Abstract
Myostatin is a TGF-β family member and a negative regulator of skeletal muscle growth. It has been proposed that reduction or elimination of myostatin could be a treatment for degenerative muscle diseases such as muscular dystrophy. Laminin-deficient congenital muscular dystrophy is one of the most severe forms of muscular dystrophy. To test the possibility of ameliorating the dystrophic phenotype in laminin deficiency by eliminating myostatin, we crossed dyW laminin α2-deficient and myostatin null mice. The resulting double-deficient dyW/dyW;Mstn−/− mice had a severe clinical phenotype similar to that of dyW/dyW mice, even though muscle regeneration was increased. Degeneration and inflammation of muscle were not alleviated. The pre-weaning mortality of dyW/dyW;Mstn−/− mice was increased compared to dyW/dyW, most likely due to significantly less brown and white fat in the absence of myostatin, and postweaning mortality was not significantly improved. These results show that eliminating myostatin in laminin-deficiency promotes muscle formation, but at the expense of fat formation, and does not reduce muscle pathology. Any future therapy based on myostatin may have undesirable side effects.
Myostatin is a TGF-β family member that has been identified as a negative muscle regulator.1 Myostatin null mutant mice and “double-muscled” cattle with spontaneous mutations in the myostatin gene have significantly greater muscle mass due to both muscle hypertrophy and hyperplasia.2–4 In vitro studies showed that myostatin inhibits cell proliferation and protein synthesis in myoblasts.5,6 Recently, it was reported that blockade of myostatin by treatment with antibodies or elimination of the myostatin by gene knock out significantly increased muscle mass, decreased muscle degeneration, and reduced fibrosis in mdx, dystrophin-deficient mice.7,8 Other agents may be used in the future to eliminate or reduce myostatin at selected times, such as the myostatin antagonists follistatin, myostatin propeptide, and growth and differentiation factor-associated serum protein-1, GASP-1.9–13 In the present study, we wanted to test if elimination of myostatin may be of benefit, not only in dystrophin-deficiency, but also in other forms of muscular dystrophy such as laminin-deficient congenital muscular dystrophy.
Congenital muscular dystrophy is a group of severe forms of muscular dystrophy, often leading to early death in humans.14–18 The majority of cases are caused by mutations in the major laminin in the muscle basement membrane, laminin containing the α2 chain (Laminin-2/Merosin). This disease has been termed merosin-deficient congenital muscular dystrophy (MCMD) or more recently MDC1A.19 Several different mutations that result in lack of laminin α2 or in the presence of a truncated form of laminin α2 have been identified in human patients.19 Several mouse models for this disease are available,20–29 including the dyW/dyW mouse generated by gene targeting in our laboratory.27,28,30 The lack of functional laminin α2 in the muscle basement membrane of dyW/dyW mice leads to severe degeneration in skeletal muscle fibers, and most of the mice die at 3 to 6 weeks of age.28,30 The deterioration in this disease is thought to be caused by the failure to form the primary laminin scaffold, which is necessary for basement membrane structure and interaction with the dystrophin-glycoprotein complex (DGC) and the integrins.31 Transgenic expression of a functional human LAMA2 gene30 or of an agrin minigene with related activities32 prevented the muscle degeneration in dyW/dyW mice. Normal muscle has a significant capacity for regeneration, and effective regeneration would be expected to improve longevity in muscular dystrophy. While the mdx, dystrophin-deficient mice have excellent muscle regeneration,33,34 the dyW/dyW mice have poor regeneration,28 which may be a factor in the poor prognosis for laminin-deficient mice. We hypothesized that elimination of myostatin may improve the regeneration in dyW/dyW mice. Indeed, dyW/dyW mice lacking myostatin showed increased muscle regeneration and had increased muscle mass. However, lack of myostatin did not improve the well-being of the mice or the pathological changes in muscle; instead, lack of myostatin had a negative effect on fat tissue and increased the postnatal mortality of the mice.
Materials and Methods
Generation of Mice
Myostatin-deficient mice of mixed C57BL and 129SV/J background were obtained from Dr. Sejin Lee (Johns Hopkins University, Baltimore, MD).1 The dyW/dyW mice30 used have been back-crossed to C57BL/6 for at least nine generations. To generate dyW/dyW, laminin-deficient mice that lack myostatin, heterozygous dyW (Lama2+/−) were bred to heterozygous (Mstn+/−) or homozygous (Mstn−/−) myostatin mutant mice. The first generation produced double-heterozygous mice (Lama2+/−/Mstn+/−), which were then used to produce laminin α2-deficient mice with or without myostatin at an expected frequency of 1/16.
PCR Genotyping
Tails were biopsied from 3-week-old mice, and genomic DNA was extracted. Four pairs of PCR primers were designed according to wild-type Lama2 (forward 5′-ACTGCCCTTTCTCACCCACCCTT-3′ and reverse 5′-GTTGATGCGCTTGGGACTG-3′); lacZ knock-in and Lama2 chimeric sequence (forward same as Lama2 forward and reverse 5′-GTCGACGACGACAGTATCGGCCTCAG-3′); 30 Mstn (forward 5′-CAGCCATGGTAGTAGACCG-3′ and reverse 5′-GATGTGCTCTCACTTCCTTG-3′); neomycin knock-in and Mstn chimeric sequence (forward 5′-TCTATCGCCTTCTTGACGAG-3′and reverse same as myostatin reverse)1 to detect all four alleles. PCR conditions were 94°C for 3 minutes, 40 cycles at 94°C for 30 seconds, 54°C for 30 seconds, 72°C for 1 minute, and 72°C for 10 minutes. PCR products were separated on an agarose gel and visualized by ethidium bromide.
Muscle Histology and Morphometric Study
Four-week-old mice were weighed and then sacrificed. Quadriceps, gastrocnemius, and triceps muscles were embedded in OCT, snap-frozen in isopentane pre-cooled in liquid nitrogen, and stored at −80°C until further processed. Ten-μm sections were cut from the mid-belly of the muscles and stained with hematoxylin and eosin (H&E). The cross-sectional area (CSA), cross-sectional fiber number (CSFN), and single fiber area (SFA) were measured and calculated using NIH image and Scion Image programs. At least four mice of each genotype were used for analysis.
Immunofluorescence
Ten-μm sections of rectus femoris muscle at mid-belly were cut on a cryostat and air-dried. Regenerated muscle fibers were detected by staining with mouse anti-embryonic myosin heavy chain (eMHC, 1:10; Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development (NICHD) and maintained by the University of Iowa, Department of Biological Science, Iowa City, IA) and slow fibers by mouse anti-MHC Type I (1:60; Sigma, St Louis, MO) using the M.O.M. kit from Vector. Myoblasts were detected by staining with rabbit anti-MyoD (1:100; Santa Cruz, Santa Cruz, CA) and macrophages with rat antibody F4/80 (1:100; Caltag, Burlingame, CA) followed by FITC-labeled goat anti-rabbit or rabbit anti-rat IgG. Leukocytes were detected with PE-Cy5-labeled anti-CD45 (1:100; eBiosciences, San Diego, CA) and mature T cells with FITC-labeled anti-CD3e (1:100; eBioscience). After washing, sections were mounted with VECTASHIELD and observed under a fluorescence microscope.
Fat Tissue Analysis
Gonadal white fat pads and interscapular brown fat pads were dissected from 4-, 8-, and 12-week-old mice according to Johnson and Hirsch.35 Fat pads from the left side were weighed and the weight expressed as percentage of total body weight. Fat pads from the right side were quick frozen, embedded in OTC, sectioned at 10 μm, and stained with H&E.
Statistics
Paired student’s t-test was used in all analyses to determine significance.
Results
dyW/dyW;Mstn−/− Mice Have a Severe Clinical Phenotype Similar to dyW/dyW Mice
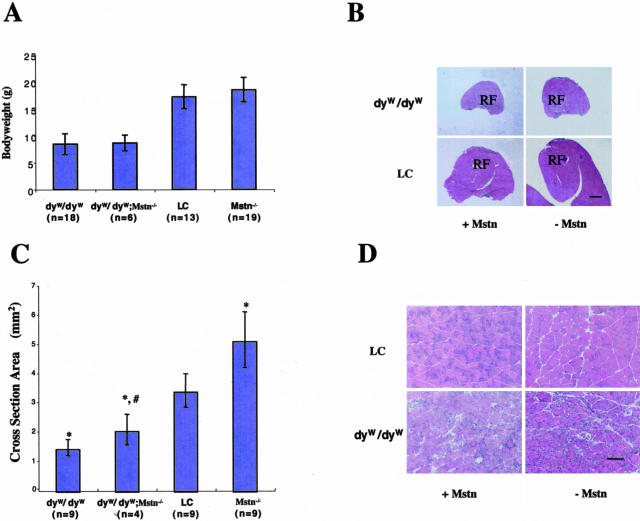
Mice were genotyped at 3 weeks and sacrificed at 4 weeks. At 4 weeks of age, the body weight of dyW/dyW;Mstn−/− was not significantly different from that of dyW/dyW mice (Figure 1A). As the body weight of the mice is a measure of muscle mass as well as of overall well-being, this result indicates that lack of myostatin did not have the same effect in the laminin-deficient mouse as it had in the dystrophin-deficient mdx mouse.7 Necrosis and inflammation, as evaluated by staining of muscle sections with H&E, were not decreased in dyW/dyW;Mstn−/− mice compared to dyW/dyW mice (Figure 1D). There was also no difference in the number of infiltrating inflammatory cells as evidenced by immunostaining for leukocytes, mature T cells, and macrophages (not shown).
Figure 1.
Phenotypic analysis. A: Presence or absence of myostatin does not significantly affect the body weight of laminin-deficient and littermate control (LC) mice at at 4 weeks of age. Graph shows mean ± SD. B: H&E-stained sections from the mid-belly of quadriceps muscle of 4-week-old mice show increased muscle area in the absence of myostatin. RF, rectus femoris muscle. Bar, 1000 μm. C: Cross-sectional area (CSA) of the rectus femoris muscle (mean ± SD). *, significant difference from LC mice; #, significant difference from dyW/dyW mice. D: High magnification H&E-stained sections from the mid-belly of the rectus femoris muscle. There is no apparent difference in inflammation or necrosis between dyW/dyW and dyW/dyW; Mstn−/− mice. Bar, 100 μm.
dyW/dyW;Mstn−/− Mice Have Larger Muscle Mass and Fiber Numbers than dyW/dyW Mice
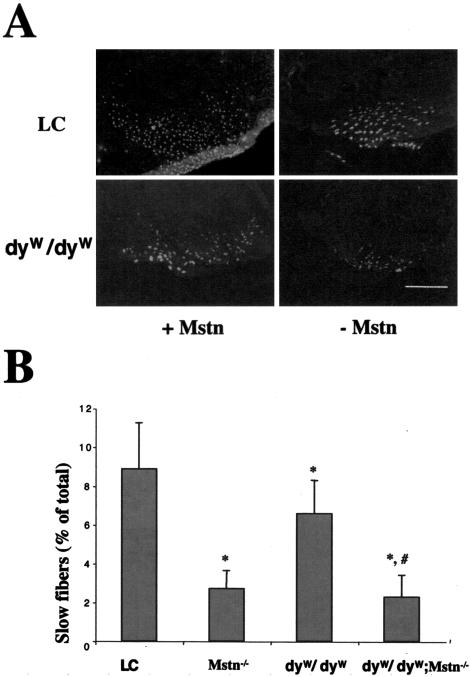
Mstn−/− mice have much larger muscles than wild-type mice.1 Both proximal limb muscle, such as triceps and quadriceps, and distal limb muscle, such as gastrocnemius, were larger in dyW/dyW;Mstn−/− than in dyW/dyW mice (Figure 1, B and C), although they were still much smaller than in wild-type mice. The cross-sectional area (CSA) of rectus femoris in dyW/dyW;Mstn−/− and Mstn−/− mice compared to dyW/dyW and littermate controls were 2.07 ± 0.52 mm2 versus 1.45 ± 0.28 mm2, P < 0.01 and 5.14 ± 0.96 mm2 versus 3.41 ± 0.58 mm2, P < 0.05 (Figure 1, B and C). The increase in CSA in dyW/dyW;Mstn−/− mice compared to dyW/dyW is attributed primarily to increased fiber number (Table 1), suggesting that the increase is due to regeneration. As has been shown before, at 8 and 12 weeks of age, the body weight of Mstn−/− mice was significantly greater than that of littermate control mice (data not shown). The dyW/dyW mice with or without myostatin were not tested at this time point, as they rarely survive to that age. Interestingly, the number of slow fibers in the rectus femoris muscle was decreased in dyW/dyW;Mstn−/− mice compared to dyW/dyW mice (Figure 2, A and B). The same was true for Mstn−/− mice compared to control mice (Figure 2, A and B).
Table 1.
Morphometry of Rectus Femoris Muscle
| dyW/ dyW | dyW/ dyW;Mstn−/− | LC | Mstn−/− | |
|---|---|---|---|---|
| CSA (mm2) | 1.45 ± 0.28 (9) | 2.07 ± 0.52 (4)* | 3.41 ± 0.58 (9) | 5.14 ± 0.96 (9)** |
| SFA (μm2) | 4004.2 ± 950.7 (8) | 4132.4 ± 348.5 (4) | 3742.6 ± 819.4 (8) | 6825.4 ± 647.9 (8)** |
| TFN | 984.3 ± 255.7 (8) | 1225.8 ± 206.2 (4)* | 2009.1 ± 635.5 (8) | 2272.0 ± 468.9 (8) |
CSA, cross section area; SFA, single fiber area; TNF, total fiber number. Results are expressed as means ± SD; number in parentheses indicates number of animals;
, statistical significance compared to dyW (P < 0.05);
, statistical significance compared to littermate control (LC) mice (P < 0.05).
Figure 2.
Reduced numbers of type I fibers in Mstn−/− mice. A: Muscle sections were stained with monoclonal anti-MHC Type I antibody in immunofluorescence. Bar, 1000 μm. B: Percentage of slow fibers in cross-sections at the mid-belly of the rectus femoris muscle (mean ± SD). *, significant difference compared to littermate control mice (LC); #, significant difference from dyW/dyW mice.
dyW/dyW;Mstn−/− Mice Have More Regenerated Muscle Fibers
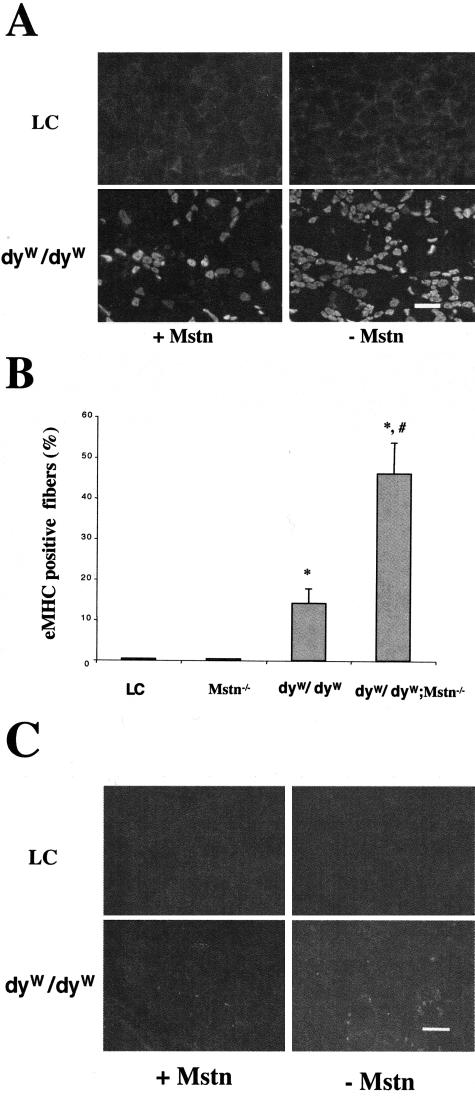
Using embryonic myosin heavy chain (eMHC) as a marker for newly formed muscle fibers, we found more positive muscle fibers in dyW/dyW;Mstn−/− mice compared to dyW/dyW mice (Figure 3, A and B). eMHC-positive fibers were not found in the Mstn−/− and control mice, suggesting that the positive fibers in dyW/dyW;Mstn−/− mice resulted from regeneration rather than growth. Since regeneration of muscle needs proliferating myoblasts, we used MyoD as a marker for such cells.36 Indeed, we found a trend of more MyoD-positive cells in dyW/dyW;Mstn−/− mice compared to dyW/dyW mice (Figure 3C).
Figure 3.
Increased numbers of regenerated muscle fibers and activated satellite cells in dyW/dyW:Mstn−/− mice compared to dyW/dyW mice. A: Sections of rectus femoris were stained with monoclonal anti-eMHC antibody in immunofluorescence. Bar, 100 μm. B: Comparison of positive fibers per high magnification field (mean ± SD). At least four mice were used in each group, and three randomly selected fields were analyzed per mouse. *, significant difference from LC; #, significant difference from dyW/dyW mice. C: Immunofluorescence staining with anti-myoD polyclonal antibody shows increased numbers of positive cells in dyW/dyW:Mstn−/− compared to dyW/dyW mice. Bar, 100 μm.
dyW/dyW;Mstn−/− Mice Have Reduced Vitality, Less Fat Tissue, and Smaller Adipocytes
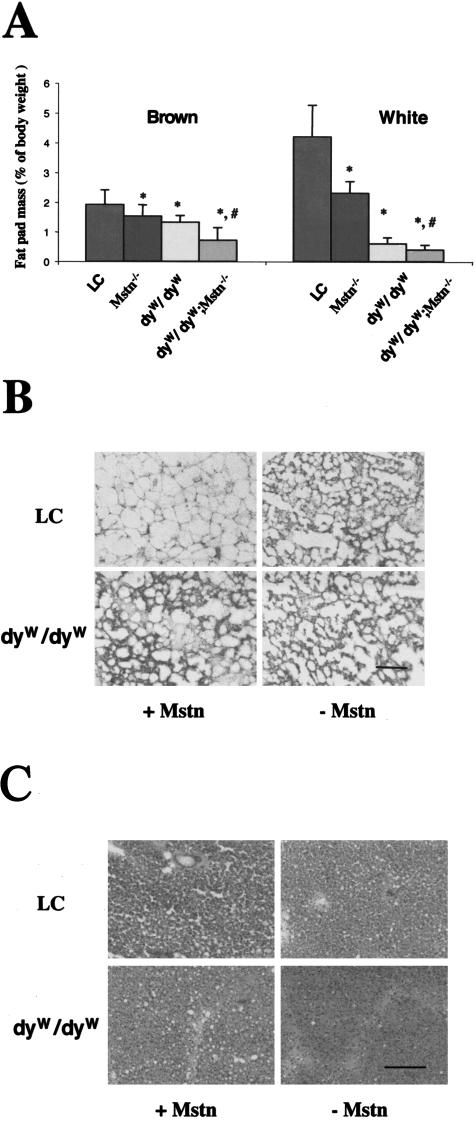
Although dyW/dyW;Mstn−/− mice had larger muscle compared to dyW/dyW mice, elimination of myostatin did not have a positive effect on postnatal survival. Fewer mice with the genotype dyW/dyW;Mstn−/− were obtained than expected according to the Mendelian frequency of 1/16 (6/279 obtained versus 17/279 expected). For dyW/dyW and Mstn−/− the number of mice was 18/279 and 17/279 as expected. We found an unusually high number of pups dead or missing a few days after birth, and some of these could be analyzed and were found to be of genotype dyW/dyW;Mstn−/−. It has been shown before that Mstn−/− mice have less fat accumulation than control mice.37 We analyzed both white and brown fat tissue in the different mice. We confirmed that Mstn−/− mice had much less white and brown fat compared to control mice as early as 4 weeks of age and up to 12 weeks of age (Figure 4A and data not shown). The dyW/dyW mice also had much less fat tissue than control mice, and the dyW/dyW;Mstn−/− mice had even less (Figure 4A). The reduction of fat mass was at least partially due to the smaller size of the adipocytes in both laminin deficiency and myostatin deficiency (Figure 4, B and C). Furthermore, there was a large variation in the size of the adipocytes in dyW/dyW mice compared to control mice, and the brown fat adipocytes were much smaller in the dyW/dyW;Mstn−/− compared to the dyW/dyW mice (Figure 4C). It appears that laminin deficiency and myostatin deficiency have additive and negative effects on fat tissue. Since brown fat is important for the neonatal animal, the lack of brown fat may contribute the increased postnatal death.
Figure 4.
Less brown and white fat in Mstn−/− mice and dyW/dyW mice. A: Interscapular brown fat and gonadal white fat pads were collected from 4-week-old mice and weighed as described in Materials and Methods. Weights are shown as percentage of total body weight. *, significant difference from littermate control (LC) mice; #, significant difference from dyW/dyW mice. B and C: Histology of white fat (B) and brown fat (C). Bar, 100 μm.
Discussion
Merosin-deficient congenital muscular dystrophy is one of the most severe forms of muscular dystrophy. In addition to muscle fiber degeneration resulting from the lack of laminin α2, the severe dystrophic phenotype is also correlated with poor regeneration28 compared to other milder forms of muscular dystrophy, such as dystrophin-deficiency in mdx mice.33,34 Regeneration is mostly dependent on satellite cells, which reside between the basement membrane and the muscle sarcolemma. On injury, the satellite cells become activated, re-enter the cell cycle, proliferate as myoblasts, and exit the cell cycle to renew the satellite cell pool or to differentiate into new myofibers. Methods to promote activation or proliferation of satellite cells may be expected to have beneficial effect on regeneration such as in muscular dystrophy.
The discovery of myostatin gave hope that it may be possible to target this negative regulator of muscle growth to increase muscle mass in degenerative diseases of muscle. It has been shown that muscle fibers in myostatin null mice are more numerous, larger, and have more activated satellite cells compared to wild-type mice,38,39 and reducing or eliminating myostatin in mdx mice resulted in improvement of the dystrophic phenotype.5,6 We show here that elimination of myostatin in dyW/dyW, laminin-deficient mice did result in a small but significant increase in size of muscle, and the increase was due primarily to hyperplasia. The increased fiber number in the dyW/dyW;Mstn−/− mice compared to dyW/dyW mice is at least partially the result of more regeneration. Nevertheless, the increased regeneration was not sufficient to improve the overall condition of the mice; the mice without myostatin were as small and had as severe muscular dystrophy as the ones with, and they had extensive necrosis, inflammation and fibrosis in muscle at 4 weeks of age. Interestingly, myostatin-negative mice had reduced numbers of type I fibers, and by inference, increased numbers of type II fibers, compared to myostatin-positive mice. This suggests that elimination of myostatin may be used to treat other myopathies resulting from non-genetic causes, such as malnutrition, cachexia, and corticosteriod excess, in which the there is selective type II fiber atrophy.40 In fact, it was shown that glucocorticoid-induced skeletal muscle atrophy is associated with up-regulation of myostatin gene expression,41 and that systemically administered myostatin could induce cachexia in mice.42
Although dyW/dyW;Mstn−/− mice have larger muscles than dyW/dyW mice, the muscles are still much smaller than those of wild-type mice, and viability was not improved by elimination of myostatin. In fact, significantly more mice died at the neonatal stage. Most surviving mice were sacrificed and used for analysis at 4 weeks of age. Based on this analysis, long-term survival was not expected to improve by the absence of myostatin and was therefore not specifically analyzed. Of eight dystrophic mice not used for analysis at 4 weeks, seven dyW/dyW mice died between day 23 and 69, and one dyW/dyW;Mstn−/− mouse lived until day 35, consistent with a lack of effect on life span.
What could explain the lack of effect of myostatin-deficiency on dy muscular dystrophy, when it was reported to be effective in mdx mice? First, the extent of regeneration in the absence of myostatin was not large enough to compensate for the degeneration of muscle fibers. This may be because laminin α2 and myostatin regulate muscle growth via different pathways. Second, although the muscle is larger in dyW/dyW;Mstn−/− mice, there was no improvement in the histopathology of the muscle; there were still signs of massive muscle fiber degeneration and infiltration of inflammatory cells. This is consistent with our previous data that laminin α2 is necessary for survival of generated myotubes.43 Without laminin, myotubes could not tolerate the forces of contraction and underwent degeneration and apoptosis.27,43 Laminin α2 is thought of as a survival signal to the cell, and a recent study showed that laminin binding increased the proliferation of C2C12 myoblast cells through the DGC and rac1 pathway.44 Third, we observed that eliminating myostatin in dyW/dyW mice led to less brown fat and smaller adipocytes. Brown fat is important for neonatal humans and mice to maintain body temperature. In combination with a congenital laminin-deficiency, lack of brown fat may be a serious problem for the newborn. Accordingly, we found excess postnatal deaths specifically among the dyW/dyW;Mstn−/− mice. It is not clear from this work whether lack of myostatin also has a negative effect on vitality in the young adult. As targeting of myostatin has been considered in the treatment of muscular dystrophy,9 it is important to note that the role of myostatin in adipogenesis. The reduction in fat tissue on myostatin elimination may be a serious side effect, even though such a side effect may be less serious in forms of myopathy with later onset or if myostatin is eliminated after birth.
Acknowledgments
We thank Dr. Sejin Lee for the myostatin knockout mice; Ling Wang for in vitro fertilization to generate Mstn−/− offspring; and Nina Humphries for morphometry.
Footnotes
Address reprint requests to Eva Engvall, The Burnham Institute 10901 N. Torrey Pines Road, La Jolla, CA 92037. E-mail: eengvall@burnham.org.
Supported by The National Institutes of Health (E.E.) and The Muscular Dystrophy Association (G.D.S. and E.E.).
References
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7:910–916. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, Fries R, Hanset R, Georges M. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;17:71–74. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275:40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- Taylor WE, Bhasin S, Artaza J, Byhower F, Azam M, Willard DH, Jr, Kull FC, Jr, Gonzalez-Cadavid N. Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am J Physiol. 2001;280:E221–E228. doi: 10.1152/ajpendo.2001.280.2.E221. [DOI] [PubMed] [Google Scholar]
- Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol. 2002;52:832–836. doi: 10.1002/ana.10385. [DOI] [PubMed] [Google Scholar]
- Khurana TS, Davies KE. Pharmacological strategies for muscular dystrophy. Nat Rev Drug Discov. 2003;2:379–390. doi: 10.1038/nrd1085. [DOI] [PubMed] [Google Scholar]
- Grobet L, Pirottin D, Farnir F, Poncelet D, Royo LJ, Brouwers B, Christians E, Desmecht D, Coignoul F, Kahn R, Georges M. Modulating skeletal muscle mass by postnatal, muscle-specific inactivation of the myostatin gene. Genesis. 2003;35:227–238. doi: 10.1002/gene.10188. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ratovitski T, Brady JP, Solomon MB, Wells KD, Wall RJ. Expression of myostatin pro domain results in muscular transgenic mice. Mol Reprod Dev. 2001;60:351–361. doi: 10.1002/mrd.1097. [DOI] [PubMed] [Google Scholar]
- Hill JJ, Qiu Y, Hewick RM, Wolfman NM. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: a novel protein with protease inhibitor and follistatin domains. Mol Endocrinol. 2003;17:1144–1154. doi: 10.1210/me.2002-0366. [DOI] [PubMed] [Google Scholar]
- Tome FM, Evangelista T, Leclerc A, Sunada Y, Manole E, Estournet B, Barois A, Campbell KP, Fardeau M. Congenital muscular dystrophy with merosin deficiency. C R Acad Sci III. 1994;317:351–357. [PubMed] [Google Scholar]
- Leyten QH, Gabreels FJ, Renier WO, Laak HJ. Congenital muscular dystrophy: a review of the literature. Clin Neurol Neurosurg. 1996;98:267–280. doi: 10.1016/0303-8467(96)00043-1. [DOI] [PubMed] [Google Scholar]
- Miyagoe-Suzuki Y, Nakagawa M, Takeda S. Merosin and congenital muscular dystrophy. Microsc Res Tech. 2000;48:181–191. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<181::AID-JEMT6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Dubowitz V. Congenital muscular dystrophy: an expanding clinical syndrome. Ann Neurol. 2000;47:143–144. doi: 10.1002/1531-8249(200002)47:2<143::aid-ana2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Tubridy N, Fontaine B, Eymard B. Congenital myopathies and congenital muscular dystrophies. Curr Opin Neurol. 2001;14:575–582. doi: 10.1097/00019052-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Allamand V, Guicheney P. Merosin-deficient congenital muscular dystrophy, autosomal recessive (MDC1A, MIM#156225, LAMA2 gene coding for alpha2 chain of laminin). Eur J Hum Genet. 2002;10:91–94. doi: 10.1038/sj.ejhg.5200743. [DOI] [PubMed] [Google Scholar]
- Michelson AM, Russell ES, Harman PJ. Dystrophia muscularis: a hereditary primary myopathy in the house mouse. Proc Natl Acad Sci USA. 1955;41:1079–1084. doi: 10.1073/pnas.41.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meie H, Southard JL. Muscular dystrophy in the mouse caused by an allele at the dy-locus. Life Sci. 1970;9:137–144. doi: 10.1016/0024-3205(70)90306-1. [DOI] [PubMed] [Google Scholar]
- Xu H, Christmas P, Wu XR, Wewer UM, Engvall E. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse. Proc Natl Acad Sci USA. 1994;91:5572–5576. doi: 10.1073/pnas.91.12.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wu XR, Wewer UM, Engvall E. Murine muscular dystrophy caused by a mutation in the laminin alpha 2 (Lama2) gene. Nat Genet. 1994;8:297–302. doi: 10.1038/ng1194-297. [DOI] [PubMed] [Google Scholar]
- Sunada Y, Bernier SM, Kozak CA, Yamada Y, Campbell KP. Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin M chain gene to dy locus. J Biol Chem. 1994;269:13729–13732. [PubMed] [Google Scholar]
- Sunada Y, Bernier SM, Utani A, Yamada Y, Campbell KP. Identification of a novel mutant transcript of laminin alpha 2 chain gene responsible for muscular dystrophy and dysmyelination in dy2J mice. Hum Mol Genet. 1995;4:1055–1061. doi: 10.1093/hmg/4.6.1055. [DOI] [PubMed] [Google Scholar]
- Besse S, Allamand V, Vilquin JT, Li Z, Poirier C, Vignier N, Hori H, Guenet JL, Guicheney P. Spontaneous muscular dystrophy caused by a retrotransposal insertion in the mouse laminin alpha2 chain gene. Neuromusc Disord. 2003;13:216–222. doi: 10.1016/s0960-8966(02)00278-x. [DOI] [PubMed] [Google Scholar]
- Kuang W, Xu H, Vachon PH, Engvall E. Disruption of the lama2 gene in embryonic stem cells: laminin alpha 2 is necessary for sustenance of mature muscle cells. Exp Cell Res. 1998;241:117–125. doi: 10.1006/excr.1998.4025. [DOI] [PubMed] [Google Scholar]
- Kuang W, Xu H, Vilquin JT, Engvall E. Activation of the lama2 gene in muscle regeneration: abortive regeneration in laminin alpha2-deficiency. Lab Invest. 1999;79:1601–1613. [PubMed] [Google Scholar]
- Miyagoe Y, Hanaoka K, Nonaka I, Hayasaka M, Nabeshima Y. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415:33–39. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- Kuang W, Xu H, Vachon PH, Liu L, Loechel F, Wewer UM, Engvall E. Merosin-deficient congenital muscular dystrophy: partial genetic correction in two mouse models. J Clin Invest. 1998;102:844–852. doi: 10.1172/JCI3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KP. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- Moll J, Barzaghi P, Lin S, Bezakova G, Lochmuller H, Engvall E, Muller U, Ruegg MA. An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature. 2001;413:302–307. doi: 10.1038/35095054. [DOI] [PubMed] [Google Scholar]
- Itagaki Y, Saida K, Iwamura K. Regenerative capacity of mdx mouse muscles after repeated applications of myo-necrotic bupivacaine. Acta Neuropathol (Berl) 1995;89:380–384. doi: 10.1007/BF00309633. [DOI] [PubMed] [Google Scholar]
- McGeachie JK, Grounds MD, Partridge TA, Morgan JE. Age-related changes in replication of myogenic cells in mdx mice: quantitative autoradiographic studies. J Neurol Sci. 1993;119:169–179. doi: 10.1016/0022-510x(93)90130-q. [DOI] [PubMed] [Google Scholar]
- Johnson PR, Hirsch J. Cellularity of adipose depots in six strains of genetically obese mice. J Lipid Res. 1972;13:2–11. [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-difficient mice. J Clin Invest. 2002;109:595–601. doi: 10.1172/JCI13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joulia D, Bernardi H, Garandel V, Rabenoelina F, Vernus B, Cabello G. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res. 2003;286:263–275. doi: 10.1016/s0014-4827(03)00074-0. [DOI] [PubMed] [Google Scholar]
- Banker BQ, Engel AG. Basic reactions of muscle. Engel AG, Franzini-Armstrong C, editors. New York: McGraw-Hill,; Mycology. 1994:838 pp. [Google Scholar]
- Ma K, Mallidis C, Bhasin S, Mahabadi V, Artaza J, Gonzalez-Cadavid N, Arias J, Salehian B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol. 2003;285:E363–E371. doi: 10.1152/ajpendo.00487.2002. [DOI] [PubMed] [Google Scholar]
- Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- Vachon PH, Loechel F, Xu H, Wewer UM, Engvall E. Merosin and laminin in myogenesis; specific requirement for merosin in myotube stability and survival. J Cell Biol. 1996;134:1483–1497. doi: 10.1083/jcb.134.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oak SH, Zhou YW, Jarrett HW. Skeletal muscle signaling pathway through the dystrophin glycoprotein complex and rac1. J Biol Chem. 278:39287–39295. doi: 10.1074/jbc.M305551200. [DOI] [PubMed] [Google Scholar]