Abstract
Peroxisome proliferator-activated receptor (PPAR)-γ controls growth, differentiation, and inflammation. PPAR-γ agonists exert anti-inflammatory effects in vitro and inhibit the activation of pancreas stellate cells, implicated in the formation and progression of fibrosis. We determined the influence of troglitazone, a ligand for PPAR-γ, on pancreatic damage and fibrosis in experimental chronic pancreatitis. Mice received six hourly intraperitoneal injections with 50 μg/kg of cerulein or saline, three times a week for 6 weeks. One week after the last injection all mice were sacrificed. Untreated mice were compared with mice treated with troglitazone either during weeks 1 to 6 or weeks 4 to 6. All mice that received cerulein injections displayed histopathological signs of chronic pancreatitis at week 7. Troglitazone treatment improved all markers for severity of pancreatitis. Moreover, early and postponed troglitazone treatments were equally effective in diminishing intrapancreatic fibrosis as quantified by Sirius red staining, hydroxyproline content, and laminin staining as well as the increased number of pancreatic stellate cells and pancreas levels of transforming growth factor-β. Thus, troglitazone attenuated pancreatic damage and inflammation in experimental chronic pancreatitis and remained beneficial in a therapeutic setting when given after initial damage had been established.
Chronic pancreatitis is characterized by progressive destruction of parenchymal tissue ultimately leading to exocrine and endocrine function loss. Clinical symptoms include abdominal pain, steatorrhea, and diabetes mellitus. The incidence of chronic pancreatitis varies from region to region, from 7 to 15 per 100,000 per year, and is rising.1 Risk factors are chronic alcohol abuse as well as genetic factors such as mutations in the cystic fibrosis gene, cationic trypsinogen gene, and serine protease inhibitor-1.2 Knowledge of the pathophysiology of chronic pancreatitis is limited. Chronic pancreatitis is considered to result from chronic repetitive inflammation within the pancreas because of alcohol abuse or recurrent bouts of even minor events of pancreatic inflammation, resulting in recurrent repair of pancreatic damage and ultimately in activation of a profibrotic cascade. Fibrosis formation in the pancreas is initiated by differentiation and activation of pancreatic stellate cells (PSCs) that produce collagen as a result.3 PSCs can be activated directly by alcohol or by transforming growth factor (TGF)-β that is produced locally in case of repetitive inflammation.4–6
Peroxisome proliferator-activated receptor (PPAR)-γ is a member of the nuclear receptor family of transcription factors.7 Considerable evidence indicates that PPAR-γ agonists inhibit inflammatory responses during inflammatory diseases.7–10 Furthermore, PPAR-γ decreases TGF-β1 production and may therefore inhibit PSC activation and fibrosis formation.11,12 Taken together, PPAR-γ ligands may have anti-inflammatory and anti-fibrotic properties that both may exert a beneficial effect on the development and course of chronic pancreatitis.9,13 In the present investigation we determined the therapeutic potential of troglitazone (a member of the glitazone family and a synthetic ligand for PPAR-γ) in a mouse model of experimental chronic pancreatitis.9,12
Materials and Methods
Animals
Female C57BL/6 mice (Harlan, Horst, The Netherlands), 10 to 12 weeks old, were used in all experiments. The Institutional Animal Care and Use Committee of the Academic Medical Center approved the protocol.
Induction of Chronic Pancreatitis
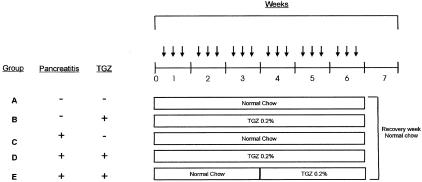
Chronic pancreatitis was induced by repeated intraperitoneal injections of the cholecystokinin analogue cerulein (Research Plus, Manasquan, NJ), as described.14 A supramaximal stimulating dose of cerulein was used for all injections (50 μg/kg). Five groups of mice (n = 10 each) were studied (Figure 1). All mice received six hourly intraperitoneal injections, three times a week for 6 weeks; groups A and B received saline injections (no induction of pancreatitis) and groups C, D, and E received cerulein injections (induction of pancreatitis). Groups A and C received normal chow throughout the entire 7-week study period. Groups B and D received chow mixed with troglitazone 0.2% (Sankyo Pharma, Tokyo, Japan) for a total of 6 weeks (weeks 1 to 6). This dose and administration route of troglitazone has been previously described.15 Group E received normal chow during the first 3 weeks (weeks 1 to 3) and chow mixed with 0.2% troglitazone for the next 3 weeks (weeks 4 to 6). All groups received normal chow in the final (7th) week, after which mice were killed. Water was administered ad libitum to all mice.
Figure 1.
Experimental design. Five groups of mice (n = 10 each) were studied. All mice received six hourly intraperitoneal injections, three times a week for 6 weeks; groups A and B received saline injections (no induction of pancreatitis), groups C, D, and E received cerulein injections (50 μg/kg; induction of pancreatitis). Groups A and C received normal chow throughout the entire 7-week study period. Groups B and D received chow mixed with troglitazone (TGZ) 0.2% for a total of 6 weeks (weeks 1 to 6). Group E received normal chow during the first 3 weeks (weeks 1 to 3) and chow mixed with 0.2% TGZ for the next 3 weeks (weeks 4 to 6). All groups received normal chow in the final (seventh) week, after which mice were killed. Arrows indicate six intraperitoneal injections with saline or cerulein.
Tissue Handling
Seven weeks after the first intraperitoneal injection mice were anesthetized with Hypnorm (Janssen Pharmaceutics, Beerse, Belgium) and midazolam (Roche, Mijdrecht, The Netherlands), and blood was collected from the vena cava inferior in heparin-coated vacutainer tubes. The pancreas was removed, and one longitudinal dissected part was frozen in liquid nitrogen to prevent degradation. Pancreas tissue was stored at −80°C until further assays were performed.
Histological Examination
For histopathological examination remaining pancreatic tissue was fixed in 10% buffered formalin and embedded in paraffin; 4-μm sections were stained with hematoxylin and eosin. All specimens were scored by a pathologist (S.F.) unaware of the origin of the specimens. Evaluation of the pancreas was performed as previously described.14 Briefly, areas of abnormal architecture were defined and quantified as 0, absent; 1, rare; 2, minimal (<10%); 3, moderate (10 to 50%); or 4, severe. Within these areas the presence of glandular atrophy, fibrosis, and pseudotubular complexes were each scored as 0, absent; 1, minimal (<10%); 2, moderate (10 to 50%); and 3, severe. For all parameters, three pancreas sections were randomly selected from each mouse. These sections were scored and a median score was calculated. In addition, the content of inflammatory cells (mainly neutrophils) and edema were scored on a 0 to 4 scale as described elsewhere.16 Furthermore, to investigate the extent of pancreatic destruction, we quantified the number of acinar cells by counting the number of acinar cells of at least three high-power fields (HPF, ×400 magnification) per pancreatic specimen. A mean number was calculated for each mouse.
Intrapancreatic Collagen Quantification
The amount of intrapancreatic collagen was quantified using image analysis of Sirius red-stained pancreatic sections as well as by quantifying hydroxyproline content. Quantitative analysis of collagen was performed by morphometric analysis.14 Three digitized pictures of each pancreatic section (10 mice per group), viewed through an Olympus BX60 microscope (Olympus, Zoeterwoude, The Netherlands) equipped with a ×20 objective lens, were transmitted by a coolsnap video camera (Roper Scientific, Vianen, The Netherlands) to a Dell 300-MHz PC equipped with Image Pro Plus software (Media Cybernetics, Gleichen, Germany). The total amount of collagen stained on each submitted section was calculated by the computer via the digitalized image as follows. In the first step, pancreas was distinguished from the background according to a difference in light density, and a measurement of the total pancreatic tissue area was performed. In the second step, the amount of collagen (stained in red) was measured and was finally expressed as a percentage of the total pancreatic surface. Furthermore, total collagen was assessed by measuring hydroxyproline content as described elsewhere.17 In brief, pancreatic samples were hydrolyzed in 12 mol/L HCl at 110°C for 16 hours. The samples were resuspended in 2 ml of deionized water and 1 ml of chloramine T dissolved in 5 mol/L sodium acetate/10% isopropanol. Next, 3 mmol/L perchloric acid and 1 ml of Ehrlich’s reagent (ICN Biochemicals, Aurora, OH) were added, mixed, and incubated at 65°C for 15 minutes. Finally absorbance was measured at 550 nm and values were compared with serial dilutions of trans-4-hydroxy-l-proline. All reagents for the hydroxyproline assay were purchased from Sigma, St. Louis, MO.
Laminin and α-Smooth Muscle Actin (SMA) Stainings
For laminin and α-SMA stainings the slides were deparaffinized and endogenous peroxidase activity was quenched by a solution of methanol/0.03% H2O2 (Merck, Darmstadt, Germany). After digestion with a solution of pepsin 0.25% (Sigma) in 0.01 mol/L HCl for laminin, and with 10 mmol/L sodium citrate solution, pH 6.0, for 10 minutes at 98°C in microwave oven for α-SMA, the sections were incubated in 10% normal goat serum (DAKO, Glostrup, Denmark) and then exposed to a rabbit anti-laminin antibody (Abcam, Cambridge, MA) or a mouse IgG2a anti-α-SMA antibody (DAKO), respectively. After washes, slides stained for laminin were probed with a goat anti-rabbit poly-horseradish peroxidase (Powervision, Immunological, Duiven, The Netherlands). Slides stained for α-SMA were incubated with a horseradish peroxidase-labeled goat anti-mouse IgG2a antibody (Southern Biotech, Birmingham, AL). Slides were finally developed using 1% H2O2 and 3,3′-diaminobenzidine-tetra-hydrochloride (Sigma) in Tris-HCl. The sections were mounted in glycerin gelatin without counterstaining. Laminin stainings were quantified using image analysis as described above. α-SMA stainings were quantified by counting the number of α-SMA-positive cells in at least three HPFs per section, positive cells located in or near blood vessel walls were ignored. Data are expressed as the number of α-SMA-positive cells per 50 acinar cells to correct for the variance in the number of cells per HPF between the groups.
Assays
For measurements of intrapancreatic amylase and active TGF-β1 concentrations, pancreas samples were homogenized in 5 vol of sterile saline with a standard tissue homogenizer (Biospec Products, Bartlesville, OK). Homogenates were lysed in lysis buffer [300 mmol/L NaCl, 15 mmol/L Tris, 2 mmol/L MgCl, 2 mmol/L Triton X-100, pepstatin A, leupeptin, aprotinin (20 ng/ml), pH 7.4] and spun at 1500 × g at 4°C for 15 minutes; the supernatant was frozen at −20°C until analysis. Protein levels in homogenates were measured using the BCA protein kit according to the manufacturer’s instructions (Pierce, Rockford, IL). Levels of pancreatic amylase and TGF-β were normalized by equal protein loading. Amylase levels in pancreas homogenates were determined with a commercially available kit (Sigma,), using a Hitachi analyzer (Boehringer Mannheim, Mannheim, Germany). Active TGF-β1 levels were measured in pancreas homogenates by enzyme-linked immunosorbent assay according to the instructions of the manufacturer, this enzyme-linked immunosorbent assay measures the active, immunoreactive, form of TGF-β1 only (R&D Systems, Minneapolis, MN). Myeloperoxidase (MPO) content was measured in pancreas homogenates as described elsewhere.18–20 The plasma concentrations of interleukin (IL)-6 (Pharmingen, San Diego, CA) and soluble tumor necrosis factor receptor type 1 (TNFR1) (R&D Systems) were measured by enzyme-linked immunosorbent assay in accordance with the instructions of the manufacturers.
Statistical Analysis
All data are expressed as means ± SE. Comparisons between groups were conducted using one-way analysis of variance followed by Dunn’s post test. Significance was set at P < 0.05.
Results
Troglitazone Treatment Attenuates Pancreatic Damage and Fibrosis
Chronic pancreatitis was induced by six hourly intraperitoneal cerulein injections, three times a week for 6 weeks. Troglitazone was given either during the whole 6-week period, or from weeks 4 to 6 (Figure 1). All mice treated with repeated cerulein injections (groups C, D, and E) displayed histopathological signs of chronic pancreatitis at the time of sacrifice (week 7), as reflected by abnormal architecture, glandular atrophy pseudotubular complexes, fibrosis, edema, and inflammatory cell infiltrate (Table 1 and Figure 2; all P < 0.05 for the comparisons with groups A and B). In mice in which chronic pancreatitis was induced and that were treated with troglitazone from either weeks 1 to 6 (group D) or from weeks 4 to 6 (group E) all markers of pancreatic damage were significantly attenuated (Table 1 and Figure 2; all P < 0.05 for the comparisons with group C). Notably, the effects of early and postponed troglitazone treatment were similar. To evaluate the degree of fibrosis in the pancreas, Sirius red-stained pancreas sections were analyzed using computer-assisted digital image analysis and pancreatic hydroxyproline content was quantified (Figure 3 and Table 2). Pancreatic collagen content dramatically increased after induction of chronic pancreatitis (P < 0.05 for the comparison between groups A and B versus groups C, D, and E). However, the increase in pancreatic collagen content was diminished by troglitazone, irrespective of the treatment schedule (Figure 3 and Table 2; P < 0.05 for the comparison between group C versus groups D and E). Furthermore, pancreas laminin content was quantified by image analysis of laminin. As expected, laminin accumulated during chronic pancreatitis induction. Independently of the treatment schedule, troglitazone treatment reduced the accumulation of laminin (Table 2; P < 0.05 A and B versus C as well as C versus D and E). α-SMA immunohistochemistry was performed to quantify the number of stellate cells, the putative cells responsible for fibrosis, in the pancreas. In line, the number of stellate cells increased during pancreatitis induction and this increase in stellate cells was attenuated by troglitazone (Table 2; P < 0.05 A and B versus C, as well as C versus D and E). This effect was similar in mice receiving early or delayed troglitazone treatment.
Table 1.
Troglitazone Reduces the Severity of Experimental Chronic Pancreatitis
| A | B | C | D | E | |
|---|---|---|---|---|---|
| Abnormal architecture | 0 ± 0 | 0 ± 0 | 2.0 ± 0.4† | 0.9 ± 0.3*† | 0.7 ± 0.2*† |
| Glandular atrophy | 0 ± 0 | 0 ± 0 | 2.7 ± 0.2† | 1.4 ± 0.3*† | 1.4 ± 0.2*† |
| Pseudotubular complexes | 0 ± 0 | 0 ± 0 | 2.6 ± 0.3† | 1.6 ± 0.2*† | 1.8 ± 0.2*† |
| Fibrosis | 0 ± 0 | 0 ± 0 | 2.9 ± 0.1† | 2.2 ± 0.2*† | 2.2 ± 0.2*† |
| Total score of above | 0 ± 0 | 0 ± 0 | 10.0 ± 0.6† | 5.7 ± 0.9*† | 6.0 ± 0.3*† |
| Edema | 0 ± 0 | 0 ± 0 | 2.4 ± 0.3† | 1.5 ± 0.2*† | 1.7 ± 0.1*† |
| Inflammation | 0 ± 0 | 0 ± 0 | 2.3 ± 0.3† | 1.3 ± 0.4*† | 1.2 ± 0.1*† |
For the description of groups A to E see Figure 1. For the description of the histopathology score see Materials and Methods. Data are mean ± SE (n = 10 mice per group).
P < 0.05 versus C;
P < 0.05 versus A and B.
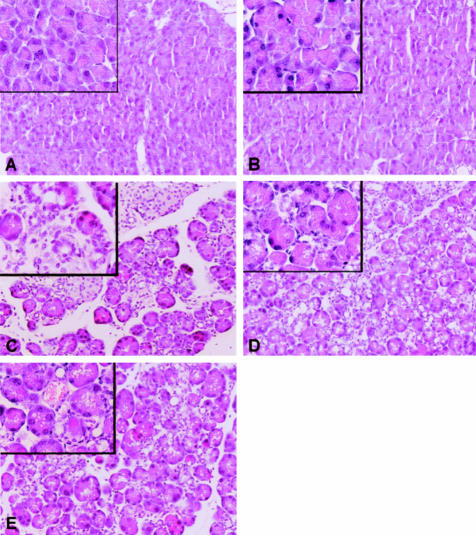
Figure 2.
Troglitazone treatment reduces pancreatic damage. Representative H&E-stained pancreas histology slides from a total of 10 mice per group. For the description of groups A to E see Figure 1. For data derived from scoring H&E-stained pancreas specimens see Table 1. H& E staining. Original magnifications: ×10; ×40 (insets).
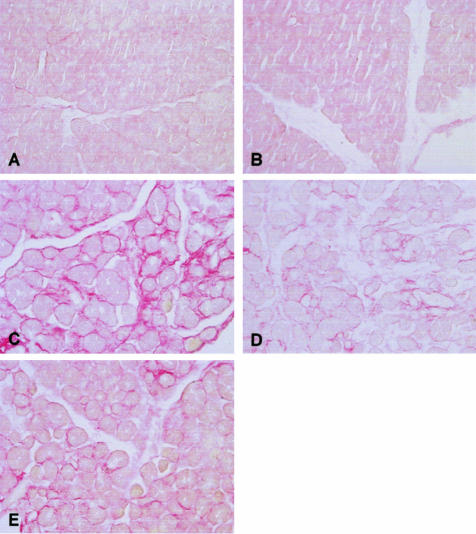
Figure 3.
Troglitazone treatment reduces pancreatic collagen content. Representative Sirius red-stained pancreas sections from a total of 10 mice per group used to quantify tissue collagen content. For the description of groups A to E see Figure 1. For data derived from image analysis of Sirius red-stained specimens see Table 2. H& E staining. Original magnifications: ×10; ×40 (insets).
Table 2.
Troglitazone Reduces Fibrosis Formation and the Number of Activated Stellate Cells during Experimental Chronic Pancreatitis
| A | B | C | D | E | |
|---|---|---|---|---|---|
| Hydroxyproline content (μg/mg protein) | 2.9 ± 0.6 | 2.7 ± 0.5 | 15.2 ± 3.1† | 6.5 ± 2.1*† | 8.1 ± 2.3*† |
| Sirius Red staining (%) | 0.8 ± 0.2 | 0.9 ± 0.2 | 10.8 ± 0.7† | 4.6 ± 0.5*† | 5.5 ± 0.7*† |
| Laminin content (%) | 8.1 ± 1.1 | 8.3 ± 1.1 | 31.2 ± 1.9† | 16.5 ± 2.3*† | 23.1 ± 1.4*† |
| α-SMA-positive cells (/50 acinar cells) | 1.2 ± 0.4 | 1.2 ± 0.4 | 11.8 ± 0.8† | 5.4 ± 0.6*† | 7.3 ± 0.5*† |
For the description of groups A to E see Figure 1. Fibrotic parameters are shown. For a detailed description of the methods, systems, and setting see the Materials and Methods section. Data are mean ± SE (n = 10 mice per group, three sections analyzed per mouse).
P < 0.05 versus C;
P < 0.05 versus A and B.
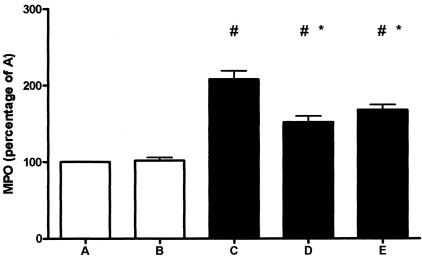
Troglitazone Reduces the Increase in Intrapancreatic MPO Content
MPO activity in pancreas homogenates was determined as a measure for neutrophil accumulation within the organ (Figure 4). Mice that received repeated cerulein injections demonstrated a profound rise in pancreatic MPO concentrations (P < 0.05 for the comparison between groups A and B versus groups C, D, and E). Troglitazone diminished this increase in pancreatic MPO levels in mice with pancreatitis irrespective of the treatment schedule (P < 0.05 for the comparison between group C versus groups D and E).
Figure 4.
Troglitazone treatment reduces MPO content. Pancreas MPO activity is expressed as a percentage of group A. Open bars represent saline-injected mice (without pancreatitis); filled bars represent cerulein-injected mice (with pancreatitis). Data are mean ± SE of 10 mice per group. For the description of groups A to E see Figure 1. #, P < 0.05 versus A and B; *, P < 0.05 versus C.
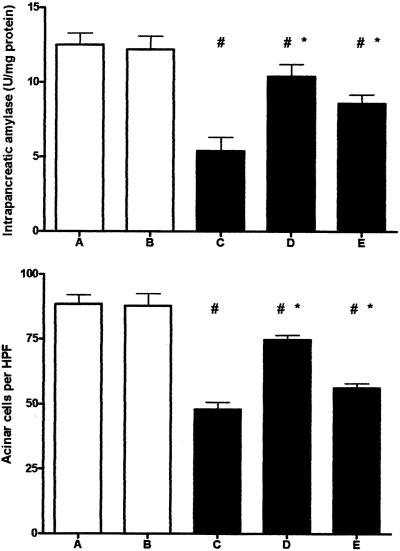
Troglitazone Partially Prevents Intrapancreatic Acinar Cell Depletion
Chronic pancreatitis is associated with a decrease in acinar cells and therefore a loss in exocrine function.2,14 To obtain insight into pancreas acinar cell content in our study, we counted the number of acinar cells per HPF and measured amylase concentrations in pancreas homogenates. The number of acinar cells per HPF as well as levels of amylase were markedly decreased in mice with experimentally induced chronic pancreatitis as compared to control mice (Figure 5; P < 0.05 for the comparison of groups A and B versus groups C, D, and E). Troglitazone partially prevented this decrease in intrapancreatic acinar cells as well as amylase concentration in mice with pancreatitis (P < 0.05 for the comparison between group C versus groups D and E). This effect was similar in mice receiving early or postponed troglitazone treatment.
Figure 5.
Troglitazone treatment reduces loss in pancreas acinar cell content. Open bars represent saline-injected mice (without pancreatitis); filled bars represent cerulein-injected mice (with pancreatitis). Data are mean ± SE of 10 mice per group. For the description of groups A to E see Figure 1. #, P < 0.05 versus A and B; *, P < 0.05 versus C.
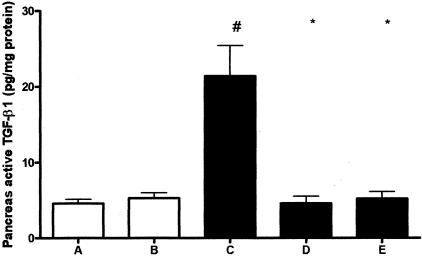
Troglitazone Treatment Prevents the Increase in Pancreatic Active TGF-β1 Concentrations
TGF-β1 has been implicated as an important mediator in the development of fibrosis in chronic pancreatitis.21,22 Because TGF-β1 is synthesized and released as a latent form that requires activation before it manifests biological activity, and the activation step appears to be as important as synthesis in determining TGF-β1 activity we measured active TGF-β1 in these studies. Induction of chronic pancreatitis was associated with an increase in active TGF-β1 concentrations in pancreas homogenates (Figure 6; P < 0.05 for the comparison between groups A and B versus group C). Remarkably, troglitazone completely prevented the rise in pancreatic active TGF-β1 levels in mice administered repeated cerulein injections (P < 0.05 for the comparison between group C versus groups D and E). This effect was similar in mice that received early or postponed troglitazone treatment.
Figure 6.
Troglitazone reduces pancreatic active TGF-β1 levels during experimental chronic pancreatitis. Open bars represent saline-injected mice (without pancreatitis); filled bars represent cerulein-injected mice (with pancreatitis). Data are mean ± SE of 10 mice per group and are expressed as pg/mg protein. For the description of groups A to E see Figure 1. #, P < 0.05 versus A and B; *, P < 0.05 versus C.
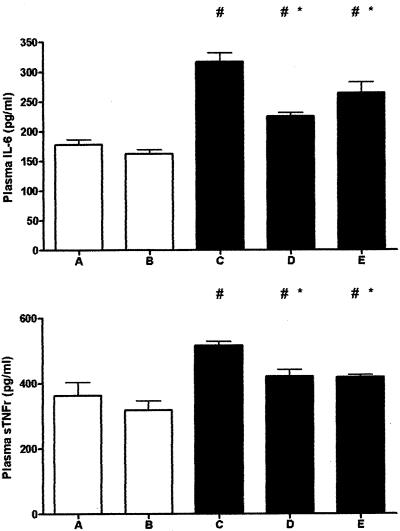
Troglitazone Attenuates Signs of Systemic Inflammation
Several plasma markers have been found to correlate with the degree of pancreas inflammation in patients, including IL-6 and soluble TNF receptors.23 Therefore, to obtain insight in the occurrence of systemic inflammation we measured the plasma concentrations of IL-6 and soluble TNF receptor type 1 (Figure 7). Chronic pancreatitis was accompanied by a rise in the plasma levels of both mediators (P < 0.05 for the comparison between groups A and B versus groups C, D, and E). Troglitazone attenuated these increases (P < 0.05 for the comparison between group C versus groups D and E).
Figure 7.
Troglitazone reduces plasma IL-6 and soluble TNFR-1 during experimental chronic pancreatitis. Open bars represent saline-injected mice (without pancreatitis); filled bars represent cerulein-injected mice (with pancreatitis). Data are mean ± SE of 10 mice per group. For the description of groups A to E see Figure 1. #, P < 0.05 versus A and B; *, P < 0.05 versus C.
Discussion
In the present investigation, we induced chronic pancreatitis in mice using a scheme in which animals received six hourly cerulein injections three times a week for 6 weeks. Mice that received cerulein injections developed chronic pancreatitis as evidenced by severe histological damage, fibrosis, and loss in acinar cell content. Mice treated with the PPAR-γ ligand troglitazone, either for the whole study period or during weeks 4 to 6 only, showed a significant attenuation of all histopathological parameters of chronic pancreatitis as well as a reduction in the loss of acinar cells. Furthermore, markers of inflammation within the pancreas (MPO) and in the circulation (IL-6, soluble TNFR1), as well as pancreatic active TGF-β1 were all decreased in troglitazone-treated mice. Therefore, troglitazone reduces pancreatic inflammation, damage, and fibrosis in experimental chronic pancreatitis and is even beneficial in a therapeutic setting when given after initial damage has been established.
The etiology of the development of chronic pancreatitis is primarily unknown. Current evidence indicates that chronic pancreatitis is the result of repetitive episodes of pancreatic inflammation and necrosis, inducing repetitive episodes of regeneration and ultimately leading to activation of PSCs by TGF-β1 and the induction of fibrosis. This necrosis-fibrosis hypothesis identifies two major components in the development of fibrosis: first the recurrent episodes of inflammation and second the activation of PSCs by TGF-β1.
PPAR-γ is a member of the nuclear receptor family of transcription factors that mediates growth, differentiation, and inflammation.7 Natural ligands of PPAR-γ are fatty acids, arachidonic acid metabolites, and prostaglandins; synthetic ligands of PPAR-γ are nonsteroidal anti-inflammatory drugs and a family of anti-diabetic drugs called glitazones, among which is troglitazone. On binding of a ligand, the PPAR-γ receptor forms a heterodimer with the retinoid X receptor and becomes activated. This heterodimer can interfere with mitogen-activated protein-kinase and the nuclear factor-κB proinflammatory pathways.7,9 Glitazones have been used clinically in the treatment of type II diabetes.24,25 The anti-diabetic properties of glitazones are contributed to the fact that these compounds, by activating PPAR-γ, counter effect the insulin resistance-generating effects of TNF-α.26 Considerable evidence indicates that PPAR-γ agonists may have beneficial effects in other diseases as well, because of their anti-inflammatory properties. PPAR-γ agonists negatively regulate proinflammatory cytokine production by mononuclear cells as well as adhesion molecule expression on endothelial cells.7–10 In this study, we observed that mice treated with troglitazone, either from the beginning of pancreatitis induction or from weeks 4 to 6 only, showed an attenuation of inflammation in the pancreas, as reflected by a reduction of MPO activity and neutrophil content estimated by semiquantitative analyses of histological sections. In the systemic compartment, markers of inflammatory activation, IL-6, and soluble TNFR1 were also diminished by troglitazone treatment, likely because of a reduction in inflammation in the pancreas itself. Indeed, the circulating levels of both mediators have been shown to correlate directly with the degree of pancreatic inflammation.27
In this study we show that inhibition of inflammatory responses by troglitazone reduced pancreatic inflammation, regeneration, and finally pancreatic damage during the bouts of repetitive inflammation induced by cerulein. It is conceivable that reduction of inflammation is because of an anti-inflammatory effect of troglitazone during the repetitive acute components of the model, indeed, previous studies have shown that PPAR-γ agonists inhibit inflammation during cerulein-induced acute pancreatitis.28,29 The positive effects of inhibition of inflammation on the development of pancreatic damage in this model are in line with another previous study, showing that mice lacking the anti-inflammatory cytokine IL-10 experienced a significant exacerbation of pancreatic damage that was related to an increase in pancreatic inflammation and a reduction of pancreatic regeneration.14
TGF-β1 is a regulator of extracellular matrix remodeling in the pancreas, and may (on activation from the latent inactive form) be an important promoting factor in the pathogenesis of chronic pancreatitis. This hypothesis is supported by findings of enhanced TGF-β1 expression in human chronic pancreatitis and development of fibrosis and inflammation in pancreata of transgenic mice overexpressing TGF-β1.30,31 In vitro data suggests that PPAR-γ agonists inhibit the activation of PSCs, cells that have been implicated in the formation and progression of fibrosis, during chronic pancreatitis by inhibiting the PSC-activating effects of TGF-β.9 In this study we show that indeed the PPAR-γ ligand troglitazone reduces the levels of active TGF-β1 in the pancreas as well as the number of PSCs that ultimately results in an impairment of fibrosis formation. These data are in line with studies in experimental hepatic fibrosis, in which glitazone treatment reduced hepatic stellate cell (HSC) activation, TGF-β1 levels, and fibrosis10,32 and with a recent study in which spontaneous chronic pancreatitis severity was reduced in male Wistar Bonn/Kobori rats by treatment with an angiotensin-converting enzyme inhibitor that attenuated TGF-β1 expression in the pancreas, resulting in the prevention of PSC activation and pancreatic fibrosis.33
Our study shows that troglitazone inhibits inflammation, pancreatic damage, and fibrosis during chronic pancreatitis. These data are in line with a previous study in WBN/Kob rats with spontaneous chronic pancreatitis in which troglitazone decreased chronic pancreatitis severity.34 In WBM/Kob rats pancreatitis develops spontaneously in the aging rat because of an incompletely understood mechanism. These changes are not restricted to the exocrine pancreas, because they are accompanied by several additional lesions such as severe endocrine pancreatic failure and diabetes, myocardiopathy, neuropathy, and bone loss.35–37 In this study, we used a different model of chronic pancreatitis that specifically reflects the development of exocrine chronic pancreatitis according to the necrosis-fibrosis hypothesis. An important finding in this study is that the positive effects of troglitazone are maintained if the treatment is delayed up until halfway through the 7-week model. At this point, mice already had experienced nine episodes of acute pancreatitis and subsequent regeneration. This approach, in which treatment was started after initial pancreatic damage had been established, was almost as effective as treatment during the whole period of pancreatitis induction. In additional experiments mice were sacrificed after 3 weeks during this model (data not shown). These experiments revealed that the fibrosis response was already present but relatively mild at this stage, suggesting that adding troglitazone after 3 weeks did block the progression of fibrosis formation rather than reversing it. However, it should be noted that the model used here is less suitable to determine whether troglitazone is able to reverse pancreas fibrosis, because the recovery week (ie, the week without cerulein injections after 6 weeks of cerulein treatment) is essential for the development of a strong fibrotic response.38 Reversal of fibrosis might in theory be possible because studies in liver fibrosis have provided a substantial body of evidence indicating that liver fibrosis is a dynamic process that can progress and regress throughout time.39 After treatment of hepatitis C with pegylated-interferon and ribavirin, it has been shown that when the virus is cleared the rate of fibrosis progression can be reversed.40 In human and rat HSCs as well as in established experimental liver fibrosis, gliotoxin, an agent that induces apoptosis in inflammatory cells, up-regulates apoptosis in HSCs which results in a regression of liver fibrosis.41 Therefore, it is conceivable that fibrosis should be regarded as a dynamic process that can be stopped and possibly even reversed. Because PSCs and HSCs share many functional characteristics, there is reason to believe that in pancreatic fibrosis similar mechanisms exist. In line with these data, we postulate that treatment with troglitazone after initial fibrosis has been established results in inhibition of PSCs which stops fibrosis progression but might also in part enhance the resolution of pancreatic fibrosis indicating that glitazones may be a valuable therapy for chronic pancreatitis. Because this is the first time that any anti-inflammatory/anti-fibrotic strategy has been shown to be effective in a therapeutic setting during experimental chronic pancreatitis, further studies evaluating the efficacy of glitazones during human chronic pancreatitis are warranted.
Footnotes
Address reprint requests to David J. van Westerloo, M.D., Academic Medical Center, Department of Gastroenterology and Hepatology, Meibergdreef 9, C2–329, 1105 AZ, Amsterdam, The Netherlands. E-mail: d.j.vanwesterloo@amc.uva.nl.
References
- Banks PA. Epidemiology, natural history, and predictors of disease outcome in acute and chronic pancreatitis. Gastrointest Endosc. 2002;56:S226–S230. doi: 10.1067/mge.2002.129022. [DOI] [PubMed] [Google Scholar]
- Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–432. doi: 10.1016/s0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- Schmid-Kotsas A, Gross HJ, Menke A, Weidenbach H, Adler G, Siech M, Beger H, Grunert A, Bachem MG. Lipopolysaccharide-activated macrophages stimulate the synthesis of collagen type I and C-fibronectin in cultured pancreatic stellate cells. Am J Pathol. 1999;155:1749–1758. doi: 10.1016/S0002-9440(10)65490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttenberger T, Schmid-Kotsas A, Menke A, Siech M, Beger H, Adler G, Grunert A, Bachem MG. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab Invest. 2000;80:47–55. doi: 10.1038/labinvest.3780007. [DOI] [PubMed] [Google Scholar]
- Jaskiewicz K, Nalecz A, Rzepko R, Sledzinski Z. Immunocytes and activated stellate cells in pancreatic fibrogenesis. Pancreas. 2003;26:239–242. doi: 10.1097/00006676-200304000-00006. [DOI] [PubMed] [Google Scholar]
- Zhang X, Young HA. PPAR and immune system—what do we know? Int Immunopharmacol. 2002;2:1029–1044. doi: 10.1016/s1567-5769(02)00057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangbam CS, Tyler RD, Lightfoot RM. Peroxisome proliferator-activated receptors in atherosclerosis and inflammation—an update. Toxicol Pathol. 2001;29:224–231. doi: 10.1080/019262301317052495. [DOI] [PubMed] [Google Scholar]
- Masamune A, Kikuta K, Satoh M, Sakai Y, Satoh A, Shimosegawa T. Ligands of peroxisome proliferator-activated receptor-gamma block activation of pancreatic stellate cells. J Biol Chem. 2002;277:141–147. doi: 10.1074/jbc.M107582200. [DOI] [PubMed] [Google Scholar]
- Galli A, Crabb DW, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, Ridolfi F, Trozzi L, Surrenti C, Casini A. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924–1940. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- Fu M, Zhang J, Zhu X, Myles DE, Willson TM, Liu X, Chen YE. Peroxisome proliferator-activated receptor gamma inhibits transforming growth factor beta-induced connective tissue growth factor expression in human aortic smooth muscle cells by interfering with Smad3. J Biol Chem. 2001;276:45888–45894. doi: 10.1074/jbc.M105490200. [DOI] [PubMed] [Google Scholar]
- Ma LJ, Marcantoni C, Linton MF, Fazio S, Fogo AB. Peroxisome proliferator-activated receptor-gamma agonist troglitazone protects against nondiabetic glomerulosclerosis in rats. Kidney Int. 2001;59:1899–1910. doi: 10.1046/j.1523-1755.2001.0590051899.x. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Demols A, Van Laethem JL, Quertinmont E, Degraef C, Delhaye M, Geerts A, Deviere J. Endogenous interleukin-10 modulates fibrosis and regeneration in experimental chronic pancreatitis. Am J Physiol. 2002;282:G1105–G1112. doi: 10.1152/ajpgi.00431.2001. [DOI] [PubMed] [Google Scholar]
- Osawa E, Nakajima A, Wada K, Ishimine S, Fujisawa N, Kawamori T, Matsuhashi N, Kadowaki T, Ochiai M, Sekihara H, Nakagama H. Peroxisome proliferator-activated receptor gamma ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology. 2003;124:361–367. doi: 10.1053/gast.2003.50067. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Saluja AK, Singh VP, Frossard JL, Lee HS, Bhagat L, Gerard C, Steer ML. Complement factor C5a exerts an anti-inflammatory effect in acute pancreatitis and associated lung injury. Am J Physiol. 2001;280:G974–G978. doi: 10.1152/ajpgi.2001.280.5.G974. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Lichtman SN, Wang J, Hummel B, Lacey S, Sartor RB. A rat model of ileal pouch-rectal anastomosis. Inflamm Bowel Dis. 1998;4:187–195. doi: 10.1097/00054725-199808000-00003. [DOI] [PubMed] [Google Scholar]
- Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, van Rooijen N, van der Poll T. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2003;167:171–179. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- van Westerloo DJ, Schultz MJ, Bruno MJ, de Vos AF, Florquin S, van der Poll T. Acute pancreatitis in mice impairs bacterial clearance from the lungs, whereas concurrent pneumonia prolongs the course of pancreatitis. Crit Care Med. 2004;32:1997–2001. doi: 10.1097/01.ccm.0000142658.22254.74. [DOI] [PubMed] [Google Scholar]
- Muller-Pillasch F, Menke A, Yamaguchi H, Elsasser HP, Bachem M, Adler G, Gress TM. TGFbeta and the extracellular matrix in pancreatitis. Hepatogastroenterology. 1999;46:2751–2756. [PubMed] [Google Scholar]
- Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, Pirola RC, McCaughan GW, Ramm GA, Wilson JS. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087–1095. doi: 10.1016/S0002-9440(10)65211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaux AC, Goldie AS, Ross JA, Carter DC, Fearon KC. Serum concentrations of inflammatory mediators related to organ failure in patients with acute pancreatitis. Br J Surg. 1996;83:349–353. doi: 10.1002/bjs.1800830317. [DOI] [PubMed] [Google Scholar]
- Parker JC. Troglitazone: the discovery and development of a novel therapy for the treatment of type 2 diabetes mellitus. Adv Drug Deliv Rev. 2002;54:1173–1197. doi: 10.1016/s0169-409x(02)00093-5. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Haring HU. Glitazones: clinical effects and molecular mechanisms. Ann Med. 2002;34:217–224. [PubMed] [Google Scholar]
- Grimble RF. Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care. 2002;5:551–559. doi: 10.1097/00075197-200209000-00015. [DOI] [PubMed] [Google Scholar]
- Brady M, Christmas S, Sutton R, Neoptolemos J, Slavin J. Cytokines and acute pancreatitis. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:265–289. doi: 10.1053/bega.1999.0024. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Britti D, Patel NS, Paola RD, Genovese T, Rosa MD, Caputi AP, Thiemermann C. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute pancreatitis induced by cerulein. Intensive Care Med. 2004;30:951–956. doi: 10.1007/s00134-004-2180-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ethridge RT, Saito H, Rajaraman S, Evers BM. The PPARgamma ligand, 15d-PGJ2, attenuates the severity of cerulein-induced acute pancreatitis. Pancreas. 2003;27:58–66. doi: 10.1097/00006676-200307000-00009. [DOI] [PubMed] [Google Scholar]
- Lee MS, Gu D, Feng L, Curriden S, Arnush M, Krahl T, Gurushanthaiah D, Wilson C, Loskutoff DL, Fox H. Accumulation of extracellular matrix and developmental dysregulation in the pancreas by transgenic production of transforming growth factor-beta 1. Am J Pathol. 1995;147:42–52. [PMC free article] [PubMed] [Google Scholar]
- Van Laethem JL, Deviere J, Resibois A, Rickaert F, Vertongen P, Ohtani H, Cremer M, Miyazono K, Robberecht P. Localization of transforming growth factor beta 1 and its latent binding protein in human chronic pancreatitis. Gastroenterology. 1995;108:1873–1881. doi: 10.1016/0016-5085(95)90152-3. [DOI] [PubMed] [Google Scholar]
- Kon K, Ikejima K, Hirose M, Yoshikawa M, Enomoto N, Kitamura T, Takei Y, Sato N. Pioglitazone prevents early-phase hepatic fibrogenesis caused by carbon tetrachloride. Biochem Biophys Res Commun. 2002;291:55–61. doi: 10.1006/bbrc.2002.6385. [DOI] [PubMed] [Google Scholar]
- Kuno A, Yamada T, Masuda K, Ogawa K, Sogawa M, Nakamura S, Nakazawa T, Ohara H, Nomura T, Joh T, Shirai T, Itoh M. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology. 2003;124:1010–1019. doi: 10.1053/gast.2003.50147. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Shiratori K, Hayashi N, Kobayashi M, Fujiwara T, Horikoshi H. Thiazolidinedione derivatives as novel therapeutic agents to prevent the development of chronic pancreatitis. Pancreas. 2002;24:184–190. doi: 10.1097/00006676-200203000-00010. [DOI] [PubMed] [Google Scholar]
- Tsuruta S, Sutani T, Masuda J, Sakaguchi Y, Tsuchihashi M, Hashimoto T, Nakamura Y, Dohi K. Mechanism of cardiac involvement in the WBN/Kob rat. J Mol Cell Cardiol. 1997;29:247–253. doi: 10.1006/jmcc.1996.0269. [DOI] [PubMed] [Google Scholar]
- Igarashi C, Maruyama T, Ezawa I, Takei I, Saruta T. WBN/Kob rat: a new model of spontaneous diabetes, osteopenia and systemic hemosiderin deposition. Bone Miner. 1994;27:133–144. doi: 10.1016/s0169-6009(08)80215-2. [DOI] [PubMed] [Google Scholar]
- Yagihashi S, Wada R, Kamijo M, Nagai K. Peripheral neuropathy in the WBN/Kob rat with chronic pancreatitis and spontaneous diabetes. Lab Invest. 1993;68:296–307. [PubMed] [Google Scholar]
- Vaquero E, Molero X, Tian X, Salas A, Malagelada JR. Myofibroblast proliferation, fibrosis, and defective pancreatic repair induced by cyclosporin in rats. Gut. 1999;45:269–277. doi: 10.1136/gut.45.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525–1528. doi: 10.1053/gast.2002.33367. [DOI] [PubMed] [Google Scholar]
- Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- Wright MC, Issa R, Smart DE, Trim N, Murray GI, Primrose JN, Arthur MJ, Iredale JP, Mann DA. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685–698. doi: 10.1053/gast.2001.27188. [DOI] [PubMed] [Google Scholar]