Abstract
Neuroblastoma (NB) tumors with abundant schwannian stroma have a differentiated phenotype, low vascularity, and are associated with a favorable prognosis. These observations suggest that cross-talk between Schwann cells and neuroblasts may influence tumor biology. To test this hypothesis, we developed a novel NB xenograft model with infiltrating mouse Schwann cells. Human SMS-KCNR NB cells were injected intrafascicularly (sciatic nerve-engrafted NB, n = 19) or outside the sciatic nerve (control, n = 12). Xenografts were harvested 4 to 12 weeks after tumor cell inoculation for histological studies. Schwann cells were immunostained with S-100 and species-specific p75NGFR, major histocompatibility complex, and human leukocyte antigen antibodies. The number of proliferating cells, infiltrating Schwann cells, apoptotic cells, differentiated neuroblasts, and blood vessels in the sciatic nerve-engrafted NB tumors were compared to controls. Significantly more Schwann cells were detected in the sciatic nerve-engrafted NB xenografts than controls (P < 0.001). The infiltrating Schwann cells were S-100-positive and reacted with anti-mouse major histocompatibility complex class Ib and p75NGFR but not anti-human p75NGFR and human leukocyte antigen class I antibodies. The sciatic nerve-engrafted tumors also had lower numbers of proliferating neuroblasts, higher numbers of differentiated neuroblasts and apoptotic cells, and decreased vascular density compared to controls. Our results indicate that infiltrating Schwann cells of mouse origin are capable of promoting human neuroblast differentiation, inducing apoptosis, and inhibiting proliferation and angiogenesis in vivo.
Neuroblastoma (NB) is the most common malignant tumor in infants and the fourth most common malignancy in children older than 1 year of age.1 This tumor arises from neural crest tissue and is remarkable for its broad spectrum of clinical behavior. NBs may regress spontaneously in infants, mature into benign ganglioneuromas in older children, or grow relentlessly and be rapidly fatal.1 Response to therapy and patient outcome directly correlate with age of presentation, stage of disease, tumor histology, and the molecular and genetic features of the tumor.2,3
NB tumors consist of two main cell populations, neuroblastic/ganglionic cells and Schwann cells, and the quantity of schwannian stroma directly correlates with the tumor maturation.4,5 A paucity of schwannian stroma is seen in undifferentiated tumors, whereas differentiating or differentiated neuroblasts that histologically resemble ganglion cells are seen in tumors with abundant schwannian stroma. The origin of the Schwann cells and their role in regulating the maturation of tumor cells remain controversial. Schwann cells in maturing NB tumors appear to lack the genetic anomalies found in the neuroblastic cells,6 suggesting that they are reactive cells recruited from the surrounding nonneoplastic tissue. On the basis of known interactions between neuroblasts and their supporting stroma, Ambros and colleagues6 speculated that the recruited Schwann cells induced neuroblast differentiation by producing anti-proliferation and prodifferentiation factors. However, other studies indicate that the stromal cells and neuroblasts may originate from a common neoplastic stem cell. In one report, shared genetic abnormalities were identified in cultured stromal-like cells and neuroblasts derived from involved bone marrow.7 Other investigators have reported identical genetic alterations in neuroblastic and nonneuronal cells derived from NB tumors.8
The favorable prognostic impact of schwannian stroma has been emphasized in the International Neuroblastoma Pathology Classification System.5 Most tumors with abundant schwannian stroma are classified as favorable histology and are associated with high rates of cure.5,9 These observations indicate that Schwann cells may be capable of influencing NB phenotype, and several laboratory studies support this hypothesis. Kwiatkowski and colleagues10 have shown that Schwann cell-conditioned medium promotes NB differentiation in vitro. NB differentiation is also induced when NB cells and Schwann cells are co-cultured.11 In addition, we and others have shown that Schwann cells produce potent inhibitors of angiogenesis that play a key role in mediating the anti-tumor activity of Schwann cell-conditioned media.12–14
To directly investigate the in vivo effects of cross-talk between Schwann cells and neuroblasts, we developed a novel NB xenograft model in which human SMS-KCNR NB cells were inoculated into mouse sciatic nerves. For negative controls, NB cells were inoculated outside the sciatic nerve. Our results demonstrate that infiltrating mouse Schwann cells are capable of influencing NB tumor proliferation, differentiation, apoptosis, and angiogenesis in vivo.
Materials and Methods
Animal Model
Female athymic nude mice (Harlan, Indianapolis, IN), 6 to 7 weeks old, were anesthetized by an intraperitoneal injection of 30 μl of a ketamine and xylazine mixture at a ratio of 5:3. The left sciatic nerve was surgically exposed. Cultured human SMS-KCNR NB cells were washed three times in media, and resuspended in RPMI 1640 complete media at a concentration of 5 × 105 cells per 5 μl. SMS-KCNR NB cells (5 × 105) were microinjected into the left sciatic nerve of 19 mice using a 10-μl Hamilton syringe as previously described.15 In control animals, the same number of NB cells were microinjected outside the sciatic nerve (n = 12). Tumor volume, [(length × width)2/2], was measured once a week. Animals were sacrificed when tumors were >500 mm3, and the tumors were harvested for histological and immunohistochemical evaluation. All animals were treated according to the National Institutes of Health guidelines for animal care and use, following protocols approved by the Animal Care and Use Committee at Northwestern University.
Tissue Processing
Xenograft tissue sections (3 mm thick) were cut at maximum diameter, fixed in 10% formaldehyde/zinc fixative (Electron Microscopy Sciences, Hatfield, PA), and embedded in paraffin. The adjacent portion of tissue was frozen with liquid nitrogen and embedded in O.C.T. compound (Sakura Finetech, Torrance, CA). Four-μm-thick serial paraffin sections were heated at 57°C for 60 minutes, deparaffinized in CitriSolv (Fisher, Pittsburgh, PA) two times for 5 minutes, and rehydrated in graded ethanol and deionized water. Sections of each tumor were stained with hematoxylin and eosin for histological evaluation. Frozen sections were fixed with cold acetone for 15 minutes and stored at −80°C until staining. Adjacent sections were used for immunohistochemistry and in situ hybridization.
Immunohistochemistry
Antigen retrieval was performed with 10 mmol/L citrate buffer (pH 6.0) for S-100, GAP-43, p75NGFR, Ki-67, human leukocyte antigen (HLA) class I, and major histocompatibility complex (MHC) class Ib antibodies, and with 1 mmol/L ethylenediaminetetraacetic acid (pH 8.0) for CD31 antibody, heated in a boiling steamer for 20 minutes, and then cooled down to room temperature for 20 minutes. Sections were incubated with the following primary antibodies: mouse monoclonal S-100 (Ab-1, clone 4C4.9, 1:100; NeoMarkers, Fremont, CA) that is reactive with both human and mouse; mouse anti-GAP-43 (clone 7B10, 1:200; Zymed Lab. Inc., San Francisco, CA) that is reactive with human and mouse; mouse anti-human p75NGFR (ab10495, clone ME20.4, 1:200; Abcam, Cambridge, MA); polyclonal rabbit anti-mouse p75NGFR (1:800, Abcam); monoclonal mouse anti-human Ki-67 (clone MIB-1, 1:200; DakoCytomation, Carpinteria, CA); monoclonal rat anti-human HLA class I (clone YTH 862.2, 1:1000; Serotec, Raleigh, NC); monoclonal hamster anti-mouse MHC class Ib (130) (1:200; Santa Cruz Biotechnology Inc., Santa Cruz, CA); and polyclonal goat anti-mouse CD31 (PECAM-1, M-20, 1:100; Santa Cruz Biotechnology Inc.). The sections were incubated in a humidity chamber overnight at 4°C, bridged with peroxidase labeled-dextran polymer to avoid nonspecific staining, and visualized with diaminobenzidine (DAKO EnVision Plus System, DakoCytomation). The HLA, MHC, and CD31 primary antibodies were linked by biotinylated rabbit anti-rat, goat anti-hamster, or horse anti-goat IgG, respectively, at a concentration of 1:200 for each secondary antibody and streptavidin (1:400; Vector Laboratories, Burlingame, CA). Sections were counterstained with Gill’s hematoxylin. The following tissues and cell lines served as positive or negative controls, respectively, for antigen expression: S-100 (human schwannoma and mouse sciatic nerve versus the human NBL-W-N NB cell line), GAP-43 (human brain and pancreas versus NBL-W-N), MHC class Ib (human Schwannoma versus mouse kidney), HLA class I (mouse tail versus human kidney), human p75NGFR (human schwannoma versus NBL-W-N), mouse p75NGFR (mouse sciatic nerve versus NBL-W-N), and CD31 (mouse kidney served as both positive and negative controls). The control tissues and cell lines were processed in the same manner as the xenografts. In additional control experiments, the primary antibodies were omitted. All immunostainings were performed on paraffin-embedded sections except the rat anti-human HLA class I antibody, which was performed on frozen sections. To ensure specificity and sensitivity, the amount of antibody used in the studies was carefully optimized to the level with maximal positivity without microscopically visible background on the control tissues described above. To ensure the consistency of the immunoassays, all 31 samples plus negative and positive controls were stained at one time for each marker using the same conditions. Brown staining in the cytoplasm, membrane, or nucleus of the neuroblasts, Schwann cells, or endothelial cells was scored as positive.
Immunofluorescence
Histological sections of the NB xenografts were double-stained with anti-S-100 and specific anti-mouse or anti-human p75NGFR antibodies. Antigens were retrieved using 10 mmol/L citrate buffer at pH 6.0 and blocked with 10% normal horse serum and CAS universal blocking reagent (Zymed) for 15 minutes each at room temperature to minimize species cross reactivity. The concentrations were 1:100 for S-100, and 1:100 and 1:400 for the p75NGFR anti-mouse and anti-human primary antibodies, respectively. A concentration of 1:200 was used for both the anti-mouse Texas Red and anti-rabbit fluorescein isothiocyanate-labeled secondary antibodies. The nuclei were counterstained with 4,6-diamidino-2-phenylindole (Vector Laboratories).
Quantification of S-100-Positive Schwann Cells
S-100-positive Schwann cells were quantified by counting 10 consecutive high-power (×400 magnification) fields starting at the area with the highest density of S-100-positive cells. The total of the mean S-100-positive Schwann cells was converted to S-100-positive cells per square millimeter.
Semiquantitation of p75NGFR and GAP-43
Cytoplasmic staining was considered as positive for GAP-43 and p75NGFR. Sections with no or ≤5% NB cells stained with GAP-43 were scored as 0. Whole tumor sections with >5% and ≤25% NB cells stained with GAP-43 were scored as 1+; >25% and ≤50% were scored as 2+; >50% were scored as 3+. For p75NGFR, tumor sections with ≤5% positive neuroblasts or Schwann cells were scored as negative. Sections with >5% p75NGFR-stained neuroblasts or Schwann cells were scored as positive.
Quantification of Apoptosis and MIB-1 (Ki-67) Labeling
In situ detection of cleaved, apoptotic DNA fragments was performed on 31 xenografts using the In Situ Cell Death Detection kit (Roche Diagnostics Corp., Indianapolis, IN) as described previously.16 Briefly, rehydrated paraffin sections of xenografts were digested with 20 μg/ml Proteinase K in 10 mmol/L Tris hydrochloride buffer (pH 7.6) at room temperature for 20 minutes and blocked with CAS (Zymed) and TSA (Perkin-Elmer Life Sciences, Boston, MA) blocking solutions at room temperature for 15 and 30 minutes, respectively. Sections were incubated with a mixture of terminal dUTP nick-end labeling (TUNEL) enzyme solution and label solution at a ratio of 1:10 in a humidity chamber at 37°C for 60 minutes. The reaction was terminated with 1% Tween 20 in 1× Tris-buffered saline buffer at room temperature for 10 minutes, and counterstained and shielded with VectaShield containing 4,6-diamidino-2-phenylindole (Vector Laboratories, Inc.). Sections of human colon mucosa processed similarly to the xenografts were included in each assay. To check for intra- and interassay consistency, the apoptotic bodies in colonic mucosa, stained as the first and the last slide of each assay, were assessed. The assays were considered adequate only when the frequencies of apoptosis on the two sections of colonic mucosa were similar. Each TUNEL in situ-labeled section was quantified at a magnification of ×400 using a Leica DM IRB inverted fluorescence microscope equipped with Image-Pro Plus Version 4.5 software (Media Cybernetics, Silver Spring, MD). The frequency of labeled apoptotic cells was obtained by quantifying 10 consecutive fields starting with areas with the highest number of TUNEL-labeled nuclei, avoiding areas of necrosis, and expressed as apoptosis per 10 high-power fields (HPFs). The MIB-1 (Ki-67)-labeled proliferating cells were quantified and expressed in the same way as TUNEL-labeled apoptosis.
Quantification of Angiogenesis
Endothelial cells were highlighted by immunostaining of CD31 on paraffin-embedded xenograft tissue sections. Mean vascular density (MVD) was quantified by counting 10 consecutive HPFs at ×400 magnification. The average MVD counted in the 10 fields was converted and expressed as MVD/mm2.
Statistical Analysis
The two-tailed Student’s t-test was used to compare the statistical significance of differences between the mean values of quantification of S-100, Ki-67, CD31, and apoptosis in the NB xenografts engrafted inside versus outside the sciatic nerve. Fisher’s exact test was used to test the significance of difference of frequency of p75NGFR and GAP-43 expression in the xenografts that developed within the sciatic nerve versus controls.
Results
Growth Rates of NB Xenografts
Tumor volumes were measured in 11 mice after intrafascicular tumor inoculation and in 6 controls. Surprisingly, the sciatic nerve-engrafted xenografts initially grew more rapidly than controls. Three weeks after microinjection of the NB tumor cells into the sciatic nerve, palpable tumors were detected in 3 of 11 mice. By 4 weeks, tumors were detected in 6 of the 11 animals. In contrast, none of the control tumors were palpable at 3 weeks, and only two of six mice had tumors by 4 weeks. At 5 weeks the average size of tumors engrafted within the nerve was 30 mm3 compared to 3 mm3 for control animals (P = 0.021). Although the sciatic nerve-engrafted tumors were also larger than controls at 6 and 7 weeks, the difference in size was no longer statistically significant (P = 0.066 and P = 0.23, respectively).
Mouse Schwann Cells Infiltrate Human NB Xenografts
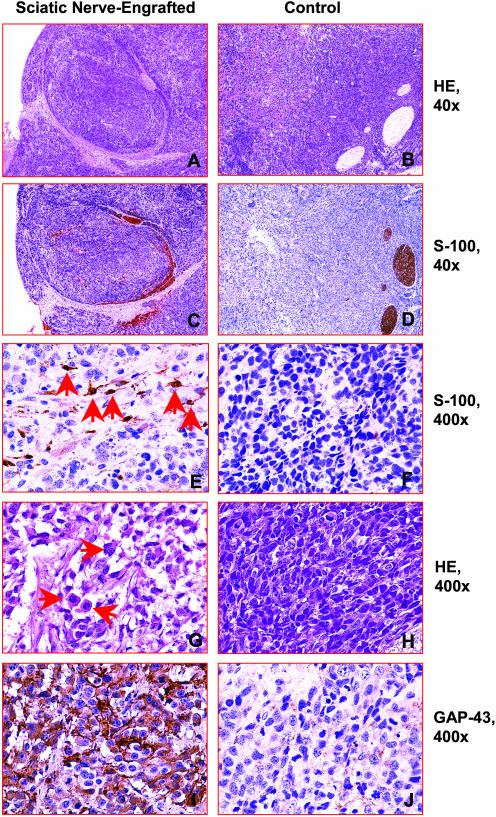
To confirm the location of the NB xenografts in relation to the mouse sciatic nerve, histological sections of the xenografts were examined. Schwann cells in the mouse sciatic nerve were identified by routine morphology and by immunohistochemical staining with S-100 antibody (Figure 1; A to D). In 12 of the 19 NB xenografts that developed after tumor cell inoculation into the sciatic nerve, the tumor was completely encased by the nerve, and in the remaining animals the sciatic nerve was in direct contact with tumor cells. All 12 control tumors grew in close proximity to the sciatic nerve, although none of the tumors were encased by the nerve tissue. Infiltrating Schwann cells were detected by immunohistochemistry in all 19 NB xenografts engrafted in the sciatic nerve, with an average of 240 ± 136 Schwann cells/mm2 (Figure 1E). In contrast, Schwann cells were not identified in 9 of the 12 control tumors, and the remaining 3 tumors contained significantly fewer numbers of Schwann cells (14 ± 13 cells/mm2, P < 0.001) (Figure 1F).
Figure 1.
Representative histological sections of tumors engrafted inside versus outside the sciatic nerve. A and B: H&E-stained sections of SMS-KCNR NB cells engrafted inside versus outside the sciatic nerve. C–F: S-100-immunostained serial sections adjacent to the respective H&E sections demonstrating NB cells encased by the sciatic nerve and Schwann cells infiltrated into the tumors. Schwann cells are highlighted by anti-S-100 antibody in C and E. Control xenografts with SMS-KCNR NB cells engrafted outside the sciatic nerve are shown in D and F. Significantly more S-100-positive Schwann cells were present in the tumor engrafted inside the sciatic nerve (arrows in E) compared to controls (F). G and H: NB cells of tumors engrafted inside the sciatic nerve were larger with abundant eosinophilic cytoplasm (arrows in G) compared to control tumors (H). I and J: Serial sections from the same tumor stained with GAP-43 show higher levels of GAP-43 expression in the NB cells engrafted in the sciatic nerve than in the controls.
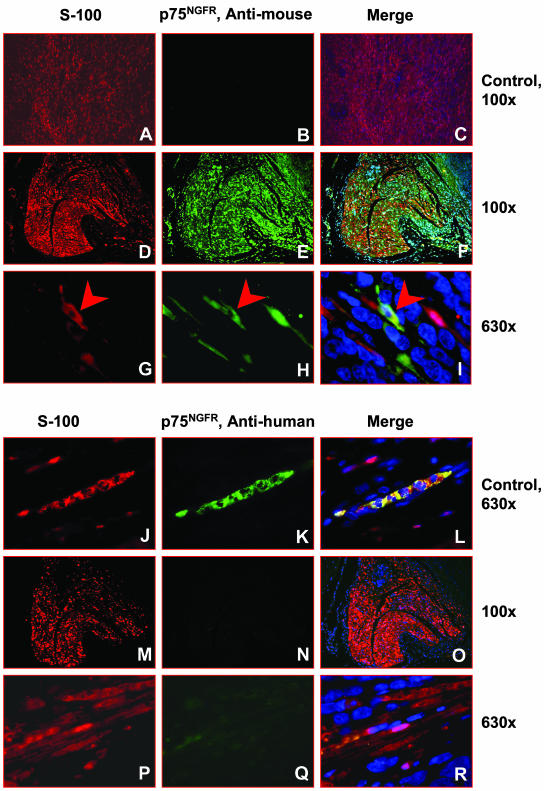
To determine the origin of the infiltrating Schwann cells, additional immunohistochemistry studies were performed with species-specific anti-mouse p75NGFR and MHC class Ib antibodies as well as anti-human HLA class I and p75NGFR antibodies. Infiltrating Schwann cells reacted with the anti-mouse p75NGFR and MHC class Ib antibodies but not with the anti-human p75NGFR and HLA class I antibodies, indicating the Schwann cells are of mouse origin (Figures 2 and 3). Double-immunofluorescence staining demonstrated that the S-100-positive Schwann cells reacted with the anti-mouse p75NGFR antibody (Figure 2, E and H), but did not react with the anti-human p75NGFR antibody (Figure 2, N and Q). The infiltrating Schwann cells were also reactive with anti-mouse MHC class Ib antibody (Figure 3C) but not reactive with anti-human HLA class I antibody (Figure 3G). Anti-human p75NGFR also reacted with a subset of the neuroblasts in 7 of the 19 sciatic nerve-engrafted xenografts. Interestingly, p75NGFR was not detected in the neuroblasts in the control tumors, further suggesting that the biology of the neuroblasts in the control versus nerve-engrafted xenografts differed.
Figure 2.
Representative photographs of tumors engrafted inside versus outside the sciatic nerve stained by double immunofluorescence. A–C: Human schwannoma serves as a negative control for anti-mouse p75NGFR antibody. Schwann cells in the human schwannoma are positive for S-100 (A) but negative for anti-mouse p75NGFR (B). A merged image is shown in C. D–I: Representative photographs taken from serial sections of a tumor engrafted inside the sciatic nerve that is stained by double immunofluorescence using S-100 (D) and p75NGFR anti-mouse antibodies (E). A merged image is shown in F. High-power views demonstrate that S-100-positive Schwann cells also react with anti-mouse p75NGFR antibody and the two antibodies are co-localized (arrowheads in G–I). J–L: Human schwannoma serves as a positive control for anti-human p75NGFR antibody. S-100-positive Schwann cells (J) were positive for anti-human p75NGFR (K). L: A merged image shows dual positivity in the Schwann cells. M–R: The S-100-positive Schwann cells do not react with anti-human p75NGFR.
Figure 3.
A–H: Representative photographs taken from anti-mouse MHC class Ib and anti-human HLA class I-immunostained histological sections from sciatic nerve-engrafted tumors and control tissues from human and mouse. Schwann cells in the human schwannoma were negative (A) and the tubular epithelial cells of mouse kidney were positive for the anti-mouse MHC class Ib antibody (B). S-100-positive infiltrating Schwann cells (D) in the sciatic nerve-engrafted tumor were positive for the anti-mouse MHC antibody (C). Tissues from mouse tail (E) and S-100-positive infiltrating Schwann cells (H) in the sciatic nerve-engrafted tumor (G) were negative but epithelial cells of human kidney glomerulus were positive (F) for the anti-human HLA class I antibody. Original magnifications, ×400.
NB Differentiation Is Induced in the Sciatic Nerve NB Xenografts
The morphological features of the NB cells in the xenografts that developed inside versus outside the sciatic nerve differed. Large differentiating neuroblasts with abundant eosinophilic cytoplasm were present in the NB xenografts engrafted within the nerve (Figure 1G). In contrast, undifferentiated NB cells with high nuclear:cytoplasmic ratios were seen in the control tumors (Figure 1H). Numerous mitotic and karyorrhectic cells were also detected in the control tumors, which are histological features of high cell turnover and one of the criteria for unfavorable histology NB.5 To further evaluate the maturation of the NB cells, tumor sections were immunostained with GAP-43, a protein synthesized at high levels during axonal outgrowth and a marker of neuronal differentiation.17,18 In the xenografts engrafted within the sciatic nerve, >50% of the tumor cells were positive for GAP-43 expression (17 of 19 scored as 2+ or 3+), whereas 0 to ≤25% of the NB cells in control xenografts weakly reacted with GAP-43 (11 of 12 scored as 0 or 1+, P < 0.001) (Table 1; Figure 1, I and J).
Table 1.
GAP-43 Expression in SMS-KCNR NB Xenografts
| GAP-43 intensity | Control tumors, n (%) | Sciatic nerve-engrafted tumors, n (%) | P value* |
|---|---|---|---|
| 0 | 2 (16.6) | 0 (0) | 0.14 |
| 1+ | 9 (75.0) | 2 (10.5) | 0.0046 |
| 2+ | 1 (8.3) | 5 (26.3) | 0.36 |
| 3+ | 0 (0) | 12 (63.3) | 0.00044 |
| Total | 12 | 19 |
Fisher’s exact test.
NB Proliferation Is Inhibited and Apoptosis Is Induced in the Sciatic Nerve NB Xenografts
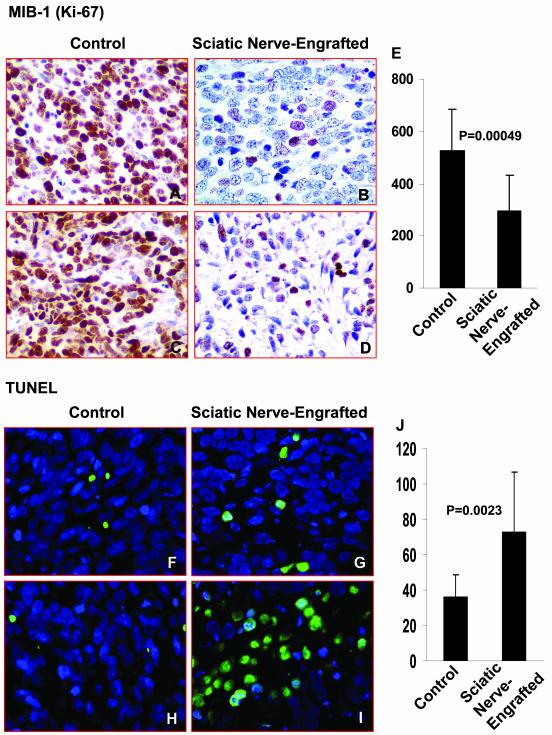
Tumor cell proliferation was examined by evaluating Ki-67 expression,19 a nuclear protein that is preferentially expressed during active phases of the cell cycle. Significantly higher rates of neuroblast proliferation were detected in the control tumors compared to the tumors that developed within the sciatic nerve (525 ± 159 per 10 HPFs versus 295 ± 139 per 10 HPFs, respectively; P = 0.00049) (Figure 4; A to E). TUNEL assays were performed on the NB xenografts to determine whether the frequency of apoptotic cells differed in the tumors engrafted inside versus outside the sciatic nerve. As shown in Figure 4, F to J, a significantly higher number of apoptotic cells were seen in the xenografts engrafted inside the sciatic nerve compared to controls (73 ± 45 per 10 HPFs versus 36 ± 13 per 10 HPFs, respectively; P = 0.0023).
Figure 4.
Representative photographs of MIB-1 (Ki-67)-immunostained tumors engrafted inside versus outside the sciatic nerve. A–D: Proliferating cells identified by MIB-1 antibody in control versus sciatic nerve-engrafted NB cells. E: The mean number of MIB-1 (Ki-67)-positive cells (brown cells) per 10 HPFs of the sciatic nerve-engrafted tumors and controls are shown in the bar graph. Representative photographs of a TUNEL assay of tumors engrafted inside versus outside the sciatic nerve. F–I: Apoptotic cells identified by TUNEL analysis (green cells) in control versus sciatic nerve-engrafted NB cells. J: The mean number of apoptotic cells per 10 HPFs of the sciatic nerve-engrafted tumors and controls are shown in the bar graph. Original magnifications, ×400.
Tumor Angiogenesis Is Inhibited in the Sciatic Nerve NB Xenografts
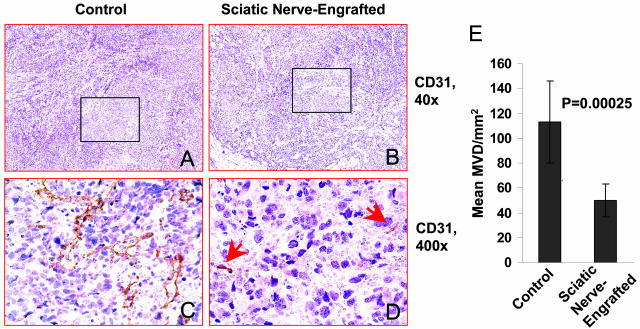
We also examined the MVDs of the NB xenografts to determine whether tumor vascularity was influenced by the infiltrating Schwann cells. A significantly lower MVD was observed in the xenografts engrafted inside the sciatic nerve compared to controls (50 ± 13.05/mm2 versus 113 ± 32.85/mm2, respectively; P = 0.00025) (Figure 5).
Figure 5.
Representative photographs taken from CD31-immunostained slides of tumors engrafted outside (A and C) versus inside the sciatic nerve (B and D). Arrows in D point to smaller vessels seen in the sciatic nerve-engrafted tumor. E: The MVDs of the sciatic nerve-engrafted tumors and controls are shown in the bar graph.
Discussion
To investigate whether Schwann cells are capable of directly influencing NB biology, we developed a novel tumor model in which mouse Schwann cells were induced to invade xenografts comprised of human NB cells. We found that endogenous mouse Schwann cells extensively infiltrated tumors that developed after intrafascicular injection of NB cells, whereas few to no Schwann cells were detected in the control tumors that grew outside the sciatic nerve. Although the cellular mechanisms that induced the migration of Schwann cells in the sciatic nerve-engrafted NB xenografts are not clear, it is possible that the penetration of the nerve sheath and subsequent injury to the sciatic nerve rendered during the inoculation of the NB cells may have played a role. Previously, others have shown that Schwann cell migration is increased on denervated sciatic nerve substrate compared to normal sciatic nerve substrate.20 Conversely, the presence of an intact nerve sheath and the lack of nerve injury may have prevented Schwann cell migration into the control tumors.
In addition to increased numbers of infiltrating Schwann cells, the sciatic nerve-engrafted xenografts contained NB cells with a more differentiated phenotype, higher numbers of apoptotic cells, lower numbers of proliferating neuroblasts, and lower numbers of blood vessels than control tumors. Because these are characteristics that are seen in NB tumors with favorable prognosis,5,21 we were surprised that the sciatic nerve-engrafted xenografts initially grew at a more rapid rate than controls. It is possible that the sciatic nerve may provide an environment that promotes tumorigenic growth. Recent studies have shown that human neurofibromatosis type 1 (NF1) Schwann cells do not form tumors when inoculated in mice subcutaneously, but they were capable of forming tumors in the sciatic nerve.22 The neuroblasts in the sciatic nerve-engrafted tumors were larger with more abundant cytoplasm than those in the control tumors. Thus, the initial increase in tumor volume in the tumors that developed within the sciatic nerve may also be consequent to the larger size of the more differentiated tumor cells.
Schwann cells secrete several potent angiogenesis inhibitors,12–14 and it is likely that these factors were responsible for the lower MVD observed in the sciatic nerve-engrafted xenografts compared to controls. Schwann cells also produce a number of neurotrophic factors including nerve growth factor, brain-derived neurotrophic factor, and ciliary neurotrophic factor23,24 that are known to induce the differentiation of neuronal cells. Others have shown that factors secreted by Schwann cells are capable of inducing NB differentiation in vitro.10,11 Thus, it is likely that the neuroblast differentiation observed in the sciatic nerve-engrafted tumors was similarly induced by these neurotrophins. Neurotrophins also regulate neuronal cell survival and death.25,26 Others have reported a marked increase in the rate of NB apoptosis after 10 days of co-culture with Schwann cells,11 and we, similarly, found an enhanced rate of NB apoptosis in the xenografts that contained infiltrating Schwann cells.
In the study reported by Ambros and co-workers,6 only NB tumors with favorable genetic features showed spontaneous development of Schwann cell stroma, suggesting that triploidy and the absence of 1p deletions in NB cells may be prerequisites for spontaneous maturation. The SMS-KCNR NB cells used in our tumor model have several unfavorable genetic features including MYCN amplification, chromosome 1p deletion, and chromosome 17q gain.27 Nevertheless, in the presence of the infiltrating Schwann cells, the phenotype of the SMS-KCNR xenografts was modified. Thus, although favorable genetic features may be a prerequisite for the NB cells to produce factors that attract Schwann cells to invade tumors, once the Schwann cells are present, they appear to be capable of influencing the biology of genetically favorable as well as unfavorable NB cells. Unfortunately, we were not able to determine the fate of the sciatic nerve-engrafted tumors in long-term studies because animals had to be sacrificed as soon as the tumors caused morbidity.
In 1889, Dr. Stephen Paget28 first suggested that interactions between tumor cells and cells in tissue microenvironments are critical in regulating tumorigenesis when he published his seminal “seed and soil” hypothesis to explain the nonrandom pattern of metastasis. More recent studies have emphasized the importance of a number of components of the “soil” in regulating tumor growth including: 1) the extracellular matrix and matricellular proteins, 2) stromal cells and their growth factors and inhibitors, 3) microvessels and angiogenesis factors, 4) immune cells and their cytokines; and 5) inflammatory cells.29 There is now substantial evidence that tumor growth and progression is dependent on cross-talk between malignant cells and their adjacent stromal compartment.30 Our results demonstrate that infiltrating Schwann cells are capable of promoting neuroblast differentiation, inducing apoptosis, and inhibiting angiogenesis and proliferation in NB xenografts. Further research investigating the molecules involved in the cross-talk between Schwann cells and neuroblasts may lead to a new paradigm for treating NB. Perhaps in addition to developing new therapies that target the “seed”, strategies should also be focused on enhancing the components of the “soil” that inhibit tumor growth.
Footnotes
Address reprint requests to Susan L. Cohn, M.D., Children’s Memorial Hospital, Division of Hematology/Oncology, 2300 Children’s Plaza, Chicago, IL 60614. E-mail: scohn@northwestern.edu.
Supported in part by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (grant NS049814), the Neuroblastoma Children’s Cancer Society, Friends for Steven Pediatric Cancer Research Fund, the Elise Anderson Neuroblastoma Research Fund, the North Suburban Medical Research Junior Board, and the Robert H. Lurie Comprehensive Cancer Center (National Institutes of Health, National Cancer Institute core grant 5P30CA60553).
References
- Brodeur GM, Maris JM. Neuroblastoma. Pizzo PA, Poplack DG, editors. Philadelphia: Lippincott-Raven,; Principles and Practice of Pediatric Oncology. 2001:pp 895–937. [Google Scholar]
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999;17:2226–2279. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- Shimada H, Chatten J, Newton WA, Jr, Sachs N, Hamoudi AB, Chiba T, Marsden HB, Misugi K. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984;73:405–416. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK, Castleberry RP. The International Neuroblastoma Pathology Classification (the Shimada System). Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- Ambros IM, Zellner A, Roald B, Amann G, Ladenstein R, Printz D, Gadner H, Ambros PF. Role of ploidy, chromosome 1p, and Schwann cells in the maturation of neuroblastoma. N Engl J Med. 1996;334:1505–1511. doi: 10.1056/NEJM199606063342304. [DOI] [PubMed] [Google Scholar]
- Valent A, Venuat AM, Danglot G, Da Silva J, Duarte N, Bernheim A, Benard J. Stromal cells and human malignant neuroblasts derived from bone marrow metastasis may share common karyotypic abnormalities: the case of the IGR-N-91 cell line. Med Pediatr Oncol. 2001;36:100–103. doi: 10.1002/1096-911X(20010101)36:1<100::AID-MPO1023>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Mora J, Cheung NK, Juan G, Illei P, Cheung I, Akram M, Chi S, Ladanyi M, Cordon-Cardo C, Gerald WL. Neuroblastic and schwannian stromal cells of neuroblastoma are derived from a tumoral progenitor cell. Cancer Res. 2001;61:6892–6898. [PubMed] [Google Scholar]
- Peuchmaur M, d’Amore ES, Joshi VV, Hata J, Roald B, Dehner LP, Gerbing RB, Stram DO, Lukens JN, Matthay KK, Shimada H. Revision of the International Neuroblastoma Pathology Classification: confirmation of favorable and unfavorable prognostic subsets in ganglioneuroblastoma, nodular. Cancer. 2003;98:2274–2281. doi: 10.1002/cncr.11773. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski JL, Rutkowski JL, Yamashiro DJ, Tennekoon GI, Brodeur GM. Schwann cell-conditioned medium promotes neuroblastoma survival and differentiation. Cancer Res. 1998;58:4602–4606. [PubMed] [Google Scholar]
- Ambros IM, Attarbaschi A, Rumpler S, Luegmayr A, Turkof E, Gadner H, Ambros PF. Neuroblastoma cells provoke Schwann cell proliferation in vitro. Med Pediatr Oncol. 2001;36:163–168. doi: 10.1002/1096-911X(20010101)36:1<163::AID-MPO1040>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Huang D, Rutkowski JL, Brodeur GM, Chou PM, Kwiatkowski JL, Babbo A, Cohn SL. Schwann cell-conditioned medium inhibits angiogenesis. Cancer Res. 2000;60:5966–5971. [PubMed] [Google Scholar]
- Chlenski A, Liu S, Crawford SE, Volpert OV, DeVries GH, Evangelista A, Yang Q, Salwen HR, Farrer R, Bray J, Cohn SL. SPARC is a key schwannian-derived inhibitor controlling neuroblastoma tumor angiogenesis. Cancer Res. 2002;62:7357–7363. [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Ranalli M, Huang X, Huang L, Volpert O, De Vries GH, Abramson LP, Bouck N. Pigment epithelium-derived factor (PEDF) in neuroblastoma: a multifunctional mediator of Schwann cell antitumor activity. J Cell Science. 2001;114:4421–4428. doi: 10.1242/jcs.114.24.4421. [DOI] [PubMed] [Google Scholar]
- Mashour GA, Moulding HD, Chahlavi A, Khan GA, Rabkin SD, Martuza RL, Driever PH, Kurtz A. Therapeutic efficacy of G207 in a novel peripheral nerve sheath tumor model. Exp Neurol. 2001;169:64–71. doi: 10.1006/exnr.2001.7641. [DOI] [PubMed] [Google Scholar]
- Liu S, Edgerton SM, Moore DH, Thor AD. Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clin Cancer Res. 2001;7:1716–1723. [PubMed] [Google Scholar]
- Changelian PS, Meiri K, Soppet D, Valenza H, Loewy A, Willard M. Purification of the growth-associated protein GAP-43 by reversed phase chromatography: amino acid sequence analysis and cDNA identification. Brain Res. 1990;510:259–268. doi: 10.1016/0006-8993(90)91376-r. [DOI] [PubMed] [Google Scholar]
- Turco A, Scarpa S, Coppa A, Baccheschi G, Palumbo C, Leonetti C, Zupi G, Colletta G. Increased TGFbeta type II receptor expression suppresses the malignant phenotype and induces differentiation of human neuroblastoma cells. Exp Cell Res. 2000;255:77–85. doi: 10.1006/excr.1999.4750. [DOI] [PubMed] [Google Scholar]
- Budke H, Orazi A, Neiman RS, Cattoretti G, John K, Barberis M. Assessment of cell proliferation in paraffin sections of normal bone marrow by the monoclonal antibodies Ki-67 and PCNA. Mod Pathol. 1994;7:860–866. [PubMed] [Google Scholar]
- Anton ES, Weskamp G, Reichardt LF, Matthew WD. Nerve growth factor and its low-affinity receptor promote Schwann cell migration. Proc Natl Acad Sci USA. 1994;91:2795–2799. doi: 10.1073/pnas.91.7.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitar D, Crawford SE, Rademaker AW, Cohn SL. Tumor angiogenesis correlates with metastatic disease, N-myc amplification, and poor outcome in human neuroblastoma. J Clin Oncol. 1996;14:405–414. doi: 10.1200/JCO.1996.14.2.405. [DOI] [PubMed] [Google Scholar]
- Muir D, Neubauer D, Lim IT, Yachnis AT, Wallach MR. Tumorigenic properties of neurofibromin-deficient neurofibroma Schwann cells. Am J Pathol. 2001;158:501–513. doi: 10.1016/S0002-9440(10)63992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros IM, Ambros PF. Schwann cells in neuroblastoma. Eur J Cancer. 1995;31A:429–434. doi: 10.1016/0959-8049(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Reynolds ML, Woolf CJ. Reciprocal Schwann cell-axon interactions. Curr Opin Neurobiol. 1993;3:683–693. doi: 10.1016/0959-4388(93)90139-p. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M, Casaccia-Bonnefil P, Carter B, Chittka A, Kong H, Yoon SO. Neurotrophin receptors: mediators of life and death. Brain Res Brain Res Rev. 1998;26:295–301. doi: 10.1016/s0165-0173(97)00036-2. [DOI] [PubMed] [Google Scholar]
- Van Roy N, Van Limbergen H, Vandesompele J, Van Gele M, Poppe B, Salwen H, Laureys G, Manoel N, De Paepe A, Speleman F. Combined M-FISH and CGH analysis allows comprehensive description of genetic alterations in neuroblastoma cell lines. Genes Chromosom Cancer. 2001;32:126–135. doi: 10.1002/gcc.1174. [DOI] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- Framson PE, Sage EH. SPARC and tumor growth: where the seed meets the soil? J Cell Biochem. 2004;92:679–690. doi: 10.1002/jcb.20091. [DOI] [PubMed] [Google Scholar]
- Rangarajan A, Weinberg RA. Opinion: comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer. 2003;3:952–959. doi: 10.1038/nrc1235. [DOI] [PubMed] [Google Scholar]