmethyl sulfonyl fluoride, 200 μmol/L Na3VO4, 200 μmol/L NaF, and 1 mmol/L ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid) at 4°C. The homogenate was centrifuged at 14,000 × g for 30 minutes at 4°C. One hundred μg of whole cell lysate was fractionated on a 7.5% sodium dodecyl sulfate (SDS)-polyacrylamide gel. The proteins were transferred to 0.45-μm Hybond-P polyvinylidene fluoride transfer membrane (Amersham, Piscataway, NJ). The membrane was then incubated with a mouse monoclonal antibody to T7 (Novagen, Madison, WI) at a 1:1000 dilution to detect the T7 epitope-tagged mouse PKC-ε. The detection signal was developed with Amersham’s electrochemiluminescence reagent.
Real-Time Quantitative PCR
Total RNA was isolated using the RNeasy RNA isolation kit (Qiagen, Valencia, CA), DNase-treated, and 1 μg was used to prepare cDNA using Ready-to-Go reverse transcription-PCR beads (Amersham). Quantitative reverse transcription-PCR was performed by monitoring in real time the increase in fluorescence of the SYBR Green dye as described using the iCycler detection system (Bio-Rad, Richmond, CA). We also quantified transcripts of the 18s RNA an endogenous RNA control, and each sample was normalized on the basis of its 18s content.
Results
PKC-ε Transgenic Mice Develop Suppurative Dermatitis and Severe Hepatomegaly and Splenomegaly with Age
The generation of PKC-ε transgenic mice has previously been described.27 To determine the in vivo functional specificity of PKC-ε in mouse skin carcinogenesis, we generated PKC-ε transgenic mouse (FVB/N) lines 224 (PKC-ε224) and 215 (PKC-ε215) that overexpress ∼8- and 18-fold, respectively, T7-epitope tagged PKC-ε immunoreactive protein in the mouse skin over endogenous levels. The level of basal PKC-ε activity in the absence of added PKC activators (PS/TPA) in epidermal extract from PKC-ε transgenic line 215 was significantly higher than the epidermal extract from line 224.27 The expression of PKC-ε was directed to the basal cells of the epidermis and cells of the hair follicle using a human cytokeratin 14 (K14) promoter.27,28 A comparison of the susceptibility of PKC-ε transgenic mouse lines (215 and 224) to the development of SCC has been reported (Wheeler DL, Li Y, Verma AK, Photochemistry and Photobiology, February, 2005). We found a dramatic difference in carcinoma latency period between the two PKC-ε transgenic mouse lines; the first carcinoma in line 215 appeared at 10 weeks whereas it took 23 weeks for the first carcinoma to appear in line 224. However, although delayed in line 224, both lines elicited similar carcinoma incidence.
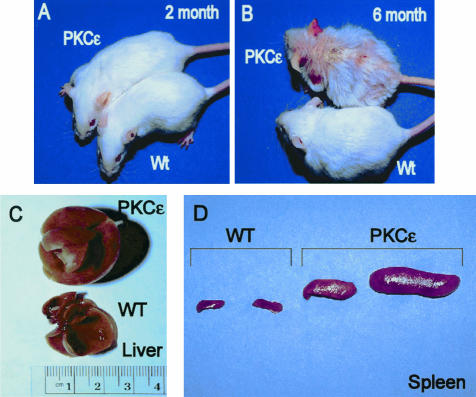
The high-expressing line 215 developed a severe MPD. To evaluate gross phenotypic and hematopathological changes, mice were allowed to age untreated, and euthanized at 2 and 6 months of age along with their corresponding wild-type littermates. At 2 months, PKC-ε transgenic mice were indistinguishable from their wild-type littermates (Figure 1A). Consistent with our previous report,27 by 6 months of age, PKC-ε transgenic mice exhibited extreme hyperkeratosis, alopecia, suppurative dermatitis, and development of scales most remarkable over the tail base, ears, face, and the dorsal skin (Figure 1B). At 6 months of age the PKC-ε transgenic mice showed marked hepatosplenomegaly (Figure 1, C and D). Both the liver and spleen weights varied from two to six times larger from their control wild-type littermates at 6 months of age (data not shown).
Figure 1.
PKC-ε transgenic mice develop suppurative dermatitis and severe hepatomegaly and splenomegaly with age. PKC-ε transgenic mice and their wild-type littermates were analyzed for external (2 and 6 months) and internal phenotype at 6 months of age. A and B: Photographs depict representative wild-type and PKC-ε transgenic mice at 2 and 6 months of age. C and D: Photographs depict representative samples of PKC-ε liver (C) and spleen (D) at 6 months of age.
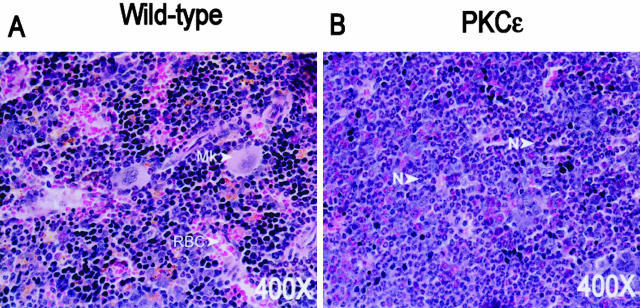
PKC-ε transgenic bone marrow exhibits increased myelopoiesis. To determine whether there were alterations in normal marrow hematopoiesis, the bone marrow of PKC-ε transgenic mice and their wild-type littermates was analyzed. Three mice from each group were euthanized at 7 months of age and the femurs were collected. This analysis of PKC-ε transgenic bone marrow at 7 months showed that normal hematopoiesis observed in wild-type bone marrow had been replaced by irregular myelopoiesis with a granulocytic predominance ie, neutrophils, eosinophils, and their early precursors (Figure 2). Furthermore, PKC-ε transgenic mice had significant increases in peripheral blood neutrophils (Table 1). PKC-ε transgenic mouse line 224 also had elevated circulating neutrophils in the peripheral blood (Table 1).
Figure 2.
PKC-ε transgenic bone marrow exhibits increased myelopoiesis with a predominance of neutrophils. The femurs from wild-type (A) and PKC-ε transgenic mice (B) were surgically removed, cut longitudinally, and fixed for 24 hours in 10% neutral-buffered formalin. The bone sections were decalcified overnight. Four-μm sections were cut and stained with H&E. Mk, megakaryocyte; N, neutrophil; RBC, red blood cell.
Table 1.
Absolute Blood Counts in the Peripheral Blood (per μl of Blood)
| Mouse | Neutrophilis | Eosinophilis | Monocytes |
|---|---|---|---|
| Four mice sampled at 18 months of age | |||
| WT | 170 ± 10 | 35 ± 5 | 90 ± 70 |
| PKCε-224 | 420 ± 87 | 17 ± 12 | 120 ± 50 |
| Four mice sampled at 6 months of age | |||
| WT | 305 ± 58 | 42 ± 14 | 107 ± 39 |
| PKCε-215 | 2345 ± 685 | 220 ± 98 | 853 ± 305 |
Aged PKC-ε Transgenic Mice Show Pronounced Neutrophilic Infiltration into Nonhematopoietic Organs
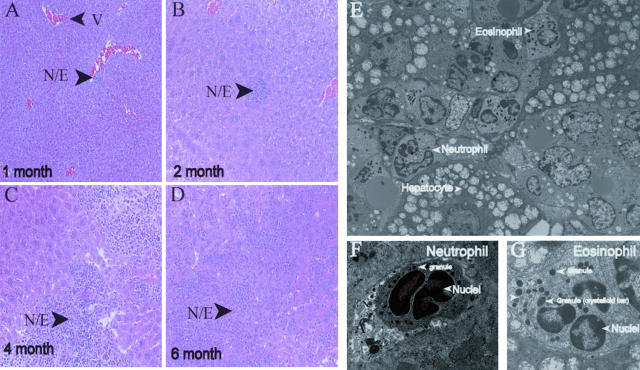
To assess the infiltration of neutrophils into various organs, histological examination of PKC-ε transgenic mouse tissues at 2, 4, 5, and 6 months of age was performed. A representative time course of morphological findings of the liver is presented in Figure 3, A to D. Neutrophil involvement in the hepatic tissue was seen as early as 1 month of age and presented with multiple perivascular foci of neutrophils and eosinophils throughout the liver. By 2 months of age, the neutrophils and eosinophils were also seen in the parenchyma as well as demonstrating increased perivascular cuffing. At 4 to 6 months of age, liver sections revealed variable foci of necrosis with large and small foci of neutrophils and eosinophils both confluent in the parenchyma and prominent within the perivascular regions. The neutrophils appeared to be intact as well as degranulating. Within regions of necrotic hepatocytes, there was associated fibrin deposition. To confirm the presence of eosinophils and neutrophils within the parenchyma of the liver, routine electron microscopy was performed (Figure 3; E to G). Neutrophils were apparent and identified by the presence of multilobed nuclei with variable sized lysosomes, whereas eosinophils were identified by the presence of the crystalloid bar within the cytoplasmic lysosomes.
Figure 3.
PKC-ε transgenic mice exhibit neutrophil invasion into the hepatic tissue. A–D: PKC-ε transgenic mice were sacrificed at 1, 2, 4, and 6 months of age. Liver was collected and fixed in 10% neutral-buffered formalin for 24 hours. Four-μm sections were cut and stained with H&E. E–G: Liver sections were collected and processed as described in Materials and Methods. N/E, neutrophil and eosinophil foci; V, vein.
Other organs with neutrophil/eosinophil involvement included the lung and the kidney. Pulmonary tissue collected from 7-month-old PKC-ε transgenic mice revealed moderate cellular infiltration in perivascular locations. Small vessels showed numerous neutrophils, often adhering to the vascular endothelium associated with marked perivascular cuffing. Collections of neutrophils were observed in peribronchial areas, although not within the bronchial lumens. In addition, lung interstitial cellular populations consisted of mononuclear cells and increased presence of neutrophils. By 6 to 8 months of age PKC-ε transgenic pulmonary tissue exhibited strong inflammatory cell infiltration and respiratory distress was clearly noted in PKC-ε transgenic mice. Examination of the kidneys revealed heavy neutrophil infiltration and their early precursors in the periarteriolar regions.
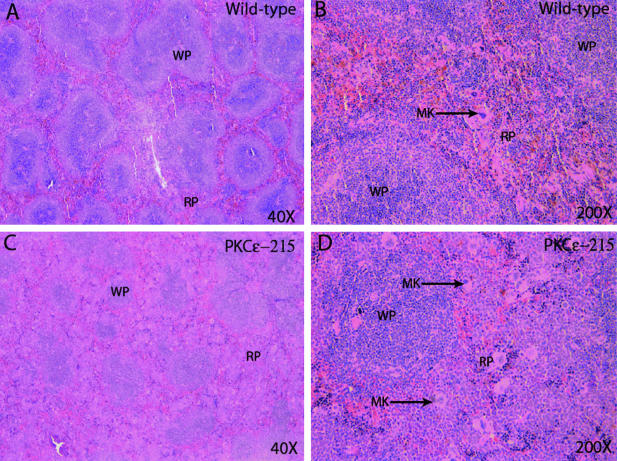
Several organs in 6-month-old PKC-ε transgenic mice did not demonstrate infiltration by neutrophils. These organs included the following: heart, brain, stomach, intestines, lymph nodes, and spleen. Wild-type spleen showed normal architecture with preserved white pulp (Figure 4). The red pulp contained slight extramedullary hematopoiesis including megakaryocytes. In contrast the spleens from PKC-ε transgenic mice (line 215) showed residual white pulp, greatly expanded red pulp by hematopoietic cells, predominately neutrophil and monocyte precursors, with increased megakaryocytes and nucleated red blood cells. Sections of 6-month-old wild-type lymph node showed normal sized lymph nodes with normal architecture. However, lymph nodes from 6-month-old PKC-ε transgenic mice (line 215) were greatly enlarged and expanded by a paracortical infiltrate composed mainly of monocytes and granulocytic precursors (data not shown).
Figure 4.
PKC-ε transgenic spleen histopathology exhibits severe extramedullary hematopoiesis. The spleens from 6-month-old wild-type (A and B) and PKC-ε transgenic mice (C and D) were removed and fixed for 24 hours in 10% neutral-buffered formalin. Four-μm sections were cut and stained with H&E. WP, white pulp; RP, red pulp; MK, megakaryocyte.
To determine whether the low-PKC-ε-expressing transgenic mouse line 224 had neutrophil infiltration into vital organs, PKC-ε transgenic mice (line 224) and their wild-type littermates were analyzed at ∼18 months of age. Complete pathological analysis of the lower expressing PKC-ε transgenic line (line 224) revealed no remarkable findings in any of the affected organs as seen in line 215.
The PKC-ε Transgene Is Not Expressed in Any Organs Other than Skin
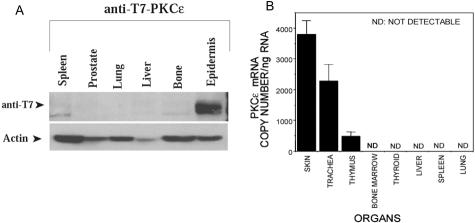
To determine whether PKC-ε protein was expressed in the affected organs (lung, liver, bone marrow, and spleen), Western analysis was performed. PKC-ε transgenic mice were collected at 7 months of age and all organs harvested and homogenized in immunoprecipitation lysis buffer. Total protein was collected, quantitated, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The immunoblot was probed with anti-T7 antibody to identify the expression of the T7-tagged PKC-ε transgene. No immunoreactive protein was detected by Western blot analysis except in the dorsal epidermal layer (Figure 5A). Although no protein was detected in any of the affected organs, it is possible that the K14 promoter drives expression of PKC-ε protein in a small subset of cells in the bone marrow itself which is below the level of detection using Western analysis. To answer this question we isolated RNA from the skin, trachea, thymus, bone marrow, thyroid, liver, spleen, and lung from PKC-ε transgenic mice. The RNA was reverse-transcribed and real-time quantitative PCR was performed. To detect only the transgene expressed T7-PKC-ε we designed a forward primer located in the T7-tag and a downstream primer oriented in the PKC-ε cDNA sequence. Transgene expression was only detected in the skin, trachea, and thymus, organs that we have previously reported to express the T7-PKC-ε protein27 (Figure 5B).
Figure 5.
T7-PKC-ε expression profile. A: Organs from untreated, 2-month-old, PKC-ε transgenic mice were collected and homogenized in immunoprecipitation lysis buffer for protein analysis. Extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with a mouse anti-T7 antibody to detect the T7-tagged PKC-ε protein. The level of actin was determined as a control for gel-loading variations. B: The indicated organs were collected from untreated PKC-ε transgenic mice and total RNA was collected. The RNA was reversed-transcribed and real-time quantitative PCR was performed using primers specific to the transgene. A standard curve was performed using serial dilutions of the K14-T7-PKC-ε vector and was used to determine the number of copies of mRNA per ng of total RNA. ND, not detected.
The Development of a Spontaneous MPD-Like Disease Is Specific to PKC-ε Transgenic Mice
To determine whether the development of this disease was specific to PKC-ε, we analyzed PKC-δ transgenic mice29 that overexpress PKC-δ under the control of the human keratin 14 promoter. Organs were analyzed from 1 to 8 months for aberrant production of cells of the myeloid lineage. There were no signs of this disease in PKC-δ mice throughout the time course noted in this study (data not shown) or in our previous work.29 In addition, we and others did not observe the development of this disease in PKC-α transgenic mice using both the human keratin 1430 and under the direction of the bovine K5 promoter.31
G-CSF, IL-5, and IL-6 Levels Are Increased in PKC-ε Transgenic Mice
Hematopoietic cytokines play critical roles in the decisions made by the myeloid stem cells, and imbalances of these cytokines have been shown to lead to nonneoplastic myeloproliferative diseases. GM-CSF and G-CSF are cytokines that promote the production, maturation of myeloid cells, and in particular, the proliferation and differentiation of neutrophil progenitors. To determine whether PKC-ε transgenic mice had imbalances of GM-CSF or G-CSF, PKC-ε transgenic mice (n = 4) and their wild-type littermates were examined at 3, 7, 9, 17, 20, and 40 weeks (GM-CSF) and 3, 7, 17, and 38 weeks (G-CSF) by ELISA. The GM-CSF levels in the serum were undetectable at all time points. However, G-CSF levels (Table 2) showed a significant increase (P < 0.05) as the mice aged. At 3 and 7 weeks of age G-CSF serum levels were not significantly different from their wild-type littermates, whereas both the 17- and 38-week time points showed significant increases in the serum (P < 0.05).
Table 2.
G-CSF Levels in the Serum
| Time | Mouse | pg/ml Serum G-CSF | P value |
|---|---|---|---|
| 3 week | WT | 76.9 ± 3.9 | = 0.4795 |
| 3 week | PKCε | 131.6 ± 34.5 | |
| 7 week | WT | 108.0 ± 16.5 | = 0.7728 |
| 7 week | PKCε | 108.7 ± 17.2 | |
| 17 week | WT | 57.0 ± 22.1 | < 0.05 |
| 17 week | PKCε | 156.4 ± 19.9 | |
| 38 week | WT | 113.6 ± 40.8 | < 0.05 |
| 38 week | PKCε | 1519.5 ± 324.2 |
To determine if keratinocytes overexpressing PKC-ε could contribute to the increases of G-CSF in the serum of PKC-ε transgenic mice, keratinocytes isolated from PKC-ε transgenic newborn pups and their wild-type littermates were plated, in triplicate, at 500,000 cells per well in six-well plates. The cells were treated with either the vehicle ethanol or 100 nmol/L of TPA for 3, 6, 12, 24, and 48 hours. At the indicated times, the media was collected and analyzed for G-CSF by ELISA (Table 3). The G-CSF released was normalized to the number of cells plated at the onset of the experiment. The results of the experiment indicated that keratinocytes from PKC-ε transgenic mice released more G-CSF into the media at all time points examined. Notable findings included the following: keratinocytes from PKC-ε transgenic mice released G-CSF within 3 hours after TPA treatment, whereas the levels in wild-type controls were not detectable; and the ethanol-treated control keratinocytes from PKC-ε transgenic mice at 48 hours had increased basal release of G-CSF without TPA stimulation.
Table 3.
G-CSF Levels Released from Cultured Keratinocytes
| Time | Keratinocytes | pg/ml Media G-CSF | P value (WT-TPA vs PKCε TPA) |
|---|---|---|---|
| 3 hours | WT-ETOH | ND* | |
| WT-TPA | ND | ||
| PKCε-ETOH | ND | ||
| PKCε-TPA | 117.5 ± 10.9 | ||
| 6 hours | WT-ETOH | ND | <0.05 |
| WT-TPA | 103.9 ± 9.4 | ||
| PKCε-ETOH | ND | ||
| PKCε-TPA | 180.9 ± 8.0 | ||
| 12 hours | WT-ETOH | ND | <0.05 |
| WT-TPA | 119.0 ± 12.9 | ||
| PKCε-ETOH | ND | ||
| PKCε-TPA | 221.3 ± 10.1 | ||
| 24 hours | WT-ETOH | ND | <0.05 |
| WT-TPA | 110.0 ± 11.9 | ||
| PKCε-ETOH | ND | ||
| PKCε-TPA | 209.9 ± 23.1 | ||
| 48 hours | WT-ETOH | ND | < 0.05 |
| WT-TPA | 415.1 ± 4.7 | ||
| PKCε-ETOH | 57.6 ± 4.4 | ||
| PKCε-TPA | 546.3 ± 26.7 |
ND, not detectable.
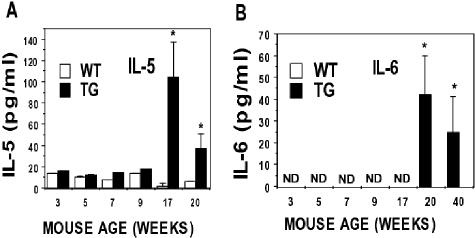
Although GM-CSF and G-CSF are the primary cytokines involved in the regulation of neutrophils, we analyzed a set of 15 cytokines to determine whether imbalances in other sets of cytokines existed. These cytokines included IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, IL-13, macrophage inflammatory protein-1α, TNF-α, RANTES, keratinocyte-derived cytokine, interferon-γ, and monocyte chemoattractant protein. Serum from PKC-ε transgenic mice and their wild-type littermates were analyzed at time points throughout their life. Only IL-5 and IL-6 were significantly (P < 0.05) increased in PKC-ε transgenic mice (Figure 6) at the later time points.
Figure 6.
PKC-ε transgenic mice have increased IL-5 and IL-6. PKC-ε transgenic mice and their wild-type littermates were sacrificed at the indicated time points and serum was collected. The serum was analyzed by Linco Diagnostics as described in Material and Methods. Serum levels of IL-5 (A) and IL-6 (B) were significantly different from their wild-type littermates at 17 and 20 and 20 and 40 weeks, respectively (P < 0.05). Serum levels of IL-6 in wild-type and PKC-ε transgenic mice from 3 to 7 weeks were not detectable (ND).
Discussion
Transgenic mice overexpressing PKC-ε in the mouse epidermis display a phenotype, increasing with age, characterized by splenomegaly, hepatomegaly, and enlarged inguinal and axillary lymph nodes associated with the development of severe dermatitis. In addition, these mice exhibit effacement of normal bone marrow hematopoiesis, with marked increases of neutrophils within the bone marrow as well as in the circulating blood. Furthermore, the aged PKC-ε, the high-PKC-ε-overexpressing transgenic mice (line 215) exhibited loss of the normal splenic architecture because of exaggerated extramedullary hematopoiesis in the red pulp, and infiltration of both neutrophils and eosinophils into nonhematopoietic organs. Analysis of G-CSF in PKC-ε transgenic mice showed elevated levels as the mice aged.
Analysis of G-CSF in PKC-ε transgenic mice revealed an increase in serum levels, which correlated with increasing age (Table 2). This finding strongly correlated with the progression of the MPD and increasing circulating neutrophils and infiltration of neutrophils in nonhematopoietic organs (Table 1 and Figure 3). Mice at 6 to 8 months of age had complete effacement of the bone marrow, which was replaced by mature neutrophils and their early precursors (Figure 2). G-CSF is a growth factor that promotes the production, maturation, and survival of myeloid stem cells. Furthermore G-CSF plays a role in the proliferation and differentiation of neutrophil progenitors as well as increasing their trafficking from the bone marrow to the blood.32–34 G-CSF is not only vital for the latter but has also been shown to have chemoattractant properties for cells of the granulocytic lineage.35 There have been several studies that have investigated and reported the role of G-CSF in granulopoiesis. The most conclusive evidence has come from gene-targeting studies involving G-CSF36 or G-CSF receptor gene knockouts.37 Both G-CSF-deficient and G-CSF-receptor-deficient mice have defective granulopoiesis with chronic neutropenia, both peripheral and in bone marrow, as well as a decrease in mature myeloid elements in their bone marrow. These studies have confirmed the hypothesis in the literature that G-CSF is a major regulator of granulopoiesis. Alternatively, studies in which murine marrow cells transduced with a retroviral vector containing the G-CSF DNA were transplanted into lethally irradiated recipients resulted in dramatic increases in neutrophilic granulocytosis in all hematopoietic tissues with neutrophilic infiltration occurring in the lung and liver.6 In addition to its roles of increasing proliferation and survival, G-CSF has also been shown to facilitate the mobilization of neutrophils from bone marrow into blood.33,34
IL-5 and IL-6 were also elevated at late time points in the life span of PKC-ε transgenic mice (Figure 6). IL-5 induces eosinophil growth, differentiation, activation, survival, and primes eosinophils to respond to chemoattractants such as eotaxin.38,39 IL-6 is a multifunctional cytokine that is produced by cells during a variety of inflammatory conditions in vivo.40–42 It stimulates activation and differentiation of B and T lymphocytes, induces fever, and regulates acute phase protein synthesis.43–46 In addition, IL-6 has been shown to be involved in various steps of hematopoiesis and has been used for ex vivo expansion of hematopoietic cells.47–49 However, given the time of onset of the disease (4 weeks) and the time of detection of these cytokines (20+ weeks), they may be produced secondarily to another primary event.
One fundamental question when analyzing a myeloproliferative disease is whether the growth advantage of the target cell is cell intrinsic. This question is addressed by transplanting bone marrow cells from PKC-ε transgenic mice into a normal, irradiated, syngeneic host. If the myeloproliferative disease persists in the transplanted mice, then a cell-intrinsic mechanism is likely. With the data presented in this communication, it is not possible to answer this question and these experiments need to be performed.
It is possible that PKC-ε is expressed in a subset of hematopoietic cells. The human keratin 14 promoter has been shown to target gene expression to the stratified squamous epithelium in transgenic mice.50 This promoter has been used to generate several different transgenic mice to understand the role of various genes in inflammatory skin disease as well as skin carcinogenesis. To date, genes driven by this promoter have been solely detected in the stratified squamous epithelium of the mouse, mainly the epidermis, trachea, thymus, and cervix. To determine whether our transgene was expressed in the affected organs of PKC-ε transgenic mice, we analyzed, by Western analysis and real-time quantitative PCR, T7-PKC-ε protein and RNA expression (Figure 5). No immunoreactivity was detected besides the skin in this experiment. Only the skin, trachea, and thymus detected expression of the transgene when analyzed using real-time quantitative PCR. These results correlate with the protein expression we have previously reported in these organs.27 These results imply that T7-PKC-ε was not driving neutrophil proliferation by aberrant production of PKC-ε in a granulocyte progenitor cell.
Another possibility for the development of this MPD is a neoplastic transformation of a precursor cell. However, given that this disease occurs with a 100% incidence in aged high-PKC-ε-overexpressing transgenic mice (line 215) it seems unlikely that such an event occurred, but cannot be ruled out with the data presented. To determine whether this MPD was because of the FVB/N background we crossed PKC-ε transgenic mice (line 215) on the FVB/N background to the SKH background for six generations. These mice presented, at 6 months of age, with severe dermatitis, scales, and ulcerations on the dorsal skin, ears, neck, and the base of the tail. On autopsy, 6-month-old PKC-ε transgenic mice on the SKH background demonstrated severe splenomegaly and hepatomegaly (data not shown). These data suggest that the development of the myeloproliferative disease is dependent on PKC-ε expression in the epidermis and not the FVB/N background itself.
During the process of transgenesis, linear DNA inserts randomly into the mouse genome. One possibility for the genesis of this disease in line 215 is insertional mutagenesis into a key gene involved in granulopoiesis. One approach to test the hypothesis that PKC-ε mediates the MPD from epidermal keratinocytes would be to generate transgenic mice expressing kinase-dead PKC-ε using the human K14 promoter and to determine whether the disease symptoms and course could be alleviated. The PKC-ε transgenic mouse line 224, which expresses eightfold more PKC-ε protein than their wild-type littermates, does not elicit histopathological changes as are observed in the line 215 that expresses 18-fold more PKC-ε protein. It is also likely that there is a threshold of PKC-ε protein, which may induce the observed phenotypic changes (hepatomegaly and splenomegaly).
The PKC-ε transgenic mice were generated to determine the role of PKC-ε in skin carcinogenesis. The PKC-ε transgenic mouse lines (215 and 224) induced papilloma-independent SCC elicited either by the initiation (DMBA)-promotion (TPA) protocol or by repeated exposure to ultraviolet radiation.51 The susceptibility of PKC-ε transgenic mice to the induction of SCC was proportional to the level of the expression of the transgene in the epidermis. We found that TNF-α52 is the key cytokine linked to the induction of SCC in these PKC-ε transgenic mice. In this context it is noteworthy that pentoxifylline, an inhibitor of TNF-α synthesis, which completely prevented the induction of SCC, failed to affect the development of the MPD (data not shown). In addition, PKC-ε transgenic mice crossed to TNF-α-null mice showed no difference in the presentation of the MPD. This finding suggests that PKC-ε-induced TNF-α is not responsible for the development of the MPD. Taken together, these data suggest that the susceptibility of PKC-ε transgenic mice to the induction of SCC and the development of MPD are unrelated. Skin carcinogenesis involves initiation (DMBA) and promotion (TPA). Tumor promotion involved chronic activation of PKC-ε and then induction of TNF-α both in the skin and serum. However, MPD develops spontaneously through basal activation of PKC-ε by mechanisms other than TPA treatment, such as generation of endogenous diacylglycerol.27 PKC-ε transgenic mice have significantly increased levels of basal PKC-ε activity. It is also noteworthy that basal PKC-ε activity is extremely low in line 224. We could not link the role of TNF-α in the development of myeloproliferative disorder.
In summary, aged PKC-ε transgenic mice develop a MPD that is characterized by massive neutrophil proliferation in the bone marrow, release to the blood, and infiltration of nonhematopoietic tissues. The spontaneous development of MPD accompanied increased serum levels of G-CSF, IL-5, and IL-6 but not of the cytokines macrophage inflammatory protein1-α, GM-CSF, monocyte chemoattractant protein, keratinocyte-derived cytokine, RANTES, interferon-γ, IL-1β, IL-2, IL-4, IL-9, IL-10, IL-12, IL-13, and TNF-α. The etiology of the development of the MPD in high-expressing PKC-ε transgenic mice is unknown. The MPD developed in PKC-ε transgenic mice have characteristics similar to that of human MPD most notably severe splenomegaly, hypercellular bone marrow, and increased circulating myeloid cells in the peripheral blood. This PKC-ε transgenic mouse model may be useful for studying the pathogenesis of myeloproliferative disease.
Acknowledgments
We thank Nancy Dreckschmidt and Marybeth Wartman for excellent technical assistance and Toshi Kinoshita for tissue processing.
Footnotes
Address reprint requests to Ajit K. Verma, Department of Human Oncology, K4/532 CSC Clinical Science Center, 600 Highland Ave., Madison, WI 53792. E-mail: akverma@facstaff.wisc.edu.
Supported by the National Institutes of Health (grant CA 35368) and the William S. Middleton VA Hospital, Madison, WI (for resources and the use of facilities).
References
- Krause DS. Regulation of hematopoietic stem cell fate. Oncogene. 2002;21:3262–3269. doi: 10.1038/sj.onc.1205316. [DOI] [PubMed] [Google Scholar]
- Lotem J, Sachs L. Cytokine control of developmental programs in normal hematopoiesis and leukemia. Oncogene. 2002;21:3284–3294. doi: 10.1038/sj.onc.1205319. [DOI] [PubMed] [Google Scholar]
- Lang RA, Metcalf D, Cuthbertson RA, Lyons I, Stanley E, Kelso A, Kannourakis G, Williamson DJ, Klintworth GK, Gonda TJ, Dunn AR. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987;51:675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- Johnson GR, Gonda TJ, Metcalf D, Hariharan IK, Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989;8:441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PM, Chung SW, Dunbar CE, Bodine DM, Ruscetti S, Nienhuis AW. Retrovirus-mediated transfer and expression of the interleukin-3 gene in mouse hematopoietic cells result in a myeloproliferative disorder. Mol Cell Biol. 1989;9:798–808. doi: 10.1128/mcb.9.2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JM, Metcalf D, Gonda TJ, Johnson GR. Long-term exposure to retrovirally expressed granulocyte-colony-stimulating factor induces a nonneoplastic granulocytic and progenitor cell hyperplasia without tissue damage in mice. J Clin Invest. 1989;84:1488–1496. doi: 10.1172/JCI114324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley RG, Fong AZ, Burns BF, Hawley TS. Transplantable myeloproliferative disease induced in mice by an interleukin 6 retrovirus. J Exp Med. 1992;176:1149–1163. doi: 10.1084/jem.176.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D, Kauvar LM. Modulating protein kinase C signal transduction. Adv Pharmacol. 1998;44:91–145. doi: 10.1016/s1054-3589(08)60126-x. [DOI] [PubMed] [Google Scholar]
- Asaoka Y, Nakamura S, Yoshida K, Nishizuka Y. Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci. 1992;17:414–417. doi: 10.1016/0968-0004(92)90011-w. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Dekker LV, Parker PJ. Protein kinase C—a question of specificity. Trends Biochem Sci. 1994;19:73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Hata A, Akita Y, Suzuki K, Ohno S. Functional divergence of protein kinase C (PKC) family members. PKC gamma differs from PKC alpha and -beta II and nPKC epsilon in its competence to mediate-12-O-tetradecanoyl phorbol 13-acetate (TPA)-responsive transcriptional activation through a TPA-response element. J Biol Chem. 1993;268:9122–9129. [PubMed] [Google Scholar]
- Cacace AM, Guadagno SN, Krauss RS, Fabbro D, Weinstein IB. The epsilon isoform of protein kinase C is an oncogene when overexpressed in rat fibroblasts. Oncogene. 1993;8:2095–2104. [PubMed] [Google Scholar]
- Mischak H, Goodnight JA, Kolch W, Martiny-Baron G, Schaechtle C, Kazanietz MG, Blumberg PM, Pierce JH, Mushinski JF. Overexpression of protein kinase C-delta and -epsilon in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993;268:6090–6096. [PubMed] [Google Scholar]
- Han EK, Cacace AM, Sgambato A, Weinstein IB. Altered expression of cyclins and c-fos in R6 cells that overproduce PKC epsilon. Carcinogenesis. 1995;16:2423–2428. doi: 10.1093/carcin/16.10.2423. [DOI] [PubMed] [Google Scholar]
- Soh JW, Lee EH, Prywes R, Weinstein IB. Novel roles of specific isoforms of protein kinase C in activation of the c-fos serum response element. Mol Cell Biol. 1999;19:1313–1324. doi: 10.1128/mcb.19.2.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacace AM, Ueffing M, Han EK, Marme D, Weinstein IB. Overexpression of PKCepsilon in R6 fibroblasts causes increased production of active TGFbeta. J Cell Physiol. 1998;175:314–322. doi: 10.1002/(SICI)1097-4652(199806)175:3<314::AID-JCP9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Ohno S, Brugge JS. Protein kinase C regulates integrin-induced activation of the extracellular regulated kinase pathway upstream of Shc. J Biol Chem. 1999;274:10571–10581. doi: 10.1074/jbc.274.15.10571. [DOI] [PubMed] [Google Scholar]
- Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco MT, Moscat J, Rapp U, Cooper GM. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997;17:732–741. doi: 10.1128/mcb.17.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueffing M, Lovric J, Philipp A, Mischak H, Kolch W. Protein kinase C-epsilon associates with the Raf-1 kinase and induces the production of growth factors that stimulate Raf-1 activity. Oncogene. 1997;15:2921–2927. doi: 10.1038/sj.onc.1201477. [DOI] [PubMed] [Google Scholar]
- Winder SJ, Allen BG, Clement-Chomienne O, Walsh MP. Regulation of smooth muscle actin-myosin interaction and force by calponin. Acta Physiol Scand. 1998;164:415–426. doi: 10.1111/j.1365-201x.1998.tb10697.x. [DOI] [PubMed] [Google Scholar]
- Reddig PJ, Dreckschmidt NE, Zou J, Bourguignon SE, Oberley TD, Verma AK. Transgenic mice overexpressing protein kinase C epsilon in their epidermis exhibit reduced papilloma burden but enhanced carcinoma formation after tumor promotion. Cancer Res. 2000;60:595–602. [PubMed] [Google Scholar]
- Jansen AP, Verwiebe EG, Dreckschmidt NE, Wheeler DL, Oberley TD, Verma AK. Protein kinase C-epsilon transgenic mice: a unique model for metastatic squamous cell carcinoma. Cancer Res. 2001;61:808–812. [PubMed] [Google Scholar]
- Reddig PJ, Dreckschmidt NE, Ahrens H, Simsiman R, Tseng CP, Zou J, Oberley TD, Verma AK. Transgenic mice overexpressing protein kinase Cdelta in the epidermis are resistant to skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1999;59:5710–5718. [PubMed] [Google Scholar]
- Jansen AP, Dreckschmidt NE, Verwiebe EG, Wheeler DL, Oberley TD, Verma AK. Relation of the induction of epidermal ornithine decarboxylase and hyperplasia to the different skin tumor-promotion susceptibilities of protein kinase C alpha, -delta and -epsilon transgenic mice. Int J Cancer. 2001;93:635–643. doi: 10.1002/ijc.1395. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Smart RC. Overexpression of protein kinase C-alpha in the epidermis of transgenic mice results in striking alterations in phorbol ester-induced inflammation and COX-2, macrophage inflammatory protein-2 and TNF-alpha expression but not tumor promotion. J Cell Sci. 1999;112:3497–3506. doi: 10.1242/jcs.112.20.3497. [DOI] [PubMed] [Google Scholar]
- Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–861. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]
- Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- Lord BI, Molineux G, Pojda Z, Souza LM, Mermod JJ, Dexter TM. Myeloid cell kinetics in mice treated with recombinant interleukin-3, granulocyte colony-stimulating factor (CSF), or granulocyte-macrophage CSF in vivo. Blood. 1991;77:2154–2159. [PubMed] [Google Scholar]
- Metcalf D. Control of granulocytes and macrophages: molecular, cellular, and clinical aspects. Science. 1991;254:529–533. doi: 10.1126/science.1948028. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746. [PubMed] [Google Scholar]
- Liu F, Wu HY, Wesselschmidt R, Kornaga T, Link DC. Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient mice. Immunity. 1996;5:491–501. doi: 10.1016/s1074-7613(00)80504-x. [DOI] [PubMed] [Google Scholar]
- Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- Rothenberg ME. Eotaxin. An essential mediator of eosinophil trafficking into mucosal tissues. Am J Respir Cell Mol Biol. 1999;21:291–295. doi: 10.1165/ajrcmb.21.3.f160. [DOI] [PubMed] [Google Scholar]
- Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today. 1997;18:428–432. doi: 10.1016/s0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- Paquet P, Pierard GE. Interleukin-6 and the skin. Int Arch Allergy Immunol. 1996;109:308–317. doi: 10.1159/000237257. [DOI] [PubMed] [Google Scholar]
- Barton BE. IL-6: insights into novel biological activities. Clin Immunol Immunopathol. 1997;85:16–20. doi: 10.1006/clin.1997.4420. [DOI] [PubMed] [Google Scholar]
- Houssiau F, Van Snick J. IL6 and the T-cell response. Res Immunol. 1992;143:740–743. doi: 10.1016/0923-2494(92)80014-c. [DOI] [PubMed] [Google Scholar]
- Seymour JF, Kurzrock R. Interleukin-6: biologic properties and role in lymphoproliferative disorders. Cancer Treat Res. 1996;84:167–206. doi: 10.1007/978-1-4613-1261-1_9. [DOI] [PubMed] [Google Scholar]
- Rifas L. Bone and cytokines: beyond IL-1, IL-6 and TNF-alpha. Calcif Tissue Int. 1999;64:1–7. doi: 10.1007/s002239900570. [DOI] [PubMed] [Google Scholar]
- Rifas L, Avioli LV. A novel T cell cytokine stimulates interleukin-6 in human osteoblastic cells. J Bone Miner Res. 1999;14:1096–1103. doi: 10.1359/jbmr.1999.14.7.1096. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Uchida N, Weissman IL. The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81:2844–2853. [PubMed] [Google Scholar]
- Ikebuchi K, Wong GG, Clark SC, Ihle JN, Hirai Y, Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci USA. 1987;84:9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Rosenberg M, Ross S, Tyner A, Fuchs E. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc Natl Acad Sci USA. 1989;86:1563–1567. doi: 10.1073/pnas.86.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DL, Wartman MB, Dreckschmidt NE, Verma AK. Protein kinase C sensitizes skin to the development of metastatic squamous cell carcinoma possibly by a paracrine mechanism involving specific cytokines. Proc Am Assoc Cancer Res. 2004;45 [Google Scholar]
- Wheeler DL, Ness KJ, Oberley TD, Verma AK. Protein kinase Cepsilon is linked to 12-O-tetradecanoylphorbol-13-acetate-induced tumor necrosis factor-alpha ectodomain shedding and the development of metastatic squamous cell carcinoma in protein kinase Cepsilon transgenic mice. Cancer Res. 2003;63:6547–6555. [PubMed] [Google Scholar]