Abstract
We describe here isolation and characterization of CD133+ cells derived from normal adult human kidney. These cells lacked the expression of hematopoietic markers and expressed PAX-2, an embryonic renal marker, suggesting their renal origin. Renal tissue-derived CD133+ cells and clones of individual cells were capable of expansion and limited self-renewal and differentiated in vitro into epithelial or endothelial cells. On subcutaneous implantation in SCID mice, the undifferentiated cells formed tubular structures expressing renal epithelial markers. At variance, when differentiated in endothelial cells, these cells formed functional vessels. On intravenous injection in SCID mice with glycerol-induced tubulonecrosis, the in vitro expanded renal-derived CD133+ cells homed into the injured kidney and integrated in tubules. We propose that CD133+ cells from kidney represent a multipotent adult resident stem cell population that may contribute to the repair of renal injury.
Nephrotoxic and ischemic insults to the kidney lead to acute renal failure and most often manifest as acute tubular necrosis. Recovery of renal function after acute renal failure is dependent on the replacement of necrotic tubular cells with functional tubular epithelium.1 In addition, a pronounced proliferative response of the glomerular and peritubular capillary endothelium2 is observed after ischemic injury. The absence or reduction of epithelial and endothelial regeneration may predispose to tubulointerstitial renal scarring and chronic renal disease. The origin of newly generated renal cells is primarily undefined, but by analogy to other organs, organ-specific pluripotent cells (ie, renal stem cells) have been suggested as precursors of new cells.3 However, the identification of adult renal stem cells is at the moment still lacking.
It has been recently demonstrated that the embryonic rat metanephric mesenchymes possess organ-specific progenitor cells capable of differentiating into epithelia, myofibroblasts, and smooth muscle cells, indicating the presence of embryonic renal stem cells.4 It is currently unknown whether these stem cells are also present in the adult human kidney. Data are also unclear regarding the possible origin of renal endothelial cells. Contradictory evidences suggest either a possible colonization of kidney by exogenous angioblasts or a common origin of renal endothelial cells with other renal cell types.5,6
The aim of the present study was to isolate and characterize a population of renal progenitor cells. As a selection marker we chose the human CD133 stem cell antigen. This pentaspan molecule, discovered for its expression on hematopoietic stem and progenitor cells,7 was shown to be also expressed by undifferentiated human intestine-derived epithelial cells in culture and by embryonic kidney.8 We therefore aimed to evaluate whether renal CD133+ cells derived from human adult kidney were capable of expansion and self-renewal and whether they could differentiate into epithelial and endothelial cells in vitro and in vivo and participate in renal tissue repair.
Materials and Methods
CD133 Isolation, Expansion, and Differentiation
Renal progenitor cells were obtained from the normal portion of cortex obtained from surgically removed kidneys. After dissection and passage through a graded series of meshes, CD133+ cells were isolated from the tubular fraction by magnetic cell sorting, using the MACS system (Miltenyi Biotec, Auburn, CA).9 CD133+ cells were plated onto fibronectin in the presence of an expansion medium, consisting of 60% DMEM LG (Invitrogen, Paisley, UK), 40% MCDB-201, with 1× insulin-transferrin-selenium, 1× linoleic acid 2-phosphate, 10−9 mol/L dexamethasone, 10−4 ascorbic acid 2-phosphate, 100 U penicillin, 1000 U streptomycin, 10 ng/ml epidermal growth factor, and 10 ng/ml platelet-derived growth factor-BB (all from Sigma-Aldrich, St. Louis, MO) and 2% fetal calf serum (EuroClone, Wetherby, UK).10 For cell cloning, single cells were deposited in 96-well plates in the presence of the expansion medium. Epithelial differentiation was obtained in the presence of fibroblast growth factor-4 (10 ng/ml) and hepatocyte growth factor (20 ng/ml, Sigma).10 Endothelial differentiations were obtained by culturing the cells in EBM medium (Cambrex Bio Science, Baltimore, MD) with vascular endothelial growth factor (10 ng/ml, Sigma) and 10% fetal calf serum on endothelial cell attachment factor (Sigma).11 CD133+ cells were also isolated from the blood of granulocyte-colony stimulating factor mobilized patients using the MACS system (Miltenyi Biotec). Mesenchymal cells were obtained from the bone marrow of healthy donors and cultured in α-minimal essential medium supplemented with 10% fetal calf serum and 10% horse serum (all from Invitrogen), as described.12 The nonadherent cells were removed by medium change at 48 hours and every 4 days thereafter. Tube formation on Matrigel was performed as described.9
Immunofluorescence and Immunocytochemistry
Cytofluorimetric analysis was performed as described9 using the following antibodies, all fluorescein isothiocyanate or phycoerythrin conjugated: anti-CD133–1 monoclonal Ab (mAb) (Miltenyi Biotec); anti-CD44 and anti-human HLA class I mAbs (Sigma); anti-CD31 and anti-CD105 mAbs (Serotec Inc., Oxford, UK); anti-KDR mAb (R&D Systems, Minneapolis, MN); anti-Muc-18 mAb (Chemicon Int., Temecula, CA); and anti-CD29, -CD33, -CD34, -CD45, -CD73, -CD90, and -CD117 mAbs (Becton Dickinson, San Jose, CA). Anti-VE cadherin mAb was kindly provided by Guido Tarone (University of Torino). Fluorescein isothiocyanate or phycoerythrin mouse nonimmune isotypic IgG (DAKO, Copenhagen, Denmark) were used as controls. Indirect immunofluorescence was performed on cells cultured on chamber slides, fixed in 4% paraformaldehyde containing 2% sucrose and, when needed, permeabilized with Hepes-Triton X-100 buffer.13 Immunofluorescence was also performed on human or mouse tissues rapidly frozen in liquid nitrogen, cut in 3-μm sections, and fixed in 3.5% paraformaldehyde containing 2% sucrose. The following antibodies were used: anti-NaCl co-transporter, anti-aminopeptidase A, and anti-alkaline phosphatase polyclonal goat Abs (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-zonula occludens (ZO)-1 polyclonal Ab (Santa Cruz Biotechnology), goat anti-von Willebrand factor (vWF) and rabbit anti-pan-cytokeratin Abs (Sigma), anti-vimentin, and anti-E cadherin mAbs (DAKO), anti-EMA mAb (Chemicon Int.), polyclonal rabbit anti-PAX-2 Ab (Covance, Princeton, NJ) phycoerythrin-conjugated anti-CD133 (Miltenyi Biotec), and anti-proliferating cell nuclear antigen, fluorescein isothiocyanate-conjugated anti-HLA I and anti-calbindin D-28K mAbs (Sigma). Control mouse, rabbit, or goat nonimmune immunoglobulins were used as controls. Fluorescein isothiocyanate-conjugated anti-mouse, -rabbit, or -goat IgGs (Sigma) were used as secondary antibodies when needed. Immunocytochemistry was performed as described14 on tissue fixed in 10% buffered formalin and embedded in paraffin. Confocal microscopy was performed using a Leica TCS SP2 model confocal microscope (Heidelberg, Germany). Hoechst 33258 dye (Sigma) was added for nuclear staining.
Quantitative Real-Time Polymerase Chain Reaction (PCR)
Quantitative real-time PCR were performed as previously described.15 Briefly first-strand cDNA was produced from 2 mg of random hexamer-primed total RNA using reverse transcription reactions (20 μl). Relative quantitation by real-time PCR was performed using SYBR-green detection of PCR products in real time using the iCycler from Bio-Rad (Cambridge, MA). In each experiment, the human b2 microglobulin (B2M) housekeeping gene was amplified as a reference standard. Primers for B2M were B2MF, 5′-AGATGAGTATGCCTGCCGTGT-3′ and B2MR, 5′-GCTTACATGTCTCGATCCCACTTA-3′. In all real-time PCR experiments, cloned Pax-2 DNA were included as positive control. RNA from human umbilical vein endothelial cells were used as negative control.14 Template RNAs were treated with DNase I before amplification. Each real-time PCR reaction (50 μl) contained 2.5 μl of cDNA were amplified using the Platinum Taq polymerase amplification system from Invitrogen (Carlsbad, CA) and primers at a final concentration of 20 μmol/L. Primers were as follows: PAX2F, 5′-CCCAGCGTCTCTTCCATCA-3′; PAX2R, 5′-GGCGTTGGGTGGAAAGG-3′. Reactions were prepared in duplicate and heated to 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds and 58°C for 60 seconds, with a final incubation at 95°C for 15 minutes. To detect the log phase of amplification, the fluorescence level (quantification of product) was determined at each cycle. The cycle at which the fluorescence reached threshold was recorded, averaged between duplicates, and normalized to the averaged cycle of threshold value for B2M. The relative expression level for PAX-2 gene was then calculated according to the manufacturer’s instructions.
Electron Microscopy
Immunogold labeling was performed on 2.5% paraformaldehyde-fixed cells16 using a primary specific antibody or an isotype-matched immunoglobulin (DAKO) and, as secondary antibody, the 5-nm gold-conjugated rabbit anti-goat Ab (BBInternational, Cardiff, UK) followed by silver enhancement (Silver enhancing kit, BBInternational). Samples were postfixed in 2.5% glutaraldehyde, dehydrated in alcohol, dried, and coated with gold by sputter coating. The specimens were examined in a scanning Jeol T300 electron microscope. Images were obtained via secondary electron at a working distance of 15 to 25 mm and at an accelerating voltage of 20 to 25 kV. Transmission electron microscopy was performed on Karnovsky’s-fixed, osmium tetraoxide-postfixed tissues and embedded in epoxy resin according to standard procedures.16 Ultra-thin sections were stained with uranyl acetate and lead citrate and were examined with a Jeol JEM 1010 electron microscope.
Transepithelial Electrical Resistance
Transepithelial electrical resistance was used as an indicator of epithelial differentiation.17 CD133+ cells undifferentiated or differentiated into epithelial cells were plated in transwells on collagen-coated polycarbonate membrane (Corning Costar Corp., Cambridge, MA) and allowed to reach confluency. An epithelial volt-ohm meter (EVOM; World Precision Instruments, Inc., Sarasota, FL) was used to determine the transepithelial electrical resistance value. All values were normalized for the area of the membrane. As a control we used an immortalized tubular epithelial cell line, previously characterized.18 All experiments were done in triplicate.
Xenograft in SCID Mice
CD133+ cells (undifferentiated or endothelial committed) were implanted subcutaneously into SCID mice (Charles River, Jackson Laboratories, Bar Harbor, ME) within Matrigel (Becton Dickinson), as described.9 Cells were harvested using trypsin-ethylenediaminetetraacetic acid, washed with phosphate-buffered saline, counted in a microcytometer chamber, and resuspended in Dulbecco’s modified Eagle’s medium (1 × 106 in 250 μl of Dulbecco’s modified Eagle’s medium). Cells were chilled on ice, added to 250 μl of Matrigel at 4°C, and injected subcutaneously into the left back of SCID mice via a 26-gauge needle using a 1-ml syringe. At day 10, mice were sacrificed and Matrigel plugs recovered and analyzed. To evaluate the ability of CD133+ cells to localize in the injured kidney, we induced acute tubular injury by intramuscle injection of glycerol in SCID mice.19,20 The peak of tubular injury was observed 3 days after glycerol injection (8 μl/g body weight of 50% glycerol solution). At this time, 106 CD133+ cells (passage III to IV) labeled with PHK2 green fluorescent dye (Sigma) were intravenously injected in treated and untreated animals, and mice were sacrificed after 3 days.
Results
Isolation and Characterization of Renal Progenitor Cells
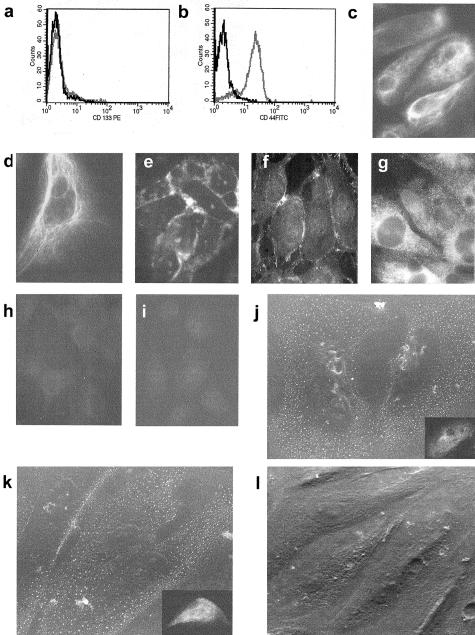
Immunostaining showed the presence of rare CD133+ cells within the cortex of normal renal tissue. Scattered cells were observed within the interstitium (Figure 1, a and b). Glomeruli were negative. The specimens we obtained did not include the medullary part of the kidney. We isolated the CD133+ cells from adult renal cortical tissue by immunomagnetic sorting. CD133-based fluorescence-activated cell sorting gated an average of 0.8 to 1.2% of the cells (Figure 1, c and d). The percentage of cells we sorted from renal tissue was 0.8 ± 0.15% of total cells extracted from the renal cell population deprived of glomeruli (n = 8). Cells were plated onto fibronectin and grown in serum-free medium with epidermal growth factor and platelet-derived growth factor-BB (expansion medium), a medium used by Jiang and colleagues10 to maintain undifferentiated multipotent adult progenitor cells.
Figure 1.
Isolation and characterization of CD133+ cells from adult human renal tissue. a and b: Micrographs representative of immunohistochemical detection of CD133+ cells in the cortex of adult normal human kidney. Cells, expressing membrane CD133, are visible within the interstitium (arrows). c and d: Flow cytometric analysis showed that ∼1% of total cells extracted from renal population deprived of glomeruli express CD133. e to i: Flow cytometric analysis of the immunosorted CD133+ cells. Almost all immunosorted cells expressed CD133 (e), CD73 (f), and CD44 (g), but not CD34 (e), CD45 (h), and CD117 (i). Eight different cell preparations were examined with similar results. j: Micrograph representative of Pax-2 nuclear staining in renal progenitor cells. k: PAX-2 mRNA expression by quantitative real-time PCR. Three different cell preparations (▪, •, ♦) were studied in duplicate, CD133+/CD34+ cells from peripheral blood were used as negative control (▴) and cloned PAX-2 as positive control (continuous line). Original magnifications: ×250 (a, j); ×400 (b). G, glomerulus.
Early characterization of the initial cell isolates by fluorescence-activated cell sorting analysis showed that virtually all sorted cells expressed CD133 (Figure 1e) as well as CD73, also named SH321 (Figure 1f), CD44 (Figure 1g), and CD29 (not shown), membrane markers commonly expressed in mesenchymal stem cells.12 HLA class I antigen was also expressed (not shown). No expression of CD34 and CD45 was detected in all isolates (Figure 1, e and h), suggesting that these cells were not derived from circulating hematopoietic CD133+ cells. In addition, renal CD133+ cells did not express other stem cell markers such as CD117 (c-kit; Figure 1i) or CD90 (not shown). To further investigate the renal origin of CD133+ cells, we evaluated the expression of the developmental renal marker PAX-2 by immunofluorescence and quantitative real-time PCR analysis. PAX-2 is expressed in induced mesenchyme and in the early epithelial derivates and it is rapidly down-regulated in mature kidney.22 By immunofluorescence we detected a typical nuclear staining for Pax-2 protein (Figure 1j). In addition, PAX-2 mRNA was highly expressed by CD133+ cells obtained from kidney, but not by CD133+ obtained from granulocyte-colony stimulating factor mobilized peripheral blood from normal donors (Figure 1k). At variance with circulating CD133+ cells, renal-derived CD133+ cells were able to adhere to fibronectin-coated plates in culture after a 6- to 8-hour incubation. Cells were maintained in expansion medium without losing CD133 expression for up to seven to nine passages, indicating that adult renal progenitor cells had a limited self-renewal. These data indicate the presence of a renal population with both stem cell and embryonic renal markers.
Cell Cloning and Differentiation into Epithelial and Endothelial Cells
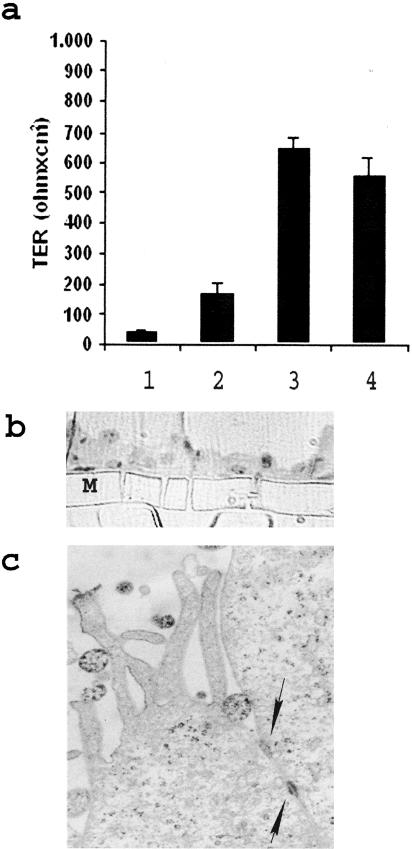
To assess the capacity of a single CD133+ cell to give rise to both an epithelial and an endothelial population, we generated clones using a limiting dilution technique. Clones were expanded in expansion medium and then cells from the same clone were plated in differentiating medium to obtain endothelial or epithelial differentiation. To obtain epithelial differentiation, cells were grown in the presence of hepatocyte growth factor and fibroblast growth factor-4. After 10 days of culture, cells lost CD133 expression but maintained CD44 expression (Figure 2, a and b) Cytokeratin expression, undetectable on undifferentiated cells, became detectable on >50% of cells after 5 days and was present on 100% of cells after 10 days of culture (Figure 2c). They also express the mesenchymal marker vimentin (Figure 2d), which is frequently co-expressed with cytokeratin in embryonic or dedifferentiated tubular cells.23 In addition, cells expressed the epithelial antigens E-cadherin (Figure 2e) and ZO-1 (Figure 2f), as well as markers characteristic of fully differentiated renal epithelia, such as alkaline phosphatase (Figure 2g), amino peptidase A (Figure 2j), mainly expressed by proximal tubular epithelial cells24,25 and the thiazide-sensitive NaCl co-transporter (Figure 2k), mainly expressed by distal tubular epithelial cells.26 Approximately 1% of the cells expressed also calbindin-D, a distal tubular marker (not shown).27 α-Smooth muscle actin was negative (Figure 2h). In vitro epithelial differentiation was also supported by polarization of the cell layer evaluated by trans epithelial electrical resistance (Figure 3a). Trans epithelial electrical resistance has been considered a marker of cell polarization and of stem cell differentiation into epithelial cells.17 Differentiated cells exhibited high trans epithelial electrical resistance in respect to undifferentiated CD133+ cells and comparable to renal proximal tubular epithelial cells used as control (Figure 3a). Moreover, epithelial differentiated cells cultured in Transwell on collagen-coated semipermeable membrane (Figure 3b) showed by transmission electron microscopy morphological aspects of cell polarization such as apical microvilli and junctional complexes (Figure 3c). To induce endothelial differentiation, cells were plated onto endothelial cell attachment factor and grown in the presence of vascular endothelial growth factor. Expression of CD133 was down-regulated after three culture passages (8 days) and undetectable thereafter (Figure 4a). In contrast, CD44, already present in undifferentiated cells, remained unaltered (Figure 4b). After 3 days of culture, cells started to express the endothelial markers Muc-18, KDR, CD105, VE-cadherin, and vWF, and the expression was maximal at day 10 (Figure 4; c to g) and persisted thereafter. In contrast, CD31 expression remained very low (not shown). When plated onto Matrigel, endothelial differentiated but not CD133-positive undifferentiated cells rapidly (4 hours) aligned to form, as mature endothelial cells, ring-like structures (Figure 4; h to j) also defined as capillary-like.28 These cords of endothelial cells expressed vWF (Figure 4k).
Figure 2.
Epithelial differentiation of renal tissue-derived CD133+ cells. a and b: Representative cytofluorimetric analysis of CD133+ cells cultured in epithelial differentiating medium. After10 days of culture, cells lost CD133 expression (a), but maintained the expression of CD44 (b). c to i: Representative immunofluorescence micrographs of cytokeratin (c), vimentin (d), E-cadherin (e), ZO-1 (f), alkaline phosphatase (g), and α-smooth muscle actin (h) expression by epithelial differentiated cells. e: Negative control. j to l: Representative micrographs of immunogold detection by scanning electron microscopy and of immunofluorescence (j, inset) of aminopeptidase A (j) and of NaCl co-transporter (k). l: Negative control. Eight cell preparations and 10 clones were studied by immunofluorescence and 2 clones by immunogold scanning electron microscopy with similar results. Original magnifications: ×400 (c-i, insets in j and k); ×1200 (j–l).
Figure 3.
Formation of a polarized layer by the epithelial differentiated renal progenitor cells evaluated by the transepithelial resistance and morphological aspects. a: Confluent CD133+ undifferentiated or differentiated cell cultures were plated on Transwell filters and assayed for the transepithelial resistance by an epithelial volt ohm meter. An immortalized tubular epithelial cell line was used as control. Column 1, Semipermeable membrane without cells; column 2, CD133+ undifferentiated cells; column 3, epithelial differentiated renal progenitor cells; column 4, immortalized tubular epithelial cells. Values are expressed as mean ± SD. Ohms/cm2 of three experiments. b: Micrograph representative of toluidine blue-stained semithin section showing a cross section of epithelial differentiated cells cultured in Transwell on a semipermeable membrane (M). c: Transmission electron microscopy micrograph of epithelial differentiated cells cultured in Transwell on a semipermeable membrane showing apical microvilli and junctional complexes (arrows). Original magnifications: ×150 (b); ×20,000 (c).
Figure 4.
Endothelial differentiation of renal tissue-derived CD133+ cells. a–f: Representative cytofluorimetric analysis of CD133+ cells cultured in endothelial differentiating medium. After 10 days of culture, cells lost CD133 expression (a); maintained the expression of CD44 (b); and acquired the endothelial markers Muc18 (c), KDR (d), CD105 (e), and VE-cadherin (f). g: The expression of vWF in the classical cytoplasmic punctuate pattern by endothelial differentiated cells, which were negative for cytokeratin (inset) (immunofluorescence micrograph). h–j: Scanning electron microscopy showing spontaneous cell organization on Matrigel. Endothelial differentiated cells (i, j), but not CD133+ undifferentiated cells (h) within 4 hours aligned to form ring-like structures. k: The expression of vWF by differentiated endothelial cells plated on Matrigel. Eight cell preparations and 10 clones were studied with similar results. Original magnifications: ×200 (h, i); × 400 (g, k); ×750 (j).
Epithelial and endothelial differentiated cells maintained PAX-2 gene expression (not shown). Similar results were obtained when the entire CD133+ cell population was differentiated instead of cell clones. Approximately 20 million cells were generated from a single clone, indicating a life span of 20 to 25 doublings. These data indicate that single progenitor cells of the adult kidney are both clonogenic and pluripotent.
Renal CD133+ cells, at variance of circulating CD133+ cells, did not differentiate in hematopoietic cells (CD33+/CD45+) after culturing in Iscove with 10% fetal calf serum, interleukin-3, and granulocyte-colony stimulating factor, nor did they differentiate in adipocytes (positive for neutral lipid vacuoles that stain with red oil) after culturing in adipocyte differentiating medium,12 at variance of bone marrow-derived mesenchymal cells (not shown).
In Vivo Implantation
Renal progenitor CD133+ cells were subcutaneously injected into Matrigel in nonimmunocompetent SCID mice to assess their differentiation in vivo. After 10 days, plugs were recovered and processed for histological and immunohistochemical analysis. Undifferentiated cells (passage III) injected into mice showed a spontaneous differentiation into epithelial tubular structures. Cells organized in tubular-like structures with morphological aspects of cell polarization and a virtual lumen containing proteinaceous material (Figure 5a). Immunohistochemical evaluation indicated that these structures were human, as they expressed HLA class I antigen (Figure 5a, inset), were positive for epithelial markers, such as cytokeratin and the EMA (Figure 5, b and d), and for the mesenchymal marker vimentin (Figure 5c), commonly expressed by regenerating renal epithelium. Moreover, tubular structures were positive for the distal tubular marker thiazide-sensitive NaCl co-transporter but not for the proximal tubular marker amino peptidase A (Figure 5, e and f). Some tubules were also positive for the alkaline phosphatase, a marker of proximal tubules (Figure 5g), and only some cells of the tubular structures expressed calbindin-D, a marker of distal tubules (Figure 5h). In addition, by immunohistochemistry cells expressed nuclear staining for Pax-2 protein (Figure 5i) but were negative for endothelial markers, such as vWF (not shown). When isotypic controls were used instead of the specific primary antibody no staining was observed (not shown). By electron microscopy, the arrangement of cells around a virtual lumen filled with dense material and the presence of short microvilli and tight junctions indicate the polarization of epithelial cells forming the tubular-like structures (Figure 6; a to c).
Figure 5.
In vivo differentiation of renal tissue-derived CD133+ cells in tubular structures. Undifferentiated CD133+ cells (2 × 106) at passage III were incorporated at 4°C within Matrigel and subcutaneously injected in SCID mice (n = 6). Mice were sacrificed after 15 days and the Matrigel plugs were excised and processed for histology and immunohistochemistry. a: Micrograph representative of a toluidine blue-stained semithin section showing a transversal section of a tubular structure formed by batiprismatic cells and a central lumen. By immunohistochemistry, the tubules were positive for human HLA class I antigen (inset), for cytokeratin (b), for vimentin (c), for EMA (d), and for NaCl co-transporter (e), but negative for aminopeptidase A (f). g: Some tubules were also positive for alkaline phosphatase. h: By immunofluorescence some tubules showed positive cells for calbindin-D. i: Pax-2 staining was mainly nuclear as observed by immunohistochemistry, in the absence of nuclear counterstaining. Original magnifications: ×150 (b); ×250 (c–i, inset in a and b); ×600 (a).
Figure 6.
In vivo differentiation of renal tissue-derived CD133+ cells in tubular structures. Undifferentiated CD133+ cells (2 × 106) at passage III were incorporated at 4°C within Matrigel and subcutaneously injected in SCID mice (n = 6). Mice were sacrificed after 15 days and the Matrigel plugs were excised and processed for electron microscopy. a: Toluidine blue-stained semithin section of a longitudinal section through a tubule showing (right) a lumen partially filled by dense material. On the left tangential section shows cells displaying different staining properties that may depend on different stage of differentiation. b: Low-power electron micrograph showing the arrangement of cells around a virtual lumen (L) filled with dense material. c: Apical part of three adjacent cells showing microvilli (*) and junction complexes (arrows). Original magnifications: ×600 (a); ×6000 (b); ×25,000 (c).
To evaluate the potential endothelial differentiation in vivo, CD133+ cells were first differentiated in vitro for 10 days, and then subcutaneously injected in growth factor-depleted Matrigel in SCID mice. Cells were not directly differentiated in vivo because the presence of vascular endothelial growth factor and other growth factors could induce the recruitment of mouse endothelial cells and formation of murine vessels. The human endothelial differentiated cells organized in vivo into functional vessels, connected with the mouse vasculature because they contained blood cells (Figure 7, a and c). The human nature of these vessels was indicated by the expression of HLA-class I antigen (Figure 7b).
Figure 7.
In vivo differentiation of renal tissue-derived CD133+ cells in functional vessels. Endothelial committed cells (2 × 106) at passage III to IV after differentiation were incorporated at 4°C within Matrigel and subcutaneously injected in SCID mice (n = 8). Mice were sacrificed after 15 days and the Matrigel plugs were excised and processed for histology and immunohistochemistry. a: Light microscopy of a trichromic-stained section of Matrigel showing several vessels with patent lumen-containing blood cells. b: The human nature of endothelial cells lining the vessels is indicated by positive immunofluorescence staining for human HLA class I antigen. Nuclei were counterstained in blue with Hoechst 33258 dye. c: Scanning electron microscopy performed on a freeze-hatched Matrigel plug showing a branched vessel containing red cells. Original magnifications: ×400 (a, b); ×1500 (c).
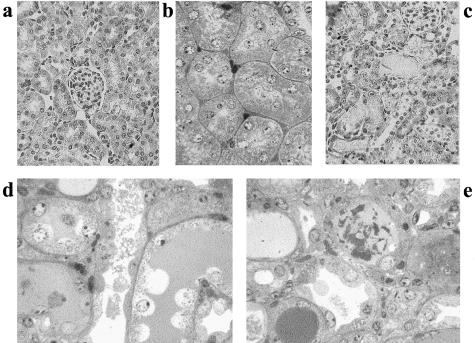
To evaluate the ability of CD133+ renal progenitor cells to localize in the injured kidney, we induced acute tubular injury by intramuscle injection of glycerol in SCID mice. Glycerol induced myolysis and hemolysis thereby exposing tissues, especially the kidney, to large amounts of myoglobin and hemoglobin.19,20 The peak of tubular injury was observed 3 days after glycerol injection. The morphological alterations observed included vacuolization and widespread necrosis of tubular epithelial cells and tubular hyaline cast formation (Figure 8). Proximal and distal tubules showed loss of brush border, cytoplasmic vacuolization, and flattening of epithelial cells with aspect of apparent denudation of tubular basal membrane (Figure 8, d and e). These lesions spontaneously recover after 10 days. Three days after glycerol injection, 106 CD133+ renal progenitor cells were intravenously injected and the mice were sacrificed after 3 days. To detect localization into the kidney of SCID mice of CD133+ renal progenitor cells in some experiments the cells were labeled with the green fluorescent dye PHK2, in others the unlabeled cells were traced by human HLA class I staining. As shown in Figure 9, a and c, human HLA class I-positive cells were detectable in proximal and distal tubules of the injured kidneys. Similar results were obtained with PHK2-labeled cells (not shown). Only rare human HLA class I-positive cells were observed in glomeruli (Figure 9a). No localization of injected renal progenitor cells was detectable into the renal medulla (not shown). When CD133+ renal progenitor cells were injected in control SCID mice without renal injury a minimal cell localization was observed in the kidney (Figure 9b). Some of the human HLA class I-positive cells also showed a positive staining for proliferating cell nuclear antigen, suggesting that once localized into tubules they may proliferate (Figure 9, d and e). In addition, the PHK2-labeled cells co-stained with cytokeratin suggesting an epithelial differentiation (Figure 9; f to h).
Figure 8.
Morphological alterations in glycerol-induced acute tubulonecrosis in SCID mice. a and b: Light micrograph showing normal renal tissue stained with H&E (a) and with toluidine blue. c–e: Tubulonecrotic injury observed 3 days after an intramuscular injection of glycerol (c, H&E-stained section; d and e, toluidine blue-stained semithin sections). Proximal and distal tubules showing loss of brush border, cytoplasmic vacuolization, and flattening of epithelial cells with aspect of apparent denudation of tubular basal membrane. Original magnifications: ×250 (a, c–e); ×400 (b).
Figure 9.
In vivo homing of renal tissue-derived CD133+ cells in the kidney of SCID mice with glycerol-induced acute tubulonecrosis. CD133+ cells were injected intravenously in SCID mice 3 days after an intramuscular treatment with glycerol (n = 6) or saline (n = 5). Mice were sacrificed 3 days after cell injection. a–c: Representative micrographs of kidney sections stained with anti-human HLA class I Ab and observed by confocal microscopy. Human HLA class I-positive cells were detected in proximal and distal tubules of mice with glycerol-induced acute tubulonecrosis injected with CD133+ cells (a and c) whereas only minimal positivity was observed in kidney without injury (b). Nuclei were counterstained in blue with Hoechst 33258 dye. d: Proliferating cell nuclear antigen staining of proliferating cells in a field correspondent to part of c. e: Merged image of c and d showing that some of the human HLA class I-positive cells were proliferating cell nuclear antigen-positive (arrows). f–h: Co-staining of cells within a tubule labeled with PHK2 green fluorescent dye before injection (f) and with cytokeratin (g). h: Merge. Original magnifications, ×400. G, glomerulus.
Discussion
In the present paper, we isolated and characterized CD133+ cells from normal adult human kidney. We show that renal tissue-derived CD133+ cells are multipotent progenitor cells, capable of expansion and potential self-renewal. These cells were competent to respond to local instructive cues, with the possible differentiation into epithelial or endothelial cells, both in vitro and in vivo, on xenograft in SCID mice. These cells lacked the expression of hematopoietic markers and expressed PAX-2, an embryonic renal marker, suggesting that they are resident renal stem cells. Finally, we show that in vitro expanded renal-derived CD133+ cells homed into injured, but not into normal kidneys when injected in SCID mice.
Adult stem cells have been isolated from several tissue sources, including the central nervous system,29 bone marrow,12 retina,30 skeletal muscle,31 and skin.32 Isolation and characterization of human adult renal stem cells has not been so far described. We identified the presence of small clusters of CD133+ cells within the interstitium of normal human kidney by immunohistochemistry and we immunomagnetically sorted and characterized these cells. CD133 antigen has been previously used to isolate stem cells from hematopoietic and nervous tissues.7,33 In hematopoietic tissues CD133 is expressed solely on CD34 bright stem and progenitor cells.34 In addition, CD133 was found on a subset of cells within both skeletal muscle and human neural tissue, the majority of which was also devoid of the hematopoietic marker CD45.35 Moreover, the discovery of alternative tissue-specific promoters may suggest the presence of different isoforms of CD133.36 We found that renal CD133+ cells were uniformly negative for the hematopoietic stem cell markers CD34 or CD45 and that they expressed PAX-2, a developmental renal marker,22 suggesting their renal origin. Alternatively, these cells may represent a bone marrow-derived population that has homed to the kidney through the circulation and has been resident long enough to have lost blood cell lineage markers. The expression of CD44, CD29, and CD73 may suggest a mesenchymal origin.12,21 The existence of mesenchymal stem cells in adult tissues was proposed by Caplan and Bruder.37 However, the cells isolated from kidney were strongly and persistently positive for CD133, a marker that is usually lacking in mesenchymal stem cells.38–40 Moreover, at variance of mesenchymal stem cells, renal CD133+ cells have limited differentiation capability.
Tissue stem cells preferentially generate differentiated cells of the same lineage as their tissue of origin: neural stem cells are biased to generate neurons and glia; bone marrow mesenchymal stem cells to generate mesodermal cell types; and hematopoietic stem cells to generate blood cells. However, several recent transplant studies indicate that at least a fraction of stem cells in these populations can generate cells of different embryonic lineages.10 We tested the capability of renal-derived CD133+ cells and of clones of individual cells to generate endothelial cells, epithelial cells, blood cells, and adipocytes. The results obtained indicate that these cells may differentiate into endothelial or epithelial cells, but not in blood cells or adipocytes. This observation suggests a partial commitment of renal CD133+ cells. The potential of kidney-derived stem cells to be highly proliferative and to generate cells with the phenotypic and functional features of endothelial and epithelial cells suggesting that these cells may be the source of the regenerative capability of the human kidney. Some acute and progressive renal diseases are characterized by a loss of the microvasculature that correlates with the development of glomerular and tubulointerstitial scarring.2 The maintenance of the microvasculature would thus seem to be critical for the recovery of acute glomerular injury41 and the prevention of progressive renal disease.2 We show that renal-derived CD133+ cells were able to differentiate in vitro in endothelial cells and to generate in vivo functional vessels. This observation suggests a potential involvement of these cells in the repair of vascular injury and in antagonizing the endothelial loss and the progression to renal failure.
At variance with the low regenerative potential of the endothelial compartment, tubular epithelial cell regeneration frequently occurs after tubulonecrotic injury.1 In this context, stem cells may participate to the epithelial cell regeneration. Several studies indicate a contribution of bone marrow-derived stem cells in tubular repair.42,43 Based on our results, we can speculate that resident CD133+ stem cells, being located in the interstitium in the proximity of tubules, may also contribute to renal repair. Indeed, renal-derived CD133+ cells and clones of individual cells differentiated in vitro into epithelial cells expressing some markers of renal proximal and distal epithelium, such as aminopeptidase A and the NaCl co-transporter. In addition, when injected subcutaneously in SCID mice these cells spontaneously formed epithelial tubular-like structures in Matrigel. These tubular structures expressed both cytokeratin and vimentin, markers of immature or regenerating tubular epithelial cells.23 In vivo, these cells expressed some markers of both distal tubules such as the NaCl co-transporter and calbindin-D and of proximal tubules such as alkaline phosphatase. However, the nonhomogeneous or focal expression of these markers probably reflects an immature phenotype. Moreover, the morphological aspects of the tubules developed subcutaneously in Matrigel are reminiscent of the renal structures because epithelial cells appeared polarized with apical microvilli and tight junctions. Finally, when renal-derived CD133+ cells were injected intravenously in SCID mice that developed glycerol-induced acute tubulonecrosis, we observed their renal homing and integration into some proximal and distal tubules. One can speculate that renal-derived CD133+ cells may contribute to the repair of renal injury either by regeneration of tissue structures or by a paracrine release of growth factors able to stimulate cell proliferation, survival, and differentiation of parenchymal cells, as described for the heart.44 The cell location and the expression of cytokeratin suggested their differentiation into tubular epithelial cells. Recent studies have also suggested the possibility that stem cells fuse with adult differentiated cells to repair damaged organs.45
In conclusion, the results of the present study demonstrate the presence in adult normal human kidney of a resident population of stem cells expressing CD133 marker and capable of expansion and potential self-renewal. These cells may respond to local environmental stimulation, with differentiation into endothelial or epithelial tubular cells, both in vitro and in vivo.
Acknowledgments
We thank Dr. Paola Cassoni for her helpful suggestions in the interpretation of morphology and Fiorella Sanavio for help in the in vivo experiments.
Footnotes
Address reprint requests to Dr. G. Camussi, Cattedra di Nefrologia, Dipartimento di Medicina Interna, Ospedale Maggiore S. Giovanni Battista, Corso Dogliotti 14, 10126, Torino, Italy. E-mail: giovanni.camussi@unito.it.
Supported by the Associazione Italiana per la Ricerca sul Cancro, the Italian Ministry of University and Research FIRB project (RBNE01HRS5-001) and COFIN, the Italian Ministry of Health (Ricerca Finalizzata 02), the Progetto S. Paolo (to G. C), and Italian Ministry of University and Research (MIUR) (ex60% to B.B.).
B.B. and S.B. contributed equally to this work.
References
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806–816. doi: 10.1681/ASN.V133806. [DOI] [PubMed] [Google Scholar]
- Al-Awqati Q, Oliver JA. Stem cells in the kidney. Kidney Int. 2002;61:387–395. doi: 10.1046/j.1523-1755.2002.00164.x. [DOI] [PubMed] [Google Scholar]
- Oliver JA, Barasch J, Yang J, Herzlinger D, Al-Awqati Q. Metanephric mesenchyme contains embryonic renal stem cells. Am J Physiol. 2002;283:F799–F809. doi: 10.1152/ajprenal.00375.2001. [DOI] [PubMed] [Google Scholar]
- Robert B, St. John PL, Abrahamson DR. Direct visualization of renal vascular morphogenesis in Flk1 heterozygous mutant mice. Am J Physiol. 1998;275:F164–F172. doi: 10.1152/ajprenal.1998.275.1.F164. [DOI] [PubMed] [Google Scholar]
- Sariola H, Peault B, LeDouarin N, Buck C, Dieterlen-Lievre F, Saxen L. Extracellular matrix and capillary ingrowth in interspecies chimeric kidneys. Cell Differ. 1984;15:433–451. doi: 10.1016/0045-6039(84)90028-9. [DOI] [PubMed] [Google Scholar]
- Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- Corbeil D, Roper K, Hellwig A, Tavian M, Miraglia S, Watt SM, Simmons PJ, Peault B, Buck DW, Huttner WB. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275:5512–5520. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Deambrosis I, Russo S, Deregibus MC, Camussi G. Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J. 2003;17:1159–1161. doi: 10.1096/fj.02-0557fje. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Mariano F, Biancone L, Foa R, David S, Cambi V, Camussi G. Interleukin-12 is synthesized by mesangial cells and stimulates platelet-activating factor synthesis, cytoskeletal reorganization, and cell shape change. Am J Pathol. 1999;154:623–632. doi: 10.1016/S0002-9440(10)65307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttiglieri S, Deregibus MC, Bravo S, Cassoni P, Chiarle R, Bussolati B, Camussi G. Role of Pax2 in apoptosis resistance and proinvasive phenotype of Kaposi’s sarcoma cells. J Biol Chem. 2004;279:4136–4143. doi: 10.1074/jbc.M306824200. [DOI] [PubMed] [Google Scholar]
- Muratovska A, Zhou C, He S, Goodyer P, Eccles MR. Paired-Box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene. 2003;22:7989–7997. doi: 10.1038/sj.onc.1206766. [DOI] [PubMed] [Google Scholar]
- Camussi G, Kerjaschki D, Gonda M, Nevins T, Rielle JC, Brentjens J, Andres G. Expression and modulation of surface antigens in cultured rat glomerular visceral epithelial cells. J Histochem Cytochem. 1989;37:1675–1687. doi: 10.1177/37.11.2809176. [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Villadsen R, Nielsen HL, Ronnov-Jessen L, Bissell MJ, Petersen OW. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 2002;16:693–706. doi: 10.1101/gad.952602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaldi PG, Biancone L, Bottelli A, Wade-Evans, Racusen LC, Orlandi V, Serra C, Camussi G, Toniolo A. HIV-1 kills renal tubular cells by a mechanism that involves fas and caspase-3 activation. J Clin Invest. 1998;102:2041–2049. doi: 10.1172/JCI3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath KA, Haggard JJ, Croatt AJ, Grande JP, Poss KD, Alam J. The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. Am J Pathol. 2000;156:1527–1535. doi: 10.1016/S0002-9440(10)65024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager RA, Andoh T, Bennett WM. Renal cholesterol accumulation. A durable response after acute and subacute renal insults. Am J Pathol. 2001;159:743–752. doi: 10.1016/S0002-9440(10)61745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry F, Boynton R, Murphy M, Haynesworth S, Zaia J. The SH-3 and SH-4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochem Biophys Res Commun. 2001;289:519–524. doi: 10.1006/bbrc.2001.6013. [DOI] [PubMed] [Google Scholar]
- Dressler GR, Douglass EC. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc Natl Acad Sci USA. 1992;89:1179–1183. doi: 10.1073/pnas.89.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R, Hage C, Thoenes W. Expression of intermediate filament proteins in fetal and adult human kidney: modulations of intermediate filament patterns during development and in damaged tissue. Lab Invest. 1991;65:74–86. [PubMed] [Google Scholar]
- Mentzel S, Dijkman HB, Van Son JP, Koenem RA, Assmann KJ. Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem. 1996;44:445–461. doi: 10.1177/44.5.8627002. [DOI] [PubMed] [Google Scholar]
- Baer PC, Tunn UW, Nunez G, Scherberich JE, Geiger H. Transdifferentiation of distal but not proximal tubular epithelial cells from human kidney in culture. Exp Nephrol. 1999;7:306–313. doi: 10.1159/000020618. [DOI] [PubMed] [Google Scholar]
- Biner HL, Arpin-Bott MP, Loffing J, Wang X, Knepper M, Hebert SC, Kaissling B. Human cortical distal nephron: distribution of electrolyte and water transport pathways. J Am Soc Nephrol. 2002;13:836–847. doi: 10.1681/ASN.V134836. [DOI] [PubMed] [Google Scholar]
- Diepens RJ, den Dekker E, Bens M, Weidema AF, Vandewalle A, Bindels RJ, Hoenderop JG. Characterization of a murine renal distal convoluted tubule cell line for the study of transcellular calcium transport. Am J Physiol. 2004;286:F483–F489. doi: 10.1152/ajprenal.00231.2003. [DOI] [PubMed] [Google Scholar]
- Grant DS, Tashiro K, Segui-Real B, Yamada Y, Martin GR, Kleinman HK. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989;58:933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- Bhatia M. AC133 expression in human stem cells. Leukemia. 2001;15:1685–1688. doi: 10.1038/sj.leu.2402255. [DOI] [PubMed] [Google Scholar]
- Shmelkov SV, Jun L, St. Clair R, McGarrigl D, Derderian CA, Usenko JK, Costa C, Zhang F, Guo X, Rafii S. Alternative promoters regulate transcription of the gene that encodes stem cell surface protein AC133. Blood. 2004;103:2055–2061. doi: 10.1182/blood-2003-06-1881. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368–374. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- Vogel W, Grunebach F, Messam CA, Kanz L, Brugger W, Buhring HJ. Heterogeneity among human bone marrow-derived mesenchymal stem cells and neural progenitor cells. Haematologica. 2003;88:126–133. [PubMed] [Google Scholar]
- Iruela-Arispe L, Gordon K, Hugo C, Duijvestijn AM, Claffey KP, Reilly M, Couser WG, Alpers CE, Johnson RJ. Participation of glomerular endothelial cells in the capillary repair of glomerulonephritis. Am J Pathol. 1995;147:1715–1727. [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002;6:1285–1290. doi: 10.1111/j.1523-1755.2002.kid569.x. [DOI] [PubMed] [Google Scholar]
- Kale S, Karihalooa A, Clark PE, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112:42–49. doi: 10.1172/JCI17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, Yang YZ, Pan C, Ge J, Phillips MI. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117:3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:485–487. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]