Abstract
Tissue regeneration on acellular matrix grafts has great potential for therapeutic organ reconstruction. However, hollow organs such as the bladder require smooth muscle cell regeneration, the mechanisms of which are not well defined. We investigated the mechanisms by which bone marrow cells participate in smooth muscle formation during urinary bladder regeneration, using in vivo and in vitro model systems. In vivo bone marrow cells expressing green fluorescent protein were transplanted into lethally irradiated rats. Eight weeks following transplantation, bladder domes of the rats were replaced with bladder acellular matrix grafts. Two weeks after operation transplanted marrow cells repopulated the graft, as evidenced by detection of fluorescent staining. By 12 weeks they reconstituted the smooth muscle layer, with native smooth muscle cells (SMC) infiltrating the graft. In vitro, the differential effects of distinct growth factor environments created by either bladder urothelial cells or bladder SMC on phenotypic changes of marrow cells were examined. First, supernatants of cultured bladder cells were used as conditioned media for marrow cells. Second, these conditions were reconstituted with exogenous growth factors. In each case, a growth factor milieu characteristic of SMC induced an SMC-like phenotype in marrow cells, whereas that of urothelial cells failed. These findings suggest that marrow cells differentiate into smooth muscle on acellular matrix grafts in response to the environment created by SMC.
Tissue regeneration on acellular extracellular matrix grafts has been studied in various experimental models and organs.1–13 Many promising features for these grafts have been reported, including escape from immunological rejection and regeneration of the native tissue on them. Despite these potential advantages, effective tissue renewal on acellular grafts is not demonstrated reproducibly in hollow organs such as the urinary and digestive tracts, or the vasculature. For reconstruction of these organs, smooth muscle regeneration is a crucial requirement, since their functions as reservoirs and/or conduits are dependent on the compliance and contraction of smooth muscle.1–5 Although smooth muscle has been shown to regenerate on some acellular grafts without any additional cell transplantation, as in tissue engineering, often this does not extend throughout the whole graft, resulting in a limited contribution of the regenerated component to functional reconstruction of the organs.3,8,14 A major drawback of these previous reports was their focus primarily on a phenomenological description of the animal experiments, with less attention given to the potential mechanisms underlying muscle regeneration.
Growth factors are believed to play important roles in muscle regeneration. The presence of multiple growth factors on small intestine submucosa (SIS), the most frequently used acellular matrix graft material, is considered to be the major advantage of the material.6,7 However, the specific contribution of each growth factor to tissue regeneration remains unclear. Another current hypothesis proposes that, during regeneration of the urinary bladder on acellular grafts, soluble factors secreted by urothelial cells (UC) within the urinary tract, induce maturation of the smooth muscle layer on the graft via epithelial-mesenchymal interaction.1,10–14 Despite this, no candidate factor from UC capable of inducing smooth muscle formation has been identified. On the other hand, we have reported that exogenous growth factors can be effectively incorporated onto acellular grafts, enabling controlled regeneration of urinary bladder tissue. However, the optimal choice and combination of growth factors to be added could not be determined as long as the functional roles of these growth factors remain unclear.8,9 Taken together, elucidation of the mechanisms underlying the regeneration of smooth muscle layer is essential to enable safe and reliable clinical application of these grafts.
Recently, novel experimental models have shed new light on this problem. Urothelium implanted ectopically into the omentum or under the renal capsule recruits mesenchymal cells positive for early smooth muscle markers, however these infiltrating cells do not mature into a functional smooth muscle layer.14,15 Another model using bone marrow-chimeric animals suggested that the cells repopulating acellular grafts are of bone marrow origin.16 Since several recent studies have described growth factor-dependent differentiation of stem or precursor cells in different experimental systems,17–26 we hypothesized in this study that dedifferentiated mesenchymal cells or circulating bone marrow-derived precursor cells, may undergo differentiation on acellular bladder patches in response to the local growth factor environment elaborated by endogenous bladder cells. To test this hypothesis, we used in vivo and in vitro models using bone marrow cells. In vivo, chimeric rats generated by transplantation of bone marrow cells expressing green fluorescent protein (GFP), were subjected to bladder patch repair and the participation of marrow cells in regeneration of the bladder smooth muscle layer was studied. In vitro, marrow-derived stromal cells (MSCs), which can potentially differentiate into various types of mature mesenchymal cells including osteoblast, chondrocyte, adipocyte, and muscle cells,27 were cultured in growth factor environments created by two kinds of bladder cells: smooth muscle cells (SMC) and urothelial cells (UC). The effects of growth factors from either bladder SMC or UC on the phenotype of marrow cells were examined.
Materials and Methods
Bladder Patch Repair with Bladder Acellular Matrix in Bone Marrow-Chimeric Rats
Rats and Bladder Acellular Matrix (BAM)
Wild-type Sprague-Dawley (SD) rat and EGFP (enhanced GFP) transgenic SD rat, generated by Dr. Okabe (Osaka University, Osaka, Japan)28 were purchased from Japan Slc. Inc (Shizuoka, Japan). BAM was processed from wild-type SD rat sacrificed for other experiments as described elsewhere.8,9 Animals were treated in accordance with NIH animal care guidelines and all animal experiments were approved by Kyoto University Animal Experiment Committee.
Generation of Bone Marrow-Chimeric Rat
Bone marrow transplantation (BMT) was carried out as described elsewhere.21 Briefly bone marrow of 10 EGFP-transgenic SD rats was harvested from tibia and femur after euthanization with diethylether overdosage. Bone marrow was flushed off from the bones with phosphate-buffered saline (PBS) containing 5% fetal bovine serum (FBS, Hyclone, Logan, UT), homogenized, and injected to 16 lethally irradiated (10 Gray) 6-week-old female SD rats. Two rats died after BMT. Flow cytometry performed 2 months after the BMT determined that both donor and chimera rat peripheral blood contained 25 to 35% of GFP-positive cells after dissolution of red blood cells,22 suggesting the establishment of stable chimerism.
Bladder Patch Repair with BAM
In chimeric animals generated above, the upper half of the bladder was resected, and either simply closed with running 8–0 polyglactin suture (Ethicon, Cornelia, GA) (chimera; partial cystectomy, n = 7) or replaced with bladder acellular matrix graft as described previously (chimera; patch repair n = 7).8 The resected bladder wall was subjected to histological examination to determine the presence of bone marrow-derived cells in the native tissue. The same patch repair was also performed in a group of wild-type SD rats (non-chimera; patch repair, n = 7). Two rats in chimera group died after patch repair. At 2, 4, and 12 weeks postoperatively, two rats from each group were evaluated, with the exception of the chimera patch group at 4 weeks (n = 1). Briefly, after the rats were sacrificed by overdose of diethylether, the bladder was filled with 0.6 ml of ice-cold PBS via 24-gauge plastic transurethral tube, and the urethra was clamped and ligated for removal of the bladder. The bladder was immediately observed under fluorescence stereomicroscope (Leica, Heerbrugg, Switzerland), under bright field or under ultraviolet lamp with green fluorescent protein filter.22 All images were recorded with a digital camera under fixed exposure to standardize the fluorescence of the image. Bladders were dissected and underwent a serial sucrose gradient, to be frozen and embedded in O.C.T compound (Sakura Finetechnical Co. Tokyo, Japan). Frozen sections of 5 μm thickness were either observed directly under fluorescence microscope, stained by hematoxylin and eosin (H&E) or double-immunostained with rabbit anti-GFP antibody (Clontech, Palo Alto, CA) and mouse anti-αSouth Muscle Actin antibody. Sections were visualized following incubation with fluorescein-conjugated anti-rabbit, immunoglobulin (Dako Japan, Kyoto, Japan) and Texas-red-conjugated anti-mouse immunoglobulin (Amersham Biosciences, Buckinghamshire, UK). The stained area was analyzed with NIH image software.29
Cell Culture
Isolation of Marrow-Derived Stromal Cells (MSC)
Bone marrow was harvested from tibia and femur dissected from 3-weeks-old male SD rats, euthanized by diethylether overdose.27 Bone marrow was flushed off the bones with α minimum essential medium (αMEM) with 15% FBS, homogenized, and incubated in 25-cm2 cell culture flasks (Corning, Corning, NY).27 Seventy-two hours later, and on alternate days thereafter, the culture medium was replaced. Adherent cells were cultured until confluent (∼1 week, on average) and were subjected to subsequent experiments. Typically, MSC were harvested from 3 to 4 rats at a time, and expanded to 2 to 3 × 107 cells in total at 1 week.
Isolation of Bladder Cells
Bladder smooth muscle and urothelial cells were isolated from eight 10-week-old female SD rats at each experiment by a combination of the procedures described elsewhere.30–33 Briefly, dissected rat bladders were incubated on a silicone dish in 1000 U/ml dispase (Godo Shusei, Tokyo, Japan) for 12 hours at 4°C. The mucosa was peeled off, minced, and digested in 0.05% trypsin and 0.02% EDTA for 10 minutes at 37°C. The cell suspension was sieved through a cell strainer, re-suspended in keratinocyte serum-free medium (Gibco, Long Island, NY) at a density of 1 × 105 cells/ml, and was seeded in 12-well cell culture dishes coated with collagen type IV (Nitta Gelatin Co., Osaka, Japan). The medium was changed 48 hours later and every other day thereafter. The remaining bladder tissue was digested with collagenase type IV (Sigma, St. Louis, MO, 250 U/ml) for 30 minutes at 37°C. Smooth muscle cells were suspended in Medium 199 (Sigma) with 10% FBS and plated in 25 cm2 cell culture flasks. The medium was changed 24 hours later, and every other day thereafter.
Culture of Bone Marrow Cells in Conditioned Media from Bladder Cells
Retrieval of Conditioned Media
When each population of bladder cells (UC or SMC) reached 50 to 70% confluency, primary culture media were changed to αMEM with 15% FBS. Each medium was retrieved after 48 hours, passed through 0.22-μm nitrocellulose filter, and used as conditioned medium (CM).34 SMC and UC after retrieval of CM were either lysed in lysis buffer (100 mmol/L Tris-HCl (pH 7.4), 75 mmol/L NaCl, 1% Triton-X 100, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 mmol/L phenylmethylsulfonyl fluoride), or trypsinized and seeded on 8-well chamber slides for immunostaining, as controls of each cellular phenotype.
Incubation of MSC in Conditioned Media
Confluent MSC were passaged and seeded at cell density of 1 × 105/ml on 4-cm culture dish (Primaria, Becton Dickinson, NJ), either in SMC-conditioned medium (SMC-CM), UC-conditioned medium (UC-CM), or αMEM containing 15% FBS as a control. The media were changed every 3 days thereafter and the culture was maintained for up to 8 days. Cells were lysed on day 0 (before first passage), day 3, 6, or 8 as described35 or passaged again onto 8-well chamber slides for immunostaining.
Cell Growth Assay
MSC were plated in 96-well culture plates at the same density and conditions as described above. At 1, 3, 4, and 6 days, the cells were washed twice with PBS and frozen at −20°C. DNA content was determined by fluorescence intensity of the DNA-binding dye, Hoechst 33258 (Nacalai tesque, Kyoto, Japan) bound to cell lysates as described elsewhere.27
Immunofluorescence Microscopy
MSC before and after incubation in control medium or bladder cell-conditioned media were seeded in chamber slides in standard culture medium, and allowed to adhere overnight. Cells were washed twice with PBS, fixed with 4% paraformaldehyde, and incubated with antibodies for αSMA (Sigma 1:100), calponin (Sigma, 1:100), and cytokeratin 18 (CK 18, Sigma 1:100). Cells were visualized by fluorescence microscopy following incubation with fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin (Dako Japan).
Western Blotting
The protein content of cell lysates was measured using the BioRad Protein Assay Kit (BioRad Laboratories, Hercules, CA). Cell lysates were resolved by sodium dodecylsulfate polyacrylamide electrophoresis (SDS-PAGE) under reducing conditions, and immunoblotted with antibodies against CK 18 (1:4000), αSMA (1:5000), smooth muscle myosin heavy chain (SM-MHC, Santa Cruz, 1:100), and calponin (1:1000) using methods described elsewhere.35 Expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was also evaluated with anti-GAPDH antibody (1:200; Chemicon, Temecula, CA) as an internal control.
Quantification of Growth Factors in Conditioned Media
The content of growth factors in the conditioned media was assessed by enzyme-linked immunosorbent assay (ELISA). The kits used were: rat hepatocyte growth factor (HGF) assay (Tokushu Men-eki Kenkyujo, Tokyo, Japan), mouse vascular endothelial growth factor (VEGF) (R&D Systems, Inc., Minneapolis, MN), mouse SCF (R&D), human bFGF (R&D), and human transforming growth factor β (TGFβ) (R & D). For mouse kits, rat VEGF (R&D) and rat stem cell factor (SCF) (Peprotech, London, UK) were used as standards. The detection of platelet-derived growth factor-BB (PDGF-BB) was carried out with antibodies kindly given from Mochida Pharmaceutical Co. (Tokyo, Japan)36 with rat PDGF-BB protein (R&D) as standard. For human kits, data are presented as percentage of control level (αMEM + 15%FBS).
Simulation of Conditioned Media by Exogenous Growth Factors
The conditions determined above were simulated by adding exogenous growth factors into αMEM with 0.5% FBS. SMC-CM was simulated by adding 40 ng/ml human recombinant HGF (kindly provided from Snow Brand, Tokyo, Japan) and 5 ng/ml recombinant TGFβ (R&D). UC-CM was simulated by adding 50 ng/ml bFGF (kindly provided from Kaken Pharmaceuticals Co., Tokyo, Japan) and 100 ng/ml rat VEGF (R&D). Other cells were cultured in αMEM with 0.5% FBS containing no or each single growth factor at the dosage as described above. MSC were incubated for 6 days, and cell growth and phenotypic changes were evaluated as described above.
Blockade of Growth Factor Activity in Conditioned Media by Neutralizing Antibodies
Growth factor activity in conditioned and control media was neutralized with blocking antibodies, and the phenotypes of MSC cultured for 6 days under these conditions, were examined by the methods described above. TGFβ and/or HGF in SMC-CM were blocked by 35 μg/ml pan-specific TGFβ antibody (R&D), 1 μg/ml anti-c-met antibody (Sigma), or both. bFGF in UC-CM and control medium (αMEM + 15% FBS) was blocked by 20 μg/ml anti-bFGF antibody (Upstate Cell Signaling Solutions, Lake Placid, NY).
Results
Bladder Patch Repair by Bladder Acellular Matrix, in Bone Marrow-Chimeric Rats
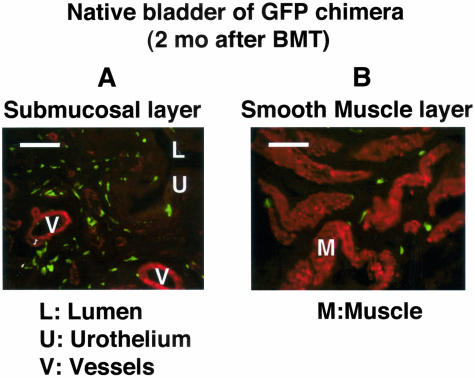
GFP-positive cells were present in the bladder wall excised at surgery 2 months after bone marrow transplantation. They were mainly distributed in the submucosal area (Figure 1A). In the smooth muscle layer, fewer cells were located at the periphery of smooth muscle fascicles (Figure 1B). No cells that were positive for both αSMA and GFP were detected.
Figure 1.
Bladder of bone marrow-chimeric rat and the distribution of transplanted marrow-derived cells in the native bladder. Two months after bone marrow transplantation, the bladder wall was excised at the surgery and was stained for αSMA (red) and GFP (green). A: Submucosal area: relatively higher numbers of transplanted GFP-positive cells, which are negative for αSMA, are distributed. B: Smooth muscle layer: fewer cells are located at the periphery of smooth muscle fascicles. Bar, 100 μm (magnification, ×400). After fixation in 4% paraformaldehyde for 3 hours at 4°C, the specimen was processed in a serial sucrose gradient, frozen and embedded in cryocompound. Frozen sections (5-μm thick) were stained with hematoxylin and eosin (H&E) or double-immunostained with rabbit anti-GFP antibody (1:200) and mouse anti-αSMA antibody (1:100), and then visualized following incubation with fluorescein-conjugated anti-rabbit immunoglobulin and Texas-red-conjugated anti-mouse immunoglobulin.
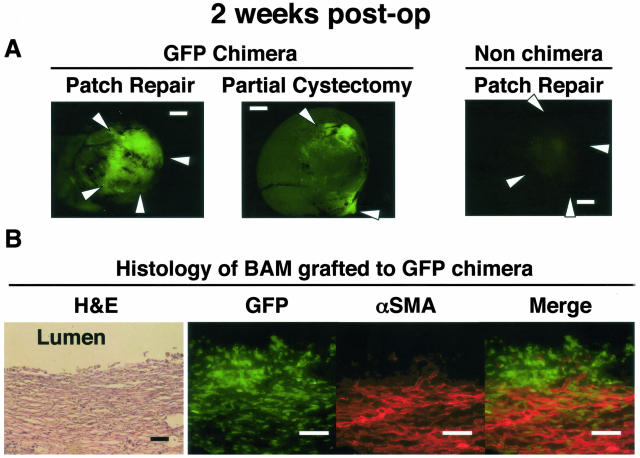
Two weeks after the bladder patch repair with acellular matrix, dense infiltration of GFP-positive cells was observed into the graft (Figure 2, A and B). Most of the cells were white blood cells, although a band of αSMA-positive cells was observed beneath the regenerating urothelium. Few cells stained positively for both GFP and αSMA, suggesting that the majority of αSMA-positive cells were of native bladder origin (Figure 2B). There were also few double-positive cells in the graft at 4 weeks.
Figure 2.
Two weeks after the bladder patch repair with acellular matrix. A: Fluorescence stereomicroscopy: in chimeric rats, fluorescence was increased in the graft in patch repair rat, or around the marking nylon suture in partial cystectomy rat. The fluorescence along the 8–0 polyglactin suture was insignificant, suggesting that inflammatory response to the anastomosing suture material may not be the chief cause for the infiltration of marrow-derived cells. No increase in fluorescence was detected in non-chimeric rats, which excludes the possibility of autofluorescence in GFP chimera. White arrows indicate the location of marking sutures. B. Histology: The specimen was processed as in Figure 1. GFP-positive marrow-derived cells infiltrated the graft. A band of αSMA-positive cells is observed beneath the regenerating urothelium. Few cells are double-stained for GFP and αSMA. Bar, 100 μm (magnification, ×400).
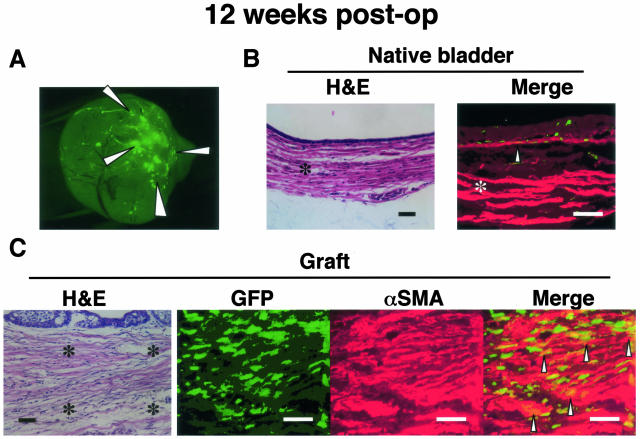
Twelve weeks after the bladder patch repair, fluorescence on the graft was still elevated compared to native bladder, but was less intense than observed at 2 weeks (Figure 3A). In the native bladder, the localization of GFP-positive cells was similar to that observed pre-operatively, but some GFP-positive cells in the submucosal area were also positive for αSMA (Figure 3B). The regenerating smooth muscle layer on the graft, which stained positive for αSMA, consisted of a mixture of GFP-positive and -negative cells (Figure 3C). GFP cells were scattered in the graft as patchy foci, where GFP signal was 100- to 200-fold more intense compared with the native bladder tissue. Double-positive cells were mainly located among the regenerating smooth muscle. The double-positive rate among GFP-positive cells in the graft area ranged from 1.2% to 4.6% per cross-section.
Figure 3.
Twelve weeks after the bladder patch repair with acellular matrix. A: Fluorescence stereomicroscopy: fluorescence on the graft was observed as patchy foci, and was still prominent at this time point, though less intense. B: Native bladder: Immunostaining for αSMA (red) and GFP (green) shows, scattered overlapping cells in the submucosal region (arrow), and few cells in the muscle layer (asterisk). C: Histology: The picture was taken at the mid-point between the border and the center of the graft. The four corners of the three right panels (immunofluorescence) are indicated by asterisks in the left panel (H&E). Regenerating smooth muscle structures were observed. The layer consisted of substantial amount of cells positive for both GFP and αSMA. The specimen was processed as in Figure 1. Bar, 100 μm (magnification, ×400).
In partial cystecomy group, clustering of GFP-positive cells was initially observed around two marking nylon sutures, but not along the anastomosing bioabsorbable sutures at any time point. In non-chimera patch repair group, little autofluorescence was observed and no cell was stained positive for anti-GFP antibody throughout the study.
Phenotypic Change of Bone Marrow-Derived Cells in Conditioned Media from Bladder Cells
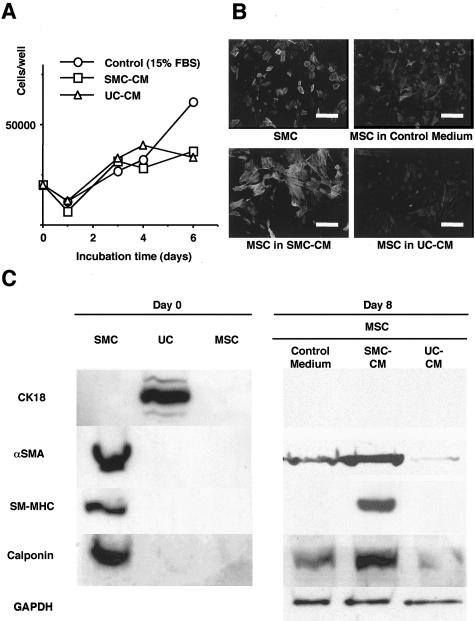
In SMC-CM and UC-CM, MSC proliferated at approximately the same rates, reaching a plateau around day 3 (Figure 4A). Before passage, MSC expressed little or no αSMA, and no other SMC markers (calponin and myosin). After 8 days of culture, MSC cultured in SMC-CM expressed elevated levels of αSMA, myosin, and calponin, which were expressed at lower levels or not at all in MSC cultured in control medium (αMEM + 15% FBS) or in UC-CM (Figure 4, B and C). These phenotypes were expressed homogeneously in the majority of MSC cultured under the same condition (Figure 4B). In contrast, the UC marker (CK 18) was not expressed in MSC cultured under any condition.
Figure 4.
Marrow cells take distinct phenotype in conditioned media of bladder cells. A: Growth curve of MSC cultured in SMC-CM, UC-CM, and control medium. MSC proliferated at equivalent rate in SMC-CM and UC-CM. B: Immunofluorescence for αSMA in cultured SMC and MSC cultured in SMC-CM, UC-CM, and control medium (magnification, ×400). The cells were fixed with 4% paraformaldehyde, incubated with αSMA antibody (1:100), and visualized with fluorescein-isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin. The nucleus was counterstained with Hoechst 33258 dye. C: Left: Cultured SMC expressed αSMA, SM-MHC and calponin. UC expressed cytokeratin. MSC expressed little or no αSMA and no other SMC markers. Right: MSC cultured in SMC-CM expressed stronger expression of all three SMC markers than MSC cultured in control medium or in UC-CM.
Quantification of Growth Factors in Conditioned Media
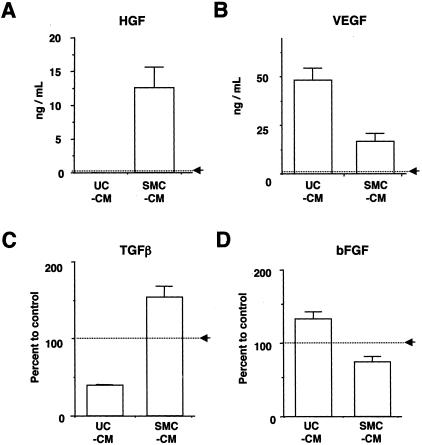
UC-CM and SMC-CM contained distinctly different levels of growth factors from each other (Figure 5). SMC-CM contained relatively higher levels of TGFβ and HGF than UC-CM, while UC-CM contained higher levels of VEGF and bFGF than SMC-CM. PDGF-BB and SCF remained below physiologically relevant levels in both media.
Figure 5.
Bladder cells create distinct growth factor environments in conditioned media. Immunoassay results for HGF, TGFβ, VEGF, and bFGF are presented. A and B: Since levels of HGF and VEGF in control medium were nearly undetectable, these data are presented as concentration of rat growth factor level. C and D: The levels of TGFβ and bFGF are presented as percentage of control level (ie, 15% FBS), because considerable levels of these factors were also detected in control medium. These growth factors could be classified in three categories. A and C: 1) Growth factors that were predominantly present in SMC-CM (HGF and TGFβ. B and D: 2) Growth factors that were predominantly present in UC-CM (VEGF and bFGF). 3) Growth factors that remained below physiological levels (<20pg/ml, PDGF-BB and SCF).
Simulation of the Effect of Conditioned Media by Exogenous Growth Factors
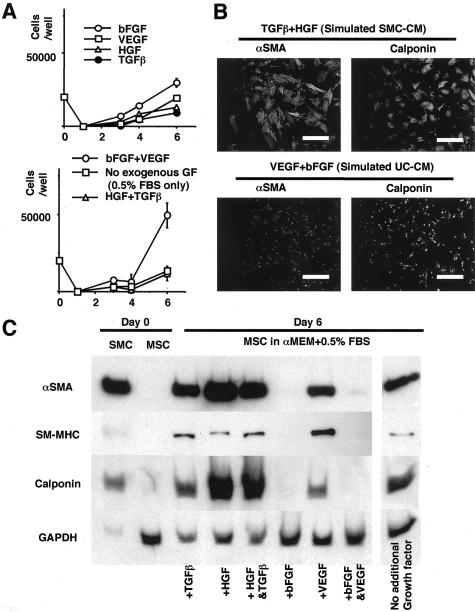
Based on these results, the conditioned media were simulated by adding exogenous growth factors into αMEM + 0.5%FBS. MSC cultured in simulated UC-CM acquired a condensed configuration, resulting in higher cell counts at confluency, compared with MSC cultured in simulated SMC-CM (Figure 6, A and B). After 6 days incubation, MSC incubated in simulated SMC-CM (TGFβ +HGF) expressed all SMC markers, while MSC incubated in simulated UC-CM (bFGF+VEGF) expressed little or no SMC markers (Figure 6, B and C). MSC incubated in αMEM + 0.5%FBS also expressed certain level of SMC markers (Figure 6C).
Figure 6.
Simulation of conditioned media by exogenous growth factors reproduced the phenotype change in marrow cells. Growth factors are added to αMEM + 0.5%FBS based on the ELISA data. A: Growth curve of MSC. MSC cultured in simulated UC-CM showed higher proliferation, which may be attributed to condensed cellular configuration. B: Immunofluorescence for SMC markers (magnification, ×400). The cells were fixed with 4% paraformaldehyde, incubated with αSMA and calponin antibodies (1:100 for each), and visualized with FITC-conjugated anti-mouse immunoglobulin. The nucleus was counterstained with Hoechst 33258 dye. C: Immunoblotting: simulated SMC-CM and UC-CM induced the similar differential phenotypic change in MSC.
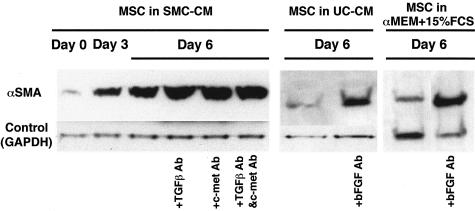
Blockade of Growth Factors in Conditioned Media by Neutralizing Antibodies
Neutralizing antibodies against TGFβ and/or HGF did not abolish the expression of αSMA in MSC cultured in SMC-CM. On the contrary, neutralization of bFGF in UC-CM and control medium (αMEM + 15% FBS) induced increased expression of αSMA in MSC (Figure 7).
Figure 7.
The effect of blocking antibody for growth factors in conditioned media. Blockade of TGFβ and/or HGF in SMC-CM did not block the expression of SMC marker. Inhibition of bFGF in UC-CM and in the control medium induced higher expression of αSMA.
Discussion
This study demonstrates the involvement of bone marrow cells in the formation of smooth muscle tissue on acellular grafts, and the differential effect of growth factors from bladder SMC and UC on phenotypic changes in marrow cells. Marrow cells acquired an SMC-like phenotype in the growth factor environment created by SMC in culture, while the growth factor milieu of UC failed to induce such a phenotype. These findings suggest that differentiation of infiltrating immature bone marrow cells to an SMC-like phenotype may contribute to regeneration of bladder muscle on acellular matrix grafts under influence from local SMC, but not from UC.
Although the present results suggest minimal involvement of the urothelium in maturation of smooth muscle tissue on acellular grafts, as postulated by other authors, our findings may not necessarily be in conflict with those reports.1,10–14 The growth factors secreted predominantly from urothelium (bFGF and VEGF) are potently angiogenic, and neovasculature created by them may generate an environment suitable for survival of infiltrating marrow-derived cells, SMC, and UC themselves (Figure 2). In addition, mature smooth muscle tissue does not regenerate beneath ectopically isolated urothelium, but only in the presence of adjacent native bladder smooth muscle tissue.14,15 This is consistent with our in vitro observations, where MSC failed to express SMC markers under growth factor conditions characteristic of the UC environment, specifically under bFGF-enriched conditions (Figures 4 and 6). Since inhibition of bFGF activity led to SMC marker expression under conditions in which it was not otherwise attained, loss of the inhibitory effects of bFGF appear necessary for smooth muscle maturation (Figure 7).
In marked contrast, medium conditioned by SMC promoted expression of multiple SMC markers in MSC (Figures 4 and 6). Despite this, the effect of principal growth factors in SMC-CM (TGFβ and HGF) on differentiation of MSC to an SMC-like phenotype is modest compared to the significant negative effect of bFGF on marker expression, as neutralization of TGFβ and/or HGF did not abolish SMC marker expression in MSC. These findings may indicate that these growth factors may not specifically promote differentiation, but that their effects may comply with the spontaneous differentiation that occurs under reduced serum conditions (Figures 6 and 7). Proliferation appears to be inversely associated with expression of the SMC-like phenotype, since MSC proliferated at a lower rate in the presence of TGFβ and HGF (SMC-CM associated factors) compared to bFGF and VEGF (Figure 6A). bFGF and VEGF are known to promote proliferation of marrow cells,24,25 while the effect of HGF on mesenchymal cells is primarily motogenic as opposed to mitogenic, and TGFβ is a known growth inhibitor for mesenchymal cells, including urinary tract SMC.37,38 However, it should also be noted that cell proliferation is not the only determinant of the cellular phenotype, since MSC proliferated at approximately the same rate in SMC-CM and UC-CM and expressed totally different phenotypes (Figure 4A). Since growth factors elicit context-dependent effects on both proliferation and differentiation, the ultimate effect on cells reflects a combination of positive and negative activities of several distinct factors. Interestingly, PDGF-BB, which is frequently associated with proliferation and differentiation of SMC,20,21,23,39 inhibited the expression of SMC markers in MSC under the same conditions as described above (data not shown), possibly suggesting negative effects of this factor on differentiation of MSC to smooth muscle, similar to the effects of bFGF.
These in vitro findings are consistent with our in vivo findings using the bladder patch repair model with acellular grafts, in which marrow cells participated in smooth muscle regeneration. Though marrow cells prevailed the graft initially, the infiltrating αSMA-positive cells at 2 weeks were mainly of native bladder origin, and not from bone marrow (Figure 2). Marrow cells acquire an SMC-like phenotype intermingled with infiltrating SMC during the time course of the experiment. Our observations with SMC-CM and medium supplemented with growth factors, suggest that the growth factor environment that promotes the observed phenotypic changes, derives from native SMC proliferating into the graft from adjacent bladder tissue, but not from UC (Figure 3).
The marrow cells may be recruited onto the graft through the circulation, or from adjacent native bladder tissues, as they are already present in native bladder after bone marrow transplantation (Figure 1). The regenerated smooth muscle layer comprised both marrow cells and non-marrow cells, in sharp contrast to the native bladder muscle layer, in which marrow-derived cells were barely detected (Figure 3). In this model, marrow cells participated in remodeling on the graft, but did not significantly remodel the native bladder tissue. This observation may reflect the need for a specific biological context in which marrow-derived cells participate in remodeling of smooth muscle tissue.19 Although such findings have also been explained as cell fusion in several models,36 our in vitro findings and other studies suggest that differentiation from marrow cells to SMC could take place without fusion.20,41 The present in vitro model has clearly shown that MSC acquire a SMC-like phenotype under the influence of soluble factors elaborated by SMC. This occurs without direct contact of the cells, although presumably cell-cell interactions could also occur in vivo, further promoting phenotypic changes.
The clinical implications of our findings remain indeterminate. The extent of αSMA expression among GFP-positive cells was modest, and the precise percentage of marrow-derived cells within regenerated smooth muscle could not be assessed because not all of the donor marrow cells expressed GFP in our model. Our observation may point to a novel method of tissue regeneration, provided that recruitment and differentiation of precursor cells that repopulate the graft can be appropriately controlled.16 However, elucidation of the mechanisms underlying recruitment of cells to the graft, that have the potential to differentiate into SMC will be crucial to achieve this goal. In addition, the function of the newly formed tissue in contributing to low-pressure storage of urine and appropriate voiding behavior remains to be determined. Bone marrow cells are known to participate in cardiac regeneration and formation of vascular neo-intimal SMC, but their contribution to organ function has been found to be either subtle (in cardiac muscle), or negative (for vessels).18,19,24,40,41 In order for acellular matrix grafts to be used successfully in bladder reconstruction, it is imperative that the newly formed smooth muscle functions equivalently to healthy native smooth muscle. Whether marrow cell-derived smooth muscle cell could attain such function should be the crucial point to be clarified in the further study.
A major finding of our study is the demonstration that specific growth factor conditions elaborated by native bladder cells are able to promote differentiation of mesenchymal cells that infiltrate the acellular graft. We should note that the matrix graft itself is another potential source of growth factors. As SIS contains growth factors such as TGFβ, VEGF, and bFGF,6,7 growth factors would remain on other animal-derived acellular grafts. Given these issues, decellularization method is critical for interpreting the results of acellular graft studies. As growth factors bind to extracellular matrix by electrostatic interactions with heparan sulfate and other charged molecules, they would be lost from matrix preparations under conditions of high ionic strength or in the presence of strong detergents.3,9 Thus, different results between previous studies may reflect different prevailing levels of growth factors within the matrix grafts. Although many promising results have been reported for bladder reconstruction using acellular matrices, it is necessary to evaluate them in the context of the underlying tissue remodeling processes. Conversely, if there exists optimal growth factor conditions for tissue regeneration, reproduction of this environment within the graft matrix would offer a reproducible and effective method for organ reconstruction.
In summary, the present findings elucidate some of unknown mechanisms in bladder regeneration process, and provide new perspectives for organ reconstruction with novel biomaterials.
Acknowledgments
We thank Dr. Chisato Fujiyama (Saga University) Dr. Momoko Yoshimoto, Dr. Hiroyuki Nakajima, Dr. Takahiro Inoue (Kyoto University), Dr. Satoshi Inada (Kyoto Prefectural University of Medicine), and Dr. Takahito Ito (Osaka University) for valuable technical support and advice.
Footnotes
Address reprint requests to Professor Osamu Ogawa, M.D., Ph.D., 54 Shogoin Kawaracho, Sakyo, Kyoto, Japan 606-8507. E-mail: ogawao@kuhp.kyoto-u.ac.jp.
Supported by Grant-in-Aid for Scientific Research, Ministry of Education, Culture, Sports, Science and Technology, Japan; Establishment of International Center of Excellence (COE) for Integration of Transplantation Therapy and Regenerative Medicine (COE program), Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- Sutherland RS, Baskin LS, Hayward SW, Cunha GR. Regeneration of bladder urothelium, smooth muscle, blood vessels, and nerves into an acellular tissue matrix. J Urol. 1996;156:571–577. doi: 10.1097/00005392-199608001-00002. [DOI] [PubMed] [Google Scholar]
- Kropp BP, Eppley BL, Prevel CD, Rippy MK, Harruff RC, Badylak SF, Adams MC, Rink RC, Keating MA. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology. 1995;46:396–400. doi: 10.1016/S0090-4295(99)80227-1. [DOI] [PubMed] [Google Scholar]
- Brown AL, Farhat W, Merguerian PA, Wilson GJ, Khoury AE, Woodhouse KA. 22 week assessment of bladder acellular matrix as a bladder augmentation material in a porcine model. Biomaterials. 2002;23:2179–2190. doi: 10.1016/s0142-9612(01)00350-7. [DOI] [PubMed] [Google Scholar]
- Malone JM, Brendel K, Duhamel RC, Reinert RL. Detergent-extracted small-diameter vascular prostheses. J Vasc Surg. 1984;1:181–191. doi: 10.1067/mva.1984.avs0010181. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Watanabe Y, Toki A. Experimental assessment of small intestinal submucosa as a small bowel graft in a rat model. J Pediatr Surg. 2003;38:1596–1601. doi: 10.1016/s0022-3468(03)00567-0. [DOI] [PubMed] [Google Scholar]
- Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478–491. [PubMed] [Google Scholar]
- Hodde JP, Record RD, Liang HA, Badylak SF. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8:11–24. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- Kanematsu A, Yamamoto S, Noguchi T, Ozeki M, Tabata Y, Ogawa O. Bladder regeneration by bladder acellular matrix combined with sustained release of exogenous growth factor. J Urol. 2003;170:1633–1638. doi: 10.1097/01.ju.0000084021.51099.8a. [DOI] [PubMed] [Google Scholar]
- Kanematsu A, Yamamoto S, Ozeki M, Noguchi T, Kanatani I, Ogawa O, Tabata Y. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials. 2004;25:4513–4520. doi: 10.1016/j.biomaterials.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Wu HY, Baskin LS, Liu W, Li YW, Hayward S, Cunha GR. Understanding bladder regeneration: smooth muscle ontogeny. J Urol. 1999;162:1101–1105. doi: 10.1016/S0022-5347(01)68082-0. [DOI] [PubMed] [Google Scholar]
- Baskin L, DiSandro M, Li Y, Li W, Hayward S, Cunha G. Mesenchymal-epithelial interactions in bladder smooth muscle development: effects of the local tissue environment. J Urol. 2001;165:1283–1288. [PubMed] [Google Scholar]
- Liu W, Li Y, Cunha S, Hayward G, Baskin L. Diffusable growth factors induce bladder smooth muscle differentiation. In Vitro Cell Dev Biol Anim. 2000;36:476–484. doi: 10.1290/1071-2690(2000)036<0476:dgfibs>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Hayward SW, DiSandro MS, Li YW, Cunha GR. Epithelial-mesenchymal interactions in the bladder: implications for bladder augmentation. Adv Exp Med Biol. 1999;462:49–61. doi: 10.1007/978-1-4615-4737-2_5. [DOI] [PubMed] [Google Scholar]
- Master VA, Wei G, Liu W, Baskin LS. Urothlelium facilitates the recruitment and trans-differentiation of fibroblasts into smooth muscle in acellular matrix. J Urol. 2003;170:1628–1632. doi: 10.1097/01.ju.0000084407.24615.f8. [DOI] [PubMed] [Google Scholar]
- Moriya K, Kakizaki H, Murakumo M, Watanabe S, Chen Q, Nonomura K, Koyanagi T. Creation of luminal tissue covered with urothelium by implantation of cultured urothelial cells into the peritoneal cavity. J Urol. 2003;170:2480–2485. doi: 10.1097/01.ju.0000095785.89925.aa. [DOI] [PubMed] [Google Scholar]
- Badylak SF, Park K, Peppas N, McCabe G, Yoder M. Marrow-derived cells populate scaffolds composed of xenogeneic extracellular matrix. Exp Hematol. 2001;29:1310–1318. doi: 10.1016/s0301-472x(01)00729-9. [DOI] [PubMed] [Google Scholar]
- Peled A, Zipori D, Abramsky O, Ovadia H, Shezen E. Expression of alpha-smooth muscle actin in murine bone marrow stromal cells. Blood. 1991;78:304–309. [PubMed] [Google Scholar]
- Saiura A, Sata M, Hirata Y, Nagai R, Makuuchi M. Circulating smooth muscle progenitor cells contribute to atherosclerosis. Nat Med. 2001;7:382–383. doi: 10.1038/86394. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003;93:783–790. doi: 10.1161/01.RES.0000096651.13001.B4. [DOI] [PubMed] [Google Scholar]
- Simper D, Stalboerger PG, Panetta CJ, Wang S, Caplice NM. Smooth muscle progenitor cells in human blood. Circulation. 2002;106:1199–1204. doi: 10.1161/01.cir.0000031525.61826.a8. [DOI] [PubMed] [Google Scholar]
- Ito T, Suzuki A, Imai E, Okabe M, Hori M. Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol. 2001;12:2625–2635. doi: 10.1681/ASN.V12122625. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Shinohara T, Heike T, Shiota M, Kanatsu-Shinohara M, Nakahata T. Direct visualization of transplanted hematopoietic cell reconstitution in intact mouse organs indicates the presence of a niche. Exp Hematol. 2003;31:733–740. doi: 10.1016/s0301-472x(03)00108-5. [DOI] [PubMed] [Google Scholar]
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fons P, Herault JP, Delesque N, Tuyaret J, Bono F, Herbert JM. VEGF-R2 and neuropilin-1 are involved in VEGF-A-induced differentiation of human bone marrow progenitor cells. J Cell Physiol. 2004;200:351–359. doi: 10.1002/jcp.20076. [DOI] [PubMed] [Google Scholar]
- Noff D, Pitaru S, Savion N. Basic fibroblast growth factor enhances the capacity of bone marrow cells to form bone-like nodules in vitro. FEBS Lett. 1989;250:619–621. doi: 10.1016/0014-5793(89)80808-7. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Tabata Y. Homogeneous seeding of mesenchymal stem cells into nonwoven fabric for tissue engineering. Tissue Eng. 2003;9:931–938. doi: 10.1089/107632703322495574. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. “Green mice” as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Kanematsu A, Marui A, Yamamoto S, Ozeki M, Hirano, Yamamoto M, Ogawa O, Komeda M, Tabata Y. Type I collagen functions as a reservoir of basic fibroblast growth factor. J Control Release. 2004;99:281–292. doi: 10.1016/j.jconrel.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Truschel ST, Ruiz WG, Shulman T, Pilewski J, Sun TT, Zeidel ML, Apodaca G. Primary uroepithelial cultures: a model system to analyze umbrella cell barrier function. J Biol Chem. 1999;274:15020–15029. doi: 10.1074/jbc.274.21.15020. [DOI] [PubMed] [Google Scholar]
- Fujiyama C, Masaki Z, Sugihara H. Reconstruction of the urinary bladder mucosa in three-dimensional collagen gel culture: fibroblast-extracellular matrix interactions on the differentiation of transitional epithelial cells. J Urol. 1995;153:2060–2067. [PubMed] [Google Scholar]
- Scriven SD, Booth C, Thomas DF, Trejdosiewicz LK, Southgate J. Reconstitution of human urothelium from monolayer cultures. J Urol. 1997;158:1147–1152. doi: 10.1097/00005392-199709000-00115. [DOI] [PubMed] [Google Scholar]
- Ma FH, Higashira-Hoshi H, Itoh Y. Functional muscarinic M2 and M3 receptors and beta-adrenoceptor in cultured rat bladder smooth muscle. Life Sci. 2002;70:1159–1172. doi: 10.1016/s0024-3205(01)01488-6. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Kitamura M, Miura H, Nonomura N, Takada S, Takahara S, Matsumoto K, Nakamura T, Matsumiya K. Prostate stromal cell-derived hepatocyte growth factor induces invasion of prostate cancer cell line DU145 through tumor-stromal interaction. Prostate. 1999;41:145–153. doi: 10.1002/(sici)1097-0045(19991101)41:3<145::aid-pros1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Iwai-Kanai E, Hasegawa K, Sawamura T, Fujita M, Yanazume T, Toyokuni S, Adachi S, Kihara Y, Sasayama S. Activation of lectin-like oxidized low-density lipoprotein receptor-1 induces apoptosis in cultured neonatal rat cardiac myocytes. Circulation. 2001;104:2948–2954. doi: 10.1161/hc4901.100381. [DOI] [PubMed] [Google Scholar]
- Todo T, Adams EF, Fahlbusch R, Dingermann T, Werner H. Autocrine growth stimulation of human meningioma cells by platelet-derived growth factor. J Neurosurg. 1996;84:852–858. doi: 10.3171/jns.1996.84.5.0852. [DOI] [PubMed] [Google Scholar]
- Ma H, Calderon TM, Kessel T, Ashton AW, Berman JW. Mechanisms of hepatocyte growth factor-mediated vascular smooth muscle cell migration. Circ Res. 2003;93:1066–1073. doi: 10.1161/01.RES.0000102867.54523.7F. [DOI] [PubMed] [Google Scholar]
- Stehr M, Estrada CR, Khoury J, Danciu TE, Sullivan MP, Peters CA, Solomon KR, Freeman MR, Adam RM. Caveolae are negative regulators of TGF-beta1 signaling in ureteral smooth muscle cells. J Urol, 2004;172:2451–2455. doi: 10.1097/01.ju.0000138084.53577.ca. [DOI] [PubMed] [Google Scholar]
- Stehr M, Adam RM, Khoury J, Zhuang L, Solomon KR, Peters CA, Freeman MR. Platelet-derived growth factor-BB is a potent mitogen for rat ureteral and human bladder smooth muscle cells: dependence on lipid rafts for cell signaling. J Urol. 2003;169:1165–1170. doi: 10.1097/01.ju.0000041501.01323.b9. [DOI] [PubMed] [Google Scholar]
- Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci USA. 2003;100:4754–4759. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]