Abstract
Wilson disease is a genetic disorder characterized by the accumulation of copper in the body due to a defect of biliary copper excretion. Although the Wilson disease gene has been cloned, the cellular localization of the gene product (ATP7B) has not been fully clarified. Therefore, the precise physiological action of ATP7B is still unknown. We examined the distribution of ATP7B using an anti-ATP7B antibody, green fluorescent protein (GFP)-ATP7B (GFP-ATP7B) and ATP7B-DsRed in various cultured cells. Intracellular organelles were visualized by fluorescence microscopy. The distribution of ATP7B was compared with that of Rab7 and Niemann-Pick C1 (NPC1), proteins that localize in the late endosomes. U18666A, which induces the NPC phenotype, was used to modulate the intracellular vesicle traffic. GFP-ATP7B colocalized with various late endosome markers including Rab7 and NPC1 but not with Golgi or lysosome markers. U18666A induced the formation of late endosome-lysosome hybrid organelles, with GFP-ATP7B localized with NPC1 in these structures. We have confirmed that ATP7B is a late endosome-associated membrane protein. ATP7B appears to translocate copper from the cytosol to the late endosomal lumen, thus participating in biliary copper excretion via lysosomes. Thus, defective copper ATPase activity of ATP7B in the late endosomes appears to be the main defect of Wilson disease.
Wilson disease is an autosomal recessive disorder characterized by the progressive accumulation of copper in the body. The failure of the hepatocytes to excrete copper into bile and decreased copper incorporation into ceruloplasmin cause the metal to accumulate in the body.1,2 The gene responsible for Wilson disease has been cloned and shown to encode a cation-transporting P-type ATPase.3–6 Wilson disease gene product, designated ATP7B, functions in copper secretion into plasma coupled with ceruloplasmin synthesis and biliary copper excretion.7,8 The proper function of ATP7B in copper homeostasis depends on the appropriate intracellular localization of this copper ATPase. The localization of ATP7B has important implications in how it functions in biliary copper excretion and copper incorporation into ceruloplasmin. However, this is now a matter of some controversy.9,10 While we have described the late endosomal localization of ATP7B,11–14 others have described ATP7B as localized in the trans-Golgi network (TGN).15–25 Therefore, the precise physiological action of ATP7B in copper metabolism within hepatocytes is still unclear.10 Elucidation of the accurate localization of ATP7B within hepatocytes is very important for the understanding of the biological mechanisms of copper homeostasis in general as well as the pathogenesis of Wilson disease.
Rab proteins are small GTPases localized at the cytoplasmic face of specific intracellular membranous compartments in the endocytic and exocytic pathways, where they regulate vesicle traffic.26 Rab7 is a representative small GTPase that is localized in the late endosome and regulates vesicle transport in the late endocytic compartments.27
Niemann-Pick C disease (NPC) is an autosomal-recessive neurodegenerative lysosomal storage disorder. The gene most commonly mutated in this disorder, NPC1, has been cloned,28 and its gene product NPC1 is a membrane protein and has been shown to be localized in a late endosomal compartment apart from the lysosome, the late endosome.29,30 NPC1 protein regulates intracellular cholesterol trafficking in the late endocytic compartments.
In the present study, we examined the relationship between ATP7B and these late endosome-associated proteins in various cultured cells. Furthermore, we examined the effect of U18666A, a sterol derivative affecting vesicle movements and causing the NPC phenotype,29 on the distribution of ATP7B. We now show that ATP7B is really a late endosome-associated membrane protein.
Materials and Methods
Cells
Huh7 (a hepatoma cell line),31 MDCK, and OUMS29 (a human hepatocyte cell line)32 cells were cultured in Dulbecco’s modified Eagle’s medium (Sigma, St. Louis, MO) supplemented with 10% fetal calf serum (Wako Pure Chemical Industries, Ltd., Osaka, Japan), penicillin (100 U/ml, crystalline penicillin G Meiji, Meiji Seika Kaisya, Tokyo, Japan) and streptomycin (0.1 mg/ml, Meiji Seika Kaisya) at 37°C in 5% CO.2 Copper concentration in the culture medium measured by direct colorimetric assay was below the detectable level (< 5 μg/dL). cDNAs were transiently transfected into cultured cells using Effecten Transfection Reagent (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s recommendations at 24 hours after plating.
Construction of cDNAs
To produce the green fluorescent protein (GFP)-ATP7B fusion protein, the BamH I-XbaI fragment, including the entire coding region of human ATP7B cDNA, was ligated into a pEGFP-C2 vector (CLONTECH Laboratories Japan Ltd., Tokyo, Japan) digested with BglII and XbaI, the site located at the 3′ end of EGFP cDNA.11–14 ATP7B-DsRed was produced by ligating the SalI-SmaI fragment of ATP7B cDNA into pDsRed-N1 (CLONTECH) digested with SalI and SmaI. Myc-Rab7 was produced by ligating the SalI -KpnI fragment of Rab7 cDNA (a kind gift from Dr. A. Wandinger-Ness)33,34 into pCMV-Myc (CLONTECH) digested with SalI and KpnI. PAsC9/flag-NPC1 was produced by insertion of a flag epitope into the ClaI site of human NPC cDNA.35
Antibodies and Reagents
The following antibodies were used: mouse anti-γ-adaptin antibody (Sigma); mouse monoclonal anti-ATP7B antibody;16 rabbit polyclonal anti-human cathepsin D antibody (Upstate Biotechnology Incorporated, New York, NY); mouse monoclonal anti-flag antibody (Sigma); mouse monoclonal anti-β1, 4-galactosyltransferase (GalT) antibody;36 mouse monoclonal anti-58-kd Golgi protein antibody (Sigma); mouse monoclonal anti-lysosome-associated membrane protein (Lamp) 1 and 2 antibodies37 (kind gifts from Prof. J.T. August, Johns Hopkins University, Baltimore, MD); mouse monoclonal anti-myc antibody (CLONTECH); fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse immunoglobulin (Dako Japan, Kyoto, Japan); tetramethylrhodamine isothiocyanate (TRITC)-conjugated rabbit anti-mouse immunoglobulin (Dako Japan); TRITC-conjugated swine anti-rabbit immunoglobulin (Dako Japan); Alexa Flour 488-conjugated rabbit anti-mouse immunoglobulin (Molecular Probes, Eugene, OR). Rhodamine-dextran (R-dextran) was used to stain the endocytic compartments (1 mg/ml, Sigma). U18666A (2 μg/ml, Biomol, Plymouth Meeting, PA) was used to manipulate the vesicle traffic in the late endocytic structures.
Immunofluorescence
At 48 hours after the transfection, cells on glass coverslips were fixed in freshly prepared 3% paraformaldehyde in phosphate-buffered saline (PBS) for 30 minutes and permeabilized in 0.2% Triton X-100 in PBS for 10 minutes or 0.1% saponin in PBS for 30 minutes. For the detection of NPC1 in flag-NPC 1-transfected cells, cells were fixed with cold ethanol (−20°C) for 20 minutes. Nonspecific binding was blocked by incubation with Protein Block Serum Free (Dako Japan) for 30 minutes followed by incubation with the primary antibodies for 1 hour and the secondary antibodies for 1 hour, respectively. A confocal laser scanning microscope (Fluoview FV 300, Olympus, Tokyo, Japan) equipped with an Argon/Krypton laser was used for observation. For double-labeling analysis, images were acquired sequentially using separate excitation wavelengths of 488 nm for GFP and FITC and 568 nm for TRITC and DsRed, and then merged.
Treatment with Copper Sulfate and a Copper Chelator
At 48 hours after seeding, the cells were treated with bathocuproine disulfonate (Sigma), a copper chelator, or copper sulfate (Wako Pure Chemical Industries, Ltd.) at the concentration of 40 μmol/L or 200 μmol/L for 2 hours, respectively.
Electron Microscopy
Cells were fixed with 1% glutaraldehyde, post-fixed in 1% osmium tetroxide, dehydrated in a graded ethanol series, and embedded in Quetol 812 (Nisshin EM, Tokyo, Japan). Ultra-thin sections were stained with uranyl acetate and lead citrate, and examined with a transmission electron microscope (JEM 2000-EX, JEOL, Tokyo, Japan).
Results
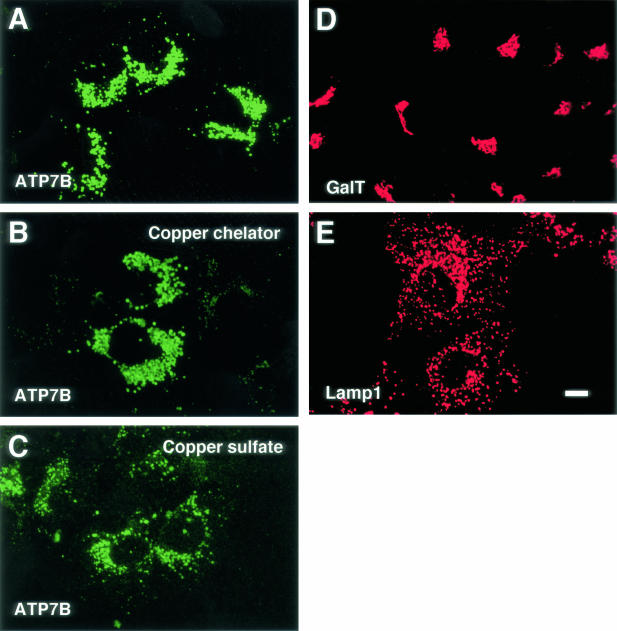
Immunofluorescent signals of ATP7B of Huh7 cells were shown in Figure 1A, they were observed as a punctate vesicular pattern around the nucleus. ATP7B was localized in the similar manner in cells treated with bathocuproine disulfonate (Figure 1B) or copper sulfate (Figure 1C). GalT has a polarized distribution toward the trans-Golgi and TGN,36 and it was visualized with the anti-GalT antibody (Figure 1D). GalT was distributed as a compact Golgi ribbon, and the morphologies of the TGN and ATP7B-containing structures were apparently different. Lamp1 is a membrane protein localized in the late endosome and lysosomes, and it was visualized with the anti-Lamp1 antibody.37 Lamp1 was distributed as a vesicular pattern similar to ATP7B, however, ATP7B was more restricted to the perinuclear region (Figure 1E). Immunofluorescent signals of ATP7B in ATP7B cDNA-transfected cells showed a juxtanuclear punctate pattern similar to endogeneous ATP7B (data not shown).11,12 In GFP-ATP7B-transfected cells, GFP signals were observed as a punctate pattern similar to endogeneous ATP7B (Figure 2A).11–14 When GFP-ATP7B transfected cells were labeled with the anti-ATP7B antibody, the GFP signals were identical to the signals produced by the anti-ATP7B antibody (data not shown).11 GFP alone was observed throughout the cells when pEGFP-C2 was transfected (data not shown).11
Figure 1.
Confocal laser scanning microscopic images of Huh7 cells. Cells were labeled with anti-ATP7B (A–C), anti-galactosyltransferase (GalT) (D) and anti-lysosome-associated membrane protein 1 (lamp1) (E) antibodies. Cells were treated with bathocuproine disulfonate (B) or copper sulfate (C). Bar, 10 μm.
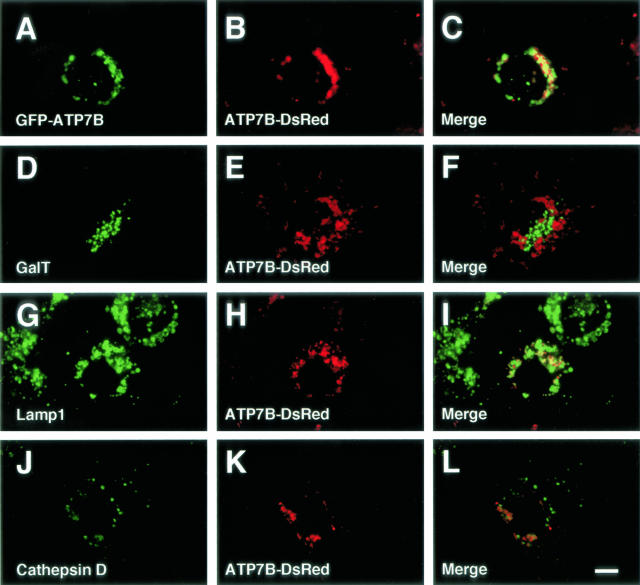
Figure 2.
Confocal laser scanning microscopic images of ATP7B-DsRed-transfected Huh7 cells (A–L). Some cells were cotransfected with GFP-ATP7B (A–C). Green: GFP-ATP7B (A), galactosyltransferase (GalT) (D), lysosome-associated membrane protein (lamp) 1 (G), cathepsin D (J). Red: ATP7B-DsRed (B, E, H, and K). Bar, 10 μm.
Previously, we examined the relation between GFP-ATP7B and various organelles using antibodies to various marker proteins of intracellular organelles and R-dextran. GFP-ATP7B was colocalized with R-dextran, and Lamp 1 and 2, but not with calnexin, γ-adaptin, GalT, or cathepsin D in primary cultured rat hepatocytes, Huh7, Hep3B, HEK293, and OUMS29 cells (Table 1).11–14
Table 1.
Relation between ATP7B and Markers for Intracellular Organelles
| Cell | Marker | Treatment | Localization of the marker | Colocalization | Reference |
|---|---|---|---|---|---|
| Isolated rat hepatocytes | |||||
| rhodamine dextran | — | endocytic structures | + | 11 | |
| galactosyltransferase | — | TGN | − | 11 | |
| lysosomal glycoprotein 8 | — | lysosome | − | 11 | |
| ZO-1 | — | tight junction | − | 11 | |
| Huh7 | |||||
| rhodamine dextran | — | endocytic structures | + | 11 | |
| galactosyltransferase | — | TGN | − | 11, 12, 13, 14 | |
| galactosyltransferase | nocodazole | ERGIC | − | 11 | |
| galactosyltransferase | brefeldin A | endoplasmic reticulum | − | 11 | |
| galactosyltransferase | U18666A | TGN | − | present study | |
| γ-adaptin | — | TGN | − | 11 | |
| 58-kd Golgi protein | — | TGN | − | present study | |
| 58-kd Golgi protein | U18666A | TGN | − | present study | |
| calnexin | — | endoplasmic reticulum | − | 13 | |
| MPR | — | TGN and late endosome | partial | 11 | |
| MPR | bafilomycin A1 | late endosome | + | 11 | |
| Lamp 1 | — | late endosome/lysosome | + | 11, 12, 13, 14 | |
| Lamp 1 | U18666A | hybrid organelle | + | present study | |
| Lamp 2 | — | late endosome/lysosome | + | 11 | |
| Lamp 2 | U18666A | hybrid organelle | + | present study | |
| NPC 1 | — | late endosome/lysosome | + | present study | |
| NPC 1 | U18666A | hybrid organelle | + | present study | |
| cathepsin D | — | lysosome | − | 11, 12, 13, 14 | |
| cathepsin D | U18666A | hybrid organelle | + | present study | |
| OUMS29 | |||||
| rhodamine dextran | — | endocytic structures | + | present study | |
| rhodamine dextran | U18666A | endocytic structures | + | present study | |
| galactosyltransferase | — | TGN | − | 14 | |
| Lamp 1 | — | late endosome/lysosome | + | 14 | |
| Lamp 1 | U18666A | hybrid organelle | + | present study | |
| Lamp 2 | — | late endosome/lysosome | + | present study | |
| Lamp 2 | U18666A | hybrid organelle | + | present study | |
| cathepsin D | — | lysosome | − | 14 | |
| NPC 1 | — | late endosome/lysosome | + | present study | |
| NPC 2 | U18666A | hybrid organelle | + | present study | |
| Hep3B | |||||
| galactosyltransferase | — | TGN | − | 14 | |
| Lamp 1 | — | late endosome/lysosome | + | 14 | |
| cathepsin D | — | lysosome | − | 14 | |
| HEK293 | |||||
| galactosyltransferase | — | TGN | − | 14 | |
| Lamp 1 | — | late endosome/lysosome | + | 14 | |
| cathepsin D | — | lysosome | − | 14 | |
| MDCK | |||||
| rhodamine dextran | — | endocytic structures | + | present study | |
| γ-adaptin | — | TGN | − | present study | |
| rab7 | — | late endosome | + | present study |
Abbreviations: ERGIC, endoplasmic reticulum-Golgi intermediate compartment; hybrid organelle, late endosome-lysosome hybrid organelle; Lamp, lysosome-associated membrane protein; MPR, mannose-6-phosphate receptor; NPC, Niemann-Pick C; TGN, trans-Golgi network.
To examine whether a tag with GFP at the N-terminus of ATP7B affects the intracellular distribution of ATP7B, we compared the distribution of GFP-ATP7B and ATP7B-DsRed, in which DsRed was ligated to the C-terminus of ATP7B. When GFP-ATP7B and ATP7B-DsRed were cotransfected to Huh7 cells, these two chimeric proteins were colocalized (Figure 2, A to C). Furthermore, ATP7B-DsRed was colocalized with Lamp 1 (Figure 2, G to I), but not with GalT (Figure 2, D to F) or cathepsin D (Figure 2, J to L). And the distribution of GFP-ATP7B was very similar to that of endogeneous ATP7B. These results indicate that both GFP-ATP7B and ATP7B-DsRed are localized in the late endosomes, and a tag with GFP at the N-terminus of ATP7B does not affect the distribution of ATP7B, indicating the validity of the use of GFP-ATP7B for dissecting the intracellular localization of ATP7B.
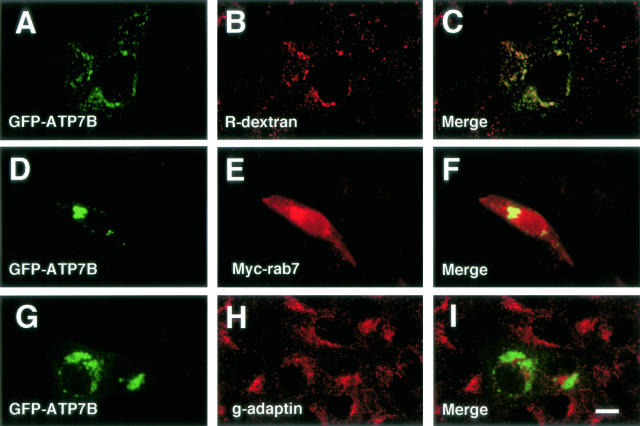
Next, we examined the relationship between GFP-ATP7B and Rab7, which was localized in the late endocytic compartments.27,33,34,38 We used MDCK cells in this analysis, because the Rab7 cDNA used in the present study was a canine cDNA.33,34 To examine the relationship between ATP7B and the endocytic compartments in MDCK cells, GFP-ATP7B-transfected cells were incubated with R-dextran, a fluid-phase marker, for 24 hours, and GFP-ATP7B was colocalized with R-dextran (Figure 3, A to C). MDCK cells were cotransfected with GFP-ATP7B and myc-Rab7, and Rab7 was detected with the anti-myc antibody. Wild-type Rab7 staining was observed in the cytoplasm and perinuclear vesicular structures, and GFP-ATP7B was colocalized with Rab7 in the perinuclear vesicular structures (Figure 3, D to F). The relationship between GFP-ATP7B and the TGN was also examined in MDCK cells. GFP-ATP7B was closely apposed but was not colocalized with γ-adaptin, a TGN marker (Figure 3, G to I).
Figure 3.
Confocal laser scanning microscopic images of GFP-ATP7B-transfected MDCK cells. Some cells were incubated with rhodamine-dextran for 24 hours (A–C). Cells in D–F were transfected with myc-Rab7. Green: GFP-ATP7B. Red: rhodamine-dextran (B), Myc-Rab7 (E), γ-adaptin (H). Bar, 10 μm.
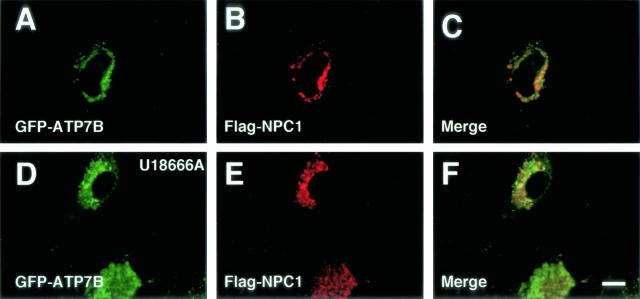
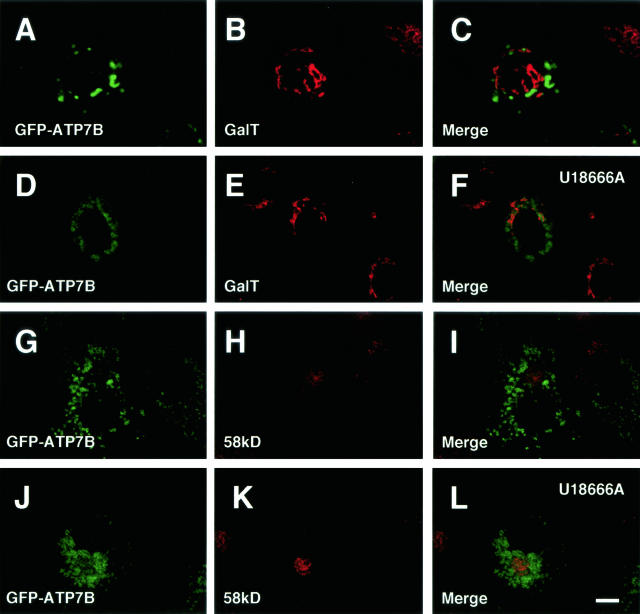
Finally, we examined the relationship between ATP7B and NPC1, the NPC1 product, in Huh7 and OUMS29 cells. NPC1 is localized in the late endosomes in ordinary conditions.29 GFP-ATP7B was colocalized with NPC1 in Huh7 cells cotransfected with GFP-ATP7B and flag-NPC1 (Figure 4, A to C). We examined the effect of U18666A on the distribution of ATP7B. U18666A is a sterol derivative that induces the NPC phenotype by inhibiting the function of NPC1 or NPC1-related proteins. This agent induces the formation of late endosome-lysosome hybrid organelles.29 In an electron microscopic examination, many electron-dense structures containing lipid-like particles were observed near the nucleus in U18666A-treated Huh7 cells (Figure 5). GFP-ATP7B-transfected OUMS29 cells were incubated with R-dextran for 24 hours, and GFP-ATP7B was colocalized with R-dextran (Figure 6A to C). When these cells were treated with U18666A, R-dextran was found in large vesicles and GFP-ATP7B was also localized in the same structures. While R-dextran was observed in expanded vesicles, GFP-ATP7B was mainly observed at the delimiting membrane as rings (Figure 6, D to F). GFP-ATP7B was colocalized with NPC1 in U18666A-treated Huh7 cells cotransfected with GFP-ATP7B and flag-NPC1 (Figure 4, D to F). We examined the relation between GFP-ATP7B and Lamp 1 (OUMS29 cells) and 2 (Huh7 cells). GFP-ATP7B was colocalized with a part but not all of Lamp 1 (Figure 7, A to C) and 2 (Figure 7, D to F)-containing vesicles in untreated cells. In U18666A-treated cells, GFP-ATP7B was almost completely colocalized with Lamp 1 (Figure 7, G to I) and 2 (Figure 7, J to L). Furthermore, we examined the relation between GFP-ATP7B and lysosomes. Although GFP-ATP7B was not colocalized with cathepsin D in untreated Huh7 cells (Figure 8, A to C), some GFP-ATP7B-bearing vesicles contained cathepsin D, a lysosomal enzyme, in U18666A-treated Huh7 cells (Figure 8, D to F). The relationship between GFP-ATP7B and the TGN was examined in U18666A-treated Huh7 cells. GFP-ATP7B was not colocalized with GalT (Figure 9, A to F) or 58-kd Golgi protein (Figure 9, G to L) before or after the treatment with U18666A. The data from the previous and present studies are summarized in Table 1.
Figure 4.
Confocal laser scanning microscopic images of GFP-ATP7B- and flag-NPC1-transfected Huh7 cells. Cells in D–F were treated with U18666A for 24 hours. Green: GFP-ATP7B. Red: flag-NPC1. Bar, 10 μm.
Figure 5.
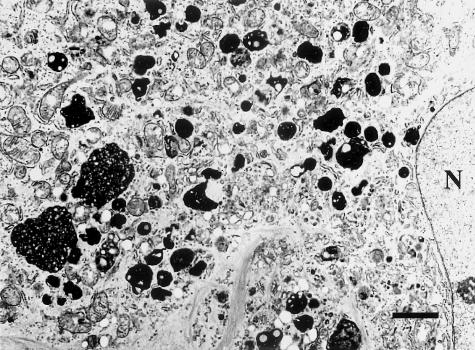
Transmission electron micrographs of Huh7 cells treated with U18666A for 24 hours. Bar, 2 μm. Many electron-dense structures containing lipid-like particles were observed near the nucleus (N).
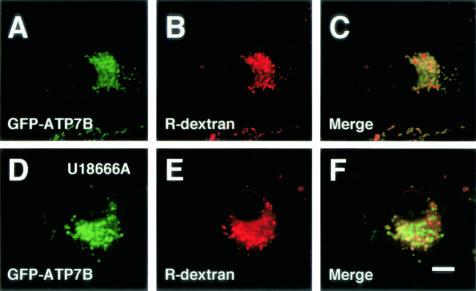
Figure 6.
Confocal laser scanning microscopic images of GFP-ATP7B-transfected OUMS29 (A–F) cells incubated with rhodamine-dextran for 24 hours. Cells in D–F were treated with U18666A for 24 hours. Green: GFP-ATP7B. Red: rhodamine-dextran. Bar, 10 μm.
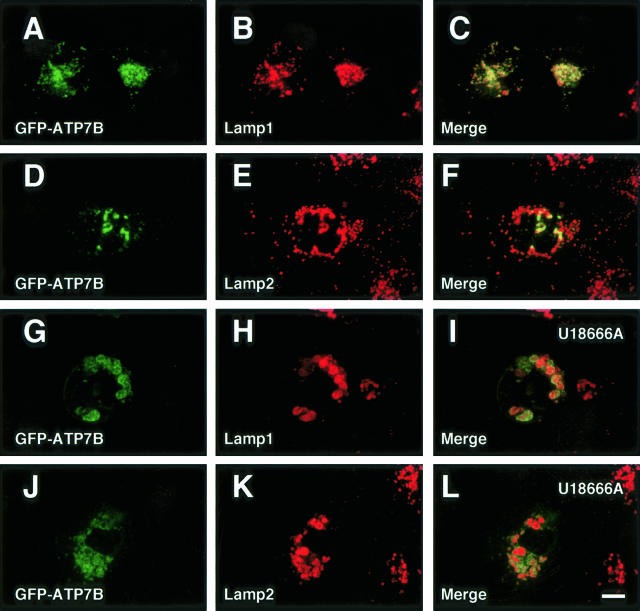
Figure 7.
Confocal laser scanning microscopic images of GFP-ATP7B-transfected OUMS29 (A—C and G–I) and Huh7 (D—F and J–L) cells. Some cells were treated with U18666A for 24 hours (G–L). Green: GFP-ATP7B. Red: (B, C, H, and I), lysosome-associated membrane protein (lamp) 1; (E, F, K, and L), lysosome-associated membrane protein (lamp) 2. Bar, 10 μm.
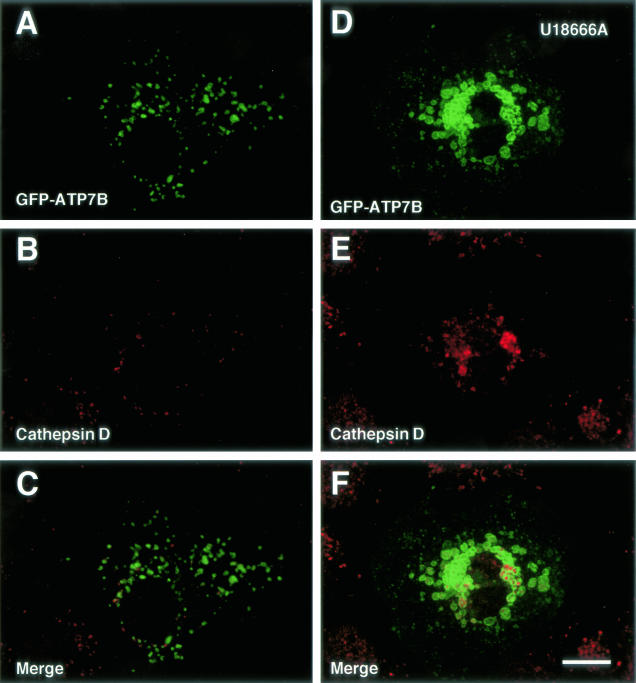
Figure 8.
Confocal laser scanning microscopic images of GFP-ATP7B-transfected Huh7 cells. Cells in D–F were treated with U18666A for 24 hours. Green: GFP-ATP7B. Red: cathepsin D. Bar, 10 μm.
Figure 9.
Confocal laser scanning microscopic images of GFP-ATP7B-transfected Huh7 cells. Cells in D–F and J–L were treated with U18666A for 24 hours. Green: GFP-ATP7B. Red: galactosyltransferase (GalT) (A-F), 58-kd Golgi protein (G-L). Bar, 10 μm.
Discussion
The gene responsible for Wilson disease encodes a copper-transporting ATPase, ATP7B.3–6 The proper function of ATP7B in copper homeostasis depends on the appropriate intracellular localization of this protein. We have reported that ATP7B was localized in the late endosomes.11–14 However, controversy still exists over the intracellular localization of ATP7B as we recently described.9,10 Initially, it was reported that copper ATPase activity was detected in the plasma membrane fraction of rat livers.39 Then, it was reported that ATP7B was localized at the TGN in HepG2 cells,15,23 ATP7B-transfected HTB9 (human bladder carcinoma) cells,16 ATP7B-transfected fibroblasts from patients with Menkes disease,17 ATP7B-transfected murine mottled fibroblasts,18 ATP7B-transfected Long-Evans Cinnamon rat hepatocytes,19 rat hepatocytes,20 ATP7B-transfected CHO cells,22,24 and human hepatocytes.21,25 The reason for the different results among the previous studies has not been clear. The difference is not likely to attribute to the difference in cells used for analysis, because we obtained the same results regarding the localization of ATP7B in various cell types, including isolated rat hepatocytes, Huh7, HEK293, Hep3B, OUMS29, and MDCK cells.11–14 Endogeneous ATP7B was detected with the anti-ATP7B antibody, and it was distributed as a punctate vesicular pattern around the nucleus. GalT, a TGN marker, was detected with the anti-GalT antibody and distributed as compact Golgi ribbons. The morphologies of the TGN and ATP7B-containing structures were apparently different. Lamp1 is a membrane protein localized in the late endosomes and lysosomes. Although Lamp1 detected with the anti-Lamp1 antibody was distributed similar to ATP7B, ATP7B seemed to be more restricted to the perinuclear region. Because these antibodies were mouse monoclonal antibodies, we could not perform the simultaneous examination for ATP7B and the marker proteins. Therefore, we examined the localization of ATP7B using GFP-ATP7B. The effect of a tag with GFP at the N-terminus of ATP7B did not influence the distribution of ATP7B, because endogeneous ATP7B and GFP-ATP7B was distributed in a similar perinuclear pattern and GFP-ATP7B and ATP7B-DsRed were colocalized in the late endosomes. Therefore, we further analyzed the intracellular localization of ATP7B by using different procedures from the previous studies.
Rab proteins, which belong to a superfamily of low molecular weight GTPases, are known to play a critical role in vesicular transport. Rabs recruit the donor vesicle membrane, where they direct targeting, docking, and fusion of the vesicles to the recipient organelles. Rab7 is localized in the late endosomes and regulates vesicle traffic in the late endocytic compartments.26,33,34,38 In the present study, GFP-ATP7B was colocalized with Rab7 in the perinuclear vesicles in MDCK cells. And GFP-ATP7B was colocalized with R-dextran but not with the TGN marker γ-adaptin in the cell line. These results further confirm the late endosomal localization of ATP7B.
NPC is an autosomal recessive neurovisceral lipid storage disorder. The prominent cellular features of this metabolic disorder are extensive sequestration and accumulation of low-density lipoprotein-derived cholesterol in lysosomes resulting from a defect in the translocation of cholesterol to other cellular membranes. The gene most commonly mutated in this disorder, NPC1, was cloned and encoded a 4.9-kb mRNA that was translated into a protein of 1278 amino acids with an estimated molecular mass of 124 kd.28,30,40 The steady-state localization of NPC1 is the late endosomes, and GFP-ATP7B was completely colocalized with NPC1.
U18666A is a hydrophobic amine, which blocks lysosomal cholesterol transport and induces the NPC phenotype. Treatment with U18666A produces a hybrid organelle having characteristics of both late endosomes and lysosomes.29,30 This organelle is called a late endosome-lysosome hybrid organelle, which contains markers of both lysosomes and late endosomes.41 It is suggested that U18666A allows fusion of NPC1 vesicles with lysosomes but blocks their subsequent fission.29 In the present study, unique electron-dense structures containing small lipid-like particles, probably late endosome-lysosome hybrid organelles, were observed in U18666A-treated cells by transmission electron microscopy. GFP-ATP7B was localized in perinuclear large vesicles and was colocalized with R-dextran, Lamp 1, Lamp 2, and NPC1 after treatment with U18666A. These results indicated that ATP7B was localized in the late endocytic structures. Interestingly, GFP-ATP7B was almost completely colocalized with Lamp 1 and 2 in U18666A-treated cells, although it was localized in a part of Lamp 1- or Lamp 2-containing vesicles in untreated cells. These findings indicate that GFP-ATP7B is located only in the late endosomes in ordinary conditions, although Lamp 1 and 2 are located in both late endosomes and lysosomes.37 In U18666A-treated cells, GFP-ATP7B, NPC1, Lamp 1, and Lamp 2 were localized in the late endosome-lysosome hybrid organelles. Interestingly, GFP-ATP7B was not colocalized with cathepsin D in untreated cells, however, some GFP-ATP7B-bearing vesicles contained cathepsin D in U18666A-treated cells. These results further confirm that ATP7B is localized in the late endosomes. Although the precise role of NPC1 in vesicle traffic has not been clear, NPC1 shuttles between the delimiting membrane and inner vesicles of multivesicular late endosomes and regulates trafficking of various substances. U18666A inhibits this movement.40,41 Therefore, NPC1 may regulate the movement of ATP7B in the late endosomes, because NPC1 regulates many substances including glycolipids and endocytosed sucrose and sterol.29 The detailed mechanism of this phenomenon will be further elucidated. After treatment with U18666A, the distributions of GalT and 58-kd Golgi protein were not altered, and GFP-ATP7B was not colocalized with GalT and 58-kd Golgi protein before or after treatment with U18666A. Furthermore, in our previous study, GFP-ATP7B was not colocalized with Golgi proteins. Treatment with brefeldin A or nocodazole affected the distribution of Golgi proteins but did not affect the perinuclear distribution of GFP-ATP7B.11 Furthermore, GFP-ATP7B was not colocalized with γ-adaptin in MDCK cells. These results indicate that ATP7B is not a Golgi resident protein, although some investigators described that ATP7B was localized at the TGN.15–25
One may be anxious about the effect of overexpression of GFP-ATP7B on its distribution. However, both GFP-ATP7B and endogeneous ATP7B were similarly distributed as perinuclear punctate patterns. Furthermore, GFP-ATP7B was localized only in the late endosomes in both low expressing- and high expressing-cells. If overexpression of the protein affects its distribution, it may be localized in the proteasomes (or aggresomes) or the autophagic vacuoles (or lysosomes).42–45 Therefore, it is unlikely that the overexpression affects the distribution of ATP7B.
In conclusion, ATP7B was certainly localized in the late endosomes. The following role of ATP7B in copper metabolism in hepatocytes is considered: ATP7B transports copper from the cytosol to the late endosomal lumen, and then copper in the late endosomes are transported to lysosomes and excreted to the bile by a process known as biliary lysosomal excretion.46,47 Some copper in the late endosomes may be transported to the TGN by mannose-6-phosphate receptor recycling vesicles, which deliver newly synthesized lysosomal enzymes from the TGN to late endosomes and recycle back to the TGN for reutilization.48 Then copper may be incorporated into apoceruloplasmin. Copper-incorporated holoceruloplasmin may be secreted to the sinusoidal space through the secretory pathway. Thus, the defective copper ATPase activity in hepatocyte late endosomes due to various types of mutations of ATP7B must be the main defect in Wilson disease.
Acknowledgments
We thank Dr. A. Wandinger-Ness for providing Rab 7 cDNA and Ms. R. Inayoshi for expert technical assistance.
Footnotes
Address reprint requests to Masaru Harada, M.D., Ph.D, Second Department of Medicine, Kurume University School of Medicine, 67 Asahi-Machi, Kurume 830-0011, Japan. E-mail: harada@med.kurume-u.ac.jp.
Supported in part by grants-in-aid (12670535, 14570528) from the Ministry of Education of Japan and a grant from the Foundation for Advancement of International Science (FAIS) (to M.H.).
References
- Linder MC, Hazegh-Azam M. Copper biochemistry and molecular biology. Am J Clin Nutr. 1996;63:797S–811S. doi: 10.1093/ajcn/63.5.797. [DOI] [PubMed] [Google Scholar]
- Camakaris J, Voskoboinik I, Mercer JF. Molecular mechanisms of copper homeostasis. Biochem Biophys Res Commun. 1999;261:225–232. doi: 10.1006/bbrc.1999.1073. [DOI] [PubMed] [Google Scholar]
- Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- Petrukhin K, Fischer SG, Pirastu M, Tanzi RE, Chernov I, Devoto M, Brzustowicz LM, Cayanis E, Vitale E, Russo JJ, Matseoane D, Boukhgalter B, Wasco W, Fifus AL, Loudianos J, Cao A, Sternlieb I, Evgrafov O, Parano E, Pavone L, Warburton D, Ott J, Penchaszadeh GK, Scheinberg IH, Gilliam TC. Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat Genet. 1993;5:338–343. doi: 10.1038/ng1293-338. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowitz LM, Devoto M, Peppercorn J, Bush AI, Sternlieb I, Pirastu M, Gusella JF, Evgrafov O, Penchaszadeh GK, Honig B, Edelman IS, Soares MB, Scheinberg IH, Gilliam TC. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Heiny ME, Gitlin JD. Isolation and characterization of a human liver cDNA as a candidate gene for Wilson disease. Biochem Biophys Res Commun. 1993;197:271–277. doi: 10.1006/bbrc.1993.2471. [DOI] [PubMed] [Google Scholar]
- Terada K, Nakako T, Yang XL, Iida M, Aiba N, Minamiya Y, Nakai M, Sakaki T, Miura N, Sugiyama T. Restoration of holoceruloplasmin synthesis in LEC rat after infusion of recombinant adeno virus bearing WND cDNA. J Biol Chem. 1998;273:1815–1820. doi: 10.1074/jbc.273.3.1815. [DOI] [PubMed] [Google Scholar]
- Terada K, Aiba N, Yang XL, Iida M, Nakai M, Miura N, Sugiyama T. Biliary excretion of copper in LEC rat after introduction of copper transporting P-type ATPase, ATP7B. FEBS Lett. 1999;448:53–56. doi: 10.1016/s0014-5793(99)00319-1. [DOI] [PubMed] [Google Scholar]
- Harada M. Wilson disease. Med Electron Microsc. 2002;35:61–66. doi: 10.1007/s007950200007. [DOI] [PubMed] [Google Scholar]
- Harada M, Kumemura H, Kawaguchi T, Sata M. Where is the site that ATP7B transports copper within hepatocytes? Gastroenterology. 2003;125:1911–1912. doi: 10.1053/j.gastro.2003.05.012. [DOI] [PubMed] [Google Scholar]
- Harada M, Sakisaka S, Terada K, Kimura R, Kawaguchi T, Koga H, Taniguchi E, Sasatomi K, Miura N, Suganuma T, Fujita H, Furuta K, Tanikawa K, Sugiyama T, Sata M. Role of ATP7B in biliary copper excretion in a human hepatoma cell line and normal rat hepatocytes. Gastroenterology. 2000;118:921–928. doi: 10.1016/s0016-5085(00)70178-8. [DOI] [PubMed] [Google Scholar]
- Harada M, Sakisaka S, Kawaguchi T, Kimura R, Taniguchi E, Koga H, Hanada S, Baba S, Furuta K, Kumashiro R, Sugiyama T, Sata M. Copper does not alter the intracellular distribution of ATP7B, a copper-transporting ATPase. Biochem Biophys Res Commun. 2000;275:871–876. doi: 10.1006/bbrc.2000.3403. [DOI] [PubMed] [Google Scholar]
- Harada M, Sakisaka S, Terada K, Kimura R, Kawaguchi T, Koga H, Kim M, Taniguchi E, Hanada S, Suganuma T, Furuta K, Sugiyama T, Sata M. A mutation of the Wilson disease protein, ATP7B, is degraded in the proteasomes and forms protein aggregates. Gastroenterology. 2001;120:967–74. doi: 10.1053/gast.2001.22543. [DOI] [PubMed] [Google Scholar]
- Harada M, Kumemura H, Sakisaka S, Shishido S, Taniguchi E, Kawaguchi T, Hanada S, Koga H, Kumashiro R, Ueno T, Suganuma T, Furuta K, Namba M, Sugiyama T, Sata M. Wilson disease protein ATP7B is localized in the late endosomes in a polarized human hepatocyte cell line. Int J Mol Med. 2003;11:293–298. [PubMed] [Google Scholar]
- Hung IH, Suzuki M, Yamaguchi Y, Yuan DS, Klausner RD, Gitlin JD. Biochemical characterization of the Wilson disease protein and functional expression in the yeast Saccharomyces cerevisiae. J Biol Chem. 1997;272:21461–21466. doi: 10.1074/jbc.272.34.21461. [DOI] [PubMed] [Google Scholar]
- Yang XL, Miura N, Kawarada Y, Terada K, Petrukhin K, Gilliam TC, Sugiyama T. Two forms of Wilson disease protein produced by alternative splicing are localized in distinct cellular compartments. Biochem J. 1997;326:897–902. doi: 10.1042/bj3260897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fontaine SL, Firth SD, Camakaris J, Englezou A, Theophilos MB, Petris MJ, Howie M, Lockhart PJ, Greenough M, Brooks H, Reddel RR, Mercer JF. Correction of the copper transport defect of Menkes patient fibroblasts by expression of the Menkes and Wilson ATPases. J Biol Chem. 1998;273:31375–31380. doi: 10.1074/jbc.273.47.31375. [DOI] [PubMed] [Google Scholar]
- Payne AS, Kelly EJ, Gitlin JD. Functional expression of the Wilson disease protein reveals mislocalization and impaired copper-dependent trafficking of the common H1069Q mutation. Proc Natl Acad Sci USA. 1998;95:10854–10859. doi: 10.1073/pnas.95.18.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano K, Nakamura K, Urakami K, Umeyama K, Uchiyama H, Koiwai K, Hattori S, Yamamoto T, Matsuda I, Endo F. Intracellular distribution of the Wilson’s disease gene product (ATP7B) after in vitro and in vivo exogenous expression in hepatocytes from the LEC rat, an animal model of Wilson’s disease. Hepatology. 1998;27:799–807. doi: 10.1002/hep.510270323. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Hopkins RG, Failla ML, Gitlin JD. Hepatocyte-specific localization and copper-dependent trafficking of the Wilson’s disease protein in the liver. Am J Physiol. 1999;276:G639–G646. doi: 10.1152/ajpgi.1999.276.3.G639. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Roelofsen H, Wolters H, Hofmann WJ, Muller M, Kuipers F, Stremmel W, Vonk RJ. Localization of the Wilson’s disease protein in human liver. Gastroenterology. 1999;117:1380–1385. doi: 10.1016/s0016-5085(99)70288-x. [DOI] [PubMed] [Google Scholar]
- Forbes JR, Cox DW. Copper-dependent trafficking of Wilson disease mutant ATP7B proteins. Hum Mol Genet. 2000;9:1927–1935. doi: 10.1093/hmg/9.13.1927. [DOI] [PubMed] [Google Scholar]
- Roelofsen H, Wolters H, Van Luyn MJ, Miura N, Kuipers F, Vonk RJ. Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology. 2000;119:782–793. doi: 10.1053/gast.2000.17834. [DOI] [PubMed] [Google Scholar]
- La Fontaine S, Theophilos MB, Firth SD, Gould R, Parton RG, Mercer JF. Effect of the toxic milk mutation (tx) on the function and intracellular localization of Wnd, the murine homologue of the Wilson copper ATPase. Hum Mol Genet. 2001;10:361–370. doi: 10.1093/hmg/10.4.361. [DOI] [PubMed] [Google Scholar]
- Huster D, Hoppert M, Lutsenko S, Zinke J, Lehmann C, Mossner J, Berr F, Caca K. Defective cellular localization of mutant ATP7B in Wilson’s disease patients and hepatoma cell lines. Gastroenterology. 2003;124:335–345. doi: 10.1053/gast.2003.50066. [DOI] [PubMed] [Google Scholar]
- Somsel Rodman J, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci. 2000;113:183–192. doi: 10.1242/jcs.113.2.183. [DOI] [PubMed] [Google Scholar]
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- Neufeld EB, Wastney M, Patel S, Suresh S, Cooney AM, Dwyer NK, Roff CF, Ohno K, Morris JA, Carstea ED, Incardona JP, Strauss JF, III, Vanier MT, Patterson MC, Brady RO, Pentchev PG, Blanchette-Mackie EJ. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J Biol Chem. 1999;274:9627–9635. doi: 10.1074/jbc.274.14.9627. [DOI] [PubMed] [Google Scholar]
- Zhang M, Dwyer NK, Neufeld EB, Love DC, Cooney A, Comly M, Patel S, Hidemichi W, Straus JF, III, Pentchev PG, Hanover JA, Blanchette-Mackie EJ. Sterol-modulated glycolipid occurs in Niemann-Pick C1 late endosomes. J Biol Chem. 2001;276:3417–3425. doi: 10.1074/jbc.M005393200. [DOI] [PubMed] [Google Scholar]
- Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- Kobayashi N, Miyazaki M, Fukaya K, Inoue Y, Sakaguchi M, Uemura T, Noguchi H, Kondo A, Tanaka N, Namba M. Transplantation of highly differentiated immortalized human hepatocytes to treat acute liver failure. Transplantation. 2000;69:202–207. doi: 10.1097/00007890-200001270-00002. [DOI] [PubMed] [Google Scholar]
- Feng Y, Press B, Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press B, Feng Y, Hoflack B, Wandinger-Ness A. Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J Cell Biol. 1998;140:1075–1089. doi: 10.1083/jcb.140.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Ninomiya H, Ohsaki Y, Higaki K, Davies JP, Ioannou YA. Accumulation of cholera toxin and GM1 ganglioside in the early endosome of Niemann-Pick C1-deficient cells. Proc Natl Acad Sci USA. 2001;98:12391–12396. doi: 10.1073/pnas.221181998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano J, Ide S, Oinuma T, Suganuma T. A protein-specific monoclonal antibody to rat liver β1,4 galactolsyltransferase and its application to immunohistochemistry. J Histochem Cytochem. 1994;42:363–369. doi: 10.1177/42.3.8308253. [DOI] [PubMed] [Google Scholar]
- Chen JW, Murphy TL, Willingham MC, Pastan I, August JT. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985;101:85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra M, Veld GIT, Berg GJVD, Muller M, Kuipers F, Vonk RJ. Adenosine triphosphate-dependent copper transport in isolated rat liver plasma membrane. J Clin Invest. 1995;95:412–416. doi: 10.1172/JCI117670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum L. Niemann-Pick type C mutations cause lipid traffic jam. Traffic. 2000;1:218–225. doi: 10.1034/j.1600-0854.2000.010304.x. [DOI] [PubMed] [Google Scholar]
- Luzio JP, Rous BA, Bright NA, Pryor PR, Mullock BM, Piper RC. Lysosome-endosome fusion and lysosome biogenesis. J Cell Sci. 2000;113:1515–1524. doi: 10.1242/jcs.113.9.1515. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R, Bebok Z, Sorscher EJ, Sztul ES. Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J Cell Biol. 1999;146:1239–0254. doi: 10.1083/jcb.146.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Kamijo K, Oda T. Degradation of overexpressed wild-type and mutant uricase proteins in cultured cells. J Histochem Cytochem. 1999;47:1133–1140. doi: 10.1177/002215549904700905. [DOI] [PubMed] [Google Scholar]
- Harada M, Kumemura H, Omary MB, Kawaguchi T, Maeyama N, Hanada S, Taniguchi E, Koga H, Suganuma T, Ueno T, Sata M. Proteasome inhibition induces inclusion bodies associated with intermediate filaments and fragmentation of the Golgi apparatus. Exp Cell Res. 2003;288:60–69. doi: 10.1016/s0014-4827(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Gross JB, Myers BM, Kost LJ, Kuntz SM, LaRusso NF. Biliary copper excretion by hepatocyte lysosomes in the rat. J Clin Invest. 1989;83:30–39. doi: 10.1172/JCI113873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Sakisaka S, Yoshitake M, Shakadoh S, Gondoh K, Sata M, Tanikawa K. Biliary copper excretion in acutely and chronically copper-loaded rats. Hepatology. 1993;17:111–117. [PubMed] [Google Scholar]
- Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]