Abstract
Pulmonary disorders are the most frequent cause of death in HIV-1-infected individuals with AIDS and remain important even in the current era of potent antiretroviral therapy. Macaques infected with Simian/Human Immunodeficiency Virus (SHIV) develop pulmonary disease and concurrent opportunistic infections similar to those observed in HIV-infected individuals, thereby providing an excellent working model to elucidate the pathogenesis of the human lung disease. Since chemokines play a crucial role in the recruitment of inflammatory cells to tissues, we investigated the relationship between respiratory disease and the levels of chemokines, monocyte chemotactic protein-1 (MCP-1) and CXCL10, in the lungs of SHIV-infected rhesus macaques. We found that lung pathology in infected macaques was closely associated with overexpression of MCP-1 and CXCL10. In addition, these chemokines could, in part, be responsible for the recruitment of inflammatory cells infiltrating into the diseased lungs as demonstrated by chemotactic assays. Lung pathology and increased levels of MCP-1 and CXCL10 correlated with high viral loads in the lung parenchyma. Using confocal microscopy, we identified SHIV-infected macrophages as the major producers of MCP-1 and CXCL10 in the diseased lungs. These data suggest that chemokine overexpression plays an important role in the pathogenesis of SHIV-associated pulmonary disease in macaques.
Pulmonary disorders remain an important complication of HIV infection, even in the current era of potent antiretroviral therapy,1 affecting 75 to 85% of AIDS patients.2 Pathological changes in these diseases are mainly associated with marked infiltration of inflammatory macrophages, T cells, and neutrophils into the tissues.3,4 These responses develop on a background of productive virus replication in the macrophage population and proliferation of opportunistic pathogens such as Pneumocystis carinii and cytomegalovirus (CMV). Chemokines are a group of small peptide cytokines that are chemotactic for various cell types.5 Chemokines usually play an important role in the control of infectious agents by recruiting effector cells to local inflammatory sites to eliminate the pathogens. However, this response fails in HIV infection since the chemokines produced by the inflammatory cells promote virus replication in macrophages and also promote recruitment of new macrophages that then become host cells for both the virus and common opportunistic pathogens.5 In contrast to self-limiting infections where chemokine production ceases after the infectious agent is eliminated, chemokine production continues throughout the course of HIV infection.6,7
We used the SHIV/rhesus macaque model to investigate the relationship between SHIV infection and changes in chemokine expression. The two chemokines, monocyte chemotactic protein-1 (MCP-1) and CXC chemokine ligand 10 (CXCL10), were chosen in the present study since both chemokines were found to be dramatically up-regulated by microarray analysis in the lungs of macaques with SHIV-pneumonia as compared to lungs of SHIV-infected macaques without pneumonia (unpublished data). SHIV-infected macaques that developed fatal lung disease had subtotal loss of CD4+T cells and severe pneumonia characterized by highly productive virus replication in macrophages, proliferation of opportunistic pathogens, such as P. carinii, CMV, or adenovirus, and massive infiltration of mononuclear cells into the lung parenchyma. Chemokines such as MCP-1 and CXCL10 play a pivotal role in the recruitment of macrophages and T cells to specific tissues such as the brain where the virus undergoes extensive replication during end-stage AIDS.8–12 Inflammatory macrophages in the tissue then provide a source of host cells for supporting viral replication. In the present study, we hypothesized that both chemokines may also be involved in the recruitment of inflammatory cells in the lungs of SHIV-infected macaques, thereby leading to the development of pneumonia. The data presented in this study showed that lung pathology was closely associated with overexpression of MCP-1 and CXCL10 and that the macrophages in the lungs were the major source of production of these two chemokines. Lung disease also correlated with high viral loads in the lung parenchyma. Based on these findings, we suggest that chemokine overexpression is crucial for the development of SHIV-associated pneumonia and could be a target for therapeutic approaches.
Materials and Methods
Studies on Archival Lung Tissues
Animals used in these studies were housed in Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited facilities and all studies were reviewed and approved by the Kansas University Medical Center’s Institutional Animal Care and Use Committee. Archival lung tissues from three uninfected and 12 SHIV (SHIV89.6P or SHIVkU-2)-infected rhesus macaques were used in this study. In addition, one rhesus macaque that died of aspiration pneumonia (not AIDS-related) was also used as a control. Of the 12 infected macaques, macaque PHP had controlled the virus and did not develop either AIDS or pulmonary disease. All of the other infected macaques developed AIDS-defining illnesses and were euthanized using pre-determined clinical criteria. Details of viral inoculation, disease course, processing of tissue samples, and histological analysis of the tissues have been described earlier.13 The animals were deeply anesthetized, exsanguinated, and perfused with lactated Ringers solution to remove blood that might interfere with quantitative virologic assays. Samples of lung tissue obtained from the apical, cardiac, and diaphragmatic lobes of each lung were fixed in 4% paraformaldehyde. Paraffin sections of lungs were stained with hematoxylin and eosin (H&E) as a preliminary screen for morphological abnormalities.
Fluorescence-Activation Cell Sorting Analysis
Samples of peripheral blood mononuclear cells were reacted with fluorescin-tagged monoclonal antibodies to CD3+, CD4+, and CD8+, (Dako, Carpenteria, CA)14 and analyzed in a fluorescence-activated cell counter FACS Calibur System (Becton-Dickinson, San Jose, CA).
Assessment of Viral RNA in Plasma
Plasma viral RNA concentrations were assessed as previously described.15 Briefly, virus was pelleted from 1 ml EDTA-anticoagulated clarified plasma for 1 hour at 4°C, at 20,000 × g. Viral RNA was then extracted with the QIAgen Viral RNA Minikit (Qiagen, Valencia, CA). The RNA was precipitated and the pellet was resuspended in 140 μl PBS. RNA samples were subjected to real-time RT-PCR using gag primers and a Taqman Probe (5′ flurescence reporter dye 6-carboxy-flurescencein and 3′quencher dye 6-carboxytetramethylrhodamine)16 with an ABI Prism 7700 Sequence Detection System (Foster City, CA). Thermal cycling conditions consisted of 60°C for 30 minutes; 95°C for 10 minutes; followed by 44 cycles of 95°C for 15 seconds and 60°C for 30 seconds. Prime RNase inhibitor was used in the reactions (7.5U, Brinkmann-Eppendorf, Westbury, NY) and reaction volume was 25 μl. Viral RNA equivalents were calculated for 1 ml plasma. The sensitivity of the analysis was 18 copies.
Viral mRNA Quantitation
Total RNA from the three regions of the lungs was extracted with Trizol (Invitrogen, Carlsbad, CA).13 Viral gag mRNA was quantified using real-time RT-PCR from total RNA isolated from lung tissues. Gag mRNA was determined using the Taqman probe and primers as described above for the plasma viral RNA determinations during 44 cycles of RT-PCR (ABI). As a measure of cellular mRNA levels, the GAPDH mRNA copy numbers in the lung RNA samples were also determined by a real-time RT-PCR Taqman assay over 40 cycles (ABI). mRNA numbers based on the use of constant standards were determined in the gag mRNA assays, and since the amplification efficiencies of the gag and GAPDH targets can be considered essentially equal [differences in the slopes (ΔS) of the standard curves was within 0.2], the gag mRNA levels were normalized to cellular GAPDH mRNA number.
RT-PCR Analysis for CXCL10 and MCP-1
The primer sets for MCP-1, CXCL10, IFN-γ, and β-actin have been described previously.17 The access RT-PCR system (Promega, Madison, WI) was used as per the manufacturers’ instructions. The reactions were carried out in a Perkin-Elmer DNA Thermal Cycler 480 with a temperature profile of 48°C for 45 minutes, one cycle; 94°C for 2 minutes, one cycle; 94°C for 30 seconds, 60°C for 1 minute, 68°C for 2 minutes, 25 cycles; 68°C for 7 minutes. RT-PCR products were resolved by electrophoresis in 1% agarose/TAE gels and visualized with ethidium bromide staining. All PCR products were normalized relative to the β-actin signal.
Lung Protein Extracts and Chemokine ELISA Analysis
Trizol reagent was used to extract protein from lung tissues according to the manufacturers’ recommendations. Briefly, pieces of rapidly frozen lung tissue were homogenized in 1 ml of Trizol, followed by protein precipitation after the RNA and DNA were removed from the samples. After extensive washing in a solution containing 0.3 mol/L guanidine hydrochloride in 95% ethanol, the protein pellet was redissolved in either PBS or 1% SDS. Protein concentration was determined by using BCA assay (Bio-Rad).
MCP-1 and CXCL10 ELISA kits (R&D Systems, Minneapolis, MN) were used to determine the MCP-1 and CXCL10 protein levels from the lung samples. The detection limit for MCP-1 and CXCL10 by ELISA was 5 pg/ml.
Chemotactic Activity of Lung Protein Extract
Disposable ChemoTX microplate (NeuroProbe, Cabin John, MD) with a lower well volume of 30 μl was used in this study. The microplate is made up of two chambers. Thirty μl of lung protein extract from either the diseased or infected but disease-free macaques was added in the bottom chamber and five million PBMC resuspended in 50 μl volume of RPMI 1640 were added to the top chamber. For blocking experiments, MCP-1 neutralizing antibody (Bethyl, Inc., Montgomery, TX) and/or CXCL10 neutralizing antibody (R&D Systems) or control mouse IgG (Sigma) antibody were pre-incubated with lung protein extracts for 1 hour before the loading of PBMCs in the upper chamber. The microplate was then incubated at 37°C with 5% CO2 for 2 hours, following which, the cells in the upper chamber were removed by washing with PBS. Cells that had migrated to the lower chamber were counted by using Cell Titer 96 Aqueous One Solution Assay (Promega). All assays were performed in triplicate. Migration index was calculated as the number of cells migrating toward the protein extract from the diseased or control macaque lungs divided by the number of cells migrating toward PBS only.
Antibodies and Immunohistochemical Analyses
Immunohistochemical analysis was carried out on paraffin-fixed sections of lung. The paraffin sections were dewaxed, rehydrated in graded ethanol solutions, and then irradiated at 750 W in a microwave oven in 0.1 mol/L sodium citrate buffer (pH 6.0) for 1.5 minutes. After blockage with 1% milk in PBS, the sections were stained with primary/secondary antibodies. To detect the presence of a macrophage-specific marker, sections were treated with mouse monoclonal antibody, HAM56, (Dako) followed by treatment with biotinylated goat anti-mouse secondary antibody and peroxidase-conjugated streptavidin (Universal LSAB kit, Dako) and Nova Red substrate (Vector Laboratories, Burlingame, CA), which yielded a reddish reaction product.18 To detect the presence of Pneumocystis carinii, mouse monoclonal antibody (clone 3F6, from Lab Vision Corporation, Fremont, CA) was used as primary antibody to stain the paraffin sections as described above. For MCP-1/CXCL10 co-localization with macrophages, goat anti-MCP-1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or mouse anti-CXCL10 monoclonal antibody (BD PharMingen, San Diego, CA) was used separately as primary antibody to stain the sections, followed by treatment with Alexa Fluor 594-conjugated secondary antibody (Molecular Probes, Eugene, OR). Macrophage-specific FITC-tagged mouse anti-CD68 antibody (Dako) was then used as the second primary antibody. For co-localization of MCP-1/CXCL10 with SHIV, the same primary antibody (goat anti-MCP-1 polyclonal antibody/mouse anti-CXCL10 monoclonal antibody) and different secondary antibody (Alexa Fluor 594-conjugated donkey anti-goat secondary antibody for MCP-1 or Alexa Fluor 488-conjugated donkey anti-mouse secondary antibody for CXCL10) were treated separately, followed by treatment with rabbit anti-Nef polyclonal antibody, which was prepared in our laboratory by immunization of rabbits with recombinant Nef protein, as the second primary antibody. Then Alexa Fluor 488-conjugated (staining MCP-1) or Alexa Fluor 594-conjugated (staining CXCL10) donkey anti-rabbit antibody was used as the second secondary antibody. After the final washing, the slides were mounted in SlowFade antifade reagent with or without DAPI (Molecular Probes) and images were captured by confocal microscopy.19 Fluorescent digital images were obtained using a Zeiss LSM510 confocal microscope equipped with a HeNe laser (1 mW) for the excitation (543 nm) and detection (long pass 560 nm filter; LP560) of the Alexa Fluor 594 and with an Argon/2 laser (25 mW) for the excitation (488 nm) and detection (band pass 505–530 nm filter; BP505–530) of the Alexa Fluor 488. Images were acquired in Multitrack channel mode (sequential excitation/emmision) with LSM510 (version 3.2) software and a Plan-Apochromat objective with a zoom factor of 1 or 2 and frame size of 1024 × 1024 pixels. Detector gain was set initially to cover the full range of all of the samples and background corrected by setting the amplifier gain, and all images were then collected under the same photomultiplier detector conditions and pinhole diameter. Control slides consisted of: mounted tissue only, no secondary antibodies for autofluorescence; red primary plus red and green secondary to check for bleed-through into green channel; green primary plus green and red secondary to check for bleed-through into red channel; and red and green secondary antibodies only to check for non-specific binding.
Statistical Analysis
Statistical analyses were performed by using Student t-tests or one-way analysis of variance analysis (independent group analysis). Statistical significance was considered to be P < 0.05.
Results
Lung Pathology
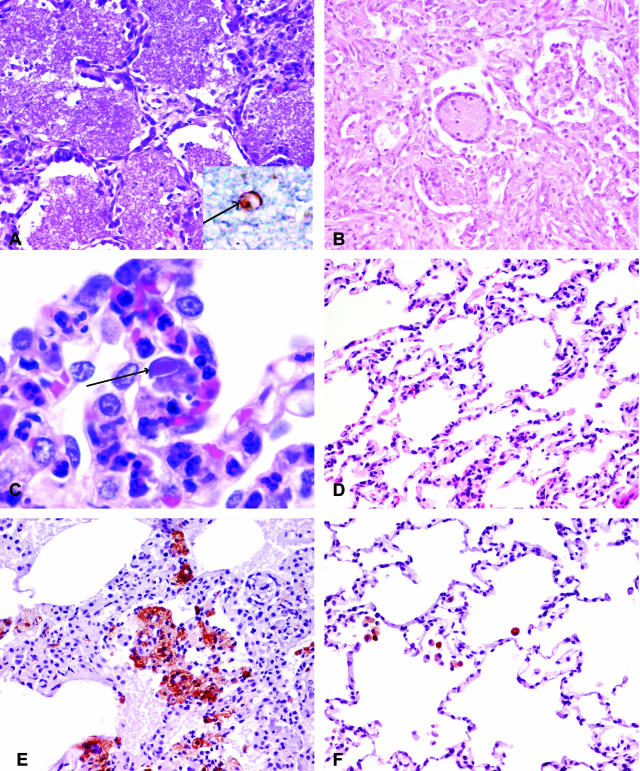
Archival lung tissues from 12 rhesus macaques infected with either SHIVKU2 or SHIV89.6P were evaluated in this study. Three uninfected normal rhesus macaques and one rhesus macaque that died of non-AIDS related pneumonia were also included in this study as controls. Of the 12 infected animals, eight (PJX, 49P1, 52C, 52D, 57W, RHU5, RMT5, and PWJ) had pathological changes in the lungs. Macaque PHP had controlled the virus and did not exhibit any pulmonary disease. Data in Table 1 show that 11 animals developed severe loss of CD4+ T cells, when compared to original pre-infection counts that exceeded 1000 cells/μl of peripheral blood. Eleven of the 12 animals also had high viral RNA concentrations in plasma and 8 of these 11 animals developed pulmonary disease. Lungs of these eight animals were characterized grossly by patchy to diffuse consolidation and patchy to uniform pale tan discoloration. Microscopic lesions in lung tissue were heterogeneous, and characterized by lesions that were pathognomonic for infections with classic opportunistic pathogens such as Pneumocystis carinii (Figure 1, A to D). Figure 1A is a section of lung from macaque RHU5 comprising of diffuse alveolar accumulations of pink flocculent material typical of Pneumocystis carinii pneumonia (PCP). A few alveoli contained syncytial giant cells, typically associated with productive replication of SHIV. Figure 1B is a lung section from macaque PJX showing diffuse interstitial and alveolar pneumonia with prominent lentiviral type giant cells. Inflammation included a proliferative stromal component as well as intense infiltration of the interstitial spaces with inflammatory macrophages. Figure 1C is a section of lung from macaque PWJ showing infiltrating foamy macrophages and septal cells containing amphophilic intranuclear inclusion bodies. Figure 1D is a section of uninflamed lung from macaque RHT5 (SHIV-infected macaque without lung disease) showing no interstitial or alveolar inflammation.
Table 1.
Viral Inoculation, CD4+ T Cell Counts, Plasma Viral RNA and Pathology in SHIV-Infected Rhesus Macaques.
| Animal names | Virus inoculation | CD4+ T cell count (cells/μl of blood)* | Plasma viral RNA (copy number/ml)* | Survival time (days p.i.) | Presence of SHIV viral protein in the lung parenchyma† | Lung pathology | Lesions other than lung |
|---|---|---|---|---|---|---|---|
| RHU5 | SHIV89.6P +SHIVKU2 | 103 | 36,545 | 323 | + | Diffuse pneumocystis carinii pneumonia | Intestine, mild chronic enteritis, flagellate parasites, low-grade viral enteritis; spinal cord, focal neuritis proximate to spinal ganglion; lymph node and nerve, suppurative neuritis with CMV infection |
| RMT5 | SHIV89.6P +SHIVKU2 | 83 | 15,180 | 345 | + | Diffuse pneumocystis carinii pneumonia | Intestine, low-grade chronic enteritis; colon, low-grade colitis; stomach, chronic gastritis with Helicobacters |
| 52C | SHIV89.6P | 47 | 74,000 | 581 | + | Diffuse interstitial pneumonia (without syncytia) | No microscopic lesions |
| 52D | SHIV89.6P | 31 | 21,000 | 622 | + | Diffuse interstitial pneumonia (without syncytia) | No microscopic lesions |
| PJX | SHIV89.6P | 166 | 40,156,805 | 288 | + | Interstitial pneumonia (with syncytia) | Brain, encephalitis; spinal cord, myelitis; intestine, mild chronic entertis, flagellate parasites; kidney, glomerulopathy |
| 49P1 | SHIV89.6P | 0 | 590,000 | 181 | + | Interstitial pneumonia (with syncytia) | Brain, encephalitis; skin, necrotizing granulotous dermatitis |
| 57W | SHIVku2 | 5 | 9,000,000 | 180 | + | Interstitial pneumonia (with syncytia) | Brain, encephalitis |
| PWJ | SHIV89.6P | 10 | 2,444,674 | 97 | + | Viral pneumonia | Brain, meningitis; spinal cord, focal poliomyelitis; gut, viral inclusions |
| RHT5 | SHIV89.6P +SHIVKU2 | 123 | 17,735,180 | 372 | – | Devoid of lung pathology | Stomach, low-grade chronic gastritis; intestine, low-grade enteritis |
| ROQ5 | SHIVku2 | 105 | 1,472,220 | 439 | – | Devoid of lung pathology | Intestine, crypt flagellate parasites; brain, focal encephalitis; lymph node, lymphadenitis |
| RJV5 | SHIV89.6P +SHIVKU2 | 15 | 84,640 | 504 | – | Devoid of lung pathology | Stomach, chronic gastritis; liver, diffuse sinusoidal amyloidosis; kidney, focal interstitial fibrosis |
| PHP | SHIV89.6P | 466 | ND‡ | 369 | – | Devoid of lung pathology | No microscopic lesions. |
, At the time of necropsy;
, detected by immunohistochemical staining with Nef antibody;
, not detected.
Figure 1.
A-D: Pathological changes in the lungs of SHIV-infected macaques stained with H&E. A: Lung section from SHIV-infected macaque with pneumonia (macaque RHU5) showing diffuse alveolar accumulations of pink flocculent material typical of Pneumocystis carinii pneumonia (×20). The inset shows the immunohistochemical staining of Pneumocystis carinii (arrow). B: Lung section from SHIV-infected macaque with pneumonia (macaque PJX) showing diffuse interstitial and alveolar pneumonia. Both the interstitium and alveoli are infiltrated with inflammatory macrophages and syncytial cells (×20). C: Higher power of the lung section from SHIV-infected macaque with pneumonia (macaque PWJ) showing inflammation with foamy macrophages and cells containing intranuclear inclusion bodies (arrow) (×100). D: Section of SHIV-infected, uninflamed lung (macaque RHT5) showing no expansion of alveolar septa and no alveolar infiltrates (×20). E: Lung section from SHIV-infected macaque with pneumonia (macaque PJX) stained with macrophage-specific antibody, Ham56, demonstrating massive influx of macrophages compared to (F) lung section of SHIV-infected macaques without pneumonia (macaque PHP) (×20).
Since macrophages and CD4+ T cells are the principal host cells for virus replication,20,21 we performed immunohistochemistry using macrophage-specific marker, HAM56, and T cell-specific marker CD3 antibodies to characterize the nature of the infiltrating cells in the lung parenchyma. As shown in Figure 1, E and F, the lungs of macaques with pneumonia had massive numbers of macrophages compared to lungs of infected macaques without pneumonia. The latter group had very few macrophages in the lungs. For CD3-positive cells, however, it was found that SHIV-infected macaques, irrespective of the pulmonary disease, had negligible numbers of them (data not shown). In the SHIV/rhesus macaque system, therefore, there was no obvious difference in the infiltrating T cell populations in the lungs of macaques with or without pneumonia. One reason for this could be the rapid loss of T cells mediated by SHIV infection.
SHIV Viral Load in the Plasma and in the Lung Parenchyma of Infected Macaques
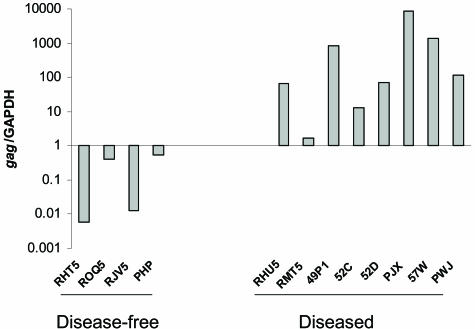
As shown in Table 1, real-time RT-PCR indicated high plasma viral RNA in 11 of the 12 monkeys. Only eight macaques with high plasma viremia developed lung disease. Thus, plasma viral load did not correlate with lung pathology. To determine whether pneumonia, irrespective of its etiology, was associated with replication of SHIV in the lung tissue, we tested for the presence of viral mRNA in the lung specimens by real-time RT-PCR using RNA extracted from lung tissues. As shown in Figure 2, lung tissues from all of the infected animals had a positive viral signal using the gag primers. However, when normalized to the GAPDH signal, it was evident that lungs from macaques with SHIV-pneumonia had a significantly higher viral load than the lungs of infected macaques that were histologically normal [P < 0.001. The median value and variance of gag RNA (log value) of the diseased group is 2.1363 ± 1.1827 and the control group is −1.175 ± 1.005]. These data therefore suggested that high pulmonary viral loads were closely associated with the pneumonic process.
Figure 2.
Viral gag mRNAs were quantified by real-time RT-PCR from RNAs extracted from lung tissues of SHIV-infected macaques with (RHU5, RMT5, 49P1, 52C, 52D, PJX, 57W, and PWJ) and without (RHT5, ROQ5, RJV5, and PHP) pneumonia. Briefly, total RNA was extracted from flash-frozen tissues, the samples were treated with DNase, and real-time RT-PCR was performed using 100 ng RNA for both, the cellular GAPDH transcript and viral gag-containing mRNA. The gag mRNA copy number was normalized to the GAPDH copy number to present the data from equivalent cell numbers.
Levels of MCP-1 and CXCL10 in the Lungs of SHIV-Infected Macaques
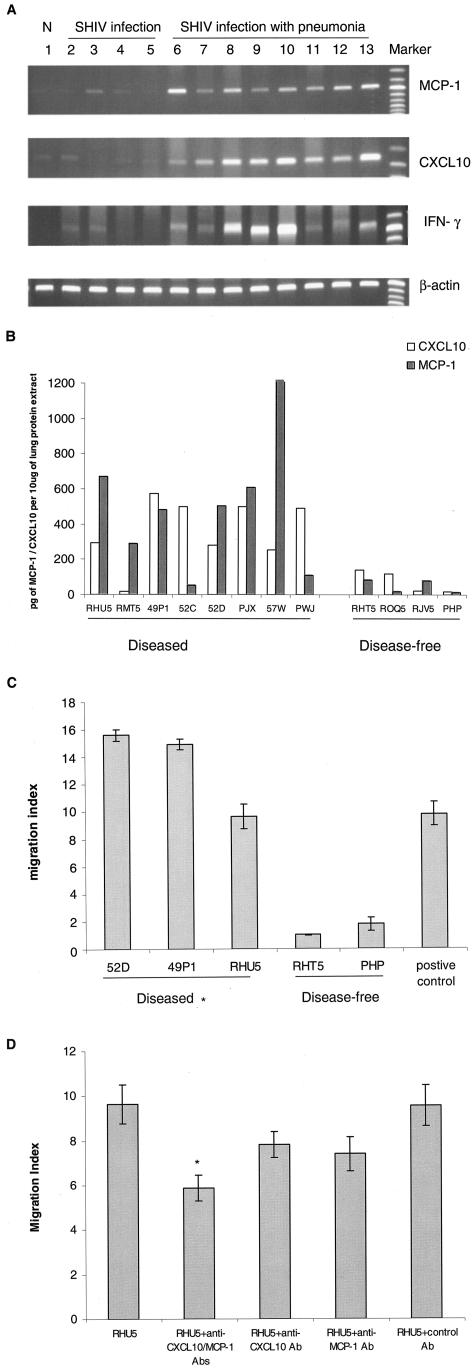
Since MCP-1 and CXCL10 are the two major modulators of monocyte/macrophage and T cell trafficking in tissues,22,23 and since these inflammatory cell types were present in the lung parenchyma of SHIV-infected macaques with pneumonia, we determined whether the expression of these chemokines was increased in the pneumonic lungs compared to lungs without disease. As shown in Figure 3A, using RT-PCR, we found that all eight animals with pneumonia had increased CXCL10 and MCP-1 RNA compared to either uninfected normal macaques and macaque with pneumonia (not AIDS-related), or SHIV-infected macaques without lung diseases. To assess whether this difference in RNA was also evident at the protein level, we quantified chemokine protein concentrations in lung homogenates by ELISA. The level of chemokines within the three control groups remained very low and unchanged. However, as shown in Figure 3B, we found significant increases in the protein levels of the chemokines: for CXCL10, P = 0.014 and the median value and variance is 363.5 ± 185.2 pg/10 μg protein extract for the diseased group, and the control group is 73.6 ± 64.2pg/10 μg protein extract, and the mean increase is 4.9-fold; for MCP-1, P = 0.011 and the median value and variance is 491.9 ± 360.6 pg/10 μg protein extract for the diseased group, and the control group is 47.8 ± 37.3 pg/10 μg protein extract, and mean increase is 10.4-fold in pneumonic lungs compared to lungs without pneumonia.
Figure 3.
A: RT-PCR analysis of MCP-1, CXCL10, and IFN-γ in the lungs of SHIV-infected macaques. Sample of lung RNA from a representative normal, uninfected macaque (lane 1), from four SHIV-infected macaques without pneumonia (lanes 2 to 5, macaques RHT5, RJV5, ROQ5, PHP, respectively); and from eight SHIV-infected macaques with pneumonia (lanes 6 to 13, macaques 57W, RMT5, PWJ, 52C, 52D, RHU5, 49P1, and PJX, respectively) were subjected to RT-PCR analysis using primers specific for macaque MCP-1, CXCL10, IFN-γ, and β-actin. B: MCP-1 and CXCL10 protein analysis in the lungs of SHIV-infected macaques. Equal amounts of proteins extracted from lung tissues were used to determine the MCP-1 and CXCL10 protein levels by ELISA kit. C: Chemotactic analysis of lung protein extract from SHIV-infected macaques. Thirty μl of lung protein extract from macaques 52D, 49P1, and RHU5 (SHIV-pneumonia group) and macaques RHT5 and PHP (SHIV-non-pneumonia group) were used for the chemotactic assay. Macaque PBMCs migrating toward the lung proteins were counted by using Cell Titer 96 Aqueous One Solution Assay. Migration index was calculated as the number of cells migrating toward the protein extract from the diseased or control macaque lungs divided by the number of cells migrating toward PBS only. 10 ng/ml of MCP-1 was used as positive control. *, SHIV-pneumonia group (52D, 49P1, or RHU5) is statistically significant compared to SHIV-non-pneumonia group (RHT5, PHP), P < 0.001. D: For blocking experiments, lung homogenate from a representative macaque with SHIV-pneumonia (RHU5, similar result was also obtained from macaque 52D) was either untreated or preincubated for 1 hour with MCP-1 and/or CXCL10 neutralizing antibodies followed by chemotactic assay. Control antibody included mouse isotype IgG. *, statistical significance of migration index of antibody-treated homogenates compared to that of the untreated homogenate.
Since IFN-γ is a known inducer of CXCL10, we inquired whether or not this cytokine was present in the lung parenchyma. An increase in the levels of IFN-γ mRNA was indeed observed in the lungs of macaques with pneumonia compared to lungs from macaques without lung disease (Figure 3A). However, we did not observe an increase in the levels of IFN-γ in the lungs of macaques with PCP, a classic TH2 opportunistic pathogen. One possible function of IFN-γ increase in the lungs could have been the induction of CXCL10 in lung macrophages. In macaques lacking increased levels of IFN-γ, IFN-γ-independent pathway, such as viral gp120-mediated up-regulation of CXCL10, may be responsible for the observed induction of the chemokine.19,24
Functional Assay of the Chemokines
After demonstrating up-regulation of MCP-1 and CXCL10 in the lungs of macaques with pneumonia, we then sought to determine whether or not this up-regulation was responsible for the recruitment of inflammatory cells into the diseased lungs. Using chemotactic assays, we demonstrated that lung homogenates from macaques with pneumonia had higher chemotactic activity for PBMCs than homogenates of pathologically unaffected lungs (Figure 3C). To determine whether the up-regulated chemokines in the lung homogenates played any roles in the observed chemotactic activity, we performed blocking experiments using MCP-1 and/or CXCL10 neutralizing antibodies. Pre-incubation of the diseased lung homogenates with the neutralizing antibodies resulted in a 30% reduction in the chemotactic activity of the migrating PBMCs (Figure 3D). This suggested that in addition to yet other unidentified chemokines, both MCP-1 and CXCL10 played a role in the recruitment of monocyte-macrophages into the diseased lungs.
Cellular Localization of Chemokines in the Lungs of Infected Macaques with Pneumonia
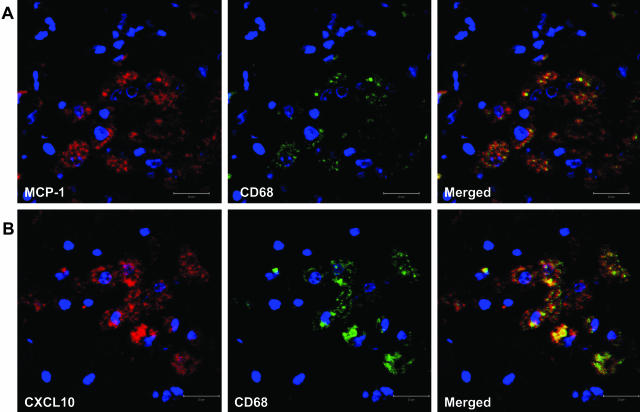
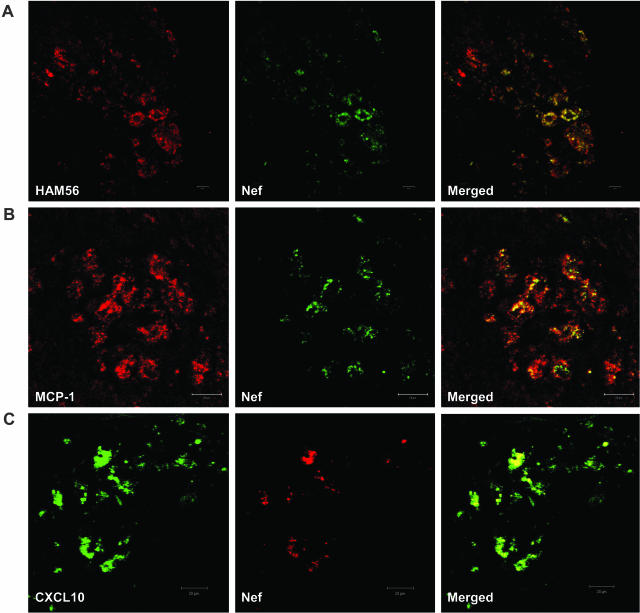
To determine whether macrophages in the pneumonic lungs were the producers of MCP-1 and CXCL10, we performed double immunohistochemisty using anti-MCP-1 (or CXCL10) and anti-CD68 (macrophage-specific) antibodies. As shown in Figure 4, A and B, both chemokines co-localized primarily with CD68-positive macrophages in the lungs. To determine whether macrophages in the lungs with pneumonia were infected with the virus, we performed double staining using antibodies directed against HAM56 (macrophage marker) and viral Nef protein (Figure 5A). As shown in Figure 5A, all of the virus-positive cells in the lungs co-localized with the macrophage marker, HAM56. To further determine whether the infected macrophages were also the source of the two chemokines, we carried out double staining of the lung sections with anti-MCP-1 (or CXCL10) and anti-Nef antibodies. Figure 5, B and C, demonstrated that infected macrophages were also the source of both MCP-1 and CXCL10, respectively. Infected T cells were not detected in the lungs of macaques with pneumonia (data not shown).
Figure 4.
A representative image of co-localization of MCP-1/CXCL10 and macrophages in the lungs of SHIV-infected macaques with pneumonia. Goat anti-MCP-1 polyclonal antibody (A) or mouse anti-CXCL10 monoclonal antibody (B) was used as primary antibody to stain the lung sections, followed by treatment with Alexa Fluor 594-conjugated secondary antibody. Macrophage-specific FITC-tagged mouse anti-CD68 antibody was then used as second primary antibody. After the final washing, the slides were mounted in SlowFade antifade reagent (with DAPI, blue stain) and images were captured by confocal microscopy.
Figure 5.
A: A representative image of co-localization of SHIV and macrophages in the lungs of SHIV-infected macaque with pneumonia. Lung sections were first stained with rabbit anti-Nef polyclonal antibody, followed by Alexa Fluor 488-conjugated anti-rabbit secondary antibody; then the sections were stained with mouse anti-HAM56 monoclonal antibody, followed by Alexa Fluor 594-conjugated anti-mouse secondary antibody. B and C: Co-localization of MCP-1/CXCL10 with SHIV-infected cells in the lungs of macaque PJX. Goat anti-MCP-1 polyclonal antibody (B) or mouse anti-CXCL10 monoclonal antibody (C) and secondary antibody (Alexa Fluor 594-conjugated donkey anti-goat secondary antibody for MCP-1 or Alexa Fluor 488-conjugated donkey anti-mouse secondary antibody for CXCL10) were used to stain the lung sections from macaque PJX, The sections were then stained with rabbit anti-Nef polyclonal antibody, followed by Alexa Fluor 488-conjugated (staining MCP-1) or Alexa Fluor 594-conjugated (staining CXCL10) donkey anti-rabbit antibody as the second secondary antibody. After the final washing, the slides were mounted in SlowFade antifade reagent and images were captured by confocal microscopy.
Discussion
Pulmonary disorders are the most frequent complication of HIV infections since approximately 75 to 85% of the AIDS patients, especially children, have related pulmonary infections.2 In this study, we provide evidence of high-level expression of two chemokines, MCP-1 and CXCL10, in lung tissues from SHIV-infected macaques with pneumonia and suggest that these chemokines played a role in the recruitment of inflammatory macrophages into the lung tissue, where they became host cells for supporting virus replication. Studies on pathogenesis of infections caused by HIV and SIV/SHIV demonstrated that CD4+ T cells and macrophages are the major cell types that support replication of the viruses. The acute phase of infection is dominated by replication of the agents in activated CD4+ T cells in lymph nodes and the gut-associated lymphoid tissues and is followed by development of cell-mediated immune responses that subsequently limit viral replication. During this stage, macrophages in the tissues become infected but replication of the virus in these cells is restricted to a minimal degree. Productive replication of the virus becomes prominent in macrophages following loss of both cell-mediated immunity and CD4+ T cells.25–27 Pulmonary cells from asymptomatic patients are known to contain proviral DNA although at lower levels than during the late stages of disease.28 The relationship between systemic virus load and the pool of viruses replicating in the lung remains poorly understood. Earlier work on the SIV/rhesus macaque model addressing this issue demonstrated that during acute infection, despite widespread virus replication in the lymphoid tissues, virus replication in the lungs proceeded only minimally and was not evident in CD68-positive macrophages.29 Furthermore, these studies also demonstrated that during chronic SIV infection, the lung tissues of macaques harbored many virus-positive CD68-positive cells that localized in large foci in diaphragmatic lobes. Similar to the findings reported by these authors,30 we found large numbers of CD68-postive, virus-positive cells in the lungs of SHIV-infected macaques with lung disease, even though the pathological changes were mainly those associated with replication of opportunistic pathogens. These cells also expressed dramatically increased levels of chemokines MCP-1 and CXCL10. Both MCP-1 and CXCL10 have been implicated as key mediators in HIV-associated CNS disease, their role being the recruitment of monocytes and/or T cells into the tissue, respectively.9 We did not observe a change in T lymphocyte trafficking in the pneumonic lungs, indicating that the primary role of these chemokines may have been the recruitment of monocytes into the lungs. The lack of T cell influx in the lungs was probably attributable to the subtotal loss of CD4+ T cells from the blood.
A possible explanation for high viral load in the lungs of macaques with associated lung disease could have been the result of concurrent infections with opportunistic pathogens that were prevalent in the lungs. This concept was illustrated in a previous report in which intratracheal inoculation of SHIV-infected macaques with S. mansoni eggs resulted in development of granulomas that comprised of macrophages that became productively infected with the virus.17 In the present study we speculate that the synergy between opportunistic pathogen(s) and SHIV could have resulted in increased numbers of virus-infected macrophages in the lungs. This, in turn, would lead to enhanced production of MCP-1 and CXCL10, both of which are known to be induced in virus-infected cells,31,32 thereby perpetuating a virus amplification response in the lung.
Our findings in the SHIV-infected rhesus macaques suggested a threshold of virus replication (gag to GAPD ratio) in the lungs of macaques with pneumonia. It is possible that the opportunistic pathogen(s) that are known to induce local activation could be the trigger for enhanced virus replication in the lung. Since all of the animals with a gag/GAPD ratio greater than 1 developed pulmonary lesions while those with a ratio lower than 1 were devoid of lung disease, we speculate that development of pulmonary pathology is a multifactorial process involving the interaction of virus, opportunistic pathogens, and host factors. Some of these host factors, such as CXCL10, have been shown to enhance virus replication in macrophages.32 Similarly, it has also been shown that endogenous MCP-1 could modulate HIV-1 replication and might act as an enhancing factor for HIV-1 spreading in monocyte-derived macrophages.33 This is the first report showing that pneumonia complications of SHIV infection in macaques, irrespective of the proliferating opportunistic pathogen, are associated with enhanced replication of the lentivirus in the lung.
Acknowledgments
We gratefully acknowledge Mingzhao Huang, Yanfen Niu, and Elizabeth Petroske for assistance with RNA extraction, real-time PCR, and confocal microscopy, respectively.
Footnotes
Address reprint requests to Shilpa J. Buch, Ph.D., Department of Microbiology, Immunology, and Molecular Genetics, Marion Merrell Dow Laboratory of Viral Pathogenesis, 5000 Wahl Hall East, University of Kansas Medical Center, 3901 Rainbow Blvd., Kansas City, KS 66160. E-mail: sbuch@kumc.edu.
Supported by grants MH-62969, NS-32203, P20RR16443, and MH068212 from the National Institutes of Health (NIH), and Biomedical Research Training Program Postdoctoral Fellowship from University of Kansas Medical Center (to Y.S.). Confocal images were acquired at Kansas University Medical Center (KUMC) core facility (http://www.kumc.edu/cic), supported by NIH Shared Resource Grant (NCRR RR14637–01) and the Kansas Biomedical Research Infrastructure network.
References
- Afessa B, Green W, Chiao J, Frederick W. Pulmonary complications of HIV infection: autopsy findings. Chest. 1998;113:1225–1229. doi: 10.1378/chest.113.5.1225. [DOI] [PubMed] [Google Scholar]
- Ashley EA, Johnson MA, Lipman MC. Human immunodeficiency virus and respiratory infection. Curr Opin Pulm Med. 2000;6:240–245. doi: 10.1097/00063198-200005000-00013. [DOI] [PubMed] [Google Scholar]
- Agostini C, Trentin L, Zambello R, Semenzato G. HIV-1 and the lung: infectivity, pathogenic mechanisms, and cellular immune responses taking place in the lower respiratory tract. Am Rev Respir Dis. 1993;147:1038–1049. doi: 10.1164/ajrccm/147.4.1038. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250:91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- Teruya-Feldstein J, Kingma DW, Weiss A, Sorbara L, Burd PR, Raffeld M, Mueller BU, Tosato G, Jaffe ES. Chemokine gene expression and clonal analysis of B cells in tissues involved by lymphoid interstitial pneumonitis from HIV-infected pediatric patients. Mod Pathol. 2001;14:929–936. doi: 10.1038/modpathol.3880414. [DOI] [PubMed] [Google Scholar]
- Agostini C, Facco M, Siviero M, Carollo D, Galvan S, Cattelan AM, Zambello R, Trentin L, Semenzato G. CXC chemokines IP-10 and mig expression and direct migration of pulmonary CD8+/CXCR3+ T cells in the lungs of patients with HIV infection and T-cell alveolitis. Am J Respir Crit Care Med. 2000;162:1466–1473. doi: 10.1164/ajrccm.162.4.2003130. [DOI] [PubMed] [Google Scholar]
- Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93:172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. [PubMed] [Google Scholar]
- Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, Clements JE. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis. 2001;184:1015–1021. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]
- Sui Y, Potula R, Pinson D, Adany I, Li Z, Day J, Buch E, Segebrecht J, Villinger F, Liu Z, Huang M, Narayan O, Buch S. Microarray analysis of cytokine and chemokine genes in the brains of macaques with SHIV-encephalitis. J Med Primatol. 2003;32:229–239. doi: 10.1034/j.1600-0684.2003.00030.x. [DOI] [PubMed] [Google Scholar]
- Johnson RP, Lifson JD, Czajak SC, Cole KS, Manson KH, Glickman R, Yang J, Montefiori DC, Montelaro R, Wyand MS, Desrosiers RC. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J Virol. 1999;73:4952–4961. doi: 10.1128/jvi.73.6.4952-4961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Niu Y, Li Z, Adany I, Pinson D, Liu Z, Berry T, Sheffer D, Jia F, Narayan O. Systemic infecion and limited replication of SHIV vaccine virus in brains of macaques inoculated intracerebrally with infectious viral DNA. Virology. 2002;301:130–135. doi: 10.1006/viro.2002.1548. [DOI] [PubMed] [Google Scholar]
- Hofmann-Lehmann R, Swenerton RK, Liska V, Leutenegger CM, Lutz H, McClure HM, Ruprecht RM. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res Hum Retroviruses. 2000;16:1247–1257. doi: 10.1089/08892220050117014. [DOI] [PubMed] [Google Scholar]
- Buch S, Pinson D, King CL, Raghavan R, Hou Y, Li Z, Adany I, Hicks A, Villinger F, Kumar A, Narayan O. Inhibitory and enhancing effects of IFN-gamma and IL-4 on SHIV(KU) replication in rhesus macaque macrophages: correlation between Th2 cytokines and productive infection in tissue macrophages during late-stage infection. Cytokine. 2001;13:295–304. doi: 10.1006/cyto.2000.0829. [DOI] [PubMed] [Google Scholar]
- Kumar A, Stipp HL, Sheffer D, Narayan O. Use of herpesvirus saimiri-immortalized macaque CD4(+) T cell clones as stimulators and targets for assessment of CTL responses in macaque/AIDS models. J Immunol Methods. 1999;230:47–58. doi: 10.1016/s0022-1759(99)00123-4. [DOI] [PubMed] [Google Scholar]
- Sui Y, Potula R, Dhillon N, Pinson D, Li S, Nath A, Anderson C, Turchan J, Kolson D, Narayan O, Buch S. Neuronal apoptosis is mediated by CXCL10 overexpression in simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;164:1557–1566. doi: 10.1016/S0002-9440(10)63714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi T, Brown CR, Endo Y, Buckler-White A, Plishka R, Bischofberger N, Hirsch V, Martin MA. Macrophages are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc Natl Acad Sci USA. 2001;98:658–63. doi: 10.1073/pnas.021551798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein JM, Fox C, Wahl SM. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio VC, Maier J, Milner R, Boztug K, Kincaid C, Moulard M, Phillipson C, Lindsley K, Krucker T, Fox HS, Campbell IL. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J Virol. 2001;75:7067–7077. doi: 10.1128/JVI.75.15.7067-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babas T, Vieler E, Hauer DA, Adams RJ, Tarwater PM, Fox K, Clements JE, Zink MC. Pathogenesis of SIV pneumonia: selective replication of viral genotypes in the lung. Virology. 2001;287:371–381. doi: 10.1006/viro.2001.1043. [DOI] [PubMed] [Google Scholar]
- Horton H, Vogel TU, Carter DK, Vielhuber K, Fuller DH, Shipley T, Fuller JT, Kunstman KJ, Sutter G, Montefiori DC, Erfle V, Desrosiers RC, Wilson N, Picker LJ, Wolinsky SM, Wang C, Allison DB, Watkins DI. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J Virol. 2002;76:7187–7202. doi: 10.1128/JVI.76.14.7187-7202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski JL, Carter DL, Spelman JP, Nealen ML, Maughan KR, Kirstein LM, Didier PJ, Adams RJ, Murphey-Corb M, Zink MC. Pathogenesis of simian immunodeficiency virus pneumonia: an immunopathological response to virus. Am J Pathol. 1998;153:1123–1130. doi: 10.1016/S0002-9440(10)65656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landay AL, Schade SZ, Takefman DM, Kuhns MC, McNamara AL, Rosen RL, Kessler HA, Spear GT. Detection of HIV-1 provirus in bronchoalveolar lavage cells by polymerase chain reaction. J Acquir Immune Defic Syndr. 1993;6:171–175. [PubMed] [Google Scholar]
- Reinhart TA, Fallert BA, Pfeifer ME, Sanghavi S, Capuano S, III, Rajakumar P, Murphey-Corb M, Day R, Fuller CL, Schaefer TM. Increased expression of the inflammatory chemokine CXC chemokine ligand 9/monokine induced by interferon-gamma in lymphoid tissues of rhesus macaques during simian immunodeficiency virus infection and acquired immunodeficiency syndrome. Blood. 2002;99:3119–3128. doi: 10.1182/blood.v99.9.3119. [DOI] [PubMed] [Google Scholar]
- Fuller CL, Choi YK, Fallert BA, Capuano S, III, Rajakumar P, Murphey-Corb M, Reinhart TA. Restricted SIV replication in rhesus macaque lung tissues during the acute phase of infection. Am J Pathol. 2002;161:969–978. doi: 10.1016/S0002-9440(10)64257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks A, Potula R, Sui YJ, Villinger F, Pinson D, Adany I, Li Z, Long C, Cheney P, Marcario J, Novembre F, Mueller N, Kumar A, Major E, Narayan O, Buch S. Neuropathogenesis of lentiviral infection in macaques: roles of CXCR4 and CCR5 viruses and interleukin-4 in enhancing monocyte chemoattractant protein-1 production in macrophages. Am J Pathol. 2002;161:813–822. doi: 10.1016/S0002-9440(10)64241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane BR, King SR, Bock PJ, Strieter RM, Coffey MJ, Markovitz DM. The C-X-C chemokine IP-10 stimulates HIV-1 replication. Virology. 2003;307:122–134. doi: 10.1016/s0042-6822(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Fantuzzi L, Spadaro F, Vallanti G, Canini I, Ramoni C, Vicenzi E, Belardelli F, Poli G, Gessani S. Endogenous CCL2 (monocyte chemotactic protein-1) modulates human immunodeficiency virus type-1 replication and affects cytoskeleton organization in human monocyte-derived macrophages. Blood. 2003;102:2334–2337. doi: 10.1182/blood-2002-10-3275. [DOI] [PubMed] [Google Scholar]