Abstract
Expression and pharmacological studies support a contribution of cyclooxygenase (COX)-2 to mammary gland tumorigenesis. In a recent transgenic study, mouse mammary tumor virus promoter-driven COX-2 expression in mouse mammary glands was shown to result in alveolar hyperplasia, dysplasia, and carcinomas after multiple rounds of pregnancy and lactation. In the study presented here, the effects of constitutive COX-2 overexpression in keratin 5-positive myoepithelial and luminal cells, driven by the keratin 5 promoter in a hormone-independent manner, was investigated. In nulliparous female mice, aberrant COX-2 overexpression correlated with increased prostaglandin (PG) E2 levels and caused cystic duct dilatations, adenosis, and fibrosis whereas carcinomas developed rarely. This phenotype depended on COX-2-mediated PGE2 synthesis and correlated with increased expression of proliferation-associated Ki67 in epithelial cells. No changes in the expression of apoptosis-related Bcl-2, caspase 3, or p53 were observed. Hyperproliferation of the mammary gland epithelial cells was associated with increased aromatase mRNA levels in this tissue. The spontaneous pathologies bear analogies to the human breast with fibrocystic changes. Intriguingly, strong COX-2 expression was observed in fibrocystic changes, as compared to low expression in normal breast epithelium. These results show for the first time that aberrant COX-2 expression contributes to the development of fibrocystic changes (FC), indicating that COX-2 and COX-2-mediated PG synthesis represent potential targets for the therapy of this most frequent benign disorder of the human breast.
The mammary gland epithelium, with its lobular-alveolar system, interlobular, and terminal ducts is primarily comprised of two epithelial cell layers: a layer of luminal cells with the potential to secrete milk and a basal layer of contractile myoepithelial cells that are involved in milk ejection during lactation. A distinguishing characteristic of these major cell types is the expression of keratin (K) 5, K14, and smooth muscle actin (SMA) in myoepithelia and the expression of K8, K18, and K19 in luminal epithelia.1 The vast majority of breast carcinomas arise from the terminal lobular-alveolar unit. Most of them express keratins that are consistent with a luminal origin and are thought to progress through a multistep sequence, ie, atypical ductal hyperplasia, ductal carcinoma in situ, invasive ductal carcinoma, and metastases.2 Breast carcinomas that show myoepithelial differentiation are rare.3,4 Although myoepithelial cells have been implicated in proliferative benign lesions such as sclerosing adenosis and intraductal papillomas,4,5 their contribution to the development of ductal carcinomas is not well understood.4,6
Within the scope of efforts in breast cancer therapy,7,8 cyclooxygenase (COX)-2, the inducible form of the COX isozymes, which catalyzes the key reaction in prostaglandin (PG) synthesis,9,10 is considered a promising pharmacological target for the prevention and treatment of certain breast malignancies.11,12 This concept is supported by growing evidence of a correlation between aberrant COX-2 overexpression and cancer development in the breast. COX-2 has been found to be overexpressed in ductal carcinomas in situ and invasive adenocarcinomas.11,13–15
Strong COX-2 overexpression, which has been found in epithelial and vascular cells, seems to be limited, however, to subsets of human breast cancers characterized by HER-2/neu overexpression and poor prognosis.13,15 Moreover, the selective COX-2 inhibitor celecoxib reduced the formation and growth of experimentally induced COX-2-positive mammary gland cancers in rodents.11,16 Transgenic overexpression of COX-2 directed by the mouse mammary tumor virus promoter to luminal cells of ducts and alveoli induced focal alveolar hyperplasia, dysplasia and ductal, as well as lobuloalveolar carcinomas in multiparous females.17 Furthermore in this transgenic model system the COX-2-mediated PGE2 synthesis was shown to be critical for turning on the angiogenic switch in breast cancer progression.18 Together these data strongly suggested that expression of COX-2 is sufficient for in situ tumor formation.17,18
Here we present the mammary gland phenotype of mice bearing the COX-2 transgene under the control of a K5 promoter. In contrast to the mouse mammary tumor virus model, transgenic COX-2 is expected to be constitutively expressed mainly in K5-expressing mammary myoepithelial cells and in a subset of luminal cells independent from estrous cycle, pregnancy, and lactation. Nulliparous mice spontaneously develop proliferative epithelial lesions, duct ectasias, cysts, and fibrosis, a phenotype that could be suppressed by a COX-2-selective inhibitor. The above abnormalities are known to be associated with FC of human breast. In fact, COX-2-overexpression was found in epithelial cells of such lesions.
Materials and Methods
Materials
Enzyme-linked immunosorbent assay-bovine serum albumin was purchased from Sigma. Polyclonal rabbit anti-mouse Ki67 and alkaline phosphatase-conjugated goat anti-rabbit IgG came from Dianova (Hamburg, Germany); rabbit polyclonal anti-EP2 receptor (SC-20675), -human HER-2 (SC-284), -ERα (SC-7207), -progesterone receptor (SC-538), goat polyclonal anti-β-actin (SC-1616), -EP3 receptor (SC-16019), -EP4 receptor (SC-16022), -human COX-2 (SC-1745), and peroxidase-conjugated goat anti-rabbit IgG came from Santa Cruz, polyclonal anti-rabbit keratin 5 (K5) was from BabCo, mouse monoclonal anti-keratin 18 was from Progen (Heidelberg, Germany) and monoclonal mouse anti-human SMA from DAKO, Glostrup, Denmark. Rabbit polyclonal anti-EP1 receptor antibody, PGE2-, and PGF2α-enzyme immunoassay kits were from Cayman (Ann Arbor, MI). Aprotinin, leupeptin, and α2-macroglobulin were from Roche Applied Sciences.
Animals
Wild-type (wt) NMRI mice (outbred strain from RCC, Füllinsdorf, Switzerland) and K5 COX-2 transgenic lines 675+/+ and 667+/− were kept under an artificial day/night rhythm and were fed Altromin standard food and water ad libitum.19 Experiments were approved by the Governmental Committee for Animal Experimentation (license 053/00).
Treatments
Biopsies were frozen immediately in liquid nitrogen or processed for histology or immunohistochemistry. To study the effect of a COX-2-selective inhibitor on the transgene-induced phenotype, a rodent diet 5010 containing 1500 ppm celecoxib (kindly provided by J. Masferrer, Pfizer, USA) was fed to transgenic mice starting on day 1 after birth for 3 to 6 months. The diet was delivered to nursing mice for 4 weeks and later on as regular chow.
Human Biopsies
Ten paraffin-embedded tissues each of breast carcinoma and FC and five of normal breasts were selected from the archives of the Institute of Pathology, University of Heidelberg, and used for immunohistochemistry. Snap-frozen tissues were used for immunoblots. Clinical data of breast cancer patients included mean age (62 ± 12 years), premenopausal (n = 2), postmenopausal (n = 8), invasive ductal carcinomas (n = 5), invasive ducto-lobular carcinoma (n = 1), and invasive lobular carcinomas (n = 4). TNM staging of carcinomas, treatment, presurgical treatment history, and HER-2, estrogen, and progesterone receptor status were recorded by I.B. (Department of Pathology, University of Heidelberg). Clinical data of patients with FC included mean age (51 ± 12 years), premenopausal (n = 6), postmenopausal (n = 4), lesions with focal ductal benign epithelial proliferation (n = 3), focal apocrine metaplasia (n = 1), interstitial fibrosis, cystic duct, and lobular dilatation, no intraductal epithelial proliferation, apocrine metaplasia, or adenosis (n = 6). Clinical data of patients with normal breast tissue included mean age (43 ± 22 years), premenopausal (n = 3), and postmenopausal (n = 2). Tissues were taken by surgical breast reduction.
Immunoblot Analysis
Immunoprecipitation of COX isozymes and immunoblot analysis were performed as described using isozyme-specific antisera.20,21 For detection of HER-2, and EP receptors, frozen skin powder was homogenized in a HP-buffer (50 mmol/L Tris/HCl, pH 7.4, 1% Tween 20, 2 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L phenylmethyl sulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 2 mmol/L α2-macroglobulin). Debris was removed by centrifugation (Beckman GPKR, 4000 rpm, 20 minutes, 4°C). Precipitated and denatured protein (120 μg) was separated by 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis and processed as described.22 Primary antibodies were used at a dilution of 1:250 to 1:1000. The secondary horseradish peroxidase-conjugated anti-IgG antibodies were used at a dilution of 1:2000. Immunodetection was performed using enhanced chemiluminescence reagent according to the manufacturer’s instructions (Amersham Pharmacia). The specificity of the immunosignals was confirmed by complete quenching of the immunosignal on preadsorption of the antiserum with a 500-fold molar excess of the respective peptide antigen from Santa Cruz or Cayman.
Histochemistry and Immunohistochemistry
Five-μm cryosections of mouse mammary gland were used for hematoxylin and eosin (H&E) staining. Immunostainings (COX-2, COX-1, Ki67) and double-immunofluorescence analysis of mouse tissue were performed with 5-μm cryosections as published.20,23 Primary antibodies were diluted 1:50 and peroxidase- or fluorochrome-conjugated secondary antibodies 1:100 in blocking solution. Ki67-positive cells were determined as the percentage of the total number of epithelial mammary gland cells counted. For staining of human paraffin-embedded specimens, the COX-2 antibodies (diluted 1:100 in phosphate-buffered saline with 1.5% normal horse serum) were incubated for 2 hours at room temperature. COX-2 detection was performed using biotinylated universal secondary antibodies and peroxidase-coupled streptavidin. The kit reagents were used according to the manufacturer’s instructions (Vector Laboratories Inc., Burlingame, CA). The nuclei were counterstained with hematoxylin. Negative controls included sections incubated without primary or with the isotype-matched primary antibody.
RNA Isolation and Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR) Analysis
Extraction of total RNA from pulverized mammary glands and RT-PCR were performed with the cDNA cycle kit (Invitrogen, Leek, The Netherlands) as described.19,21 Using primers 5′-ATTCCTGCAGCCCAACATCTGT-3′ and 5′-AGGTTC-TGCTTTGGGTTTGGG-3′ (set 1), a 460-bp fragment diagnostic for 3′utr of transgenic mRNA was amplified. Using primers 5′-ACTCACTCAGTTTGTTGAGTCATTC-3′ and 5′-TTTGATTAGTACTGTAGGGTTAATG-3′ (set 2), a 583-bp fragment diagnostic for COX-2 mRNA was amplified. β-actin (fragment size, 430 bp) was amplified with primers 5′-AAACTGGAACGGTGAAGGC-3′ and 5′-GCTGCCTCAACACCTCAAC-3′.
Quantitative RT-PCR Analysis
cDNA was synthesized in a 20-μl RT reaction containing 2 μg of total RNA, 0.5 μg oligo(dT)18-primer, 1× RT buffer, 500 μmol/L dNTP each, 10 U RNase inhibitor, and 4 U Omniscript reverse transcriptase (Qiagen), and was incubated at 37°C for 1 hour. cDNA was PCR-amplified in a 20-μl reaction using the QuantiTect SYBR Green PCR kit (Qiagen), combining 1× QuantiTect SYBR Green PCR Master Mix, 20 pmol aromatase sense 5′-TTAATGAAAGCATGCGGTACC-3′ and anti-sense 5′-GGAAGTACTCGAGCCTGTGC-3′ primer each and 2 μl of the RT reaction. Thermal cycling was performed using the Mx3000P real-time PCR system (Stratagene) with an initial activation step of 15 minutes at 95°C, and 50 cycles of 15 seconds denaturation at 95°C, 30 seconds primer annealing at 55°C, and 30 seconds extension at 72°C. Amplification rates were measured automatically and the number of cycles needed to cross the threshold (Ct) determined. To confirm amplification specificity, the PCR products were subjected to a melting curve analysis and subsequent agarose gel electrophoresis.
Flow Cytometric Analysis
Multiparametric flow cytometry was performed as previously published24 on a Galaxy pro flow cytometer using the Flow-Max program (Partec, Münster, Germany) and the Multi Cycle Software for cell cycle analysis (Phoenix Flow Systems, San Diego, CA). To analyze the expression of apoptosis markers phycoerythrin-labeled anti-caspase 3 and anti-p53 antibodies (Apotech, c/o Alexis, Germany), and fluorescein isothiocyanate-labeled anti-Bcl-2 antibody (oncogene PC68) were used. For quantitations of DNA content, cells were counterstained with 4,6-diamidino-2-phenylindole. According to the published protocol,24 2.1% citric acid containing 0.5% Tween 20 was used to isolate single cells from frozen mammary gland biopsies (n = 5, each of wt and transgenic animals). For each measurement a minimum of 10,000 cells was analyzed. Cut-off negative and positive cells resulted from measurements of fluorescein isothiocyanate fluorescence and phycoerythrin fluorescence isotype controls for each sample.
Determination of PG Levels in Mammary Gland Tissue
PGE2 contents were determined using a commercially available enzyme immunoassay kit, as described.23 Concentrations (pg/mg protein) represent mean ± SD (n = 5). Statistical analysis was done with Student’s t-test. P values <0.05 were regarded as significant.
Results
Phenotype of K5 COX-2 Transgenic Mammary Glands
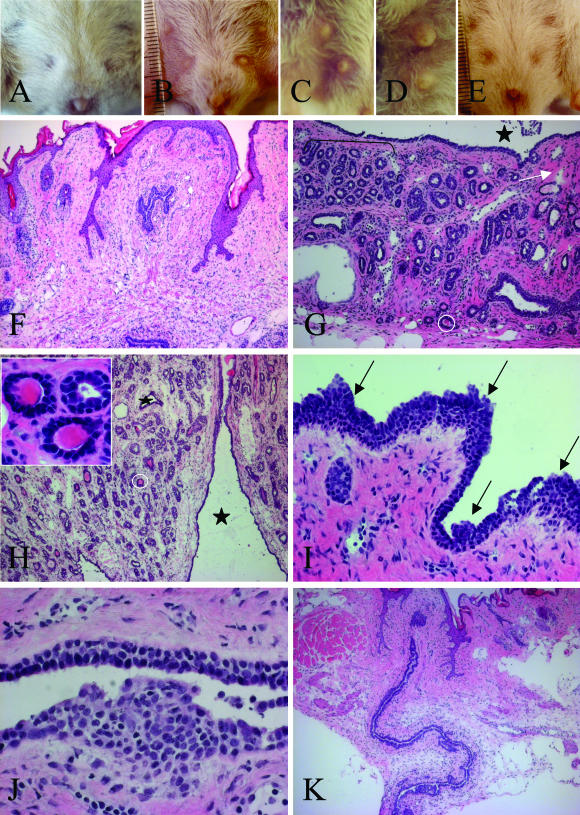
We previously reported that K5 promoter-driven COX-2 overexpression in basal cells of interfollicular and follicular epidermis caused a preneoplastic skin phenotype in heterozygous transgenic mouse lines.19,23 An additional transgene-dependent phenotype, which is more pronounced in homozygous than in heterozygous mouse lines, was observed in mammary glands as reported here. Showing a normal appearance at birth, transgenic homozygous virgins (K5 COX-2/675+/+) began to differ from wt littermates at the age of 5 weeks, in that thoracic and inguinal mammary glands were enlarged. The remarkable size differences, shown for a 6-month-old (Figure 1B) and an 11-month-old transgenic (Figure 1, C and D) compared to a 6-month-old wt virgin (Figure 1A), were independent of any pregnancy or lactogenic hormones. Histological examination of mammary glands from 7-week-old to 6-month-old transgenic virgins revealed cystic duct dilatations and adenosis as the predominant changes (Figure 1, G and H). Some alveoli were filled with eosin-stainable secretion (Figure 1H, inset). Focally, intraductal hyperplasia (Figure 1, I and J) and periductal low-grade interstitial fibrosis were detected (Figure 1, G and I). Invasive ductal carcinomas were seen at a rare frequency (1 of 1020) beyond 12 months of age and only after a pregnancy. Tumors with myoepithelial differentiation were never found. Overall the major morphological alterations diagnosed in K5-COX-2 transgenic mammary glands resembled those which are associated with FC in human breast tissue.5,25
Figure 1.
Phenotype of mammary glands in K5 COX-2/675+/+ transgenic and wt virgin mice. A–E: Gross phenotype. Mammary glands of a 6-month-old wt (A), a 6-month-old (B) and an 11-month-old transgenic virgin (C, D), and a 6-month-old transgenic virgin fed with celecoxib throughout life (E). Inguinal (B, C) and thoracic (D) glands of transgenics were increased in size as compared to wt (A). F–K: Histology. H&E-stained sections of mammary glands of a 6-month-old wt (F) and a transgenic virgin (G, I), a 10-month-old transgenic (H, J), and a 6-month-old transgenic virgin fed with celecoxib diet throughout life (K). Note in transgenics the cystic transformations of the ducts (black asterisks in G and H), adenosis (bracket in G), periductal low-grade interstitial fibrosis (G, white arrow), and secreting alveoli (G and H, white circles; H, inset) as well as focal intraductal hyperplasia (I, arrows, and J). Original magnifications: ×10 (F, H, K), ×16 (G), ×40 (I), ×63 [H (inset), J].
Expression and Activity of Transgenic COX-2
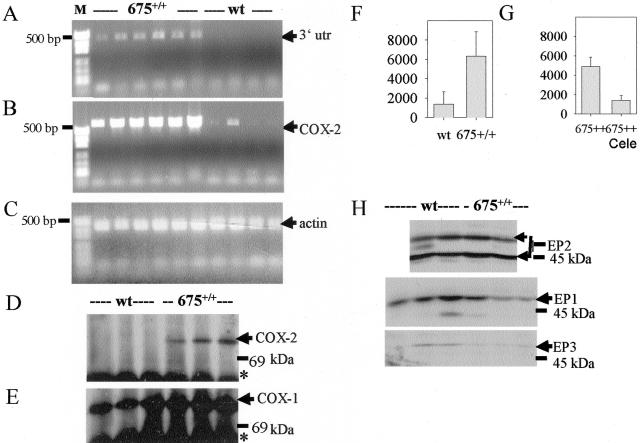
Because the K5 promoter is known to be active in mammary gland cells,1 the histopathological changes in transgenics were expected to correlate with COX-2 overexpression. Transgene expression was detected by RT-PCR using the primer set 1 (Figure 2A). The diagnostic COX-2 3′utr DNA amplicon of 480 bp in size was consistently amplified using RNA of mammary glands of 7-week-old transgenic (six of six) but not from age-matched wt virgins (none of six) confirming the specificity of the primers. RT-PCR with the primer set 2 generated an amplicon of 580 bp, which is diagnostic for transgenic and endogenous COX-2 mRNA and verified COX-2 expression in the same mammary glands from transgenics (six of six) and rarely (one of six) from wt virgins (Figure 2B).
Figure 2.
Expression of COX isozymes, PGE2-levels, and expression of EP receptors. A: RT-PCR of transgenic COX-2 mRNA from mammary glands (n = 6) of six 7-week-old transgenic virgins (675+/+) and from mammary glands (n = 4) of four age-matched wt virgins using primer set no. 1. B: RT-PCR of transgenic and endogenous COX-2 mRNA of the same samples as in A using primer set no. 2. C: As an internal control β-actin was amplified. A–C: A negative control (PCR without cDNA) was included and gave no signals. D and E: COX isozyme levels in mammary glands of wt and transgenic (675+/+) virgins. Homogenates of mammary glands (n = 3) were immunoprecipitated and immunoblotted using COX-2-specific (D) and COX-1-specific (E) antibodies. IgG (*) cross-reacted with the secondary antibody. F: PGE2 levels in mammary glands of 7-week-old transgenic (675+/+) and wt virgins (n = 5, P < 0.01). G: PGE2 levels in mammary glands of 3-month-old transgenic virgins fed control diet (675+/+, n = 5) or diet containing celecoxib (675+/+Cele, n = 3) for 3 months after birth (P < 0.05). H: Immunoblot detection of EP1-EP3 receptors in mammary glands from 10-month-old transgenic and wt virgins (n = 2 to 3). Arrows indicate specific immunosignals.
Immunoblot analysis of mammary glands revealed COX-2 protein in all transgenic biopsies (n = 8 biopsies from eight 7-week-old virgins), whereas corresponding wt tissues were COX-2-negative (representatively shown in Figure 2D). COX-1 expression was similar in transgenic and wt mammary glands (Figure 2E). Concomitantly, with the increased COX-2 protein levels, approximately threefold increased PGE2 levels were found in transgenic as compared to wt mammary glands (Figure 2F). PGE2 may act via four G protein-coupled 7-transmembrane receptors EP1 to EP4. Expression levels of PGE2 receptor isoforms EP2 (Figure 2H) were similar, whereas EP1 and EP3 protein levels tended to be reduced in transgenic as compared to wt mammary glands (Figure 2H).
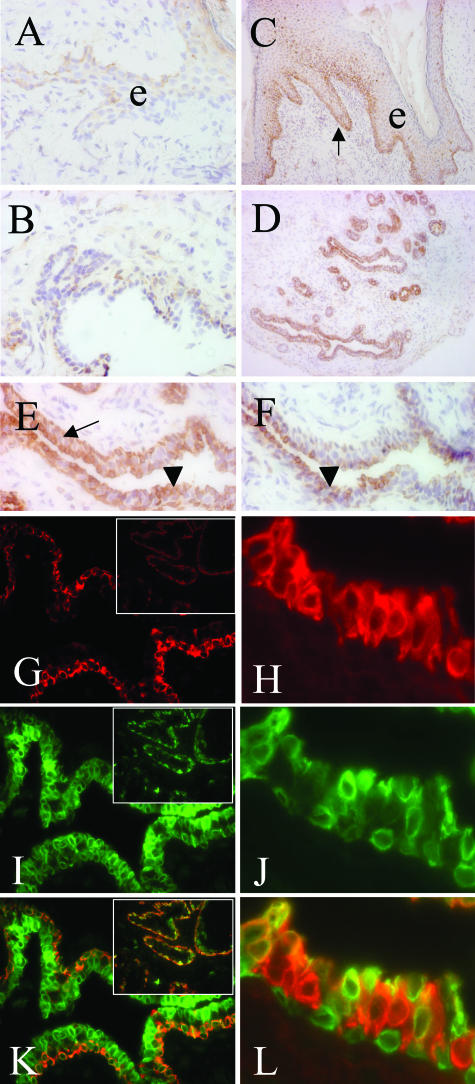
Immunohistochemical analysis of mammary gland cryosections from transgenics revealed COX-2-specific immunosignals in basal keratinocytes of the surface epithelium as well as in basal and luminal cells of the mammary gland epithelium, whereas mesenchymal and endothelial cells were COX-2-negative (Figure 3; C to E). This distribution corresponded to the described K5 promoter activity1 and K5 expression in mammary glands of transgenics (Figure 3, G and K, and insets), except for the widespread luminal localization of COX-2 (Figure 3; D, E, I, K). COX-2 co-localized in the basal compartment with K5 (Figure 3K, and inset) and with smooth muscle actin (data not shown). In luminal epithelium COX-2 was found to co-localize with subpopulations of K18-expressing cells (Figure 3; H, J, L), suggesting that endogenous COX-2 was up-regulated in these cells. In mammary gland tissue of wt mice, however, COX-2 was detectable only in a few keratinocytes of the superficial epithelium and in a few duct cells (Figure 3, A and B). COX-1 expression was mainly restricted to luminal cells of transgenic (Figure 3F) and wt mammary epithelium (not shown). Preadsorption of the antibodies with sequence-specific blocking peptides suppressed the immunosignals.
Figure 3.
Localization of COX isozymes. Immunoperoxidase staining of cryosections from 10-month-old COX-2 transgenic (C–F) and wt virgins (A, B) with anti-COX-2 (A–E) and anti-COX-1 (F) antisera. Note strong COX-2 expression in basal cells (C, arrow) of superficial epithelium (e) in basal (E, black arrow) and luminal cells (E, black arrowheads) of transgenic mammary gland (D, E); COX-1 was found predominantly in luminal cells (F, black arrowheads). Double-immunofluorescence analysis of transgenic specimens (G–L) using anti-K5 and anti-COX-2 antisera or anti-K18 and anti-COX-2 antisera revealed that K5 (G and inset, red signals) and COX-2 (I and inset, J; green signals) co-localize in the myoepithelium and in K5-positive luminal cells (K and inset, yellow/orange signals). Moreover, note that expression of K18 (H, red signals) and COX-2 (J, green signals) co-localize in some luminal epithelial cells (L, yellow/orange signals). Original magnifications: ×10 (insets G, I, K); ×16 (C, D); ×63 (A, B, E, F, G, I, K); ×150 (H, J, L).
Development of the Mammary Gland Phenotype of K5 COX-2 Transgenic Virgins Depends on COX-2-Mediated PGE2 Synthesis
When transgenic mice were fed the COX-2-selective inhibitor celecoxib26 for 3 to 6 months postnatally PGE2 accumulation in mammary gland was reduced to wt levels, whereas transgenic littermates fed with a drug-free control diet for the same period had highly elevated PG levels (Figure 2G). Moreover, the transgene-dependent phenotype for the mammary gland epithelium of the virgins, ie, cystic duct dilatations and adenosis (Figure 1, G and H), did not develop under celecoxib treatment (Figure 1K). Instead, mammary gland morphology resembled that of wt virgins (Figure 1F) or wt mice with involuted mammary glands after a round of pregnancy and lactation (data not shown). The results confirm that the development of the transgenic phenotype was causally related to the COX-2-dependent PGE2 synthesis.
Development of a Proliferative Phenotype in K5 COX-2 Transgenic Mammary Glands
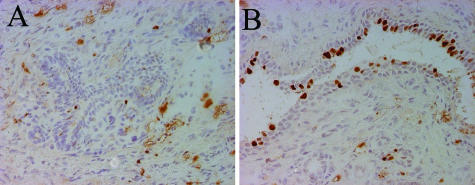
The proliferation index in mammary gland epithelium, as revealed by Ki67 staining, was markedly increased in transgenics in both ducts and lobuli as compared to wt mammary glands [10.8% ± 2 SEM (n = 4 mice; total cell count, 4387) versus 3.5% + 2 SEM (n = 4 mice; total cell count, 2333). In transgenics, proliferating cells were found both in the myoepithelial and luminal layers, whereas only a few proliferating cells distributed throughout both layers were seen in wt tissue (Figure 4).
Figure 4.
Proliferative active mammary glands in K5 COX-2/675+/+ transgenic mice. Immunoperoxidase staining of cryosections from 10-month-old COX-2 transgenic (B) and wt virgins (A) with anti-Ki67 antiserum. Note increased number of Ki67-positive basal and luminal cells in transgenic as compared to wt tissue (B). Original magnifications, ×40.
The levels of apoptosis-related proteins Bcl-2, caspase 3, and p53 as analyzed by multiparametric flow cytometry were similar in mammary glands of wt and transgenic virgins, ie, the percentage of positive cells in wt and transgenic samples (n = 5, each) was 5.4 ± 1.7 versus 3.3 ± 1.1 for Bcl-2; 3.7 ± 0.3 versus 3.3 ± 1.9 for caspase 3, and 5.1 ± 2.2 versus 3.6 ± 1.9 for p53, respectively.
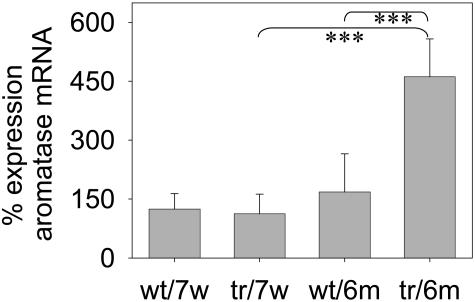
Estrogen is known to be mitogenic for mammary gland epithelial cells.27 The final step of estrogen biosynthesis is catalyzed by the enzyme aromatase, which is encoded by the Cyp19 gene.28 Therefore we next looked for aromatase levels in transgenic mammary glands. Using quantitative RT-PCR, a significant increase in aromatase mRNA expression was observed in 6-month-old transgenic samples as compared to wt virgins (Figure 5), whereas comparable aromatase mRNA levels were found in mammary glands of 7-week-old wt and transgenic virgins.
Figure 5.
Increased levels of aromatase mRNA in K5 COX-2/675+/+ transgenic mice. Quantitative RT-PCR analysis of aromatase gene expression in mammary glands of 7-week-old (7w) and 6-month-old (6m) wt and transgenic (tr) mice. Normalization was done for each specimen to housekeeping gene β-actin. Values represent mean values from three to six mammary glands each and two experiments. Student’s t-test, P < 0.002 (***).
COX-2 Expression in Benign as Compared to Malignant Human Breast Diseases
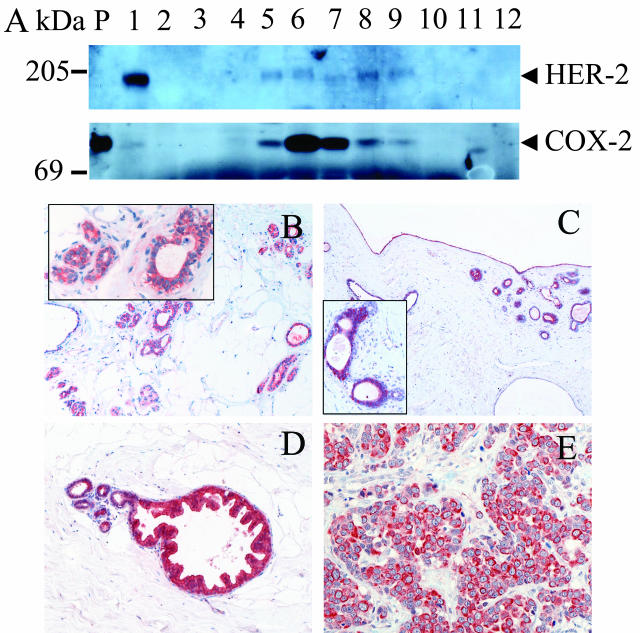
Given that the mammary glands of transgenic mice exhibited signs of FC, we next addressed the question as to whether COX-2 expression is associated with benign breast diseases of humans. Therefore, tissue biopsies exhibiting focal ductal epithelial proliferation, focal apocrine metaplasia, interstitial fibrosis, cystic duct and lobular dilatation, intraductal epithelial proliferation, apocrine metaplasia, or adenosis versus normal breast tissue and breast carcinomas as controls were analyzed for COX-2 expression. Confirming previous studies,11,13–15 COX-2 expression was found in HER-2-positive ductal carcinoma in situ and breast carcinomas (Figure 6A; lanes 1, 5 to 9) but not in HER-2-negative breast carcinomas and normal breast tissue (Figure 6A, lanes 2 and 12). Only faint or no COX-2-specific signals could be detected in biopsies from benign breast diseases (Figure 6A; lanes 3, 4, and 10). However, when using the more sensitive immunohistochemical analysis, 10 of 10 biopsies from benign breast diseases were COX-2-positive. Intermediate to high levels of COX-2 expression were found predominantly in epithelial cells of cysts and areas of apocrine metaplasia and intraductal epithelial proliferations (Figure 6, C and D). In contrast, in normal human breast tissue only a focal slight or moderate COX-2 immunoreactivity was detected in ductal and some lobular epithelial cells. Mesenchymal and endothelial cells were COX-2-negative (Figure 6B). Invasive ductal or lobular carcinomas and ductal carcinomas in situ showed a moderate to strong cytoplasmic COX-2 immunoreactivity in the atypic epithelium (representatively shown for a poorly differentiated ductal carcinoma in Figure 6E).
Figure 6.
HER-2 receptor and COX-2 expression in normal, malignant, and fibrocystically changed human breast. A: Immunoblot analysis of extracts from normal mammary gland (lane 12), ductal carcinoma in situ (lane 9), invasive ductal carcinomas (lanes 1, 2, 5 to 8), and mammary glands with FC (lanes 3, 4, 10) using anti-human HER-2 receptor antibodies (top) and of protein immunoprecipitated with an anti-COX-2 antibody from the same samples (bottom). Marker proteins (lane 11) are given in kd. Purified COX-2 protein was run as a positive control (P). B–E: COX-2 immunostainings of normal breast (B), FC exhibiting cysts (C), apocrine metaplasia (D), and a poorly differentiated invasive ductal carcinoma (E). Note weak COX-2 signals in ducts and alveoli of normal, but strong COX-2 signals in fibrocystically changed breast and breast carcinoma. Original magnifications: ×26 (B, C); ×52 [B and C (insets), D, E].
Discussion
Several genetic studies document a cause and effect relationship between carcinogenesis and COX-2.17–19,23,29–32 Previously we showed that K5 promoter-driven COX-2 overexpression in the proliferative compartment of the epidermis, ie, in basal keratinocytes caused a hyperplastic and dysplastic tail epidermis because of abnormal terminal differentiation.19 Although transgenic COX-2 overexpression was insufficient for spontaneous tumor induction in this organ it transformed epidermis into an autopromoted state, ie, sensitized the tissue dramatically for the carcinogenic activity of dimethylbenz[a]anthracene and as a consequence papillomas, squamous cell carcinomas, and sebaceous gland adenomas developed at a high frequency.23
An additional phenotype that critically depended on COX-2 expression and activity emerged in the mammary gland of the mice. Transgenic COX-2 expression, which was associated with high tissue levels of PGE2, occurred in K5- and smooth muscle actin-positive myoepithelial and K5-positive luminal cells. In addition, COX-2 signals became apparent in some K18-positive luminal cells, indicating an up-regulation of endogenous COX-2. Duct dilatations in young transgenic virgins (up to 7 weeks) developed although secreted material was not seen in the ductal or alveolar structures. The cystic transformation of ducts that was observed with increasing age of nulliparous females may indicate an abnormal differentiation of luminal cells.
Along with cystic duct dilatations we recognized an increased number of glandular elements that were enlarged in size and surrounded focally by fibrosis. The mild intraductal hyperplasia that grew out focally may be regarded as the development of an initial stage in the hypothesized multistep sequence toward breast carcinomas.33 However, subsequent preneoplastic stages, including atypical ductal hyperplasia or ductal carcinoma in situ or myoepithelial hyperplasia, were not found indicating that high COX-2 expression and activity in the mentioned cell types alone are not sufficient for tumor development. Invasive ductal carcinomas were only noticed in females with at least one round of pregnancy and developed with rare frequency in virgins, indicating that additional effects are required. An increased incidence of invasive ductal and lobuloalveolar carcinomas was observed in another transgenic model engineered to overexpress COX-2 under the control of the mouse mammary tumor virus promoter, which is known to be activated in a hormone-dependent manner during pregnancy and lactation, but only after multiple rounds of pregnancy and lactation.17 Obviously tumor development depended on the high COX-2 expression in luminal epithelium of alveoli and ducts, because the low COX-2 levels in virgins, which were below the detection limit in Western blot analysis and which correlated to marginally stimulated PGE2 levels as compared to wt controls did not cause neoplastic lesions but a precocious mammary gland differentiation.17 In breast carcinogenesis, the role of high levels of estradiol as a hormone stimulating cell proliferation and as a procarcinogen inducing genetic damage is still under debate.34–36 Therefore, a synergistic stimulation of the carcinogenic process under repeated exposure to estrogen or other hormones and COX-2 cannot be ruled out in the mouse mammary tumor virus-COX-2 transgenic model.
As shown here, the K5 promoter-driven overexpression of COX-2 and the increased PGE2 levels in mammary glands of transgenic virgins correlated with an increased Ki67 proliferation index of the ductal and alveolar epithelium as compared to age-matched wt virgins. Although COX-2 overexpression has been causally related to an inhibition of apoptosis,37 such an effect does not seem to contribute to the observed transgenic phenotype, because the expression of anti-apoptotic Bcl-2 and proapoptotic Bax (immunoblot data not shown), or of active caspase 3 and of p53 were unaffected.
Previously, PGE2 was shown to stimulate proliferation of mammary epithelial cells via EP receptor38-cAMP-dependent induction of aromatase in adipocytes subsequently leading to enhanced estrogen biosynthesis.39,40 Moreover, aromatase and COX–2 gene expression have been found to correlate positively in human breast cancer tissues.41 Here in our transgenic approach, we present strong evidence for the association between increased COX-2 expression/PGE2 levels and increased aromatase mRNA levels in vivo. Together with the observation that EP1 to EP3 receptors were expressed in these lesions, our observations may suggest, that local COX-2-mediated PGE2 production leads via the activation of the protein kinase A and/or protein kinase C signaling cascades to the expression of the aromatase gene in an autocrine or paracrine manner,42 and subsequently, the local estrogen biosynthesis and estrogen-dependent growth of mammary gland epithelium in vivo as well.43 However, the fact that there is no coincidence between increased aromatase mRNA expression and elevated PGE2 levels 7 weeks after birth may point to a more complex mechanism of aromatase induction. The expression of ER-α, thought to render cells susceptible to the mitogenic stimulus of estrogen44 was similar in wt and transgenic virgins (data not shown). In previous studies, it was shown that mouse mammary tumor virus-driven overexpression of aromatase caused ductal and alveolar hyperplasia and nuclear abnormalities in the mammary glands of virgins, similar to our K5 COX-2 transgenic virgins. Again no spontaneous tumor development was observed in this model.45
The spontaneously developing mammary gland phenotype in K5 COX-2 transgenics bears analogy to that of a woman with a benign FC. This disease includes a heterogeneous group of lesions such as nonproliferative lesions (small or large cysts, fibroadenosis, apocrine changes, duct ectasia, and mild epithelial hyperplasia), proliferative lesions without atypia (sclerosing adenosis, moderate to florid epithelial hyperplasia), and atypical hyperplasia of ducts and lobuli.5,46 FC affects an estimated 50 to 90% of women during the reproductive years and has been assumed to be because of imbalances of hormones such as estrogen, progesterone, or prolactin.46–48 It is associated with a significantly increased risk of breast cancer, especially if the benign breast disease is of the proliferative and atypical type.49 Here it is shown for the first time that aberrant COX-2 overexpression and elevated PGE2 levels as well as increased aromatase expression levels in mammary glands are associated with various abnormalities of FC, including nonproliferative (cysts and duct ectasia) and proliferative abnormalities (moderate ductal and lobular hyperplasia). Intriguingly, COX-2 was also found to be overexpressed in HER-2-negative lesional epithelium of women with FC, indicating that aberrant COX-2 overexpression may be involved in the development of this disease in humans as well. Moreover, the preclinical data with the COX-2-selective inhibitor, which suppressed the phenotype of FCs in transgenics, points to a potential clinical application of COX-selective inhibitors in patients with FC.
Acknowledgments
We thank J. Büchele, D. Kucher, S. Pfrang, A. Pohl-Arnold, and B. Steinbauer for excellent technical assistance; and S. Edwards for excellent editorial assistance.
Footnotes
Address reprint requests to Karin Müller-Decker, Deutsches Krebsforschungszentrum, INF 280, 69120 Heidelberg, Germany. E-mail: k.mueller-decker@dkfz-heidelberg.de.
Supported by the Deutsche Krebshilfe, eV, Bonn, Germany.
References
- Page JR, Amess B, Townsend RR, Parekh R, Herath A, Brusten L, Zvelebil MJ, Stein RC, Waterfield MD, Davies SC, O’Hare JO. Proteomic definition of normal human luminal and myoepithelial breast cells purified from reduction mammoplasties. Proc Natl Acad Sci USA. 1999;96:12589–12594. doi: 10.1073/pnas.96.22.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X-J, Salunga R, Tuggle JT, Gaudet J, Enright E, McQuary P, Payette T, Pistone M, Stecker K, Zhang BM, Zhou YX, Varnholt H, Smith B, Gadd M, Chatfield E, Kessler J, Baer TM, Erlander MG, Sgroi DC. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci USA. 2003;100:5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foschini MP, Eusebi V. Carcinomas of the breast showing myoepithelial cell differentiation. A review of the literature. Virchows Arch. 1998;432:303–310. doi: 10.1007/s004280050170. [DOI] [PubMed] [Google Scholar]
- Lakhani SR, O’Hare MJ. The mammary myoepithelial cell—Cinderella or ugly sister? Breast Cancer Res. 2001;3:1–4. doi: 10.1186/bcr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin C, Mertens F. Cytogenetics of benign breast lesions. Breast Cancer Res Treatment. 1998;51:1–15. doi: 10.1023/a:1006009531378. [DOI] [PubMed] [Google Scholar]
- Xiao G, Liu YE, Gentz R, Sang QA, Ni J, Goldberg ID, Shi YE. Suppression of breast cancer growth and metastasis by a serpin myoepithelium-derived serine proteinase inhibitor expressed in the mammary myoepithelial cells. Proc Natl Acad Sci USA. 1999;96:3700–3705. doi: 10.1073/pnas.96.7.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PH, Lippman SM. Chemoprevention of breast cancer. Breast Cancer Res. 2000;62:1–17. doi: 10.1023/a:1006484604454. [DOI] [PubMed] [Google Scholar]
- Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- Howe LR, Subbaramaiah K, Brown AMC, Dannenberg AJ. Cyclooxygenase-2: a target for the prevention and treatment of breast cancer. Endocr Relat Cancer. 2001;8:97–114. doi: 10.1677/erc.0.0080097. [DOI] [PubMed] [Google Scholar]
- Singh-Ranger G, Mokbel K. Role of cyclooxygenase-2 (COX-2) in breast cancer, and implications of COX-2 inhibition. Eur J Surg Oncol. 2002;28:729–737. doi: 10.1053/ejso.2002.1329. [DOI] [PubMed] [Google Scholar]
- Ristimäki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- Half E, Tang XM, Gwyn K, Sahin A, Wathen K, Sinicrope FA. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;62:1676–1681. [PubMed] [Google Scholar]
- Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer. J Biol Chem. 2002;277:18649–18657. doi: 10.1074/jbc.M111415200. [DOI] [PubMed] [Google Scholar]
- Howe LR, Subbaramaiah K, Patel J, Masferrer JL, Deora A, Hudis C, Thaler HT, Muller WJ, Du B, Brown AMC, Dannenberg AJ. Celecoxib, a selective COX-2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res. 2002;62:5405–5407. [PubMed] [Google Scholar]
- Liu CH, Chang SH, Narko K, Trifan OC, Wu M-T, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- Chang S-H, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, Lane TF, Hla T. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci USA. 2004;101:591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufang G, Fürstenberger G, Heidt M, Marks F, Müller-Decker K. Abnormal differentiation of epidermis in transgenic mice constitutively expressing cyclooxygenase-2 in skin. Proc Natl Acad Sci USA. 2001;98:7629–7634. doi: 10.1073/pnas.121574098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Decker K, Kopp-Schneider A, Seibert K, Marks F, Fürstenberger G. Localization of prostaglandin H synthase isoenzymes in murine epidermal tumors: suppression of skin tumor promotion by inhibition of prostaglandin H synthase-2. Mol Carcinog. 1998;23:36–44. doi: 10.1002/(sici)1098-2744(199809)23:1<36::aid-mc5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Müller-Decker K, Reinerth G, Krieg P, Zimmermann R, Heise H, Bayerl C, Marks F, Fürstenberger G. Prostaglandin H synthase isozyme expression in normal and neoplastic human skin. Int J Cancer. 1999;82:648–656. doi: 10.1002/(sici)1097-0215(19990827)82:5<648::aid-ijc6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Müller-Decker K, Scholz K, Marks F, Fürstenberger G. Differential expression of prostaglandin H synthase isozyme expression during multistage carcinogenesis in mouse epidermis. Mol Carcinog. 1995;12:31–41. doi: 10.1002/mc.2940120106. [DOI] [PubMed] [Google Scholar]
- Müller-Decker K, Neufang G, Berger I, Neumann M, Marks F, Fürstenberger G. Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc Natl Acad Sci USA. 2002;99:12483–12488. doi: 10.1073/pnas.192323799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehemann V, Sykora J, Vera-Delgado J, Lange Adelheid, Otto HF. Flow cytometric detection of spontaneous apoptosis in human breast cancer using the TUNEL-technique. Cancer Lett. 2003;194:125–131. doi: 10.1016/s0304-3835(03)00054-5. [DOI] [PubMed] [Google Scholar]
- Dupont WD, Page MD. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- Talley JJ, Brown DL, Carter JS, Graneto MJ, Koboldt CM, Masferrer JL, Perkins WE, Rogers RS, Shaffer AF, Zhang YY, Zweifel BS, Seibert K. 4-[5-Methyl-3-phenylisoxazol-4-yl]-benzenesulfonamide, valdecoxib: a potent and selective inhibitor of COX-2. J Med Chem. 2000;43:775–777. doi: 10.1021/jm990577v. [DOI] [PubMed] [Google Scholar]
- Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgroe MW, Hinselwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR, Bulun S. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trazaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, Tiano HF, Morham SG, Smithies O, Langenbach R. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 2000;60:4705–4708. [PubMed] [Google Scholar]
- Tiano HF, Loftin CD, Akunda J, Lee CA, Spalding J, Sessoms A, Dunson DB, Rogan EG, Morham SG, Smart RC, Langenbach R. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002;62:3395–3401. [PubMed] [Google Scholar]
- Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105:1589–1594. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani SR. The transition from hyperplasia to invasive carcinoma of the breast. J Pathol. 1999;187:272–278. doi: 10.1002/(SICI)1096-9896(199902)187:3<272::AID-PATH265>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Yue W, Santen RJ, Wang JP, Verderame MF, Bocchinfuso WP, Korach KS, Devanesan P, Todorovic R, Rogan EG, Cavalieri EL. Genotoxic metabolites of estradiol in breast: potential mechanism of estradiol induced carcinogenesis. J Steroid Biochem Mol Biol. 2003;86:477–486. doi: 10.1016/s0960-0760(03)00377-7. [DOI] [PubMed] [Google Scholar]
- Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocrine Rev. 2000;21:40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- Liehr JG. Role of DNA adducts in hormonal carcinogenesis. Reg Toxicol Pharmacol. 2000;32:276–282. doi: 10.1006/rtph.2000.1432. [DOI] [PubMed] [Google Scholar]
- Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Purohit A, Reed MJ. Regulation of estrogen synthesis in postmenopausal women. Steroids. 2002;67:979–983. doi: 10.1016/s0039-128x(02)00046-6. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;137:5739–5742. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- Davies G, Martin L-A, Sacks N, Dowsett M. Cyclooxygenase-2 (COX-2), aromatase and breast cancer: a possible role for COX-2 inhibitors in breast cancer chemoprevention. Ann Oncol. 2002;13:669–678. doi: 10.1093/annonc/mdf125. [DOI] [PubMed] [Google Scholar]
- Richards JA, Brueggemeier RW. Prostaglandin E2 regulates aromatase activity and expression in human adipose stromal cells via two distinct receptor subtypes. J Clin Endocrinol Metab. 2003;88:2810–2816. doi: 10.1210/jc.2002-021475. [DOI] [PubMed] [Google Scholar]
- Johnston SRD, Dowsett M. Aromatase inhibitors for breast cancer: lessons from the laboratory. Nat Rev Cancer. 2003;3:821–831. doi: 10.1038/nrc1211. [DOI] [PubMed] [Google Scholar]
- Shaaban AM, Sloane JP, West CR, Foster CS. Breast cancer risk in usual ductal hyperplasia is defined by estrogen receptor α and Ki67 expression. Am J Pathol. 2002;160:597–604. doi: 10.1016/s0002-9440(10)64879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekmal RR, Ramachandra N, Gubba S, Durgam VR, Mantione J, Toda K, Shizuta Y, Dillehay DL. Overexpression of int-5/aromatase in mammary glands of transgenic mice results in the induction of hyperplasia and nuclear abnormalities. Cancer Res. 1996;56:3180–3185. [PubMed] [Google Scholar]
- Donegan WL. Donegan WL, Spratt JS, editors. St. Louis: Saunders,; Cancer of the Breast. (ed 5) 2002:pp 67–110. [Google Scholar]
- Selim AA, Wells CA. Immunohistochemical localization of androgen receptor in apocrine metaplasia and apocrine adenosis of the breast: relation to oestrogen and progesterone receptors. J Clin Pathol. 1999;52:838–841. doi: 10.1136/jcp.52.11.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan-Chiu E, Wang J, Costantino JP, Paik S, Butch C, Wickerham DL, Fisher B, Wolmark N. Effects of tamoxifen on benign breast disease in women at high risk for breast cancer. J Natl Cancer Inst. 2003;95:302–307. doi: 10.1093/jnci/95.4.302. [DOI] [PubMed] [Google Scholar]
- Minami Y, Ohuchi N, Taeda Y, Takano A, Fukao A, Satomi S, Hisamichi S. Risk of breast cancer in Japanese women with benign breast disease. Jpn J Cancer Res. 1999;90:600–606. doi: 10.1111/j.1349-7006.1999.tb00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]