Abstract
CCL28 is a recently identified chemokine ligand for CCR10 and CCR3 that has been identified in mucosal epithelial surfaces in diverse tissues. CCL28-mediated eosinophil chemotaxis and peroxidase release were inhibited by preincubation of cells with anti-CCR3. CCL28 was constitutively expressed in lung tissue collected from nonsensitized control mice but increased levels were found in mice sensitized and rechallenged with cockroach antigen (CRA). CCL28 levels peaked in the lungs 24 hours after intratracheal challenge with CRA, whereas eotaxin expression peaked at 8 hours. Increased expression of CCR3 but not CCR10 could be detected during the induction of the CRA-induced pulmonary inflammation. To investigate the role of CCL28 in allergic airway responses, mice were treated with CCL28 antiserum 1 hour before receiving the final CRA challenge. The level of airway hyperresponsiveness in mice treated with anti-CCL28 was significantly reduced at 24 hours, but not 8 hours, compared to mice receiving control serum. This reduction was not related to decreased Th2 cytokine, chemokine, or leukotriene levels at 24 hours although peribronchial eosinophilia was significantly reduced. Thus, CCL28 appears to play a role in regulating eosinophil recruitment to peribronchial regions of the lung possibly by coordinated temporal production with eotaxin.
Chemokines are a large family of peptide chemoattractant cytokines with diverse functions during inflammatory and immune responses, including controlling cellular recruitment, activation, and differentiation. Chemokines have been shown to play a critical role in a number of pathophysiological conditions including allergic airway disease, autoimmune disease, acute and chronic inflammation, and infectious diseases.1–4 The chemokines have been divided into four subfamilies, CXCL, CCL, CL, and CXXXCL on the basis of their sequence homology and the position of cysteine residues within the protein. Chemokines mediate their effects via interaction with 7-transmembrane-spanning G-protein-coupled receptors and their diverse role in disease may stem from the complexity of these interactions in which single chemokines may bind promiscuously to multiple receptors and individual receptors can bind to multiple chemokines.
Numerous studies have established a role for chemokines in controlling the various stages of allergic airway disease.2,4–6 Cellular sources of the chemokines within the asthmatic airway include alveolar macrophages and airway epithelial cells and these resident cells may have a profound and rapid effect on the environment within and surrounding the airways. Chemokines previously identified in the airways of asthmatics include CCL5 (RANTES), CCL7 (MCP-1), and CCL13 (MCP-4), each of which is thought to play a role in regulating eosinophil recruitment and activation.7–11 These same chemokines may also influence recruitment of Th2 lymphocytes to the lungs, in addition to activating or modulating the phenotype of chemokine receptor-expressing structural cells within the airways.12
CCL28 (also known as MEC), the most recently identified CC chemokine, shares 40% homology with CCL27.13,14 It is encoded on chromosome 5 and human CCL28 has been identified as having at least four exons separated by large introns. Human and murine CCL28, like CCL6 (C10), CCL9 (MIP-1γ), and CCL23 (MPIF-1) have six cysteines, two more than the more commonly found four conserved cysteines. CCL28 mRNA expression has been demonstrated in multiple tissues although it seems to be particularly associated with mucosal epithelial surfaces including the trachea.14 CCL28 mRNA has been detected within normal and asthmatic lungs whereas in vitro studies have identified CCL28 expression by bronchus-associated epithelial cells, but not fibroblasts or endothelial cells.13 Although little is known about the regulation of CCL28, it has been shown to mediate both eosinophil and T-cell recruitment in vitro, in addition to playing a role in mucosal immunity because of potent broad-spectrum antimicrobial activity.13–15 CCL28, like CCL27, has been identified as a ligand for CCR10/GPR213 however Pan and colleagues14 have demonstrated that the functional effects of CCL28 are also mediated by CCR3. We investigated the pattern of CCL28 expression within normal and allergen-challenged lungs and examined the role of CCL28 in a murine model of allergic airway inflammation.
Materials and Methods
Animals and Antibodies
Dr. Fred Lewis at the Biomedical Research Laboratory (Frederick, MD) supplied Swiss Albino mice, infected heavily with Schistosoma mansoni helminth parasite. These mice displayed severe infection and very significant eosinophilia with >50% of circulating granulocytes as eosinophils. CBA/J mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and housed in University of Michigan animal facilities under pathogen-free conditions. Chemokines and antibodies used for enzyme-linked immunosorbent assay (ELISA) and immunostaining were purchased from R&D Systems (Minneapolis, MN).
Generation of Murine CCL28 Antiserum
Rabbit anti-murine CCL28 antibody was prepared by multiple-site immunization of New Zealand White rabbits with recombinant murine CCL28 (R&D Systems, Rochester, MN) in CFA. Polyclonal antibodies were titered by direct ELISA and specifically verified by the failure to cross-react to mouse interleukin (mIL)-3, mIL-1, mTNF, mIL-4, mIL-10, mIL-12, mMIP-1α, mJE, mRANTES, mC10, and mCCL27. The IgG portion of the serum was purified over a protein A column and tested in a sandwich ELISA. Whole serum (0.5 ml/mouse) was used in vivo to block CCL28 during the cockroach allergen (CRA) challenge.
CRA Sensitization Protocol
Mice were sensitized and rechallenged with purified CRA as previously described.5 Briefly, 6- to 8-week-old female CBA/J mice were immunized intraperitoneally and subcutaneously with 10 μg of CRA (Bayer Corp., Elkhart, IN) in incomplete Freund’s adjuvant. Fourteen days later the mice received an intranasal challenge of 10 μg of CRA to localize the inflammatory response to the airways. Mice were then rechallenged 7 days later (day 42) by intratracheal administration of 10 μg of CRA in a 40-μl volume. In chemokine depletion studies, mice were pretreated intraperitoneally with murine CCL28 antiserum 1 hour before intratracheal CRA challenge.
Measurement of Airway Hyperreactivity
Airway hyperreactivity was measured using a Buxco mouse plethysmograph and software for calculation of the measurements (Buxco, Troy, NY) as previously described.16 Briefly, mice were anesthetized with sodium pentobarbital, intubated via cannulation of the trachea with an 18-gauge metal tube, and ventilated with a Harvard pump ventilator (0.3 ml tidal volume; 120 breaths per minute). Mice were sealed within the plethysmograph for 5 minutes before baseline resistance readings were taken (via the division of tracheal pressure by the change in box volume). A dose-response curve to intravenous methacholine was performed to determine the optimal dose required to induce airway hyperresponsiveness. Mice were subsequently injected with 150 μg/kg of methacholine and peak airway resistance was recorded. The change in airway resistance was assessed by subtracting the baseline resistance from the peak methacholine-induced airway resistance. Data presented are mean ± SEM change in airway resistance (cm H2O/ml/second) for five animals and are representative of data obtained in three separate experiments for a total of 12 to 15 mice per group.
Histological Analysis of Lung Inflammation
Whole lungs were dissected from the thoracic cavity and fixed by inflation with formalin. The lungs were maintained in formalin for a further 24 hours before being processed into paraffin using standard histological techniques. Five-μm lung tissue sections were stained with hematoxylin and eosin for analysis of peribronchial eosinophil accumulation. Slides were blinded and coded. Peribronchial eosinophil counts were analyzed by counting the number of eosinophils surrounding the airways in 50 high-power fields (×200).
RNA Isolation and Analysis of Gene Expression
RNA was isolated from lung tissue after homogenization in Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Five μg of total RNA was reverse-transcribed in a 25-μl volume. mRNA expression was determined in 1 μl of cDNA by TaqMan real-time polymerase chain reaction using a Prism 7700 sequence detection system (PE Biosystems, Foster City, CA) using gene-specific primers and probes labeled with 5′-6-FAM and 3′-TAMRA. The primers and probes used for the detection of murine CCL28mRNA were: forward primer 5′-TTTTCAAACCTCAGAAGCCATACTT-3′; reverse primer 5′-GGATGCTGCATGAACTCACTCTT-3′; probe 5′-CCATGGCCTCCAGCTCTTGCACT. CCR3 mRNA expression was determined using a predeveloped primer/probe set (PE Biosystems). Reactions were incubated for 2 minutes at 50°C, denatured for 10 minutes at 95°C, and subjected to 40 two-step amplification cycles with annealing/extension at 60°C for 1 minute followed by denaturation at 95°C for 15 seconds. A predeveloped primer/probe set for murine GAPDH (PE Biosystems) was used as an internal control for quantitation of the total amount of cDNA used in the reaction. Results are normalized to GAPDH expression and presented as fold increase in mRNA expression compared to the level detected at 0 hours after intratracheal CRA challenge. CCR10 mRNA expression was measured by gene array analysis using the Chemokine and Receptor GEArray Q Series kit (SuperArray, Bethesda, MD). Briefly, RNA (5 μg) was reverse-transcribed and assayed according to the manufacturer’s instructions. Results are normalized to β-actin expression and presented as fold increase in CCR10 mRNA compared to the level detected at 0 hours after intratracheal CRA challenge.
ELISA Analysis
Lung samples were prepared for ELISA analysis by homogenization in buffer containing Complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) and 0.05% Triton X (Sigma Chemical Co., St. Louis, MO). Murine cytokine and chemokine concentrations were determined using a standardized sandwich ELISA technique.5 Briefly, Maxisorp ELISA plates (Fisher Scientific, Pittsburgh, PA) were coated with 1 to 5 μg/ml polyclonal capture antibody (R&D Systems) in coating buffer (0.6 mol/L NaCl, 0.26 mol/L H3B04, 0.08 mol/L NaOH; pH 9.6) overnight at 4°C and washed with phosphate-buffered saline (PBS) containing 0.05% Tween-20. Nonspecific binding sites were blocked with 2% bovine serum albumin in PBS for 1 hour at 37°C. After washing, cell-free lung homogenate or standard was added to each well and incubated for 1 hour at 37°C. Plates were subsequently washed and incubated with biotinylated affinity-purified polyclonal detection antibody (0.1 to 3.5 μg/ml) for 45 minutes at 37°C. After thorough washing, streptavidin-horseradish peroxidase (BD Pharmingen, San Diego, CA) was added to each well for 30 minutes at 37°C. Plates were washed again, and after addition of chromogen substrate the optical density readings were measured at 492 nm. Recombinant cytokines and chemokines (R&D Systems) were used to generate standard curves and the limit of detection for the assays was 50 pg/ml.
CCL28 Immunostaining
Formalin-fixed lung tissue sections (5 μm) were deparaffinized and rehydrated before antigen retrieval by microwaving in 10 mmol/L citric acid buffer. Endogenous peroxidase activity in the sections was blocked by treatment with 50% methanol/1.5% H2O2 for 15 minutes at room temperature. Nonspecific binding was blocked using avidin-, biotin-, and serum-blocking reagents from the Cell and Tissue staining kit according to the manufacturer’s protocol (R&D Systems). After overnight incubation with 10 μg/ml of goat α-mouse CCL28 or control antibody at 4°C, sections were stained with reagents contained in the AEC tissue staining kit according to the manufacturer’s instructions (Vector Laboratories, Burlingame, CA). Antibody incubations were performed in staining buffer containing PBS/5% nonfat dried milk/0.1% saponin. Slides were washed in PBS containing 0.2% Tween-20 (Sigma Chemical Co.)
Analysis of Lung LTC4
LTC4 and LTE4 levels were assessed in lung homogenates prepared as described above in the ELISA analysis section. The leukotriene concentrations were determined using LTC4 or LTE4-specific ACE competitive enzyme immunoassay kits (Cayman Chemical Co., Ann Arbor, MI).
Purification and Stimulation of Eosinophils
At 7 weeks after infection with S. mansoni, mice were injected intraperitoneally with 3000 freshly isolated S. mansoni eggs in 4% thioglycollate containing 50 ng of murine IL-5. After 48 hours, peritoneal fluid was collected and the cells resuspended in Dulbecco’s modified Eagle’s medium containing 15% fetal calf serum, T-cell stimulation medium (BD Biosciences, Bedford, MA). Adherent cell populations were removed by culturing the cell suspension for 1 hour at 37°C. The nonadherent cells were washed and resuspended in phosphate-buffered saline (PBS)/bovine serum albumin. Eosinophils were purified from the remaining leukocyte suspension using the MACS cell-sorting system by negative depletion with anti-MHCclII-, anti-CD45R-, and anti-CD90-coated magnetic microbeads according to the manufacturer’s protocol (Miltenyi Biotec Inc., Auburn, CA). After the plate adherence and MACS separation, the population of cells contained >97% eosinophils with contaminating neutrophils (1%) and mononuclear cells (1 to 2%). Eosinophils were used immediately or cultured overnight in Dulbecco’s modified Eagle’s medium containing 15% fetal calf serum, T-cell stimulation medium, and 5 ng/ml IL-5. Eosinophils were resuspended in Dulbecco’s phosphate-buffered saline (DPBS)/bovine serum albumin at 2 × 106/ml and stimulated with murine CCL28 at concentrations ranging from 1 to 100 ng/ml. In CCR3 blocking experiments, purified eosinophils were incubated with α-murine CCR3 (R&D Systems) for 20 minutes and washed twice in DPBS/bovine serum albumin before stimulation with chemokines.
Eosinophil Chemotaxis
Chemotaxis assays using a 12-well microchemotaxis chamber (Neuroprobe, Pleasanton, CA) were performed as previously described.17 Briefly, the bottom wells of the chemotaxis chamber were filled with CCL28 at concentrations ranging from 5 to 100 mg/ml or assay medium as negative control. The upper and lower chambers were separated by a polycarbonate polyvinylpyrrolidone-free PVPF filter with a 5-μm pore size (Poretics, Livermore, CA), and eosinophils (2 × 106 cells/ml) were placed in the upper wells. The chamber was incubated at 37°C/5% CO2 for 60 minutes at which time the filters were carefully removed and the upper surface scraped clean. Chemotaxis filters were stained with Diff-Quik (Baxter, Deerfield, IL) and migration was quantified by counting the number of eosinophils that had completely migrated through the filter in 10 high-powered fields (×1000). The data are expressed as the average number of migrated cells per high-powered field (±SEM).
Eosinophil Peroxidase Assay
Cell-free bronchoalveolar lavage (BAL) supernatants, collected as previously described,5 were analyzed for eosinophil peroxidase as a marker of eosinophil degranulation. Serial dilutions of peroxidase (Sigma) were used as a positive control. A total of 50 μl of each BAL sample or peroxidase control were mixed with 100 μl of substrate (0.2 mg/ml o-phenylenediamine in Tris, pH 8, containing 0.1% Triton and 0.02% H2O2) in 96-well microtiter plates Maxisorp ELISA plates (Fisher Scientific). The reaction was allowed to progress for 30 minutes before quenching with 50 μl of 4 mol/L sulfuric acid. Optical density readings were measured at 490 nm and data presented as mean optical density reading minus the optical density of a well that did not contain peroxidase.
Statistical Analysis
All results are expressed as mean ± SEM. Statistical significance was calculated by analysis of variance or unpaired Student’s t-test to calculate the two-tailed P value. Significance was determined as values of P < 0.05.
Results
Chemokine and Chemokine Receptor Expression during CRA
Lung samples were collected 0, 8, 24, 48, and 72 hours after intratracheal administration of CRA and processed for RNA, protein, and histological analysis. Real-time polymerase chain reaction analysis compared fold differences in CCL28 mRNA expression at various time points to the level of expression seen at 0 hours after intratracheal CRA. The level of CCL28 mRNA in nonsensitized control mice was not significantly different (1.3 ± 0.23) from that seen at 0 hours in mice sensitized to CRA. CCL28 mRNA expression was increased after CRA challenge with a 5.3 ± 0.97-fold increase in expression detected at 24 hours (Figure 1a). As with the pattern of CCL28 mRNA expression, the CCL28 protein concentration in the lung was also increased after CRA challenge, peaking at 24 hours, before returning to baseline levels by 72 hours (Figure 1b). ELISA analysis revealed a constitutive level of CCL28 of ∼6 ± 0.65 ng/lung in nonsensitized control mice. Eotaxin was also constitutively expressed in the lung (1.62 ± 0.16 ng/lung) but was up-regulated after CRA challenge. The lung eotaxin concentration peaked at 8 hours after CRA challenge before returning to baseline levels by 24 hours (Figure 2a). Analysis of chemokine receptor expression in the lungs showed a 4.3-fold increase in CCR3 expression 8 hours after challenge in comparison to the level detected at 0 hours (Figure 2b). CCR3 expression returned to baseline levels by 24 hours. In contrast, although CCR10 mRNA expression was detected in the lungs, it was not modulated by allergen challenge (Figure 2b). Thus, we were not able to establish that there was an increased expression of pulmonary CCR10 during allergen activation.
Figure 1.
CCL28 expression in a murine model of allergic airway disease. Pulmonary levels of CCL28 mRNA (a) and protein (b) were analyzed 0 to 72 hours after administration of intratracheal CRA. Levels of CCL28 in nonsensitized control lung are represented by dashed line. Analysis of CCL28 mRNA from whole lungs was compared in allergen-challenged mice to control mice at each time point. ELISA data are representative of three separate experiments and presented as mean ± SEM (n = 5 mice in each experiment).
Figure 2.
Chemokine and chemokine receptor expression after allergen challenge. Eotaxin levels (a) and expression of CCR3 and CCR10 mRNA (b) were determined in lungs collected 0 to 24 hours after allergen challenge. Real-time polymerase chain reaction analysis was assessed from five mice per group and compared to appropriate control mice at each time point to ascertain the fold increase. ELISA data are representative of three separate experiments and presented as mean ± SEM (n = 5 mice per group).
Sources of CCL28 during CRA
Immunohistochemical staining identified CCL28 protein expression primarily in airway epithelial cells in nonsensitized control mice with a low number of CCL28-positive leukocytes also visible, most likely interstitial macrophages (Figure 3A). A similar pattern of staining was observed immediately after CRA challenge (Figure 3B). Twenty-four hours after CRA challenge the most striking observation was the high level of CCL28 expression in both the peribronchial and perivascular leukocytes (Figure 3C). CCL28 expression was highest at 24 hours after CRA challenge corresponding to the peak of inflammatory cell recruitment into the lung tissue, and decreased as the inflammation resolved. CCL28-positive peribronchial and perivascular leukocytes were clearly visible under high power and as this inflammatory infiltrate is primarily composed of mononuclear cells and eosinophils, the staining indicates that both cell types may be capable of expressing CCL28 after CRA challenge (Figure 3G). CCL28-positive granulocytes were also identified in contact with the endothelial surfaces within blood vessels (Figure 3H). The primary difference observed as the allergic response progressed was in the staining of CCL28 within the infiltrating leukocyte populations, suggesting that leukocytes may be capable of producing this chemokine.
Figure 3.
Immunohistochemical localization of CCL28 in the lung. The cellular sources of CCL28 were examined in nonsensitized control lungs (A) and after administration of intratracheal CRA challenge at 0 hours (B), 24 hours (C), 48 hours (D), and 72 hours (E). Nonspecific staining was assessed with the use of control IgG (F). Cellular localization of CCL28 staining in peribronchial (G) and perivascular (H) regions of the lung in animals 24 hours after challenge. Original magnifications: ×200 (A–F); ×1000 (G, H).
Functional Effects of CCL28 on Eosinophils
CCL28 has previously been identified as a chemoattractant for human eosinophils.14 We confirmed that murine CCL28 also induced a dose-dependent increase in murine eosinophil migration with significant increases greater than the baseline levels being detected in the presence of 1 to 50 ng/ml CCL28 (Figure 4a). In these same studies when we blocked CCR3 with a specific antibody we observed an abrogation of eosinophil chemotaxis. The anti-CCR3 monoclonal antibody did not block CxCL10/CxCR3-induced chemotaxis, indicating that the effect was specific for CCR3-mediated responses (data not shown). The ability of CCL28 to induce eosinophil peroxidase release from purified eosinophils was also investigated in cells stimulated with chemokine concentrations ranging from 1 to 100 ng/ml. CCL28 caused a dose-dependent increase in eosinophil peroxidase release from the cells, which was significantly reduced by preincubation of the eosinophils with α-CCR3 antibody (Figure 4b). Previous studies with isolated eosinophils demonstrated that CCL28 functionally binds CCR3.14 We therefore performed studies to examine Ca++ mobilization in fura-2-loaded eosinophils and found that CCL28 could completely block eotaxin-induced Ca++ flux (data not shown), verifying previous data.14 Furthermore, no CCR10 mRNA could be detected in isolated eosinophils using quantitative polymerase chain reaction-specific primers as in Figure 2 (data not shown). Thus, it appears that CCL28 is primarily functioning via activation of CCR3 on these isolated eosinophils.
Figure 4.
In vitro effects of CCL28 on eosinophils. Eosinophils were elicited to the peritoneum and isolated from S. mansoni-infected mice. a: Isolated eosinophils were placed in Boyden chambers with increasing doses of CCL28 to assess migration in the presence or absence of anti-CCR3. Eosinophil peroxidase release was measured in response to stimulation with CCL28 in the presence or absence of α-CCR3 antibody after 30 minutes of stimulation. Horizontal dotted line indicates the level from unstimulated eosinophils for comparison. Data are representative of three separate experiments and depicted as mean ± SEM (**, P < 0.01; *, P < 0.05).
Role of CCL28 in Allergic Lung Inflammation
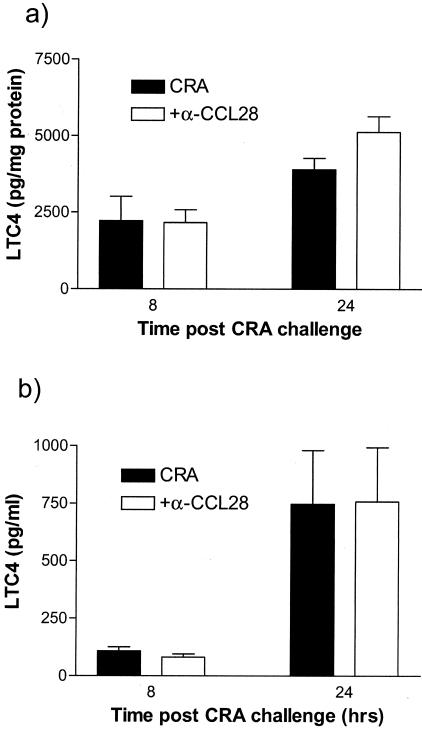
To assess the role of CCL28 in allergic airway inflammation, allergen-sensitized mice were pretreated with CCL28 antiserum 1 hour before the intratracheal CRA challenge. Control mice received normal rabbit serum. ELISA analysis confirmed that at 24 hours after CRA challenge, lung CCL28 concentration was significantly reduced from 9.46 ± 0.66 ng/lung in the control serum-treated mouse to 4.8 ± 0.68 ng/lung in the CCL28 antiserum-treated mice, a level similar to that seen in nonsensitized control mice. In addition to CCL28 levels, we examined both Th2-type cytokines and other CC chemokines that are induced during allergic responses. The data in Figure 5 demonstrates that there was no significant alteration in the level of these cytokines or chemokines. However, treatment with CCL28 antiserum resulted in a significant decrease in methacholine-induced airway hyperresponsiveness at 24, but not 8 hours after allergen challenge (Figure 6a). We also investigated the airway hyperresponsiveness at 48 hours but in both the control and anti-CCL28, the response had returned to background in these studies (data not shown). The decrease in airway resistance at 24 hours was accompanied by a significant reduction in peribronchial eosinophil accumulation (Figure 6b). BAL eosinophils were decreased, but variable and did not reach statistical significance (data not shown), similar to other leukocytes examined in the BAL fluid. To assess levels of eosinophil activation we examined leukotriene levels and although leukotriene levels were elevated in the lungs and BAL of CRA-challenged mice, no significant difference in LTC4 concentration was detected between control and CCL28 antiserum-treated mice (Figure 7). No increase in LTE4 was detected at 8 or 24 hours after allergen challenge whereas levels of eosinophil peroxidase were very low and nearly undetectable in the BAL in both control and anti-CCL28-treated animals (data not shown). Thus, although the reduced response appeared to correlate fully with the accumulation and presence of eosinophils around the airways of allergic mice, little difference could be found in mediators often associated with eosinophil activation.
Figure 5.
Effect of CCL28 antiserum on lung cytokine and chemokine levels. Mice were treated with CCL28 antiserum 1 hour before challenge with intratracheal CRA and lungs were collected 24 hours later. Cytokine (a) and chemokine (b) levels were assessed in whole lung homogenates by ELISA. Data are representative of three separate experiments and presented as mean ± SEM (n = 5 mice per treatment group; P < 0.05).
Figure 6.
Effect of CCL28 antiserum on allergic lung inflammation. Mice were treated with anti-CCL28 or control serum 1 hour before challenge with intratracheal CRA and airway hyperresponsiveness (a) and peribronchial eosinophilia (b) were measured 8 or 24 hours later. As indicated, the dose of methacholine chosen for these studies induced minimal increases in control, unchallenged mice while inducing a significant response in allergen-challenged mice. Data are representative of three separate experiments and presented as mean ± SEM (n = 4 to 5 mice per treatment group; P < 0.05).
Figure 7.
LTC4 levels in lungs and BAL are not different in control versus anti-CCL28-treated animals. Levels of LTC4 were measured in whole lung homogenates (a) and in cell-free BAL fluid (b) collected at 8 or 24 hours after challenge using a specific RIA detection kit. Data depicts the mean ± SE from five mice per group.
Discussion
Recruitment of leukocytes to the airways during allergic inflammation results from the coordinated release of multiple chemokines and cytokines.2,6,16,18 This process is controlled by site-specific production of chemokines and the availability of multiple ligands and receptors resulting in a complex and diverse allergic response. A number of chemokine receptors have been implicated in allergic airway disease.4 The most widely studied of these is CCR3, which is highly expressed on eosinophils19,20 and has also been detected on Th2 lymphocytes.21,22 There are currently 11 chemokines that have been shown to bind and signal through CCR3. Initially described as the receptor for CCL11 (eotaxin), it has since been identified as one of the most promiscuous chemokine receptors.23 Although CCL11 −/− mice had little effect on eosinophils,24,25 Humbles and colleagues26 reported that trafficking of activated eosinophils to the lungs of sensitized individuals is primarily, although not completely, dependent on CCR3.24,25 CCR3-deficient mice exhibited a significant reduction in eosinophil trafficking both to the airway lumen and into lung tissue.26 However, because eotaxin −/− mice and initial studies with anti-eotaxin demonstrated little defect in eosinophil accumulation,25 other CCR3 ligands likely contribute to the migration of eosinophils to the peribronchial region. One of the most recently identified CCR3 ligands is CCL28, which has primarily remained uncharacterized.
CCL28 was originally identified as a ligand for CCR10 and was considered to be primarily an epithelial cell-associated chemokine.13,14 However, expression was also detected at the mRNA level in a wide range of cell types and tissues. Wang and colleagues13 detected CCL28 mRNA in the lungs and also indicated that there was increased expression in some asthmatic patients. The present studies further examined the pattern and level of CCL28 expression in both sensitized and nonsensitized lungs, and aimed to determine the role of the chemokine in the development of allergic airway disease using a murine model. CCL28 was constitutively expressed in nonsensitized control lungs but the level was increased at both protein and mRNA levels in allergen-challenged mice. Immunohistochemical analyses identified epithelial cells as the primary source of CCL28 in control lungs although a number of interstitial macrophages also stained for the chemokine. After sensitization and rechallenge with CRA, little change in CCL28 levels was detected in the lungs at 8 hours after challenge but levels were significantly increased by 24 hours. Interestingly, the peak of CCL28 expression in the lung was not associated with an increase in epithelial cell-derived CCL28, but rather with the recruitment of CCL28-positive leukocytes. Eosinophils have previously been shown to express high levels of CCL28 mRNA whereas expression was absent in highly polarized Th2 cells from C57BL/6 mice.13 Treatment with CCL28 antiserum resulted in a reduction in peribronchial inflammation and in particular caused a significant decrease in peribronchial eosinophil accumulation. No evidence of decreases in Th2 responses was observed and therefore CCL28 appears to play a role primarily in eosinophil recruitment. Using in vitro analyses CCL28 has previously been shown to activate eosinophils.14 In the present studies CCR3 expression was increased after challenge with CRA, whereas no increase in pulmonary CCR10 was observed after allergen challenge and we could not find CCR10 expression in isolated eosinophils. In addition, our data indicates that anti-CCR3 blocked CCL28-induced eosinophil accumulation. Altogether, the evidence indicates that CCL28 is likely functioning primarily through CCR3.
Previous studies had identified eosinophil and lymphocyte chemoattractant properties of CCL28 using human cells.13,14 Our in vitro studies with eosinophils isolated from S. mansoni-infected mice confirmed that CCL28 was a chemoattractant for murine eosinophils. There appears to be a temporal relationship of CCL28 production with eotaxin in the CRA model. Perhaps these two chemokines, along with other CCR3 ligands, together play an important role for the localization of eosinophils around the airway. This latter notion is supported in the literature by studies that have targeted other CCR3 ligands, such as MCP-3 (CCL7), and demonstrated decreased eosinophilia and airway pathophysiology.27 Inhibiting CCR3 during allergic asthmatic responses to reduce eosinophil accumulation is likely the most logical approach to reduce eosinophil-mediated disease. This strategy has been successfully used using anti-CCR3 treatment and in CCR3 −/− mice, reducing eosinophilia and the corresponding airway hyperreactivity.28,29 It may be that different CCR3 ligands are important for various stages of eosinophil migration and/or activation within the airway. Interestingly, the anti-CCL28-treated animals had reduced peribronchial eosinophil accumulation but we could neither find significant evidence of reduced airway eosinophil accumulation nor reduced activation parameters (LTC4) within BAL fluid samples. Thus, other CCR3 ligands, such as eotaxin, may be operative for the localization/migration of the eosinophils into the airway. It may be the combined efforts of the multiple CCR3 ligands that is important for the continued extravasation from vessel to airway.
The reduction in airway hyperresponsiveness and peribronchial eosinophilia appears to be directly linked to the absence of CCL28 because numerous other downstream mediators of allergic airway disease including Th2 type cytokines, chemokines, LTC4, and IgE (data not shown) were not modulated in the absence of CCL28. Thus, our data indicate that CCL28 plays an important role in localizing eosinophil recruitment to the airway. Future studies will attempt to identify whether the coordinated release of other CCR3 ligands, in addition to CCL28 and eotaxin, play a role in the temporal regulation of eosinophil accumulation and activation within the airways of mice.
Footnotes
Address reprint requests to Nicholas W. Lukacs, Department of Pathology, University of Michigan Medical School, 1301 Catherine Rd., Ann Arbor MI 48109-0602. E-mail: nlukacs@umich.edu.
Supported by the National Institutes of Health (grants AI36302 and HL3196).
References
- Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- Gonzalo JA, Lloyd CM, Wen D, Albar JP, Wells TN, Proudfoot A, Martinez AC, Dorf M, Bjerke T, Coyle AJ, Gutierrez-Ramos JC. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs NW, Kunkel SL. Chemokines and their role in disease. Int J Clin Lab Res. 1998;28:91–95. doi: 10.1007/s005990050025. [DOI] [PubMed] [Google Scholar]
- Lukacs NW. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol. 2001;1:108–116. doi: 10.1038/35100503. [DOI] [PubMed] [Google Scholar]
- Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161:7047–7053. [PubMed] [Google Scholar]
- Teran LM. CCL chemokines and asthma. Immunol Today. 2000;21:235–242. doi: 10.1016/s0167-5699(00)01634-0. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Weiss ST, Israel E, Luster AD, Drazen JM, Lilly CM. Eotaxin and impaired lung function in asthma. Am J Respir Crit Care Med. 1999;160:1952–1956. doi: 10.1164/ajrccm.160.6.9811089. [DOI] [PubMed] [Google Scholar]
- Lamkhioued B, Garcia-Zepeda EA, Abi-Younes S, Nakamura H, Jedrzkiewicz S, Wagner L, Renzi PM, Allakhverdi Z, Lilly C, Hamid Q, Luster AD. Monocyte chemoattractant protein (MCP)-4 expression in the airways of patients with asthma. Induction in epithelial cells and mononuclear cells by proinflammatory cytokines. Am J Respir Crit Care Med. 2000;162:723–732. doi: 10.1164/ajrccm.162.2.9901080. [DOI] [PubMed] [Google Scholar]
- Mattoli S, Stacey MA, Sun G, Bellini A, Marini M. Eotaxin expression and eosinophilic inflammation in asthma. Biochem Biophys Res Commun. 1997;236:299–301. doi: 10.1006/bbrc.1997.6958. [DOI] [PubMed] [Google Scholar]
- Teran LM, Noso N, Carroll M, Davies DE, Holgate S, Schroder JM. Eosinophil recruitment following allergen challenge is associated with the release of the chemokine RANTES into asthmatic airways. J Immunol. 1996;157:1806–1812. [PubMed] [Google Scholar]
- Holgate ST, Bodey KS, Janezic A, Frew AJ, Kaplan AP, Teran LM. Release of RANTES, MIP-1 alpha, and MCP-1 into asthmatic airways following endobronchial allergen challenge. Am J Respir Crit Care Med. 1997;156:1377–1383. doi: 10.1164/ajrccm.156.5.9610064. [DOI] [PubMed] [Google Scholar]
- Stellato C, Brummet ME, Plitt JR, Shahabuddin S, Baroody FM, Liu MC, Ponath PD, Beck LA. Expression of the C-C chemokine receptor CCR3 in human airway epithelial cells. J Immunol. 2001;166:1457–1461. doi: 10.4049/jimmunol.166.3.1457. [DOI] [PubMed] [Google Scholar]
- Wang W, Soto H, Oldham ER, Buchanan ME, Homey B, Catron D, Jenkins N, Copeland NG, Gilbert DJ, Nguyen N, Abrams J, Kershenovich D, Smith K, McClanahan T, Vicari AP, Zlotnik A. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2). J Biol Chem. 2000;275:22313–22323. doi: 10.1074/jbc.M001461200. [DOI] [PubMed] [Google Scholar]
- Pan J, Kunkel EJ, Gosslar U, Lazarus N, Langdon P, Broadwell K, Vierra MA, Genovese MC, Butcher EC, Soler D. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J Immunol. 2000;165:2943–2949. doi: 10.4049/jimmunol.165.6.2943. [DOI] [PubMed] [Google Scholar]
- Hieshima K, Ohtani H, Shibano M, Izawa D, Nakayama T, Kawaski Y, Shiba F, Shiota M, Katou F, Saito T, Yoshie O. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol. 2003;170:1452–1461. doi: 10.4049/jimmunol.170.3.1452. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Warmington K, Lincoln P, Chensue SW, Kunkel SL. Differential recruitment of leukocyte populations and alteration of airway hyperreactivity by C-C family chemokines in allergic airway inflammation. J Immunol. 1997;158:4398–4404. [PubMed] [Google Scholar]
- Lukacs NW, Standiford TJ, Chensue SW, Kunkel RG, Strieter RM, Kunkel SL. C-C chemokine-induced eosinophil chemotaxis during allergic airway inflammation. J Leukoc Biol. 1996;60:573–578. doi: 10.1002/jlb.60.5.573. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Oliveira SH, Hogaboam CM. Chemokines and asthma: redundancy of function or a coordinated effort? J Clin Invest. 1999;104:995–999. doi: 10.1172/JCI8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo JA, Newman W, Gutierrez-Ramos JC, Mackay CR. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, Sirotina A, Springer MS. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–2354. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- Lilly CM, Daugherty BL. A novel LPS-inducible CCR3 activator: why so many CCR3 ligands? Am J Respir Cell Mol Biol. 2001;25:673–675. doi: 10.1165/ajrcmb.25.6.f222. [DOI] [PubMed] [Google Scholar]
- Yang Y, Loy J, Ryseck RP, Carrasco D, Bravo R. Antigen-induced eosinophilic lung inflammation develops in mice deficient in chemokine eotaxin. Blood. 1998;92:3912–3923. [PubMed] [Google Scholar]
- Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbles AA, Lu B, Friend DS, Okinaga S, Lora J, Al-Garawi A, Martin TR, Gerard NP, Gerard C. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci USA. 2002;99:1479–1484. doi: 10.1073/pnas.261462598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford S, Li H, Forsythe PA, Ryan M, Bravo R, Alam R. Monocyte chemotactic protein-3 (MCP-3)/fibroblast-induced cytokine (FIC) in eosinophilic inflammation of the airways and the inhibitory effects of an anti-MCP-3/FIC antibody. J Immunol. 1997;158:4953–4960. [PubMed] [Google Scholar]
- Ma W, Bryce PJ, Humbles AA, Laouini D, Yalcindag A, Alenius H, Friend DS, Oettgen HC, Gerard C, Geha RS. CCR3 is essential for skin eosinophilia and airway hyperresponsiveness in a murine model of allergic skin inflammation. J Clin Invest. 2002;109:621–628. doi: 10.1172/JCI14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JP, Borchers MT, Crosby JR, Hines EM, Shen HH, Ochkur SI, McGarry MP, Lee NA, Lee JJ. Ablation of eosinophils leads to a reduction of allergen-induced pulmonary pathology. Am J Physiol. 2003;284:L169–L178. doi: 10.1152/ajplung.00260.2002. [DOI] [PubMed] [Google Scholar]