Abstract
Although erythropoietin (Epo) is a known stimulator of erythropoiesis, recent evidence suggests that its biological functions are not confined to hematopoietic cells. To elucidate the role of Epo and erythropoietin receptor (EpoR) in melanoma, we examined the expression and function of these proteins in melanocytes and melanoma cells. We found increased expression of Epo in melanoma cells compared to melanocyte in vitro. EpoR was also strongly expressed in all of the melanoma cell lines and two of the three melanocyte cell lines examined. Epo expression was significantly higher in melanoma than in benign nevi as determined by immunohistochemistry. Although melanoma cells secreted Epo in normoxic condition in vitro, hypoxia and CoCl2 treatment increased Epo secretion. EpoR in melanoma cells was functional, because exogenous Epo increased melanoma resistance to hypoxic stress, pretreatment of melanoma cells with Epo significantly increased resistance to dacarbazine treatment, and Epo increased the phosphorylation of EpoR, RAF, and MEK. In conclusion, we demonstrated constitutive expression of Epo and EpoR as well as autonomous secretion of Epo by melanoma cells, indicating a novel autocrine loop of Epo in melanoma. The results suggest that the autocrine and paracrine functions of Epo might play a role in malignant transformation of melanocytes and in the survival of melanoma cells in hypoxia and other adverse conditions.
Erythropoietin (Epo), a glycoprotein hormone, was considered to be a specific stimulator of erythropoiesis, acting via its specific receptor. Erythropoietin receptor (EpoR) stimulation in erythroblasts results in proliferation, differentiation, and inhibition of apoptosis.1,2 However, EpoR expression is not restricted to hematopoietic cells; it is expressed in a variety of other cell types and Epo modulates a host of cellular signal transduction pathways to perform multiple functions other than erythropoiesis.2,3 In the brain, there is a paracrine Epo/EpoR system in which neurons express EpoR and astrocytes produce Epo.4,5 Epo is a potent inhibitor of neuronal apoptosis induced by ischemia and hypoxia.6 In the uterus, estradiol stimulates Epo production, which is thought to play a role in the cyclical endometrial angiogenesis via EpoR expressed in vascular endothelial cells.7 Epo and EpoR are also expressed in trophoblasts8,9 and mammary epithelial cells.10–12 Epo prevents endothelial cell apoptosis and promotes neovascularization in tissue.7,13
Epo and EpoR expression is present not only in various nonhematopoietic tissues, but also in malignant tumors.11,12,14,15 Both Epo and EpoR are expressed in breast cancers with the highest levels of expression observed in the most hypoxic areas.12 Epo and EpoR are also expressed in malignant cervical, endometrial, and ovarian cancers, and their expression correlates with tumor progression.14,16,17 Exogenous Epo stimulates tyrosine phosphorylation and inhibits the cytotoxic effect of cis-platin in HeLa cervical carcinoma cells.15 These data indicate that Epo might play a role during tumor growth and progression.
Epo activates several downstream signal transduction pathways, which may be responsible for its growth-promoting effects. Binding of Epo to EpoR, a member of the superfamily of cytokine receptors, induces homodimerization of EpoR and the subsequent activation of Janus kinase 2 (Jak2), phosphorylation of EpoR and signal transducer and activator of transcription 5 (STAT5) through tyrosine phosphorylation.2 Epo mediates the activation of RAF-1 and extracellular signal-regulated kinase 1/2 (ERK1/2) in various cell lines expressing endogenous or transfected EpoRs.18 Epo has also been shown to stimulate the c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) pathways in erythroleukemia cells.19,20
Epo is widely used in oncological patients to treat anemia. However, two recent clinical trials of Epo in cancer patients suggested that Epo had negative effects on patient survival.21,22 Epo has been used in melanoma patients who develop anemia after chemotherapy. However, little is known about Epo and EpoR expression or their functions in melanoma. In this study, we show that Epo and EpoR are not only expressed in melanoma, but that there is an autocrine loop of Epo that might contribute to melanocyte transformation, and melanoma survival and progression.
Materials and Methods
All chemicals were purchased from Sigma (St. Louis, MO). Human recombinant erythropoietin (rHuEpo, Epogen) was purchased from Amgen (Thousand Oaks, CA).
Cell Culture and Hypoxia Treatment
The melanocyte (FOM103, 115, 117) culture was described previously.23 The melanoma cell lines used in this study were derived from different stages of human melanoma progression, radial growth phase: WM35, WM3211, and Sbcl2; vertical growth phase: WM793, WM1366, and WM298; and metastatic melanoma: 1205 Lu, 451Lu, and WM9. Tumor cells were cultured in a MCDB153/L15 medium (v/v: 4/1) supplemented with 2% fetal bovine serum, insulin (5 μg/ml), CaCl2 (2 mmol/L), 100 U/ml penicillin, and 100 mg/ml streptomycin. All cells were cultured in a 5% CO2 incubator at 37°C, except during hypoxia treatment. For hypoxic treatment, tumor cells were plated at a density of 1 × 106 cells per well. Twenty-four hours before hypoxia exposure or rHuEpo treatments, cells were switched to a serum-free medium. Hypoxia treatment was performed using a well-characterized chamber system as described previously.15 A 1% O2, 5% CO2, and 94% N2 mixed gas was used in the hypoxia experiments. CoCl2 (100 μmol/L) was also used to stimulate Epo secretion.
Western Blotting
Cells were washed with ice cold (1×) phosphate-buffered saline (PBS) and then lysed in a RIPA buffer [50 mmol/L Tris buffer, pH 7.4, 150 mmol/L NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 1 mmol/L ethylenediaminetetraacetic acid, 1× protease inhibitor cocktail (Sigma) and 10 mmol/L phenylmethyl sulfonyl fluoride], incubated for 30 minutes on ice, and then centrifuged at 10,000 rpm for 15 minutes. Whole cell lysates were normalized for protein concentration. Fifty μg of proteins were separated in NuPAGE 4 to 12% Bis-Tris gel (Invitrogen, Carlsbad, CA) and transferred to a polyvinylidene difluoride membrane (Hybond-P; Amersham Biosciences, Little Chalfont, UK). Proteins were detected using antibodies to Epo (H-162, 1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA); EpoR (C-20, 1:1500; Santa Cruz); phos-EpoR [p-EpoR (Tyr479)-R,1:1000; Santa Cruz]; phos-RAF, phos-MEK, phos-MAPK, and phos-ELK (1:1000; Cell Signaling Technology, Beverly, MA). As a loading control, a mouse monoclonal antibody β-actin (clone AC-74, 1:10,000; Sigma) was used. Membranes were incubated with the primary antibodies overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (Bio-Rad Laboratories, Hercules, CA). Immunoreactive bands were visualized using chemiluminescence (ECL Western blotting detection system; Amersham Biosciences). Bands were scanned and quantified using a ChemiDoc XRS system (Bio-Rad Laboratories).
Detection of Epo in Conditioned Medium
To demonstrate Epo secretion by melanoma cells, 2 × 105 cells were plated in 100-mm culture plates and incubated for 24 hours in a regular medium. The medium was then replaced with a serum-free medium and cells were incubated for an additional 24 hours. Ten ml of conditioned medium was collected and concentrated to a volume of ∼500 μl using Amicon Ultra centrifuge filters (10 kd molecular weight cutoff pore size; Millipore, Bedford, MA). Twenty μl of the concentrated conditioned medium was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted with anti-Epo and anti-β-actin antibodies as described above.
Mitochondrial Membrane Potential Assay
To determine the mitochondrial membrane potential, we used the mitochondria-specific cationic dye JC-1 (Molecular Probes, Eugene, OR), which undergoes membrane potential-dependent accumulation in the mitochondria. The JC-1 dye exists as a monomer when the membrane potential (Δψ) is lower than 140 mV and emits green light (540 nm) after excitation by blue light (490 nm). At higher membrane potentials, the JC-1 monomers convert to J-aggregates that emit red light (590 nm) after excitation with green light (540 nm). For the measurement of mitochondrial membrane potential, 1 × 105 WM35 cells were plated in 12-well plates, and cultured for 24 hours. The culture medium was then replaced by a serum-free medium and cells were treated with rHuEpo (2 U/ml) for 48 hours. After the incubation period, tumor cells were treated with 2 μg of JC-1 in a prewarmed phosphate buffer for 30 minutes at 37°C, followed by washing with the PBS buffer. Fluorescence emission was observed under a Leitz DMIRB fluorescence microscope equipped with a dual-emission filter for green (515 to 545 nm) and red (585 to 615 nm) fluorescence (Leica, McHenry, IL).
Immunohistochemistry
Fourteen cases of benign compound nevi and nine cases of melanoma were randomly selected from the surgical pathology files at the University of Pennsylvania Medical Center. Two of these cases were desmoplastic melanoma. Immunohistochemical assays were performed on formalin-fixed, paraffin-embedded sections as described previously.24 Briefly, 5-μm-thick sections were cut and deparaffinized in xylene and rehydrated in graded alcohols. Sections were steamed in a 0.01mol/L sodium citrate buffer (pH 6.0) for 18 minutes. Endogenous peroxidase activity was blocked by a 3% hydrogen peroxide solution for 20 minutes. Slides were incubated with the polyclonal antibody against Epo (H-162, 1:200; Santa Cruz Biotechnology Inc.) and EpoR (C-20, 1:400), overnight at 4°C. Slides were washed five times with Tris-buffered saline containing Tween 20 (TBST, pH 7.6; DAKO, Carpinteria, CA) and incubated for 30 minutes at room temperature with horseradish peroxidase-labeled dextran polymer coupled to anti-rabbit (DAKO EnVision + System HRP, DAKO). Slides were then washed three times with TBST, developed with diaminobenzidine, and counterstained with hematoxylin. For Epo and EpoR, slides of fetal liver and placenta were used as positive controls. A negative control was achieved by omitting the primary antibody. The specificity of the Epo and EpoR antibodies was confirmed previously.11,12,15
Interpretation of Immunohistochemical Stains
Immunohistochemical stains for Epo and EpoR were interpreted semiquantitatively by assessing the intensity and extent of staining on the entire tissue sections present on the slides according to a four-tiered (0 to 3) scale.11,15 For Epo cytoplasmic, for EpoR cytoplasmic, and/or membrane immunoreactivity were considered positive. First, the total percentage of positively staining tumor cells was determined. Then the percentage of weakly (1), moderately (2), and strongly (3) staining cells was determined, so that the sum of these categories equated with the overall percentage of positivity. A staining score was then calculated as follows: score (out of a maximum of 300) = the sum of 1 × percentage of weak, 2 × percentage of moderate, and 3 × percentage of strong staining.
Trypan Blue Dye Exclusion Assay
Cell survival was determined by bright-field microscopy using the 0.4% trypan blue dye exclusion method. Melanoma cells (WM 35, WM 793, and 1205 Lu) were serum-starved for 24 hours and then treated with Epo (10 U/ml) and/or hypoxia (1% O2) for an additional 24 hours under serum-free condition at 37°C. Then, the cells were trypsinized and cell survival was determined by counting the viable cells in a randomly selected nonoverlapping four fields using a hemacytometer. Experiments were performed in triplicate.
MTT Assay
The MTT cell viability assay was performed according to the manufacture’s instructions (Promega Corporation, Madison, WI). Briefly, melanoma cells (WM35, WM 793, and 1205 Lu) were washed with PBS and suspended in a final concentration of 1 × 105/ml in an assay medium. Fifty μl of cell suspension (5000 cells) were dispensed into 96-well plates. Total volume in the wells adjusted to 100 μl with regular 2% tumor media. The plates were incubated at 37°C for 24 hours in a humidified CO2 incubator. Then the media was aspirated from the wells and 80 μl of serum-free media with different concentrations of EPO (0, 10, and 100 U/ml) were added to the wells and incubated for 2 hours. Twenty μl of serum-free medium containing various concentrations of cis-platin (Bedford Laboratories, Bedford, OH) and dacarbazine (DTIC) (0.5, 1.0, 10, and 100 μg/ml; American Pharmaceutical Partners, Schaumburg, IL) were added, and the plates were further incubated at 37°C for 24 hours. For the color development, 15 μl of dye solution was added to each well and the plates were incubated at 37°C for 4 hours. After 4 hours, 100 μl of solubilization/stop solution were added to each well. The plates were kept at 4°C overnight and, using a 96-well plate reader, an absorbance rate at 570 nm wavelength was recorded. Experiments were performed in triplicate.
Statistical Analysis
A one-way analysis of variance with a subsequent Tukey’s multiple comparison test were used to analyze the effect of Epo on cell viability. A Student’s t-test was used to compare the results of the MTT assay after DTIC and cis-platin treatment as well as Epo expression in melanomas and nevi and EpoR phosphorylation. Statistical significance was determined if the two-sided P value of a test was less than 0.05.
Results
Epo and EpoR Expression in Human Melanocyte and Melanoma Cell Lines in Vitro
We examined the expression of Epo and EpoR in three human melanocyte and nine melanoma cell lines derived from normal skin and melanoma at various stages of tumor progression by Western blotting. Melanocyte and melanoma cell lines were grown in normoxic conditions, harvested, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The Western blots showed that low levels of Epo were detectable in cultured human melanocytes (FOM103, 115, and 117). Epo expression increased significantly in melanoma cell lines derived from radial growth phase, vertical growth phase, and metastatic melanomas compared to melanocyte cell lines (Figure 1). EpoR was highly expressed in all of the melanoma cell lines and two of three melanocyte cell lines (Figure 1). These results indicate that melanoma cells constitutively express Epo and EpoR. Epo expression was increased in melanoma cells compared to melanocytes.
Figure 1.
Epo and EpoR protein expression in melanocyte and melanoma cell lines. A representative blot for Epo and EpoR expression by melanocytes (FOM103, 115, 117), radial growth phase melanoma (WM35, WM3211, and sbcl2), vertical growth phase melanoma (WM793, WM1366, and WM298), metastatic melanoma (1205Lu, 451Lu, and WM9). Representative blot from three independent experiments. Epo expression was increased in melanoma cell lines compared to melanocyte cell lines.
Epo and EpoR Expression in Human Melanocytic Lesions
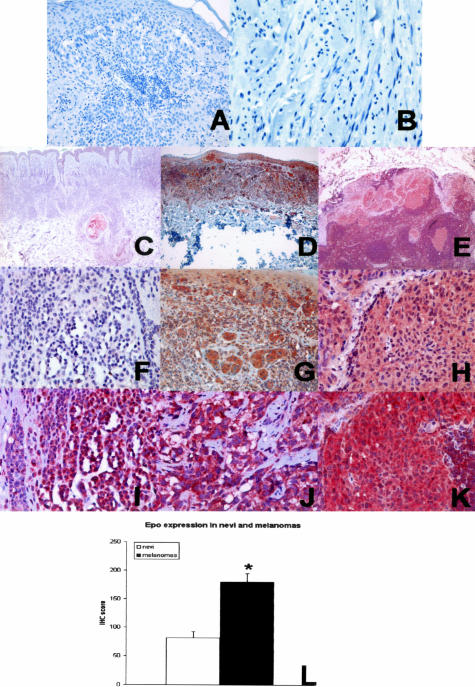
To confirm the expression of Epo and EpoR in human tissue, we performed immunohistochemistry on achieved human tissues. Normal melanocytes in the skin weakly expressed Epo and EpoR (data not shown). Cytoplasmic granular Epo immunostaining was seen in 13 of 14 compound nevi and all melanomas (Figure 2; C to H). The staining was variable in compound nevi, but more intense and uniform in primary melanomas. Epo expression was significantly increased in epithelioid primary melanomas compared to compound nevi (P < 0.001; Figure 2L). Two desmoplastic melanomas, which are known to have different immunohistochemical staining pattern than conventional epithelioid melanomas, showed diffuse and weak Epo staining (data not shown). Cytoplasmic and/or membrane immunostaining for EpoR was present in all samples, and EpoR expression levels were similar in melanomas and compound nevi (Figure 2; I to K). Prominent EpoR expression was also present in the endothelial and smooth muscle cells of blood vessels (not shown). These data confirm Epo and EpoR expression in melanocytic lesions, and demonstrate that Epo and EpoR are constitutively expressed in melanoma. There is significantly increased Epo expression in melanomas compared to compound nevi, suggesting that Epo might play a role during malignant transformation of melanocytes.
Figure 2.
Epo and EpoR expression in human tissue. A: Negative control for Epo. B: Negative control for EpoR. C and F: Epo expression in a compound nevus (Epo staining), weak cytoplasmic staining was present. D and G: Epo expression in a primary epithelioid melanoma (Epo staining), strong cytoplasmic staining was present. E and H: Epo expression in metastatic melanoma (Epo staining), strong cytoplasmic staining similar to that in primary melanoma. I: EpoR expression in nevus (EpoR staining), strong diffuse cytoplasmic/membrane staining. J: EpoR expression in primary melanoma (EpoR staining), strong diffuse cytoplasmic/membrane staining. K: EpoR expression in metastatic melanoma (EpoR staining), strong diffuse cytoplasmic/membrane staining. L: Quantification of immunohistochemical staining in nevi and primary epithelioid melanomas. Epo expression was significantly increased in melanoma cells (*, P < 0.001). Original magnifications: ×400 (A, B, F–K); ×50 (C–E).
Melanoma Cells Secrete Epo under Normoxic and Hypoxic Conditions
Because melanoma cells expressed both Epo and EpoR, we examined whether there was an autocrine loop of Epo in melanoma. Melanoma cell lines were grown to 80% confluence, regular medium was replaced with serum-free medium, and the cells were incubated under normoxic conditions for an additional 24 hours. The conditioned medium was collected and concentrated. Melanoma cell lines derived from various stages (radial growth phase, vertical growth phase, and metastatic) all secreted detectable Epo into the conditioned medium (Figure 3, A and B), indicating a constitutive activation of Epo in melanoma under normoxic conditions. β-Actin was used to normalize the loading of samples. There was less β-actin in the concentrated media from 1205Lu cells, which probably represent less apoptotic activity in this cell line in vitro.
Figure 3.
A: Effects of hypoxia on Epo secretion by melanoma. Tumor cells were incubated in normoxic and hypoxic conditions; the conditioned medium was collected and concentrated. β-Actin in the medium was used as a loading control. Representative blots from at least three independent experiments. Epo secretion was significantly increased under hypoxic conditions. B: Effects of CoCl2 on Epo secretion by melanoma. Representative blots from at least three independent experiments. β-Actin in the medium was used as a loading control. Epo secretion was significantly increased after treatment with CoCl2.
Because Epo production is controlled by hypoxia. We examined the effects of low O2 concentration on Epo secretion by melanoma cells. Melanoma cells were grown to 80% confluence, and then serum-starved for 24 hours. For hypoxic treatment, tumor cells were exposed to 1% O2, 5% CO2, and 94% N2 using the modular incubator chamber method as previously described15 and incubated at 37°C for 24 hours. The conditioned medium was collected, concentrated, and analyzed. Under hypoxic conditions, Epo secretion was significantly increased in all three cell lines examined (Figure 3A). CoCl2 is known to activate HIF-1α and mimic the effects of hypoxia in the cells.25 CoCl2 was used at a concentration of 100 μmol/L in our experiments. Similar to the effects of hypoxia, CoCl2 increased Epo secretion in melanoma cell lines in vitro (Figure 3B).
Epo Increases Melanoma Resistance to Hypoxia and Chemotherapeutic Drugs in Vitro
We first tested the effects of Epo on melanoma cell survival under moderate hypoxia (1% O2), using a trypan blue dye exclusion assay. We found that melanoma cells (WM35, WM793, and 1205Lu) were sensitive to moderate hypoxia (P < 0.01) and addition of Epo (10 U/ml) to the medium significantly increased tumor cell resistance to hypoxia (P < 0.01, Figure 4A). We then studied the effects of Epo on melanoma cell death induced by chemotherapeutic agents such as DTIC and cis-platin. DTIC and cis-platin are commonly used clinically to treat patients with melanoma. The tumor cells were pretreated with Epo (10 and 100 U/ml) for 2 hours before addition of various concentrations of DTIC and cis-platin (0, 0.5, 1, 10, 100 μg/ml). The MTT assay was used to estimate the remaining viable cells. We found that there were dose-dependent effects on cell viability after treatment with DTIC and cis-platin. Epo (10 U/ml and 100 U/ml) significantly increased cell viability in DTIC-treated cells by 10 to 50% in certain doses. In general, a high concentration of Epo (100 U/ml) had a more pronounced effect. The effect of Epo on cis-platin-treated tumor cells was much less. Therefore, the Epo protective effect appears to be chemotherapeutic agent- and dose-dependent (Figure 4B).
Figure 4.
A: Effects of Epo on hypoxia-induced cell death. Tumor cells were incubated in normoxic, hypoxic, and hypoxic with Epo (10 U/ml) for 24 hours. Values represent the means with SE from at least three independent experiments. Hypoxia significantly reduced cell survival (comparison between control and hypoxia treatment; *, P < 0.01) and the effect was prevented by addition of Epo (comparison between hypoxia and hypoxia plus Epo treatment; #, P < 0.01). B: Effects of Epo on DTIC and cis-platin-induced cell death. The tumor cells were pretreated with Epo (0, 10, and 100 U/ml) for 2 hours before the addition of chemotherapeutic agents. Values represent the means with SE from at least three independent experiments. Epo significantly increased cell viability in response to DTIC treatment at certain doses. The effects of Epo on cis-platin-treated cells were less prominent. *, Significant difference between 100 U/ml Epo and controls (P < 0.05); #, significant difference between 10 U/ml Epo and controls (P < 0.05). C: Epo increases mitochondria potential in melanoma. a: WM 793 was in a serum-free medium for 72 hours. b: WM 793 was in a serum-free medium for 24 hours and then supplemented with 2 U/ml of Epo for an additional 48 hours. The red fluorescence indicates high mitochondrial potential and aggregates of JC-1. There is an increase of mitochondria membrane potential after Epo treatment.
Epo Modulates Mitochondrial Membrane Potential
To assess whether Epo protects cell survival by affecting mitochondrial membrane potential, the fluorescent dye JC-1, a cationic membrane potential indicator, was used. Compared to the untreated control (in serum-free medium), Epo-treated cells (2 U/ml) had a significant increase in their red fluorescence intensity, indicating that Epo increased the mitochondrial membrane potential in these cells, which might contribute to its anti-apoptotic effect (Figure 4C).
Epo Stimulates Phosphorylation of EpoR in Melanoma
Binding of Epo to EpoR, which has no tyrosine kinase domain, induces homodimerization of EpoR and phosphorylation of the receptor via activation of JAK2 through tyrosine phosphorylation. To assess phosphorylation of EpoR, WM35 was incubated in normoxic conditions and serum-starved for 24 hours before being stimulated with rHuEpo (0, 0.1, 1, and 10 U/ml) for 5 minutes. The results showed that there was basal phosphorylation of the EpoR, and exogenous Epo stimulation significantly increased the phosphorylation of EpoR at 1 and 10 U/ml (Figure 5, A and B). The basal EpoR phosphorylation was probably related to the autocrine secretion of Epo by WM35.
Figure 5.
Epo stimulates phosphorylation of EpoR in melanoma. WM35 cells were treated with Epo (0, 0.1, 1, and 10 U/ml) for 5 minutes. A: Representative blots for EpoR phosphorylation. β-Actin and total EpoR were used as loading controls. B: The bands were quantified by laser densitometry after being corrected to the load controls. Values represent the means with SE from three independent experiments. Epo significantly increased EpoR phosphorylation at 1 and 10 U/ml compared to the control (*, P < 0.05).
Epo Stimulates Phosphorylation of RAF and MEK
B-RAF mutation is the most common mutation in melanoma and the RAS-RAF-MEK pathway is known to be involved in melanoma proliferation and progression. To examine whether Epo can stimulate this pathway, WM35 cells were serum-starved for 24 hours and treated with rHuEpo (10 U/ml) for 5 minutes. Equal amounts of cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotted with antibodies specific to phosphorylated RAF, MEK, MAPK, and ELK antibodies. Epo significantly increased the phosphorylation of RAF and MEK (Figure 6), and the phosphorylation of RAF appeared to be dose-dependent. There was a high basal level phosphorylation of MAPK and ELK, and Epo (10 U/ml) stimulation did not significantly increase the phosphorylation of these two kinases, which was likely because of the constitutive activation of MAPK in the melanoma cells.
Figure 6.
Epo stimulates phosphorylation of RAF and MEK in melanoma. WM35 cells were serum-starved for 24 hours and treated with rHuEpo (0, 0.1, 1, and 10 U/ml) for 5 minutes. Representative blot from at least three independent experiments. RAF and MEK phosphorylations were increased after Epo stimulation.
Discussion
In this study, we examined the expression and function of Epo and EpoR in melanoma. We found that Epo and EpoR are constitutively expressed in melanocyte and melanoma cell lines derived from foreskin and various stages of melanoma progression. Epo expression levels were significantly higher in melanoma cells compared to cultured melanocytes in vitro. Similarly, Epo expression was also increased in melanoma compared to benign nevi and melanocytes in human tissues, suggesting that Epo might be involved in the malignant transformation of melanocytes. Melanoma cells not only expressed Epo and EpoR, but also secreted Epo under normoxic conditions in vitro, suggesting that constitutive activation of HIF-1α in melanoma and Epo might be acting as a growth factor for melanoma cells in normoxic conditions. Exogenous Epo reversed the cytotoxic effects of moderate hypoxia and increased melanoma resistance to DTIC. The results suggest that Epo is involved in the hypoxic adaptation and resistance to chemotherapy of melanomas.
It is well known that some solid tumors can be associated with paraneoplastic polycythemia. Elevated serum Epo levels have been recognized in patients with renal cell carcinomas, Wilms’ tumors, hepatomas, cerebellar hemangioblastomas, tumors associated with common mutations of the VHL tumor suppressor genes, and tumors arising in anatomical sites in which Epo is normally expressed in low levels.26,27 EpoR expression was also reported in cases of renal cell carcinoma, and a potential paracrine or autocrine role for Epo, which promotes the growth of renal carcinomas, has been suggested.28 We have recently described that cultured human breast, cervix, and endometrial cancers express high levels of Epo and EpoR mRNA and protein.11,15 Our current data shows that both Epo and EpoR are constitutively expressed in human melanoma in vitro and in vivo.
Growth of melanocytes and their malignant counterparts is regulated by a variety of cytokines and other polypeptides.29,30 Under physiological conditions, melanocytes depend for their survival on paracrine stimulatory factors provided by the surrounding keratinocytes and fibroblasts.31,32 Transformed melanocytes have a decreased dependence on paracrine stimulation through de novo expression of some growth factors and stimulate proliferation of tumor cells in an autocrine loop.33 Our immunohistochemical stains showed that benign nevi and melanomas in the tissue uniformly expressed high levels of EpoR. On the other hand, Epo expression was significantly increased comparing melanomas to benign nevi, suggesting that Epo might be one of the factors involved in the malignant transformation of melanocytes.
Matching oxygen consumption and supply represents a fundamental challenge to multicellular organisms. Hypoxia is invariably present in solid tumors.34 Hypoxia induces a variety of responsive genes to allow cells to adapt to the hypoxic stress35 through activation of HIF-1α.36 HIF-1α is known to regulate Epo expression.37 Epo stimulated the proliferation and migration of cultured human umbilical vein endothelial cells,7 human and bovine endothelial cells,13 and microvascular endothelial cells isolated from rat mesentery in vitro.38 Angiogenesis is involved in melanoma progression.39,40 We now demonstrate that melanoma cells can respond to hypoxia or CoCl2 stimulation by up-regulating Epo secretion, suggesting that in addition to the autocrine function, Epo might also induce angiogenesis via its paracrine function during melanoma progression.
Epo was shown to stimulate breast cancer cell proliferation and also enhance the resistance of cervical cancer cells to chemotherapeutic drug.11,15 Epo-treated ovarian cancer cells became resistant to paclitaxel and survived longer than non-Epo-treated cells (unpublished data). Similar to these findings, we showed that Epo not only reversed the cytosolic effects of moderate hypoxia on melanoma cells, but pretreatment with Epo increased melanoma resistance to DTIC resulting in 10 to 50% increase of tumor cell survival at certain doses. However, the increase in cell survival was not dramatic, and the effects of Epo on cis-platin-induced cell death were minimal. Therefore, the biological relevance of Epo-induced tumor resistance to chemotherapeutic agents should be further investigated.
We also demonstrate that EpoR is biologically functional in melanoma cells. Epo (1 U/ml and 10 U/ml) significantly increased EpoR phosphorylation. In addition, there is a basal level phosphorylation of EpoR in melanoma in vitro, indicating constitutive activation of the receptors in these cells. The constitutive activation most likely resulted from the presence of Epo in the culture medium. These data further supported an autocrine function of Epo in these cells. Epo activation of the RAF-MEK pathway is thought to be critical in Epo-responsive cell proliferation.18 Epo increased the phosphorylation of RAF and MEK in this pathway in melanoma, however, phosphorylation of MAPK and ELK were not significantly increased, which was likely because of the high basal level phosphorylation of MAPK.41,42
rHuEpo is frequently used to treat or prevent anemia in patients receiving chemotherapy for various cancers, including melanoma. However, two recent trials of Epo in cancer patients showed that it negatively affected patient survival. There was a slight but significantly higher mortality rate in cancer patients treated with Epo than patients taking placebos.21,22 The authors suggest that precaution should be taken when using Epo in patients with EpoR-expressing tumors. All of the melanoma cell lines and tissues we examined expressed detectable EpoR. Therefore, our findings might also have important clinical implications. However, further clinical studies are necessary to elucidate the effects of Epo on melanoma patients.
In summary, we demonstrated a functional Epo autocrine loop in melanoma and showed that Epo inhibited hypoxia- and DTIC-induced cell death. Our data suggest that autocrine and paracrine functions of Epo may be involved in the melanocyte transformation, and melanoma survival and progression.
Acknowledgments
We thank Dr. Frank Lee for his advice and for allowing us to use the hypoxia chamber.
Footnotes
Address reprint requests to Xiaowei Xu, M.D., Ph.D., Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, 3400 Spruce Street, Philadelphia, PA 19104. E-mail: xug@mail.med.upenn.edu.
Supported in part by the McCabe Foundation (fellowship to X.X.).
References
- Lappin T. The cellular biology of erythropoietin receptors. Oncologist. 2003;8(Suppl 1):15–18. doi: 10.1634/theoncologist.8-suppl_1-15. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. The erythropoietin receptor. Semin Oncol. 2001;28:19–23. doi: 10.1016/s0093-7754(01)90208-8. [DOI] [PubMed] [Google Scholar]
- Erbayraktar S, Yilmaz O, Gokmen N, Brines M. Erythropoietin is a multifunctional tissue-protective cytokine. Curr Hematol Rep. 2003;2:465–470. [PubMed] [Google Scholar]
- Nagai A, Nakagawa E, Choi HB, Hatori K, Kobayashi S, Kim SU. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J Neuropathol Exp Neurol. 2001;60:386–392. doi: 10.1093/jnen/60.4.386. [DOI] [PubMed] [Google Scholar]
- Knabe W, Knerlich F, Washausen S, Kietzmann T, Siren AL, Brunnett G, Kuhn HJ, Ehrenreich H. Expression patterns of erythropoietin and its receptor in the developing midbrain. Anat Embryol (Berl) 2004;207:503–512. doi: 10.1007/s00429-003-0365-y. [DOI] [PubMed] [Google Scholar]
- Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Presta M, Vacca A, Ria R, Giuliani R, Dell’Era P, Nico B, Roncali L, Dammacco F. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–2636. [PubMed] [Google Scholar]
- Conrad KP, Benyo DF, Westerhausen-Larsen A, Miles TM. Expression of erythropoietin by the human placenta. FASEB J. 1996;10:760–768. doi: 10.1096/fasebj.10.7.8635693. [DOI] [PubMed] [Google Scholar]
- Fairchild BD, Conrad KP. Expression of the erythropoietin receptor by trophoblast cells in the human placenta. Biol Reprod. 1999;60:861–870. doi: 10.1095/biolreprod60.4.861. [DOI] [PubMed] [Google Scholar]
- Juul SE, Zhao Y, Dame JB, Du Y, Hutson AD, Christensen RD. Origin and fate of erythropoietin in human milk. Pediatr Res. 2000;48:660–667. doi: 10.1203/00006450-200011000-00018. [DOI] [PubMed] [Google Scholar]
- Acs G, Acs P, Beckwith SM, Pitts RL, Clements E, Wong K, Verma A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61:3561–3565. [PubMed] [Google Scholar]
- Acs G, Zhang PJ, Rebbeck TR, Acs P, Verma A. Immunohistochemical expression of erythropoietin and erythropoietin receptor in breast carcinoma. Cancer. 2002;95:969–981. doi: 10.1002/cncr.10787. [DOI] [PubMed] [Google Scholar]
- Anagnostou A, Lee ES, Kessimian N, Levinson R, Steiner M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc Natl Acad Sci USA. 1990;87:5978–5982. doi: 10.1073/pnas.87.15.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda Y, Fujita Y, Masuda S, Musha T, Ueda K, Tanaka H, Fujita H, Matsuo T, Nagao M, Sasaki R, Nakamura Y. Erythropoietin is involved in growth and angiogenesis in malignant tumours of female reproductive organs. Carcinogenesis. 2002;23:1797–1805. doi: 10.1093/carcin/23.11.1797. [DOI] [PubMed] [Google Scholar]
- Acs G, Zhang PJ, McGrath CM, Acs P, McBroom J, Mohyeldin A, Liu S, Lu H, Verma A. Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression. Am J Pathol. 2003;162:1789–1806. doi: 10.1016/S0002-9440(10)64314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda Y, Musha T, Tanaka H, Fujita Y, Fujita H, Utsumi H, Matsuo T, Masuda S, Nagao M, Sasaki R, Nakamura Y. Inhibition of erythropoietin signalling destroys xenografts of ovarian and uterine cancers in nude mice. Br J Cancer. 2001;84:836–843. doi: 10.1054/bjoc.2000.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acs G, Xu X, Chu C, Acs P, Verma A. Prognostic significance of erythropoietin expression in human endometrial carcinoma. Cancer. 2004;100:2376–2386. doi: 10.1002/cncr.20244. [DOI] [PubMed] [Google Scholar]
- Chen C, Sytkowski AJ. Erythropoietin regulation of Raf-1 and MEK: evidence for a Ras-independent mechanism. Blood. 2004;104:73–80. doi: 10.1182/blood-2003-04-1340. [DOI] [PubMed] [Google Scholar]
- Jacobs-Helber SM, Ryan JJ, Sawyer ST. JNK and p38 are activated by erythropoietin (EPO) but are not induced in apoptosis following EPO withdrawal in EPO-dependent HCD57 cells. Blood. 2000;96:933–940. [PubMed] [Google Scholar]
- Nagata Y, Takahashi N, Davis RJ, Todokoro K. Activation of p38 MAP kinase and JNK but not ERK is required for erythropoietin-induced erythroid differentiation. Blood. 1998;92:1859–1869. [PubMed] [Google Scholar]
- Leyland-Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol. 2003;4:459–460. doi: 10.1016/s1470-2045(03)01163-x. [DOI] [PubMed] [Google Scholar]
- Powles T, Shamash J, Liu W. Erythropoietin to treat anaemia in patients with head and neck cancer. Lancet. 2004;363:82. doi: 10.1016/S0140-6736(03)15189-6. [DOI] [PubMed] [Google Scholar]
- Quong RY, Bickford ST, Ing YL, Terman B, Herlyn M, Lassam NJ. Protein kinases in normal and transformed melanocytes. Melanoma Res. 1994;4:313–319. doi: 10.1097/00008390-199410000-00008. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhang PJ, Elder DE. Tyrosinase expression in malignant melanoma, desmoplastic melanoma, and peripheral nerve tumors. Arch Pathol Lab Med. 2003;127:1083–1084. doi: 10.5858/2003-127-1083a-LTTE. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Gu J, Huang LE, Park JW, Zhu H. Erythropoietin: a model system for studying oxygen-dependent gene regulation. J Exp Biol. 1998;201:1197–1201. doi: 10.1242/jeb.201.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesener MS, Seyfarth M, Warnecke C, Jurgensen JS, Rosenberger C, Morgan NV, Maher ER, Frei U, Eckardt KU. Paraneoplastic erythrocytosis associated with an inactivating point mutation of the von Hippel-Lindau gene in a renal cell carcinoma. Blood. 2002;99:3562–3565. doi: 10.1182/blood.v99.10.3562. [DOI] [PubMed] [Google Scholar]
- Richards FM. Molecular pathology of von Hippel Lindau disease and the VHL tumour suppressor gene. Expert Rev Mol Med. 2001;2001:1–27. doi: 10.1017/S1462399401002654. [DOI] [PubMed] [Google Scholar]
- Wood L, Swanepoel C, du TA, Jacobs P. Clinically silent renal tumour producing erythropoietin. S Afr Med J. 2003;93:128–129. [PubMed] [Google Scholar]
- Rodeck U, Herlyn M. Growth factors in melanoma. Cancer Metastasis Rev. 1991;10:89–101. doi: 10.1007/BF00049407. [DOI] [PubMed] [Google Scholar]
- Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–922. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- Valyi-Nagy IT, Murphy GF, Mancianti ML, Whitaker D, Herlyn M. Phenotypes and interactions of human melanocytes and keratinocytes in an epidermal reconstruction model. Lab Invest. 1990;62:314–324. [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Morisaki N, Kimura M. Biological characterization of human fibroblast-derived mitogenic factors for human melanocytes. Biochem J. 1998;330:1235–1239. doi: 10.1042/bj3301235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R. The regulation of normal melanocyte proliferation. Pigment Cell Res. 2000;13:4–14. doi: 10.1034/j.1600-0749.2000.130103.x. [DOI] [PubMed] [Google Scholar]
- Denko NC, Giaccia AJ. Tumor hypoxia, the physiological link between Trousseau’s syndrome (carcinoma-induced coagulopathy) and metastasis. Cancer Res. 2001;61:795–798. [PubMed] [Google Scholar]
- Denko NC, Fontana LA, Hudson KM, Sutphin PD, Raychaudhuri S, Altman R, Giaccia AJ. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22:5907–5914. doi: 10.1038/sj.onc.1206703. [DOI] [PubMed] [Google Scholar]
- Park SK, Dadak AM, Haase VH, Fontana L, Giaccia AJ, Johnson RS. Hypoxia-induced gene expression occurs solely through the action of hypoxia-inducible factor 1alpha (HIF-1alpha): role of cytoplasmic trapping of HIF-2alpha. Mol Cell Biol. 2003;23:4959–4971. doi: 10.1128/MCB.23.14.4959-4971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleadle JM, Ratcliffe PJ. Induction of hypoxia-inducible factor-1, erythropoietin, vascular endothelial growth factor, and glucose transporter-1 by hypoxia: evidence against a regulatory role for Src kinase. Blood. 1997;89:503–509. [PubMed] [Google Scholar]
- Ashley RA, Dubuque SH, Dvorak B, Woodward SS, Williams SK, Kling PJ. Erythropoietin stimulates vasculogenesis in neonatal rat mesenteric microvascular endothelial cells. Pediatr Res. 2002;51:472–478. doi: 10.1203/00006450-200204000-00012. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez OC, Herlyn M. The vascular phenotype of melanoma metastasis. Clin Exp Metastasis. 2003;20:229–235. doi: 10.1023/a:1022987201264. [DOI] [PubMed] [Google Scholar]
- Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res. 2003;63:1684–1695. [PubMed] [Google Scholar]
- Smalley KS. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int J Cancer. 2003;104:527–532. doi: 10.1002/ijc.10978. [DOI] [PubMed] [Google Scholar]