Abstract
Multiple system atrophy (MSA) is a progressive neurodegenerative disorder characterized by parkinsonism unresponsive to dopaminergic therapy, cerebellar ataxia, and dysautonomia. Neuropathology shows a characteristic neuronal multisystem degeneration that is associated with widespread oligodendroglial α-synuclein (α-SYN) inclusions. Presently no animal model completely replicates the specific neuropathology of MSA. Here we investigated the behavioral and pathological features resulting from oligodendroglial α-SYN overexpression in transgenic mice exposed to mitochondrial inhibition by 3-nitropropionic acid. In transgenic mice 3-nitropropionic acid induced or augmented motor deficits that were associated with MSA-like pathology including striatonigral degeneration and olivopontocerebellar atrophy. Widespread astrogliosis and microglial activation were also observed in the presence of α-SYN in oligodendrocytes. Our results indicate that combined mitochondrial inhibition and overexpression of oligodendroglial α-SYN generates a novel model of MSA that may be useful for evaluating both pathogenesis and treatment strategies.
Multiple system atrophy (MSA) is a sporadic neurodegenerative disorder that presents with levodopa-resistant parkinsonism, cerebellar ataxia, and dysautonomia in any combination.1,2 Neuropathologically there is multisystem neuronal loss affecting striatum and substantia nigra pars compacta (striatonigral degeneration, SND), cerebellum, pons, inferior olives (olivopontocerebellar atrophy), and the intermediolateral column of the spinal cord.3 Microglial activation,4 astrogliosis,5 and demyelination6 appear to be associated with the degeneration process. (Oligodendro)glial cytoplasmic inclusions (GCIs) represent the neuropathological hallmark lesion of MSA.7 The importance of GCIs for the pathogenesis of MSA is generally acknowledged, however, the relation of GCI formation to glial and neuronal cell death remains unclear. The discovery of α-synuclein (α-SYN) as the major component of GCIs8–10 expanded the molecular understanding of MSA pathology. Reactive oxygen species derived from dysfunctional mitochondria in the presence of environmental toxins may contribute to the pathogenesis of MSA.11,12 Indeed, oxidative damage of α-SYN was detected in GCIs.13
MSA carries a serious prognosis because of rapid progression of motor disabilities.2 In addition, effective therapy for MSA patients is lacking. Therefore, there is a strong need for experimental models as preclinical test beds for novel treatment strategies in MSA. We have developed several animal models of SND, the neuropathological substrate of MSA-associated parkinsonism, based on neurotoxic lesions of striatum and substantia nigra pars compacta (SNc) by either direct unilateral delivery in rats14–19 or systemic exposure in mice and primates.20–23 Distinct behavioral and morphological abnormalities have been characterized in these models. However, oligodendroglial α-SYN pathology was not present in any of them. Thus, transgenic (Tg) mouse models were generated in which human α-SYN was specifically expressed in oligodendrocytes.24 In Tg mice overexpressing human wild-type α-SYN under control of the proteolipid protein (PLP) promotor, both GCI-like aggregates and biochemical markers of MSA were observed, including detergent insolubility25–27 and phosphorylation at serine-12928 of the transgenic α-SYN. In the present work we aimed to establish whether oligodendroglial α-SYN overexpression in (PLP)-α-SYN mice24 combined with mitochondrial inhibition by 3-nitropropionic acid (3-NP)22 replicates the combined glial and neuronal pathology of MSA.
Materials and Methods
Animals and Treatment
The generation and characterization of the (PLP)-α-SYN mice was previously described.24 The following in vivo protocols were approved by the Federal Ministry of Science and Transport of Austria. In the present study we used a total of 41 homozygous (PLP)-α-SYN mice (Tg) and 38 C57BL/6 mice (control) older than 10 months of age. Animals were maintained in a temperature-controlled room under 12-hour light/dark cycle with free access to food and water. Mice of each genotype (Tg or control) were subgrouped into saline-treated, low-dose 3-NP, and high-dose 3-NP groups. The 3-NP intraperitoneal treatment was done according to the following scheme22: low-dose 3-NP: 4 × 10 mg/kg, 4 × 20 mg/kg, 4 × 40 mg/kg, 3 × 50 mg/kg, total dose 430 mg/kg; high-dose 3-NP: 8 × 40 mg/kg, 3 × 80 mg/kg, total dose 560 mg/kg. 3-NP was dissolved in saline and pH 7.4 was adjusted with 1 mol/L NaOH. The concentration was calculated to keep the injected volume (∼250 μl) stable. Intraperitoneal injections were done every 12 hours during the intoxication period.
Behavioral Tests
Standardized Motor Behavioral Scale for Assessment of the Severity of 3-NP-Induced Motor Disability in Mice
We used a previously described rating scale22 for evaluation of hindlimb clasping, general locomotor activity, hindlimb dystonia, truncal dystonia, and postural challenge response (0, normal; 1, slightly disturbed; and 2, markedly disabled). The rating was performed every 12 hours before the injection during the intoxication period and every day during the period after intoxication. The total score for each session was determined.
Pole Test
The pole test22,29 was performed at baseline and on day 13 after the beginning of intoxication. Each mouse was habituated to the test the day before. A wooden vertical pole with rough surface, 1 cm wide and 50 cm high was applied. The mouse was placed with the head up at the top of the pole and the time for turning downwards (Tturn) as well as the total time for climbing down the pole until the mouse reached the floor with the four paws (Ttotal) was taken in five trials. The best performance of all of the five trials was kept for the statistical analysis.22 If a mouse was unable to perform the test, a default value of 120 seconds was taken into account.
Open Field Activity
To test the locomotor activity of the mice we applied the Flex Field Activity System (San Diego Instruments, San Diego, CA), which allows monitoring and real-time counting of horizontal and vertical locomotor activity by 544 photo-beam channels. Mice were placed in the center of the open field (40.5 × 40.5 × 36.5 cm) and tested for a 15-minute period always at the same time of the day (5:00 p.m.). The tests were performed in a dark room that was completely isolated from external noises and light during the test period. The sum of counts in the horizontal and vertical plane at baseline and on day 13 after the first injection was further analyzed.
Stride Length
The stride length22 of the forelimbs and hindlimbs of the mice was measured at baseline and before sacrificing them after a habituation to the test for 3 days before its performance. The limbs of each animal were wetted with a nontoxic food color and the mouse was let to run on a strip of paper (42 cm long, 4.5 cm wide) down a bright corridor toward a dark goal box. After three runs, the stride length of the forelimbs and the hindlimbs on each side was measured, excluding the beginning (7 cm) and the end (7 cm) of the run. The mean stride length for each forelimbs and hindlimbs was determined.
Tissue Processing
Animals were perfused under thiopental anesthesia (day 15 after the first 3-NP injection) with 5 ml of phosphate-buffered saline followed by 20 ml of 4% paraformaldehyde (pH 7.4). Brains were quickly removed, postfixed in the same fixative for 1 hour at 4°C, and then kept in 25% sucrose solution until they sank. Brains were slowly frozen with 2-methylbutan and kept at −80°C until processing. Four series of two adjacent 20-μm sections (directly mounted on slides) and six series of 50-μm free-floating sections throughout the whole brain were cut on a freezing microtome (Leica, Nussloch, Germany) and either dried or kept in assorter buffer at 4°C, respectively. The series on slides were used for standard cresyl violet and Klüver-Barrera stainings and the free-floating sections were used for immunohistochemical stainings. A group of mice was decapitated under thiopental anesthesia, brains were quickly removed, fixed in 4% formaldehyde in phosphate-buffered saline, and embedded in paraffin. Sections (4 μm) were stained with hematoxylin and eosin, Gallyas silver, Campbell-Switzer silver, thioflavin S, and Luxol fast blue-periodic acid-Schiff. The proteinase K-digested paraffin-embedded tissue blot was performed as described previously.30
Immunocytochemistry
The following antibodies were used in this study: monoclonal anti-DARPP-32 (1:20000; generous gift from Prof. H. Hemmings, New York Presbyterian Hospital, NY), monoclonal anti-TH (1:500; Sigma, St. Louis, MO), monoclonal anti-calbindin D28k (1:1000, Sigma), monoclonal anti-GFAP (1:100; Roche, Vienna, Austria), monoclonal anti-CNP (1:100, Sigma), monoclonal anti-CD68 (1:100, MCA 1957; Serotec, Oxford, UK), monoclonal anti-human-α-synuclein (1:10, 15G731), monoclonal anti-MBP (1:100, mAb 381; Chemicon, Temecula, CA), monoclonal anti-MOG (1:100, 8.18-C532), monoclonal anti-ubiquitin (1:300, mAb 1510; Chemicon). Secondary antibodies were biotinylated goat anti-mouse IgG or goat anti-rat IgG (Vector, Burlingame, CA).
Image Analysis
All morphometric analyses were done by a blinded observer on Olympus BX60 microscope (Olympus, Hamburg, Germany) supplied with computerized image analysis system (Sony 3CCD video camera, Image Pro Plus software; Media Cybernetics, Silver Spring, MD, and Neurolucida software, MicroBrightField, Inc., Colchester, VT). Stereological methods33 were used to count neurons in the striatum, substantia nigra pars compacta, locus ceruleus, pontine nuclei, deep cerebellar nuclei, and inferior olive. The entire cerebellar hemisphere was outlined on serial sections to determine cerebellar volume. Purkinje cells were counted in a region outlined to include only the Purkinje cell layer.34 The density per mm2 of 2′3′-cyclic nucleotide 3′-phosphodiesterase (CNP)-positive oligodendrocytes in the striatum was determined. The optical density of the dopaminergic terminals in the striatum was measured in serial sections and a mean density score was determined for each mouse.
Statistics
Behavioral data were analyzed with Kruskal-Wallis nonparametric analysis and posthoc Dunn’s test. After confirming normality of distribution, the histological morphometric data were subjected to two-way analysis of variance to compare control and Tg mice after intoxication with different doses of 3-NP. Posthoc Bonferroni test was applied where appropriate. P < 0.05 was used to determine statistical significance.
Results
Locomotor Dysfunction
Both (PLP)-α-SYN and C57BL/6 control mice developed progressive motor disability after treatment with 3-NP in a dose-dependent manner as established by the Bordeaux motor behavioral scale for assessment of the severity of 3-NP-induced motor disorders in mice.22 In the low-dose treatment group only 43% of the control mice developed motor disability evaluated by this scale, whereas all of the Tg mice in the low-dose group (100%) showed signs of motor impairment. In low-dose 3-NP-treated mice the mean motor behavioral score was significantly greater in Tg compared to control mice at day 15 (Figure 1a). In contrast, motor disability increased in both high-dose 3-NP-treated Tg and control mice reaching a maximum on the last test day (day 15) before sacrifice (Figure 1a). Comparison of overall motor disability scores in Tg and control mice revealed dose dependence (Figure 1b). Forty-six percent of the high-dose 3-NP-treated Tg mice died before the end of the test period, whereas none of the control mice died after the high-dose intoxication. The 3-NP dose-dependent motor impairment was confirmed by open field activity (Figure 1, c and d), pole (Figure 1, e and f) and stride length tests (Figure 1, g and h). The general locomotor activity in an open field arena decreased because of 3-NP intoxication both in the horizontal and vertical plane (Figure 1, c and d). Further, the rearing scores (vertical movements) were significantly more reduced in Tg than in control mice receiving low-dose 3-NP (Figure 1d).
Figure 1.
Behavioral motor impairment. a: Mean daily motor impairment scores increased substantially after day 8 in control and Tg mice receiving high-dose 3-NP. In contrast, low-dose 3-NP induced significant behavioral impairment in Tg, but not in control mice at day 15. b: Significant dose-dependent effect of 3-NP treatment on the total motor impairment score was observed (total for 15 days). c: Open-field horizontal activity was significantly impaired both by low- and high-dose 3-NP without dose dependency. d: However, when analyzing rearing as one of the open-field activity measures 3-NP showed a clear dose-response effect that was more marked in Tg mice exposed to low-dose 3-NP. Tturn (e) and Ttotal (f) of the pole test showed similar results suggesting a combined overall genotype and 3-NP effect. Forelimb (g) and hindlimb (h) stride length decreases confirmed 3-NP and overall genotype effects. Further, even without treatment a significantly shorter hindlimb stride length was observed in Tg mice. Significant changes are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001 for 3-NP treatment effects within the control or Tg group; °, P < 0.05; °°, P < 0.01 for comparisons of control and Tg mice. For all tests, n > 6 per group. Flex field activity test and pole test were performed on day 13. Stride length measurements were performed on day 14.
The time that mice needed to turn their head and body down (Tturn) and to descend along a pole (Ttotal) significantly increased with escalating dose levels of 3-NP (Figure 1, e and f). Additionally, Tturn and Ttotal were significantly longer in 3-NP-treated Tg compared to control mice indicating a more severe motor deficit with disturbance of balance and coordination. Exposure to 3-NP induced a dose-dependent shortening of the stride length in both control and Tg mice (Figure 1, g and h). In addition, hindlimb stride length was significantly reduced in untreated Tg compared to control mice (Figure 1h).
Neuropathology
Striatonigral Neuronal Pathology
Striatal neuronal cell loss was induced by treatment with 3-NP in both control and Tg mice as shown by cresyl violet staining and DARPP-32 immunohistochemistry. Neuronal loss was most prominent in the dorsolateral striatum with well-circumscribed symmetrical striatal lesions in mice treated with high-dose 3-NP (Figure 2, a and b). DARPP-32+ neurons in the striatum were lost dose dependently after 3-NP intoxication (Figure 2c). In addition, Tg mice showed greater striatal neuronal loss than control mice after low-dose 3-NP exposure. Neuronal loss was accompanied by striatal shrinkage as measured by decrease in striatal volume (Figure 2d). Again, striatal volume loss was greater in Tg compared to control mice receiving low-dose 3-NP. In addition to the loss of DARPP-32+ neurons in the striatum, 3-NP intoxication also caused a dose- and genotype-dependent loss of dopaminergic innervation of the striatum, as evidenced by reduced TH immunoreactivity (Figure 2e). A significant reduction of TH-immunoreactive neurons was observed in the SNc of Tg mice at baseline (Figure 2f), suggesting that the presence of α-SYN in oligodendrocytes alone induced dopaminergic neuron loss. 3-NP exposure exacerbated dopaminergic cell loss in both Tg and control mice (Figure 2, g and h) in a dose- and genotype-dependent manner (Figure 2f).
Figure 2.
Striatonigral neuropathology. a and b: DARPP-32 immunohistochemistry of striatum in a control saline-treated mouse and in a Tg mouse after treatment with high-dose 3-NP. Arrows point to the lesion in the dorsolateral striatum. Insets represent higher magnification of the striatum. In Tg + 3NP mice the center of the lesion is deprived of cells and the periphery of the lesion shows a few remaining degenerating cell bodies. c: Neuronal cell counts in the striatum revealed dose-dependent loss of DARPP-32-labeled neurons in C57BL/6 (control) and Tg mice that was more pronounced in the latter when exposed to low-dose 3-NP. d: Analysis of striatal volume showed a similar 3-NP and genotype effect. e: The density of TH immunoreactivity in the striatum of control and Tg mice was significantly decreased after 3-NP intoxication. There was also a significant overall group effect of Tg versus control mice (P < 0.01). f: There was a reduction of TH-positive neurons in SNc in untreated Tg versus control mice. After 3-NP administration neuronal loss in SNc increased dose dependently with greater loss in Tg mice receiving low-dose 3-NP. g and h: Photomicrographs showing TH-immunopositive dopaminergic neurons in SNc of control (saline-treated) and 3-NP-treated Tg mice. Significant changes are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001 for 3-NP treatment effects within the control or Tg group; °, P < 0.05; °°, P < 0.01; °°°, P < 0.001 for comparisons of control and Tg mice. Scale bars: 1 mm (a); 50 μm (insets in a, b); 100 μm (g, h).
Cerebellar, Pontine, and Inferior Olive Neuronal Pathology
In the low-dose and high-dose 3-NP regimens there was no consistent effect of the toxin on cerebellar neurons in control mice. In contrast, Tg mice showed dose-dependent 3-NP-induced cerebellar Purkinje cell loss as measured by cresyl violet staining (Figure 3, a and b) and calbindin D28K immunohistochemistry (data not shown). 3-NP treatment induced dose-dependent loss of the cerebellar volume in Tg mice that was not observed in control mice (Figure 3b). The deep cerebellar nuclei of neither control nor Tg mice were affected by 3-NP intoxication (Figure 3b).
Figure 3.
Cerebellar, pontine, and olivary neuropathology. a: Cresyl violet staining demonstrated Purkinje cell loss in 3-NP-treated Tg but not in control mice. b: Morphometric analysis confirmed significant loss of Purkinje cells and of cerebellar volume in Tg mice treated with high-dose 3-NP. In contrast, deep cerebellar nuclei were preserved. c: Representative photomicrographs of TH-immunopositive neurons in locus ceruleus (LC) in control and Tg mice, without or with 3-NP treatment. d: Morphometric analysis showed neuronal loss of LC in untreated Tg compared to control mice. 3-NP induced dose-dependent cell loss in both control and Tg mice, with a significant difference between both groups. Cell counts revealed loss of neurons in the pontine nuclei and inferior olivary complex of Tg but not control mice exposed to low- and high-dose 3-NP. Significant changes are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001 for 3-NP treatment effects within the control or Tg group; °, P < 0.05; °°, P < 0.01; °°°, P < 0.001 for comparisons of control and Tg mice. Scale bar, 100 μm (c).
Analysis of the neuronal loss in the pons demonstrated TH+ neuron loss in the locus ceruleus (LC) already at baseline in Tg mice (Figure 3, c and d). After 3-NP intoxication, TH+ cell loss was evident in both control and Tg mice, and this was more marked in Tg than in control mice (Figure 3, c and d). Furthermore, neuronal loss in the pontine nuclei and in the inferior olivary nucleus on 3-NP treatment was observed in Tg but not in control mice (Figure 3d).
Astrogliosis
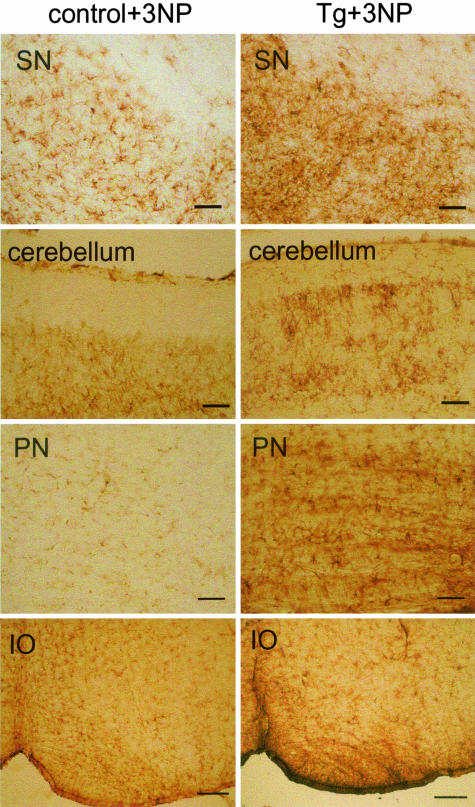
There was no apparent difference between the GFAP staining pattern in untreated control and Tg mice. As a result of the 3-NP intoxication we observed astroglial activation in the striatum of both control and Tg mice forming a scar around the lesion in the dorsolateral striatum (data not shown). In SN the astroglial activation was more prominent in Tg than in control mice treated with 3-NP (Figure 4). Further, 3-NP induced astroglial activation in cerebellum, pons, and inferior olives was only seen in Tg but not in control mice (Figure 4).
Figure 4.
Astrogliosis. GFAP immunohistochemistry demonstrating widespread astrogliosis in Tg mice after 3-NP intoxication including substantia nigra (SN), cerebellum, pontine nuclei (PN), and inferior olive (IO). In contrast, 3-NP-treated control C57BL/6 mice show weak astroglial activation in SN but not in the other brain structures presented. Scale bars: 50 μm (SN, cerebellum, PN); 0.2 mm (IO).
Microglial Activation
Prominent microglial activation was observed in the striatum, SNc, and in the white matter throughout the brain of the untreated Tg but not the control mice (Figure 5). Intoxication with 3-NP increased microglial activation in the striatum and substantia nigra of Tg mice and to a lesser degree also induced microglial activation in these areas in control mice (data not shown). In addition the activation of microglia in the white matter of the Tg mice was markedly intensified by 3-NP, whereas in control mice it was absent or just single CD68-positive cells were seen (Figure 5). CD68-positive cells were observed also in the cerebellar cortex and deep cerebellar nuclei, the pontine nuclei, and the inferior olive of 3-NP-treated Tg mice but not in control mice (data not shown).
Figure 5.
Microglial activation. CD68 immunohistochemistry demonstrated microglial activation in several brain regions. Untreated Tg mice showed diffuse microglial infiltration in white matter tracts [capsula interna (delineated by arrows), cerebellum] that was not observed in untreated control mice. In Tg mice, intoxication with 3-NP augmented microglial activation in the sites mentioned above but remained absent in control mice. Scale bars, 0.2 mm.
Oligodendroglial Pathology
Oligodendrocytes of Tg mice showed human α-SYN-positive profiles (Figure 6, a and b), as previously described.24 Morphometric analysis of the density of oligodendrocytes revealed a significant cell loss because of high-dose, but not low-dose, 3-NP intoxication (Figure 6c). No widespread specific positive inclusions were detectable with ubiquitin antibodies (data not shown). Argyrophilic thioflavin S-positive or proteinase K-resistant α-SYN was not detected in oligodendrocytes of Tg mice (data not shown). Luxol-fast blue staining as well as MOG and MBP immunohistochemistry did not reveal significant loss of myelin in Tg mice compared to control mice (data not shown).
Figure 6.
Oligodendroglial pathology. a: Immunohistochemistry for α-SYN with 15G7 antibody, demonstrating the density of α-SYN-positive profiles in oligodendrocytes in the striatum of a Tg mouse. b: Immunohistochemistry for α-SYN with 15G7 antibody, demonstrating a single oligodendrocyte of a Tg mouse with a typical polar accumulation of α-SYN reminiscent of a GCI (arrow). c: Semiquantitative analysis of the density of oligodendrocytes in the striatum of control C57BL/6 and Tg mice showed loss of oligodendrocytes in response to 3-NP intoxication regardless of the mouse genotype.
Discussion
We here characterize a novel MSA mouse model resulting from oligodendroglial α-SYN overexpression in transgenic mice exposed to mitochondrial inhibition by 3-NP. Neither oligodendroglial α-SYN overexpression nor 3-NP intoxication alone was sufficient to induce the full spectrum of MSA-like neuropathology. As previously reported, 3-NP induced selective neuronal cell loss with astrogliosis in the striatum and loss of dopaminergic neurons in SNc of control mice.22,35 Although significant suppression of succinate dehydrogenase in cerebellum and many other brain regions is known to result from systemic 3-NP,36 no cell loss has ever been reported outside of the striatonigral system. In the present study, our observations in control mice intoxicated with 3-NP confirm the selective loss of neurons in striatum, and SNc with sparing of the cerebellum, pontine nuclei, and inferior olive, although for the first time we were able to show 3-NP-induced neuronal loss in LC of C57BL/6 mice older than 10 months of age. 3-NP alone although widely used to model Huntington’s disease37 or SND,22,35 is not able to replicate olivopontocerebellar atrophy pathology that is typically associated with MSA.
Ectopic oligodendroglial expression of α-SYN in Tg mice has been shown to replicate the oligodendroglial pathology of MSA. In the first report of these mice GCI-like profiles and serine-129 phosphorylated insoluble α-SYN were demonstrated.24 These markers characterize oligodendrocytes of MSA patients.28 However, Tg mice of up to 10 months of age did not show motor disturbances on the rotarod. Our present study applied additional locomotor tests to extend the behavioral characterization of these mice. We observed a subtle but significant shortening of the stride length of Tg mice but no other changes in their motor activity. This behavioral change was associated with a 23% TH+ cell loss in SNc that may not have been detected by the other motor tests because of threshold effects.38 Further, we detected TH+ cell loss in the LC of untreated Tg mice. Similar to SNc, LC is composed of monoamine-containing neurons that appear to be particularly sensitive to ectopic α-SYN expression in oligodendrocytes. Abnormal microglial activation may have also contributed to neuronal loss in untreated Tg mice, but it cannot readily explain the selective loss of monoaminergic neurons in these animals.
In contrast to the limited effects of isolated 3-NP exposure or oligodendroglial overexpression of α-SYN in mice both strategies combined resulted in MSA-like features including complex locomotor impairment and neuropathological changes such as SND, olivopontocerebellar neuronal loss, astrogliosis, and microglial activation, along with the GCI-like pathology.24 The severe motor impairment phenotype included stiffness and flexion of the trunk, hindlimb dystonia, prominent poverty of spontaneous movements, reduction of stride length, and poor balance and coordination. Although similar signs were observed also in control mice treated with 3-NP, performance of the 3-NP-treated Tg mice was significantly worse, consistent with increased vulnerability to oxidative stress when combined with oligodendroglial α-SYN overexpression. Unfortunately, the present study had to be terminated on day 15 because of the severe morbidity of the high-dose 3-NP-treated mice. However, between days 14 and 15 we observed a significant difference in the motor behavior of control and Tg mice in the low-dose 3-NP group, suggesting that long-term studies need to be performed to confirm and follow the motor deterioration. The behavioral observations were further supported by the neuropathological findings. Interestingly, oligodendroglial overexpression of α-SYN exacerbated 3-NP-induced SND and it mediated 3-NP-induced olivopontocerebellar pathology that was absent in control mice. This observation suggests that in the presence of α-SYN-overexpressing oligodendrocytes, systemic 3-NP-induced oxidative stress results in a MSA-like neuropathological lesion pattern. Importantly, environmental factors leading to oxidative stress have been associated with the aetiopathogenesis of MSA by several investigators.12,39 The origin of the neuronal protein α-SYN in MSA oligodendrocytes remains uncertain.11 α-SYN released from dying neurons may be taken up by oligodendroglial cells; alternatively, oligodendroglial α-SYN may be overexpressed as a result of noxious stimuli. Increased α-SYN mRNA levels in GCI-positive oligodendrocytes have recently been reported in brains of patients with the cerebellar, but not the parkinson presentation of MSA.40 Serine-129 phosphorylated insoluble α-SYN is present in the oligodendrocytes of Tg mice24 similar to the human disease. Argyrophilia and proteinase resistance of the α-SYN filamentous inclusions are other features typically seen in human GCIs derived from end-stage MSA.26 These features were absent in our study, however, the short survival time may have limited further development of GCI-like inclusions. The lack of widespread specific positive inclusions detectable with ubiquitin antibodies argues against a role of ubiquitination of α-SYN in this mouse model at the stage studied. This finding corresponds to neuropathological data suggesting that ubiquitination of α-SYN is not required for inclusion formation and might be a late secondary phenomenon.41 We observed oligodendroglial cell loss in both control and Tg mice after treatment with 3-NP. It has been previously shown that 3-NP induces calpain-mediated neuronal and oligodendroglial cytoskeletal break down.42,43 More detailed studies are needed to address whether α-SYN overexpression alters the pattern of oligodendroglial cell death in Tg mice with features of apoptosis similar to the human disease.44 Microglial activation, again reminiscent of the human pathology,4 was pronounced in 3-NP-treated Tg mice. Whether α-SYN oligodendroglial pathology itself or chronically activated microglia or both drive the selective neuronal degeneration process in MSA remains to be studied further in the Tg mouse model that reproduces both features. Further, demyelination is observed in the end-stage MSA brain tissue,5 but we could not prove the same in the Tg mice. The lack of demyelination in the present study (like several other features discussed above) could have contributed to the short survival time that did not let the scattered oligodendroglial pathology and microgliosis to induce marked myelin pathology. It cannot be excluded that all of the effects documented for 3-NP in (PLP)-α-SYN mice are caused by a subtly impaired functional oligodendrocyte-neurite contact,6 which is still permissive for myelination. Alternatively, myelin pathology in the Tg mice might have been undetectable at the chosen stage with the stains used in previous studies of human MSA.6
In conclusion, the present study describes the first animal model of MSA that reproduces both the specific neuronal and glial pathology of the disorder with a corresponding motor phenotype. Our data demonstrate that oligodendroglial α-SYN overexpression is critical for the increased susceptibility to oxidative stress. Further, this model could be applied to study the pathogenic mechanisms and pathways of selective neurodegeneration underlying MSA, as well as serve as an invaluable test bed for future therapeutic strategies including restoration of levodopa response and neuroprotection at different stages of the disease progression.
Acknowledgments
We thank Monika Hainzer for the excellent technical assistance.
Footnotes
Address reprint requests to Gregor K. Wenning, Clinical Department of Neurology, Innsbruck Medical University, Anichstrasse 35 A-6020, Innsbruck, Austria. E-mail: gregor.wenning@uibk.ac.at.
Supported by the Austrian Science Foundation (grants P14633-B05 and P16128) and the Deutsche Forschungsgemeinschaft (SFB596 project A1).
References
- Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, Kaufmann H, Klockgether T, Lang AE, Lantos PL, Litvan I, Mathias CJ, Oliver E, Robertson D, Schatz I, Wenning GK. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163:94–98. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Colosimo C, Geser F, Poewe W. Multiple system atrophy. Lancet Neurol. 2004;3:93–103. doi: 10.1016/s1474-4422(03)00662-8. [DOI] [PubMed] [Google Scholar]
- Daniel S. Autonomic failure. Bannister R, Mathias CJ, editors. Oxford: University Press; The Neuropathology and Neurochemistry of Multiple System Atrophy. 1999:pp 321–328. [Google Scholar]
- Ishizawa K, Komori T, Sasaki S, Arai N, Mizutani T, Hirose T. Microglial activation parallels system degeneration in multiple system atrophy. J Neuropathol Exp Neurol. 2004;63:43–52. doi: 10.1093/jnen/63.1.43. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Seppi K, Tison F, Jellinger K. A novel grading scale for striatonigral degeneration (multiple system atrophy). J Neural Transm. 2002;109:307–320. doi: 10.1007/s007020200025. [DOI] [PubMed] [Google Scholar]
- Matsuo A, Akiguchi I, Lee GC, McGeer EG, McGeer PL, Kimura J. Myelin degeneration in multiple system atrophy detected by unique antibodies. Am J Pathol. 1998;153:735–744. doi: 10.1016/S0002-9440(10)65617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Gai WP, Power JHT, Blumbergs PC, Blessing WW. Multiple system atrophy: a new α-synuclein disease? Lancet. 1998;352:547–548. doi: 10.1016/s0140-6736(05)79256-4. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. α-Synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998;249:180–182. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- Burn DJ, Jaros E. Multiple system atrophy: cellular and molecular pathology. Mol Pathol. 2001;54:419–426. [PMC free article] [PubMed] [Google Scholar]
- Hanna PA, Jankovic J, Kirkpatrick JB. Multiple system atrophy: the putative causative role of environmental toxins. Arch Neurol. 1999;56:90–94. doi: 10.1001/archneur.56.1.90. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by α-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Granata R, Laboyrie PM, Quinn NP, Jenner P, Marsden CD. Reversal of behavioural abnormalities by fetal allografts in a novel rat model of striatonigral degeneration. Mov Disord. 1996;11:522–532. doi: 10.1002/mds.870110507. [DOI] [PubMed] [Google Scholar]
- Puschban Z, Scherfler C, Granata R, Laboyrie P, Quinn NP, Jenner P, Poewe W, Wenning GK. Autoradiographic study of striatal dopamine re-uptake sites and dopamine D1 and D2 receptors in a 6-hydroxydopamine and quinolinic acid double-lesion rat model of striatonigral degeneration (multiple system atrophy) and effects of embryonic ventral mesencephalic, striatal or co-grafts. Neuroscience. 2000;95:377–388. doi: 10.1016/s0306-4522(99)00457-1. [DOI] [PubMed] [Google Scholar]
- Puschban Z, Waldner R, Seppi K, Stefanova N, Humpel C, Scherfler C, Levivier M, Poewe W, Wenning GK. Failure of neuroprotection by embryonic striatal grafts in a double lesion rat model of striatonigral degeneration (multiple system atrophy). Exp Neurol. 2000;164:166–175. doi: 10.1006/exnr.2000.7422. [DOI] [PubMed] [Google Scholar]
- Scherfler C, Puschban Z, Ghorayeb I, Goebel GP, Tison F, Jellinger K, Poewe W, Wenning GK. Complex motor disturbances in a sequential double lesion rat model of striatonigral degeneration (multiple system atrophy). Neuroscience. 2000;99:43–54. doi: 10.1016/s0306-4522(00)00171-8. [DOI] [PubMed] [Google Scholar]
- Ghorayeb I, Puschban Z, Fernagut PO, Scherfler C, Rouland R, Wenning GK, Tison F. Simultaneous intrastriatal 6-hydroxydopamine and quinolinic acid injection: a model of early-stage striatonigral degeneration. Exp Neurol. 2001;167:133–147. doi: 10.1006/exnr.2000.7535. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Lundblad M, Tison F, Poewe W, Cenci MA, Wenning GK. Effects of pulsatile L-DOPA treatment in the double lesion rat model of striatonigral degeneration (multiple system atrophy). Neurobiol Dis. 2004;15:630–639. doi: 10.1016/j.nbd.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Ghorayeb I, Fernagut PO, Aubert I, Bezard E, Poewe W, Wenning GK, Tison F. Toward a primate model of L-dopa-unresponsive parkinsonism mimicking striatonigral degeneration. Mov Disord. 2000;15:531–536. doi: 10.1002/1531-8257(200005)15:3<531::AID-MDS1017>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ghorayeb I, Fernagut PO, Stefanova N, Wenning GK, Bioulac B, Tison F. Dystonia is predictive of subsequent altered dopaminergic responsiveness in a chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine+3-nitropropionic acid model of striatonigral degeneration in monkeys. Neurosci Lett. 2002;335:34–38. doi: 10.1016/s0304-3940(02)01137-0. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Diguet E, Stefanova N, Biran M, Wenning GK, Canioni P, Bioulac B, Tison F. Subacute systemic 3-nitropropionic acid intoxication induces a distinct motor disorder in adult C57Bl/6 mice: behavioural and histopathological characterisation. Neuroscience. 2002;114:1005–1017. doi: 10.1016/s0306-4522(02)00205-1. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Puschban Z, Fernagut PO, Brouillet E, Tison F, Reindl M, Jellinger KA, Poewe W, Wenning GK. Neuropathological and behavioral changes induced by various treatment paradigms with MPTP and 3-nitropropionic acid in mice: towards a model of striatonigral degeneration (multiple system atrophy). Acta Neuropathol (Berl) 2003;106:157–166. doi: 10.1007/s00401-003-0717-y. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Spooren W, Fuss B, Mallon B, Macklin WB, Fujiwara H, Hasegawa M, Iwatsubo T, Kretzschmar HA, Haass C. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3:583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Lin W, Liu WK, Yen SH. Multiple system atrophy: a sporadic synucleinopathy. Brain Pathol. 1999;9:721–732. doi: 10.1111/j.1750-3639.1999.tb00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BC, McLean CA, Culvenor JG, Gai WP, Blumbergs PC, Jakala P, Beyreuther K, Masters CL, Li QX. The solubility of alpha-synuclein in multiple system atrophy differs from that of dementia with Lewy bodies and Parkinson’s disease. J Neurochem. 2001;76:87–96. doi: 10.1046/j.1471-4159.2001.00021.x. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. Alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 1997;73:45–48. doi: 10.1016/s0165-0270(96)02211-x. [DOI] [PubMed] [Google Scholar]
- Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Muller V, Odoy S, Fujiwara H, Hasegawa M, Iwatsubo T, Trojanowski JQ, Kretzschmar HA, Haass C. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van Der Putten H, Probst A, Kremmer E, Kretzschmar HA, Haass C. Subcellular localization of wild-type and Parkinson’s disease-associated mutant α-synuclein in human and transgenic mouse brain. J Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner C, Lassmann H, Waehneldt TV, Matthieu JM, Linington C. Differential ultrastructural localization of myelin basic protein, myelin oligodendroglial glycoprotein, and 2′,3′-cyclic nucleotide 3′-phosphodiesterase in the CNS of adult rats. J Neurochem. 1989;52:296–304. doi: 10.1111/j.1471-4159.1989.tb10930.x. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Gundersen HJ. If you assume, you can make an ass out of u and me: a decade of the disector for stereological counting of particles in 3D space. J Anat. 1996;188:1–15. [PMC free article] [PubMed] [Google Scholar]
- German DC, Quintero EM, Liang C, Xie C, Dietschy JM. Degeneration of neurons and glia in the Niemann-Pick C mouse is unrelated to the low-density lipoprotein receptor. Neuroscience. 2001;105:999–1005. doi: 10.1016/s0306-4522(01)00230-5. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Diguet E, Bioulac B, Tison F. MPTP potentiates 3-nitropropionic acid-induced striatal damage in mice: reference to striatonigral degeneration. Exp Neurol. 2004;185:47–62. doi: 10.1016/j.expneurol.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Scallet AC, Nony PL, Rountree RL, Binienda ZK. Biomarkers of 3-nitropropionic acid (3-NPA)-induced mitochondrial dysfunction as indicators of neuroprotection. Ann NY Acad Sci. 2001;939:381–392. doi: 10.1111/j.1749-6632.2001.tb03647.x. [DOI] [PubMed] [Google Scholar]
- Beal MF. Neurochemistry and toxin models in Huntington’s disease. Curr Opin Neurol. 1994;7:542–547. doi: 10.1097/00019052-199412000-00012. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Nee LE, Gomez MR, Dambrosia J, Bale S, Eldridge R, Polinsky RJ. Environmental-occupational risk factors and familial associations in multiple system atrophy: a preliminary investigation. Clin Auton Res. 1991;1:9–13. doi: 10.1007/BF01826052. [DOI] [PubMed] [Google Scholar]
- Miller DW, Johnson JM, Solano SM, Standaert DG, Young AB. Expression of alpha synuclein mRNA in multiple system atrophy. Func Neurol. 2004;19:145–146. doi: 10.1007/s00702-005-0378-1. [DOI] [PubMed] [Google Scholar]
- Sampathu DM, Giasson BI, Pawlyk AC, Trojanowski , Lee M-Y. Ubiquitination of α-synuclein is not required for formation of pathological inclusions in α-synucleinopathies. Am J Pathol. 2003;163:91–100. doi: 10.1016/s0002-9440(10)63633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizat N, Hermel JM, Boyer F, Jacquard C, Creminon C, Ouary S, Escartin C, Hantraye P, Kajewski S, Brouillet E. Calpain is a major cell death effector in selective striatal degeneration induced in vivo by 3-nitropropionate: implications for Huntington’s disease. J Neurosci. 2003;23:5020–5030. doi: 10.1523/JNEUROSCI.23-12-05020.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken E, Dewar D, Hunter AJ. White matter damage following systemic injection of the mitochondrial inhibitor 3-nitropropionic acid in rat. Brain Res. 2001;892:329–335. doi: 10.1016/s0006-8993(00)03266-2. [DOI] [PubMed] [Google Scholar]
- Probst-Cousin S, Rickert CH, Schmid KW, Gullotta F. Cell death mechanisms in multiple system atrophy. J Neuropathol Exp Neurol. 1998;57:814–821. doi: 10.1097/00005072-199809000-00002. [DOI] [PubMed] [Google Scholar]