Abstract
Cytochrome P450 enzymes of the 4A family (CYP4A) convert arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE) in blood vessels of several vascular beds. The present study examined the effects of inhibiting the formation of 20-HETE with N-hydroxy-N′-(4-butyl-2-methylphenol) formamidine (HET0016) on the mitogenic response of vascular endothelial growth factor (VEGF) in human umbilical vein endothelial cells (HUVECs) in vitro, and on growth factor-induced angiogenesis in the cornea of rats in vivo. HET0016 (10 μmol/L and 20 μg, respectively) abolished the mitogenic response to VEGF in HUVECs and the angiogenic response to VEGF, basic fibroblast growth factor, and epidermal growth factor in vivo by 80 to 90% (P < 0.001). Dibromododecenyl methylsulfonimide (DDMS), a structurally and mechanistically different inhibitor of 20-HETE synthesis, also abolished angiogenic responses when tested with VEGF. Additionally, administration of the stable 20-HETE agonist, 20-hydroxyeicosa-6(Z) 15(Z)-dienoic acid (WIT003) induced mitogenesis in HUVECs and angiogenesis in the rat cornea in vivo. We studied the ability of HET0016 to alter the angiogenic response in the rat cornea to human glioblastoma cancer cells (U251). When administered locally into the cornea, HET0016 (20 μg) reduced the angiogenic response to U251 cancer cells by 70%. These results suggest that a product of CYP4A product, possibly 20-HETE, plays a critical role in the regulation of angiogenesis and may provide a useful target for reduction of pathological angiogenesis.
Arachidonic acid is acutely released from membrane phospholipids in response to a variety of stimuli, including vascular endothelial growth factor (VEGF),1 fibroblast growth factor (FGF)-2,2 and epidermal growth factor (EGF).3–5 Arachidonic acid can be metabolized in blood vessels by enzymes of the cytochrome P450 4A (CYP4A) family to 20-hydroxyeicosatetraenoic acid (20-HETE) and by CYP 2C and 2W to epoxyeicosatrienoic acid (EETs). Both of these factors have been implicated in the regulation of angiogenesis. CYP4A products, in particular 20-HETE, have been reported to serve as second messengers for the vasoactive and mitogenic responses of a number of compounds in blood vessel cells and other cells, including angiotensin II (ang II) norepinephrine, endothelin, vasopressin, 5-hydroxy-tryptamine, and EGF. Blockade of the formation of 20-HETE attenuated the growth response to serum, norepinephrine, and EGF.6–9
Recently, Amaral and colleagues10 reported that CYP4A, and by extension 20-HETE, plays a critical role in the angiogenesis induced by electrical stimulation of skeletal muscle. This angiogenic response is ang II- and VEGF-dependent. Jiang and colleagues11 have shown that overexpression of CYP4A1 in smooth muscle promotes endothelial sprouting in renal arterial microvessels. Norepinephrine, ang II, and many growth factors stimulate cytosolic phospholipase A2 (cPLA2) and the release of arachidonic acid.1,2,12,13 This promotes the formation of CYP metabolites of arachidonic acid (EET and/or 20-HETE) in the microvasculature. In addition, activation of the VEGF receptor 2 (VEGF-R2) stimulates phospholipase C and the downstream Raf-MAP kinase pathway.14 Indeed, Muthalif and colleagues15 reported that activation of MAPK by norepinephrine, ang II, and EGF is dependent on the formation of 20-HETE, which is generated after stimulation of cPLA2 by calcium/calmodulin-dependent protein kinase II. Activation of the Ras/MAPK pathway by 20-HETE amplifies cPLA2 activity and additional release of arachidonic acid by a positive feedback mechanism. Muthalif and colleagues15 proposed that this mechanism may play a central role in the regulation of other cellular signaling molecules involved in cell proliferation and growth. Both Ras and MAPK activation are associated with angiogenesis.16,17 Thus, there may be an association between the actions of angiogenic growth factors metabolites of arachidonic acid derived from CYP4A activity.
VEGF, FGF-2, and EGF are all prominent agonists of members of the membrane-bound tyrosine kinase receptor family. These growth factors play a pivotal role in the regulation of angiogenesis and in the pathology of disorders associated with angiogenesis including the growth of solid tumors. Thus, the present studies were designed to test the hypothesis that the metabolism of arachidonic acid by CYP4A enzymes plays a central role in tyrosine kinase-dependent growth factors involved in angiogenesis. To address this hypothesis, we studied whether inhibition of CYP4A activity using two chemically dissimilar inhibitors, HET0016 and dibromododecenyl methylsulfonimide (DDMS), decreased the angiogenic response in the cornea of rats in vivo and the mitogenic response in human umbilical vein endothelial cells (HUVECs) in vitro to VEGF, a prototypical angiogenic growth factor. In addition, we extended the in vivo studies using HET0016 to include FGF-2 and EGF. We also studied whether HET0016 would affect cancer-induced angiogenesis in the cornea of rats in vivo.
Materials and Methods
Reagents
HET0016 [N-hydroxy-N′-(4-butyl-2 methylphenyl) formamidine] was synthesized as previously described18,19and was provided by Taisho Pharmaceuticals Corp. (Satiama, Japan). The CYP4A inhibitor, DDMS, and the stable 20-HETE agonist, WIT003 [20-hydroxyeicosa-6(Z), 15(Z)-dienoic acid], were synthesized by one of the authors (J.R.F.)20 and have been used previously.21–23 VEGF165, FGF-2, and EGF were purchased from R&D Systems (Minneapolis, MN) and Hydron type NCC was obtained from Interferon (New Brunswick, NJ). Primers for polymerase chain reaction (PCR) were synthesized from Qiagen (Valencia, CA). HUVECs and related culture reagents were purchased from Cambrex (Walkersville, MD). All other cell culture reagents were purchased from Invitrogen (Carlsbad, CA). Palmitic acid and all other reagents were purchased from Sigma Chemical Corp. (St. Louis, MO).
Animals
Experiments were performed in 7- to 8-week-old male Sprague-Dawley rats weighing 200 to 225 g (Charles River Laboratories, Wilmington, MA). Rats were housed in a 12 hour/12 hour light/dark cycle environment and provided with food and water ad libitum. All procedures complied with the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research. Approval for the use of animals was obtained from the Institutional Animal Care and Use Committee of Henry Ford Health System, Detroit, MI.
HUVEC Proliferation Assay
HUVECs were seeded onto a 96-well plate at 1 × 104 cells/well. Cultures were grown overnight and then exposed to either 10 μmol/L HET0016, 1 μmol/L WIT003, or 250 ng/ml VEGF alone or combined with either HET0016 or WIT003. HET0016 and WIT003 were both dissolved in ethanol. Control cultures had ethanol only. Organic solvent concentration never exceeded 0.1% of total culture volume. The dose of HET0016 was defined on a single experiment that showed that 10 μmol/L gave maximal inhibition. Cell proliferation was measured 24 hours later using CellTiter96 Aqueous One reagent (Promega, Madison, WI). This assay is based on the reduction of a tetrazolium compound by cells into a colored soluble product. This appears to be accomplished by NADPH or NADH produced by dehydrogenase enzymes in metabolically active cells (see Promega technical bulletin 245). This is a reliable colorimetric method for determining the number of viable cells in proliferation. Twenty μl of Aqueous One reagent was added to the 100-μl medium in each well. The plates were incubated for 2 hours at 37°C in a humidified incubator. Absorbance was recorded at 490 nm using a 96-well Bio Kinetics Reader EL340 (Bio-Tek, Winooski, VT). The data represent changes in percent absorbance of treated cultures compared with control cells. Three different experiments were performed, and each point was determined in triplicate.
Cornea Pocket Angiogenesis Assay
Preparation of Sustained-Release Polymer
Sustained-release polymer pellets were prepared by making a 1:1 mix of a 12% solution in ethanol of the polymer (Hydron polyhydroxyethylmethacrylate), with saline containing the growth factors. The growth factors FGF-2, VEGF, and EGF were dissolved at a concentration of 125 ng/μl. HET0016 and WIT00321 were dissolved in ethanol to a concentration of 10 μg/μl whereas DDMS was dissolved in ethanol to a concentration of 5 μg/μl. Two μl of these solutions were added to each pellet. Thus, a pellet containing 250 ng of a single growth factor was implanted at random in the right or left eye. The other eye was implanted with a pellet containing the same dose of growth factor plus HET0016. In some rats the pellet contained VEGF alone and VEGF plus DDMS. Twenty μg of the stable 20-HETE agonist analog WIT003 was implanted in one eye to determine whether it induces angiogenesis. Because ethanol was the vehicle for HET0016, DDMS, and WIT003, ethanol was added as a control to all other pellets. For FGF-2, we used sucralfate (0.2 mg/pellet) to stabilize and allow sustained release of this growth factor.24 Nine μl of the 1:1 Hydron/treatment mixture was placed on the ends of individual rods and the pellets allowed to dry at room temperature. Dry pellets were implanted into the corneal stroma of rats.
Pellet Implantation
The rats were anesthetized intramuscularly with ketamine (80 mg/kg) and xylazine (10 mg/kg). The eyes were topically anesthetized with 0.5% proparacaine (Ophthetic; Alcon, Fort Worth, TX) and the globes proptosed with a jeweler’s forceps. Using an operating microscope, a central intrastromal linear keratotomy, ∼1.5 mm in length, was performed with a surgical blade (Bard-Parker no. 11; Becton Dickinson, Franklin Lake, NY) parallel to the insertion of the lateral rectus muscle. A curved iris spatula (no. 10093-13; Fine Science Tools, Belmont, CA) ∼1.5 mm wide and 5 mm long was inserted under the lip of the incision and gently pushed through the stroma toward the temporal limbus of the eye. The distance between the limbus and the base of the pocket was kept at 1.0 ± 0.1 mm. The pellet was advanced to the temporal end of the pocket. Antibiotic ointment (erythromycin) was applied to the anterior surface of the eye.
U251 Human Glioma Cell Spheroids
U251 human glioma cells were generously provided by Dr. Stephen Brown (Dept. of Radiation Oncology, Henry Ford Health System, Detroit, MI). The cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum, penicillin (10 IU/ml), streptomycin (10 μg/ml), and 10% nonessential amino acids and grown at 37°C in a humidified incubator containing 5% CO2. U251 spheroids were obtained by a modification of the method of Carlsson and Yuhas.25 Briefly, tumor cell spheroids were prepared by seeding a single-cell suspension (5 × 106 cells), over a layer of 0.8% Noble agar (Difco, Livonia, MI). Cells were grown for 2 to 3 days until spheroids formed. Spheroids of similar diameter were selected and then transferred onto a cell culture dish and washed with phosphate-buffered saline to eliminate traces of serum. Spheroid diameter was measured using a dissection microscope equipped with a ruler. Five to eight spheroids, each ∼200 μm in diameter, were aspirated into a syringe attached to a blunt 27-gauge needle, and inserted into the corneal pocket. In this experiment, one eye contained the spheroids and a pellet containing ethanol (HET0016 solvent) whereas the other eye received spheroids and a pellet containing 20 μg of HET0016.
Quantitation of Corneal Neovascularization
Seven days after pellet implantation, the rats were deeply anesthetized as previously described with ketamine and xylazine. The left ventricle was cannulated and the animal was perfused with 200 to 250 ml of saline through the left ventricle followed by 20 to 25 ml of India ink (waterproof drawing ink; Sanford, Bellwood, IL). The eyes were marked for orientation, enucleated, and placed in 4% formalin for 24 hours. The cornea was dissected free from the surrounding globe and underlying iris, bisected, and loosely mounted between two glass slides, thus gently flattening the cornea. These flat mounts were examined microscopically using a Nikon Diaphot Epi-fluor 2 microscope (Japan) attached to a Sony (Japan) charge-coupled device video camera and the images were digitized and saved using a computer. Neovascularization was quantitated by comparing total vessel length in the control and experimental eyes. Vessel length was determined by tracing all and each vessel from the limbus to the pellet. Total length is the sum of these values in pixels and was determined using conventional image analysis software (Sigma Scan Pro; SPSS, Chicago, IL).
Groups
In all cases pellets were implanted in both eyes. One eye served as control and the other was the experimental group. The following groups were studied.
Group 1
Controls. Pellets containing only 2 μl of ethanol were implanted in the rat corneas. In some experiments we tested for nonspecific effects caused by the mere presence of fatty acids in the pellets. In these experiments, the pellet contained up to 40 μg of palmitic acid (n = 4). There was no difference in angiogenic responses to VEGF in eyes treated with only ethanol versus ethanol containing palmitic acid.
Group 2
Effects of the CYP4A inhibitor HET0016. Control versus 20 μg of HET0016 or 10 μg of DDMS. These doses were chosen based on dose-response studies described below (n = 6). This group was included to determine whether the inhibitors had any visibly toxic or proangiogenic effects. In some rats, the effects of DDMS (10 μg/pellet) were also tested.
Group 3
Dose response for HET0016 inhibition of VEGF angiogenic responses: 1) VEGF versus VEGF + 5 μg HET0016 (n = 4); 2) VEGF versus VEGF + 20 μg HET0016 (n = 4); 3) VEGF versus VEGF + 40 μg HET0016 (n = 4).
Group 4
Anti-angiogenic effects of HET0016. In these rats we tested the effects of HET0016 in the neovascularization response to VEGF, FGF-2, and EGF: 1) VEGF versus VEGF + 20 μg HET0016 (n = 6); 2) FGF-2 versus FGF-2 + 20 μg HET0016 (n = 6); 3) EGF versus EGF + 20 μg HET0016 (n = 8).
Group 5
Anti-angiogenic effects of a second CYP4A inhibitor. In these rats we tested the effects of DDMS on the neovascularization response to VEGF. VEGF versus VEGF + 10 μg DDMS (n = 7). This dose was selected after pilot experiments indicated it was as effective as 20 μg of HET0016.
Group 6
Proangiogenic effect of 20-HETE. In these rats we tested whether the stable 20-HETE analog WIT003 was angiogenic. Control versus 20 μg WIT003 (n = 7).
Group 7
Anti-angiogenic effects of HET0016. In these rats, we studied HET0016 effects on cancer-induced angiogenic responses. For these experiments, we used the human glioblastoma cancer cell line U251, known to be angiogenic.26 The spheroids and the pellet containing HET0016 were implanted together in the same cornea pocket. Angiogenic responses were assessed 14 days after implantation of the spheroids. U251 cells spheroids versus U251 spheroids + 20 μg HET0016 (n = 8).
Cornea CYP4A1 mRNA Expression
mRNA Extraction and cDNA Synthesis
The expression of CYP4A1 mRNA in the cornea during neovascularization was determined using reverse transcriptase-PCR. The corneas were rapidly removed and snap-frozen in liquid nitrogen. The corneas were later thawed and homogenized in TRIzol. Total RNA was extracted from TRIzol according to the manufacturer’s protocol (Invitrogen). Quality of the RNA was assessed by using the 260/280-nm absorbance ratio. Only samples within the 1.8 to 2.0 range were used. A total of 1 to 3 μg of mRNA was reverse-transcribed using the First Strand synthesis kit (Invitrogen). One μg of the cDNA was amplified by PCR.
PCR Analysis
We amplified the CYP4A1 mRNA using the following specific CYP4A1 mRNA primers: sense, TTCCAGGTTTGCACCAGACTCT; and anti-sense, TTCCTCGCTCCTCCTGAGAAG. Amplification of β-actin was used as an internal control. The primers were designed using Primer Express software from Applied Biosystems (Foster City, CA). The mRNA sequences of CYP4A1 were obtained from GenBank with accession number NM_175837 and for rat β-actin with accession number NM_031144.
The PCR conditions used to amplify CYP4A1 and β-actin comprised a precycle of 95°C for 3 minutes followed by 35 cycles consisting of 95°C for 45 seconds, 57°C for 1 minute, and 72°C for 1 minute, and then a final extension at 72°C for 10 minutes. PCR products were subjected to electrophoresis in 10% acrylamide gels and visualized by ethidium bromide. Appropriate controls were used to ensure that amplified samples contained no genomic DNA.
Statistical Analysis
The statistical significance of the differences in control and experimental eyes within rats was determined by paired t-test. The differences in responses between groups were determined by analysis of variance followed by posthoc test. A P < 0.05 was considered significant.
Results
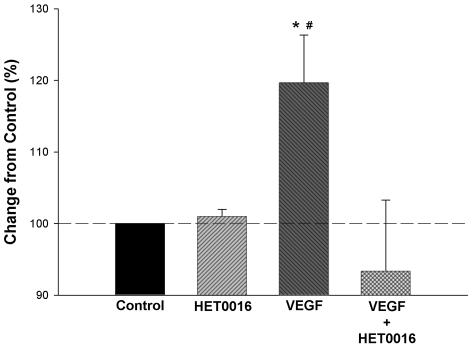
The effects of VEGF on the growth of HUVECs are presented in Figure 1. VEGF stimulated proliferation in these cells, and this response was abolished in cultures simultaneously treated with HET0016. HET0016 did not alter basal proliferation rate of HUVECs. We next studied the effects of HET0016 on the angiogenic response to VEGF in vivo using the rat cornea pocket angiogenesis assay. Neither HET0016 nor DDMS alone elicited a response different from saline (Figure 2B and Figure 5B). To determine the optimal dose of HET0016, we performed a dose-response study in which pellets containing different doses of HET0016 together with VEGF were implanted in the corneas. HET0016 at a dose of 20 μg and 40 μg per pellet almost completely abolished the angiogenic response to VEGF. Five μg of HET0016 reduced angiogenic responses to VEGF by ∼50%. We therefore selected 20 μg per pellet of HET0016 or other related compounds because all have comparable molecular weight and solubility. As a control for nonspecific effects of fatty acids, we used palmitic acid in some experiments. Palmitic acid had no effect by itself and showed no ability to influence angiogenic responses to VEGF or any of the other angiogenic factors studied (not shown).
Figure 1.
Effects of HET0016 on the proliferative response of VEGF in endothelial cells. HUVECs were incubated with 250 ng/ml of VEGF165 alone or in the presence of 10 μmol/L HET0016 and proliferation was assayed 24 hours later. HET0016 abolished proliferative responses to VEGF (n = 3, each by triplicate), but did not alter basal proliferation rate of HUVECs because there was no difference between HET003 alone and saline. *, P < 0.05, control versus VEGF; #, P < 0.05, VEGF versus VEGF + HET0016.
Figure 2.
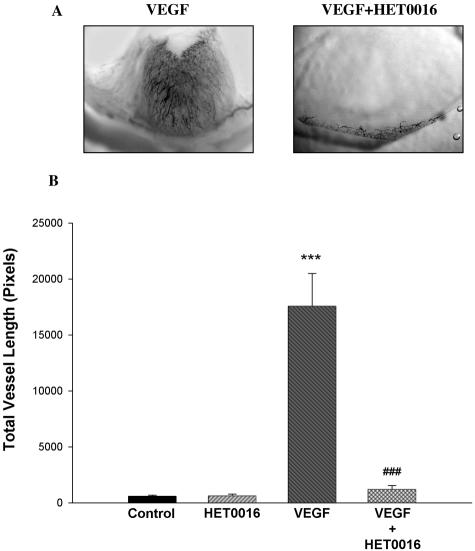
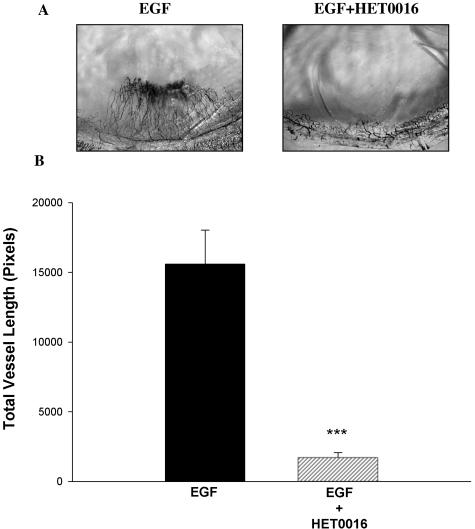
A and B: Effects of HET0016 on the angiogenic responses elicited by VEGF in vivo. Changes in neovascularization were assayed using the rat cornea pocket angiogenesis assay. Pellets containing VEGF alone (250 ng/pellet) or VEGF and HET0016 (20 μg) were implanted into the stroma of the cornea. Rats were sacrificed 7 days later and neovascularization visualized using India ink. Total vessel length, a quantitative estimation of the angiogenic response, was measured by retracing every visible vessel, and using appropriate software to obtain a numerical value. A: Representative cornea flat mounts in the region of the pellet implant. B: Mean ± SEM for the total vessel length for all experimental groups (***P < 0.001, VEGF versus controls; ###P < 0.001, VEGF versus VEGF + HET0016).
Figure 5.
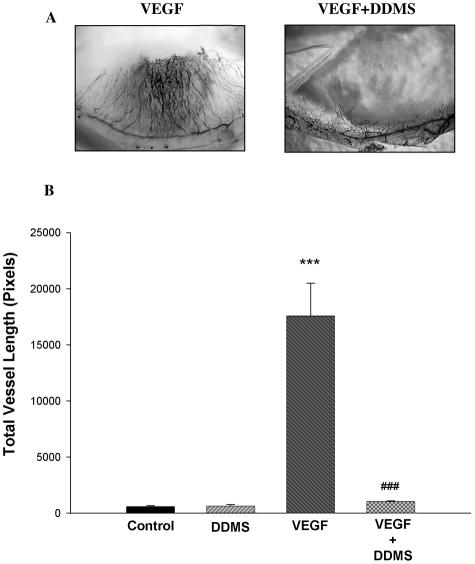
A and B: Effects of DDMS on the angiogenic response to VEGF in vivo. Pellets containing VEGF alone (250 ng/pellet) or VEGF and DDMS (10 μg) were implanted into the stroma of the rat cornea. A: Representative example. B: Difference in the angiogenic response (n = 6. ***P < 0.001, VEGF versus controls; ###P < 0.001, VEGF versus VEGF + DDMS).
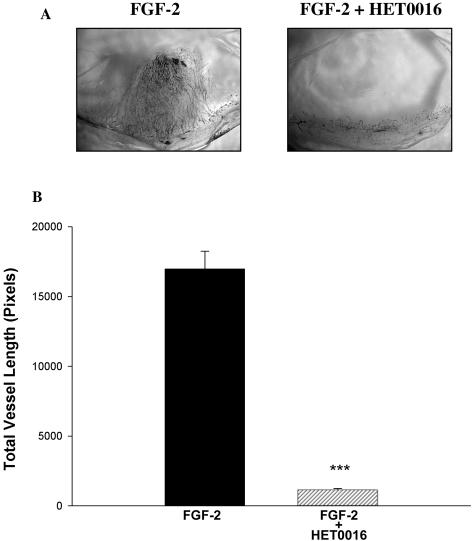
Inclusion of VEGF in the pellets resulted in a marked neovascularization response, which was obliterated by HET0016. Figure 2A shows representative micrographs of corneas treated with VEGF alone and VEGF + HET0016, whereas Figure 2B shows the results in graphic form. In other experiments, we examined the effect of blocking CYP4A activity on the angiogenic response to other growth factors. We studied whether the anti-angiogenic effect of HET0016 is constrained to VEGF or also suppresses responses to FGF-2 and EGF. Inclusion of HET0016 in the pellet drastically decreased the angiogenic response to both FGF-2 (Figure 3, A and B) and EGF (Figure 4, A and B). These data suggest that CYP4A activity is necessary for angiogenic growth factors to elicit an angiogenic response. To reinforce this concept, we tested whether a chemically dissimilar inhibitor of CYP4A, DDMS, also suppresses the angiogenic response to VEGF in the rat cornea pocket angiogenesis assay. The results of these experiments are presented in Figure 5, A and B, and indicate that DDMS also completely inhibits the angiogenic response to VEGF.
Figure 3.
A and B: Effects of HET0016 on the angiogenic responses elicited by FGF-2 in vivo. Pellets containing FGF-2 alone (250 ng/pellet) or FGF-2 and HET0016 (20 μg) were implanted into the stroma of the rat cornea. A: Representative cornea flat mounts. B: Changes in the angiogenic response as in Figure 2 (n = 6; P < 0.001, FGF-2 versus FGF-2 + HET0016).
Figure 4.
A and B: Effects of HET0016 on the angiogenic responses elicited by EGF in vivo. Pellets containing EGF alone (250 ng/pellet) or EGF and HET0016 (20 μg) were implanted into the stroma of the rat cornea. A: Representative cornea flat mounts. B: Changes in the angiogenic response (n = 7; P < 0.001, EGF versus EGF + HET0016).
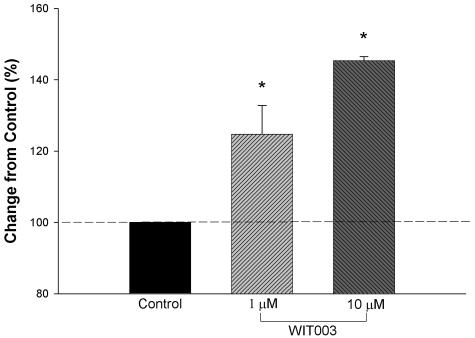
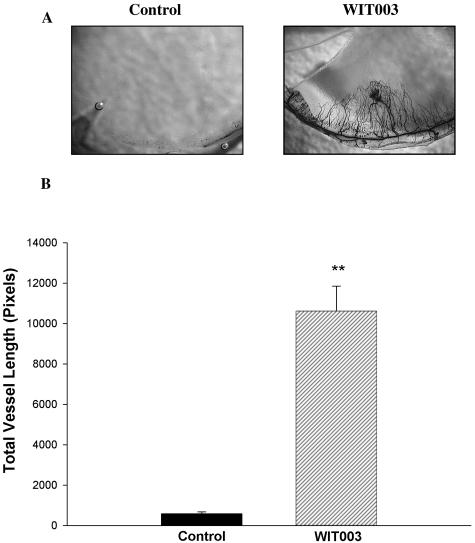
Because CYP4A is a ω-hydroxylase that synthesizes 20-HETE from arachidonic acid, HET0016 may act by inhibiting the synthesis of 20-HETE. To determine whether 20-HETE synthesis inhibition may contribute to the anti-angiogenic activity of CYP4A inhibitors, we studied the effects of the stable 20-HETE analog, WIT003, on the proliferation of HUVECs in vitro and the growth of new vessels in vivo. WIT003 increased the proliferation rate of HUVECs (Figure 6), and induced an angiogenic response in the rat cornea pocket angiogenesis assay (Figure 7, A and B).
Figure 6.
Effects of WIT003, a 20-HETE agonist analog, on proliferation of HUVECs. HUVECs were incubated with either 40 μmol/L palmitic acid or solvent alone (ethanol controls). There was no difference and therefore control data were combined. In the experimental group, cells were incubated with 1 μmol/L WIT003 for 48 hours and proliferation was assayed. WIT003 increased proliferation in HUVECs (n = 3, each by triplicate; *, P < 0.05 and #, P < 0.01, controls versus 1 and 10 μmol/L WIT003).
Figure 7.
A and B: Angiogenic effects of the 20-HETE analog, WIT003, in vivo. Pellets containing 20 μg of WIT003 were implanted into the stroma of the cornea. Rats were sacrificed 7 days later and neovascularization measured. A: Representative flat mounts. B: Angiogenic response to WIT003 (n = 6; P < 0.01, controls versus WIT003).
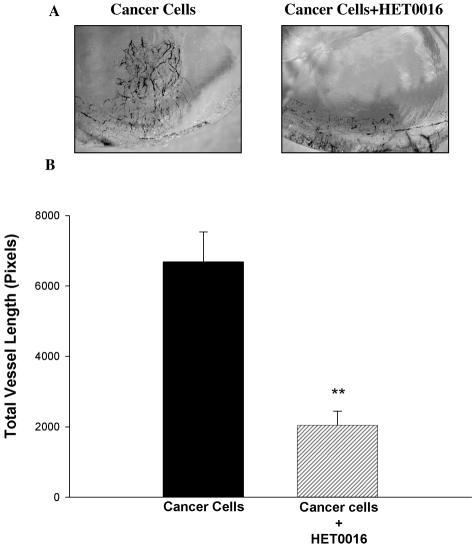
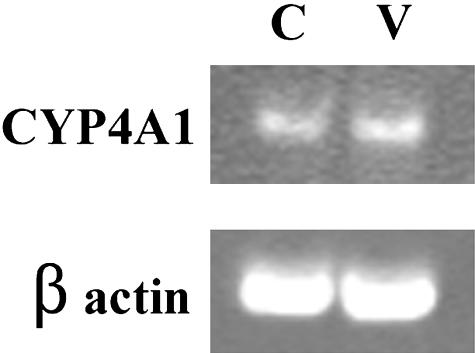
We also studied whether HET0016 inhibited the angiogenesis induced by tumor cells. Three-dimensional spheroids of the human glioblastoma cell line U251 implanted in the cornea pocket led to marked angiogenesis after 2 weeks. Corneal neovascularization was significantly inhibited in the presence of HET0016 (P < 0.01) (Figure 8, A and B). The results of the experiments to assess the expression of CYP4A mRNA in the rat corneas are presented in Figure 9. A band of the expected size was detected in control corneas. No detectable changes were observed in the VEGF-treated corneas.
Figure 8.
A and B: Effects of HET0016 on the angiogenic response of U251 cancer cells in vivo. Spheroids of the human glioblastoma cancer cell line U251 were generated by seeding single-cell suspensions at low densities over a layer of 0.8% agar. A total of five to eight spheroids, ∼200 μm each, were inserted into a corneal pocket carved in both eyes. A pellet containing either 20 μg of HET0016 or vehicle (ethanol), for control, was placed adjacent to the spheroids. Rats were sacrificed 2 weeks after implantation and neovascularization responses measured. A: Corneas in the region of the spheroid/pellet implant for all rats in this series. B: Changes in angiogenic response (n = 8; P < 0.01, control spheroids versus spheroids + HET0016).
Figure 9.
Gene expression of CYP4A1 in the corneas of control and VEGF-treated rats. The presence of mRNA for CYP4A1 was determined by semiquantitative reverse transcriptase-PCR. After electrophoresis in a 10% polyacrylamide gel, the PCR products were visualized using ethidium bromide. Bands from corneas containing saline (C) and VEGF (V) are of the predicted size. β-actin was used as a loading control.
Discussion
The present study examined the effects of inhibitors of CYP4A on the mitogenic response of HUVECs to VEGF and in the growth factor-induced angiogenesis in the cornea in vivo. The results indicated that blockade of CYP4A activity with HET0016 blocked the known proliferative response to VEGF in HUVECs.27 Further evidence that a metabolite of arachidonic acid may play a role in VEGF-induced proliferation was suggested by experiments using the stable 20-HETE analog, WIT003. Treatment of HUVECs with WIT003 increased proliferation of the cells in a manner similar to the changes after treatment of the endothelial cells with VEGF. However, compared with VEGF, WIT003 was a weak angiogenic factor because we needed to use μm concentrations.
Our findings that a CYP4A metabolite of fatty acids plays a role in the mitogenic responses of HUVECs are consistent with studies in vascular smooth muscle cells.15 These authors concluded that CYP4A, and in particular 20-HETE, plays a central role in the regulation of other cellular signaling molecules involved in cell proliferation and growth. The present results suggest a more general involvement of CYP4A products in growth responses to angiogenic mitogens, of which VEGF is the prototypical representative.
The angiogenic response in vivo involves much more than just an increase in endothelial cell proliferation because other processes are involved including cell migration, matrix degradation, endothelial cell differentiation, and recruitment of perimural cells. Given the complexity of the angiogenic process, it is essential to demonstrate that any potential inhibitor of angiogenesis is able to affect the formation of fully formed blood vessels in vivo. Because we hypothesized that inhibition of CYP4A would alter angiogenic responses in vivo, we tested this hypothesis in the rat cornea pocket angiogenesis assay, an in vivo model of angiogenesis. This assay involves placing a pellet containing an angiogenic inducer (in our case angiogenic growth factors or cancer cells) into a pocket carved into the corneal stroma. The pellet slowly and continuously releases the angiogenic factor, and this in turn stimulates outgrowth from the peripherally located limbal vasculature toward the pellet, following the concentration gradient. In comparison to other in vivo assays, it has the advantage of measuring only new blood vessels, because the cornea is initially avascular and transparent.28
Vigorous angiogenic responses were observed when VEGF, FGF-2, or EGF was implanted into the rat cornea. The angiogenic response to all of these growth factors was almost obliterated by the presence of HET0016. This suggests that CYP4A is a crucial regulator of angiogenesis, because angiogenic responses were practically abolished when a potent inhibitor of CYP4A was present. The olefinic compound, DDMS, has been reported to be a potent and highly selective inhibitor of CYP4A whose structure and mechanism of action is unrelated to that of HET0016.23 The effects of VEGF on corneal neovascularization were also abolished by DDMS. Thus, two different inhibitors of CYP4A abolished the angiogenic responses induced by VEGF, consequently strengthening the concept that these inhibitors affect an essential step in the angiogenic process. Because inhibition of CYP4A is apparently the common link between HET0016 and DDMS, we conclude that one product of the enzymatic activity of CYP4A may be either a mediator or a necessary component (perhaps a gatekeeper or permissive factor) without which the angiogenic process does not proceed.
With regard to the cornea pocket assay we used, corneal angiogenesis is thought by some to be caused by inflammation and thus driven by VEGF released by invading white blood cells responding to the foreign pellet.29 However, we saw no angiogenesis or signs of inflammation in the corneas implanted with a pellet alone, so the pellet, per se, is not inflammatory or angiogenic in our hands. We cannot exclude that FGF-2 and EGF have acted by attracting macrophages to the cornea.30,31
The present findings are consistent with the results of previous studies indicating that 20-HETE is involved in regulation of angiogenesis. Amaral and colleagues32 examined the role of 20-HETE in the angiogenesis induced by electrical stimulation in skeletal muscle. Electrical stimulation significantly increased 20-HETE formation and angiogenesis in the muscle, and this increase was blocked by chronic treatment with HET0016. Relevant to the present work, anti-VEGF antibodies blocked the increased 20-HETE formation induced by electrical stimulation. The authors suggested that 20-HETE acted downstream from VEGF in the signaling pathway for angiogenesis. Because we administered the growth factors directly, HET0016 and DDMS must have acted downstream from VEGF. In other studies, renal microvessels transfected with an adenovirus vector inducing smooth muscle-specific overexpression of CYP4A produced higher levels of 20-HETE and exhibited endothelial sprouting.11 Thus, overexpression of the CYP4A1 gene induced angiogenesis, presumably by increased production of 20-HETE.6,9,15 Therefore, 20-HETE is a prime candidate to explain the effects of inhibiting CYP4A on angiogenic responses.
Mobilization of arachidonic acid from cellular membrane glycerolipid pools is an integral part of cellular response to many stimuli such as cytokines, growth factors, and hormones. Arachidonic acid can be oxidized by cyclooxygenase (COX), lipoxygenase(LOX), or P450 epoxygenase (EOX) to form a variety of eicosanoids involved in several homeostatic biological functions and inflammation. The COX enzymes, COX1 and COX2, catalyze a key step in the conversion of arachidonate to an endoperoxide precursor, prostaglandin H2 (PGH2), which is subsequently converted by isomerases to form prostaglandins, prostacyclin, or thromboxane. There are data suggesting that COX-2 activity, reflected by PGE2 production, is involved in hypoxia-induced VEGF expression, and thus angiogenesis. Arachidonate metabolism by LOXs leads to the formation of regioisomeric cis-/trans-conjugated hydroxyeicosatetraenoic acids (HETEs), leukotrienes, lipoxins, and hepoxilins. Among them, 12(S)-HETE is biologically very active. 12(S)-HETE can elicit several angiogenic responses such as migration and mitogenesis of endothelial cells.33 In addition, 12-HETrE is also angiogenic.34 EOX products, such as 11,12-epoxyeicosatrienoic acid (EET), stimulate endothelial cell proliferation, and it has been reported that CYP 2C9-derived EETs stimulate angiogenesis by a mechanism involving the activation of the EGF receptor.35 We now report that 20-HETE is also angiogenic and that CYP4A may be a crucial factor in regulation of angiogenic responses. It is clear that eicosanoids have emerged as key regulators for angiogenesis. It is obvious that further studies are necessary on the role that CYP4A-derived metabolites and, among them 20-HETE, play in pathological angiogenesis and growth.
It remains to be determined how inhibitors of CYP4A are so effective at reducing the angiogenic responses to different growth factors. It would be reasonable to ascribe their anti-angiogenic activity to blockade of 20-HETE, which by itself is angiogenic. We questioned whether corneas of control rats show CYP4A1 gene expression and whether VEGF would alter these basal levels. The mRNA coding for CYP4A1 was present in vehicle-treated rat corneas. We were unable to detect changes in mRNA levels in corneas treated with VEGF for 7 days. Thus, it appears that growth factors do not act by chronically increasing CYP4A gene expression. The precise interaction between growth factor induced angiogenesis and CYP4A-derived metabolites remains to be elucidated.
The present results point to 20-HETE synthesis inhibition as being responsible for the anti-angiogenic effects of the CYP4A inhibitors. However, further confirmation is needed because we cannot exclude the possibility that HET0016 and DDMS have other targets besides CYP4A and inhibition of 20-HETE synthesis. According to Miyata and colleagues,18 HET0016 inhibits CYP4A enzymes in human renal microsomes at ∼0.01 μmol/L concentration. The concentrations of HET0016 we needed to inhibit responses to VEGF in the in vitro studies were in the order of 10 μmol/L. This may be because of barriers for HET0016 to reach CYP4A within the cell and/or metabolism of HET0016, which was incubated with the cells for 24 hours. Yet it can also suggest that HET0016 is acting on targets other than CYP4A, which are inhibited at high HET0016 concentrations. In addition we have not shown in this work that HET0016 acts by inhibiting the synthesis of discrete eicosanoids, including 20-HETE.
Further, it is not clear which of the described biological effects of 20-HETE may explain its angiogenic activity. Numerous reported results suggest that 20-HETE activates multiple signaling cascades that impact on ion channel activity, vascular tone, and growth in various cell types.6 20-HETE causes blockade of Ca2+-activated K+ channels (BKCa), thereby promoting Ca2+ entry by depolarizing vascular smooth muscle cells. If this were the mechanism behind the angiogenic effects of 20-HETE, then angiogenic growth factors should act by similar mechanisms. However, FGF-2, a potent angiogenic polypeptide, has been reported to activate BKCa.36 Thus it seems unlikely that prevention of angiogenic responses by HET0016 and DDMS is because of activation of BKCa secondary to 20-HETE removal.
VEGF is a strong activator of ERK1/2 (extracellular signal regulated protein kinases 1 and 2) via tyrosine phosphorylation of kinase insert domain-containing receptor (KDR) and specific inhibitors of MEK1/2 (mitogen-activated protein kinase/ERK kinase 1/2), the kinase responsible for ERK activation, reduce endothelial tubulogenesis in vitro. The major pathway through which receptor tyrosine kinases activate ERKs involves Ras activation, leading to activation of the Raf-1/MEK/ERK cascade. There is strong evidence, however, that KDR activates ERK via a Ras-independent pathway involving PLC-γ. VEGF induces strong PLC-γ tyrosine phosphorylation and activation, leading to generation of diacylglycerol and inositol 1,4,5-trisphosphate and subsequent activation of protein kinase C (PKC) and Ca2+ mobilization. VEGF induces Ras-independent induction of the ERK pathway whereas PKC inhibitors block VEGF-induced activation of ERK1/2 and MEK.37 Several studies also show that PKC inhibition, using either pharmacological inhibitors or anti-sense oligonucleotides to PKC-α and PKC-ζ isoforms, blocks VEGF mitogenic signaling and diverse PKC inhibitors block VEGF-induced angiogenesis in vitro.38 ERK mediates VEGF-induced activation of cPLA2, a key rate-limiting step in release of arachidonic acid and its subsequent conversion to diverse metabolites such as 20-HETE via CYP and prostanoids via COX. PKC inhibitors block VEGF-induced and ERK-mediated cPLA2 activation and PGI2 production.39 20-HETE activates PKC, and inhibitors of PKC block the vasoconstrictor response to 20-HETE in cat cerebral vascular smooth muscle cells40 and renal arterioles.15,41 Thus the linkage between receptor tyrosine kinase signaling and 20-HETE may involve Ca2+ mobilization, cPLA2 activation, and activation of the PKC and Ras-Raf mitogen-activated protein kinase (MAPK) pathway. Interestingly, tyrosine kinase inhibitors block the effects of 20-HETE.6 This suggests that how CYP4A inhibitors affect tyrosine-kinase receptors signaling deserves further study.
Angiogenesis is a crucial event in physiological conditions such as wound healing and the female reproductive cycle and also in pathological situations such as diabetic retinopathy, macular degeneration, and chronic inflammatory diseases. In particular, tumor expansion is dependent on angiogenesis, which is critical for the growth of cancers greater than 1 to 2 mm. Consequently, suppressing a tumor’s ability to generate new vessels is an appealing therapeutic target.42
Therefore, we explored whether HET0016 would affect tumor-induced angiogenesis. For this, we selected a malignant glioma cell model known to be highly angiogenic, the glioblastoma cell line U251.43 This is clinically relevant because glioblastoma multiforme is distinguished by intense angiogenesis.26,44 We implanted U251 spheroids into the rat corneal stroma together with a pellet containing either HET0016 or controls (palmitic acid or saline). All control eyes showed marked corneal neovascularization, however, HET0016 significantly decreased angiogenic responses by ∼70%. This suggests that CYP4A may be a key regulator of cancer-induced angiogenesis and therefore cancer progression. The elucidation of the role of CYP4A, as well as other arachidonic acid-metabolizing enzymes (such as COX, LOX, and EOX), brings about a potentially important opportunity to develop novel inhibitors targeted to reduce pathological angiogenesis.
In summary, CYP4A enzymes metabolize arachidonic acid to 20-HETE. We demonstrated that 20-HETE is mitogenic in endothelial cells in vitro and angiogenic in vivo. The highly selective CYP4A inhibitor HET0016 blocked the mitogenic activity of VEGF in endothelial cells. DDMS is another selective inhibitor of CYP4A and both HET0016 and DDMS inhibit angiogenic responses to VEGF in vivo. HET0016 also blocked the angiogenic response to FGF-2 and EGF. HET0016 decreased angiogenesis induced by U251, a human glioblastoma cell line. This is consistent with a metabolite produced by CYP4A having a crucial role in the regulation of angiogenesis, either as a mediator or an essential permissive factor. Further studies are needed to determine whether or not this metabolite is 20-HETE or whether or not HET0016 and DDMS indeed inhibited angiogenesis via CYP4A inhibition. The results shown here suggest that inhibitors of CYP4A may be useful tools to manipulate angiogenic responses.
Acknowledgments
We thank Taisho Pharmaceuticals for donating the HET0016.
Footnotes
Address reprint requests to A. Guillermo Scicli, Eye Care Services, Henry Ford Health System, One Ford Place, 4 D, Detroit, MI 48202-3450. E-mail: gscicli1@hfhs.org.
Supported by grants from the National Institutes of Health (EY014385 to A.G.S., GM31278 to J.R.F., and HL 036279 to R.J.R.), the Robert A. Welch Foundation (to J.R.F.), and the American Heart Association (to P.C.).
References
- Wheeler-Jones C, Abu-Ghazaleh R, Cospedal R, Houliston RA, Martin J, Zachary I. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase A2 in endothelial cells via p42/p44 mitogen-activated protein kinase. FEBS Lett. 1997;420:28–32. doi: 10.1016/s0014-5793(97)01481-6. [DOI] [PubMed] [Google Scholar]
- Sa G, Murugesan G, Jaye M, Ivashchenko Y, Fox PL. Activation of cytosolic phospholipase A2 by basic fibroblast growth factor via a p42 mitogen-activated protein kinase-dependent phosphorylation pathway in endothelial cells. J Biol Chem. 1995;270:2360–2366. doi: 10.1074/jbc.270.5.2360. [DOI] [PubMed] [Google Scholar]
- Warner LC, Hack N, Egan SE, Goldberg HJ, Weinberg RA, Skorecki KL. RAS is required for epidermal growth factor-stimulated arachidonic acid release in rat-1 fibroblasts. Oncogene. 1993;8:3249–3255. [PubMed] [Google Scholar]
- Thorsen VA, Vorland M, Bjorndal B, Bruland O, Holmsen H, Lillehaug JR. Participation of phospholipase D and alpha/beta-protein kinase C in growth factor-induced signalling in C3H10T1/2 fibroblasts. Biochim Biophys Acta. 2003;1632:62–71. doi: 10.1016/s1388-1981(03)00063-5. [DOI] [PubMed] [Google Scholar]
- Banan A, Zhang LJ, Shaikh M, Fields JZ, Farhadi A, Keshavarzian A. Key role of PLC-gamma in EGF protection of epithelial barrier against iNOS upregulation and F-actin nitration and disassembly. Am J Physiol. 2003;285:C977–C993. doi: 10.1152/ajpcell.00121.2003. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Zhao X, Imig JD. Kidney CYP450 enzymes: biological actions beyond drug metabolism. Curr Drug Metab. 2003;4:73–84. doi: 10.2174/1389200033336892. [DOI] [PubMed] [Google Scholar]
- Sacerdoti D, Gatta A, McGiff JC. Role of cytochrome P450-dependent arachidonic acid metabolites in liver physiology and pathophysiology. Prostaglandins Other Lipid Mediat. 2003;72:51–71. doi: 10.1016/s1098-8823(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Lin F, Rios A, Falck JR, Belosludtsev Y, Schwartzman ML. 20-Hydroxyeicosatetraenoic acid is formed in response to EGF and is a mitogen in rat proximal tubule. Am J Physiol. 1995;269:F806–F816. doi: 10.1152/ajprenal.1995.269.6.F806. [DOI] [PubMed] [Google Scholar]
- Amaral SL, Maier KG, Schippers DN, Roman RJ, Greene AS. CYP4A Metabolites of arachidonic acid and VEGF are mediators of skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol. 2003;284:H1528–H1535. doi: 10.1152/ajpheart.00406.2002. [DOI] [PubMed] [Google Scholar]
- Jiang M, Mezentsev A, Kemp R, Byun K, Falck JR, Miano JM, Nasjletti A, Abraham NG, Laniado-Schwartzman M. Smooth muscle-specific expression of CYP4A1 induces endothelial sprouting in renal arterial microvessels. Circ Res. 2004;94:167–174. doi: 10.1161/01.RES.0000111523.12842.FC. [DOI] [PubMed] [Google Scholar]
- Hack N, Margolis B, Schlessinger J, Skorecki K. Interaction of epidermal growth factor with vasoactive hormones in the regulation of phospholipase A2. J Basic Clin Physiol Pharmacol. 1991;2:161–182. doi: 10.1515/jbcpp.1991.2.3.161. [DOI] [PubMed] [Google Scholar]
- Boccellino M, Giovane A, Servillo L, Balestrieri C, Quagliuolo L. Fatty acid mobilized by the vascular endothelial growth factor in human endothelial cells. Lipids. 2002;37:1047–1052. doi: 10.1007/s11745-002-0999-7. [DOI] [PubMed] [Google Scholar]
- Shibuya M. Vascular endothelial growth factor receptor-2: its unique signaling and specific ligand, VEGF-E. Cancer Sci. 2003;94:751–756. doi: 10.1111/j.1349-7006.2003.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthalif MM, Benter IF, Karzoun N, Fatima S, Harper J, Uddin MR, Malik KU. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci USA. 1998;95:12701–12706. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranenburg O, Gebbink MF, Voest EE. Stimulation of angiogenesis by Ras proteins. Biochim Biophys Acta. 2004;1654:23–37. doi: 10.1016/j.bbcan.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Mazure NM, Brahimi-Horn MC, Pouyssegur J. Protein kinases and the hypoxia-inducible factor-1, two switches in angiogenesis. Curr Pharm Des. 2003;9:531–541. doi: 10.2174/1381612033391469. [DOI] [PubMed] [Google Scholar]
- Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, Kameo K. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol. 2001;133:325–329. doi: 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Ishii T, Kobayashi-Matsunaga Y, Amada H, Taniguchi K, Miyata N, Kameo K. Discovery of a N′-hydroxyphenylformamidine derivative HET0016 as a potent and selective 20-HETE synthase inhibitor. Bioorg Med Chem Lett. 2001;11:2993–2995. doi: 10.1016/s0960-894x(01)00614-x. [DOI] [PubMed] [Google Scholar]
- Capdevila JH, Falck JR. Biochemical and molecular properties of the cytochrome P450 arachidonic acid monooxygenases. Prostaglandins Other Lipid Mediat. 2002;68–69:325–344. doi: 10.1016/s0090-6980(02)00038-2. [DOI] [PubMed] [Google Scholar]
- Yu M, Cambj-Sapunar L, Kehl F, Maier KG, Takeuchi K, Miyata N, Ishimoto T, Reddy LM, Falck JR, Gebremedhin D, Harder DR, Roman RJ. Effects of a 20-HETE antagonist and agonists on cerebral vascular tone. Eur J Pharmacol. 2004;486:297–306. doi: 10.1016/j.ejphar.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol. 1999;277:F790–F796. doi: 10.1152/ajprenal.1999.277.5.F790. [DOI] [PubMed] [Google Scholar]
- Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther. 1998;284:966–973. [PubMed] [Google Scholar]
- Volkin DB, Verticelli AM, Marfia KE, Burke CJ, Mach H, Middaugh CR. Sucralfate and soluble sucrose octasulfate bind and stabilize acidic fibroblast growth factor. Biochim Biophys Acta. 1993;1203:18–26. doi: 10.1016/0167-4838(93)90031-l. [DOI] [PubMed] [Google Scholar]
- Carlsson J, Yuhas JM. Liquid-overlay culture of cellular spheroids. Recent Results Cancer Res. 1984;95:1–23. doi: 10.1007/978-3-642-82340-4_1. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Volpert OV, Steck PA, Mikkelsen T, Polverini PJ, Rao S, Chou P, Bouck NP. Inhibition of angiogenesis in human glioblastomas by chromosome 10 induction of thrombospondin-1. Cancer Res. 1996;56:5684–5691. [PubMed] [Google Scholar]
- Lang I, Hoffmann C, Olip H, Pabst MA, Hahn T, Dohr G, Desoye G. Differential mitogenic responses of human macrovascular and microvascular endothelial cells to cytokines underline their phenotypic heterogeneity. Cell Prolif. 2001;34:143–155. doi: 10.1046/j.1365-2184.2001.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D’Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- Staton CA, Stribbling SM, Tazzyman S, Hughes R, Brown NJ, Lewis CE. Current methods for assaying angiogenesis in vitro and in vivo. Int J Exp Pathol. 2004;85:233–248. doi: 10.1111/j.0959-9673.2004.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T, Ishida S, Yamashiro K, Kaji Y, Poulaki V, Moore J, Moore T, Amano S, Horikawa Y, Dartt D, Golding M, Shima DT, Adamis AP. VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest Ophthalmol Vis Sci. 2004;45:368–374. doi: 10.1167/iovs.03-0106. [DOI] [PubMed] [Google Scholar]
- Shaw JP, Chuang N, Yee H, Shamamian P. Polymorphonuclear neutrophils promote rFGF-2-induced angiogenesis in vivo. J Surg Res. 2003;109:37–42. doi: 10.1016/s0022-4804(02)00020-3. [DOI] [PubMed] [Google Scholar]
- Amaral SL, Papanek PE, Greene AS. Angiotensin II and VEGF are involved in angiogenesis induced by short-term exercise training. Am J Physiol. 2001;281:H1163–H1169. doi: 10.1152/ajpheart.2001.281.3.H1163. [DOI] [PubMed] [Google Scholar]
- Nie D, Tang K, Diglio C, Honn KV. Eicosanoid regulation of angiogenesis: role of endothelial arachidonate 12-lipoxygenase. Blood. 2000;95:2304–2311. [PubMed] [Google Scholar]
- Stoltz RA, Conners MS, Gerritsen ME, Abraham NG, Laniado-Schwartzman M. Direct stimulation of limbal microvessel endothelial cell proliferation and capillary formation in vitro by a corneal-derived eicosanoid. Am J Pathol. 1996;148:129–139. [PMC free article] [PubMed] [Google Scholar]
- Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR). FASEB J. 2003;17:770–772. doi: 10.1096/fj.02-0640fje. [DOI] [PubMed] [Google Scholar]
- Wiecha J, Reineker K, Reitmayer M, Voisard R, Hannekum A, Mattfeldt T, Waltenberger J, Hombach V. Modulation of Ca2+-activated K+ channels in human vascular cells by insulin and basic fibroblast growth factor. Growth Horm IGF Res. 1998;8:175–181. doi: 10.1016/s1096-6374(98)80108-1. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Gliki G, Wheeler-Jones C, Zachary I. Vascular endothelial growth factor induces protein kinase C (PKC)-dependent Akt/PKB activation and phosphatidylinositol 3′-kinase-mediates PKC delta phosphorylation: role of PKC in angiogenesis. Cell Biol Int. 2002;26:751–759. doi: 10.1016/s1065-6995(02)90926-1. [DOI] [PubMed] [Google Scholar]
- Uchiba M, Okajima K, Oike Y, Ito Y, Fukudome K, Isobe H, Suda T. Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ Res. 2004;95:34–41. doi: 10.1161/01.RES.0000133680.87668.FA. [DOI] [PubMed] [Google Scholar]
- Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272:27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- Sun CW, Falck JR, Harder DR, Roman RJ. Role of tyrosine kinase and PKC in the vasoconstrictor response to 20-HETE in renal arterioles. Hypertension. 1999;33:414–418. doi: 10.1161/01.hyp.33.1.414. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Oku N. Cancer anti-angiogenic therapy. Biol Pharm Bull. 2004;27:599–605. doi: 10.1248/bpb.27.599. [DOI] [PubMed] [Google Scholar]
- Schmidek HH, Nielsen SL, Schiller AL, Messer J. Morphological studies of rat brain tumors induced by N-nitrosomethylurea. J Neurosurg. 1971;34:335–340. doi: 10.3171/jns.1971.34.3.0335. [DOI] [PubMed] [Google Scholar]
- Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36:91–102. doi: 10.1023/a:1005805203044. [DOI] [PubMed] [Google Scholar]