Abstract
Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) is a member of the tumor necrosis factor superfamily. TWEAK acts on responsive cells via binding to a small cell surface receptor named Fn14. Recent studies have demonstrated that TWEAK can stimulate numerous cellular responses including cell proliferation, migration, and proinflammatory molecule production, but the role of this cytokine in cardiovascular disease and stroke has not been established. The present study investigated whether TWEAK or Fn14 expression was regulated in a murine model of cerebral ischemia and whether TWEAK played a role in ischemia-mediated cell death. We found that TWEAK and Fn14 were expressed by primary mouse cerebral cortex-derived astrocytes and neurons cultured in vitro. Also, both the TWEAK and Fn14 proteins were present at elevated levels in the ischemic penumbra region after middle cerebral artery occlusion. Finally, we report that intracerebroventricular injection of a soluble Fn14-Fc decoy receptor immediately after middle cerebral artery occlusion significantly reduced infarct volume and the extent of microglial cell activation and apoptotic cell death in the ischemic penumbra. We conclude that the cytokine TWEAK may play an important role in ischemia-induced brain injury and that inhibition of TWEAK expression or function in the brain may represent a novel neuroprotective strategy to treat ischemic stroke.
Stroke is the second most common cause of death in the world after heart disease and a leading cause of disability.1 Stroke is caused by an occlusion of a cerebral blood vessel, which if not quickly resolved, leads to a reduction in blood flow and irreversible ischemic brain injury. Ischemia triggers a cascade of pathophysiological events, including energy depletion, excitotoxicity, peri-infarct depolarization, inflammation, and apoptotic cell death, that occur in a precise temporal sequence and result in brain injury.2,3 The inflammatory response, a complex process coordinated by numerous proinflammatory molecules expressed by neurons, glia, endothelial cells, and leukocytes recruited from the peripheral circulation, contributes to secondary ischemic brain damage by multiple mechanisms.2–4 Inflammation in the ischemic brain and its role in neurotoxicity is presently an area of considerable interest, and although significant progress has been made, additional studies investigating this process may lead to the identification of new therapeutic targets for patients suffering ischemic brain injury.2–4
Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) is a member of the tumor necrosis factor (TNF) superfamily.5 It is a type II transmembrane protein that can be cleaved to generate an ∼17-kd soluble factor with biological activity.5 Soluble TWEAK has been shown to stimulate various cellular responses when it is added to cells in culture, including cell proliferation, migration, survival, apoptosis, and differentiation.6,7 Also, TWEAK is a proinflammatory factor5,8–12 and may play a role in neuroinflammation in vivo.13 TWEAK activity is mediated via binding to Fn14, a member of the TNF receptor (TNFR) superfamily of cell surface receptors.14 The TWEAK-Fn14 signal transduction pathway is not fully understood, but it has been shown that adaptor proteins known as TNFR-associated factors can bind the Fn14 cytoplasmic tail14–16 and that TWEAK binding to Fn14 activates the nuclear factor-κB (NF-κB),11,15–18 extracellular signal-regulated kinase (ERK)17 and c-Jun NH2-terminal kinase (JNK)17 signal transduction pathways. In the present study we investigated whether the TWEAK-Fn14 signaling system could be involved in ischemia-induced brain injury.
Materials and Methods
Cortical Cell Culture and Immunofluorescence
Mixed cortical cell cultures were isolated from embryonic day 15 CD1 mice and cultured as described.19 One week after plating, some of the cells were washed twice with phosphate-buffered saline (PBS), fixed in 3% formaldehyde in PBS, and then permeabilized and blocked in 0.1% saponin and 5% bovine serum albumin in PBS for 1 hour. Other cell cultures were enriched for astrocytes by maintaining them in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum, 2 mmol/L glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin for an additional 2 weeks. These cells were washed and fixed in the same manner as the 1-week-old cultures. All antibodies were diluted in the blocking buffer described above and coverslips were washed three times in between each step. Cells were incubated with either a 1:100 dilution of rabbit anti-Fn14 IgG20 in combination with a 1:500 dilution of anti-neuron-specific nuclear protein (NeuN) monoclonal antibody (Chemicon, Temecula, CA) or a 1:50 dilution of antigen-purified goat anti-TWEAK polyclonal antibody (Cell Sciences, Canton, MA) in combination with a 1:100 dilution of rabbit anti-glial fibrillary acidic protein (GFAP) polyclonal antibody (DAKO, Carpinteria, CA) for 1 hour at room temperature. Goat anti-rabbit secondary antibodies conjugated to Alexa 488 (Molecular Probes, Eugene, OR) and donkey anti-mouse or anti-goat secondary antibodies conjugated to Rhodamine Red-X (Jackson ImmunoResearch, West Grove, PA) were diluted to 1:500 and applied for 1 hour at room temperature. In some cases the cells were counterstained with 4,6-diamidino-2-phenylindole (Molecular Probes) for 2 to 3 seconds. Coverslips were rinsed with PBS and mounted on glass slides with Gel/Mount (Biomedia, Foster City, CA).
Construction and Stable Transfection of the Fn14-Fc and Fc Expression Plasmids, Purification of the Fn14-Fc and Fc Proteins
The plasmid pSecTag2/Fn14-Fc was constructed and a stably transfected human embryonic kidney (HEK) 293T clonal cell line was isolated as described.17 To construct the plasmid pSecTag2/Fc, the plasmid pSecTag2/OPG-Fc (provided by M. Tondravi, National Institutes of Health, Bethesda, MD) was digested with SfiI to release the OPG cDNA insert. The DNA ends were filled in using T4 DNA polymerase and then the plasmid was self-ligated using T4 DNA ligase. DNA sequence analysis was performed to confirm the identity of the construct. This plasmid was transfected into HEK293T cells and a stably transfected cell line (a pooled population) was isolated by drug selection as described.17 The soluble Fn14-Fc and Fc proteins were purified from conditioned medium by affinity chromatography as described.17
Characterization of the Fn14-Fc and Fc Proteins Expressed in Transiently Transfected HEK293T Cells
Approximately 106 HEK293T cells (American Type Culture Collection, Manassas, VA) were grown as described17 and then transfected with 4 μg of either the pSecTag2/Fn14-Fc or pSecTag2/Fc plasmids using Lipofectamine Plus (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. At 48 hours after transfection the conditioned medium was collected and centrifuged to remove any contaminating cells. Protein A-Sepharose beads (Amersham Pharmacia, Piscataway, NJ) were added to the conditioned medium and bound proteins subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 4 to 12% NuPage gradient gel (Invitrogen). Proteins were transferred to nitrocellulose membranes (Schleicher and Schuell, Keene, NH) and then visualized using Ponceau S stain (Sigma, St. Louis, MO). The membranes were blocked for 1 hour at 37°C in TBST (25 mmol/L Tris/HCl, pH 7.5, 150 mmol/L NaCl, 0.1% Tween-20) containing 5% nonfat dry milk and then incubated for 1 hour at room temperature in TBST containing 5% bovine serum albumin and a 1:500 dilution of anti-myc monoclonal antibody 9E10 (gift of Sue Robinson, University of Maryland School of Medicine, Baltimore, MD). The membranes were then washed three times with TBST, and incubated for 1 hour in TBST containing 5% nonfat dry milk and a 1:10,000 dilution of horseradish peroxidase-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were washed three times in TBST and bound secondary antibodies were detected using the Supersignal West Pico kit (Pierce, Rockford, IL).
Animal Model
Animal experiments were conducted according to the guidelines of the Institutional Animal Care and Use Committee. Focal cerebral ischemia was induced by permanent middle cerebral artery occlusion (MCAO) as described elsewhere.21,22 Briefly, C57BL/6J male mice, weighing 20 to 30 g, were anesthetized by intraperitoneal injection of ketamine (2 mg) and xylazine (0.2 mg). Atropine (1 mg/kg) was administered intramuscularly, and body temperature was maintained by keeping the animals on a heating pad. Brain temperature was monitored with a thermometer placed into the left masseter muscle. A U-shaped incision was made between the left ear and left eye. The skull was exposed by retraction of the temporal muscle and a small opening (1 to 2 mm in diameter) was made with a handheld drill, with saline superfusion to prevent heat injury over the middle cerebral artery (MCA) region. The meninges were removed with forceps, and the MCA was occluded by ligation with 10-0 nylon thread and transected distally to the ligation point. Finally, the temporal muscle and skin were sutured back in place. At either 0, 24, 48, or 72 hours after MCAO animals were transcardially perfused with PBS and 4% paraformaldehyde. Brains were removed and TWEAK and Fn14 expression levels were assayed as described below.
A separate group of animals was placed on a stereotaxic frame immediately after MCAO and 2 μl of either PBS, soluble Fn14-Fc decoy receptor (1 μg/μl), or soluble Fc protein (1 μg/μl) was injected intracerebroventricularly throughout a 60-second period using a Hamilton syringe. The coordinates for the injection were: bregma, −2 mm; medial-lateral, 0 mm; and dorso-ventral, 2 mm.23 The animals were allowed to recover before being returned to their cages. After 72 hours, the animals were anesthetized and perfused as described above. The brains were removed, embedded in paraffin, and coronal sections, 20 μm thick, were cut through the rostrocaudal extent of the brain. The sections were stained with hematoxylin and eosin and infarction volume was calculated by the integration of the areas of eight chosen sections and the distances between them using the NIH Scion Image Analyzer System as described.24 The rostral and caudal limits for the integration were set at the frontal and occipital poles of the cortex.25 Statistical significance between groups was evaluated by unpaired Student’s t-test for comparison between two means. Differences were considered statistically significant at a probability value of P < 0.05.
RNA Isolation and Real-Time Quantitative Reverse Transcription (RT)-Polymerase Chain Reaction (PCR) Analysis
Total RNA was isolated from mouse ipsilateral (ischemic) or contralateral (nonischemic) hemisphere brain tissue using RNA Stat-60 (Tel-Test, Friendswood, TX) according to the manufacturer’s instructions. The integrity of each RNA sample was confirmed by denaturing gel electrophoresis followed by ethidium bromide staining. One μg of each RNA sample was converted to cDNA using TaqMan reverse transcription reagents according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). Each PCR reaction was performed in triplicate using an ABI Prism 7900HT sequence detector system. The reactions contained 5 μl of each cDNA, 1× TaqMan Universal PCR Master Mix, and murine TWEAK-, murine Fn14-, or rodent GAPDH-specific primers and fluorescence-labeled probes (Applied Biosystems Assay-On-Demand Products) in 100-μl total volume according to the manufacturer’s instructions (Applied Biosystems). The thermal cycling conditions comprised an initial denaturation step at 95°C for 10 minutes and 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. Threshold cycle (Ct) was obtained from the PCR reaction curves and TWEAK and Fn14 mRNA levels were quantitated using the comparative Ct method with GAPDH mRNA serving as the reference. Statistical significance was evaluated as described above.
Immunohistochemistry
All immunohistochemistry was performed on 5-μm deparaffinized embedded sections. The sections were first immersed in 100% methanol/0.3% H2O2 for 30 minutes to exhaust endogenous peroxidase activity and then preincubated with either 10% goat serum (TWEAK and Fn14 staining) or 10% rabbit serum (Mac-1 staining) for 20 minutes at room temperature. Sections were then incubated with either a 1:50 dilution of rabbit anti-TWEAK IgG (gift of Timothy Zheng, Biogen Idec Inc Cambridge, MA.), a 1:10 dilution of rabbit anti-Fn14 IgG,20 a 1:100 dilution of rat anti-Mac-1 antibody M170 (gift of Li Zhang, University of Maryland School of Medicine, Baltimore, MD) or an equivalent amount of normal control rabbit or rat IgG (Sigma) for 1 hour at room temperature. After a wash with PBS, a 1:200 dilution of biotinylated anti-rabbit or anti-rat secondary antibody (Vector Laboratories, Burlingame, CA) was added for 30 minutes at room temperature. The sections were then washed in PBS, incubated with a 1:100 dilution of ABC Elite reagent (Vector Laboratories), developed with 3,3′-diaminobenzidine for 4 minutes, and counterstained with Mayer’s hematoxylin for 2 minutes.
Terminal dUTP Nick-End Labeling (TUNEL) Staining
Paraffin-embedded sections from vehicle and Fn14-Fc-treated animals (72 hours after MCAO) were prepared and TUNEL reactivity was measured using the ApopTag Plus Fluorescein In Situ Apoptosis Detection kit (Chemicon) according to the manufacturer’s instructions.
Results
TWEAK and Fn14 Expression in Primary Neuronal and Astrocyte Cell Cultures
We first determined whether TWEAK and Fn14 protein expression could be detected in mouse cerebral cortex-derived neurons or astrocytes by indirect immunofluorescence analysis. In these experiments, we identified neurons by staining for the neuronal nuclear marker NeuN and astrocytes by staining for GFAP. We detected TWEAK and Fn14 expression in both neurons and astrocytes; however, TWEAK expression was significantly more pronounced in astrocytes (Figure 1; A to C) whereas Fn14 expression was more pronounced in neurons (Figure 1; D to F).
Figure 1.
Indirect immunofluorescence analysis of TWEAK and Fn14 expression in mouse cerebral cortex-derived cell cultures. Astrocyte-enriched 3-week-old cell cultures were incubated with anti-TWEAK antibodies in combination with anti-GFAP antibodies. TWEAK staining is shown in A, GFAP staining is shown in B, and a merged image is shown in C. Neuron-enriched 1-week-old cell cultures were incubated with anti-Fn14 antibodies in combination with anti-NeuN antibodies. Fn14 staining is shown in D, NeuN staining is shown in E, and a merged image is shown in F. Original magnifications, ×60.
TWEAK and Fn14 Expression after Ischemic Stroke
We then investigated TWEAK and Fn14 gene expression in the murine MCAO model of cerebral ischemia. This model produces a very reproducible area of infarct and changes in both the ischemic penumbra region of the ipsilateral hemisphere and the corresponding region of the nonischemic contralateral hemisphere can be monitored. The ischemic penumbra is defined as the region bordering the necrotic core with moderately reduced blood flow and partially preserved energy metabolism and is an area within the injured brain where significant inflammation and apoptosis occur.2,3
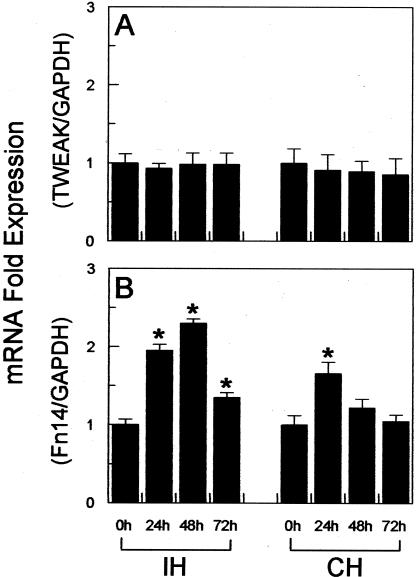
We first determined whether TWEAK or Fn14 mRNA expression levels were regulated in the brain after MCAO in the mouse. Ipsilateral (ischemic) or contralateral (nonischemic) hemisphere brain tissue was obtained from mice that were sacrificed either immediately after MCAO (0-hour time point) or at 24, 48, and 72 hours after MCAO and RNA was isolated. Real-time quantitative RT-PCR analysis revealed that TWEAK mRNA levels in both brain hemispheres remained relatively constant during the course of the experiment (Figure 2A). In contrast, we observed a statistically significant increase in Fn14 mRNA levels in the ipsilateral, ischemic hemisphere at all three time points, with peak induction at 48 hours (2.3-fold increase greater than baseline) (Figure 2B). An increase in Fn14 mRNA expression was also detected in the contralateral, nonischemic hemisphere at the 24-hour time point only (1.7-fold increase greater than baseline).
Figure 2.
Real-time quantitative RT-PCR analysis of TWEAK and Fn14 mRNA expression in mouse brain after cerebral infarction. Mice were subjected to MCAO and then sacrificed at the indicated time points. RNA was isolated from the ipsilateral hemisphere (IH) and contralateral hemisphere (CH) regions dissected from the same brain specimen and real-time RT-PCR was performed to analyze TWEAK mRNA (A) or Fn14 mRNA (B) expression. TWEAK and Fn14 mRNA expression levels were normalized to GAPDH mRNA expression, and then the IH and CH 24-, 48-, and 72-hour values were normalized to the corresponding 0-hour value. The values shown are mean ± SEM from one cDNA preparation analyzed in triplicate. Similar results were obtained with a second cDNA preparation. *, P < 0.05 versus the corresponding 0-hour value.
We next compared TWEAK and Fn14 protein expression levels and distribution in the ipsilateral and contralateral hemispheres by immunohistochemical staining. MCAO was performed, mice were sacrificed at the same time points as those listed above, and tissue sections were prepared. TWEAK immunoreactivity was detected in the ipsilateral hemisphere in the region surrounding the necrotic core (ie, the ischemic penumbra). This staining was most intense at 48 hours after MCAO (Figure 3, A and C) and TWEAK immunoreactivity was much lower in the corresponding region of the nonischemic contralateral hemisphere (Figure 3, B and D). Fn14 immunoreactivity was also detected in the ipsilateral hemisphere and again staining was most intense in the ischemic penumbra at 48 hours after MCAO (Figure 4, A and C). Fn14 immunoreactivity was significantly lower in the corresponding region of the contralateral hemisphere (Figure 4, B and D). Staining of the ischemic penumbra region was not detected when control rabbit IgG was used instead of anti-TWEAK or anti-Fn14 IgG as the primary immunological reagent (data not shown).
Figure 3.
Immunohistochemical analysis of TWEAK expression in mouse brain at 48 hours after cerebral infarction. Mice were subjected to MCAO and sacrificed 48 hours later. Tissue sections were prepared from the area surrounding the necrotic core in the ipsilateral hemisphere (A and C) and from a corresponding area in the healthy, nonischemic contralateral hemisphere (B and D) and stained with anti-TWEAK antibodies. Abbreviations: IP, ischemic penumbra; HT, healthy tissue. Original magnifications: ×20 (A, B); ×40 (C, D).
Figure 4.
Immunohistochemical analysis of Fn14 expression in mouse brain at 48 hours after cerebral infarction. Mice were subjected to MCAO and sacrificed 48 hours later. Tissue sections were prepared from the area surrounding the necrotic core in the ipsilateral hemisphere (A and C) and from a corresponding area in the healthy, nonischemic contralateral hemisphere (B and D) and stained with anti-Fn14 antibodies. Abbreviations: IP, ischemic penumbra; HT, healthy tissue. Original magnifications: ×20 (A, B); ×40 (C, D).
Effect of a Soluble Fn14-Fc Decoy Receptor on Stroke Volume
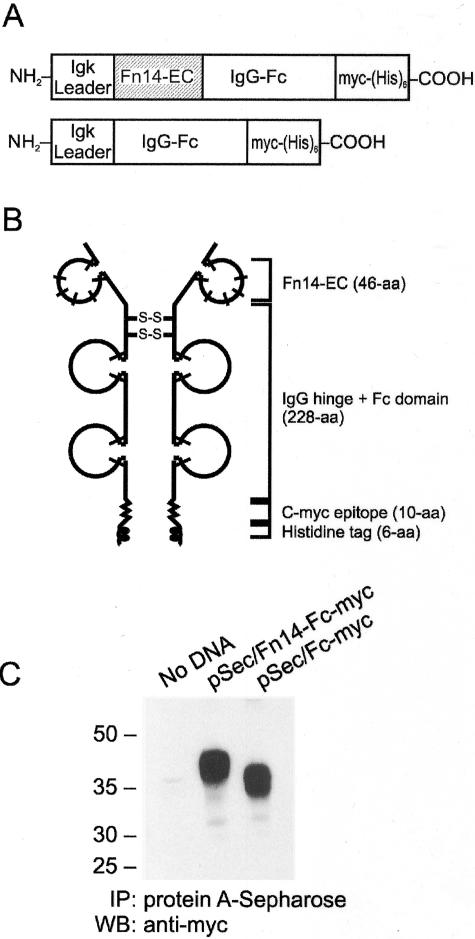
We next investigated whether TWEAK-Fn14 interactions played a role in cerebral ischemia-mediated brain injury using a soluble Fn14-Fc decoy receptor. This protein consists of the extracellular ligand-binding domain of murine Fn14 fused to the Fc portion and hinge region of the mouse IgG1 heavy chain17 (Figure 5, A and B). We have previously shown that Fn14-Fc can bind TWEAK with high affinity and inhibit TWEAK-stimulated cellular responses in vitro.17,26 For the present studies, we also constructed an additional plasmid that expresses only the Fc portion and hinge region of the mouse IgG1 heavy chain (Figure 5A). Initial transient transfection experiments demonstrated that the Fc protein was the appropriate apparent molecular mass and was efficiently secreted from cells (Figure 5C). This Fc protein, which does not bind TWEAK (data not shown), was purified from the conditioned medium of stably transfected cells in the same manner as the Fn14-Fc decoy receptor.17 PBS vehicle, the soluble Fn14-Fc decoy receptor, and the soluble Fc protein were each administered via intracerebroventricular injection immediately after MCAO. Mice were sacrificed 72 hours later and the volume of the ischemic area was calculated. Fn14-Fc decoy receptor administration resulted in a 37% reduction in infarct volume; in contrast, the Fc protein had no detectable effect (Figure 6).
Figure 5.
Structural properties and characterization of the murine Fn14-Fc and Fc proteins. A: The structural features of the pSecTag2 vector-based expression constructs encoding a soluble Fn14-Fc chimeric protein (top) or a soluble Fc protein (bottom) are shown. The murine Ig κ chain signal peptide region (Igk leader), the murine Fn14 extracellular domain (Fn14-EC, shaded area), the murine Ig hinge and Fc domain (IgG-Fc), and the region containing the myc epitope and polyhistidine tags [myc-(His)6] are indicated. B: A schematic depiction of the Fn14-Fc decoy receptor is shown. The cysteine-rich domains are represented by loops and the bars represent cysteine residues. C: Conditioned medium was collected from untransfected (no DNA), pSec/Fn14-Fc-myc-transfected, and pSec/Fc-myc-transfected HEK293T cells and incubated with protein A-Sepharose beads. Bound proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis using an anti-myc antibody.
Figure 6.
Effect of Fn14-Fc or Fc administration on infarct volume. Mice were subjected to MCAO and then vehicle (PBS), Fn14-Fc protein, or Fc protein was injected into the third ventricle. Mice were sacrificed 72 hours later and stroke volumes were measured. N = 5 per group and the values shown are mean ± SEM. *, P < 0.05 versus animals injected with PBS or Fc protein.
Effect of Fn14-Fc Administration on Microglial Cell Activation and Apoptotic Cell Death in the Ischemic Penumbra Region
Early after the onset of cerebral ischemia there is activation of microglial cells and a strong inflammatory reaction in the area surrounding the necrotic core (ischemic penumbra),2–4,27,28 followed by the appearance of apoptotic cell death in the same area.2,29 Because TWEAK appears to be a proinflammatory cytokine,5,8–12 we postulated that the Fn14-Fc decoy receptor could be acting, at least in part, by inhibiting TWEAK-stimulated brain inflammation. To test this hypothesis, PBS vehicle and the soluble Fn14-Fc decoy receptor were each administered via intracerebroventricular injection immediately after MCAO. Mice were sacrificed 72 hours later and tissue sections were prepared. Immunohistochemistry was then performed using an antibody that recognizes the integrin Mac-1 (αMβ2), a marker for microglial activation.30 TUNEL staining was also performed to examine apoptotic cell death. We found that Fn14-Fc treatment decreased both ischemia-induced microglial cell activation (Figure 7) and cellular apoptosis (Figure 8) in the area of the ischemic penumbra.
Figure 7.
Effect of Fn14-Fc administration on ischemia-induced microglial activation in the ischemic penumbra. Mice were subjected to MCAO and treated with either PBS (A and B) or with the Fn14-Fc decoy receptor (C and D) and sacrificed 72 hours later. Tissue sections were prepared from the area surrounding the necrotic core in the ipsilateral hemisphere (A and C) and from a corresponding area in the healthy, nonischemic contralateral hemisphere (B and D) and stained with anti-MAC-1 antibodies. Original magnifications, ×40.
Figure 8.
Effect of Fn14-Fc administration on ischemia-induced apoptotic cell death in the ischemic penumbra. Mice were subjected to MCAO and treated with either PBS (A and B) or with the Fn14-Fc decoy receptor (C and D) and sacrificed 72 hours later. Tissue sections were prepared from the area surrounding the necrotic core in the ipsilateral hemisphere (A and C) and from a corresponding area in the healthy, nonischemic contralateral hemisphere (B and D) and stained for apoptotic cells using a TUNEL-based fluorescence assay. Abbreviations: IP, ischemic penumbra; HT, healthy tissue. Original magnifications, ×20.
Discussion
In this study we investigated whether TWEAK, a member of the TNF superfamily that signals via the Fn14 receptor, played a role in ischemia-mediated brain injury in the mouse. Initial immunofluorescence experiments revealed that TWEAK and Fn14 were expressed by both murine neurons and astrocytes cultured in vitro. Our astrocyte data are consistent with a previous study demonstrating TWEAK and Fn14 mRNA expression in murine astrocytes.13 We found that TWEAK staining was most intense on astrocytes while Fn14 staining was most intense on neurons.
We then determined whether TWEAK and/or Fn14 gene expression was regulated in response to focal cerebral ischemia using a mouse MCAO model and both real-time quantitative RT-PCR and immunohistochemical assays. TWEAK mRNA was detected at similar levels in both brain hemispheres at all time points after MCAO; nevertheless, an elevated amount of TWEAK protein was found in the ischemic penumbra region of the ipsilateral hemisphere using an immunohistochemical approach. There are several possible explanations for this apparent discrepancy. First, TWEAK mRNA expression may be rapidly induced in the ischemic penumbra region and then return to basal levels by 24 hours, the first time point we examined using the real-time RT-PCR assay. If TWEAK is a relatively stable protein, then increased TWEAK immunoreactivity might be apparent at 24, 48, and 72 hours after MCAO. Second, the tissue specimen used for RNA isolation represented the entire ipsilateral hemisphere and contained multiple cell types, including endothelial cells, which express relatively high levels of TWEAK (data not shown). This could make it difficult to detect any changes in TWEAK mRNA expression that may only occur in the neurons or glia residing in the ischemic penumbra. Third, it is possible that TWEAK mRNA levels do not change at all in response to cerebral ischemia, but ischemia itself promotes either the redistribution of TWEAK or a conformational change in TWEAK in such a manner that antibody recognition is increased.
We found that Fn14 mRNA levels transiently increased in both the ipsilateral and contralateral hemispheres. In the ipsilateral, ischemic hemisphere, an increase in Fn14 mRNA levels was first detected at 24 hours, peak expression was at 48 hours, and the level was still greater than baseline at 72 hours after MCAO. In contrast, in the contralateral, nonischemic hemisphere, increased Fn14 mRNA expression was noted at only one time point (24 hours). The increase we observe in this hemisphere may reflect cellular stress because of the surgical procedure. The molecular mechanism responsible for increased Fn14 mRNA (and protein) expression in the ipsilateral hemisphere ischemic penumbra after MCAO is not known. Because the penumbra is an area of moderate hypoxia,31 Fn14 may be a hypoxia-inducible factor-1 target gene,32 but this possibility has not yet been investigated. However, we have shown that several growth factors can induce Fn14 gene expression in vitro.14,17,20,33 Two of these growth factors, FGF-234 and VEGF-A,31 are expressed at elevated levels in the ischemic border after focal cerebral ischemia and thus one or both could play a role in Fn14 gene up-regulation after MCAO.
Finally, we investigated whether TWEAK-Fn14 signaling within the brain could contribute to focal ischemic injury by blocking the interaction between endogenously-expressed TWEAK and Fn14 using a previously characterized soluble Fn14-Fc decoy receptor.17,26 Intracerebroventricular injection of this protein immediately after MCAO significantly reduced infarct volume. This result implies that TWEAK is acting as a neurotoxic factor in the ischemic brain. We propose that this could occur via two alternative mechanisms. First, TWEAK may be directly stimulating apoptosis. Although there are reports that TWEAK treatment of certain human tumor cell lines can induce apoptosis,35 this does not appear to be its major biological activity.6 Indeed, of particular relevance to this study, TWEAK treatment of rat PC12 cells,36 human glioma cells,26 human astrocytes,8 or mouse astrocytes13 does not cause cell death. Second, TWEAK may be indirectly stimulating apoptosis by contributing to the focal ischemia-associated inflammatory response.2–4,27,28 This is the mechanism that we favor at the present time because TWEAK treatment of several different cell types, including astrocytes, has been shown to stimulate the expression of various proinflammatory molecules.5,8–12 Our results demonstrating that Fn14-Fc decoy receptor treatment results in a significant decrease in microglial cell activation and apoptotic cell death in the area of the ischemic penumbra support this hypothesis.
It has been reported that TWEAK binding to Fn14 activates the NF-κB signal transduction pathway,11,15–18 and this pathway is a key regulator of numerous inflammatory response genes.37 TWEAK regulation of NF-κB function is of particular interest in the present context because this transcription factor is activated during ischemic brain injury and contributes to neuronal cell death.38,39 It should be noted that TWEAK also activates the ERK and JNK signal transduction pathways.17 TWEAK-stimulated ERK and JNK pathway activation in the brain may also be involved in TWEAK neurotoxicity in consideration of recent studies demonstrating that specific inhibitors of these pathways reduce infarct volume in rodent models of focal cerebral ischemia.40–43
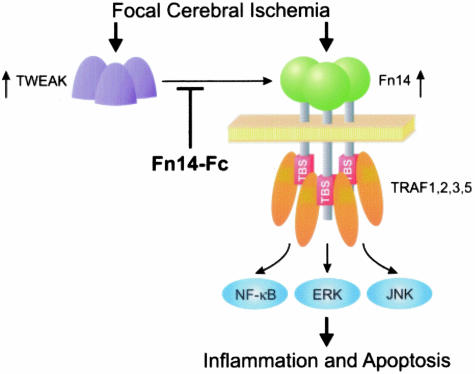
In summary, we have shown that TWEAK and Fn14 are present at elevated levels in the ischemic penumbra in a murine model of stroke. We hypothesize that TWEAK binding to Fn14 receptors promotes the activation of signal transduction pathways, the secretion of proinflammatory and proapoptotic cytokines, and cell death (Figure 9). Our findings suggest that the TWEAK-Fn14 signaling pathway might be a potential target for therapeutic strategies aimed at promoting neuronal cell survival during acute cerebral ischemia.
Figure 9.
The TWEAK-Fn14 signaling system may play a role in stroke pathophysiology. We have shown here that focal cerebral ischemia promotes an increase in TWEAK and Fn14 expression in the ischemic penumbra. We propose that TWEAK binding to Fn14 cell surface receptors would then induce TNFR-associated factor (TRAF) 1, 2, 3, and 5 binding to the TRAF binding site (TBS) on the Fn14 cytoplasmic tail.15 NF-κB, ERK, and/or JNK pathway activation17 would then stimulate cellular production of proinflammatory cytokines and apoptotic factors, resulting in neurotoxicity and brain injury. We hypothesize that administration of the soluble Fn14-Fc decoy receptor into the brain prevents endogenous TWEAK from binding to Fn14 cell surface receptors and thereby reduces ischemia-mediated cell death.
Acknowledgments
We thank Ms. Hong Vu, Dr. Elena Loukinova, and Ms. Heather Hanscom for technical assistance; and Dr. Li Zhang and Dr. Timothy Zheng for providing the antibodies.
Footnotes
Address reprint requests to Jeffrey A. Winkles, Ph.D., Department of Surgery, University of Maryland School of Medicine, 15601 Crabbs Branch Way, Rockville, MD 20855. E-mail: jwinkles@som.umaryland.edu.
Supported in part by the National Institutes of Health (grants NS-02223 to M.Y., HL-55374 and HL-55747 to D.A.L., and HL-39727 to J.A.W.).
References
- Jamison DT, Creese A, Prentice T. Geneva: The World Health Organization,; The World Health Report. 1999:pp 1–121. [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Barone FC, Parsons AA. Therapeutic potential of anti-inflammatory drugs in focal stroke. Expert Opin Invest Drugs. 2000;9:2281–2306. doi: 10.1517/13543784.9.10.2281. [DOI] [PubMed] [Google Scholar]
- Zhang W, Stanimirovic D. Current and future therapeutic strategies to target inflammation in stroke. Curr Drug Targets Inflamm Allergy. 2002;1:151–166. doi: 10.2174/1568010023344689. [DOI] [PubMed] [Google Scholar]
- Chicheportiche Y, Bourdon PR, Xu H, Hsu Y, Scott H, Hession C, Garcia I, Browning JL. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- Wiley SR, Winkles JA. TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor Rev. 2003;14:241–249. doi: 10.1016/s1359-6101(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Polek TC, Talpaz M, Darnay BG, Spivak-Kroizman T. TWEAK mediates signal transduction and differentiation of RAW264.7 cells in the absence of Fn14/TweakR. Evidence for a second TWEAK receptor. J Biol Chem. 2003;278:32317–32323. doi: 10.1074/jbc.M302518200. [DOI] [PubMed] [Google Scholar]
- Saas P, Boucraut J, Walker PR, Quiquerez A, Billot M, Desplat-Jego S, Chicheportiche Y, Dietrich P. TWEAK stimulation of astrocytes and the proinflammatory consequences. Glia. 2000;32:102–107. [PubMed] [Google Scholar]
- Chicheportiche Y, Chicheportiche R, Sizing I, Thompson J, Benjamin CB, Ambrose C, Dayer J. Proinflammatory activity of TWEAK on human dermal fibroblasts and synoviocytes: blocking and enhancing effects of anti-TWEAK monoclonal antibodies. Arthritis Res. 2002;4:126–133. doi: 10.1186/ar388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Nakayama M, Nakano H, Fukuchi Y, Yagita H, Okumura K. Pro-inflammatory effect of TWEAK/Fn14 interaction on human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2002;299:488–493. doi: 10.1016/s0006-291x(02)02670-0. [DOI] [PubMed] [Google Scholar]
- Xu H, Okamoto A, Ichikawa J, Ando T, Tasaka K, Masuyama K, Ogawa H, Yagita H, Okumura K, Nakao A. TWEAK/Fn14 interaction stimulates human bronchial epithelial cells to produce IL-8 and GM-CSF. Biochem Biophys Res Commun. 2004;318:422–427. doi: 10.1016/j.bbrc.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kang YJ, Kim WJ, Woo DK, Lee Y, Kim DI, Park YB, Kwon BS, Park JE, Lee WH. TWEAK can induce pro-inflammatory cytokines and matrix metalloproteinase-9 in macrophages. Circ J. 2004;68:396–399. doi: 10.1253/circj.68.396. [DOI] [PubMed] [Google Scholar]
- Desplat-Jego S, Varriale S, Creidy R, Terra R, Bernard D, Khrestchatisky M, Izui S, Chicheportiche Y, Boucraut J. TWEAK is expressed by glial cells, induces astrocyte proliferation and increases EAE severity. J Neuroimmunol. 2002;133:116–123. doi: 10.1016/s0165-5728(02)00368-5. [DOI] [PubMed] [Google Scholar]
- Wiley SR, Cassiano L, Lofton T, Davis-Smith T, Winkles JA, Lindner V, Liu H, Daniel TO, Smith CA, Fanslow WC. A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity. 2001;15:837–846. doi: 10.1016/s1074-7613(01)00232-1. [DOI] [PubMed] [Google Scholar]
- Brown SA, Richards CM, Hanscom HN, Feng SL, Winkles JA. The Fn14 cytoplasmic tail binds tumour-necrosis-factor-receptor-associated factors 1, 2, 3 and 5 and mediates nuclear factor-kappaB activation. Biochem J. 2003;371:395–403. doi: 10.1042/BJ20021730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Yoon K, Lee K, Kim K, Jang H, Lee NK, Hwang K, Young LS. TNF-related weak inducer of apoptosis receptor, a TNF receptor superfamily member, activates NF-kappa B through TNF receptor-associated factors. Biochem Biophys Res Commun. 2003;305:789–796. doi: 10.1016/s0006-291x(03)00852-0. [DOI] [PubMed] [Google Scholar]
- Donohue PJ, Richards CM, Brown SA, Hanscom HN, Buschman J, Thangada S, Hla T, Williams MS, Winkles JA. TWEAK is an endothelial cell growth and chemotactic factor that also potentiates FGF-2 and VEGF-A mitogenic activity. Arterioscler Thromb Vasc Biol. 2003;23:594–600. doi: 10.1161/01.ATV.0000062883.93715.37. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Nakayama M, Nakano H, Yagita H, Yamamoto N, Yamaoka S. TWEAK induces NF-kappaB2 p100 processing and long lasting NF-kappaB activation. J Biol Chem. 2003;278:36005–36012. doi: 10.1074/jbc.M304266200. [DOI] [PubMed] [Google Scholar]
- Yepes M, Moore E, Brown SA, Hanscom HN, Smith EP, Lawrence DA, Winkles JA. Progressive ankylosis (Ank) protein is expressed by neurons and Ank immunohistochemical reactivity is increased by limbic seizures. Lab Invest. 2003;83:1025–1032. doi: 10.1097/01.lab.0000075640.49586.e6. [DOI] [PubMed] [Google Scholar]
- Meighan-Mantha RL, Hsu DKW, Guo Y, Brown SAN, Feng SY, Peifley KA, Alberts GF, Copeland NG, Gilbert DJ, Jenkins NA, Richards CM, Winkles JA. The mitogen-inducible Fn14 gene encodes a type I transmembrane protein that modulates fibroblast adhesion and migration. J Biol Chem. 1999;274:33166–33176. doi: 10.1074/jbc.274.46.33166. [DOI] [PubMed] [Google Scholar]
- Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. San Diego: CA Academic Press, Inc.,; The Mouse Brain in Stereotaxic Coordinates. 2001:pp 1–93. [Google Scholar]
- Yepes M, Sandkvist M, Wong MK, Coleman TA, Smith E, Cohan SL, Lawrence DA. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000;96:569–576. [PubMed] [Google Scholar]
- Osborne KA, Shigeno T, Balarsky AM, Ford I, Mcculloch J, Teasdale GM, Graham DI. Quantitative assessment of early brain damage in a rat model of focal cerebral ischaemia. J Neurol Neurosurg Psychiatry. 1987;50:402–410. doi: 10.1136/jnnp.50.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NL, McDonough WS, Donohue PJ, Winkles JA, Berens TJ, Ross KR, Hoelzinger DB, Beaudry C, Coons SW, Berens ME. The human Fn14 receptor gene is up-regulated in migrating glioma cells in vitro and overexpressed in advanced glial tumors. Am J Pathol. 2003;162:1313–1321. doi: 10.1016/S0002-9440(10)63927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison JA, Barone FC, Feuerstein GZ. Matrix remodeling after stroke. De novo expression of matrix proteins and integrin receptors. Ann NY Acad Sci. 1999;890:204–222. doi: 10.1111/j.1749-6632.1999.tb07996.x. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62:329–339. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- Kloss CU, Bohatschek M, Kreutzberg GW, Raivich G. Effect of lipopolysaccharide on the morphology and integrin immunoreactivity of ramified microglia in the mouse brain and in cell culture. Exp Neurol. 2001;168:32–46. doi: 10.1006/exnr.2000.7575. [DOI] [PubMed] [Google Scholar]
- Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- Feng SY, Guo Y, Factor VM, Thorgeirsson SS, Bell DW, Testa JR, Peifley KA, Winkles JA. The Fn14 immediate-early response gene is induced during liver regeneration and highly expressed in both human and murine hepatocellular carcinomas. Am J Pathol. 2000;156:1253–1261. doi: 10.1016/S0002-9440(10)64996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TN, Te J, Lee M, Sun GY, Hsu CY. Induction of basic fibroblast growth factor (bFGF) expression following focal cerebral ischemia. Brain Res Mol Brain Res. 1997;49:255–265. doi: 10.1016/s0169-328x(97)00152-6. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Ishidoh K, Kojima Y, Harada N, Kominami E, Okumura K, Yagita H. Fibroblast growth factor-inducible 14 mediates multiple pathways of TWEAK-induced cell death. J Immunol. 2003;170:341–348. doi: 10.4049/jimmunol.170.1.341. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Bonilla I, Winkles JA, Strittmatter SM. Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth. J Neurosci. 2003;23:9675–9686. doi: 10.1523/JNEUROSCI.23-29-09675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- Xu L, Zhan Y, Wang Y, Feuerstein GZ, Wang X. Recombinant adenoviral expression of dominant negative IkappaBalpha protects brain from cerebral ischemic injury. Biochem Biophys Res Commun. 2002;299:14–17. doi: 10.1016/s0006-291x(02)02573-1. [DOI] [PubMed] [Google Scholar]
- Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Natl Acad Sci USA. 1999;96:12866–12869. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namura S, Iihara K, Takami S, Nagata I, Kikuchi H, Matsushita K, Moskowitz MA, Bonventre JV, Alessandrini A. Intravenous administration of MEK inhibitor U0126 affords brain protection against forebrain ischemia and focal cerebral ischemia. Proc Natl Acad Sci USA. 2001;98:11569–11574. doi: 10.1073/pnas.181213498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang H, Xu L, Rozanski DJ, Sugawara T, Chan PH, Trzaskos JM, Feuerstein GZ. Significant neuroprotection against ischemic brain injury by inhibition of the MEK1 protein kinase in mice: exploration of potential mechanism associated with apoptosis. J Pharmacol Exp Ther. 2003;304:172–178. doi: 10.1124/jpet.102.040246. [DOI] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]