Abstract
Mammalian pregnancy is thought to be a state of immunological tolerance. The mechanisms underlying this phenomenon are still poorly understood. Here, we determined whether an inappropriate function of T regulatory (Treg) cells is involved in the pathogenesis of spontaneous abortion. We evaluated spleen and decidual lymphocytes from CBA/J mice undergoing immunological abortion (DBA/2J-mated) or having normal pregnancy (BALB/c-mated) on day 14 of gestation for ex vivo cytokine production after PMA or paternal antigen (alloantigen) stimulation. Treg activity was characterized by quantifying CD4+CD25+ cells, foxp3 expression, and interleukin-10 secretion. Decidual lymphocytes from abortion CBA/J mice contained a significantly higher frequency of interferon-γ-producing T cells specific for paternal antigens compared to those from normal pregnancy (7.8% versus 2.7%, P < 0.05). Compared to virgin CBA/J females, normal pregnant mice showed strongly elevated numbers of CD4+CD25+ and interleukin-10+ Treg cells in the thymus whereas significantly lower frequencies of Treg cells were observed in abortion mice. Very interestingly, CD4+CD25+ Treg cells from normal pregnant and nonpregnant CBA/J mice could inhibit both proliferation and interferon-γ secretion of lymphocytes from abortion mice in vitro whereas in vivo prevention of fetal rejection could only be achieved after adoptive transfer of Treg cells from normal pregnant mice. Our data suggest that pregnancy-induced Treg cells play a vital role in maternal tolerance to the allogeneic fetus.
Mammalian pregnancy remains an open question to immunologists because the mechanisms allowing the maternal immune system to support its semiallogeneic fetus are still poorly understood. Medawar first proposed the similarities of a growing fetus with an allograft.1 Because normally the maternal immune system does not reject its semiallogeneic conceptus, pregnancy has been thought to be a state of immunological tolerance.1,2 Having learned why the fetus is tolerated at the feto-maternal interface, it would be very helpful not only to design therapeutic approaches for immunological pregnancy complications but also to understand how an allograft can be tolerated in a foreign milieu.
Local mechanisms may play an important role in evading immune attack because maternal alloreactive lymphocytes are not systemically depleted. The specialized fetal tissue in contact with maternal uterine tissue might contribute to tolerance by several mechanisms, such as depleting tryptophan,3 by inactivating natural killer cells through HLA-G expression,4 or by provoking apoptosis of activated maternal lymphocytes.5 Incomplete tolerance might therefore result in disturbed pregnancy such as spontaneous abortion and pre-eclampsia. Further, Th1/Th2 cytokine balance has been seen as a very important mechanism determining the survival of the fetus in the maternal uterus6–10 because the production of Th2-type cytokines such as interleukin (IL)-4 and IL-10 locally at the feto-maternal interface would favor the maintenance of mammalian pregnancy11,12 and a Th1/proinflammatory predominant pattern [up-regulation of IL-2, IL-6, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α] would mediate the fetal rejection.13–17 However, IL-4 and IL-10 genetically deficient mice do not show disturbed pregnancy,18 suggesting that Th2 cells are not essential for normal pregnancy and alloreactive Th1 cells must be differently regulated, as already proposed.19–21 Similar challenges of the Th1/Th2 concept have been made for organ allografts as tolerance induction in IL-4−/− mice develops normally.22
CD4+CD25+ Treg cells were recently described as a unique subpopulation of T cells, 23–25 known to play a major role in preventing autoimmunity and tolerating allogeneic organ grafts.26,27 The role of Treg cells in physiology and pathophysiology of pregnancy is still unclear. The acceptance of paternally derived tumors cells during pregnancy2 supports the involvement of systemic regulatory processes in pregnancy, and encourages one to design studies aiming to clarify the role of Treg cells during pregnancy.
The CBA/J × DBA/2J murine model offers the possibility to elucidate mechanisms underlying immunological spontaneous abortion because the mating of CBA/J females (H2k) with DBA/2J males (H2d) provokes spontaneously high abortion rates, whereas mating CBA/J females with BALB/c mice, which also bear H2d antigens, ends in completely normal pregnancies.28 Here, we wondered whether a functional deficiency of Treg cells contributes to the spontaneous abortion in this model and whether fetal rejection could be prevented by adoptive transfer of Treg cells from normal pregnant or nonpregnant mice into pregnant abortion-prone mice.
Materials and Methods
Animals and Pregnancy Outcome
Male DBA/2J mice were purchased from Charles River (Wilmington, MA) and female CBA/J were purchased from Charles River (Les Oncins, France) through Charles River, Sulzfeld, Germany. BALB/c males were acquired from Harlan Winkelmann (Borchen, Germany). All animals were housed in a barrier facility. Animal care and experimental procedures were followed according to institutional guidelines and conformed to requirement of the state authority for animal research conduct (LaGetSi, Berlin, 0070/03). An immunological model of abortion was used, in which the mating combination CBA/J × DBA/2J represents the abortion group, using the combination CBA/J × BALB/c as a normal pregnancy control.28 Two-month-old CBA/J females were paired with 2- to 4-month-old BALB/c or DBA/2J males, checked twice a day for vaginal plugs and separated from the males if mated. The day of the vaginal plug was considered as day 0 of pregnancy. The animals included in the first part of the study received no treatment.
In the second part of the study, pregnant CBA/J females mated with BALB/c were considered as group 1 and received phosphate-buffered saline (PBS) intravenously (n = 28). DBA/2J-mated females were then randomized and divided in the following groups: group 2, abortion group + PBS intravenously (n = 28); group 3, abortion group + 2.105 Treg cells from nonpregnant CBA/J virgin females intravenously (n = 7); and group 4, abortion group + 2.105 Treg cells from 14-day BALB/c-pregnant CBA/J females intravenously (n = 10). All injections were made intravenously on day 0 to 2 of pregnancy. The data presented in the present study are a mean of two experiments. In a third set of experiments, BALB/c-mated CBA/J female were injected intraperitoneally with anti-CD25 (rat IgG1, clone PC615.3, 125 μg/mouse, n = 8; Exbio Antibodies, Prag, Czech Republic) or with PBS (n = 7).
On day 14 of pregnancy, the females were sacrificed, the uteri removed, and the implantation sites were documented. The abortion sites were identified by their small size accompanied by a necrotic, hemorrhagic appearance, compared with normal embryos and placentas. The percentage of abortions was calculated as the ratio of resorption sites and total implantation sites (resorption plus normal implantation sites) as described previously.17 For in vitro studies, 14-day pregnant normal (BALB/c-mated) or abortion (DBA/2J-mated) CBA/J mice as well as age-matched nonpregnant CBA/J females were included. Four experiments have been performed in which at least three animals have been used.
Treg Cell Isolation
CD4+CD25+ Treg cells were isolated from a mixture containing spleen and thymus cells from nonpregnant or 14-day normal pregnant mice by using magnetic beads following the instructions by the manufacturer (MACS, Miltenyi Biotech, Germany). The purity of the preparations was between 96% and 98% in all experiments. After isolation, Treg cells were washed twice with cold PBS, counted, diluted at 2.105 in 200 μl of PBS, and intravenously injected into pregnant mice or used for the in vitro approaches.
Sample Collection
Spleen and thymus samples were kept in RPMI at 4°C. The uterine horns were opened longitudinally, and the feto-placental unit separated from the uterine implantation sites. The whole placental and decidual unit was separated individually from the respective embryo and its implantation site. Further, placenta and deciduas were carefully separated from each other for flow cytometry and molecular biology but kept together for immunohistochemistry. For flow cytometry, decidual samples were cut in small pieces and collected in Hanks’ balanced salt solution containing no Ca2+ and no Mg2+ (Sigma, Taufkirchen, Germany). For RNA isolation or Western blot, placental and decidual tissues were carefully washed with cold sterile PBS, pH 7.40, snap-frozen, and kept at −80°C until use. For RNA isolation, we exclusively harvested tissues from healthy implantation sites because previous studies from our laboratory failed in obtaining RNA from resorption sites. For immunohistochemistry, tissues were fixed with ethanol 96°C at 4°C.
Flow Cytometry
We isolated mononuclear cells from spleen or thymus by disaggregating the tissue, filtering it through a sterile net (100 μm; BD Biosciences, Heidelberg, Germany), and lysing the erythrocytes with a NHCl4/NaCl solution. Decidual immune cells were obtained as previously described.17 Spleen, thymus, or decidual cells were incubated for 4 hours with 50 ng/ml of phorbol myristate acetate (PMA) (Sigma), 1 μg/μl ionomycin (Sigma), and 20 μmol/L monensin (Sigma) in RPMI (Gibco, Invitrogen, Karlsruhe, Germany) with 10% fetal calf serum (Biochrom AG, Berlin, Germany) in a humidified incubator at 37°C and 5% CO2. Thereafter, the cells were washed, incubated with the surface antibodies for 10 minutes at 4°C in darkness, and fixed with a 1% paraformaldehyde solution overnight at 4°C. On the following day, after permeabilizing the cells with 0.2% saponin, intracellular antibodies were added for 20 minutes at 4°C in darkness. The cells were washed and read on a FACScan flow cytometer from BD Biosciences. Washing steps were performed using washing buffer [1% bovine serum albumin (Sigma) and 0.1% sodium azide (Merck, Darmstadt, Germany)]. The following monoclonal antibodies (mAbs) (purchased from BD Biosciences) were used: fluorescein isothiocyanate-conjugated rat anti-mouse CD4 (rat IgG1), Cy5-conjugated anti-mouse CD8, phycoerythrin-labeled anti-mouse CD25, IL-4, IL-10, IFN-γ, and TNF-α (rat IgG2b). Fluorescein isothiocyanate-, Cy5-, or phycoerythrin-conjugated rat IgG1 or Ig2b were used as negative control in separate tubes.
Real-Time Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR)
One hundred mg of tissue (placenta or deciduas) were treated with 1 ml of Trizol (Gibco, Life Technologies) and disaggregated using a homogenizator (Ultra Turrax T8; Ika, Germany). The RNA was then extracted with chloroform, precipitated with absolute ethanol, washed, and finally diluted in RNase-free water. The RNA was quantified by reading ultraviolet absorbance at 260 nm. Two μg of total RNA were placed for 2 minutes on ice and added with dNTPs (2.5 mmol/L, Amersham Pharmacia), DNase I (2 U/ml, Stratagene), and RNase inhibitor (40 U/ml, Promega) mixed in reaction buffer. The mix was incubated for 30 minutes at 37°C and further heated to 75°C for 5 minutes. The addition of the reverse transcriptase (200 U/ml, Amersham) and RNase inhibitor in diethyl pyrocarbonate water (diethyl pyrocarbonate from Sigma) started the reverse transcription. This reaction mixture was incubated at 42°C for 60 minutes followed by incubation at 94°C for 5 minutes.
Amplification reactions (13 μl) for foxp3, IL-10, and CD3 consisted of 2 μl of cDNA, 6.25 μl of mastermix containing PCR buffer, dNTPs, MgCl2, and Ampli-Taq DNA polymerase (Eurogentec, Berlin, Germany), 3 μl of the primer mix, 1.25 μl of water, and 0.5 μl of the fluorescent probes. PCR reaction was performed as follows: 2 minutes at 50°C followed by an initial denaturation step of 10 minutes at 95°C, followed by 15 seconds at 95°C, and 1 minute at the appropriate annealing temperature for 40 cycles. All samples were normalized regarding their β-actin content. All reactions were performed on the ABI Prism 7700 sequence detection system (Perkin Elmer Applied Biosystems). Primer and probe sequences are described in Table 1.
Table 1.
Primer and Probe Sequences
| Molecule | Forward primer | Reverse primer | FAM-labeled probe |
|---|---|---|---|
| IL-10 | GAAGACCCT CAGGATGCGG | CCTGCTCCA CTGCCTTGCT | CGCTGTCATCGA TTTCTCCCCTGTGA |
| Foxp3 | CCCAGGAAAG ACAGCAACCTT | TTCTCACAACC AGGCCACTTG | ATCCTACCCACTGC TGGCAAATGGAGTC |
| CD3 | ATTGCGGGACAG GATGGAG | CTTGGAGATGGC TGTACTGGTCA | TCGCCAGTCAAGAGC TTCAGACAAGCA |
| β-Actin | GCTTCTTTGCAG CTCCTTCGTT | GTTGTCGACGA CCAGCGC | CAGCCTTCCTTCTTG GGTATGGAATCCT |
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Western Blot
Proteins were extracted from frozen placentas by resuspending them in 350 μl of lysis buffer containing 25 mmol/L CHAPS, 250 mmol/L HEPES, and 25 mmol/L dithiothreitol. It is important to remark that placental extracts were obtained exclusively from healthy implantation sites, because it is impossible to distinguish between placenta and fetus in resorption tissues. The samples were homogenized and protein concentration was assessed in the supernatants using the Bio-Rad Protein Assay (Bio-Rad, Munich, Germany) following the instructions given by the manufacturer. Sixty μg of protein were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel by electrophoresis and transferred to a nitrocellulose membrane (Bio-Rad) 1 hour at 125 mA and 10 W. Membranes were blocked 2 hours at room temperature with Tris-buffered saline (TBS) containing 0.05% Tween and 5% skim milk and thereafter incubated with the first antibody (goat polyclonal antibody against foxp3; Abcam, Hiddenhausen, Germany) at a concentration of 1 μg/ml O.N. at 4°C. After washing three times with TBS + 0.05% Tween, the membranes were incubated with the biotinylated secondary antibody (rabbit anti-goat; DAKO, Hamburg, Germany) for 1 hour at room temperature. After washing, the blots were incubated for 45 minutes with avidin-biotin peroxidase complex. Bands were revealed by chemiluminescence (Amersham, Freiburg, Germany) and exposed onto Kodak Miomax MR Imaging film (Sigma). As a positive control, we used human peripheral blood mononuclear cell extract as suggested by the manufacturer.
Immunohistochemistry
Ethanol-fixed placentas were embedded in paraffin as described before,17 cut at 5 to 7 μm, and conserved at 4°C in darkness. The sections were deparaffinized before immunohistochemistry. After washing with TBS, the sections were heated in 10 mmol/L citrate buffer (pH 6) by pressure cooker and further incubated for 20 minutes with 3% H2O2 in methanol (Sigma) to quench the endogenous peroxidase activity. Nonspecific binding was reduced by blocking the sections with bovine serum albumin (10% bovine serum albumin from Sigma in TBS). The sections were incubated overnight in a humidified chamber at 4°C with the first antibody diluted in 10% bovine serum albumin. The antibodies used were: polyclonal rabbit anti-human progesterone receptor (PR, DAKO) and mouse anti-human estrogen receptor (ER)-α (DAKO) as suggested by Kurita and colleagues.29 On the following day, the sections were washed in TBS and incubated at room temperature for 1 hour with the secondary antibody (biotinylated goat anti-rabbit or horse anti-mouse; Vector, Peterborough, UK). After washing, the samples were incubated with an avidin-biotin peroxidase complex solution (DAKO) for 30 minutes at room temperature. 3-amino-9-ethyl-carbazole (AEC) (DAKO for HO-1 and iNOS) or diaminobenzidine (for HO-2 and eNOS, DAKO) were used to visualize the specific immunostaining. The sections were counterstained with hemalaun. The diaminobenzidine-stained sections were then dehydrated and mounted using HistoKit (Roth, Karlsruhe, Germany). The AEC-stained sections were mounted with ultramount medium (DAKO). Negative controls were performed by replacing the first antibody with 10% bovine serum albumin, or 10% nonimmune serum. All sections were analyzed under the light microscope by two independent and blind observers and the expression levels were set by using scores from 0 (no staining) to 5 (very intense staining).
Mixed Lymphocyte Culture: Cytokine Secretion Assay
The MLC was established by mixing 3.5 × 105 spleen or pooled decidual cells from pregnant CBA/J females with 3.5 × 105 3000 rad γ-irradiated BALB/c or DBA/2J male spleen cells in 0.2-ml cultures in 96-well flat-bottom plates. Previously, effector cells were stained with CFDA-SE (CFSE; Molecular Probes, Eugene, OR) at 1 μmol/L, 107 cells/ml for 3.5 minutes and washed twice with medium containing 10% fetal calf serum. Cells were cultured in RPMI medium (Gibco, Invitrogen) supplemented with 10% fetal calf serum (Biochrom) and antibiotics (Gibco, Invitrogen) and cultured at 37°C for 72 hours. On the third day of culture, cells were added with 50 ng/ml PMA (Sigma) and 1 μg/μl ionomycin (Sigma) for 1 hour. Monensin (20 μmol/L, Sigma) was also added to the culture and the cells were incubated for further 4 hours. Cells were washed, permeabilized with saponin 0.1%, and stained with phycoerythrin-labeled mAb against IFN-γ or IL-4 (BD Pharmingen) as previously described and read in a FACS-Calibur.
Co-Culture of T Effector and Treg Cells
The MLC was established as described above. Here, 0.15 × 105, 1.5 × 105, or 15.0 × 105 spleen or pooled decidual cells from pregnant CBA/J females were incubated with equal quantities of 3000 rad γ-irradiated DBA/2J males spleen cells in 0.2-ml cultures in 96-well flat-bottom plates. Cultures were added with 15.0 × 105, 1.5 × 105, or 0.15 × 105 Treg cells obtained from normal pregnant or nonpregnant mice (ratios of T effector:Treg cells were 10:1, 1:1, and 1:10, respectively). Before co-culture, effector cells (spleen or decidual from pregnant CBA/J females) were stained with CFDA-SE (Molecular Probes). Cells were cultured in RPMI medium (Gibco, Invitrogen) supplemented with 10% fetal calf serum (Biochrom) and antibiotics (Gibco, Invitrogen) and cultured at 37°C for 72 hours. On the third day of culture, cells were processed as described previously and analyzed for their proliferation and IFN-γ production.
Data Analysis and Statistics
All data are presented as medians or medians ± 75% quartiles. As suggested by our statistical advisor because of the characteristics of the groups and the irregular number of samples between the groups, analysis of the differences between all groups was performed using the nonparametric Kruskal-Wallis test. The Mann-Whitney U-test was applied for analyzing differences between two particular groups. The in vitro data are illustrated as mean ± SD from four independent experiments performed. When using pooled samples, significances were analyzed using the χ2 test and α-adjusted with Bonferroni’s test. In all cases, P < 0.05 was considered a statistically significant difference.
Results
The Abortion-Prone Mating Combination Presented Abnormal Pregnancies
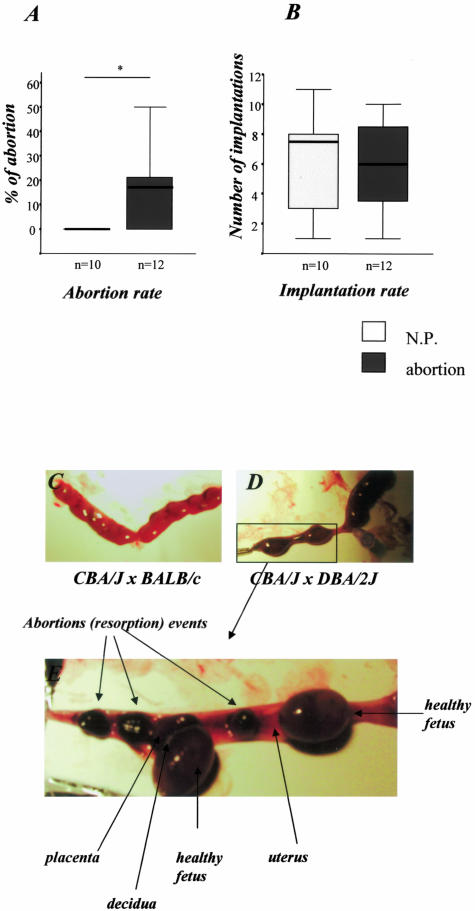
As already described by other groups and us,17,28,30 we observed a statistically significant higher abortion rate in the CBA/J × DBA/2J compared to the CBA/J × BALB/c combination (18% versus <1%, P < 0.05; Figure 1A) although both allogeneic male strains share H2d antigens. Implantation rates were comparable in both combinations (Figure 1B). Syngeneic mating combinations, ie, CBA/J × CBA/J matings have no abortions (unpublished observations in n = 9, A.C.Z.).
Figure 1.
Abortion rates. A: DBA/2J-mated CBA/J mice (abortion mice) showed enhanced abortion rates compared to control mice (CBA/J × BALB/c, normal pregnant, N.P.) as analyzed by the nonparametric Mann-Whitney U-test (P < 0.05) in two independent experiments. B: Comparable implantation rates between both experimental groups. The data are presented as median ± 75% quartiles. C and D: Representative pictures of uteri from normal pregnant mice (C) and from abortion mice (D). E: The left uterus from abortion mice is amplified and five implantation sites can be observed. Three of them are abortions whereas the other two are normal implantation sites where healthy fetuses and placentas can be perfectly identified from each other.
Enhanced Frequency of IFN-γ-Producing Paternal Antigen-Specific T Cells in Decidua but Not Spleen of Abortion Mice Compared to Normal Pregnant Mice
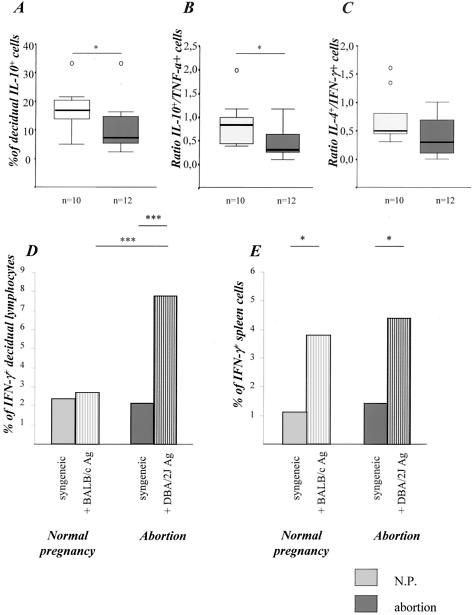
Real-time RT-PCR analysis of decidual and placental samples revealed comparable levels of CD3 mRNA in abortion and normal pregnant mice, suggesting a similar T-cell intrauterine infiltration in both combinations (data not shown). Next, we studied the functionality of the decidual T cells by ex vivo stimulation. Decidual cells were harvested and analyzed for their cytokine production after unspecific stimulation with PMA/ionomycin, which is known to stimulate almost all memory/effector T cells to produce cytokines. Decidual lymphocytes from abortion mice produced significantly less IL-10 (Figure 2A) and more TNF-α than decidual cells from normal pregnant mice resulting in significantly diminished ex vivo IL-10/TNF-α ratio (Figure 2B). In addition, there were comparable frequencies of IFN-γ-producing T cells but slightly lower frequencies of IL-4-producing T cells in the decidua of abortion mice resulting in a marginal but statistically not significant diminution of the IL-4/IFN-γ ratio (Figure 2C).
Figure 2.
Cytokine production. Abortion mice produced significantly less IL-10 (A) and presented therefore a statistically significant diminution in the IL-10/TNF-α ratio when compared to normal pregnant mice (B, P < 0.05) as analyzed by the nonparametric Mann-Whitney U-test (P < 0.05). The IL-4/IFN-γ (C) ratio was slightly but not significantly diminished as analyzed in decidual cells after ex vivo stimulation with PMA/ionomycin. The data are presented as median ± 75% quartiles. D and E: Ag-specific cytokine production. Decidual (D) but not spleen (E) cells from abortion mice secreted more IFN-γ when stimulated with male APCs compared to normal pregnant mice (P < 0.001). IFN-γ production was analyzed in pooled decidual (D) or spleen (E) immune cells from at least three normal pregnant or abortion mice when co-cultured with CBA/J antigen (syngeneic control) or male antigens (BALB/c or DBA/2J). The data are representative of two independent experiments. Statistical significances were analyzed by the χ2 test and α-adjusted by Bonferroni’s test.
Notably, we found significantly enhanced frequencies of IFN-γ-expressing Th1 memory/effector decidual T cells after stimulation with paternal irradiated splenocytes but not with irradiated syngeneic cells in abortion but not in normal pregnant mice (Figure 2D). In contrast, spleen cells of both combinations showed comparable ex vivo cytokine secretion after both PMA/ionomycin (data not shown) and paternal alloantigen stimulation (Figure 2E). These data confirm an enhanced intrauterine accumulation of paternal alloantigen-reactive memory/effector Th1 cells in abortion mice.
Abortion Mice Presented Diminished Number of CD4+CD25+ and IL-10+ Treg Cells in Thymus When Compared to Normal Pregnant and Nonpregnant Mice
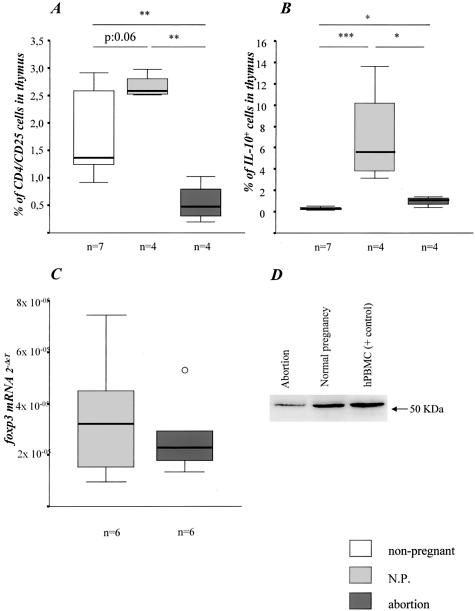
We further wondered whether the accumulation of paternal alloantigen-specific Th1 cells at the feto-maternal interface of abortion mice might be related to diminished Treg activity. We analyzed the number of natural CD4+CD25+ Treg in spleen, thymus, and decidua from normal pregnant and abortion mice by flow cytometry. Interestingly, we found comparable proportion of this T-cell subset in spleen and decidua from both animals groups (data not shown), but a statistically significant increased percentage of Treg in thymus from normal pregnant mice when compared to abortion mice (Figure 3A). The latter group expressed even lower levels of CD4+CD25+ T cells than nonpregnant age-matched control mice (Figure 3A). These data suggest that high levels of CD4+CD25+ Treg have been generated in the thymus during normal pregnancy, which could not be achieved in the abortion combination. Remarkably, the thymus of abortion mice presented less CD8+CD25+ T cells than normal pregnant and normal nonpregnant mice (0.26% versus 1.86% versus 1.78%, respectively; P < 0.05). This cell population may also have regulatory properties. Decidual tissue from both combinations presented high numbers of CD4+CD25+ T cells (median, 5%). However, activated effector CD4+ cells also express CD25, therefore this marker may not be useful for quantifying Treg at the site of immune activation.
Figure 3.
Treg activity. A: Normal pregnant mice presented more Treg cells than nonpregnant mice (P = 0.06). DBA/2J-mated CBA/J females undergoing abortion showed significantly decreased numbers of Treg cells when compared to BALB/c-mated CBA/J females (P < 0.01). B: Thymocytes from normal pregnant mice produced significantly more IL-10 when compared to nonpregnant (P < 0.001) or normal pregnant mice (P < 0.05). The data are presented as median ± 75% quartiles. Significances were calculated using the nonparametric Kruskall-Wallis test followed by the Mann-Whitney U-test. Abortion mice presented lower placental foxp3 mRNA (C) and protein levels (D) as analyzed by real-time RT-PCR and Western blot, respectively. In Western blot human PBMCs were used as a positive control.
To characterize the function of Treg, we analyzed the ability of thymocytes to synthesize IL-10 after ex vivo PMA/ionomycin stimulation. Interestingly, in the thymus from normal pregnant but not abortion mice we found an enhanced frequency of IL-10-producing T cells confirming the intrathymic expansion of Treg subset in normal pregnant but not in abortion-prone mice (Figure 3B). In addition, the diminished frequency of ex vivo IL-10-secreting decidual T cells (Figure 2, A and B) suggests a Treg deficiency in the decidua from abortion mice. A lower foxp3 mRNA and protein expression—an essential transcription factor of naturally occurring Treg—in placenta of abortion mice supports this view (Figure 3, C and D).
CD4+CD25+ Treg Cells Inhibit Paternal Alloantigen-Induced Proliferation and IFN-γ Production by Decidual T Cells from Abortion Mice in Vitro
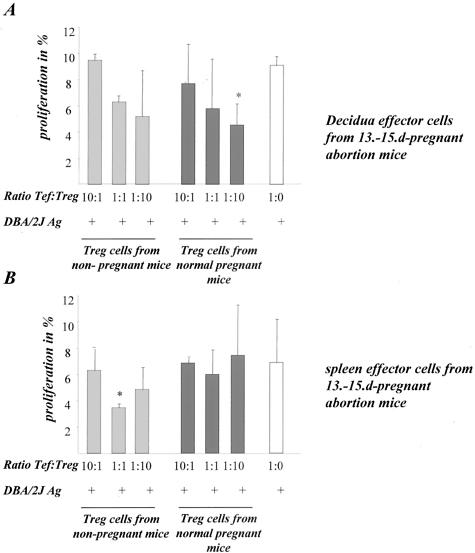
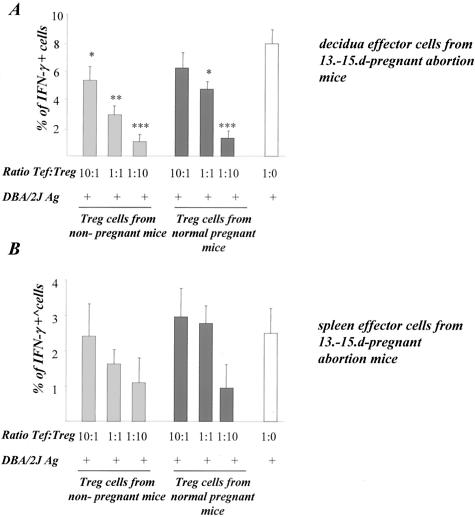
Next, we addressed the question whether the addition of Treg might reverse the cellular abnormal cell response in abortion-prone mice. To analyze the in vitro effect of Treg on T-cell proliferation and cytokine production, we stimulated decidual or spleen cells from abortion CBA/J mice with male (DBA/2J) APCs and added to the culture Treg cells from nonpregnant or normal pregnant CBA/J mice in different proportions. Very interestingly, we observed a Treg number-dependent inhibition of alloantigen-induced proliferation of decidual cells (Figure 4A). Additionally, a less inhibited proliferation of spleen lymphocytes could be observed (Figure 4B). The inhibitory effect could be observed after addition of Treg from both nonpregnant or normal pregnant mice. Similarly, the frequency of IFN-γ-secreting allostimulated decidual (Figure 5A) was significantly inhibited after addition of Treg from both nonpregnant and normal pregnant mice. Spleen cells produced less IFN-γ after allostimulation if co-cultured with Treg cells (Figure 5B).
Figure 4.
Treg modulated the in vitro proliferation. A to C: The results of our in vitro assays by co-culturing pooled decidual (A) or spleen (B) effector cells with Treg cells isolated from nonpregnant (gray bars) or normal pregnant mice (black bars) and male antigens (DBA/2J APCs). White bars show the proliferation controls without Treg cells addition. Pooled effector cells were stained with CFSE and their proliferation was analyzed 3 days after culture. The addition of Treg cells led to a significant inhibition in the proliferation rates by decidual cells (A) and a slight diminution by spleen cells (B). Data are a mean of three independent experiments, in which pooled cells from at least three animals were analyzed. Statistical significances were analyzed by the χ2 test and α-adjusted by Bonferroni’s test.
Figure 5.
Treg modulated the in vitro IFN-γ production. The addition of Treg cells led to a significant inhibition in the IFN-γ production by effector cells. The in vitro assays were performed by co-culturing pooled decidual (A) or spleen (B) effector cells with Treg cells isolated from nonpregnant (gray bars) or normal pregnant mice (black bars) and male antigens (DBA/2J APCs). White bars show IFN-γ secretion controls without Treg cell addition. IFN-γ secretion was analyzed 3 days after culture. Data are a mean of three independent experiments, in which pooled cells from at least three animals were analyzed. Statistical significances were analyzed by the χ2 test and α-adjusted by Bonferroni’s test.
The Adoptive Transfer of Treg Cells from NP Mice Significantly Diminished the Abortion Rate
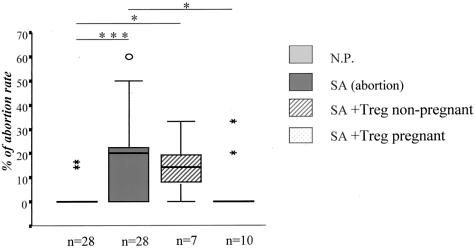
To verify the hypothesis that Treg cells play a protective role during pregnancy as suggested by our in vitro data, we isolated CD4+CD25+ cells from thymus and spleen of 14-day normal pregnant mice (BALB/c-mated CBA/J females) or age-matched nonpregnant CBA/J female mice as for the in vitro experiments described above. Treg cells (2 × 105) were adoptively transferred into 0- to 2-day pregnant abortion-prone mice. Very interestingly, the transfer of CD4+CD25+ Treg cells from normal pregnant but not from nonpregnant CBA/J mice completely prevented spontaneous abortion (Figure 6). Notably, transfer of spleen Treg or thymus Treg had the same effect than the mixture (n = 3 each, data not shown). Additionally, the Treg transfer failed to prevent abortion if made on days 4 to 5 of pregnancy (data not shown), suggesting that Treg cells are necessary locally for successful implantation to take place.
Figure 6.
NP-Treg transfer avoided abortion. The injection of 2 × 105 Treg cells from normal pregnant, but not from nonpregnant mice led to a statistically significant diminution in the abortion rate (P < 0.05). The abortion rate from NP-Treg cell-transferred mice was comparable to the normal pregnant controls. The data are presented as median ± 75% quartiles and the statistically significant differences were analyzed by Kruskall-Wallis test followed by Mann-Whitney U-test.
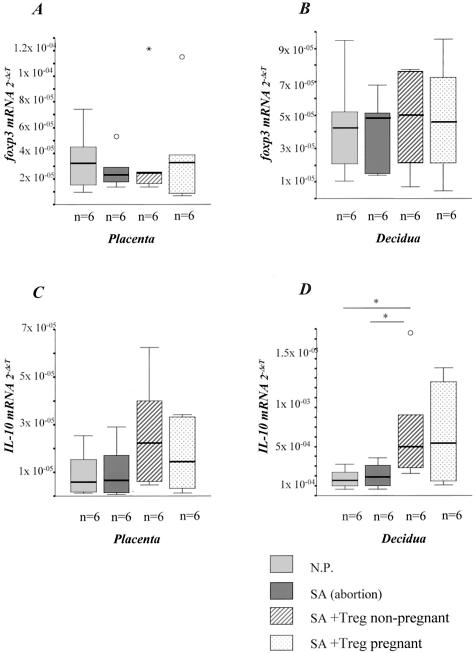
Our in vivo data are in contrast to the in vitro results in which both Treg cells from normal pregnant and nonpregnant mice possessed regulatory potency. Therefore, we wondered whether Treg from normal pregnant mice have an advantage in infiltrating placenta/decidua compared to natural Treg from nonpregnant mice, which might explain their superior efficacy in vivo. To address this issue, we analyzed the intrauterine foxp3 and IL-10 mRNA expression after Treg transfer. Indeed, there was a marginal up-regulation of foxp3 (Figure 7, A and B) and a significant up-regulation (twofold to fivefold) of IL-10 mRNA expression (Figure 7, C and D) at day 14 in decidua and placenta of abortion-prone mice after application of Treg from normal pregnant mice suggesting an accumulation of Treg at the fetal-maternal interface. However, a similar up-regulation of both foxp3 (1.3-fold) and IL-10 (1.5- to 5-fold) was observed in placenta and decidua after transfer of Treg from nonpregnant female mice, which did not prevent abortion (Figure 7; A to D). These data suggest that Treg from both normal pregnant and nonpregnant mice are able to infiltrate the feto-maternal interface but only those Treg cells previously exposed to paternal alloantigens have protective regulatory activity in vivo.
Figure 7.
Treg transfer up-regulated foxp3 and IL-10 mRNA. Abortion mice presented diminished or comparable foxp3 (A and B) and IL-10 (C and D) mRNA levels in placenta and decidua when compared to normal pregnant mice. The injection of 2 × 105 Treg cells from both normal pregnant or nonpregnant mice led to augmented foxp3 (slightly) and IL-10 mRNA levels (significantly). The data are presented as median ± 75% quartiles and the statistically significant differences were analyzed by Kruskall-Wallis test followed by Mann-Whitney U-test.
Blocking Treg before Implantation by Anti-CD25 mAb Application Reduced the Percentage of Pregnant Mice per Total Mated Mice
To test the hypothesis that Treg cells are necessary before the implantation period—probably by suppressing potentially harmful cells locally—we treated normal pregnant mice (CBA/J × BALB/c) with a monoclonal antibody against CD25 or with PBS on day 0 of pregnancy. The anti-CD25 treatment resulted in a decrease in the percentage of pregnant mice per total mated mice (37.5%) as compared to the controls (100%, Table 2).
Table 2.
The Application of a Monoclonal Antibody against CD25 Significantly Diminished the Percentage of Pregnant Mice in Mated Females
| Anti-CD25 | PBS | |
|---|---|---|
| Plugged female | 8 | 7 |
| Animals with visible implantations on day 14 of pregnancy | 3/8 (37.5%) | 7/7 (100%) |
Transfer of Treg Cells from Both Nonpregnant and Pregnant Mice into Abortion Restored the Levels of Progesterone Receptor Expression at the Feto-Maternal Interface
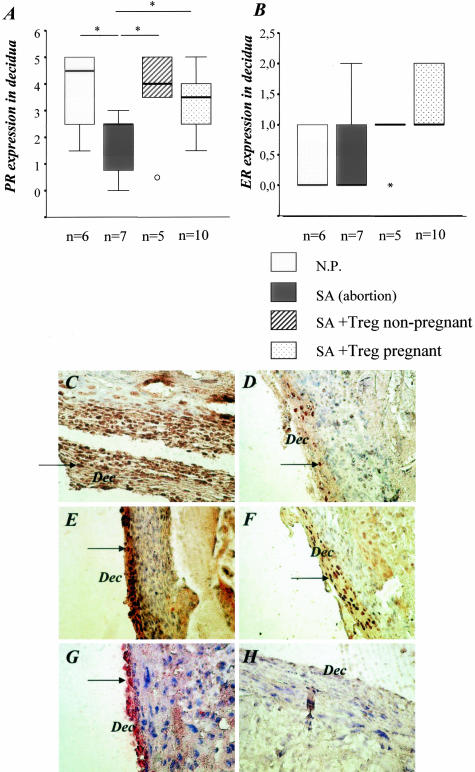
Because immune dysfunction may contribute to infertility through the negative effects on the reproductive endocrine system, we decided to analyze the impact of our treatment on the hormonal receptivity of the mice by semiquantifying the levels of the receptors for progesterone (PR) and estrogen (ER). As shown in Figure 8, treatment of the animals with Treg from normal pregnant animals but also with Treg from nonpregnant animals led to increased expression of progesterone receptor, suggesting that not only hormonal changes are necessary for pregnancy success. The estrogen receptor expression was not modified by the application of Treg.
Figure 8.
Treg transfer up-regulated the expression of progesterone receptors and had no effect on the estrogen receptor expression. A: Abortion mice presented lower PR levels than normal pregnant mice. Treg transfer restored the receptor expression to levels comparable with those observed in normal pregnant mice. B: No differences were observed between any groups regarding the expression of ER. C to F: Representative PR staining in normal pregnant mice (C), abortion-prone mice (D), and in mice transferred with Treg from nonpregnant (E) or pregnant animals (F). G is a typical field for ER staining while H depicts our negative control.
Discussion
During pregnancy, the maternal immune system has to tolerate the persistence of paternal alloantigens without affecting anti-infectious immune responsiveness. Failure of these tolerance mechanisms might result in abnormal pregnancy, such as spontaneous abortion and pre-eclampsia.31 Here, we demonstrate for the first time the important consequences of a deficient Treg activity during pregnancy, namely immunological spontaneous abortion. Abortion-prone mice show a diminished Treg cell activity and fetal rejection could be completely prevented by adoptive transfer of Treg exclusively from normal pregnant mice.
To study the key role of Treg in pregnancy, we used the murine CBA/J × DBA/2J abortion combination, which has been used for years to investigate immunological pathways leading to spontaneous abortion.28 Certified strains of CBA/J mice (H2k) originally from the Jackson Laboratories, but not CBA/C spontaneously show high abortion rates when mated with H2d-bearing DBA/2J but do not abort if mated with other H2d-bearing male such as BALB/c suggesting a role of minor MHC antigens.9,28,30 The abortion rate seems also to be related to the characteristics and conditions of the animal husbandry, because differences in abortion rates between laboratories have been reported.28 In our facilities, CBA/J × DBA/2J combination shows 18 to 30% abortion, whereas CBA/J × BALB/c usually presents no abortion events with comparable implantation rates in both combinations. Thus, the CBA/J × DBA/2J model is a reliable model for analyzing mechanisms underlying immunological spontaneous abortions.
Cytokines were proposed to play a crucial role in mammalian pregnancy success. The Th1/Th2/Th3 paradigm proposes that Th2- and Th3-type cytokines such as IL-4, IL-10, and transforming growth factor (TGF)-β may favor the maintenance of mammalian pregnancy,6,10–12 whereas the excessive production of Th1 cytokines (IL-2, IFN-γ, TNF-α) would mediate the rejection of the fetus at the feto-maternal interface.10,13–17 Nevertheless, this hypothesis was questioned in the last 3 years, because 1) the combination of IL-4 and IL-10 knockout mice resulted in normal pregnancies, suggesting that neither maternal, nor fetal production of IL-4 or IL-10 is crucial for the completion of pregnancies;18 2) the placental cells from the abortion-prone mating combination expressed and secreted less IL-18 than the nonabortion mating combination;20 and 3) blood and decidual cells from patients suffering from spontaneous abortion produced less IL-12 than cells from normal pregnant patients.21
The fact that IL-10- and IL-4-deficient mice are having normal pregnancies points out that none of these cytokines are crucial for fetal survival.18 Unfortunately, no data on possible abortion events (resorptions) were provided in the study by Svensson and colleagues,18 because they documented only living neonates after spontaneous delivery which do not rule out resorptions/abortions. Although IL-4 and IL-10 are related to successful pregnancy outcome in humans and mice6,9,32–34 and the administration of IL-10 inhibited the abortion rate in the CBA/J × DBA/2J model.32 Other immunosuppressive molecules, especially TGF-β, have been proposed to play an important role during pregnancy.35,36 It is tempting to speculate that in the IL-10/IL-4 knockout mice, the lack of IL-10 or IL-4 could be covered or by-passed by high levels of other immunosuppressive molecules, ie, TGF-β. No reports confirm this hypothesis. Interestingly, TGF-β2 and -β3 play very important roles in development as observed in TGF-β2- and -β3-deficient mice.37 Accordingly, White and colleagues38 pointed out that although IL-10 is not essential for tolerance or successful pregnancy irrespective of MHC disparity in the fetus, maternal IL-10 is a determinant of growth trajectory in progeny in uterus and after birth.38
Here, we analyzed the ex vivo ability of spleen and decidual lymphocytes from abortion and normal mice on day 14 of pregnancy to produce Th1 and Th2 cytokines after unspecific stimulation with PMA/ionomycin as well as after specific stimulation with male antigens. Previous data indicated diminished IL-4 and IL-10 production by whole placenta cultures as well as low IL-4 and IL-10 expression in placental tissues from the CBA/J × DBA/2J combination when compared to CBA/J × BALB/c.32–34 High ex vivo placental levels of TNF-α and IL-6 have been reported for this murine abortion model,14,17 being placental and stroma decidual cells the major producers of these cytokines. Further, it has been reported that placental cells derived from the abortion-prone combination CBA/J × DBA/2J are able to induce a Th1-like response in responder lymphocytes and furthermore, that when these stimulated lymphocytes are adoptively transferred to normal pregnant mice, they induce fetal resorption.39 Although, no studies concentrated on the ex vivo ability of spleen and decidual immune cells to produce Th1/Th2 cytokines as an in vivo mirror of immune cell imprinting. We found comparable CD3+ T-cell infiltration at the feto-maternal interface as well as PMA/ionomycin inducible Th1 memory/effector T cells in the two mating combinations but an enhanced frequency of paternal alloantigen-specific Th1 memory/effector lymphocytes in abortion mice, suggesting, as Tangri and colleagues39 did, production of detrimental cytokines by Ag-specific T cells as a cause of abortion. On the contrary, spleen cells showed comparable polyclonal and paternal alloantigen-specific Th1 frequencies in both combinations. These data demonstrate that the generation of polyclonal Th1 memory/effector cells and their homing to the tissues including the uterus is not influenced by pregnancy. In addition, systemic generation of paternal alloantigen-specific Th1 memory/effector cells is not inhibited during normal pregnancy but their activity is down-regulated at the feto-maternal interface. This fact suggests the existence of specific regulatory processes, which would control Th1 cells that are specific against paternal alloantigen. The diminished frequency of IL-10- and IL-4-producing decidual T cells after polyclonal stimulation and the significantly lower proportion of CD4+CD25+ and IL-10+ T cells in the thymus of abortion mice suggest a deficient immunoregulation.
There are a lot of evidences from human and mice studies that CD4+CD25+ T cells have immunoregulatory activity: they are named as T regulatory cells (Treg). Treg cells have a role in preventing graft-versus-host disease and their adoptive transfer could protect skin grafts from rejection.40 Further, tolerance could be adoptively transferred by transferring Treg from long-time surviving cardiac allograft.41 Treg cells were shown to in vitro inhibit Th1 but not Th2 responses42 as well as proliferation of Th1 effector cells by secreting IL-10 and TGF-β.43 Because IL-10 is produced by activated Treg cells, a lower frequency of IL-10-producing T cells in abortion mice may be an indicator of diminished Treg frequency/activity. Treg can exert their actions not only by secreting IL-10 and other immunomodulatory cytokines but also by contact-dependent inhibition, particularly of APC.43 Thus, the lower number of IL-10+ decidual T cells as well as the lower foxp3 mRNA and protein expression in our model might reflect disturbed Treg generation. The lower decidual IL-4 production in abortion mice might further contribute to diminished Treg activity because IL-4 is not produced by Treg but it acts synergistically with Treg in organ transplant models.44
Very recently, while preparing this article, Aluvihare and colleagues45 described the role of natural Treg in mediating maternal tolerance to the fetus in a normal pregnancy model. They observed an expansion of CD4+CD25+ T cells in almost all tissues of pregnant compared to nonpregnant female mice independently on the paternal MHC difference. Like in our study, in vitro analysis showed an inhibitory capacity of CD4+CD25+ T cells on maternal alloresponses. CD25+ T-cell depletion led to gestation failure suggesting an active role of Treg in mediating maternal tolerance to the fetus.45 However, CD25 antibody may inhibit not only Treg but also other immune cells, such as activated lymphocytes in a late pregnancy state. In the present study, we demonstrate for the first time the pathophysiological consequences of diminished Treg activity in pregnancy, namely spontaneous abortion. The successful prevention of abortion by adoptive transfer of Treg from normal pregnancy in our model first confirmed the impact of Treg for pregnancy outcome and is very relevant clinically. Additionally, two very recent human studies support the important role of Treg in human pregnancy as well.46,47
Next, we wondered what could be the reason for diminished Treg generation and activity in abortion-prone mice, which could help to elucidate diminished Treg levels observed in patients undergoing abortion.47 Decidual tissue presented relatively high levels of CD4+CD25+ T cells and no differences could be observed between controls and abortion-prone mice, maybe because of the fact that CD25 may also mark activated cells in decidua. We observed comparable numbers of Treg cells in spleen from both experimental groups but a significant diminution in the number of CD4+CD25+ and IL-10+ Treg cells in thymus from abortion mice when compared to normal pregnant mice. The latter group showed a dramatic increase in the number of CD4+CD25+ thymocytes when compared to age-matched nonpregnant mice suggesting an enhanced Treg generation in the thymus during normal pregnancy. Moreover, CD4+CD25+ T cells from both normal pregnant and nonpregnant mice were able to in vitro suppress the maternal alloresponse of lymphocytes from abortion-prone mice and accumulated at the feto-maternal interface of these mice after adoptive transfer as shown by enhanced decidual foxp3 and IL-10 expression. Remarkably, however, Treg from normal pregnant but not from nonpregnant mice could protect fetal rejection in vivo. The data suggest that alloantigen (H2d in our model) stimulation of Treg is required for mediating their protective effects in vivo. Similar observations were made in organ transplantation models. Natural CD4+CD25+ T cells as well in vitro generated genetically modified alloreactive IL-10-expressing T cells suppressed the alloresponse of naive T cells in vitro but were unable to prolong allograft survival in vivo (Brandt C and colleagues, unpublished observations).25
In the light of our results, in vivo (allo)antigen priming of Treg may be required for optimal Treg activity protecting allogeneic fetuses from rejection, as described for allotransplants. As we observed dramatic differences in the number of Treg in the thymus of normal pregnant versus abortion-prone mice it is tempting to speculate that the thymus plays a role in generation of both natural Treg43 as well as pregnancy-induced Treg. This would require fetal antigens, passing through the thymus. Previous data suggest an immunomodulatory role for the thymus during pregnancy.48 In mice, the weight of the thymus falls by 75% because of a massive depletion of cortical thymocytes while medullary thymocytes remain unaffected.49,50 However, the thymus remains functional.51,52 Moreover, the thymic involution seems to be a physiological process necessary for the success of normal pregnancy52 and the placenta was proposed to be a crucial player in thymic involution by affecting thymocyte properties during their maturation in the thymic microenvironment.48,53 Using an in vitro co-culture system, Savion and Toder48 were able to show that placental or decidual explants from normal pregnant mice had inhibitory effect on thymocyte proliferation. The bi-directional transfer of cells between mother and fetus has been a matter of debate but it is now accepted that such an exchange is in fact taking place in the placenta.54,55 Accordingly with these observations and our data on up-regulated levels of Treg cells in thymus from normal pregnant mice but not from pregnant mice undergoing abortion, it is very probably that placenta-derived antigens enter the thymus and provoke the generation of immunoregulatory cells, which would avoid fetal rejection. Our data would further explain why the transfer of thymocytes from multiparous mice provoked tolerance to male skin grafts.56 The in vitro co-culture of thymocytes and placental/decidual extracts further led to a lower IL-6 production.48 Accordingly with the in vitro data and because increased IL-6 levels are known to be associated with abortion17 it is tempting to speculate that placental antigens prime the cytokine production by thymocytes to a regulatory profile. We propose that placental antigens generated in the CBA/J × BALB/c but not CBA/J × DBA/2J combination regulate the generation of Treg as well as the suppressive cytokine production, ie, IL-10. Which factor is generating Treg cells in one combination and not in the other is still unknown.
Whether the immunoregulatory activity of Treg cells is antigen-specific, it is still a matter of discussion,23,42,56,57,58 but at least in stronger systems such as allotransplantation there are evidences for their antigen specificity.59 As both mating combinations share the paternal MHC type, Treg generated to BALB/c can specifically protect maternal anti-DBA/2J responses as well, both by direct or indirect allorecognition which might be the dominant pathway for immunoregulation by CD4+CD25+ cells.58,59 Interestingly, Chaouat and colleagues60 reported in 1983 that abortion rates in CBA/J female mice mated with DBA/2J males could be dramatically reduced by vaccination with BALB/c male spleen cells, but not by CBA/J or DBA/2 J male spleen cells. Further, this effect correlated with the ability of BALB/c spleen cells to induce MLR suppressor activity in CBA/J female mice.60 In the light of our results, we can speculate that in the experiments by Chaouat and colleagues60 the transfer of BALB/c male splenocytes generated Treg cells in DBA/2J-mated CBA/J females and avoided therefore immunological abortion.
Recently, Erlebacher and colleagues61 showed very nicely that an immune failure of mouse pregnancy by anti-CD40 treatment was mediated by impaired progesterone synthesis by the ovary as well as by TNF-α production. In our preliminary analysis of hormonal receptivity, we found that the transfer of Treg from both nonpregnant and pregnant mice restored the expression levels of PR as analyzed on day 14 of pregnancy. ER expression was not influenced by Treg transfer. These data suggest that solely hormonal changes are not enough to revert abortion events. Ag specificity by Treg may be indispensable for pregnancy success.
Finally, it appears that Treg cells are effective before implantation takes place as suggested by the experiments blocking Treg cells by anti-CD25 on day 0 of pregnancy as well as by the fact that pregnancy-induced Treg are not effective if applied on days 4 to 5 of pregnancy. Which mechanisms underlie these phenomena remain to be elucidated and should be the focus of next studies.
In summary, our study indicates that normal pregnant mice show no differences in the generation of polyclonal and paternal antigen-specific Th1 response compared to abortion-prone mice. However, the decidual accumulation of paternal alloantigen-specific but not of polyclonal Th1 memory/effector cell is significantly inhibited in successful pregnancy. The accumulation of paternal alloantigen-specific Th1 cells at the decidua of abortion mice seems to be because of insufficient generation of pregnancy-induced Treg. Here, we show for the first time that adoptive transfer of Treg from normal pregnancy protects against fetal rejection in abortion-prone mice. This understanding might have an important impact on specific immunotherapy of fetal loss by spontaneous abortion and other immunological-related pregnancy complication, such as pre-eclampsia.
Acknowledgments
We thank Dr. Brigitte Wegner (Institute of Medical Biometry) for her advice in statistics, Dr. Stefan Fest for critical reading of the manuscript, Annelie Dernier and Frauke Ringel for their excellent assistance and expertise in real-time RT-PCR, Katrin Vogt for designing the foxp3 primers, Dr. Isabela Schmitt-Knosalla for excellent advice and assistance in the intravenous injections, and Dr. Miloslav Suchanek for providing the mAb against CD25.
Footnotes
Address reprint requests to Dr. Ana Claudia Zenclussen, Institut für Medizinische Immunologie, Biomedizinisches Forschungszentrum, Raum 2.0534, Charité, Campus Virchow Klinikum, Augustenburger Platz 1, D-13353, Berlin, Germany. E-mail: ana.zenclussen@charite.de.
Supported by grants from the Charité (UFF 2003-109 and 2003-230 to A.C.Z. and a Ph.D. fellow to M.L.Z.).
References
- Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;44:320–338. [Google Scholar]
- Tafuri A, Alferink J, Moller P, Hammerling G, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–633. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- Munn D, Zhou M, Attwood J, Bondarev I, Conway S, Marshall B, Brown C, Mellor A. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Rouas-Freiss N, Goncalves RM, Menier C, Dausset J, Carosella ED. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci USA. 1997;94:11520–11525. doi: 10.1073/pnas.94.21.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makriagiannis A, Zoumakis E, Kalantaridou C, Coutifaris C, Margioris AN, Coukos G, Rice KC, Gravansi A, Chrousos GP. Corticotropin-releasing hormone promotes blastocytes implantation and early maternal tolerance. Nat Immunol. 2001;18:367–391. doi: 10.1038/ni719. [DOI] [PubMed] [Google Scholar]
- Lin H, Mossmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the feto-maternal interface. J Immunol. 1993;151:4562–4573. [PubMed] [Google Scholar]
- Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18:478–482. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- Raghupathy R, Makhseed M, Azizieh F, Hassan N, Al-Azemi M, Al-Shamali E. Maternal Th1- and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol. 1999;196:122–130. doi: 10.1006/cimm.1999.1532. [DOI] [PubMed] [Google Scholar]
- Clark DA, Arck PC, Chaouat G. Why did your mother reject you? Immunogenetic determinants of the response environmental selective pressure expressed at the uterine level. Am J Reprod Immunol. 1999;41:5–22. doi: 10.1111/j.1600-0897.1999.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Saito S. Cytokine network at the feto-maternal interface. J Reprod Immunol. 2001;47:87–103. doi: 10.1016/s0165-0378(00)00060-7. [DOI] [PubMed] [Google Scholar]
- Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both, leukemia inhibitor factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4:1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- Joachim RA, Zenclussen AC, Polgar B, Douglas AJ, Fest S, Knackstedt M, Klapp BF, Arck PC. The progesterone derivative dydrogesterone abrogates murine stress triggered abortion by inducing a Th2 biased local immune response. Steroids. 2003;68:931–940. doi: 10.1016/j.steroids.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Krishnan L, Guilbert LJ, Wegmann TG, Belosevic M, Mossmann TR. T helper-1 response against Leishmania major in pregnancy C57BL/6 mice increases implantation failure and fetal resorptions. Correlation with increased IFN-gamma and TNF-alpha and reduced IL-10 production by placenta cells. J Immunol. 1996;15:653–662. [PubMed] [Google Scholar]
- Joachim RA, Hildebrandt M, Oder J, Klapp BF, Arck PC. Murine stress-triggered abortion is mediated by increase of CD8+ TNF-alpha+ decidual cells via substance P. Am J Reprod Immunol. 1999;45:303–309. doi: 10.1111/j.8755-8920.2001.450506.x. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Kortebani G, Mazzolli A, Margni R, Malan Borel I. Interleukin-6 and soluble interleukin-6 receptor serum levels in recurrent spontaneous abortion women immunized with paternal white cells. Am J Reprod Immunol. 2000;44:22–29. doi: 10.1111/j.8755-8920.2000.440104.x. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Fest S, Sehmsdorf US, Hagen E, Klapp BF, Arck PC. Upregulation of decidual P-selection expression is associated with an increased number of Th1 cell populations in patients suffering from spontaneous abortion. Cell Immunol. 2001;213:94–103. doi: 10.1006/cimm.2001.1877. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Blois S, Stumpo R, Olmos S, Arias K, Malan Borel I, Roux ME, Margni RA. Murine abortion is associated with enhanced interleukin-6 levels at the feto-maternal interface. Cytokine. 2003;24:150–160. doi: 10.1016/j.cyto.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Svensson L, Arvola M, Sallstrom MA, Holmdahl R, Mattsson R. The Th2 cytokines IL-4 and IL-10 are not crucial for the completion of allogeneic pregnancy in mice. J Reprod Immunol. 2001;51:3–7. doi: 10.1016/s0165-0378(01)00065-1. [DOI] [PubMed] [Google Scholar]
- Margni RA, Zenclussen AC. During pregnancy, in the context of a Th2 type cytokine prolife, serum IL-6 levels might condition the quality of the synthesized antibodies. Am J Reprod Immunol. 2001;46:181–187. doi: 10.1034/j.1600-0897.2001.d01-1.x. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Zourbas S, Ostojic S, Lappree-Delage G, Dubanchet S, Ledee N, Martal J. A brief review of recent data on some cytokine expressions at the materno-foetal interface which might challenge the classical Th1/Th2 dichotomy. J Reprod Immunol. 2002;53:241–256. doi: 10.1016/s0165-0378(01)00119-x. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Fest S, Busse P, Joachim R, Klapp BF, Arck PC. Questioning the Th1/Th2 paradigm in reproduction: peripheral levels of IL-12 are down-regulated in miscarriage patients. Am J Reprod Immunol. 2002;48:245–251. doi: 10.1034/j.1600-0897.2002.01136.x. [DOI] [PubMed] [Google Scholar]
- Li XC, Roy-Chaudhury P, Hancock WW, Manfro R, Zand MS, Li Y, Zheng XX, Nickerson PW, Steiger J, Malek TR, Strom TB. IL-2 and IL-4 double knockout mice reject islet allografts: a role for novel T cell growth factors in allograft rejection. J Immunol. 1998;161:890–896. [PubMed] [Google Scholar]
- Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- Waldmann H, Graca L, Cobbold S, Adams E, Tone M, Tone Y. Regulatory T cells and organ transplantation. Semin Immunol. 2004;16:119–126. doi: 10.1016/j.smim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunological self-tolerance maintained by activated T-cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various auto-immune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Kingsley CL, Karim M, Bushell AR, Wood K. CD25+CD4+ regulatory T cells prevent graft rejection: cTLA4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Clark DA, Wegmann TG. Genetic aspects of the CBA x DBA/2 and B10 x B10. A model of murine abortion and its prevention by lymphocytes immunisation. 18th RCOG Study Group. Allen WR, Clark DA, Gill TJ, Mowbray JF, Robertson WE, editors. London: RCOG Press,; Early Pregnancy LossMechanisms and Treatment. 1988:pp 89–102. [Google Scholar]
- Kurita T, Lee K, Saunders PT, Cooke PS, Taylor JA, Lubahn D, Zhaeo C, Mäkelä S, Gustafsson GA, Dahija R, Cunha G. Regulation of progesterone receptors and decidualization in uterine stroma of the estrogen-receptor-alpha knock out mouse. Biol Reprod. 2001;64:272–283. doi: 10.1095/biolreprod64.1.272. [DOI] [PubMed] [Google Scholar]
- Clark DA, McDermott MR, Szewczuk MR. Impairment of graft versus host reaction in pregnant mice: II) selective suppression of cytotoxic cell generation correlates with soluble suppressor activity and with successful allogeneic pregnancy. Cell Immunol. 1980;52:106–118. [PubMed] [Google Scholar]
- Mellor A, Munn D. Extinguishing maternal immune responses during pregnancy: implications for immunosuppression. Semin Immunol. 2001;13:213–218. doi: 10.1006/smim.2000.0317. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Meliani A, Martal J, Raghupathy R, Elliot J, Mosmann TR, Wegmann T. IL-10 prevents naturally occurring fetal loss in the CBA x DBA/2 mating combination, and local defect on IL-10 production in this abortion combination is corrected in vivo injection of IFN-τ. Am J Reprod Immunol. 1995;154:4261–4268. [PubMed] [Google Scholar]
- Chaouat G, Cayol V, Mairovitz V, Dubanchet S. Localization of the Th2 cytokines IL-3, IL-4, IL-10 at the fetomaternal interface during human and murine pregnancy and lack of requirement for Fas/Fas ligand interaction for a successful allogeneic pregnancy. Am J Reprod Immunol. 1999;42:1–13. doi: 10.1111/j.1600-0897.1999.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Dealtry G, O’Farrell M, Fernandez N. The Th2 cytokine environment of the placenta. Int Arch Allergy Immunol. 2000;123:107–119. doi: 10.1159/000024441. [DOI] [PubMed] [Google Scholar]
- Clark DA, Flanders KC, Banwatt D, Millar-Book W, Manuel J, Stedronska-Clark J, Rowley B. Murine pregnancy decidua produces a unique immunosuppressive molecule related to transforming growth factor beta-2. J Immunol. 1990;144:3008–3014. [PubMed] [Google Scholar]
- Gorivodsky M, Torchinsky A, Zemliak I, Savion S, Fein A, Toder V. TGF beta 2 mRNA expression and pregnancy failure in mice. Am J Reprod Immunol. 1999;42:124–133. [PubMed] [Google Scholar]
- Dünker N, Krieglstein K. TGF-β2−/− and 3−/− double knockout mice display severe midline fusion defects and early embryonic lethality. Anat Embryol. 2002;206:73–83. doi: 10.1007/s00429-002-0273-6. [DOI] [PubMed] [Google Scholar]
- White CA, Johansson M, Roberts CT, Ramsay AJ, Robertson SA. Effect of interleukin-10 null mutation on maternal immune response and reproductive outcome in mice. Biol Reprod. 2004;70:123–131. doi: 10.1095/biolreprod.103.018754. [DOI] [PubMed] [Google Scholar]
- Tangri S, Wegmann T, Lin H, Raghupathy R. Maternal anti-placental reactivity in natural immunologically-mediated fetal resorptions. J Immunol. 1994;152:4903–4911. [PubMed] [Google Scholar]
- Zelenika D, Adams E, Humm S, Lin CY, Waldmann H, Cobbold SP. The role of CD4+ T-cell subsets in determining transplantation rejection or tolerance. Immunol Rev. 2001;182:164–79. doi: 10.1034/j.1600-065x.2001.1820113.x. [DOI] [PubMed] [Google Scholar]
- Hall BM, Pearce NW, Gurley KE, Dorsch SE. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. III. Further characterization of the CD4+ suppressor cell and its mechanisms of action. J Exp Med. 1990;171:141–157. doi: 10.1084/jem.171.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmi L, Liotta F, Angeli R, Mazzinghi B, Santarlasci V, Manetti R, Lasagni L, Vanini V, Romagnani P, Maggi E, Annunziato F, Romagnani S. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood. 2004;103:3117–3121. doi: 10.1182/blood-2003-09-3302. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- Ke B, Ritter T, Kato H, Zhai Y, Li J, Lehmann M, Busuttil RW, Volk HD, Kupiec-Weglinski JW. Regulatory cells potentiate the efficacy of IL4 gene transfer by upregulating Th2-dependent expression of protective molecules in the infectious tolerance pathway in transplant recipients. J Immunol. 2000;164:5739–5745. doi: 10.4049/jimmunol.164.11.5739. [DOI] [PubMed] [Google Scholar]
- Aluvihare V, Kallikourdis M, Betz A. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;3:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- Savion S, Toder V. Pregnancy-associated effect on mouse thymocytes in vitro. Cell Immunol. 1995;162:282–287. doi: 10.1006/cimm.1995.1080. [DOI] [PubMed] [Google Scholar]
- Phuc LH, Papiernik M, Berrih S, Duval D. Thymic involution in pregnant mice. I. Characterization of the remaining thymocyte subpopulations. Clin Exp Immunol. 1981;44:247–252. [PMC free article] [PubMed] [Google Scholar]
- Phuc LH, Papiernik M, Dardenne M. Thymic involution in pregnant mice. II. Functional aspects of the remaining thymocytes. Clin Exp Immunol. 1981;44:253–261. [PMC free article] [PubMed] [Google Scholar]
- Clarke AG, Kendall MD. Histological changes in the thymus during mouse pregnancy. Thymus. 1989;14:65–78. [PubMed] [Google Scholar]
- Clarke AG, Gil AL, Kendall MD. The effects of pregnancy on the mouse thymic epithelium. Cell Tissue Res. 1994;275:309–318. doi: 10.1007/BF00319429. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Kolb JP, Wegmann TG. The murine placenta as an immunological barrier between the mother and the fetus. Immunol Rev. 1983;75:31–60. doi: 10.1111/j.1600-065x.1983.tb01090.x. [DOI] [PubMed] [Google Scholar]
- Kirby DR, Billington WD, Bradbury S, Goldstein DJ. Antigen barrier of the mouse placenta. Nature. 1964;204:548–549. doi: 10.1038/204548a0. [DOI] [PubMed] [Google Scholar]
- Billington WD. The normal fetomaternal immune relationship. Baillieres Clin Obstet Gynaecol. 1992;6:417–438. doi: 10.1016/s0950-3552(05)80004-5. [DOI] [PubMed] [Google Scholar]
- Smith RN, Powell AE. The adoptive transfer of pregnancy-induced unresponsiveness to male skin grafts with thymus-dependent cells. J Exp Med. 1977;146:899–904. doi: 10.1084/jem.146.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Kingsley C, Niimi M, Read S, Turvey S, Bushell A, Morris PJ, Powrie F, Wood K. IL-10 is required for regulatory cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- Kupiec-Weglinski JW, Onodera K, Volk HD. The “infectious” tolerance pathway in organ allograft recipients. Transplant Proc. 1998;4:1595–1597. doi: 10.1016/s0041-1345(98)00365-0. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Kiger N, Wegmann TG. Vaccination against spontaneous abortion in mice. J Reprod Immunol. 1983;5:389–392. doi: 10.1016/0165-0378(83)90248-6. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, Zhang D, Parlow AF, Glimcher LH. Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system. J Clin Invest. 2004;114:39–48. doi: 10.1172/JCI20645. [DOI] [PMC free article] [PubMed] [Google Scholar]