Abstract
Organ-specific tumor cell adhesion to extracellular matrix (ECM) components and cell migration into host organs often involve integrin-mediated cellular processes that can be modified by environmental conditions acting on metastasizing tumor cells, such as shear forces within the blood circulation. Since the focal adhesion kinase (FAK) appears to be essential for the regulation of the integrin-mediated adhesive and migratory properties of tumor cells, its role in early steps of the metastatic cascade was investigated using in vitro and in vivo approaches. Human colon and hepatocellular carcinoma cells were used to study adhesive properties under static conditions and in a parallel plate laminar flow chamber in vitro. In addition, intravital fluorescence microscopy was used to investigate early interactions between circulating tumor cells and the microvasculature of potential target organs in vivo. Shear forces caused by hydrodynamic fluid flow induced Tyr-hyperphosphorylation of FAK in cell monolayers. Reduced expression of FAK or its endogenous inhibition by FAK-related non-kinase (FRNK) interfered with early adhesion events to extracellular matrix components under flow conditions. In contrast, tumor cell adhesion to endothelial cells under these conditions was not affected. Furthermore, down-regulation of FAK inhibited metastatic cell adhesion in vivo within the liver sinusoids. In summary, FAK appears to be involved in early events of integrin-mediated adhesion of circulating carcinoma cells under fluid flow in vitro and in vivo. This kinase may take part in the establishment of definitive adhesive interactions that enable adherent tumor cells to resist fluid shear forces, resulting in an organ-specific formation of distant metastases.
Most carcinomas often show organ preference of metastasis formation.1,2 In particular, the liver and lung are sites often colonized by metastatic carcinoma cells. Organ-specific sites of metastatic lesions are determined, in part, by adhesive interactions of malignant cells with organ microvessel endothelial cells (EC) and underlying extracellular matrix (ECM).1,2 Important in these interactions is the role played by integrin-mediated adhesion of malignant cells to ECM components.3 For example, different adhesive and migration properties of poorly and highly metastatic human colon carcinoma cells to microvascular EC obtained from various organs have been described.4 Recently, we found that subclones of HT-29 colon carcinoma cells with different metastatic properties possessed different patterns of integrin-mediated adhesion to various ECM components in static and dynamic adhesion assays.5,6 However, these differences were not caused by differences in the expression of integrin subunits, because both cell lines expressed similar patterns of integrins.5
Under specific conditions, such as the presence of fluid flow within the microcirculation of host organs, distinct intracellular events during the adhesion of carcinoma cells to ECM components may be required for the adhesive process and its stabilization. For example, blockade of different integrins with antibodies can specifically inhibit metastatic tumor cell adhesion within the liver sinusoids in vivo suggesting direct interactions with ECM components, such as collagens, in the space of “Disse”. These experiments indicated that metastasizing colon or hepatocellular carcinoma cells can arrest in target organs without size restriction. Cell adhesion of circulating tumor cells occurred in metastatic target organs only, where specific interactions appear to be required for successful arrest. Furthermore, invasion of colon carcinoma cells into target organs correlated with their metastatic potential. (Schlüter et al, submitted) Moreover, the integrity of microtubules and actin filaments can modulate this tumor cell adhesion within the hepatic microcirculation.7 Since integrins appear to be directly involved in early steps of metastasis formation, cell signaling and regulatory processes that modulate their affinity and/or avidity may also influence metastatic tumor cell adhesion or migration into host organs.
Signal transduction is apparently required both for the adhesion event itself and for subsequent steps involved in host organ colonization.8 These cell-ECM interactions that lead to signal transduction are mediated mainly through integrins, which are intracellularly linked to cytoskeleton components9 and other protein molecules.10,11 The functional status of integrins is regulated by complex interactions with a number of these cytosolic, cytoskeletal, and membrane-bound proteins.12 For example, cells can modulate integrin-binding affinities and kinetics of interactions between integrin receptors and their adhesive ligands through inside-out signaling.13,14 After binding and clustering of integrins at the cell surface, interactions between integrins and intracellular proteins, often involving protein phosphorylation, are induced.10,15 One of the most important components of the integrin-linked focal contacts is the focal adhesion kinase (FAK) that can bind directly to integrins and mediate various signal transduction events.16,17,18
In transformed cells and in clinical analyses of human tumors, elevated FAK expression and activity have been correlated with the progression to a highly malignant and metastatic phenotype (reviewed in19). In carcinoma cells, FAK-mediated signaling appears to be required for epithelial to mesenchymal transition, invasive properties, and the formation of β1-integrin focal contact sites.20 Additionally, studies using antisense to reduce FAK expression in these cells also found that FAK was a required component for optimal cell motility.21 Notably, changes in cell invasion were independent from changes in either cell motility or transformation. FAK has been linked to the formation of focal contacts and FAK-null fibroblasts exhibited motility defects as a result of increased focal contact formation and the inability to remodel contact sites in response to various motility stimuli22 suggesting an additional role of FAK in focal adhesion disassembly.23 Besides its role in focal adhesion turnover, FAK has been implicated in adhesion stabilization and mechanotransduction. The interactions of the integrin receptors with ECM ligands can stabilize cell adhesions by recruiting signaling proteins involving FAK, among others, and cytoskeletal components.24,25 The mechanistic role of FAK, however, seems to be highly context-dependent. For example, for integrin-stimulated cell motility the FAK Tyr397 site and its kinase activity were required.26 In contrast, growth factor-stimulated cell motility of FAK-null fibroblasts was unaffected by the expression of kinase-inactive FAK.27
The majority of studies on adhesion-mediated signal transduction has been performed using assays where cell adhesion was conducted under static conditions, and the influence of fluid flow in the microcirculation was not considered.28 However, it is known that shear forces alone can mediate various cellular events in endothelial cells, leukocytes or platelets, such as phosphorylation,29 cytoskeletal alterations, or secretion of soluble factors.30 To counteract the shear forces of blood flow that appears to be required for cell adhesion of circulating tumor cells to vessel walls during metastasis formation several adhesion systems acting in parallel are probably necessary to form definitive adhesions that ultimately allow adherent tumor cell stabilization, migration, and invasion into the host organ.1 Hydrodynamic adhesion assays are capable of mimicking hemodynamic conditions in the microcirculation.30,31 Various studies have shown that tumor cells, such as colon carcinoma cells, can interact with EC and ECM under dynamic conditions of fluid flow.32,33 Instead of measuring the complex of initial cell-surface interactions and adhesion stabilization using microtiter plate assays, we have used specific parallel plate laminar flow chambers for hemodynamic adhesion assays34 to monitor the primary events of adhesion between circulating cells and vascular surfaces (EC or ECM) and their stabilization separately.35 With this flow chamber assay we recently demonstrated that the initial dynamic interactions between colon carcinoma cells and various ECM components were mostly nonspecific, whereas definitive adhesion and its stabilization were specifically mediated, in part, by integrins.36
The C-terminal non-catalytic region of FAK (FRNK: FAK-related non-kinase) can be expressed as a separate RNA transcript in a variety of embryonic tissues,37 in chicken fibroblasts,38 and also in smooth muscle cells after vascular injury.39 When expressed in various cell types by transfection methods FRNK exerts distinct effects on cell migration, cell adhesion, and cell spreading in vitro.39,40 As a dominant-negative inhibitor of FAK, FRNK can negatively affect FAK-mediated cell functions, such as cell migration.41 It has been suggested that FRNK disrupts associations of FAK with other intracellular proteins, such as pp60src,42,43 prevents the localization of FAK to focal adhesions and thus antagonizes their formation.44 FAK undergoes autophosphoryation as a reaction to cell adhesion and integrin-mediated stimulation on several Tyr residues, one of which is the crucial residue Tyr397.45 This autophosphorylation can also be inhibited by FRNK overexpression.44 In addition, there are different reports as to whether ectopic FRNK expression can induce apoptosis.44,46
Furthermore, most studies investigating the role of FAK in adhesive interactions have been carried out on fibroblasts,40–42 myocytes,39,44 and embryonic tissues.37 Since the ability of various carcinoma cells to migrate and adhere on ECM components appears to correlate with their invasiveness and organ-specific metastatic potential, biophysical forces can modulate cell signaling in circulating tumor cells, and integrins can directly mediate metastatic tumor cell adhesion within host organs, we focused on adhesive and migratory properties under these conditions and the regulation of these processes. We hypothesized that FAK is involved in these early integrin-dependent steps of metastasis formation. The purpose was to investigate the involvement of FAK in the regulation of static and dynamic cell adhesion to ECM components in vitro and in metastatic tumor cell adhesion within the hepatic sinusoids in vivo. First, we found that hydrodynamic shear forces can increase Tyr-phosphorylation of FAK in these cells. In addition, we demonstrated that reduced expression of FAK or its endogenous inhibition using FRNK can interfere with integrin-mediated cell adhesion to collagen and adhesion stabilization under the influence of hydrodynamic shear forces, but not under static conditions or to EC as adhesive substrate. Finally, blockade of FAK resulted in a loss of metastatic tumor cell adhesion within the liver microcirculation in vivo.
Materials and Methods
Materials and Cells
Fetal bovine serum (FBS), RPMI 1640, and minimal essential medium (MEM) were purchased from Life Technologies (Karlsruhe, Germany); type I collagen (C I) and type IV collagen (C IV) were obtained from Collaborative Biomedical Products (Bedford, MA). Genistein is a broad range inhibitor of protein tyrosine kinase (PTK) (IC50 = 1 to 100 μmol/L). Erbstatin analog (inhibitor of EGFR-kinase: IC50 = 0.78 μmol/L), Tyrphostin25 (IC50 = 3 to 150 μmol/L) and Tyrphostin47 (broad-range PTK inhibitor with a different spectrum of inhibitory activity compared to Genistein: IC50 = 3 to 640 μmol/L) served as control substances. All inhibitors were purchased from Calbiochem (Bad Soden, Germany) and reconstituted in dimethyl-sulfoxide (DMSO). The monoclonal antibodies (mAb) against P-Tyr (Py20), paxillin and FAK were obtained from Transduction Laboratories, and CalceinAM from Molecular Probes (Eugen, Netherlands). All other chemicals were purchased from Sigma (Taufkirchen, Germany).
We used highly metastatic HT-29LMM colon carcinoma cells or liver-metastatic Hep-G2 cells (hepatocellular carcinoma) to study cell adhesion properties. Cells were cultured in RPMI medium containing 10% FBS (HT-29LMM) or MEM medium containing 20% FBS (Hep-G2), respectively, without antibiotics in humidified 5% CO2/95% air at 37°C. Only cells of less than 20 passages were used, and they were harvested with Trypsin/EDTA during the log-phase of growth. After trypsinization, the cells were resuspended in adhesion medium (RPMI [1:1], bovine serum albumin [BSA] 1%) at room temperature for 45 minutes for reconstitution of surface proteins and washed extensively in calcium-magnesium-free phosphate-buffered solution (CMF-PBS).47 HUVEC (Cambrex Bioproducts, Apen, Germany) were grown on glass slides or in microtiter plates in endothelial cell medium to confluence.
Static Adhesion Assays
Microtiter plates (96-well, Corning) were coated with C I (50 μg/ml), C IV (50 μg/ml), or 1% BSA (negative control) at 37°C for 3 hours (150 μl/well). Effective concentrations to completely coat the wells were determined in previous experiments.6 Blocking of nonspecific binding sites was performed using 1% BSA (37°C, 30 minutes, 200 μl/well). During reconstitution of surface proteins in adhesion medium, cells were fluorescence-labeled with CalceinAM (20 μg/15 ml). After washing, cells were resuspended in adhesion medium at a final concentration of 0.5 × 106 cells/ml.
Adhesion experiments were performed with 150-μl single-cell suspension/well for different times (10 to 120 minutes) as previously described.5 In some experiments, cells were incubated with Genistein (1 to 100 μmol/L), Erbstatin analog (0.1 to 1 μmol/L), Tyrphostin25 (1 to 10 μmol/L), Tyrphostin47 (1 to 10 μmol/L), or with the same amount of DMSO alone (10 μl/ml). Pretreatment was done at 37°C for 1 hour before adhesion assays. Cell viability was assessed by the trypan blue exclusion method, and pretreatment or CalceinAM labeling did not affect the number of viable cells (>95%). The effects of PTK inhibitors were calculated by comparing adhesion of untreated (set to 100% adhesion) and treated cells. All experiments were performed in triplicate and repeated twice.
Flow Experiments
The laminar flow chamber was constructed as previously described.36 Briefly, using an ECM-coated glass support and a polycarbonate shear deck (CoverWell Grace Bio-Labs) to form a uniform channel with a small height-to-width ratio a parallel plate chamber was formed that allowed an approximation of laminar flow. The distance between shear deck and collagen- (0.5 μg/cm2) or EC-coated glass slide was ensured by a medical-grade silicon gasket. The flow chamber was mounted on the stage of an upright microscope (magnification 1:63.5). BSA-coated glass slides were used as negative controls. A uniform fluid supply containing a single-cell suspension was maintained using a standard syringe pump (Fresenius, Germany). The temperature of the cell suspension was kept at 37°C using a water bath. The fluid flow Q could be varied between 0.17 to 0.83 ml/min (shear rates 1.6 to 5.6 dynes/cm2) as a laminar flow with a parabolic velocity within the chamber (Reynolds number <2). Therefore, the wall shear stress τ was calculated using the equation for Newtonian fluids. The viscosity for H2O at 37°C was assumed to be 0.7 cP.
Analysis of dynamic cell adhesion was performed as described previously.36 Briefly, tumor cells were resuspended in adhesion medium at a concentration of 1 × 105 cells/ml. In some experiments cells were pretreated with different concentrations of PTK inhibitors as described above. Untreated cells were allowed to adhere to BSA-coated surfaces (negative control) or collagen-coated slides (positive controls) and the effects of the inhibitors were assessed using C I or C IV as specific adhesive substrate. Alternatively, confluent EC were used. Flow experiments were performed with variable fluid flow starting at a wall shear stress (WSS) of 5.6 dynes/cm2 which is above flow conditions in the normal microcirculation (<3 dynes/cm2). Flow rates were decreased in 30 seconds intervals so that the WSS equaled 4.8, 4.0, 3.2, or 2.4 dynes/cm2. The WSS at the point of initial cell adhesion events was used to measure wall shear adhesion threshold (WSAT). The dynamic adhesion rate (DAR) represented the total number of adherent cells after 1 minute at a low flow rate of 2.4 dynes/cm2, which was approximately 50% of WSAT and in the range of physiological conditions in the microcirculation (1.6 dynes/cm2 for EC-coated slides). Following this low-flow interval, the flow rate was increased immediately to 4.8 dynes/cm2 in an attempt to detach adherent cells that had not achieved adhesion stabilization during the low-flow interval. The relative percentage of cells remaining adherent after 60 seconds in relation to DAR was evaluated as adhesion stabilization rate (ASR). All experiments were repeated five times and results were shown as mean ± SD.
Western Blotting of Phosphotyrosine, Paxillin, and Focal Adhesion Kinase
Adhesion assays were performed as described above in microtiter plates (6-well, Corning) using a final concentration of 1 × 106 cells/ml. After different adhesion time periods, the plates were placed on ice and adhesion medium was carefully removed by aspiration. The cells were lysed in ice-cold modified RIPA buffer (10 mmol/L Tris-HCl, 150 mmol/L NaCl, 1.0% NP40, 0.25% deoxycholate, 0.1 mmol/L PMSF, 0.1 mmol/L TLCK, 0.1 mmol/L iodoacetamide, 10 μg/ml leupeptin, and 10 μg/ml aprotinin; pH 7.4) at 4°C for 30 minutes. Cell lysates were centrifuged at 13,000 × g for 10 minutes to remove insoluble material and lysates were added to sodium dodecyl sulfate (SDS) sample buffer and boiled at 95°C for 5 minutes. Twenty μg of protein was added to each lane. After SDS-PAGE electrophoresis (10% gel), proteins were blotted onto polyvinylidene difluoride (PVDF) membranes (100 V for 1 hour), and the membranes were blocked in incubation buffer (10 mmol/L Tris-HCl, 50 mmol/L NaCl, 0.05% Tween 20, pH 7.5 containing 3% BSA) overnight at 4°C. Membranes were incubated with anti-P-Tyr, anti-paxillin, or anti-FAK mAb in incubation buffer containing 1% BSA at room temperature for 1 hour while rocking, and after washing three times they were incubated with anti-mouse specific anti-IgG-alkaline phosphatase for 1 hour at room temperature. For detection of bound antibodies, the membranes were incubated with BCIP/NBT solution (Zymed, Heiden, Germany) and the color reaction was stopped with distilled H2O. Paxillin or total FAK were used as control blots for equal loading.
Immunoprecipitation of Phosphorylated Proteins
Cell lysates were obtained as described above. Pre-clearance was performed by adding 15-μl anti-mouse-IgG-agarose to the lysate and incubating at 4°C for 2 hours. Supernatants were then incubated with 5-μl anti-P-Tyr mAb at 4°C overnight. Immunoprecipitation was carried out using 15-μl anti-mouse-IgG-agarose (4°C for 2 hours). After washing three times with RIPA-buffer, SDS sample buffer was added to the agarose-beads, and the mixture was boiled at 95°C for 5 minutes. The beads were removed by centrifugation, and the supernatants were subjected to SDS-PAGE electrophoresis (10% gel). Proteins were subsequently blotted onto PVDF membranes. Phosphorylated proteins were identified using Western blotting as described above.
Suppression of FAK Expression with Antisense-Oligonucleotides
For inhibition of FAK expression phosphorothioate oligonucleotides (ODN) against sequences of human pp125FAK were used. Antisense-FAK (FAK-AS2: 5′-ATAATCCAGCTTGAACCAAG-3′) representing the nucleotides 1016 to 1035 of the human FAK sequence and a mismatch control ODN (FAK-AS control: 5′-ATAATCGACTGTCAAGCAAG-3′) were checked for sequence homologies using Blast Search and the GenBank. There were no cross-reactions to other genes, and FAK-AS control did not show any significant sequence homologies. The ODN were synthesized and HPLC-purified by Life Technologies. HT-29LMM cells were grown to 80% confluence. After removal of medium and washing with CMF-PBS, cells were incubated with serum-free DME/F12 medium at 37°C for 12 hours. Using fresh serum-free medium, 0.5 to 1 μg ODN/ml medium were mixed with 10 μl/ml Lipofectin (Life Technologies) according to the manufacturer’s instructions. ODN containing liposomes were added to the cells; and HT-29 cells were incubated with FAK-AS2, FAK-AS control, or Lipofectin without ODN at 37°C for 12 to 36 hours. For further experiments, cells were lysed in modified RIPA-buffer or harvested using trypsin-EDTA as described above. Quantification of FAK expression was performed using Western blotting as described above. Detection of paxillin was used as an internal control. Toxic effects of ODN and serum-free conditions were evaluated using the Trypan blue exclusion method.
Overexpression of FRNK
Total mRNA was isolated from HT-29 cells (RNeasy Mini Kit, Qiagen, Hilden, Germany) and transcribed into cDNA (Omniscript RT Kit, Qiagen). The FRNK gene sequence was amplified and restriction sites were added via PCR. A Kozak sequence was added as well. The resulting fragment was sequenced and cloned into the mammalian expression vectors pcDNA3.1 (Invitrogen, Karlsruhe, Germany) and pEGFP-C3 (Clontech, Heidelberg, Germany) using TaqPCR Core Kit (Qiagen), TOPO TA Cloning Kit (Invitrogen) and appropriate restriction enzymes (Promega, Heidelberg, Germany). The constructed expression vectors were transformed into the bacterial strain XL Blue (Stratagene, Amsterdam, Netherlands). From overnight cultures the plasmids were isolated and purified using a plasmid preparation system (QIAfilter Plasmid Midi Kit, Qiagen).
For transfection, Hep-G2 or HT-29LMM cells were used. The constructed plasmids were transiently transfected into the cells using a liposome-based method (Lipofectamine 2000, Invitrogen) according to manufacturer’s instructions. The genuine vectors pcDNA3.1 (Promega) or pEGFP-C3 (Clontech) were used as negative control. The day after transfection cell proliferation rates were determined using a BrdU test (Boehringer, Mannheim, Germany). Apoptosis rate was determined 3 days after transfection using an Annexin assay (Coulter-Immunotech, Krefeld, Germany) and evaluated by FACS analysis.
In Vivo Observation of Metastatic Tumor Cell Adhesion
Male Sprague-Dawley rats (200 to 250 g, Charles River) were cared for in accordance with standards of the German Council on Animal Care, under an approved protocol of the local Animal Welfare Committee. Rats were anesthetized using inhalation of isofluorane (Curamed, Karlsruhe, Germany) and N2O and prepared as previously described.7,48 For intravital observation of adhesive interactions between circulating tumor cells and the hepatic microcirculation, single-cell suspensions (1 × 106 cells) were injected intra-arterially over 60 seconds. In some experiments cells were pretreated as described above. This technique did not interfere with cardiocirculatory or pulmonary functions of the animals.
Various parameters were used for further investigation and semi-quantitative analysis of these interactions. The localization of stable tumor cell adhesions within the vascular tree and in relation to the diameter of the involved vessels were evaluated. If tumor cells were able to arrest within the sinusoids, the diameter of the involved vessel was determined compared to the diameter of the adherent tumor cell. Furthermore, remaining blood flow within this vessel or its occlusion was investigated. A semi-quantitative analysis of tumor cell adhesions and extravasation was performed over a 30- minute observation period and the numbers of adherent cells were counted for each of the 5-minute intervals. Using a standardized procedure, all fields were analyzed in each observation period and the average number of adherent cells, migrated cells, and total cells observed in 30 microscopic fields was counted. Relative migration rates were calculated as percentage of cells within the hepatic parenchyma in relation to the total number of arrested cells.
Statistical Analysis
Statistical analysis was performed using the StatMost32 statistical program (DataMost Corp., Los Angeles, CA). P values were calculated according to Student’s t-test. Significant differences were accepted for P < 0.05.
Results
Fluid Flow Induces Tyr-Phosphorylation of FAK
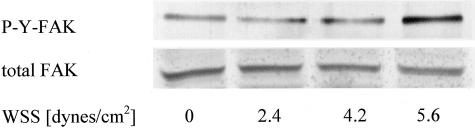
To investigate the influence of fluid flow on the phosphorylation status of immobilized cells we used the laminar flow chamber to expose cells grown on tissue culture slides to hydrodynamic shear forces. After 60 minutes of different WSS, hydrodynamic shear forces induced an increase in Tyr-phosphorylation of FAK. Highest stimulation was observed if high wall shear stress (WSS = 5.6 dynes/cm2) was applied (Figure 1). Tyr-phosphorylation of FAK was increased 2.3-fold at high WSS compared to control cells that were kept under static conditions and not exposed to shear forces. Therefore, further experiments were performed to investigate the potential role of FAK for tumor cell adhesion under hydrodynamic flow conditions comparable to the conditions found in potential metastatic organs, such as within liver sinusoids. FAK was either blocked using antisense oligonucleotides or its competitive inhibitor FRNK.
Figure 1.
Stimulation of Tyr-phosphorylation by hydrodynamic shear forces. HT-29 cells grown on glass slides were subjected to different rates of WSS in a laminar flow chamber. Tyr-phosphorylated FAK were measured after 60 minutes of flow exposure. Experiments were performed three times and one representative example is shown.
Reduced Expression of FAK Using Antisense Oligonucleotides
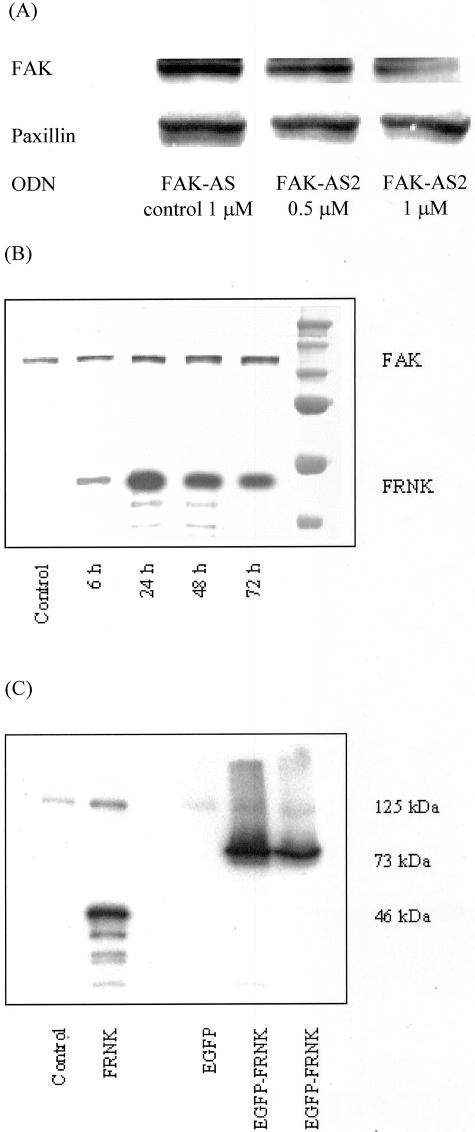
Transient transfection was performed with different concentrations of ODN and various incubation times. Using 1 μg/ml FAK-AS2, a decrease of FAK expression was found starting after 24 hours, but maximal effects were seen after 36 hours of incubation. Longer incubation (72 hours) resulted in an occurrence of cell death (>10%, determined by dye exclusion method) without further reduction of FAK expression. In contrast, FAK-AS control led only to minimal effects on FAK expression. Densitometrical comparison of FAK protein expression after FAK-AS2 treatment revealed protein expression of FAK was reduced to less than 30% after 36 hours, whereas FAK-AS control resulted in 88% of FAK expression. These effects were concentration-dependent (Figure 2A). As an internal control the expression level of the focal adhesion protein paxillin was not significantly influenced. For further experiments, cells were pretreated with 1 μg/ml ODN for 36 hours in serum-free medium using Lipofectin. This was consistent with antisense-mediated reduction of FAK using ODN against a different part of the FAK sequence.49
Figure 2.
Inhibition of FAK function or expression. A: HT-29 cells were incubated with 0.5–1 μmol/L FAK-AS2 or FAK-AS control oligonucleotides using Lipofectin (10 μl/ml) in serum-free DME-F12 medium for 36 hours. Expression of FAK was determined using Western blotting. In antisense-treated cells expression of FAK was significantly reduced to less than 30% compared to untreated cells (P < 0.05 from four experiments), whereas mismatch-control treatment had only slight effects (88%) on FAK protein amounts. In contrast, the focal adhesion protein paxillin was not affected. B: Transient transfection of Hep-G2 cells resulted in overexpression of FRNK after 24 to 72 hours as determined by Western blot using monoclonal antibodies against the C-terminal region of FAK. Endogeneous FRNK has not been detected in these cells. C: The 46 kd-FRNK fragment was found using pcDNA3.1 plasmids, where the EGFP-FRNK plasmid produced a 73-kd fragment. Control cells were transfected with genuine plasmids.
Overexpression of FRNK and EGFP-FRNK
A transient transfection with a successful expression of FRNK and EGFP-FRNK in Hep-G2 or HT-29LMM cells was performed to avoid clonal selection for further experiments. For detection of transfection effectivity, an anti-FAK antibody was used that cross-reacts with FAK (125 kd) as well as FRNK (46 kd) and EGFP-FRNK (73 kd). In Figure 2B, the time-dependence of FRNK expression for 6 to 72 hours is shown for Hep-G2 cells. FRNK and EGFP-FRNK were well expressed after 24 hours which was stable for approximately 48 hours. (Figure 2C). Transfection rates for EGFP-FRNK were 60 to 70% as verified by FACS analysis (data not shown). Endogenous FRNK was not detected in cell lysates.
Toxicity of FRNK expression was determined using BrdU-proliferation assays and annexin-apoptosis detection after 48 and 72 hours. Transient transfection slightly reduced cell division, but differences between transfection with the genuine EGFP-plasmid and the EGFP-FRNK transfection were not found. Similarly, apoptosis was unspecifically affected by the plasmid (3.3 ± 0.8% versus 12.9 ± 1.7%), but overexpression of FRNK did not result in further effects (11.9 ± 1.2%) (not shown).
FAK Is Not Required for Static Cell Adhesion to Collagens
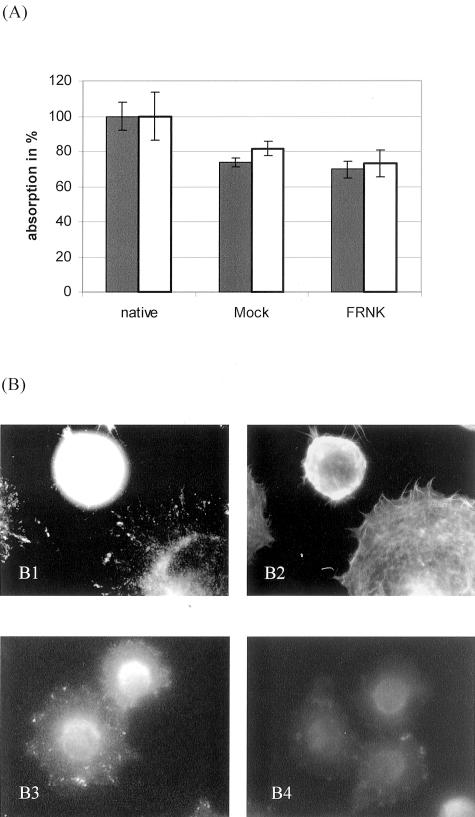
Using the microtiter plate assay, HT-29LMM cells showed substantial time-dependent adhesion to collagens under static conditions. Maximum values were reached after 60 to 90 minutes. After more than 120 minutes, adhesion began to decrease, probably because of the release of proteases and degradation of ECM.5 Similar results were obtained using HT-29LMM cells treated with antisense or mismatch-control ODN. Significant differences in adhesive properties to C I or C IV under these conditions were not observed if expression of FAK was reduced (not shown). If FRNK was hyperexpressed in Hep-G2 cells the adhesive behavior to collagen was not significantly altered. Similar adhesion rates to C I and C IV under static conditions were found compared to control transfected cells after 10 to 60 minutes. (Figure 3A) Some of these experiments were repeated using laminin or fibronectin as adhesive substrates and differences between the ECM components were not found (not shown). Furthermore, FRNK-transfected cells did not show cell spreading or adhesion-mediated hyperphosphorylation of FAK within 10 to 60 minutes (Figure 3B).
Figure 3.
Static cell adhesion and spreading of FRNK-transfected cells. A: Hep-G2 cells transfected with EGFP-plasmid (//) or pcDNA3.1-plasmid () containing FRNK or in its genuine form (mock) were allowed to adhere to collagen IV in microtiter plate assays for 30 minutes. Significant differences in the numbers of adherent cells were not found between FRNK- and control-transfected cells. Values are shown as mean ± SD from quadruplicate experiments. Comparable results were obtained using collagen I or colon carcinoma cells (not shown). B: Hep-G2 cells adherent to collagen IV for 30 minutes were fluorescence-stained for FAK (epitope within FRNK-fragment, B1) and F-actin (phalloidin, B2) in a double-labeling technique. The cell expressing FRNK remained round without cell spreading and reorganization of the actin cytoskeleton. In addition, FRNK-transfected cells did not show adhesion-specific phosphorylation of FAK (B4), whereas mock-transfected cells demonstrated typical focal adhesions with phosphorylated Tyr397 residues of FAK (B3). This inhibition was confirmed by Western blot (not shown).
FRNK and Reduced Expression of FAK Decrease Dynamic Cell Adhesion
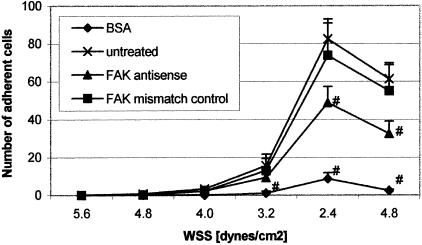
For investigation of FAK-related alterations of dynamic adhesive properties, we examined adhesion of HT-29LMM cells where FAK expression was reduced or endogenously inhibited. Treatment of cells with FAK-AS2 or FAK-AS control did not affect initial cell-ECM interactions and rolling of cells under flow conditions. Short-term cell sticking without definitive adhesion was also found to be similar. The flow rates or WSS, respectively, where FAK-AS2 and FAK-AS control-treated cells began to adhere to C I- or C IV-coated surfaces were similar, and significant differences between WSAT values were not found. However, using lower fluid flow or reduced shear forces, which represented lower WSS, the numbers of adherent cells were significantly reduced if expression of FAK was inhibited (Figure 4). The rates of adherent cells at low wall shear stress WSS (dynamic adhesion rates DAR) but not adhesion stabilization rates (ASR) demonstrated highly significant differences between FAK-AS2 and control cells (Table 1). Almost identical results were obtained if HT-29LMM were pretreated with the PTK-inhibitor Genistein using different concentrations that were able to inhibit adhesion-mediated Tyr-phosphorylation of FAK (Table 2). In contrast, other PTK inhibitors, such as the Erbstatin analog, Tyrphostin25 or Tyrphostin47, that were unable to modify FAK phosphorylation did not demonstrate any effect on dynamic adhesion of HT-29LMM cells (not shown).
Figure 4.
Effect of reduced FAK expression on dynamic cell adhesion to ECM. HT-29 cells treated with FAK antisense or mismatch-control oligonucleotides were used for adhesion assays in a laminar flow chamber. BSA was used as nonspecific adhesion substrate. Values are shown as mean ± SD from five different experiments. Numbers of adherent cells were compared to dynamic adhesion of untreated cells to C I using Student’s t-test (#, P < 0.05). Similar results were obtained using C IV as adhesive substrate.
Table 1.
Reduced Expression of FAK Decreases Dynamic Adhesion Rate to Collagen
| Substrate | Oligonucleotide | WSAT [dynes/cm2] | DAR [cells] | ASR [%] |
|---|---|---|---|---|
| BSA | 2.9 ± 0.5* | 9 ± 3* | 18 ± 19* | |
| C I | 4.6 ± 0.4 | 83 ± 10 | 74 ± 4 | |
| C I | FAK-AS2 | 4.2 ± 0.4† | 49 ± 9‡ | 66 ± 3† |
| C I | FAK-AS control | 4.2 ± 0.4† | 74 ± 17† | 74 ± 6† |
Wall shear adhesion threshold (WSAT), dynamic adhesion rate (DAR) and adhesion stabilization rate (ASR) were calculated as described. Values are given as mean ± SD from five different experiments. Expression of FAK in HT-29 cells was reduced using antisense ODN (FAK-AS2) or mismatch control ODN (FAK-AS control). Statistical analysis (Student’s t-test) was performed for BSA-versus C I-coated surfaces, and untreated versus treated cells.
P < 0.001;
not significant;
P < 0.05.
Table 2.
Genistein-Mediated Reduced Dynamic Adhesion Rate to Collagen
| Substrate | Inhibitor | WSAT [dynes/cm2] | DAR [cells] | ASR [%] |
|---|---|---|---|---|
| BSA | 3.4 ± 0.7* | 20 ± 13* | 35 ± 25* | |
| C I | 4.5 ± 0.7 | 148 ± 43 | 73 ± 12 | |
| C I | Genistein 10 μM | 4.3 ± 0.7† | 63 ± 14* | 79 ± 9† |
| C I | Genistein 100 μM | 4.1 ± 0.8† | 68 ± 39‡ | 72 ± 15† |
Wall shear adhesion threshold (WSAT), dynamic adhesion rate (DAR) and adhesion stabilization rate (ASR) were calculated as described. Values are given as mean ± SD from five different experiments. Cells were pretreated using different concentrations of Genistein. Statistical analysis (Student’s t-test) was performed for BSA-versus C I-coated surfaces, and untreated versus treated cells.
P < 0.001;
not significant,
P < 0.05.
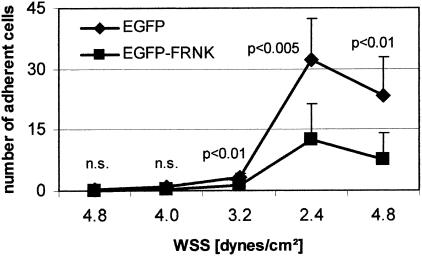
Comparable to the antisense-mediated down-regulation of FAK expression of FRNK resulted in a significantly reduced dynamic cell adhesion (Figure 5), although initial interactions were not affected (WSAT: 3.87 ± 0.6 versus 3.3 ± 0.3 dynes/cm2; P = 0.086). The ability of cells to stabilize their adhesions to collagen, however, was significantly inhibited by FRNK expression (ASR: 71 ± 8% versus 55 ± 15%; P < 0.05), and crawling of cells along the coated surfaces was regularly seen in FRNK-transfected cells.
Figure 5.
FRNK negatively regulates dynamic cell adhesion to collagen. Hep-G2 cells transfected with genuine EGFP-vector or EGFP-FRNK were used for dynamic adhesion assays on C I. Values are shown as mean ± SD from six different experiments. WSAT was not significantly affected (4.7 ± 0.6 dyn/cm2 versus 4.1 ± 0.3 dyn/cm2), but DAR (7.8 ± 6.8 cells; P < 0.005) and ASR (71 ± 7% versus 55 ± 15%; 0.05) were significantly reduced in FRNK-transfected cells. Similar results were obtained using C IV, but total numbers of cells were smaller, if this ECM component was used (not shown).
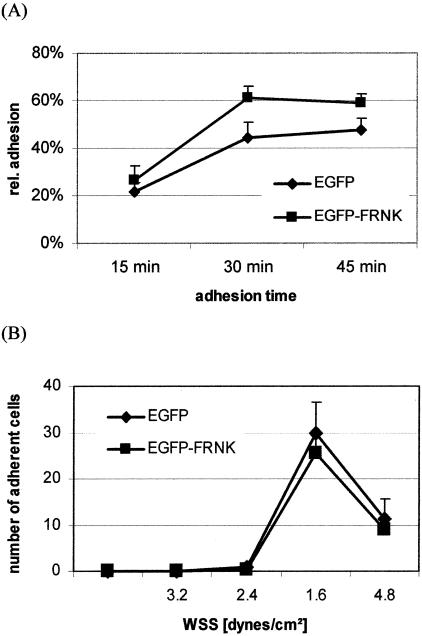
FAK-Independent Cell Adhesion to Endothelial Cells
FRNK-transfected Hep-G2 cells were compared for their static cell adhesion to HUVEC-coated surfaces. After 15 to 45 minutes FRNK-expressing cells and EGFP-control transfected cells showed comparable adhesion rates, but FRNK cells were slightly more adherent to HUVEC after 30 and 45 minutes (P < 0.005) (Figure 6A). Under hydrodynamic conditions Hep-G2 cells demonstrated cell adhesion only at low flow conditions representing the fact that selectin-mediated adhesive bonds are less stable than integrin-mediated contacts. Therefore, the low flow interval was used at WSS of 1.6 dynes/cm2. However, the adhesive behavior under these conditions was also comparable between FRNK-transfected and control cells and wall shear adhesion threshold WSAT, dynamic adhesion rates DAR or adhesion stabilization rates ASR did not show significant differences (Figure 6B).
Figure 6.
FAK is not required for tumor cell adhesion to endothelial cells. Hep-G2 cells transfected with genuine EGFP-vector or EGFP-FRNK were used for (A) static or (B) dynamic adhesion assays on HUVEC in vitro. Values are shown as mean ± SD from five different experiments. FRNK did not affect dynamic adhesion, but cell adhesion under static conditions was significant higher in FRNK-transfected cells (P < 0.005).
Regulation of Metastatic Cell Adhesion in Vivo by FAK
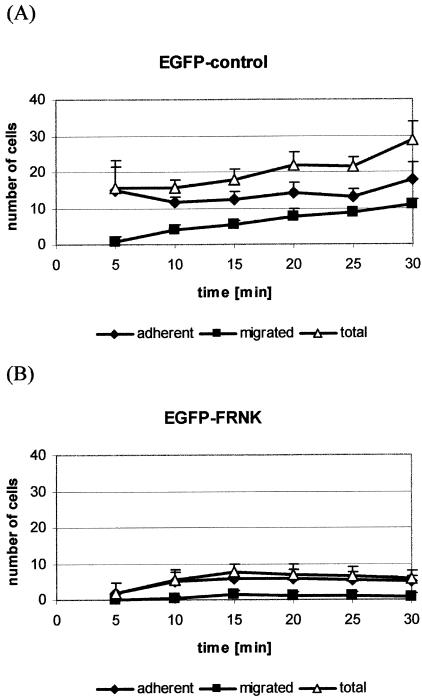
This results suggested that FAK is required for integrin-mediated cell adhesion under the influence of shear forces comparable to the conditions for metastatic cell adhesion during hetogenous formation of distant metastases. Since metastatic tumor cell adhesion in vivo can be mediated by integrin interactions with ECM components that are directly accessible for circulating tumor cells in the space of “Disse”, we used our in vivo model to evaluate the role of FAK for cell arrest in the hepatic microcirculation under physiological conditions. Considering the transfection rates of Hep-G2 cells expressing EGFP, adhesive interactions of these cells within the liver microcirculation was slightly reduced compared to untreated cells. However, the localization of cell adhesions, remaining vessel lumen of affected sinusoids and the time course of increasing numbers of adherent cells during the first ∼15 minutes and early migration into the hepatic parenchyma starting within 5 minutes after cell injection were not influenced by EGFP transfection. In contrast, if cells expressed FRNK we observed a dramatic reduction in their ability to arrest within the sinusoids. The numbers of adherent and total numbers of arrested cells were highly significantly lower than EGFP-transfected cells (P < 0.0001). In addition, cell migration into the parenchyma was completely impaired by FRNK-expression (P < 0.001) (Figure 7).
Figure 7.
In vivo cell adhesion within the hepatic microcirculation. Hep-G2 cells transfected with (A) genuine EGFP-vector or (B) EGFP-FRNK were used for the intravital observation of circulating tumor cells within the liver sinusoids. Numbers of adherent cells, migrated cells and the total number of arrested cells (adherent + migrated) are shown as mean ± SD from seven animals in each group. Expression of FRNK almost completely inhibited adhesive interactions of circulating Hep-G2 cells within the liver.
Discussion
Various studies have shown that integrin-mediated interactions between tumor cells and ECM components within host organs appear to be crucial for the organ-specific formation of distant metastases.6,50,51 For example, integrin expression can profoundly influence metastatic tumor progression in various tumor entities.52–55 The mechanisms by which integrins take part in various steps of these processes in vivo during blood-borne metastasis formation are only partially understood and appear to involve changes in tumor cell binding affinities and signal transduction into the cells.56,57 Intravital microscopy technologies have been recently used to investigate this metastatic tumor cell adhesion within host organ microcirculation, such as in liver and lung, and their invasion into the host organ parenchyma.58–62 In these studies contradictory results were reported regarding the type of entrapment (mechanical entrapment versus active cell adhesion) and the requirement of invasion into host organ parenchyma (invasion versus intravascular proliferation). Using colon carcinoma cells our group observed a lack of mechanical entrapment in rat liver sinusoids and a rapid extravasation into the liver parenchyma,48 suggesting that active regulation of adhesion molecules is involved in organ-specific colonization of host organs. As described above circulating tumor cells appear to be able to directly bind to ECM components in the space of “Disse” via different types of integrins (laminin- and fibronectin-binding integrins), whereas other integrin heterodimers seem to be mainly involved in the migration into host organ parenchyma (collagen-binding integrins). These observations suggested that the regulation of integrin function, their affinity and/or avidity, might have an important role in the organ-specific colonization of distant organs by metastasizing tumor cells. FAK is a very important regulator of “outside-in” integrin-mediated cell signaling and may also regulate these “inside-out” integrin functions.
In our study we provide in vitro and in vivo evidence that FAK is required for integrin-mediated direct tumor cell adhesion within the liver, a major metastatic target organ. Since down-regulation of FAK in different tumor cell entities did not inhibit adhesive interactions between these tumor cells and EC, the loss of cell adhesions in FAK-inhibited cells appears to support our previous findings that direct integrin-mediated tumor cell adhesions with ECM components in the space of “Disse” can occur that are regulated by integrin-mediated signaling. We have previously found that adhesive interactions of colon carcinoma cells with ECM-coated surfaces under laminar flow conditions proceed in discrete steps: cell rolling, cell sticking or initial adhesion, and stabilization of cell adhesion.36 Initial steps seem to be severly influenced by biophysical factors, and modulation of cell deformability and rigidity can determine the effectiveness of tumor cell arrest within host organ microcirculation.7 Later events showed increasing receptor specificity and were apparently mediated, at least in part, by the same integrins as demonstrated in static adhesion assays.5
The functional status of integrins, which are known for their activation or affinity to bind to ECM components, is regulated by complex interactions with a number of cytosolic, cytoskeletal, and membrane-bound proteins.63 Cells can modulate integrin-binding affinity and kinetics of interactions between integrin receptors and their adhesive ligands.9 Using different integrin chimeras it was shown that this “activation” of integrins is related to only a limited number of intracellular factors, such as FAK,9 but signal transduction is apparently required both for the adhesion itself and for further steps during tumor cell host organ colonization.64 Surprisingly, reduced expression of FAK or its competitive inhibition by FRNK did not influence adhesive properties under static conditions. However, similar to the in vivo results with the almost complete loss of metastatic cell adhesions the numbers of adherent cells and adhesion stabilization were significantly reduced under hydrodynamic fluid flow in vitro, whereas initial cell binding was not different. Fewer cells were able to withstand shear forces and form definite cell attachments, even if the WSS was in the lower range of microcirculatory fluid flow (<3 dynes/cm2). These results were confirmed by inhibition of FAK kinase activity using Genistein in our experiments and by others,65 whereas other PTK inhibitors did not change adhesive properties.
In contrast to static cell adhesion, biophysical factors can influence adhesion processes under flow conditions as they occur within the microcirculation of host organs.66 First, flow velocity determines available time for cell adhesion. The initial areas of cell contacts to the vessel walls are very small because of the more or less spherical shape of flowing tumor cells and the fact that initial cell contacts may occur via cell protrusions or filopodia. Alterations in cell shape and morphology (flattening of cells) are important for increasing the areas of cell-cell contacts. Additionally, cell flattening enables adherent cells to avoid high shear forces closer to the center of the parabolic curve of fluid flow. These factors as well as the character of flow are responsible for the strength of the shear forces acting against cell adhesion. Flow rate and viscosity of the perfusate are the main factors determining strength of cell shear stress.67 In addition to these biophysical factors, shear stress and tension alone can induce functional reactions in circulating cells, such as leukocytes and platelets, or EC.30,68
In accordance with these reports in hematological cells, our results suggest that hydrodynamic fluid flow comparable to the microcirculation can induce changes in cell signaling in adherent epithelial tumor cells. The focal adhesion protein FAK showed increased Tyr-phosphorylation after a relatively short exposure to low shear forces. Similar results were previously found for EC and other circulating blood cells, but most studies used longer application times or higher shear forces up to 100 dynes/cm2. For example, shear stress can directly activate different signaling cascades in EC.69–71 Using bovine aortic EC, Li et al72 provided evidence that this phosphorylation of FAK appears to have an important role in shear-mediated signaling. Moreover, the organization and structure of cytoskeletal components, such as actin, can also be modified by shear stress.73 Other studies demonstrated differences in adhesive properties of platelets between static and dynamic conditions.74 In a previous study we have also shown similar differences for tumor cells adhesion to ECM components.5 Our results suggest that comparable to cells physiologically exposed to shear forces of blood flow, circulating tumor cells can also respond to wall shear stress under hydrodynamic conditions by changes in Tyr-phosphorylation of FAK which mainly occur at the Tyr397-residue.7 This responsiveness of FAK to external forces pointed to a specific role of this kinase for early steps of metastasis formation. FAK may therefore combine detection of external forces from the environment75 in metastasizing tumor cells with their cellular response for effective adhesion stabilization providing the basis for further steps of metastasis formation. Comparable to its role for cell motility and cell migration where FAK is an important regulator of mechanotransduction and focal adhesion turnover, this kinase also appears to be required for adhesion stability in circulating tumor cells.
In this study we presented the first evidence that FAK seems to be directly involved in early events of integrin-mediated metastatic tumor cell adhesion under dynamic conditions of fluid flow in vitro and in vivo. Our experiments suggested that FAK might be an important PTK that is not only involved in adhesion-mediated down-stream signaling and focal adhesion disassembly, but also responsible for the establishment of stabilized adhesive interactions. These interactions enable circulating tumor cells to resist shear forces within the microcirculation of metastatic target organs that is likely a prerequisite for successful formation of distant metastases.
Footnotes
Address reprint requests to J. Haier, Molecular Biology Lab, Department of General Surgery, University Hospital Münster, Waldeyerstr. 1, 48149 Münster, Germany. E-mail: haier@uni-muenster.de.
Supported by grants to J.H. by the Deutsche Forschungsgemeinschaft (HA 2636/5–1) and the Westfälische Wilhelm-University Münster (IMF-project HA 1 2 01 01).
References
- Nicolson GL. Cancer metastasis: tumor cell and host properties important in metastasis to specific secondary sites. Biochim Biophys Acta. 1988;948:175–224. doi: 10.1016/0304-419x(88)90010-8. [DOI] [PubMed] [Google Scholar]
- Nicolson GL. Tumor and host molecules important in the organ preference of metastasis. Semin Cancer Biol. 1991;2:143–154. [PubMed] [Google Scholar]
- Nicolson GL. Metastatic tumor cell interactions with endothelium, basement membrane, and tissue. Curr Opin Cell Biol. 1989;1:1009–1019. doi: 10.1016/0955-0674(89)90073-2. [DOI] [PubMed] [Google Scholar]
- Sawada H, Wakabayashi H, Nawa A, Mora E, Cavanaugh PG, Nicolson GL. Differential motility stimulation but not growth stimulation or adhesion of metastatic human colorectal carcinoma cells by target organ-derived liver sinusoidal endothelial cells. Clin Exp Metastasis. 1996;14:308–314. doi: 10.1007/BF00053904. [DOI] [PubMed] [Google Scholar]
- Haier J, Nicolson GL. Tumor cell adhesion of human colon carcinoma cells with different metastatic properties to extracellular matrix under dynamic conditions of laminar flow. J Cancer Res Clin Oncol. 2000;126:699–706. doi: 10.1007/s004320000161. [DOI] [PubMed] [Google Scholar]
- Haier J, Nasralla M, Nicolson GL. Different adhesion properties of highly and poorly metastatic HT-29 colon carcinoma cells with extracellular matrix components: role of integrin expression and cytoskeletal components. Br J Cancer. 1999;80:1867–1874. doi: 10.1038/sj.bjc.6690614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb T, Schlüter K, Enns A, Spiegel HU, Senninger N, Nicolson GL, Haier J. Integrity of actin fibres and microtubules influences metastatic tumor cell adhesion. Exp Cell Res. 2004;299:236–247. doi: 10.1016/j.yexcr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Chen YP, O’Toole TE, Shipley T, Forsyth J, LaFlamme SE, Yamada KM. “Inside-out” signal transduction inhibited by isolated integrin cytoplasmic domains. J Biol Chem. 1994;269:18307–18310. [PubMed] [Google Scholar]
- Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Ann Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Richardson A, Parsons JT. Signal transduction through integrins: a central role for focal adhesion kinase? Bioessays. 1995;17:229–236. doi: 10.1002/bies.950170309. [DOI] [PubMed] [Google Scholar]
- Motamed K, Sage EH. SPARC inhibits endothelial cell adhesion but not proliferation through a tyrosine phosphorylation-dependent pathway. J Cell Biochem. 1998;70:543–552. doi: 10.1002/(sici)1097-4644(19980915)70:4<543::aid-jcb10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hemler ME, Elices MJ, Chan BMC, Zetter B, Matsuura N, Takada Y. Multiple ligand binding functions for VLA-2(α2β1) and VLA-3 (α3β1) in the integrin family. Cell Differ Dev. 1990;32:229–238. doi: 10.1016/0922-3371(90)90035-u. [DOI] [PubMed] [Google Scholar]
- Jewell K, Kapron-Bras C, Jeevaratnam P, Dedhar S. Stimulation of tyrosine phosphorylation of distinct proteins in response to antibody-mediated ligation and clustering of α3 and α6 integrins. J Cell Sci. 1995;108:1165–1174. doi: 10.1242/jcs.108.3.1165. [DOI] [PubMed] [Google Scholar]
- Guan JL, Trevithick JE, Hynes RO. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120 kDa protein. Cell Regul. 1991;2:951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F, Pepe A, Wary KK, Spinardi L, Mohammadi M, Schlessinger J. Signal transduction by the α6β4 integrin: distinct β4 subunit sites mediate recruitment of Shc/Grb2 and association with cytoskeleton of hemidesmosomes. EMBO J. 1995;14:4470–4481. doi: 10.1002/j.1460-2075.1995.tb00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Ino Y, Ochiai A, Kanai Y, Akimoto S, Hirohashi S. Formation of focal adhesion and spreading of polarized human colon cancer cells in association with tyrosine phosphorylation of paxillin in response to phorbol ester. Lab Invest. 1996;74:199–208. [PubMed] [Google Scholar]
- Gabarra-Niecko V, Schaller MD, Dunty JM. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003;22:359–374. doi: 10.1023/a:1023725029589. [DOI] [PubMed] [Google Scholar]
- Avizienyte E, Wyke AW, Jones RJ, McLean GW, Westhoff MA, Brunton VG, Frame MC. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat Cell Biol. 2002;4:632–638. doi: 10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Sieg DJ, Hsia DA, Loftus JC, Gaarde WA, Monia BP, Schlaepfer DD. Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factor-stimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res. 2001;61:7079–7090. [PubMed] [Google Scholar]
- Ren X, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113:3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK, and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci USA. 2001;98:11295–11300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Butler P, Wang Y, Hu Y, Han DC, Usami S, Guan JL, Chien S. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci USA. 2002;99:3546–3551. doi: 10.1073/pnas.052018099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Lallier TE. Measurement of cell-cell and cell-extracellular matrix interactions: a quantitative cell attachment assay. Celis JE, editor. New York: Academic Press; Cell BiologyA Laboratory Handbook. 1998:pp 302–309. [Google Scholar]
- Bierbaum S, Notbohm H. Tyrosine phosphorylation of 40 kDa proteins in osteoblastic cells after mechanical stimulation of α1-integrins. Eur J Cell Biol. 1998;77:60–67. doi: 10.1016/s0171-9335(98)80102-7. [DOI] [PubMed] [Google Scholar]
- Lawrence MB, McIntire LV, Eskin SG. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70:1284–1290. [PubMed] [Google Scholar]
- Lawrence MB, Smith CW, Eskin SG, McIntire LV. Effect of venous shear stress on CD18-mediated neutrophil adhesion to cultured endothelium. Blood. 1990;75:227–237. [PubMed] [Google Scholar]
- Giavazzi R, Foppolo M, Dossi R, Remuzzi A. Rolling and adhesion of human tumor cells on vascular endothelium under physiological flow conditions. J Clin Invest. 1993;92:3038–3044. doi: 10.1172/JCI116928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tözeren A, Kleinman HK, Wu S, Mercurio AM, Byers SW. Integrin α6β4 mediates dynamic interactions with laminin. J Cell Sci. 1994;107:3153–3163. doi: 10.1242/jcs.107.11.3153. [DOI] [PubMed] [Google Scholar]
- Menter DG, Patton JT, Updyke TV, Kerbel RS, Maamer M, McIntire LV, Nicolson GL. Transglutaminase stabilizes melanoma adhesion under laminar flow. Cell Biophys. 1991;18:123–143. doi: 10.1007/BF02989810. [DOI] [PubMed] [Google Scholar]
- Yun Z, Smith TW, Menter DG, McIntire LV, Nicolson GL. Differential adhesion of metastatic RAW117 large-cell lymphoma cells under static or hydrodynamic conditions: role of the αvβ3 integrin. Clin Exp Metastasis. 1997;15:3–11. doi: 10.1023/a:1018451616309. [DOI] [PubMed] [Google Scholar]
- Haier J, Nasralla M, Nicolson GL. α1-integrin mediated dynamic adhesion of colon carcinoma cells to extracellular matrix under laminar flow. Clin Exp Metastasis. 1999;17:377–388. doi: 10.1023/a:1006658414040. [DOI] [PubMed] [Google Scholar]
- Nolan K, Lacoste J, Parsons JT. Regulated expression of focal adhesion kinase-related nonkinase, the autonomously expressed C-terminal domain of focal adhesion kinase. Mol Cell Biol. 1999;19:6120–6129. doi: 10.1128/mcb.19.9.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Parsons JT. Autonomous expression of a nonkatalytic domain of the focal adhesion-associated protein tyrosine kinase pp125FAK. Mol Cell Biol. 1993;13:785–791. doi: 10.1128/mcb.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, Mack CP, Nolan K, Regan CP, Owens GK, Parsons JT. Selective expression of an endogenous inhibitor of FAK regulates proliferation and migration of vascular smooth muscle cells. Mol Cell Biol. 2001;21:1565–1572. doi: 10.1128/MCB.21.5.1565-1572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Shannon JD, Adams RB, Schaller MD, Parsons JT. Identification of integrin-stimulated sites of serine phosphorylation in FRNK, the separately expressed C-terminal domain of focal adhesion kinase: a potential role for protein kinase A. Biochem J. 1997;324:141–149. doi: 10.1042/bj3240141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Hsia DA, Puente XS, Cheresh DA, Schlaepfer DD. FRNK blocks v-Src-stimulated invasion and experimental metastases without effects on cell motility or growth. EMBO J. 2002;21:6289–6302. doi: 10.1093/emboj/cdf631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Malik RK, Hildebrand JD, Parsons JT. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpession of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidkamp MC, Bayer AL, Kalina JA, Eble DM, Samarel AM. GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes. Circ Res. 2002;90:1282–1289. doi: 10.1161/01.res.0000023201.41774.ea. [DOI] [PubMed] [Google Scholar]
- Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- Slack JK, Adams RB, Rovin JD, Bissonette EA, Stoker C, Parsons JT. Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene. 2001;20:1152–1163. doi: 10.1038/sj.onc.1204208. [DOI] [PubMed] [Google Scholar]
- Yamada KM. Cell adhesion. Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz, Yamada KM, editors. New York: Wiley & Sons; Current Protocols in Cell Biology. 1998 [Google Scholar]
- Haier J, Korb T, Hotz B, Spiegel HU, Senninger N. An intravital model to monitor steps of metastatic tumor cell adhesion within the hepatic microcirculation. J Gastrointest Surg. 2003;7:507–515. doi: 10.1016/S1091-255X(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Naruse K, Yamada T, Sai XR, Hamaguchi M, Sokabe M. pp125FAK is required for stretch-dependent morphological response of endothelial cells. Oncogene. 1998;17:455–463. doi: 10.1038/sj.onc.1201950. [DOI] [PubMed] [Google Scholar]
- Kemperman H, Wijnands YM, Meijne AM, Roos E. TA3/St, but not TA3/Ha, mammary carcinoma cell adhesion to hepatocytes is mediated by alpha 5 beta 1 interacting with surface-associated fibronectin. Cell Adhes Commun. 1994;2:45–58. doi: 10.3109/15419069409014201. [DOI] [PubMed] [Google Scholar]
- Kemperman H, Wijnands YM, Roos E. αv integrins on HT-29 colon carcinoma cells: adhesion to fibronectin is mediated solely by small amounts of αvβ6, and αvβ5 is codistributed with actin fibers. Exp Cell Res. 1997;234:156–164. doi: 10.1006/excr.1997.3599. [DOI] [PubMed] [Google Scholar]
- Gulubova MV. Expression of cell adhesion molecules, their ligands and tumour necrosis factor alpha in the liver of patients with metastatic gastrointestinal carcinomas. Histochem J. 2002;34:67–77. doi: 10.1023/a:1021304227369. [DOI] [PubMed] [Google Scholar]
- Liu LX, Jiang HC, Liu ZH, Zhou J, Zhang WH, Zhu AL, Wang XQ, Wu M. Integrin gene expression profiles of human hepatocellular carcinoma. World J Gastroenterol. 2002;8:631–637. doi: 10.3748/wjg.v8.i4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejjari M, Hafdi Z, Gouysse G, Fiorentino M, Beatrix O, Dumortier J, Pourreyron C, Barozzi C, D’errico A, Grigioni WF, Scoazec JY. Expression, regulation, and function of alpha V integrins in hepatocellular carcinoma: an in vivo and in vitro study. Hepatology. 2002;36:418–426. doi: 10.1053/jhep.2002.34611. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, Mueller BM. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci USA. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- Horwitz AR, Werb Z. Cell-to-cell contacts and extracellular matrix. Cell adhesion and the extracellular matrix: recent progress and emerging themes. Curr Opin Cell Biol. 1998;10:563–565. [Google Scholar]
- Chambers AF, Schmidt EE, MacDonald IC, Morris VL, Groom AC. Early steps in hematogenous metastasis of B16F1 melanoma cells in chick embryos studied by high-resolution intravital microscopy. J Natl Cancer Inst. 1992;84:797–803. doi: 10.1093/jnci/84.10.797. [DOI] [PubMed] [Google Scholar]
- Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2000;6:100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- Koop S, MacDonald IC, Luzzi K, Schmidt EE, Morris VL, Grattan M, Khokha R, Chambers AF, Groom AC. Fate of melanoma cells entering the microcirculation: over 80% survive and extravasate. Cancer Res. 1995;55:2520–2523. [PubMed] [Google Scholar]
- Naumov GN, Wilson SM, MacDonald IC, Schmidt EE, Morris VL, Groom AC, Hoffman RM, Chambers AF. Cellular expression of green fluorescence protein, coupled with high-resolution in-vivo microscopy, to monitor steps in tumor metastasis. J Cell Science. 1999;112:1835–1842. doi: 10.1242/jcs.112.12.1835. [DOI] [PubMed] [Google Scholar]
- Ito S, Nakanishi H, Ikehara Y. Real-time observation of micrometastasis formation in the living mouse liver using green fluorescent protein-tagged rat tongue carcinoma cell line. Int J Cancer. 2001;93:212–217. doi: 10.1002/ijc.1318. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol. 1995;7:681–689. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]
- Schmidt CE, Horwitz AF, Lauffenburger DA, Sheetz MP. Integrin-cytoskeletal interactions in migrating fibroblasts are dynamic, asymmetric, and regulated. J Cell Biol. 1993;123:977–991. doi: 10.1083/jcb.123.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan R, Kyle E, Nguyen P, Trepel J, Ingui C, Neckers L. Genistein-stimulated adherence of prostate cancer cells is associated with the binding of focal adhesion kinase to β1-integrin. Clin Exp Metastasis. 1996;14:389–398. doi: 10.1007/BF00123398. [DOI] [PubMed] [Google Scholar]
- McIntire LV. Bioengineering and vascular biology. Ann Biomed Engineering. 1994;22:2–13. doi: 10.1007/BF02368217. [DOI] [PubMed] [Google Scholar]
- Weiss L. Biomechanical interactions of cancer cells with the microvasculature during hematogenous metastasis. Cancer Metastasis Rev. 1992;11:227–235. doi: 10.1007/BF01307179. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, Sheetz MP. Forces on adhesive contacts affect cell function. Curr Opin Cell Biol. 1998;10:566–571. doi: 10.1016/s0955-0674(98)80030-6. [DOI] [PubMed] [Google Scholar]
- Ishida T, Peterson TE, Kovach NL, Berk BC. MAP kinase activation by flow in endothelial cells: role of beta1 integrins and tyrosine kinases. Circ Res. 1996;79:310–316. doi: 10.1161/01.res.79.2.310. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Berk BC. Mitogen-activated protein kinase (ERK1/2) activation by shear stress and adhesion in endothelial cells: essential role for a herbimycin-sensitive kinase. J Clin Invest. 1996;98:2623–2631. doi: 10.1172/JCI119083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Ishida T, Traub O, Corson MA, Berk BC. Mechanotransduction in endothelial cells: temporal signaling events in responses to shear stress. J Vasc Res. 1997;34:212–219. doi: 10.1159/000159225. [DOI] [PubMed] [Google Scholar]
- Li S, Kim M, Hu YL, Jalali S, Schlaepfer DD, Hunter T, Chiem S, Shyy YY. Fluid shear stress activation of focal adhesion kinase: linking to mitogen-activated protein kinases. J Biol Chem. 1997;272:30455–30462. doi: 10.1074/jbc.272.48.30455. [DOI] [PubMed] [Google Scholar]
- Okuyama M, Ohta Y, Kambayashi J, Monden M. Fluid shear stress induces actin polymerization in human neutrophils. J Cell Biochem. 1996;63:432–441. doi: 10.1002/(SICI)1097-4644(19961215)63:4%3C432::AID-JCB5%3E3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Verkleij MW, Morton LF, Knight CG, de Groot PG, Barnes MJ, Sixma JJ. Simple collagen-like peptides support platelet adhesion under static but not under flow conditions: interaction via alpha2 beta1 and von Willebrand factor with specific sequences in native collagen is a requirement to resist shear forces. Blood. 1998;91:3808–3816. [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]