Abstract
Missense mutations of the tau gene cause autosomal dominant frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17), an illness characterized by progressive personality changes, dementia, and parkinsonism. There is prominent frontotemporal lobe atrophy of the brain accompanied by abundant tau accumulation with neurofibrillary tangles and neuronal cell loss. Using a hamster prion protein gene expression vector, we generated several independent lines of transgenic (Tg) mice expressing the longest form of the human four-repeat tau with the R406W mutation associated with FTDP-17. The TgTauR406W 21807 line showed tau accumulation beginning in the hippocampus and amygdala at 6 months of age, which subsequently spread to the cortices and subcortical areas. The accumulated tau was phosphorylated, ubiquitinated, conformationally changed, argyrophilic, and sarcosyl-insoluble. Activation of GSK-3β and astrocytic induction of mouse tau were observed. Astrogliosis and microgliosis correlated with prominent tau accumulation. Electron microscopic examination revealed the presence of straight filaments. Behavioral tests showed motor disturbances and progressive acquired memory loss between 10 to 12 months of age. These findings suggested that TgTauR406W mice would be a useful model in the study of frontotemporal dementia and other tauopathies such as Alzheimer’s disease (AD).
Frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) is a familial neurodegenerative disease characterized by autosomal dominant inheritance, personality change, progressive dementia, and parkinsonism. Extensive tau accumulation with neurofibrillary tangles (NFT) and loss of neurons are characteristic pathological changes and are associated with frontotemporal lobe atrophy.1 Following the initial discovery of missense mutations in the tau gene,2–4 numerous exonic and intronic mutations have been reported.5 The majority of mutations are clustered within or close to the microtubule (MT)-binding domains, or in the 5′-splice site of exon 10.5 Most of the exonic mutations lead to tau proteins with a decreased ability to promote MT assembly and an increase in self-aggregation.6 Some of the exonic, and all of the intronic, mutations cause an increase in the level of four-repeat tau.3,4 An increase in four-repeat tau is hypothesized to promote tau self-aggregation and decrease MT assembly.7 These gain-of-function effects have been suggested to cause tau accumulation leading to NFT formation and neuronal cell death.
Several neurodegenerative diseases that display tau accumulation, such as Alzheimer’s disease, frontotemporal dementia, Pick’s disease, progressive supranuclear palsy, and corticobasal degeneration are now classified as tauopathies.8 It is therefore important to clarify the mechanism by which mutant tau accumulates and leads to NFT formation and to determine whether or not there is a single common pathological pathway of tauopathies. Although pedigrees segregating the tau R406W mutation have a variety of clinical and pathological characteristics, consistent pathological features include frontotemporal atrophy, abundant tau accumulation, and neurofibrillary tangles containing both paired helical filaments and straight filaments.9–11 The tau R406W mutation has far a weaker effect on MT assembly-promoting activity than that of tau P301L and tau V337M mutations,6 and stable or transient transfection of tau R406W in non-neuronal cell lines showed that tau R406W was less phosphorylated than wild-type tau.12 While the sarcosyl-soluble tau R406W was less phosphorylated than sarcosyl-soluble wild-type tau, sarcosyl-insoluble tau R406W was as highly phosphorylated as the insoluble wild-type tau.13 These results suggested unique molecular effects of tau R406W on NFT formation. For this reason, we generated transgenic (Tg) mice expressing tau R406W (TgTauR406W). These mice were then followed using behavioral, neuropathological, and neurochemical methods. While the majority of these mice did not develop an overt behavioral or neuropathological phenotype, a small proportion of these mice (<20%) developed a behavioral, neuropathological, and neurochemical phenotype that displayed several features reminiscent of human tauopathies. The cause of this variation in expressivity/penetrance is being explored and remains unknown (possibly the effects of genetic background modifiers although environmental effects cannot yet be excluded). Nevertheless, in its most highly expressed form, the TgTauR406W mice developed an illness characterized by extensive accumulation of tau and subsequent alterations in the neocortex, hippocampus, and amygdala associated with motor and memory disturbances.
Materials and Methods
Transgene Construction, Generation of Transgenic Mice, and Analysis of RT-PCR
The longest isoform of wild-type human four-repeat tau cDNA containing a eukaryotic Kozak initiation sequence (GCCGCCACC)14,15 upstream of the start codon was ligated into the SalI restriction site of the cos.tet expression vector containing the Syrian hamster prion protein promoter gene,16 packaged in vitro and plated on E. coli DH1 to obtain a bacterial stock containing the recombinant cosmid clone. To generate the tau R406W mutation, wild-type human four-repeat tau cDNA was mutated by an oligonucleotide-mediated method with a proofreading DNA polymerase (“Quick change”, Stratagene, La Jolla, CA). Following confirmation of the site-directed mutagenesis by direct sequencing, the mutated tau R406W cDNA was reintroduced into the cos.tet expression vector. The transgenes were purified and microinjected into fertilized oocytes of FVB/N mice as previously described.17,18 Positive founders were subsequently bred with FVB wild-type mice and offspring were genotyped using a human tau cDNA fragment radiolabeled by the random-primer method.
To analyze gene expression of human tau, RT-PCR was performed using 2 μl of mRNA isolated using the QuickPrep Micro mRNA purification kit (Amersham Biosciences, UK) from brains of Tg mice (21807) and non-Tg mice brains at 6 and 10 months old (n = 3, respectively) in the reaction tube of Ready-To-Go RT-PCR Beads (Amersham Biosciences, UK) with PCR primers sets as follows: mouse tau exon 9F (5′-CACCAAAATCCGGAGAACGA-3′) and mouse exon 11R (5′-CTTTGCTCAGGTCCACCGGC-3′): human tau exon 9F (5′-CTCCAAAATCAGGGGATCGC-3′) and human tau exon 11R (5′-CCTTGCTCAGGTCAACTGGT-3′). PCR conditions included 30 cycles of 94°C for 30 seconds, 62°C for 30 seconds, 72°C for 45 seconds with a final 72°C extension phase for 10 minutes, as in a previous report.19 For semi-quantification, RT-PCR of β-actin was performed as internal control.20 Ten μl of PCR products were analyzed by 2.5% agarose gel elecrophoresis. The intensity of ethidium-stained bands was analyzed by Scion Image (Scion Corporation, USA).
Transgenic founders were used to create Tg mice lines bearing the tau R406W mutation: TgTauR406W 21807, TgTauR406W 21783, and TgTauR406W 21768. Twenty-one positive Tg progenies, 16 F1 Tg and 98 F2 Tg were analyzed. The TgTauR406W 21807 line (n = 11) was used for the rotarod and passive avoidance tests. All animal experiments were performed according to guidelines established in the “Guide for the Care and Use of Laboratory Animals.”
Antibodies
The following antibodies were used: two human tau-specific antibodies, anti-tau154 (1:200; antibody to synthetic amino acids of 154–168 of human tau 441), E1 (1:1000)21 and two human and mouse tau antibodies, PHF-1 (1:100, kindly provided by Dr. P. Davies, Albert Einstein College of Medicine),22 and anti-tau-C against C-terminal tau (1:200, 422–438 amino acid of human tau 441),23 a conformation-dependent tau antibody Alz-50 (1:100, kindly provided by Dr. P Davies);24 an anti-glial fibrillary acidic protein antibody (GFAP, 1:20,000, DAKO, Denmark), an anti-microglia antibody (F4/80, 1:20, BMA Biomedicals, Switzerland), an anti-ubiquitin antibody (1:500, kindly provided by Dr. D. Dickson, Mayo Clinic Jacksonville); phosphorylation-site specific tau antibodies: anti-PS199 (phosphorylated serine 199, 1:500),25,26 CP13 (phosphorylated at serine 202, 1:100, kindly provided by Dr. P. Davies),27 AT8 (phosphorylated serine 202/threonine 205, 1:2000),28 anti-PT205 (phosphorylated threonine 205, 1:100),26 anti-PT231/PS235 (phosphorylated threonine 231/serine 235, 1:500),26 anti-PS396 (phosphorylated serine 396, 1:500)26 and anti-PS413 (phosphorylated serine 413, 1:100)26; antibodies against kinase for tau phosphorylation: anti-glycogen synthase kinase-3α (anti-GSK-3α, 1:50),25,26 anti-GSK-3β (1:100),25,26 anti-PY216 (anti-activated GSK-3β, 1:250),25,26,29 anti-PS9 (anti-inactive GSK-3β, 1:25),25,26 anti-Cdk5 antibody (anti-cyclin-dependent kinase 5, 1:100)25,26 and anti-MAPK (anti-mitogen-activated protein kinase, 1:250).25,26
Tissue Preparation and Staining
After mice were sacrificed under ether anesthesia, brains were removed and cut sagittally at the midline. One hemisphere was fixed in 4% paraformaldehyde with 0.1 mol/L phosphate buffer (PB, pH 7.6) for 1 week and embedded in paraffin. Five μm-thick sections were prepared for staining. Sections were immersed in 0.5% periodic acid and treated with 99% formic acid for 3 minutes for tau immunostaining. After blocking with 5% normal goat serum in 50 mmol/L phosphate-buffered saline (PBS) containing 0.05% Tween 20 and 4% Block Ace (Snow Brand, Japan), sections were incubated for 6 hours with the primary antibodies. The specific labeling was visualized using a Vectastain Elite ABC kit (Vector Labs, Burlingame, CA). These tissue sections were counterstained with hematoxylin. Conventional Gallyas-Braak silver staining for NFT and Nissl staining for neuronal cell counts were performed. We adopted the terminology defined based on an atlas of the mouse brain.30 The number of neurons/mm2 was counted in three Nissl-stained 5-mm sections from the piriform cortex and amygdala of 11 Tg mice, including no. 8859 and no. 9731, and 11 age-matched 10-month-old non-Tg mice.
Electron Microscopic Study
The brain tissues were immersed in a fixative solution (2.5% glutaraldehyde, 0.1 mol/L phosphate buffer, pH 7.4) for 4 hours and washed several times in 0.1 mol/L PB containing 7% sucrose. Blocks were then post-fixed in 2% osmium tetroxide, dehydrated in ethanol and propylene oxide, and embedded in Quetol 812 (Nisshin EM, Japan). Ultra-thin sections were stained with uranyl acetate and lead acetate before observation with an electron microscope.
For immuno-electron microscopic study, PBS-washed pellets, after sarcosyl extraction of Tg mice brains, were applied to carbon-coated 400 mesh EM grids (VECO, Holland). The samples were incubated with an anti-tau154 antibody at room temperature for 3 hours. Each specimen was washed with PBS and incubated with 12-nm colloidal gold conjugated anti-rabbit IgG (Jackson Immunoresearch Labs, PA) at room temperature for 1 hour, then examined by electron microscopy (EM).
Western Blot Analysis
Half of the brains from Tg or non-Tg mice at 5 and 13 months of age were weighed and homogenized using a Teflon-homogenizer in nine volumes of Tris-saline buffer (TS) with protease inhibitors (TS inhibitors: 50 mmol/L Tris-HCl and 150 mmol/L NaCl, pH 7.6, 0.5 mmol/L DIFP, 0.5 mmol/L PMSF, 1 μg/ml TLCK, 1 μg/ml antipain, 1 μg/ml leupeptin, 0.1 μg/ml pepstatin, 1 mmol/L EGTA). The homogenate was centrifuged at 55,000 rpm for 60 minutes at 4°C and the supernatant was analyzed as the TS-soluble fraction. Then, the pellets were homogenized again in four volumes of 1% sarcosyl in TS inhibitors, incubated on ice for 30 minutes, and centrifuged at 55,000 rpm for 60 minutes at 4°C. The supernatant and pellet were analyzed as sarcosyl-soluble or sarcosyl-insoluble fractions, respectively. Each 2 μl of sample was boiled at 70°C in four volumes of sodium dodecylsulfate (SDS) sample buffer and separated on 4 to 12% NuPAGE Bis-Tris Gel (Invitrogen Corp., Carlsbad, CA). The signal intensity was detected using the ECL-Plus system (Amersham Bioscience Corp., NJ) and a luminoimage analyzer (LAS 1000-Mini, Fuji Film, Tokyo).
Rotarod Test
Tg mice (n = 11) and age-matched non-Tg control mice (n = 11) at 10 and 12 months old were assessed for how long they could stay on a rotating rod treadmill apparatus (Ugo Basile, Biological Research Apparatus, Milan, Italy). Mice were placed on the rod rotating at a speed of 16 rpm for 30 seconds, and the time they stayed on the rotating rod was measured. The trial was performed three times and then repeated three additional times after 10 minutes of rest for every mouse. Statistical analysis was conducted by the Mann-Whitney test.
Step-Through Passive Avoidance Test
Tg mice (n = 11) and age-matched non-Tg control mice (n = 11) at 10 months of age were examined. The apparatus (AP model, O’Hara Co., Tokyo, Japan) for the step-through passive avoidance test consisted of two compartments; one was illuminated [light at the top of the compartment (27 watt, 3000 lux)] and the other was a dark compartment. After the mouse was placed in the illuminated safe compartment, the compartment was lit, and the mouse stepped through an open guillotine door into the dark compartment. The time spent in the illuminated compartment was defined as the latency time. Three seconds after the mouse entered the dark compartment, a foot-shock (0.3 mA, 50 V, 50 Hz AC, for 3 seconds) was given. The retention of avoidance memory trials was carried out once a week for 9 weeks after 5 days of serial acquisition trials. The retention latency time was measured for up to 300 seconds without delivering a foot-shock.31,32 Statistical analysis was performed by a two-way repeated measure analysis of variance (SPSS Version 11).
Results
Progression and Distribution of Tau Accumulation in Brains of Tg Mice
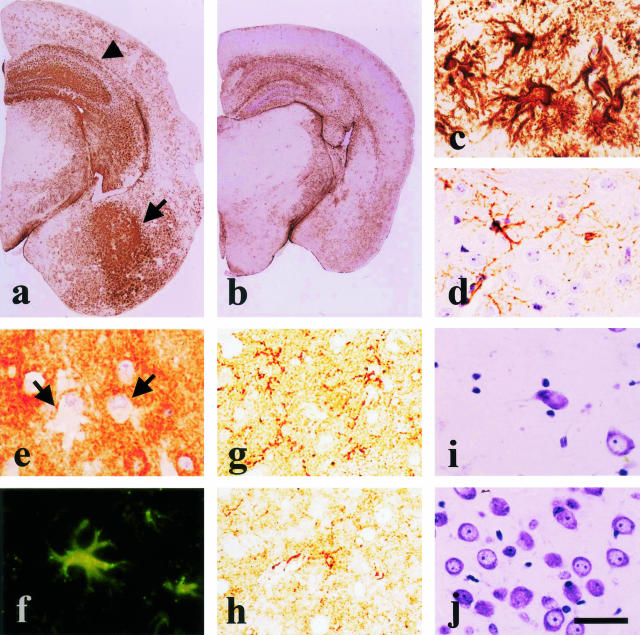
At 3 months of age (n = 5), there was no detectable accumulation of human tau by immunocytochemistry. At 6 months of age (n = 5), tau 154 labeled some neurons and processes in the dentate gyrus of the hippocampus (Figure 1a, arrowhead) and neurons in the amygdala (Figure 1a, arrow). At 8 months of age (n = 5), tau deposition was observed in the cell bodies and processes of neurons of the neocortex, hippocampus, and amygdala (Figure 1b). At 14 months old (n = 4), accumulation of tau protein progressively extended to the caudate putamen, white matter, and cerebellar cortex (Figure 1c). This tau immunoreactivity was enhanced by formic acid pretreatment. Human tau was not detected in the brains of non-Tg control mice at 14 months of age (Figure 1d).
Figure 1.
Progress and distribution of human tau accumulation. In 6-month-old TgTauR406W 21807 mice, human tau is detected in the hippocampus and the amygdala (arrowhead ▴, hippocampus; arrow →, amygdala) (a). Accumulation of human tau has spread to the neocortex, which corresponded to the neocortex in mouse pathology, and caudate putamen, in addition to the hippocampus at 8 months of age (b). In 14-month-old TgTauR406W 21807 mice, human tau is widely accumulated in the neocortex and subcortical regions (c). No human tau accumulation is found in age-matched non-Tg mice brains (d and f). Tau accumulated in the cell bodies (e, neocortex; g, anterior horn cells) and processes (h) of neuronal cells. Accumulation of human tau is prominent in the neocortex and the piriform cortex (i to k), hippocampus (k and l), amygdala (l), cerebellum (m), and spinal cord (n and o) at 10 months of age. Anti-tau154 antibody staining. Bar, 0.5 mm (a–d and i–o); 12.5 μm (e–h).
Accumulation of human tau was intensely observed in the neocortex and the piriform cortex, amygdala, and hippocampus in 10-month-old Tg mice brains (n = 13) (Figure 1, i to k). In the neocortex, accumulation of tau was prominent in layer II and IV-VI (Figure 1k). Moderate accumulation of tau was observed in the entorhinal cortex, caudate putamen, anterior and posterior horn of the spinal cord, brainstem and cortex of the cerebellum (Figure 1, l to o). The staining of tau in the olfactory bulb, thalamus, and hypothalamus was weak (Figure 1, i to l). Tau was also observed in subcortical areas, such as the corpus callosum, internal capsule, white matter of the cerebellum, and white matter of the spinal cord. No atrophy was observed in skeletal muscles. The accumulated tau localized predominantly in the cell bodies and processes of the neocortical neurons (Figure 1e). Granular dot-like stainings were frequently observed all around the neurons (Figure 1e). No human tau was observed in non-Tg control mice (Figure 1f). Tau accumulated in the cell bodies and dendrites of neurons in the anterior horn of the spinal cord (Figure 1g) and in neuronal processes in the neocortex (Figure 1h).
Reactive Changes following Tau Accumulation in Tg Mice Brains
In 10-month-old Tg mice, GFAP immunostaining demonstrated marked astrogliosis in the neocortex, amygdala (Figure 2a, arrow), and hippocampus (Figure 2a, arrowhead) compared with non-Tg mice (Figure 2b). Numerous large GFAP-positive bizarre astrocytes were prominent in the amygdala and hippocampus (Figure 2c). These reactive astrocytes were not detected in non-Tg control mice (Figure 2d), and they were not stained with anti-tau 154 (Figure 2e, arrow). However, anti-tau-C antibody labeled these astrocytes (Figure 2f, arrow), suggesting that intrinsic murine tau was induced in these glial cells. F4/80 staining showed more prominent microgliosis in the neocortex, amygdala and hippocampus of Tg mice (Figure 2g) compared with non-Tg control mice (Figure 2h). In a TgTauR406W 21807 (no. 8859) mouse that presented with rapidly progressive and severe akinesia, and which had the most severe pathological alterations, there was a subjective impression that number of neuronal cells was decreased in the amygdala (Figure 2i) and the piriform cortex in comparison with those of non-Tg control mice (Figure 2j).
Figure 2.
Reactive changes. In TgTauR406W 21807 mice, extensive astrogliosis is detected in the hippocampus, amygdala and neocortex (arrowhead ▴, hippocampus; arrow →, amygdala) (a) compared with non-Tg controls (b). At high magnification of the amygdala (a), numerous large bizarre astrocytes are shown in the Tg mice (c), but not in the non-Tg mice brains (d). In the bizzare astrocytes, human tau R406W is not observed (→) (e). However, these astrocytes express endogenous mouse tau (→) (f). Microgliosis is more prominent in the Tg mice brains (g) compared with the brain of a control mouse (h). In the amygdala of 21807 mice, the number of neuronal cells is decreased (i) compared with the non-Tg mice brains (j). Bar, 1 mm (a and b); 50 μm (c–j).
Although formal stereological cell counts have not been performed, there did not appear to be any massive and consistent patterns of neuronal cell loss. A subset of Tg mice appeared to have reduced neuronal cell number that did not reach significance when overall quantification of neurons was performed. However, no Aβ amyloid deposition was induced in the brains of Tg mice (data not shown).
Phosphorylation and Ubiquitination of Accumulated Tau in Tg Mice Brains
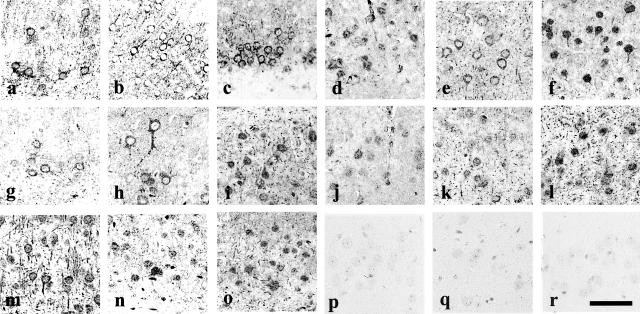
To investigate whether the accumulated tau was phosphorylated and ubiquitinated, we analyzed 10-month-old Tg mice brains by immunocytochemistry using several antibodies, which identify the PHF of Alzheimer’s disease.33 The accumulated human tau R406W in the cell bodies and neuronal processes of the piriform cortices was labeled by the human tau-specific antibodies anti-tau 154 (Figure 3a) and E1 (Figure 3b). PHF-1 (Figure 3c) and antibody CP13 (Figure 3d) labeled cell bodies mainly. Alz-50 (Figure 3e) similarly immunostained neuronal cell bodies and processes. The anti-ubiquitin antibody (Figure 3f) labeled cell bodies with nuclear staining and processes. Phosphorylation site-specific tau antibodies, PS199, AT8(Ser202/Thr205), PT205, PT231/PS235, PS396, and PS413 similarly labeled neuronal cell bodies and processes (Figure 3, g to l). These findings suggested that the accumulated tau was phosphorylated and ubiquitinated as is seen in PHF tau in Alzheimer’s disease. To investigate what kind of kinase was activated in forming phosphorylated tau in Tg mice, kinases associated with PHF tau were examined. Anti-GSK-3β labeled the axons, dendrites, and cell bodies of neuronal cells in neocortices and the hippocampus area (Figure 3m). Anti-PY216 labeled the cell bodies of neuronal cells (Figure 3n). The anti-Cdk5 antibody labeled the processes and cell bodies of neuronal cells (Figure 3o), while non-Tg mice did not show any such immunostaining (not shown). Anti-PS9, anti-GSK-3α, and anti-MAPK did not label any neuronal cells in either Tg or non-Tg mice (Figure 3, p to r). These findings suggested that activated GSK-3β and Cdk5 were major kinases for phosphorylation of accumulated tau in this Tg mouse.
Figure 3.
Phosphorylation of accumulated tau. In the Tg mice brains, anti-tau154 antibody-labeled tau accumulations are seen in neurons and processes and as small round dot-like stains (a). These structures are labeled by both E1 (b) and PHF-1 antibodies (c). Antibody CP13 (d), Alz-50 (e) labeled neuronal cell bodies and dendrites. Anti-ubiquitin antibody stained neuronal cell bodies and dendrites (f). Phosphorylated tau antibodies PS199, AT8 and PT205, PT231/PS235, PS396, PS413 labeled neuronal cell bodies and dendrites (g–l). Anti-GSK-3β antibody labeled cell bodies of neurons (m). Anti-PY216 antibody against activated GSK-3β immunostained cell bodies of neurons (n), which are also stained by Cdk5 (o). Neither anti-PS9 against non-activated GSK-3β, anti-GSK-3α, nor anti-MAPK labeled these neurons (p to r). Bar, 50 μm.
Gallyas-Braak Staining
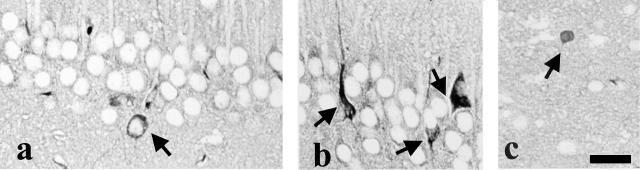
To detect the formation of neurofibrillary tangles, Gallyas-Braak staining was performed. This staining labeled occasional cell bodies and the processes of the pyramidal cells of the hippocampus (Figure 4, a and b, arrow) and amygdala (Figure 4c, arrow) in 14-month-old Tg mice.
Figure 4.
Gallyas-Braak staining. In 14-month-old Tau tauR406W 21807 mice, some granular cells of the hippocampus (a and b, arrow →) and neurons in the amygdala (c, →) are labeled by Gallyas-Braak staining. Bar, 20 μm.
Electron Microscopic Study
EM studies of the piriform cortex revealed massive filaments in the cell bodies of neurons (Figure 5a). Other aggregates were observed near the nucleus of neurons in the amygdala (Figure 5b). At higher magnifications, these filaments were straight and their diameters were 15 to 30 nm (Figure 5c). These filaments were present in the sarcosyl-insoluble fraction by negative staining (Figure 5d). By immunogold EM, these filaments were labeled by the anti-tau154 antibody (Figure 5e), suggesting that they consisted of accumulated human tau R406W.
Figure 5.
Electron microscopic study. Numerous filamentous aggregates are detected in the cell bodies near the nucleus of neurons in the piriform cortex (a, ×27,000). Other filamentous aggregates were observed near the nucleus of neurons in the amygdala (b, ×11,900). At higher magnification (c, ×93,000), and following negative staining of sarcosyl-insoluble fractions (d, ×130,000), 15- to 30-nm straight filaments are observed. Immunogold staining using anti-tau154 confirmed that these were aggregated tau filaments in the sarcosyl-insoluble fraction (e, ×130,000). Bar, 330 nm (a), 750 nm (b), 97 nm (c), and 69 nm (d and e).
Analyses of RT-PCR Transcripts and Western Blot
RT-PCR showed that expression of human tau mRNA transcripts (exon 9–11) was detected only in Tg mice brains, but not in non-Tg mice brains, and revealed that the level of transcription was equivalent at 6 months and 10 months of age (Figure 6a, upper panel). The expression of mouse tau mRNA transcripts was detected in both Tg mice brains and non-Tg mice brains (Figure 6a, lower panel). Semi-quantification of RT-PCR showed that the expression level of human tau R406W mRNA transcript was 119% at 6 months old and 121% at 10 months old compared to the levels of endogenous mouse tau.
Figure 6.
Analyses of RT-PCR transcripts and Western blot. Human tau mRNA transcripts (exon 9–11) were detected as 390 bp only in Tg mice brains, but not in non-Tg mice brains, and the intensity of PCR products was at the same level as 6- and 10-month-old Tg mice brains (a, upper panel). Mouse tau mRNA transcripts were detected in both Tg and non-Tg mice brains at the same level at 6 and 10 months of age. The signal intensities of the bands of the human tau mRNA transcripts expressed more than those of mouse tau (a, lower panel). The expression level of human tau R406W in a TS-soluble fraction was relatively higher in cerebral cortical areas, the hippocampus, caudate putamen, thalamus and cerebellum, in addition to the brainstem-spinal cord (b, upper panel). The expression of TS-soluble tau is not observed in non-Tg control mice (b, lower panel). In the TS-soluble fraction of the brain, the expression rate of total tau protein detected by antibody tau-C is 1.8 times higher in the Tg mouse at 6 months of age compared with an age-matched non-Tg mouse (c, upper panel). A Western blot using AT8 showed that tau in a TS-soluble fraction of Tg mice was phosphorylated (c, lower panel). In two 10-month-old TgTau(R406W) brains (Tg1, 21807 and Tg2, 21783), accumulated tauR406W was recognized in the sarcosyl-insoluble fractions and corresponded to the highest 67-kd band of recombinant tau 6 isoforms. Smear tau was detected in the brain of an AD patient; however, no accumulation of sarcosyl-insoluble tau is observed in non-Tg control mice (d). Accumulated tau was detected by PHF-1, Alz-50, and AT8, which corresponded to the highest 67 kd band of recombinant tau 6 isoforms (e, upper panel). Several phosphorylated tau antibodies showed a 67-kd band corresponding to the longest tau isoform, and accumulated tau was phosphorylated at several sites of human tau (PS199, PT205, PT231/PS235, PS396, PS413) (e, middle panel). GSK-3β, especially activated GSK-3β (PY216), showed high accumulation to 48kD in Tg mice brains. On the contrary, PS9, an inactivated GSK-3β antibody, did not show any bands (e, lower panel).
Tris-saline (TS)-soluble human tau distributed in all neocortical regions, the hippocampus, caudate putamen, and thalamus, in addition to the brainstem-spinal cord in 5-month-old Tg mice (Figure 6b). In the TS-soluble fraction, the level of total tau protein detected by antibody tau-C was 1.8 times higher than age-matched non-Tg control mice, suggesting that the expression level of human tau R406W was about 80% that of endogenous mouse total tau protein (Figure 6c, upper panel). The Western blot findings by AT8 showed tau in the TS-soluble fractions of Tg mice was phosphorylated (Figure 6c, lower panel). Accumulated tau R406W was recognized in sarcosyl-insoluble fractions and corresponded to the highest 67-kd band of recombinant human tau 6 isoforms 33,34 (Figure 6d). The accumulation of TS-soluble or sarcosyl-insoluble tau was not observed in non-Tg control mice (Figure 6, b to d). The Western blot with AT8, Alz-50, and PHF-1 showed a band at 67 kd in the sarcosyl-insoluble fractions of Tg mice brains (Figure 6e, upper panel). Accumulated tau was phosphorylated at serine 199, threonine 205, serine 202/threonine 205, threonine 231/serine 235, serine 396, and serine 413 in Tg mice brains (Figure 6e, middle panel). The anti-GSK-3β antibody and anti-PY216 showed a 48-kd size band. Anti-PS9 did not show any bands (Figure 6e, lower panel).
Rotarod and Step-Through Passive Avoidance Tests
There was no significant difference in rotarod test between 5-month-old Tg and non-Tg mice. The rotarod test revealed a significant decrease in retention time on the rotating rod in 10-month-old (P < 0.0001) and 12-month-old Tg mice (P < 0.0001) compared with their age-matched non-Tg mice littermates. However, there was no significant difference in performance between 10- and 12-month-old Tg mice (Figure 7a).
Figure 7.
Behavioral examination. a: Rotarod test. There was no significant difference in rotarod test between 5-month-old Tg and non-Tg mice. The retention time of Tg mice (n = 11) and non-Tg mice (n = 11) on a rotoring rod is significantly decreased in Tg mice at 10 and 12 months of age compared with non-Tg mice (P < 0.0001). b: Step-through passive avoidance test. Learning stages of Tg mice (closed circle) and non-Tg mice (open circle) at 10 months of age were not significantly different for 5 days (left). The latency time of Tg mice became shorter than that of non-Tg mice from 2.5 weeks and a significant decrease was shown from 7 weeks (P < 0.05) and from 8 to 10 weeks (P < 0.001) by two-way repeated measure analysis of variance.
The step-through passive avoidance test at 10 months of age revealed that both the Tg and non-Tg control mice learned to avoid electrical stimuli in the dark compartment within five serial trials in 1 week, with no significant difference during the learning phase. However, the retention time for the avoidance reaction in the Tg mice began to decrease 7 weeks later. This behavioral disturbance continued to worsen during the subsequent eighth, ninth, and tenth weeks compared with non-Tg control mice (P < 0.0001) (Figure 7b).
Discussion
Several groups have reported wild-type tau Tg mice35–39 and mutant-type tau Tg mice.40–44 Tg mice expressing the wild-type of four-repeat human tau have exhibited somatodendritic localization and hyperphosphorylation of tau,35 and prominent axonal tau accumulation in the brain and spinal cord. The shortest human three-repeat tau showed age-dependent emergence, and progression of tauopathy with Congophilic or Gallyas-Braak stain-positive, NFT-like intraneuronal inclusions in the brainstem and spinal cord.36,37 Tau P301L Tg mice exhibited neurofibrillary tangles and neuronal loss in the anterior horn of the spinal cord.40,41 Tau V337M Tg mice were reported to form phosphorylated and ubiquitinated tau aggregations labeled by Congo red.42 Tau P301S Tg mice also showed neuronal loss in the spinal cord, presenting with abundant filaments consisting of hyperphosphorylated tau protein.43 Tau R406W Tg mice under a CaMKII promoter showed Congophilic and argyrophilic tau inclusion in hippocampal neurons, and impaired associative memory during cued and contexual tests.44
Compared with these mice, TgTauR406W mice showed a unique distribution of tau accumulation initiating from neuronal processes to the cell bodies of neurons in the hippocampus, amygdala, and neocortices, which was accompanied by astrocytosis and microgliosis. The accumulated tau was ubiquitinated and was phosphorylated at serine 199, serine 202, threonine 205, threonine 231/serine 235, serine 396, and serine 413. These sites are also phosphorylated in Alzheimer’s disease.45 In FTDP-17 patients with tau R406W, the accumulated tau is phosphorylated at serine 202, threonine 205, 231, and threonine 396.10–12 Recently, in immortalized mouse cortical cells that express low levels of endogenous tau and which were stably transfected with human tau R406W or wild-type human tau, the tau R406W was more highly phosphorylated at numerous epitopes, and showed decreased microtubule binding compared to wild-type human tau.46 The accumulated tau R406W in our Tg mouse model therefore has phosphorylated sites in common with those observed in FTDP-179–13 and Alzheimer’s disease.23,47
The expression level of tau R406W was about 80% that of the endogenous mouse tau levels by Western blot analysis. The human tau was expressed at relatively similar levels in all neocortical regions of the mice brains. Human tau R406W was clearly detected in the sarcosyl-insoluble fraction in Tg mice brains. Phosphorylated tau observed by immunostaining was revealed to accumulate in sarcosyl-insoluble fractions by Western blot.
The antibody to activated GSK-3β (PY216) detected prominent activated GSK-3β in TgTauR406W mice brains by immunocytochemistry. These findings were in agreement with similar results from Western blot analysis of the sarcosyl-insoluble fraction, indicating the close biochemical relationship between tau accumulation and the activity of GSK-3β in Tg mice brains, which is likely to occur in the brains of FTDP-17 patients with tau R406W 9–13 and tauopathies including AD.23,47
EM analysis showed straight 15- to 30-nm filaments in the cell bodies of neurons composed of expressed human mutant tau R406W. FTDP-17 patients with tau R406W showed both paired helical filaments (with a maximum diameter of 20 to 24 nm and with periodic constrictions at 70- to 80-nm intervals) and a few straight filaments of about 12-nm diameter.9 These filaments were located in sarcosyl-insoluble fractions.10 In the AD brain, paired helical filaments with a diameter of 8 to 20 nm and straight filaments were also typically recovered from the sarcosyl-insoluble fraction. However, although the distribution and characteristics of the accumulated tau in TgTauR406W mice brains resembled those in Dutch patients with the same FTDP-17 (tau R406W) (Dutch 4 family),10 we did not observe PHFs, but rather straight filaments, in Tg mice brains. It is conceivable that this may have arisen because our mutant transgene expressed only a single isoforms of tau. It will be necessary to undertake additional studies with transgenes allowing alternative splicing of exons 2, 3, and 10 to determine whether accumulation of all six isoforms of tau R406W can cause the typical PHF as seen in AD patients.
A prominent pathological findings in the brains of patients with the TauR406W mutation has been reactive astrocytosis and microgliosis.9–11 We observed prominent astrocytosis in the neocortex, amygdala, and hippocampus. Interestingly, these reactive astrocytes expressed endogenous murine tau in their cell bodies. These findings suggest that accumulated mutant human tau induced reactive astrocytosis and microgliosis, and may then exacerbate the tauopathy by further modulating the metabolism of endogenous tau. Recent studies reported the appearance of filamentous tau in oligodendrocytes and astrocytes in JNPL3 lines expressing mutant tau P301L using a murine prion promoter.48
In addition to pathological and biochemical findings, TgTauR406W mice demonstrated aberrant behavior. The decreased retention time in the rotarod test was due to a slow movement or a slow response causing postural instability on the rotating rod. The slow response, slow movement, and paucity of mobility resemble the parkinsonian motor deficits observed in some FTDP-17 patients, and suggests that tau accumulation in subcortical areas, especially the caudate and putamen in Tg mice brains, may cause these behavioral abnormalities. A motor deficit detected by the rotarod test started from at least 10 months and was also detected at 12 months of age (P < 0.005). In the passive avoidance test, TgTauR406W mice failed to remember the noxious stimulus and moved actively from the light compartment into the dark compartment. This selective loss of acquired memory in TgTauR406W mice may have been caused by massive accumulation of phosphorylated, conformationally changed, and insoluble aggregated tau in neurons and their processes.
Taken together, all these findings indicate that the present animal model reproduced many of the principal neuropathological, biochemical, and behavioral features of FTDP-17 with the tau R406W mutation, not achieved by expressing tagged tau,44 indicating the first convincing R406W tau mouse model. As a result, these TgTauR406W mice may be useful for investigating the pathogenesis of not only FTDP-17, but also other tauopathies including Alzheimer’s disease,49,50 and for developing possible therapeutic agents.
Acknowledgments
We thank Dr. M. Morishima-Kawashima and Dr. Y. Ihara for technical advice, Dr. P. Davies and Dr. D. Dickson for provision of antibodies, and Dr. M. Goedert and Dr. T. Bird for valuable discussions.
Footnotes
Supported by Scientific Research on Priority Areas (C)-Advanced Brain Science Project-(M.I. no. 15016018) and Grants-in-Aid for Scientific Research (C) (2) (M.I. no. 15590879), Grants-in-Aid for Primary Amyloidosis Research Committee (M.S.), and by Grants-in-Aid for Scientific Research (B) (M.S. no. 16390251) and Scientific Research on Priority Areas (C)-Advanced Brain Science Project-(M.S.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, the Canadian Institutes for Health Research, the Howard Hughes Medical Institute, the Canadian Genetic Diseases Network, and the Alzheimer Society of Ontario (P.S.G.-H.).
References
- Foster NL, Wilhelmsen K, Sima AA, Jones MZ, D’Amato CJ, Gilman S. Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Ann Neurol. 1997;41:706–715. doi: 10.1002/ana.410410606. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LA, Wszolek ZK, Hutton M. Phenotypic correlations in FTDP-17. Neurobiol Aging. 2001;22:89–107. doi: 10.1016/s0197-4580(00)00202-5. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Smith MJ, Goedert M. Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett. 1998;437:207–210. doi: 10.1016/s0014-5793(98)01217-4. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M, Crowther RA, Murrell JR, Farlow MR, Ghetti B. Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci USA. 1997;94:4113–4118. doi: 10.1073/pnas.94.8.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 1998;21:428–433. doi: 10.1016/s0166-2236(98)01337-x. [DOI] [PubMed] [Google Scholar]
- Reed LA, Grabowski TJ, Schmidt ML, Morris JC, Goate A, Solodkin A, Van Hoesen GW, Schelper RL, Talbot CJ, Wragg MA, Trojanowski JQ. Autosomal dominant dementia with widespread neurofibrillary tangles. Ann Neurol. 1997;42:564–572. doi: 10.1002/ana.410420406. [DOI] [PubMed] [Google Scholar]
- van Swieten JC, Stevens M, Rosso SM, Rizzu P, Joosse M, de Koning I, Kamphorst W, Ravid R, Spillantini MG, Niermeijer MF, Heutink P. Phenotypic variation in hereditary frontotemporal dementia with tau mutations. Ann Neurol. 1999;46:617–626. doi: 10.1002/1531-8249(199910)46:4<617::aid-ana10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Saito Y, Geyer A, Sasaki R, Kuzuhara S, Nanba E, Miyasaka T, Suzuki K, Murayama S. Early-onset, rapidly progressive familial tauopathy with R406W mutation. Neurology. 2002;58:811–813. doi: 10.1212/wnl.58.5.811. [DOI] [PubMed] [Google Scholar]
- Matsumura N, Yamazaki T, Ihara Y. Stable expression in Chinese hamster ovary cells of mutated tau genes causing frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). Am J Pathol. 1999;154:1649–1656. doi: 10.1016/S0002-9440(10)65420-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka T, Morishima-Kawashima M, Ravid R, Heutink P, van Swieten JC, Nagashima K, Ihara Y. Molecular analysis of mutant and wild-type tau deposited in the brain affected by the FTDP-17 R406W mutation. Am J Pathol. 2001;158:373–379. doi: 10.1016/S0002-9440(10)63979-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984;12:857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991;115:887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MR, Kohler R, Foster D, Prusiner SB. Chimeric prion protein expression in cultured cells and transgenic mice. Protein Sci. 1992;1:986–997. doi: 10.1002/pro.5560010804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St. George Hyslop P, Selkoe DJ. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- Duff K, Knight H, Refolo LM, Sanders S, Yu X, Picciano M, Malester B, Hutton M, Adamson J, Goedert M, Burki K, Davies P. Characterization of pathology in transgenic mice overexpressing human genomic and cDNA tau transgenes. Neurobiol Dis. 2000;7:87–98. doi: 10.1006/nbdi.1999.0279. [DOI] [PubMed] [Google Scholar]
- Elliott JL. Cytokine upregulation in a murine model of familial amyotrophic lateral sclerosis. Brain Res Mol Brain Res. 2001;95:172–178. doi: 10.1016/s0169-328x(01)00242-x. [DOI] [PubMed] [Google Scholar]
- Crowe A, Ksiezak-Reding H, Liu WK, Dickson DW, Yen SH. The N terminal region of human tau is present in Alzheimer’s disease protein A68 and is incorporated into paired helical filaments. Am J Pathol. 1991;139:1463–1470. [PMC free article] [PubMed] [Google Scholar]
- Greenberg SG, Davies P, Schein JD, Binder LI. Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J Biol Chem. 1992;267:564–569. [PubMed] [Google Scholar]
- Yamaguchi H, Ishiguro K, Uchida T, Takashima A, Lemere CA, Imahori K. Preferential labeling of Alzheimer’s neurofibrillary tangles with antisera for tau protein kinase (TPK) I/glycogen synthase kinase-3β and cyclin-dependent kinase 5, a component of TPK II. Acta Neuropathol (Berl) 1996;92:232–241. doi: 10.1007/s004010050513. [DOI] [PubMed] [Google Scholar]
- Wolozin BL, Pruchnicki A, Dickson DW, Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986;232:648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Tomidokoro Y, Ishiguro K, Harigaya Y, Matsubara E, Ikeda M, Park J-M, Yasutake K, Kawarabayashi T, Okamoto K, Shoji M. Aβ amyloidosis induces the initial stage of tau accumulation in APPsw mice. Neurosci Lett. 2001;299:169–172. doi: 10.1016/s0304-3940(00)01767-5. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Sato K, Takamatsu M, Park J, Uchida T, Imahori K. Analysis of phosphorylation of tau with antibodies specific for phosphorylation sites. Neurosci Lett. 1995;202:81–84. doi: 10.1016/0304-3940(95)12206-0. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Berenfeld B, Davies P. Sequence requirements for formation of conformational variants of tau similar to those found in Alzheimer’s disease. J Neurosci Res. 1999;55:713–723. doi: 10.1002/(SICI)1097-4547(19990315)55:6<713::AID-JNR6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett. 1995;189:167–169. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- Planel E, Yasutake K, Fujita SC, Ishiguro K. Inhibition of protein phosphatase 2A overrides tau protein kinase I/glycogen synthase kinase 3 beta and cyclin-dependent kinase 5 inhibition and results in tau hyperphosphorylation in the hippocampus of starved mouse. J Biol Chem. 2001;276:34298–34306. doi: 10.1074/jbc.M102780200. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. Paxinos G, Franklin KB, editors. San Diego: Academic Press,; The Mouse Brain in Stereotaxic Coordinates. (ed 2) 2001 [Google Scholar]
- Ikarashi Y, Kuribara H, Shiobara T, Takahashi A, Ishimaru H, Maruyama Y. Learning and memory in mice treated with choline oxidase, a hydrolytic enzyme for choline. Pharmacol Biochem Behav. 2000;65:519–522. doi: 10.1016/s0091-3057(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Ikarashi Y, Harigaya Y, Tomidokoro Y, Kanai M, Ikeda M, Matsubara E, Kawarabayashi T, Kuribara H, Younkin SG, Maruyama Y, Shoji M. Decreased level of brain acetylcholine and memory disturbance in APPsw mice. Neurobiol Aging. 2004;25:483–490. doi: 10.1016/S0197-4580(03)00122-2. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Cairns NJ, Crowther RA. Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron. 1992;8:159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Expression of separate isoforms of human tau protein: correlation of the tau pattern in brain and effects on tubulin polymerazation. EMBO J. 1990;9:4225–4230. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Probst A, Spillantini MG, Schafer T, Jakes R, Burki K, Goedert M. Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J. 1995;14:1304–1313. doi: 10.1002/j.1460-2075.1995.tb07116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Zhang B, Higuchi M, Yoshiyama Y, Trojanowski JQ, Lee VM. Age-dependent induction of congophilic neurofibrillary tau inclusions in tau transgenic mic. Am J Pathol. 2001;158:555–562. doi: 10.1016/S0002-9440(10)63997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spittaels K, Van den Haute C, Van Dorpe J, Bruynseels K, Vandezande K, Laenen I, Geerts H, Mercken M, Sciot R, Van Lommel A, Loos R, Van Leuven F. Prominent axonopathy in the brain and spinal cord of transgenic mice overexpressing four-repeat human tau protein. Am J Pathol. 1999;155:2153–2165. doi: 10.1016/S0002-9440(10)65533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst A, Gotz J, Wiederhold KH, Tolnay M, Mistl C, Jaton AL, Hong M, Ishihara T, Lee VM, Trojanowski JQ, Jakes R, Crowther RA, Spillantini MG, Burki K, Goedert M. Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol (Berl) 2000;99:469–481. doi: 10.1007/s004010051148. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Murphy M Paul, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Gotz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. J Biol Chem. 2001;276:529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- Tanemura K, Murayama M, Akagi T, Hashikawa T, Tominaga T, Ichikawa M, Yamaguchi H, Takashima A. Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J Neurosci. 2002;22:133–141. doi: 10.1523/JNEUROSCI.22-01-00133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi Y, Miyasaka T, Chui DH, Akagi T, Mishima K, Iwasaki K, Fujiwara M, Tanemura K, Murayama M, Ishiguro K, Planel E, Sato S, Hashikawa T, Takashima A. Tau filament formation and associative memory deficit in aged mice expressing mutant (R406W) human tau. Proc Natl Acad Sci USA. 2002;99:13896–13901. doi: 10.1073/pnas.202205599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y. Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem. 1995;270:823–829. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy PK, Johnson GV. Mutant (R406W) human tau is hyperphosphorylated and does not efficiently bind microtubules in a neuronal cortical cell model. J Biol Chem. 2004;279:7893–7900. doi: 10.1074/jbc.M311203200. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Barrachina M, Puig B. Glycogen synthase kinase-3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer’s disease, Pick’s disease, progressive supranuclear palsy, and corticobasal degeneration. Acta Neuropathol (Berl) 2002;104:583–591. doi: 10.1007/s00401-002-0587-8. [DOI] [PubMed] [Google Scholar]
- Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Filamentous tau in oligodendrocytes and astrocytes of transgenic mice expressing the human tau isoform with the P301L mutation. Am J Pathol. 2003;162:213–218. doi: 10.1016/S0002-9440(10)63812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]