Abstract
The pro-form of nerve growth factor (pro-NGF) has been shown to be a high affinity ligand for p75NTR and to induce apoptosis through this receptor. It has been reported that pro-NGF, rather than mature NGF, is the predominant form of this neurotrophin in human brain. In the present work we studied the potential involvement of pro-NGF purified from human brains affected by Alzheimer’s disease (AD), where it is especially abundant, in the neuronal apoptosis observed in this disease. Western blot analysis of human brain tissue showed the existence of several pro-NGF forms. Some of these pro-NGF forms were significantly increased in AD brain cortex in a disease stage-dependent manner. Pro-NGF, purified by chromatography from human AD brains, induced apoptotic cell death in sympathetic neurons and in a p75NTR stably transfected cell line. Blocking p75NTR in cell culture abolished neuronal apoptosis caused by pro-NGF. p75NTR-transfected cells underwent apoptosis in the presence of pro-NGF while control wild-type cells did not. Taken together, these results indicate that pro-NGF purified from AD human brains can induce apoptosis in neuronal cell cultures through its interaction with the p75NTR receptor.
Alzheimer’s disease (AD) is a degenerative disease of old age characterized by the presence of senile plaques, composed of Aβ amyloid, and neurofibrillary tangles containing hyperphosphorylated tau. This is accompanied by degeneration of synapses and dendrites, and by cell death and loss of neurons.1 Several studies have shown that Aβ amyloid induces neuronal apoptosis in several manners including oxidative stress mediation, changes in intracellular calcium homeostasis,2 and JNK pathway activation.3,4 Multiple Aβ amyloid receptors have been involved in causing neurotoxicity.5 Among them, p75NTR has been shown to directly bind Aβ amyloid inducing apoptosis in vitro.6,7 The intracellular pathway involved in this apoptosis comprises JNK activation, caspases-9/3 and caspase-2 activation,8 and induction of FasL.3 In addition to Aβ amyloid, other molecules may also be involved in neuronal death in AD.
Several pieces of evidence support the idea that p75NTR can mediate cell death in different models, including hippocampal neurons.9 Furthermore, complete deletion of p75NTR leads to a long-lasting increase in the number of basal forebrain cholinergic neurons (BFCN).10 It has also been reported that, in the developing retina, cell death is caused by NGF/p75NTR interaction.11 In this model, NGF produced by microglia induces apoptosis in ganglion cells expressing p75NTR, with this effect being significantly reduced in the p75NTR knock out. In oligodendrocytes, spinal cord injury induces an increase in p75NTR expression and an increase in mature NGF (mNGF) and pro-NGF levels,12 suggesting a role for this neurotrophin in the induction of apoptosis through p75NTR. It is classically accepted that the ability of p75NTR to induce cell death under mNGF activation depends on the ratio of p75NTR/TrkA expression in a given cell model.13
Since 2001, general knowledge about the p75NTR receptor has greatly improved. It has been shown that the pro-form of NGF (pro-NGF), classically considered as an inactive form of the neurotrophin, is the more specific and higher affinity ligand for p75NTR and can induce apoptotic cell death even in the presence of TrkA.14 In this context, the ratio of pro-NGF/mNGF emerges as a critical regulatory event for the maintenance of survival and death balance.15 Pro-NGF (rather than mNGF) has been shown to be the predominant form of this neurotrophin in human brain.16 Synthesis of precursors and processing by proteolysis is a common feature for most neurotrophins. Several pro-NGF forms with apparent molecular weights (MW) ranging from 16 to 60 kd have been described.17–20 The combinations of two different transcript products,21,22 together with the existence of several potential targets for convertases and glycosidases, provide several possible pro-NGF forms that can vary from one tissue to another. The relevance of a given pro-NGF form and their relative stability, binding properties and/or physiological effects still remain to be determined.
Recent studies have shown increased levels of this pro-neurotrophin (32-kd pro-NGF) in AD brains,16,23 although It is not currently known which are the mechanisms involved in this phenomenon. In the present work we describe the existence of several pro-NGF forms in human brain significantly increased, in a steadily dependent manner, in the frontal and entorhinal cortex of brains affected by AD. We also show that pro-NGF purified from AD brains is effective in inducing apoptotic cell death through p75NTR in vitro. These results suggest a physiological role for pro-NGF in the neuronal death observed in AD.
Materials and Methods
Cell Cultures and Treatments
Superior cervical ganglion (SCG) neuron cultures were prepared by isolating ganglia from 0 to 2 post-natal day Sprague Dawley rats. After dissection, ganglia were incubated with collagenase type IV (20 U/ml) (Worthington, Lakewood, NJ) for 30 minutes at 37°C followed by incubation with trypsin (0.25%, Gibco, BRL, Paisley, Scotland) for 25 minutes at 37°C and washed with Minimum Essential Medium (MEM) (Gibco) supplemented with 10% horse serum (HS) and antibiotics (complete medium). Mechanical dissociation was performed by passing the ganglia through a 200-μl pipette tip. Cells were plated at 12,500 cells/cm2 in collagen (0.1 mg/ml) (Becton Dickinson, Franklin Lakes, NJ)/poly-L-lysine (10 μg/ml) (Sigma) pre-coated 24-well plates and maintained in complete medium supplemented with nerve growth factor (NGF, 100 ng/ml) and L-glutamine (2 mmol/L) (Gibco, BRL). To prevent the growth of fibroblasts, cells were cultured in the presence of 5-fluoro-2′-deoxyuridine (50 nmol/L) and uridine (50 nmol/L) (Sigma). Treatments were performed after 5 days in culture. The cells were washed three times with serum-free medium and the reagents indicated were added in each case.
Cells of the 3T3 cell line, stably transfected to express human p75NTR (3T3-p75st) (kindly provided by M.V. Chao), were grown in collagen pre-coated 24-well plates at 10,000 cells/cm2 with DMEM (Gibco, BRL) supplemented with 10% fetal bovine serum (FBS). Before treatment, cells were washed twice with serum-free medium and treatments were carried out in 0.5% FBS medium.
Rat pheochromocytoma cell line, PC12 cells, were grown in 24-well plates in DMEM supplemented with 6% FBS, 6% HS and antibiotics. PC12 cells were used for NGF-induced neuritogenesis studies and were treated as indicated.
Human Samples
Human cases are summarized in Table 1. Brain samples were obtained from the Institut de Neuropatologia, Hospital de Bellvitge. At autopsy, half of the brain was fixed in 10% formalin for no less than 3 weeks, whereas the other half was cut in coronal sections 1-mm thick, frozen on dry ice and stored at −80°C until use. The neuropathological study was carried out in formalin-fixed, paraffin-embedded sections of the frontal, primary motor, primary somatosensory, posterior parietal, primary and association visual, temporal superior, temporal inferior, anterior insular, anterior cingulate, and entorhinal cortices; subiculum, and anterior and posterior levels of the hippocampus; the caudate and putamen, nucleus pallidus, amygdala, Meynert nucleus, and medial and posterior levels of the thalamus; midbrain (two levels); pons (two levels, including the locus ceruleus), and medulla oblongata (two levels); and upper vermis, lateral hemisphere, and dentate nucleus of the cerebellum. De-waxed sections were stained with hematoxylin and eosin, luxol fast blue-Klüver Barrera or processed for immunohistochemistry following the streptavidin-peroxidase method (Supersensitive Kit, Menarini) or with the EnVision + System Peroxidase (DAB) procedure (Dako, Carpinteria, CA) to βA4 amyloid (M. Sarasa, Zaragoza; Boehringer-Mannheim), tau (Sigma) phospho-tau (phospho-specific antibodies Thr181, Ser202, Ser214, Ser262, Ser396, Ser422: Calbiochem), αB-crystallin (Novocastra), α-synuclein (Chemicon), ubiquitin (Dako), phosphorylated neurofilament epitopes (Boehringer, Ingelheim, Germany), and α- and β-tubulin (Sigma). The neuropathological diagnosis and staging were carried out following Braak and Braak classification.1,24 A summary of the cases examined in the present study is shown in Table 1. Control and diseased cases were processed in parallel. The frontal cortex (area 8), and the entorhinal cortex and anterior hippocampus were used for further immunohistochemical and biochemical studies. Cerebrospinal fluid (CSF) was obtained at autopsy following in situ-intraventricular aspiration. Biochemical and cytological studies were carried out to eliminate CSF samples contaminated during the process of extraction.
Table 1.
Summary of Cases Examined in the Present Study
| No. | Age | Gender | Diagnostic | Braak stages beta amyloid | Braak stages tau pathology | Post-mortem delay (hours) |
|---|---|---|---|---|---|---|
| 1 | 82 | F | No lessions | A | 0 | 11 |
| 2 | 63 | M | No lessions | A | 0 | 17 |
| 3 | 46 | F | No lessions | A | 0 | 20 |
| 4 | 80 | F | No lessions | A | 0 | 21 |
| 5 | 49 | F | No lessions | A | 0 | 7 |
| 6 | 74 | M | AD | B | I | 24 |
| 7 | 85 | F | AD | B | II | 12 |
| 8 | 85 | M | AD | B | III | 14 |
| 9 | 81 | F | AD | B | IV | 14 |
| 10 | 82 | F | AD | C | V | 10 |
| 11 | 69 | M | AD | C | V | 20 |
| 12 | 86 | F | AD | C | V | 10 |
| 13 | 69 | M | AD | C | V | 6 |
| 14 | 82 | F | AD | C | V | 5 |
AD, Alzheimer’s disease; F, female; M, male; A, B and C, Braak and Braak’s classification of AD stages depending on amyloid plaques; 0–V, Braak and Braak’s classification of AD stages depending on the distribution and amount of neurofibrillary tangles.
Antibodies
The antibody against pro-NGF pro-domain was made as described previously by Beattie et al.12 In brief, GST-fusion protein containing asp23-arg81 peptide from human pro-NGF was used to immunize New Zealand rabbits (Charles River Laboratories, Wilmington, MA). Specific anti-sera was purified by first incubating whole serum with GST to adsorb GST-specific immunoreactivity and then followed by adsorption to, and elution from, a glutathione column to which GST-pro-NGF was immobilized. Anti-pre-pro-NGF directed against the amino acid sequence −144 to −166 of pro-NGF was obtained from Pro-Hormone Science.17,18 Antibodies against mNGF, H20, and anti-2.5S NGF were purchased from Santa Cruz and Cedarlane Laboratories, respectively. Anti-β-actin antibody (AC-15) was obtained from Sigma and anti-GFAP antibody from Dako. Secondary antibodies (anti-mouse IgG-HRP and anti-rabbit IgG-HRP) were obtained from Amersham. Pro-NGF activity was blocked by pre-incubating either 25 μg/ml of anti-pro-NGF antibody or 20 μg/ml of anti-β-NGF (Sigma) with 25 ng/ml hbi-pro-NGF for 2 hours at room temperature before addition to the cell cultures. Blocking of p75NTR was performed by adding 50 ng/ml of anti-p75NTR (REX) antibody (kindly provided by L.F. Reichardt, Howard Hughes Medical Institute, University of California) to the medium 2 hours before treatment. The following antibodies were used in the neuropathological classification of the human brain samples: βA4 amyloid (M. Sarasa, Zaragoza; Boehringer-Mannheim), tau (Sigma) phospho-tau (phospho-specific antibodies Thr181, Ser202, Ser214, Ser262, Ser396, Ser422: Calbiochem), αB-crystallin (Novocastra), α-synuclein (Chemicon), ubiquitin (Dako), phosphorylated neurofilament epitopes (Boehringer), and α- and β-tubulin (Sigma).
Pro-NGF Immunohystochemistry
Sections of frontal and entorhinal cortex and hippocampus 30-μm thick were processed free-floating with the LSAB method (Dako LSAB (labeled streptavidinbiotin + Kit) following the instructions of the supplier. Briefly, after blocking endogenous peroxidases, the sections were incubated with normal serum for 2 hours and then incubated overnight at 4°C with the primary antibodies. The antibody to pro-NGF was used at a dilution of 1:1000. After washing, the sections were then incubated with link solution (LSAB) and with streptavidin-peroxidase solution for 15 minutes each at room temperature. The peroxidase reaction was then visualized, as a dark blue precipitate, with NH4NiSO4 (0.05 mol/L) in phosphate buffer (0.1 mol/L), 0.05% diaminobenzidine, NH4Cl, and 0.01% H2O2. Blank sections stained only with the secondary antibodies were used as negative controls. Double-labeling immunohistochemistry was carried out following a two-step protocol and the streptavidin LSAB method (Dako). Paraformaldehyde-fixed, cryostat sections, 15-μm thick, were incubated with methanol and normal serum, processed free-floating for pro-NGF immunohistochemistry, and then incubated with the corresponding secondary antibody. Immediately afterward, the sections were incubated with anti-GFAP, followed by the corresponding secondary antibody. Controls were carried out by changing the order of the primary antibodies and by incubating the sections with only the secondary antibodies. Blockage of antibody immunoreactivity was performed by incubating the antigenic peptide with the antibody in a proportion 10:1, for 2 hours at room temperature, previous to the immunohistochemistry procedure.
Western Blotting
Protein extracts from the frontal and entorhinal cortex were homogenized as described.25 Pieces weighing between 0.3 to 1.0 g were mechanically disrupted in 8.8 mmol/L HEPES pH 7.4, 6.3 mmol/L CaCl2, 15 mmol/L MgCl2 (Scharlab, Barcelona, Spain) supplemented with sodium orthovanadate (100 μmol/L) and protease inhibitors aprotinin (10 μg/ml), PMSF (1 mmol/L), leupeptin (20 μg/ml), and benzamidine (100 μmol/L) (Sigma). Homogenates were centrifuged at 12,000 × g for 10 minutes and protein concentration in the supernatant was determined by DC-Protein Assay (Bio-Rad). Thirty μg of total protein was resolved in 12% SDS-PAGE, transferred to Immobilon-P membranes (Millipore) and blocked for 1 hour at room temperature in TBS-T (50 mmol/L Tris, pH 8.0; 133 mmol/L NaCl, 0.2% Tween 20) with 5% skim milk. For immunodetection of the pro-NGF forms, membranes were incubated with either anti-pro-NGF antibody or H20 antibody (1:1000 in TBS-T) at 4°C overnight. After washing in TBS-T, membranes were incubated with HRP-conjugated anti-rabbit antibody (1:5000 in TBS-T) at room temperature for 1 hour. For detection, an ECL chemiluminescence system (Amersham-Pharmacia) was used in accordance with the manufacturer’s instructions. Membranes were stripped and re-blotted with anti-β-actin antibody (Sigma) (1:5000 in TBS-T) to assess correct protein loading.
Isolation of Pro-NGF from Human Brain
Methodology was based on the protocol for isolation of mNGF from mice submaxillar gland described by Longo et al.26 Briefly, frozen human brain tissue from AD-affected frontal cortex (6 to 10 g) was homogenized in 20 ml sterile water using a Polytron device on ice. After centrifugation of homogenates (2500 × g for 1 hour at 4°C), supernatants were dialyzed against 20 mmol/L Na2HPO4/NaH2PO4 (pH 6.8) overnight using a 12- to 14-kd MWCO membrane (SERVA). The samples were loaded on a DEAE-Sepharose CL-4B column (Pharmacia) pre-equilibrated in the same buffer. Eluted fractions having absorbance A280 >0.5 were equilibrated by a second dialysis against 20 mmol/L Na2HPO4/NaH2PO4 (pH 6.8) overnight. Salt concentration was adjusted to 0.4 mol/L NaCl in 50 mmol/L CH3COONa (pH 4.0). The sample was centrifuged at 2500 × g for 30 minutes and supernatant was loaded on a DEAE-Sepharose CL-4B column previously equilibrated with the same buffer. All the procedures were performed at 4°C. Eluted fractions with 50 mmol/L CH3COONa and 0.4 mol/L NaCl (pH 4.0), having absorbance A280> 0.1 were collected and analyzed by Western blot using antibodies against either mNGF (H20, Santa Cruz) or pro-NGF. mNGF protein was undetectable in all of the fractions obtained using H20 anti-mNGF antibody. For gel filtration chromatography, the peak corresponding to pro-NGF fraction was concentrated using Amicon Ultra 10,000 MWCO (Millipore) and loaded to a Sephacryl S100 column (Pharmacia Biotech). The column was calibrated before and after each pro-NGF chromatography using as MW standards BSA for 67 kd, ovalbumin for 43 kd and quimotripsin A for 25 kd (Sigma). All of the procedures were performed at 4°C, using 50 mmol/L CH3COONa and 0.4 mol/L NaCl (pH 4.0) buffer. Assay of TNF-α and IL-1β was performed using ELISA Kits (Biosource Europa, Nivelles, Belgium).
Detection of Apoptosis in the Cultures
Twenty-four hours after treatment, cells were fixed with 4% paraformaldehyde and processed for TUNEL assay using the in situ Cell Detection Kit (Roche Diagnostics) following the manufacturer’s protocol. Additionally, cell nuclei were stained with Hoechst 33258 (Sigma). The fluorescence was analyzed using an Olympus LCPlan ×20 objective and documented with an Olympus DP70 camera. Fluorescent signals were overlapped and the percentage of apoptosis was determined. At least 500 nuclei in random, non-overlapping fields per condition were counted in each experiment.
Densitometry and Statistical Analysis
The density of the immunoreactive bands was determined by densitometry of the films using an Arcus II image analysis system (AGFA). Pro-NGF pixel values for diseased samples were compared to control values in at least four separate Western blots. Statistical significance between groups was calculated using Student’s t-test.
Results
Pro-NGF Is Present in Human Brain Cortex and Increases in Alzheimer’s Disease
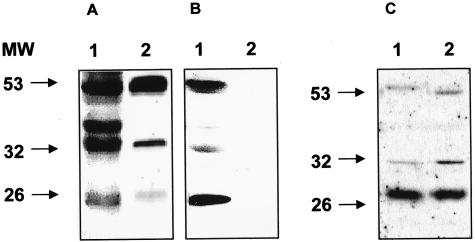
It has been shown, using an anti-mNGF antibody, that the predominant form of this neurotrophin in the brain is a 32-kd form corresponding to pro-NGF rather than mNGF.16 The presence of mNGF was not detectable in our human brain samples using an anti-NGF antibody (Figure 1, A and B). We used a different polyclonal antibody raised against the pro-domain of pro-NGF, as described in Materials and Methods, to study the pattern of bands in AD and control human frontal and entorhinal cortex at different stages of the disease (Figure 2, A and C). The use of this antibody showed the presence of several immunoreactive bands (53, 37, 32, and 26 kd) that appeared to be specific as the immunoreactivity was abolished by the immunogenic peptide (Figure 2B). We were also able to detect a similar pattern of bands in parallel Western blots (WBs), using antibodies raised against different NGF regions recognizing different epitopes of the protein (Figure 1). All of the antibodies tested recognize 53-, 37-, and 32-kd bands, although 26-kd bands are only faintly detectable in some cases (Figure 1, D and C, respectively). The 32-kd form showed an increase in AD samples of about two times, which corresponds to that previously described.16,23 The 53-kd band was the most apparent and immunoreactive, as observed in control brain (Figure 2, A and C). In addition, this 53-kd pro-NGF form was significantly increased in AD, even more than the previously described 32-kd form (Figure 2, A and C).
Figure 1.
The different antibodies anti-pro-NGF and anti-mNGF recognize high molecular weight forms of pro-NGF in human brain tissue and cerebrospinal fluid. Human samples from AD brain tissue or cerebrospinal fluid (CSF) (30 μg per lane) were analyzed by Western blot using two different antibodies directed against either the pro-domain of pro-NGF (C: anti-pre-pro-NGF, Prohormone Sciences; D: anti-pro-NGF, see Materials and Methods) or the mature part of the molecule (A: H20, Santa Cruz; B: anti-mNGF, Cederlane Labs.).
Figure 2.
Western blotting analysis of pro-NGF forms in human brain samples from frontal and entorhinal cortex. High molecular weight forms of pro-NGF (53, 37, 32, and 26 kd) were immunodetected with an antibody directed against the pro-domain of pro-NGF (A). Pre-incubation of anti-pro-NGF with the antigenic peptide (1:20) blocks immunoreactivity in human brain samples (B: lane 1, AD; lane 2, antigenic peptide blockage). The content of pro-NGF forms is clearly increased in Alzeimer’s disease (AD)-affected brains (A, lanes AD) compared to controls (A, lanes C). β-actin was used as loading control. Densitometry analysis of anti-pro-NGF immunodetected bands shows a significant increase of several bands in AD-affected tissue in a disease stage-dependent manner. Tissue samples obtained from human frontal and entorhinal brain cortex affected by AD were classified into A, B, and C stages according to Braak and Braak24 (see Table 1 and Materials and Methods). Bars represent the mean of four stage B and five stage C samples as percentage of the mean of five stage A samples (controls) (B). (*, P < 0.05; **, P < 0.01; Student’s t-test).
Densitometry analysis of the bands revealed differences between the frontal and entorhinal cortex. In entorhinal cortex, only the 53-kd band was increased, whereas in frontal cortex this increment was extended to other forms of pro-NGF (Figure 2C). As can be observed, the increase in pro-NGF levels was clearly more evident in frontal cortex than in entorhinal cortex. We also observed that the increase in pro-NGF levels was stage-dependent as the highest amount of pro-NGF corresponded to the most dramatically affected brains: values were higher in stage C than in stage B, following the staging of Aβ amyloid burden of Braak and Braak.24 Furthermore, using a set of four different antibodies in parallel WBs of human cerebrospinal fluid (CSF) samples, we mainly detected the 53- and 26-kd pro-NGF forms, and the lack of mNGF, indicating that it is able to be secreted as a stable pro-form (Figure 2).
Cellular Distribution of Pro-NGF in Human Cortex
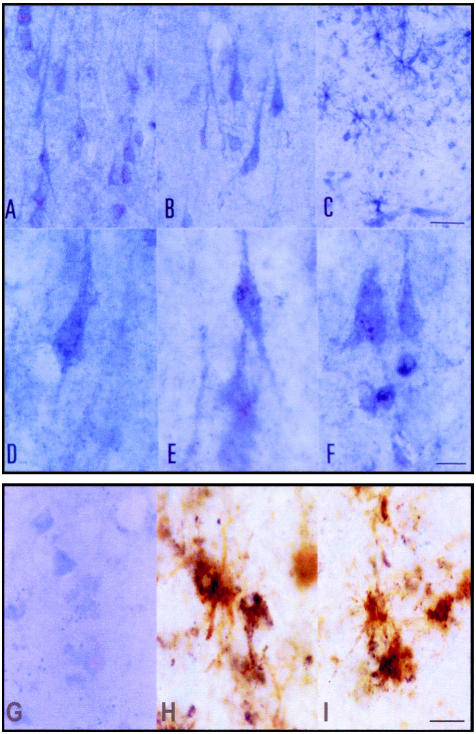
Immunohistochemistry showed widespread distribution of pro-NGF in the frontal neocortex, entorhinal cortex, and hippocampus (Figure 3, A and B). Pro-NGF was also expressed in glia (Figure 3, C, H and I) and the immunostaining of these cells was particularly prominent in the white matter (Figure 3C). Pro-NGF was recognized as a fine granular deposition in the cytoplasm and large dendrites of the majority, if not all, of the frontal and entorhinal cortex and hippocampal neurons (Figure 3, D and E). In addition, pro-NGF immunoreactivity was present in scattered nuclei of the frontal cortex and hippocampus (Figure 3, E and F). Localization in astrocytes of pro-NGF was demonstrated by double-labeling immunohistochemistry to pro-NGF and GFAP (Figure 3, H and I).
Figure 3.
Pro-NGF immunoreactivity in the frontal cortex (A, D, and F), CA1 area of the hippocampus (B and E), and subcortical white matter of the frontal cortex (C) in control cases. Pro-NGF is expressed in neurons (A, B, D, and E) and in glia (C). Pro-NGF immunoreactivity is present as a fine granular precipitate in the cytoplasm of neurons and main dendritic branches (D and E), but also in scattered nuclei (F). H and I: Double-staining immunohistochemistry showing pro-NGF expression (dark blue precipitate) in GFAP-immunoreactive (brown) astrocytes. G: Anti-pro-NGF blocked immunoreactivity with the antigenic peptide (1:10). A–C, bar in C = 25 μm; D–F, bar in F = 10 μm. G–I, bar in I = 10 μm.
Pro-NGF Obtained from AD-Affected Human Brains Is Functional and Stable
To use a more physiological approach we designed an experiment to obtain pro-NGF from AD human brain as reported in the experimental procedures. This was named human brain isolated pro-NGF (hbi-pro-NGF). WB analysis of hbi-pro-NGF with anti-pro-NGF antibody, showed the presence of three main bands of 53, 32, and 26 kd (Figure 4A, lane 2). These bands appeared to be specific since hbi-pro-NGF blocked the pro-NGF antibody immunoreactivity in a WB of an AD human brain lysate (Figure 4B). Among them, 53-kd band appears to be glycosylated, as its apparent MW was lowered after the treatment with N-glycanase (Figure 4C, lane 2). Taking into account that the obtaining of a functional, well-formed protein is one of the major problems of protein expression,27 the next aim was to assess whether it was possible to obtain mNGF from hbi-pro-NGF which could induce survival and differentiation of PC12 cells, which are known to be highly dependent on this neurotrophin.28,29 To this end, a similar approach to that of Rattenholl et al,30 using human wild-type recombinant pro-NGF, was set up. As can be seen in the Figure 5A, the product of a 10-second trypsin treatment of hbi-pro-NGF (25 ng/ml) sustained PC12 cell survival to a similar extent as mNGF (25 ng/ml). The same digestion product was also able to differentiate PC12 cells to a similar degree as 25 ng/ml of mNGF (Figure 5B). This indicated that the mNGF obtained by partial trypsinization of hbi-pro-NGF was fully functional. A WB of the medium from the above-described experiment showed a 14-kd band corresponding to mNGF. Yet this 14-kd band was not detected in the medium from PC12 cells treated with non-trypsinized hbi-pro-NGF (data not shown). This indicated that in these conditions hbi-pro-NGF is resistant to degradation by proteases arising from the cells. IL-1β and TNF-α ELISA assays were done in two different preparations of hbi-pro-NGF. Only low levels of these cytokines were detected at the limit of the procedure (50 pg/ml for IL-1β and 20 pg/ml for TNF-α).
Figure 4.
Analysis by Western blots of Pro-NGF isolated from frontal cortex AD-affected tissue. Protein extracts from post-mortem AD-affected tissue were processed for ion-exchange chromatography (see Materials and Methods). In the final purification fraction (hbi-pro-NGF), different pro-NGF isolated forms of 53, 32, and 26 kd (A, lane 2) were detected by Western blotting with anti-pro-NGF antibody. Hbi-pro-NGF blocks anti-pro-NGF immunodetection of pro-NGF forms in human brain tissue homogenates (B, lane 2). B, lane 1: total lysate of human brain AD-affected tissue. The 53-kd band immunodetected in hbi-pro-NGF is a glycosylated form of the pro-neurotrophin as the treatment with N-glycanase (C, lane 2) results in a decrease in its apparent MW (C, lane 1, untreated hbi-pro-NGF).
Figure 5.
Trypsin pre-treatment of hbi-pro-NGF generates mature NGF which protects PC12 cells from deprivation-induced apoptotic death. PC12 cells were serum-deprived and treated with NGF (100 ng/ml), hbi-pro-NGF (25 ng/ml), or with trypsin-digested hbi-pro-NGF (25 ng/ml hbi-pro-NGF treated with 50 mg/ml trypsin for 10 seconds at 37°C) for 48 hours. Apoptotic nucleus morphology was detected by Hoechst staining (A). Differentiated cells were counted as positive when neurite extensions were longer than a cell body (B). Results are the mean ± SD of 900 to 1600 cells counted in a representative experiment carried out in triplicate. Statistic was done by comparing between treatments and deprived cells. **, P < 0.05, Student’s t-test.
Human Brain Pro-NGF Induces Apoptotic Cell Death in Neuronal Cultures
It has been reported that recombinant cleavage-resistant pro-NGF expressed in 293 cells can induce apoptosis in SCG cultures. The same authors have shown that pro-NGF induced this neuronal apoptosis due to its interaction with the p75NTR receptor.14 Based on these findings, we tested whether a more physiological source of pro-NGF (hbi-pro-NGF) could induce apoptosis in two different culture systems expressing p75NTR:SCG primary neurons31 and the 3T3-p75st cell line.
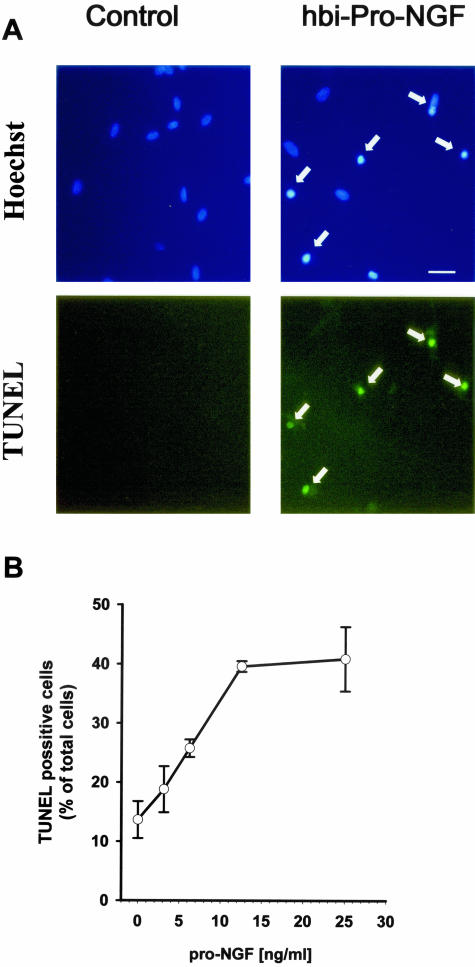
SCG cells were treated with hbi-pro-NGF (25 ng/ml) for 24 hours and counted by means of the TUNEL assay (Figure 6A). The percentage of TUNEL-positive cells was 40% at the hbi-pro-NGF concentration of 15 ng/ml reaching saturation levels (Figure 6B). Pre-incubation of hbi-pro-NGF with either an anti-pro-NGF or an anti-β-NGF antibody before its addition to the cultures completely blocked SCG cell death (Figure 7A).
Figure 6.
Hbi-pro-NGF induces apoptosis in SCG neurons. Cultured SCG neurons were treated for 24 hours with hbi-pro-NGF (25 ng/ml) and apoptotic cell death was evidenced by means of the TUNEL method and staining with Hoechst (A). Quantification of TUNEL-positive cells shows that the cell death increases with the increased concentration of hbi-pro-NGF (B). Bar = 50 μm.
Figure 7.
Anti-pro-NGF antibody blocks hbi-pro-NGF-induced apoptosis in SGC cells and in 3T3-p75st. Induction of apoptotic cell death in SCG cells treated with hbi-pro-NGF is prevented by pre-incubation with anti-p75 antibody (REX, 50 ng/ml), anti-pro-NGF (20 μg/ml), or anti-β-NGF (25 μg/ml) (A). Apoptotic cell death was evidenced by means of the TUNEL assay. Hbi-pro-NGF also induces apoptotic nucleus morphology determined by Hoechst staining in the non-neuronal cell line 3T3-p75st. Apoptotic nuclei reach ∼25% of total cells (B) and the pre-incubation of hbi-pro-NGF with anti-pro-NGF for 2 hours before its addition to cell cultures completely blocks cell death induced by 30-hour treatment with hbi-pro-NGF (25 ng/ml). 3T3 wild-type cells are not affected by the treatment with hbi-pro-NGF (C). **, P < 0.01, Student’s t-test.
Similar studies were then carried out with 3T3-p75st cells. In this system, following a 72-hour treatment, hbi-pro-NGF induced 40% cell death (Figure 7B). The pre-incubation of hbi-pro-NGF with the anti-pro-NGF antibody completely blocked the cell death caused by a 30-hour pro-NGF treatment in 3T3-p75st cells (Figure 7B). Hbi-pro-NGF did not induce apoptosis in wild-type 3T3 cells under any conditions (Figure 7C). Therefore, the lack of effect of hbi-pro-NGF in wild-type 3T3, which do not contain p75NTR receptors, ruled out any non-specific effect of potential toxic elements that could be present in pro-NGF purifications.
As three predominant MW forms of pro-NGF were present in hbi-pro-NGF, we wanted to assess whether all of them were efficient in causing cell death. We used gel filtration chromatography to separate these forms. Fractions containing either 53-, 32-, or 26-kd pro-NGF were used for 24-hour treatment of 3T3-p75st cells and induction of apoptosis measured by Hoechst staining (Figure 8). The three fractions were able to cause apoptosis to a similar extent when expressed per μg of protein added to the culture.
Figure 8.
Hbi-pro-NGF forms separated by gel filtration chromatography induce cell death in 3T3-p75st cells. Three fractions of gel filtration chromatography of hbi-pro-NGF (8 μg total protein of each fraction) containing pro-NGF forms of 54, 32, and 26 kd, were added to 3T3-p75st for 30 hours. Apoptotic nuclei morphology was determined by Hoechst staining. Three μg of Hbi-pro-NGF was used as control of death induction in deprived cells. Apoptotic cell nuclei were counted as percentage of the total cells in each treatment. Bars are median ± SD of triplicates.
Human Brain Pro-NGF Induces Apoptosis through p75NTR
Since it has been shown that p75NTR is involved in cell death in AD and pro-NGF is a high affinity ligand for p75NTR,14 we wanted to elucidate whether the cell death induced by hbi-pro-NGF was due to the interaction of pro-NGF with the p75NTR receptor. For this purpose, we used the anti-p75NTR receptor antibody raised against the extracellular domain of p75NTR (REX) as previously described,32 and studied whether this blockade had any effect on the cell death caused by hbi-pro-NGF. In SCG cells, 50 ng/ml of the anti-p75NTR antibody was added to the culture medium 2 hours before cell treatment. After 24 hours, the percentage of apoptosis in cells pre-treated with REX and hbi-pro-NGF was 28.9 ± 4.5%, which is significantly lower that that observed for REX non-pre-treated cells (40.8 ± 5.4%) (Figure 7A). The same occurred with 3T3-p75st cells. In this case, blocking the p75NTR receptor reduced cell death after a 30-hour treatment with hbi-pro-NGF to 11.2 ± 0.2%. When p75NTR was not blocked, 22.5 ± 1.6% of the cells died (Figure 7B). Hbi-pro-NGF did not induce apoptosis in wild-type 3T3 cells, further indicating that the effect was mediated by p75NTR (Figure 7C).
Discussion
Our results indicate that pro-NGF is present in human brain in considerable amounts in glial cells and in cortical and hippocampal neurons. We have also demonstrated that pro-NGF levels are increased in AD in a stage-dependent manner according to the progression of the disease. Finally we have shown that pro-NGF can induce a high proportion of apoptotic neuronal death mediated by its interaction with p75NTR.
Classically, p75NTR, activated by NGF in the absence of TrkA, has been considered to be a good candidate for mediating neuronal apoptosis. Actually, neuronal apoptosis induced by mNGF/p75NTR interaction has been reported in several models. However, this induction seems to be weak and highly dependent on the cellular model.15,33,34 Recently, new insights have been provided since it has been shown that pro-NGF binds to p75NTR more strongly than mNGF.14 Moreover, as reported by the same authors, activation of p75NTR through recombinant, cleavage-resistant pro-NGF expressed in the 293 cell line, causes a higher proportion of apoptotic cell death compared to mNGF. However, other published reports contradict these results. The use of cleavage-resistant pro-NGF expressed in insect cells has been described to promote neuronal survival and differentiation instead of apoptosis.35 The results that we present in this work, using pro-NGF extracted from human AD-affected brains reinforce the apoptotic-inducing role of pro-NGF/p75NTR interaction. The pathophysiological relevance of pro-NGF as a death-inducing ligand has recently been reported in rat CSF, as the induced and secreted active form after neuronal injury is able to induce apoptosis in primary oligodendrocytes.36 In human CSF, we detected pro-NGF but not mNGF. Following this observation, it is conceivable that pro-NGF, which is secreted to the extracellular space and then to the CSF, may act as a circulating p75NTR ligand, and promote neuronal death. However, the extracellular concentration of pro-NGF in brain could be under the detection limits of immunohistochemistry method. The presence of pro-NGF in the human CSF is in favor of neurosecretion, albeit determination of pro-NGF in the extracellular compartment escapes the threshold of immunohistochemical probes.
Several Pro-NGF Forms Are Present in Human Brain and Some of Them Increase in AD
It has been shown that classical procedures (ie, extraction from mouse submaxillar glands) used for the obtaining of mNGF give rise to small amounts of different MW forms of pro-NGF.18 Only a 32-kd form of pro-NGF had been described as being present in human brain, using an anti-mNGF antibody.16 However, the present results of WB using either two different anti-pro-NGF (anti-proNGF and anti-pre-proNGF) or two anti-mNGF antibodies(H20 and anti-mNGF), showed several bands of 53, 37, 32, and 26 kd. The 53-kd form of pro-NGF, which immunoreacts with all of the antibodies mentioned above, has also been found in human and rat tissues.17,18 The lack of a 14-kd band corresponding to mNGF, confirm the previously reported observation.16 Several pro-protein convertases have been described to possibly act on pro-NGF.14 All of them are members of the subtilisin/kexin family. Among them, furin in Golgi and plasmin in synapses release the mNGF of 14 kd. MMP-7, PACE4, and PC5/6B can give rise to higher MW fragments.37 Despite the presence of all these proteases, pro-NGF is not degraded as the pro-form is found many tissues.15 Different explanations could be given for the increase in pro-NGF levels in AD human brain. Some authors16 suggest a decrease in processing of proNGF. Accordingly, plasmin has been described to diminish in AD.38 Finally, as also described by others,18 we show in the present work that the 53-kd form of pro-NGF which increases markedly in AD, appears to be an N-glycosylated form of pro-NGF. A 43-kd form of pro-NGF present in other cellular models has also been described to be N-glycosylated.18,21 Interestingly, a high degree of glycosylation in some proteins such as acetylcholinesterase, APP,39,40 and tau41 has been reported in AD. Since glycosylation may protect from proteolytic hydrolysis, this could also provide an explanation for the predominance of high molecular weight glycosylated forms of pro-NGF in AD human brain samples. All these possibilities remain to be explored and they will be the aim of our further work.
Interestingly, the amount of pro-NGF in AD increases in the entorhinal and frontal cortex with disease progression, accounting for about four times the 53-kd form in stage C in frontal cortex. Yet regional differences do exist, as the increase in the frontal cortex is higher than in the entorhinal cortex. A similar consideration could be made regarding stages of neurofibrillary degeneration (see Table 1).
To further analyze whether pro-NGF increases were specific and not general for other pro-neurotrophins, similar assays were carried out using an antibody raised against the pro-domain of pro-BDNF. The pattern of immunoreactive bands obtained (data not shown) was different from the one given by the pro-NGF antibody. Furthermore, as previously reported with the use of an anti-mBDNF antibody,42 pro-BDNF was not increased in AD human cortex thus indicating that the increase observed in pro-NGF might be specific for this pro-neurotrophin.
Pro-NGF Extracted from AD Human Brain Induced Apoptotic Cell Death in Neuronal Cultures through p75NTR
It has recently been reported in SCG cultures that a recombinant cleavage-resistant pro-NGF expressed in 293 cells induces apoptosis.14 In oligodendrocyte primary cultures, either the same recombinant pro-NGF or injured spinal cord extracts containing pro-NGF12 have also been shown to induce similar levels of apoptotic cell death. This represents about five times the percentage obtained with a similar concentration of mNGF (0.5 nmol/L).
The aim of this study was to see whether pro-NGF purified from AD human brain could be functional in inducing apoptosis in neurons. Routinely, protocols used for mNGF isolation from the mouse submaxillar gland give rise to the presence of some pro-NGF.18 Based on this, we essentially used this protocol to isolate pro-NGF from human brain samples. The fraction resulting from this purification (hbi-pro-NGF) contains three pro-NGF MW forms that could be detected by WB with antibodies recognizing different domains of the molecule. Furthermore, hbi-pro-NGF was able to block all of the pro-NGF immunoreactive bands, indicating the specificity of the fraction. Hbi-pro-NGF was particularly stable probably due to glycosylation that could play a role in its protection from proteolysis. Previous work performed with the wild-type bacterially expressed pro-NGF30 showed the functionality of the protein by partially hydrolyzing the pro-domain with trypsin and showing that the mNGF resulting from the hydrolysis could induce the survival of dorsal root ganglia neurons (DRG) at a similar rate as would the same concentration of mNGF.30 Based on this, a survival and differentiation assay on PC12 cells was performed to assess whether hbi-pro-NGF was potentially functional. Hbi-pro-NGF could not sustain the survival and/or differentiation of PC12 cells. However, when hbi-pro-NGF was partially digested by trypsin, PC12 survival and differentiation were observed. Moreover trypsin partially digested hbi-pro-NGF protected PC12 cells from deprivation to the same extent as mNGF, thus indicating that mNGF is obtained as a product of trypsin digestion. This indicates that the chromatographic purification from human AD brain gives rise to stable forms of pro-NGF, as it is susceptible to give functional mNGF when partially digested by trypsin.
The present study has also shown that hbi-pro-NGF induces apoptosis in SCG and in 3T3-p75st, but not in wild-type 3T3 cells. The percentage of apoptosis induced by hbi-pro-NGF was significantly high and very similar for SCG and 3T3-p75st. Since either anti-pro-NGF or anti-β-NGF blocked apoptosis in both SCG and 3T3-p75st cells, isolated hbi-pro-NGF is likely to be the factor responsible for causing apoptosis in these models.
In SCG cultures, hbi-pro-NGF-induced apoptosis was blocked by pre-treating the cells with an anti-p75NTR antibody raised against the extracellular domain of p75NTR (REX). These results indicate that the activation of p75NTR by pro-NGF could be, at least in part, responsible for the death observed in neuronal cells in AD.
An important issue to be studied was whether the 53-, 37-, and 32-kd pro-NGF forms were able to cause apoptotic death independently. Several studies describe biological activity of the 32-kd pro-NGF form.12,43 As little work has been done showing 53-kd pro-NGF biological activity,44 and as it clearly increased in human frontal cortex affected by AD, more than the rest of the pro-forms, it was relevant to isolate this form and show whether it was effective in inducing apoptosis. In the present work, we separate the three pro-NGF MW forms by gel filtration chromatography, and show that all are capable of inducing apoptosis in 3T3-p75st cells. Furthermore, the effect of gel filtration fractions containing pro-NGF reinforces the specificity of hbi-pro-NGF effect discussed above. The possible presence of apoptosis inducers such as IL-1β and TNF-α in hbi-pro-NGF preparations, acting synergistically with β-amyloid peptides in some models in vitro,45 was also ruled out.
Taken together, our results show that pro-NGF is increased in AD. This increase could be relevant in the neuronal cell death observed in AD since pro-NGF directly purified from human AD brain induces apoptosis in cultured neurons through its interaction with the p75NTR. The complete set of elements needed by the neurons to enter the apoptotic program in response to pro-NGF/p75NTR interaction remains to be determined. Further work is needed to understand the regulation of this pro-neurotrophin to prevent massive cell death in the AD-affected brain.
Acknowledgments
We thank Isabel Sanchez for technical assistance; the generous donations of REX antibody from Dr. L.F. Reichardt; Dr. E. Schwarz and Dr. E.M. Johnson, Jr. for helpful suggestions; and Dr. Neil Goodman for manuscript proofreading.
Footnotes
Address reprint requests to Carme Espinet, Ph.D., Laboratori de Neuropatología Molecular, Departament de Ciències Mèdiques Bàsiques, Universitat de Lleida, C/ Montserrat Roig 2, 25008 Lleida, Spain. E-mail: carme.espinet@cmb.udl.es.
Supported by Instituto de Salud Carlos III, Fondu de Investigaciones Sanitarias (FIS) grants PI020128 and Fundació Roviralta (to C.E.) and FIS grants PI02/0004 and C03–006, Brain Net II (to I.F.), and National Institutes of Health (NIH) (NS30687) (to B.H.). P.P. is recipient of a pre-doctoral fellowship from Universitat de Lleida.
C.E.P. and P.P. contributed equally to this work.
References
- Duychaerts CH, Dickson DW. Neuropathology of Alzheimer’s disease. Dickson DW, editor. Basel: ISN Neuropathology Press; Neurodegenerationthe molecular pathology of dementia and movement disorders. 2003:pp 47–65. [Google Scholar]
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, Davis RJ, Shirasaki Y, Greenberg ME. Beta amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci. 2001;21:7551–7560. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy CM, Rabacchi SA, Xu Z, Maroney AC, Connors TJ, Shelanski ML, Greene LA. Beta amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J Neurochem. 2001;77:157–164. doi: 10.1046/j.1471-4159.2001.t01-1-00218.x. [DOI] [PubMed] [Google Scholar]
- Tsukamoto E, Hashimoto Y, Kanekura K, Niikura T, Aiso S, Nishimoto I. Characterization of the toxic mechanism triggered by Alzheimer’s amyloid-beta peptides via p75 neurotrophin receptor in neuronal hybrid cells. J Neurosci Res. 2003;73:627–636. doi: 10.1002/jnr.10703. [DOI] [PubMed] [Google Scholar]
- Yaar M, Zhai S, Pilch PF, Doyle SM, Eisenhauer PB, Fine RE, Gilchrest BA. Binding of beta-amyloid to the p75 neurotrophin receptor induces apoptosis: a possible mechanism for Alzheimer’s disease. J Clin Invest. 1997;100:2333–2340. doi: 10.1172/JCI119772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaar M, Zhai S, Fine RE, Eisenhauer PB, Arble BL, Stewart KB, Gilchrest BA. Amyloid beta binds trimers as well as monomers of the 75-kDa neurotrophin receptor and activates receptor signaling. J Biol Chem. 2002;277:7720–7725. doi: 10.1074/jbc.M110929200. [DOI] [PubMed] [Google Scholar]
- Troy CM, Rabacchi SA, Friedman WJ, Frappier TF, Brown K, Shelanski ML. Caspase-2 mediates neuronal cell death induced by beta-amyloid. J Neurosci. 2000;20:1386–1392. doi: 10.1523/JNEUROSCI.20-04-01386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20:6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann T, Casademunt E, Hollerbach E, Hofmann J, Dechant G, Frotscher M, Barde YA. Complete deletion of the neurotrophin receptor p75NTR leads to long-lasting increases in the number of basal forebrain cholinergic neurons. J Neurosci. 2002;22:2409–2418. doi: 10.1523/JNEUROSCI.22-07-02409.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Genetic evidence for cell death mediated by nerve growth factor and the neurotrophin receptor p75 in the developing mouse retina and spinal cord. Development. 1999;126:683–690. doi: 10.1242/dev.126.4.683. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. Pro-NGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Chao MV, Bothwell M. Neurotrophins: to cleave or not to cleave. Neuron. 2002;33:9–12. doi: 10.1016/s0896-6273(01)00573-6. [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol Cell Neurosci. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Sinicropi DV, Boucher TJ, Bennett DL, McMahon SB, Anand P. Molecular forms of NGF in human and rat neuropathic tissues: decreased NGF precursor-like immunoreactivity in human diabetic skin. J Peripher Nerv Syst. 2002;7:190–197. doi: 10.1046/j.1529-8027.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- Reinshagen M, Geerling I, Eysselein VE, Adler G, Huff K, Moore GP, Lakshmanan J. Commercial recombinant human beta-nerve growth factor and adult rat dorsal root ganglia contain an identical molecular species of nerve growth factor prohormone. J Neurochem. 2000;74:2127–2133. doi: 10.1046/j.1471-4159.2000.0742127.x. [DOI] [PubMed] [Google Scholar]
- Mowla SJ, Pareek S, Farhadi HF, Petrecca K, Fawcett JP, Seidah NG, Morris SJ, Sossin WS, Murphy RA. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J Neurosc. 1999;19:2069–2080. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH, Selby MJ, Garcia PD, Rutter WJ. Processing of the native nerve growth factor precursor to form biologically active nerve growth factor. J Biol Chem. 1988;263:6810–6815. [PubMed] [Google Scholar]
- Ullrich A, Gray A, Berman C, Dull TJ. Human beta-nerve growth factor gene sequence is highly homologous to that of mouse. Nature. 1983;303:821–825. doi: 10.1038/303821a0. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Selby MJ, Rutter WJ. Differential RNA splicing predicts two distinct nerve growth factor precursors. Nature. 1986;319:784–787. doi: 10.1038/319784a0. [DOI] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol. 2004;63:641–649. doi: 10.1093/jnen/63.6.641. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Peters A, Morrison JH, editors. New York, Boston, Dordrecht, London, Moscow: Kluwer Academic/Plenum Press Publishers; 1999:475–512. [Google Scholar]
- Ferrer I, Barrachina M, Puig B. Anti-tau phospho-specific Ser262 antibody recognizes a variety of abnormal hyper-phosphorylated tau deposits in tauopathies including Pick bodies and argyrophilic grains. Acta Neuropathol (Berl) 2002;104:658–664. doi: 10.1007/s00401-002-0600-2. [DOI] [PubMed] [Google Scholar]
- Longo FM, Woo JE, Mobley WC. Purification of nerve growth factor. Rush RA, editor. Chicago: John Wiley and Sons Ltd; Nerve Growth Factors. 1989:pp 3–30. [Google Scholar]
- Edwards RH, Selby MJ, Mobley WC, Weinrich SL, Hruby DE, Rutter WJ. Processing and secretion of nerve growth factor: expression in mammalian cells with a vaccinia virus vector. Mol Cell Biol. 1988;8:2456–2464. doi: 10.1128/mcb.8.6.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein DE, Blumberg PM, Greene LA. Nerve growth factor-induced neuronal differentiation of PC12 pheochromocytoma cells: lack of inhibition by a tumor promoter. Brain Res Rev. 1982;247:115–119. doi: 10.1016/0006-8993(82)91033-2. [DOI] [PubMed] [Google Scholar]
- Rattenholl A, Lilie H, Grossmann A, Stern A, Schwarz E, Rudolph R. The pro-sequence facilitates folding of human nerve growth factor from Escherichia coli inclusion bodies. Eur J Biochem. 2001;268:3296–3303. doi: 10.1046/j.1432-1327.2001.02232.x. [DOI] [PubMed] [Google Scholar]
- Ma Y, Campenot RB, Miller FD. Concentration-dependent regulation of neuronal gene expression by nerve growth factor. J Cell Biol. 1992;117:135–141. doi: 10.1083/jcb.117.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel PS, Smith SG, Vining ER, Valletta JS, Mobley WC, Reichardt LF. The extracellular domain of p75NTR is necessary to inhibit neurotrophin-3 signaling through TrkA. J Biol Chem. 2001;276:11294–11301. doi: 10.1074/jbc.M005132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL. The many faces of p75NTR. Curr Opin Neurobiol. 2002;12:260–267. doi: 10.1016/s0959-4388(02)00321-5. [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Yu G, Michalski B, Mathew S, Colquhoun A, Ross G, Coulghlin D. The nerve growth factor precursor proNGF exhibits neurotrophic activity but is less active than mature growth factor. J Neurochem. 2004;89:581–592. doi: 10.1111/j.1471-4159.2004.02360.x. [DOI] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Mörl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM. From the cover: secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci USA. 2004;101:6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Pareek S, Savaria D, Hamelin J, Goulet B, Laliberte J, Lazure C, Chretien M, Murphy RA. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem J. 1996;314:951–960. doi: 10.1042/bj3140951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma M, Da Silva JS, Crassaerts K, Delacourte A, De Strooper B, Dotti CG. Brain plasmin enhances APP alpha-cleavage and Abeta degradation and is reduced in Alzheimer’s disease brains. EMBO Rep. 2000;1:530–535. doi: 10.1093/embo-reports/kvd107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Valero J, Mok SS, Small DH. An unusually glycosylated form of acetylcholinesterase is a CSF biomarker for Alzheimer’s disease. Acta Neurol Scand Suppl. 2000;176:49–52. doi: 10.1034/j.1600-0404.2000.00307.x. [DOI] [PubMed] [Google Scholar]
- Fodero LR, Saez-Valero J, McLean CA, Martins RN, Beyreuther K, Masters CL, Robertson TA, Small DH. Altered glycosylation of acetylcholinesterase in APP (SW) Tg2576 transgenic mice occurs prior to amyloid plaque deposition. J Neurochem. 2002;81:441–448. doi: 10.1046/j.1471-4159.2002.00902.x. [DOI] [PubMed] [Google Scholar]
- Buée L, Bussière T, Buée-Cherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation, and role in neurodegenerative disorders. Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Marin C, Rey MJ, Ribalta T, Goutan E, Blanco R, Tolosa E, Martí E. BDNF and full-length and truncated TrkB expression in Alzheimer disease: implications in therapeutic strategies. J Neuropathol Exp Neurol. 1999;58:729–739. doi: 10.1097/00005072-199907000-00007. [DOI] [PubMed] [Google Scholar]
- Srinivasan B, Roque CH, Hempstead BL, Al-Ubaidi M, Roque RS. Microglia-derived pronerve growth factor promotes photoreceptor cell death via p75 neurotrophin receptor. J Biol Chem. 2004;279:41839–41845. doi: 10.1074/jbc.M402872200. [DOI] [PubMed] [Google Scholar]
- Lakshmanan J, Beattie GM, Hayek A, Burns C, Fisher DA. Biological actions of 53 kDa nerve growth factor as studied by a blot and culture technique. Neurosci Lett. 1989;99:263–267. doi: 10.1016/0304-3940(89)90457-6. [DOI] [PubMed] [Google Scholar]
- Perini G, Della-Bianca V, Politi V, Della Valle G, Dal-Pra I, Rossi F, Armato U. Role of neurotrophin receptor in the neurotoxicity by beta-amyloid peptides and synergistic effect of inflammatory cytokines. J Exp Med. 2002;7:907–918. doi: 10.1084/jem.20011797. [DOI] [PMC free article] [PubMed] [Google Scholar]