Abstract
The mucin-type glycoprotein podoplanin is specifically expressed by lymphatic but not blood vascular endothelial cells in culture and in tumor-associated lymphangiogenesis, and podoplanin deficiency results in congenital lymphedema and impaired lymphatic vascular patterning. However, research into the biological importance of podoplanin has been hampered by the lack of a generally available antibody against the human protein, and its expression in normal tissues and in human malignancies has remained unclear. We generated a human podoplanin-Fc fusion protein and found that the commercially available mouse monoclonal antibody D2-40 specifically recognized human podoplanin, as assessed by enzyme-linked immunosorbent assay and Western blot analyses. We found that, in addition to lymphatic endothelium, podoplanin was also expressed by peritoneal mesothelial cells, osteocytes, glandular myoepithelial cells, ependymal cells, and by stromal reticular cells and follicular dendritic cells of lymphoid organs. These findings were confirmed in normal mouse tissues with anti-podoplanin antibody 8.1.1. Podoplanin was also strongly expressed by granulosa cells in normal ovarian follicles, and by ovarian dysgerminomas and granulosa cell tumors. Although podoplanin was primarily absent from normal human epidermis, its expression was strongly induced in 22 of 28 squamous cell carcinomas studied. These findings suggest a potential role of podoplanin in tumor progression, and they also identify the first commercially available antibody for the specific staining of a defined lymphatic marker in archival human tissue sections, thereby enabling more widespread studies of tumor lymphangiogenesis in human cancers.
Lymphatic vessels play an important role in the maintenance of tissue homeostasis1 and in the transport of immune cells,2 but they also serve as the primary conduit for malignant tumor cell metastasis to regional lymph nodes.3 Although there is considerable evidence, obtained in genetic and xenotransplant tumor models, that tumor lymphangiogenesis promotes lymphatic tumor spread,3,4 it has remained controversial whether human tumors might actively induce lymphangiogenesis, and whether the degree of intra- or peritumoral lymphangiogenesis might serve as a prognostic indicator of tumor progression.5,6 Several new markers for the specific detection of human lymphatic endothelium versus blood vascular endothelium have been recently identified;7–9 however, there have been no commercially available antibodies against these lymphatic-specific proteins and, therefore, large-scale studies of tumor lymphangiogenesis are still lacking.
The mucin-type transmembrane glycoprotein podoplanin is one of the most highly expressed lymphatic-specific genes in cultured human lymphatic endothelial cells (LECs),10 and we have previously shown that podoplanin is a target gene of the homeobox gene Prox1, a master gene that controls the development of lymphatic progenitors from embryonic veins.11 In vivo expression of podoplanin in lymphatic endothelium was first reported by Wetterwald and colleagues,12 who named it “E11 antigen.” It was further characterized under the name “podoplanin,” because of its low-level expression in kidney podocytes.13 However, podoplanin is homologous to T1alpha, which was found to encode an antigen that is expressed at the apical surface of alveolar type I cells in rat lung.14,15 Expression of podoplanin has also been detected in the choroid plexus in the rat brain and the ciliary epithelium in the rat eye.16 Other podoplanin homologs include OTS-8,17 RTI40,18 gp38,19 canine gp40,20 human gp36,21 and murine PA2.26.22 However, little is understood about the biological function of podoplanin. Recently, we found that mice deficient in podoplanin develop congenital lymphedema and that they have defects in lymphatic vessel, but not blood vessel, pattern formation.23 Moreover, our in vitro studies indicated that podoplanin is involved in mediating cell motility by promoting rearrangement of the actin cytoskeleton.23
In this study, we aimed to identify an anti-human podoplanin antibody suitable for immunostains of archival paraffin-embedded human tissues, and to comprehensively characterize the cell type-specific expression of podoplanin in normal tissues and its potential involvement in tumor progression. We show that the commercially available antibody D2-40, originally raised against an unidentified M2A protein derived from germ cell tumors,24 specifically recognizes human podoplanin and that it can be used for routine immunohistochemical studies of tumor lymphangiogenesis. Using normal human tissue arrays, we found that podoplanin is also expressed by bile duct cells of the liver, peritoneal mesothelial cells, osteocytes, glandular myoepithelial cells, ependyma cells, and by stromal reticular cells and follicular dendritic cells of lymphoid organs. These findings were confirmed in tissue arrays of normal mouse tissues. Importantly, podoplanin was also strongly expressed by granulosa cells in normal ovarian follicles and by dysgerminomas and granulosa cell tumors. Although podoplanin was primarily absent from normal human epidermis, its expression was strongly induced in 22 of 28 squamous cell carcinomas (SCCs) studied. These findings suggest a potential role of podoplanin in tumor progression, and they also identify the first commercially available antibody for the specific staining of a defined lymphatic marker in human archival tissue sections, thereby enabling more widespread studies of tumor lymphangiogenesis and its role in tumor progression.
Materials and Methods
Immunostains
Immunofluorescence stainings were performed on 6-μm cryostat sections of neonatal human foreskin or on 6-μm paraffin sections of human malignant melanoma as described previously,6,10 using the mouse monoclonal antibody D2-40 (Signet, Dedham, MA), rabbit polyclonal antibodies against the lymphatic markers LYVE-17 and Prox125 (kindly provided by Dr. K. Alitalo, University of Helsinki, Helsinki, Finland), CD34, CD31 (BD Pharmingen, San Diego, CA), and corresponding secondary antibodies labeled with Alexa Fluor 488 or Alexa Fluor 594 (Molecular Probes, Eugene, OR). Nuclei were counterstained with 20 μg/ml of Hoechst bisbenzimide (Molecular Probes). Additional immunohistochemical stains were performed on tissue arrays of normal mouse (MaxArray mouse tissue microarray slides; Zymed, San Francisco, CA) and human tissues (MaxArray human normal tissue microarray slides, Zymed), human skin tumors (IMH-323; Imgenex, San Diego, CA) and ovary tumors (IMH-347, Imgenex) as described previously.6 Briefly, the primary antibodies D2-40 or LYVE-1 were applied, followed by incubation with conjugated anti-mouse or anti-rabbit immunoglobulin using the 3-amino-9-ethylcabazole peroxidase kit (Vector Laboratories, Burlingame, CA). The D2-40 antibody only stains human tissues, but not mouse tissues. For mouse tissues, the hamster monoclonal antibody 8.1.1 (Developmental Studies Hybridoma Bank, University of Iowa, Ames, IA) was used. We have previously shown that this antibody, originally raised against a mouse gp38 antigen,19 specifically recognizes podoplanin expressed by lymphatic vessels in wild-type mice, but not in podoplanin-deficient mice.23 The 8.1.1 antibody does not recognize human podoplanin. Sections were examined by using a Nikon E-600 microscope (Nikon, Melville, NY) and images were captured with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI).
Cell Transfection
Immortalized rat L6 myoblasts26 were maintained in Dulbecco’s modified Eagle’s medium that contained 10% fetal bovine serum, 2 mmol/L l-glutamine, and antibiotics (Life Science, Grand Island, NY). The human podoplanin coding sequence was cloned into the pCMV6-XL5 vector (Origene, Rockville, MD). Rat myoblasts were transfected either with the full-length human podoplanin cDNA or with the pCMV6-XL5 vector alone using the SuperFect transfection reagent (Qiagen, Chatsworth, CA). Specific binding of D2-40 antibody to human podoplanin was investigated by immunofluorescence staining of the transiently transfected cells after paraformaldehyde fixation (Fluka, Buchs, Germany).
Expression of Podoplanin as a Soluble IgFc Fusion Protein
For the amplification of full length human podoplanin, RNA was isolated from cultured human dermal microvascular endothelial cells (Promocell, Heidelberg, Germany) using a Qiagen RNeasy kit, according to the manufacturer’s instructions (Qiagen, Valencia, CA). First strand synthesis was performed by oligo-dT priming using 3 μg of total RNA. The podoplanin coding sequence was amplified from 2 μl of this product by polymerase chain reaction with the primers hPodo156FHindIII (GTCAGCAGGAAGCTTCCAGGAGAGCAACAACTCAAC) and hPodo570RBamHI (TCGGCTCCGGATCCACTGTTGACAAACCATCTTTCTC)using Pfu DNA polymerase (Stratagene, La Jolla, CA). After digestion with HindIII and BamHI, the product was cloned into the HindIII/BamHI-digested IgFc vector pCDM7Ig,27 to yield a construct encoding podoplanin fused at the COOH terminus to the Fc region of human IgG1.7 For expression and purification of the podoplanin-Fc fusion protein, the expression vector was transfected into human 293T cells using the calcium phosphate method. Transfectants were grown in serum-free UltraCHO medium (Bio-Whittaker, Walkersville, MD) for 3 days before harvesting culture supernatants. The fusion protein was purified by affinity chromatography on a column of 1 ml of protein A Sepharose (Sigma, St. Louis, MO). Fractions containing the fusion protein were neutralized and the purity was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Enzyme-Linked Immunosorbent Assay (ELISA)
Binding of the mouse monoclonal antibody D2-40 to immobilized podoplanin-Fc fusion protein was tested in 96-well ELISA plates (Nalge Nunc, Rochester, NY). Plates were coated with 5 μg of either human podoplanin-Fc or human LYVE-1-Fc.7 After overnight incubation, wells were washed and blocked using 1% bovine serum albumin (Sigma) and 0.05% Tween (Sigma). D2-40 antibody was applied at a series of dilutions from 1:50 to 1:50,000, each in triplicates and incubated for 1 hour. Unbound antibody was removed by washing with phosphate-buffered saline; bound antibody was detected by incubation with horseradish peroxidase-conjugated goat anti-mouse IgG (Pierce, Rockford, IL) followed by O-phenylenediamine substrate (Sigma), and the absorbance was measured at 490 nm in a microplate reader (Bio-Rad, Hercules, CA).
Cell Transfection with Human Podoplanin siRNAs and Western Blot Analyses
The following small inhibitory RNA (siRNA) oligonucleotides were synthesized by Dharmacon (Lafayette, CO): R1, 5′-GCGAAGACCGCUAUAAGUCdTdT-3′ and R2, 5′-AAGAUGGUUUGUCAACAGUdTdT-3′.23 Primary human LECs10 were transfected or not with siRNA oligonucleotides (500 nmol) or with equimolar concentrations of control plasmid vector by using the Nucleofector kit (Amaxa, Cologne, Germany) according to the manufacturer’s instructions. Cells were harvested at 4 days after transfection. For Western analyses, cell lysates were obtained as described11 and 30 ng of protein per sample were immunoblotted with the D2-40 antibody. For detection of podoplanin-Fc fusion protein, 200 ng of human podoplanin-Fc and human LYVE-1-Fc7 as a control were electrophoresed on a polyacrylamide sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and were transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ). The blots were incubated with D2-40 (0.5 μg/ml) and were developed by using a chemiluminescent detection kit (Pierce).
Results
Monoclonal Antibody D2-40 Recognizes Human Podoplanin
Immunofluorescence staining of rat myoblasts that were transiently transfected with a human podoplanin overexpression vector, with the monoclonal antibody D2-40 revealed strong cytoplasmic labeling of podoplanin-transfected cells (Figure 1B) whereas control vector-transfected cells were unstained (Figure 1A). The D2-40 antibody specifically detected a human podoplanin-Fc fusion protein, but not a LYVE-1 fusion protein, as assessed by Western blotting (Figure 1C). ELISAs further demonstrated specific binding of the D2-40 antibody to the immobilized human podoplanin-Fc fusion protein, whereas we only found low nonspecific binding to the human LYVE-1-Fc fusion protein (Figure 1D) that also contains glycosylated sialoglycoproteins with O-linked carbohydrate structures. Because the D2-40 antibody recognizes human but not mouse podoplanin, its specificity could not be further tested in lymphatic cells obtained from podoplanin-deficient mice that we previously described.23 Therefore, we studied siRNA-mediated knock-down of podoplanin in human LECs. We found that siRNA-mediated podoplanin knockdown resulted in reduced podoplanin detection by D2-40, by 66% and 36% (Figure 1E), as compared with control LECs.
Figure 1.
The D2-40 antibody specifically recognizes human podoplanin. A and B: Rat myoblasts transiently transfected with human podoplanin-cDNA demonstrated labeling with D2-40 antibody (B, green; blue = nuclear Hoechst stain) whereas control-transfected myoblasts are negative (A). C: D2-40 antibody specifically recognizes the human podoplanin-Fc fusion protein, but not a human LYVE-1-Fc fusion protein, as detected by Western blotting. D: ELISA shows specific binding of the D2-40 antibody to immobilized human podoplanin-Fc fusion protein, as compared to low nonspecific binding to human LYVE-1-Fc fusion protein. E: Four days after anti-human podoplanin-siRNA transfection of human primary LECs, Western blots using the D2-40 antibody reveal reduced levels of endogenous podoplanin protein, using two different siRNA oligonucleotides (R1 and R2), as compared with control cells (C1, control vector cDNA; C2, sham-transfected cells). Scale bar, 50 μm (B).
Podoplanin Expression in Normal Human Tissues
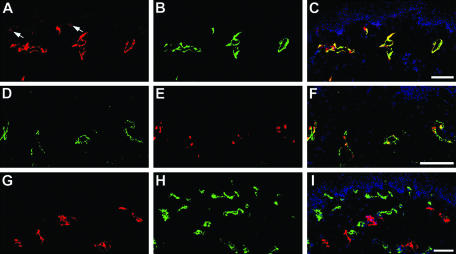
Immunofluorescence double stains of normal human skin with the D2-40 antibody and with antibodies against the lymphatic-specific hyaluronan receptor LYVE-17 or the lymphatic homeobox protein Prox128 revealed complete overlap of immunoreactivity, confirming specific podoplanin expression by lymphatics, but not by blood vessels (Figure 2; A to F). Double immunostains with D2-40 and with an antibody against the blood vascular-specific marker CD3410 further demonstrated mutually exclusive expression of podoplanin and CD34 by cutaneous lymphatic vessels and blood vessels, respectively (Figure 2; G to I). Occasionally, focal expression of podoplanin was also detected on basal epidermal keratinocytes (Figure 2A).
Figure 2.
Specific detection of podoplanin expression by LECs in normal human skin using the D2-40 antibody. A to C: Double-immunofluorescence stains for podoplanin (A) and for the lymphatic-specific hyaluronan receptor LYVE-1 (B) revealed co-localization on LECs (C merge, nuclei are labeled blue). In addition, podoplanin was focally expressed by basal epidermal keratinocytes (arrows). Lymphatic endothelial cells stained by D2-40 (D) also expressed the lymphatic-specific homeobox protein Prox1 (E, F merge). In contrast, double-immunofluorescence stains for podoplanin (G) and for the blood vessel-specific marker CD34 (H) demonstrated mutually exclusive expression by lymphatics and blood vessels. Scale bars, 100 μm.
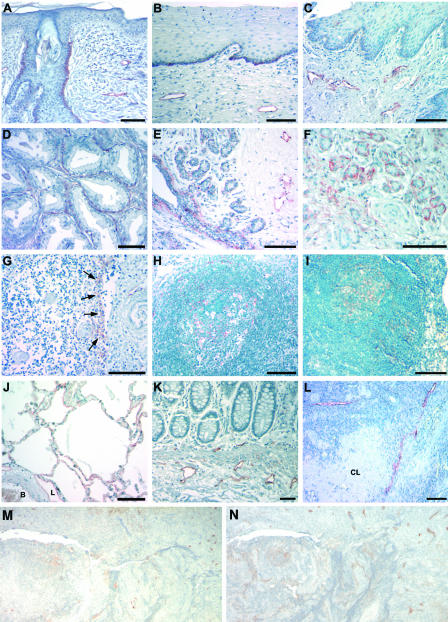
We next studied whether podoplanin might also serve as a specific marker for lymphatic vessels in other human organs, in addition to the skin, and whether other cell types might also express podoplanin, using human multiple tissue arrays. In all human organs examined, podoplanin was detected on LECs that also expressed the lymphatic-specific hyaluronan receptor LYVE-1 (see below and data not shown). Moreover, basal epithelial keratinocytes of the skin, cervix, and esophagus also showed focal podoplanin expression (Figure 3; A to C). Surprisingly, strong podoplanin expression was detected on myoepithelial cells of the breast glands and of salivary glands, as well as on myofibroblasts of the prostate (Figure 3; D to F). Moreover, podoplanin-positive stromal reticular cells and follicular dendritic cells were found in the follicular germinal centers of lymphoid organs, including the thymus, tonsils, and lymph nodes (Figure 3; G to I). Staining of serial sections for the follicular dendritic cell marker CD21 (Figure 3M) and for podoplanin (Figure 3N) confirmed that CD21-positive follicular dendritic cells express podoplanin. In the lung, podoplanin was expressed by alveolar type I cells (Figure 3J), as well as by LECs that were also stained in the colon (Figure 3K) and the ovary (Figure 3L). In contrast, blood vascular endothelial cells were negative for podoplanin.
Figure 3.
Podoplanin expression in normal human tissues. In the skin (A), cervix (B), and esophagus (C), basal keratinocytes and LECs express podoplanin, whereas blood vascular endothelial cells are not labeled. Podoplanin is also expressed by fibromyocytes in the prostate (D) and by myoepithelial cells of breast glands (E) and salivary glands (F). G: Podoplanin expression by stromal cells in the medulla of the thymus near invaginations of the capsule that penetrates through the thymic cortex (arrows). H and I: Expression of podoplanin by follicular dendritic cells of germinal centers of the tonsils (H) and of lymph nodes (I). J: In the lung, alveolar type I cells and LECs (L, lymphatic vessel; B, blood vessel) express podoplanin. Lymphatic endothelial expression in the colon (K) and the ovary (L); a corpus albicans (CL) is unlabeled. M: Follicular dendritic cells express CD21 and also express podoplanin (N). Scale bars, 100 μm.
Expression of Podoplanin in Normal Mouse Tissues
All organs examined, except for the central nervous system, showed strong labeling of LECs with the anti-podoplanin antibody 8.1.1 (Figure 4, A to L; Table 1), as confirmed by staining of serial sections for LYVE-1 (data not shown). Myoepithelial cells of the salivary glands and fibromyocytes of the testis (Figure 4, C and F) also expressed podoplanin. In the central nervous system, podoplanin was expressed by ependymal cells lining the ventricles (Figure 4D), by choroid plexus cells (Figure 4D), and by meningeal cells (Figure 4I). In the peripheral nervous system, perineural cells expressed podoplanin, as detected in spinal nerve roots (Figure 4E) and in peripheral nerves of the skin, the tongue, and the skeletal muscle (data not shown). In agreement with the findings in human tissues, stromal reticular cells and dendritic reticular cells of the follicular germinal centers in the spleen and in lymph nodes (Figure 4, G and H), as well as osteocytes (Figure 4I) and alveolar type I cells (Figure 4J) also expressed podoplanin. Remarkably, the germinal epithelium and granulosa cells of primary and secondary ovarian follicles showed strong podoplanin expression (Figure 4L), whereas podoplanin was only weakly expressed by tertiary follicles and was absent from corpora lutea (for summary of results obtained in human and murine tissues, see Table 1). No major differences of staining patterns were observed between human and mouse tissues.
Figure 4.
Podoplanin expression in normal mouse tissues. Immunohistochemical staining demonstrated podoplanin expression by lymphatic vessels of the skin (A) and the tongue (B). D: In the central nervous system, the ependyma lining the ventricular system and the choroid plexus expressed podoplanin. E: The perineurium demonstrated podoplanin expression around spinal nerve roots. Myoepithelial cells of the salivary gland (C) and fibromyocytes of the testis (F) also express podoplanin. In lymphoid organs, podoplanin is expressed by endothelial cells lining lymphatic vessels (L) and by follicular dendritic cells of the germinal centers in the spleen (G, arrows). H: In lymph nodes, reticulum cells of the cortex around follicles express podoplanin. Expression of podoplanin in osteocytes (I; arrows), the periosteum (P), meninges (M) and lung alveolar type I cells (J). K: Only lymphatic vessels were labeled in the colon. L: In the ovary, granulosa cells of primary (PF) and secondary follicles (SF) express higher levels of podoplanin than tertiary (developed) follicles (DF). The corpus luteum (CL) does not express podoplanin, whereas the germinal epithelium covering the ovary (GE) shows strong podoplanin expression. Scale bars, 100 μm.
Table 1.
Expression of Podoplanin in Normal Tissues (Combined Results of Human and Murine Tissues)
| Organ | Cell type |
|---|---|
| Skin | Focal expression in basal keratinocytes; lymphatic endothelium |
| Lung | Type I alveolar cells; lymphatic endothelium; pleura |
| Kidney | Glomerular podocytes; parietal epithelial cells of Bowman’s capsule; lymphatic endothelium |
| Liver | Bile ducts; lymphatic endothelium; peritoneum |
| Esophagus | Basal keratinocytes; lymphatic endothelium |
| Intestine | Lymphatic endothelium; peritoneum |
| Central nervous system | Choroid plexus; ependyma; meninges |
| Peripheral nervous system | Perineurium; lymphatic endothelium |
| Ovary | Follicular granulosa cells; lymphatic endothelium; germinal epithelium |
| Cervix | Basal keratinocytes; lymphatic endothelium |
| Breast | Myoepithelial cells; lymphatic endothelium |
| Prostate | Myofibroblasts; lymphatic endothelium |
| Testis | Fibromyocytes; lymphatic endothelium |
| Salivary gland | Myoepithelial cells; lymphatic endothelium |
| Bone | Osteocytes; periosteum |
| Lymphoid organs | Stromal reticular cells; follicular dendritic cells; lymphatic endothelium |
Podoplanin Expression in Human Tumors
We next investigated whether the D2-40 anti-podoplanin antibody might reliably detect tumor-associated lymphatic vessels and whether tumor cells themselves might express podoplanin, using human tumor microarrays. Because of the observed occasional expression of podoplanin in epidermal keratinocytes of the skin, and in distinct cells of the ovary, we focused our study on SCCs and ovarian tumors. All tumor-associated lymphatic vessels expressed podoplanin, as confirmed by staining of serial sections for LYVE-1 (see below and data not shown). We also found strong podoplanin expression by tumor cells of dysgerminomas of the ovary (four of four tumors; Figure 5A), whereas serial sections of those tumors revealed that LYVE-1 labeling was restricted to lymphatic vessels only (Figure 5B). One of three granulosa cell tumors also expressed podoplanin (Figure 5C), whereas podoplanin was absent from all other ovarian tumors studied, including adenocarcinomas (n = 5; Figure 5D), thecomas (n = 5), and Sertoli-Leydig cell tumors (n = 2; data not shown). Importantly, podoplanin was expressed by the majority of primary SCCs of the skin studied (22 of 28 tumors). Moderately differentiated SCCs (n = 12) expressed podoplanin predominantly within the basal tumor cell layer (Figure 5E) with enhanced membrane-staining pattern (Figure 5F), whereas less-differentiated SCCs (n = 3) showed additional podoplanin expression beyond the basal cell layer with frequent cytoplasmic staining (Figure 5G). In contrast, well-differentiated SCCs (n = 13) did not express podoplanin (Figure 5H). All recurrent SCCs (three of three tumors) showed high podoplanin expression levels (data not shown).
Figure 5.
Podoplanin expression in ovarian and skin cancers. Dysgerminomas of the ovary showed a very strong podoplanin expression (A), whereas serial sections of the same tumors revealed that LYVE-1 expression was restricted to lymphatic vessels (B). Granulosa cell tumors (C) but not adenocarcinomas of the ovary (D) showed podoplanin expression by tumor cells. Moderately differentiated SCCs of the skin showed positive staining for podoplanin predominantly within the basal tumor cell layer (E) with enhanced membrane staining pattern (F, higher magnification of E). G: In less differentiated SCCs, podoplanin is also expressed beyond the basal cell layer with cytoplasmic staining. H: In contrast, well-differentiated SCCs do not express podoplanin. I: In malignant melanomas, podoplanin expression by peritumoral lymphatic vessels is detected by staining with the D2-40 antibody. The identity of podoplanin-positive lymphatics was confirmed in serial sections of the same tumors (J) by fluorescent labeling for LYVE-1 (green) and CD31 (red), combined with nuclear staining (blue). L, lymphatic vessel; B, blood vessel. Scale bars, 100 μm.
We have recently demonstrated that the extent of tumor lymphangiogenesis can serve as a new prognostic indicator for the risk of lymph node metastasis in human malignant melanomas of the skin, as evaluated by immunostains for the hyaluronan receptor LYVE-1.6 We found that staining of archival paraffin sections of human melanomas with the anti-podoplanin antibody D2-40 also resulted in strong labeling of melanoma-associated lymphatic vessels (Figure 5I). The specificity of podoplanin expression for lymphatics was confirmed by immunofluorescence labeling for LYVE-1 in serial sections of the same tumors (Figure 5J).
Discussion
Our findings reveal that human podoplanin, a recently identified marker for LECs,8 is specifically recognized by the monoclonal antibody D2-40, that distinct nonendothelial cell types express podoplanin in normal tissues, and that podoplanin is likely involved in epithelial tumor progression. The monoclonal antibody D2-40 was originally raised against a glycoprotein named oncofetal M2A antigen that is expressed by testicular germ cell neoplasias.24 Because purified M2A antigen was shown to be a 40-kd surface sialoglycoprotein with extensive O-glycosylation, similar to podoplanin, and because M2A was also found to stain lymphatic vessels,29 we hypothesized that the M2A antigen recognized by the D2-40 antibody might be identical to human podoplanin. In fact, we found that the D2-40 antibody specifically recognized a recombinant human podoplanin-Fc fusion protein, as confirmed by Western blotting and by ELISA, and that siRNA-mediated knock-down of podoplanin in human LECs specifically reduced the 40-kd protein recognized by the D2-40 antibody. Together, these results identify the D2-40 antibody as a specific antibody against human podoplanin.
Our study of the tissue distribution of podoplanin in normal human and murine tissues revealed that podoplanin is expressed by lymphatic vessels, but not by blood vessels, in all organs examined. Surprisingly, however, we found that podoplanin is also expressed by a number of nonendothelial cell types in normal tissues, in particular by several cell types that are exposed to a fluid interphase. Within the central nervous system, podoplanin is expressed by ciliated epithelial ependymal cells that line the ventricular system and the central canal, and by the cuboidal epithelial cells of the choroid plexus that represent a modified ependyma covering a highly vascular connective tissue core.30 This finding is in agreement with the reported expression of the lung type I alveolar cell marker T1alpha in epithelial cells of the choroid plexus16 because T1alpha and podoplanin are in fact identical proteins.23 It is of interest that we also found podoplanin expression in the epithelial cells of the hepatic bile ducts that are exposed to the alkaline bile. Together with the expression of podoplanin/T1alpha in alveolar type I cells (our data and Rishi et al15) and in glomerular podocytes of the kidney,13 these findings indicate that the extensively O-glycosylated mucin-type glycoprotein podoplanin with its high content of sialic acid and its negative charged structure might have a protective function toward these external and internal fluid compartments.
In a recent study, we found that podoplanin overexpression in cultured vascular endothelial cells promoted the formation of elongated cell extensions and significantly increased endothelial cell adhesion, migration, and tube formation, indicating an important role in cytoskeletal reorganization.23 This potential function of podoplanin is supported by our results that myofibroblasts of the prostate, myoepithelial cells of the mammary and salivary glands, fibromyocytes of the testis, and cells of the perineurium strongly express podoplanin. All these cell types are contractile with frequent changes of their cell shape mediated by myofilaments.31 Therefore, podoplanin might play an important role in mediating cellular contractile properties and cytoskeletal reorganization.
Surprisingly, we found podoplanin to be also expressed by granulosa cells, a stratified epithelium surrounding the oocyte, of developing ovarian follicles. Whereas primary and secondary follicles showed very strong podoplanin expression, its expression was reduced in developed follicles and was completely absent from the corpus luteum or corpus albicans stage. Thus, podoplanin might play a role in early granulosa cell differentiation, but its direct biological function in follicle maturation remains to be established. It is of interest that podoplanin was strongly expressed by all tumor cells of ovarian dysgerminomas and a fraction of granulosa cell tumors, but not by other ovarian neoplasias, indicating its potential use as a specific marker for these tumor entities.
In agreement with our previous study in mouse skin,23 we occasionally detected weak focal podoplanin expression in basal epidermal keratinocytes of normal human skin, although the majority of keratinocytes was podoplanin-negative. Importantly, however, we found that the majority of human SCCs of the skin strongly expressed podoplanin, with a particularly strong expression in recurrent SCCs. Moreover, we found that podoplanin expression was absent from well-differentiated SCCs, with increasing expression in moderately differentiated SCCs and with further enhanced expression in poorly differentiated SCCs that are characterized by enhanced propensity for tumor progression. Together with the observed up-regulation of the podoplanin homologue PA2.26 during murine epidermal carcinogenesis22 and with our previous findings that podoplanin overexpression promoted cell motility and migration,23 these results indicate a possible role for podoplanin in epithelial tumor progression, possibly by enhancing tumor cell spread via lymphatic vessels. We currently investigate whether enhanced podoplanin expression might also be involved in mediating the metastatic spread of experimental tumors.
Lymph node metastasis is a major determinant for the staging and clinical management of human cancers. In contrast to the extensive studies on tumor-associated angiogenesis, however, little is known about the mechanisms by which tumor cells gain entry into the lymphatic system. Recent studies in experimental tumor metastasis models have suggested an important role of tumor-associated lymphatic vessels in promoting cancer spread to lymph nodes,3,4,32,33 and an increasing number of clinicopathological studies have shown a direct correlation between tumor expression of the lymphangiogenesis factors VEGF-C or VEGF-D and metastatic tumor spread in many human cancers.34 Importantly, tumor lymphangiogenesis has been recently identified as a new prognostic parameter for the risk of lymph node metastasis of human cutaneous malignant melanomas and of head and neck cancers.6,35 Antibodies against the lymphatic hyaluronan receptor LYVE-1 have been used to visualize lymphatic vessels in these studies; however, the specificity of LYVE-1 for LECs has been questioned by some investigators36 and antibodies against LYVE-1 are not generally available. Our results reveal that podoplanin, detected by the D2-40 antibody, is a specific marker for human tumor-associated lymphatic vessels, as confirmed by co-expression of podoplanin, LYVE-1 and Prox1 in melanoma-associated peritumoral lymphatic vessels. Because podoplanin is also expressed by a variety of nonendothelial cell types, as demonstrated in this study, double stains for the pan-vascular marker CD31 and for podoplanin should be used to confirm the identity of lymphatic vessels. Taken together, the commercially available antibody D2-40, specifically detecting podoplanin in archival paraffin-embedded human tissues, represents a promising tool for more widespread studies of tumor lymphangiogenesis and its role in human cancer progression.
Acknowledgments
We thank B. Ma and M. Constant for technical assistance, Dr. K. Alitalo for the gift of the Prox1 antibody, and Satoshi Hirakawa and Rainer Kunstfeld for helpful discussions.
Footnotes
Address reprint requests to Michael Detmar, M.D., Institute of Pharmaceutical Sciences, Swiss Federal Institute of Technology Zurich, Wolfgang-Pauli-Str. 10, HCI H303, CH-8093 Zurich, Switzerland. E-mail: michael.detmar@pharma.ethz.ch.
Supported by the National Institutes of Health (National Cancer Institute grants CA69184, CA86410, and CA92644 to M.D. and Pathology Training grant 5T32CA09216 to S.S.D.), the Susan G. Komen Breast Cancer Foundation (to M.D.), the American Cancer Society (program project grant 99-23901 to M.D.), the Deutsche Forschungsgemeinschaft (to V.S.), and the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co. Ltd. Agreement (to M.D.).
References
- Witte MH, Bernas MJ, Martin CP, Witte CL. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech. 2001;55:122–145. doi: 10.1002/jemt.1163. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- Straume O, Jackson DG, Akslen LA. Independent prognostic impact of lymphatic vessel density and presence of low-grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res. 2003;9:250–256. [PubMed] [Google Scholar]
- Dadras SS, Paul T, Bertoncini J, Brown LF, Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC, Detmar M. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162:1951–1960. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Soleiman A, Horvat R, Amann G, Kowalski H, Kerjaschki D. Podoplanin—a specific marker for lymphatic endothelium expressed in angiosarcoma. Verh Dtsch Ges Pathol. 1999;83:270–275. [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- Wetterwald A, Hoffstetter W, Cecchini MG, Lanske B, Wagner C, Fleisch H, Atkinson M. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone. 1996;18:125–132. doi: 10.1016/8756-3282(95)00457-2. [DOI] [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Matsui K, Soleiman A, Meraner P, Poczewski H, Kalt R, Schaffner G, Kerjaschki D. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- Dobbs LG, Williams MC, Gonzalez R. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim Biophys Acta. 1988;970:146–156. doi: 10.1016/0167-4889(88)90173-5. [DOI] [PubMed] [Google Scholar]
- Rishi AK, Joyce-Brady M, Fisher J, Dobbs LG, Floros J, VanderSpek J, Brody JS, Williams MC. Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev Biol. 1995;167:294–306. doi: 10.1006/dbio.1995.1024. [DOI] [PubMed] [Google Scholar]
- Williams MC, Cao Y, Hinds A, Rishi AK, Wetterwald A. T1 alpha protein is developmentally regulated and expressed by alveolar type I cells, choroid plexus, and ciliary epithelia of adult rats. Am J Respir Cell Mol Biol. 1996;14:577–585. doi: 10.1165/ajrcmb.14.6.8652186. [DOI] [PubMed] [Google Scholar]
- Nose K, Saito H, Kuroki T. Isolation of a gene sequence induced later by tumor-promoting 12-O-tetradecanoylphorbol-13-acetate in mouse osteoblastic cells (MC3T3–E1) and expressed constitutively in ras-transformed cells. Cell Growth Differ. 1990;1:511–518. [PubMed] [Google Scholar]
- Gonzalez RF, Dobbs LG. Purification and analysis of RTI40, a type I alveolar epithelial cell apical membrane protein. Biochim Biophys Acta. 1998;1429:208–216. doi: 10.1016/s0167-4838(98)00231-3. [DOI] [PubMed] [Google Scholar]
- Farr AG, Berry ML, Kim A, Nelson AJ, Welch MP, Aruffo A. Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. J Exp Med. 1992;176:1477–1482. doi: 10.1084/jem.176.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer G, Lottspeich F, Maisner A, Klenk HD, Herrler G. Molecular characterization of gp40, a mucin-type glycoprotein from the apical plasma membrane of Madin-Darby canine kidney cells (type I). Biochem J. 1997;326:99–108. doi: 10.1042/bj3260099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer G, Oeffner F, Von Messling V, Tschernig T, Groness HJ, Klenk HD, Herrler G. Cloning and characterization of gp36, a human mucin-type glycoprotein preferentially expressed in vascular endothelium. Biochem J. 1999;341:277–284. [PMC free article] [PubMed] [Google Scholar]
- Gandarillas A, Scholl FG, Benito N, Gamallo C, Quintanilla M. Induction of PA2.26, a cell-surface antigen expressed by active fibroblasts, in mouse epidermal keratinocytes during carcinogenesis. Mol Carcinog. 1997;20:10–18. doi: 10.1002/(sici)1098-2744(199709)20:1<10::aid-mc3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks A, Sutherland DR, Bailey D, Iglesias J, Law J, Lei M, Yeger H, Banerjee D, Baumal R. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br J Cancer. 1999;80:569–578. doi: 10.1038/sj.bjc.6690393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Kanai M, Goke M, Tsunekawa S, Podolsky DK. Signal transduction pathway of human fibroblast growth factor receptor 3. Identification of a novel 66-kDa phosphoprotein. J Biol Chem. 1997;272:6621–6628. doi: 10.1074/jbc.272.10.6621. [DOI] [PubMed] [Google Scholar]
- Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem. 2001;276:19420–19430. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn HJ, Marks A. A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest. 2002;82:1255–1257. doi: 10.1097/01.lab.0000028824.03032.ab. [DOI] [PubMed] [Google Scholar]
- Fuller GN, Burger PC. Central nervous system. Sternberg SS, editor. New York: Raven Press, Ltd.,; Histology for Pathologists. 1997:pp 145–168. [Google Scholar]
- Ross MH, Romrell LJ. Histology: A Text and Atlas. Baltimore: Williams & Wilkins,; 1989 [Google Scholar]
- Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, Baldwin ME, Achen MG. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16:922–934. doi: 10.1096/fj.01-0945rev. [DOI] [PubMed] [Google Scholar]
- Maula SM, Luukkaa M, Grenman R, Jackson D, Jalkanen S, Ristamaki R. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res. 2003;63:1920–1926. [PubMed] [Google Scholar]
- Carreira CM, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, Jain RK. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079–8084. [PubMed] [Google Scholar]