Abstract
The homing of lymphocytes to peripheral lymph nodes is initiated by an adhesive interaction between L-selectin on lymphocytes and PNAd, a set of sialomucins that are constitutively displayed on high endothelial venules of lymph nodes. PNAd is defined by monoclonal antibody MECA-79 that recognizes a sulfated oligosaccharide carried by the sialomucins. This epitope overlaps with 6-sulfo sialyl Lewis x, a recognition determinant for L-selectin. Previous work has shown that administration of a L-selectin monoclonal antibody blocks both late-phase airway responses and airway hyperresponsiveness in a sheep model of asthma. We show here that airway-associated lymphoid collections from lungs of allergic sheep exhibited PNAd+ venules as detected by immunostaining with MECA-79. The same vessels also expressed a GlcNAc-6-O-sulfotransferase known as HEC-GlcNAc6ST, which is known to contribute to the formation of the MECA-79 epitope in high endothelial venules of mouse lymph nodes. Intravenous administration of MECA-79 to allergic sheep significantly blunted both the late-phase airway response and airway hyperresponsiveness induced by airway allergen challenge. Furthermore, MECA-79 inhibited the accumulation of all classes of leukocytes in bronchoalveolar lavage fluid. These findings represent the first demonstration that targeting of PNAd has therapeutic efficacy in an inflammatory disease.
It is widely accepted that airway inflammation is a major contributing factor to the chronic airway hyperresponsiveness (AHR) of asthma in which there is an enhanced bronchoconstrictor response to allergens and other stimuli.1,2 As seen in human asthmatics in the laboratory, certain animal models of allergic asthma develop both early airway responses (EARs) and late airway responses (LARs) to inhaled antigen.1–4 LARs are associated with the influx of inflammatory cells into the airways and are often followed by a sustained period of AHR.5 Thus, the development of antigen-induced LARs and AHR are thought to be physiological indicators of a heightened inflammatory response initiated by a single antigen challenge.1 Agents that interfere with these inflammatory processes may therefore prevent the physiological responses. Interrupting the inflammatory cascade becomes important, because continued exposure to allergens incites further inflammation and can lead to airway remodeling and long-term airway damage.2,6
The prominent role played by the recruitment, activation, and retention of inflammatory cells in the pathophysiology of asthma has fostered a great deal of interest in the molecular events that initiate and amplify the antigen-induced inflammatory response. Receiving a great deal of attention in this regard are the leukocyte-endothelial adhesion mechanisms responsible for the recruitment of leukocytes into airways of allergic patients. A widely held belief is that controlling leukocyte recruitment into the lungs is a means to ameliorate disease severity.7 This has led to the use of a number of anti-adhesion strategies to counteract the mechanisms responsible for inflammatory cell recruitment and retention in the airways.
The selectins are lectin-like adhesion receptors that participate in the initial interactions of leukocytes with the vascular endothelium.8,9 E-selectin and P-selectin are expressed on the surface of activated endothelium where they can interact with ligands (eg, PSGL-1) on leukocyte subpopulations, thereby initiating and sustaining rolling interactions. These two vascular selectins have been implicated in various animal models of allergic airway disease through antibody blockade studies and through utilization of selectin-null mice.10–12 Furthermore, correlative evidence exists from the study of tissue samples from human asthmatics. Thus, E-selectin is up-regulated on blood vessels within bronchial biopsies13 and serum levels of soluble E-selectin increase during asthma attacks.14 Recently, a series of findings have focused attention on L-selectin, which is broadly expressed on leukocytes and mediates rolling interactions through recognition of specific ligands on vascular endothelium.15 L-selectin is best known for its role in the initial interaction of lymphocytes with high endothelial venules (HEVs) of lymph nodes during the process of lymphocyte homing.15 Pertinent to airway inflammation, L-selectin-null mice that are sensitized to ovalbumin are protected from the development of AHR in allergic airway disease after ovalbumin challenge.16 In a sheep model of allergic asthma, airway administration of a L-selectin mAb blocked the LAR and subsequent AHR.4 A series of small molecular weight selectin inhibitors also showed therapeutic efficacy in this model.4 In clinical trials a single intravenous dose of one of these compounds (TBC 1269) was found to be ineffective,17 but a subsequent study using multiple doses given by aerosol indicated some protection against the LAR.18 These data complement the first evidence for the potential importance of L-selectin ligands in the disease provided by immunohistological evaluation of peribronchial biopsies from asthmatic patients.19 Using a panel of mAbs that react with carbohydrate-based epitopes pertinent to L-selectin ligands, Toppila and colleagues19 observed staining of capillaries and venules in inflamed submucosal regions (peribronchial regions) of bronchi. Reactivity was absent in specimens from normal individuals. The best characterized of these antibodies was MECA-79, a widely used reporter antibody for a major class of L-selectin ligands in HEVs and activated endothelium.15,20 MECA-79 recognizes a specific set of sialomucins carrying 6-sulfo sLex [ie, Sia2→3Galβ1→4(Fucα1→3)(SO3→6)GlcNAc] on extended core 1 (ie, GlcNAcβ1→3Galβ1→3GalNAc) O-glycans.15,21 The set of glycoproteins is usually referred to as peripheral node addressin or PNAd. MECA-79 staining has also been reported for HEV-like vessels within bronchus-associated lymphoid tissue of normal mice.22 Although bronchus-associated lymphoid tissue is present in mice and other rodents, it is not a feature of normal lung in humans.22,23
In this study, we tested whether interfering with L-selectin ligands had therapeutic efficacy in an inflammatory disease model. Using the sheep model of allergic asthma referred to above, we identified MECA-79+ vessels within lymphoid collections that were adjacent to bronchi and bronchioles. We demonstrate the presence in these vessels of a sulfotransferase (ie, HEC-GlcNAc6ST) that is involved in the formation of L-selectin ligands in secondary lymphoid organs. We show further that treatment of sheep with MECA-79 had a protective effect against allergen-induced LAR, AHR, and airway inflammation. Our findings represent the first example in which a class of L-selectin ligands has been targeted with therapeutic benefit in a chronic model of inflammation.
Materials and Methods
Immunohistochemistry
Paraffin blocks were prepared from the lungs of sheep that had been exposed to Ascaris suum every 2 weeks for 28 weeks and showed evidence of airway remodeling.6 Paraffin sections from four control sheep (1 to 2 years of age) (MaxArray sheep tissue array) were purchased from Zymed Laboratories (South San Francisco, CA). The paraffin sections were rehydrated in xylenes, followed by a graded series of ethanol and water. The slides were microwaved in Unmasking Solution (Vector Laboratories, Burlingame, CA) bringing the solution to boil four times throughout 30 minutes. Staining was performed with MECA-79 or rat IgM (BD Biosciences Pharmingen, San Diego, CA) at 3 μg/ml in Dulbecco’s phosphate-buffered saline (PBS) containing 5% goat serum. The sections were sequentially exposed to biotinylated mouse anti-rat kappa, streptavidin-horseradish peroxidase (Caltag Laboratories, Burlingame, CA), and the substrate NovaRed (Vector Laboratories). Finally, the sections were counterstained with Harris hematoxylin (Sigma-Aldrich, St. Louis, MO). To enumerate the extent of MECA-79+ airway segments in a range of allergic sheep, paraffin sections from six different allergic sheep from the same cohort described above, were stained with MECA-79 as above. The percentage of large airways (bronchioles and bronchi) with positive epithelial segments was computed relative to the total number of airways in the section. A minimum of 40 airways was counted for each sheep. For dual-immunofluorescent staining experiments, we harvested tissue from the lung of an euthanized allergic sheep similar to those used in the functional studies. Specimens were subjected to staining with MECA-79 and an affinity-purified, peptide-specific antibody to HEC-GlcNAc6ST as previously described.24 Briefly, 10-μm cryostat sections were prepared from O.C.T. (Tissue Tek; Sakura Finetek USA, Torrance, CA)-embedded tissue and allowed to dry for 1 hour. The sections were fixed in ice-cold acetone for 5 minutes followed by air-drying for 30 minutes. All staining steps were performed in blocking solution consisting of PBS with 3% bovine serum albumin and 5% normal mouse serum. Primary antibodies were incubated on slides for 1 hour at room temperature at the following concentrations: anti-murine HEC-GlcNAc6ST (0.5 μg/ml) and MECA-79 (1.0 μg/ml). Anti-HEC-GlcNAc6ST was detected by biotinylated goat anti-rabbit IgG (1.3 μg/ml; Jackson ImmunoResearch, West Grove, PA) followed by Cy2-conjugated streptavidin (1.8 μg/ml, Jackson ImmunoResearch). MECA-79 was detected by Cy3-conjugated goat anti-rat IgM (1.5 μg/ml, Jackson ImmunoResearch). Sections were counterstained with Harris hematoxylin and mounted with FluorSave (EMD Biosciences, San Diego, CA). As a specificity control in some experiments, the anti-HEC-GlcNAc6ST antibody was incubated with the peptide immunogen at 10 μg/ml.24 Archival lung specimens (paraffin sections) from two humans who died from acute asthmatic paroxysm (collected with Institutional Review Board approval by Dr. Walter Finkbeiner, Department of Pathology, University of California at San Francisco, San Francisco, CA) were also processed for MECA-79 immunocytochemistry as described above.
Airway Responses
Animal Preparation
A total of eight sheep (28 to 44 kg; mean, 35 ± 1.7 kg), which had previously been shown to develop EAR, LAR, and AHR in response to inhaled A. suum antigen were used. The sheep were conscious and were restrained in a modified shopping cart in the prone position with their heads immobilized. The animals remained in this state throughout the course of the day for as long as measurements were being obtained. For the airway function studies the sheep were instrumented under local anesthesia as previously described.25,26 To avoid discomfort, the cuff of the endotracheal tube was inflated only during measurements of pulmonary mechanics and during aerosol delivery of agents. The Mount Sinai Medical Center Animal Research Committee, responsible for assuring the humane care and use of experimental animals, approved the procedures used in this study.
Airway Mechanics
Breath by breath determination of mean pulmonary airflow resistance (RL) was measured with the esophageal balloon technique described extensively by us.25,26 The mean of at least five breaths, free of swallowing artifact, were used to obtain RL in cm H2O/L/second.
Aerosol Delivery Systems
All aerosols were generated using a disposable medical nebulizer (RaindropR; Nelcor-Puritan Bennett, Carlsbad, CA). The nebulizer was connected to a dosimeter system, consisting of a solenoid valve and a source of compressed air (20 psi). The output of the nebulizer was directed into a plastic T-piece, one end of which was connected to the inspiratory port of a piston respirator (Harvard Apparatus, Mills, MA). The solenoid valve was activated for 1 second at the beginning of the inspiratory cycle of the respirator. Aerosols were delivered at a tidal volume of 500 ml and a rate of 20 breaths per minute as previously described.25,26
Concentration Response Curves to Carbachol Aerosol
Airway responsiveness was determined from cumulative concentration response curves to carbachol inhaled as previously described. RL was measured immediately after inhalation of phosphate-buffered saline (PBS) and after each consecutive administration of 10 breaths of increasing concentrations of carbachol (0.25, 0.5, 1.0, 2.0, and 4.0% w/v PBS). The provocation test was discontinued when RL increased more than 400% from the value after PBS or after the highest carbachol concentration had been administered. The cumulative carbachol concentration [in breath units (BU)] that increased RL by 400% more than the value after PBS (PC400) was calculated by interpolation from the dose-response curve. One BU was defined as one breath of a 1% w/v carbachol aerosol solution.25,27
Antibody Administration
The MECA-79 and the TIB-146 hybridomas were obtained from the American Type Culture Collection (Manassas, VA). The antibodies were prepared by Ligocyte Pharmaceuticals (Bozeman, MT). IgM antibodies were purified and contained less than 4 EU/ml of endotoxin using a Sigma endotoxin kit. TIB-146, like MECA-79, is a rat IgM. It reacts with the mouse B-cell antigen B22028 but does not cross-react with sheep lymphocytes (unpublished observation); thus, it served as a class-matched control for MECA-79. To examine the effects of the MECA-79 and TIB-146 (control) antibodies on antigen-induced airway responses, we measured baseline airway responsiveness (ie, PC400) to carbachol 2 to 3 days before antigen challenge. Antibodies (MECA-79 or TIB-146 in PBS) were injected intravenously at both 24 hours and 0.5 hours before antigen challenge at 12.2 mg/kg for each dose. On the antigen challenge day, RL was measured and then the sheep were administered the last dose of the experimental antibody. RL was remeasured 30 minutes later and then animals were challenged with A. suum antigen. RL was remeasured immediately after, hourly from 1 to 6 hours after and half hourly from 6.5 to 8 hours after antigen challenge as previously described.3,4,25,26 After challenge determinations of airway responsiveness (PC400) were made 24, 48, and 72 hours after antigen challenge to assess the development and persistence of AHR. The responses to treatment with the active and control mAbs were compared to responses after allergen challenge when the sheep were given PBS.
Bronchoalveolar Lavage (BAL)
Lung lavage was performed by the infusion and aspiration of PBS (pH 7.4, 39°C) 1 day before antigen challenge (baseline) and then 8, 24, 48, and 72 hours after antigen challenge. For each time point, the lavages from three different subsegmental bronchi were pooled. The lavage fluid was strained through two layers of gauze to remove mucus. The total number of cells was counted in a hemacytometer from a sample of unconcentrated lavage fluid by phase contrast microscopy. The effluent was then centrifuged at 250 × g for 15 minutes at 4°C. The cell pellet was resuspended in PBS from which cytospin preparations were made. Differential counts were made from slides stained with May-Grunwald’s (metachromatic cells) and Giemsa.
Plasma Levels
An enzyme-linked immunosorbent assay was performed on plasma samples to determine the level of MECA-79 present at different time points after its administration. The test antigen consisted of human PNAd, the complex of glycoproteins isolated from human tonsils by immunoaffinity chromatography with MECA-79.29,30 PNAd diluted in PBS was coated onto Immulon 2HB microtiter plates (Thermo Labsystems, Franklin, MA) overnight at 4°C. After washing with PBS with 0.1% Tween 20, the plate was blocked with 3% bovine serum albumin in PBS for 2 hours at room temperature. Serial dilutions of sheep plasma diluted into block were then added for 1 hour at room temperature. The plates were treated sequentially with biotinylated mouse anti-rat kappa, streptavidin alkaline phosphatase (Caltag Laboratories) and the substrate PNPP (Pierce Chemical Co., Rockford, IL). The optical density was determined at 405 nm. The antibody levels were determined by comparing the values obtained for plasma with a standard curve generated from known amounts of MECA-79.
Statistical Analysis
Comparisons of allergen-induced airway responses with active and control antibodies were made with historical control runs performed on the same sheep in which physiological saline was injected instead of antibody.26 A paired Student’s t-test (two-tailed) was used to compare responses in the same sheep at each time point. Comparisons of the changes in airway responsiveness and BAL values between MECA-79 and control antibody-treated sheep were evaluated for statistical significance at each time point with a Student’s t-test (two-tailed). All values in the text and figures are reported as means ± SEM.
Results
Immunohistochemistry with MECA-79 mAb
Based on the finding that L-selectin antagonists prevent allergic bronchoconstriction in the sheep model,4 we performed immunochemistry on the lungs of allergic sheep to look for expression of MECA-79 reactivity (ie, PNAd) on vessels. Bronchioles with associated lymphoid aggregates frequently demonstrated MECA-79+ venules or capillaries in close proximity (Figure 1). Bronchioles that lacked aggregates were devoid of PNAd+ vessels. Lymphoid aggregates around bronchi also generally exhibited positive vessels. Stained vessels exhibited a range of endothelial thickness from flat to plump. Staining was absent when we substituted a rat IgM as a class-matched control for MECA-79 (not shown).
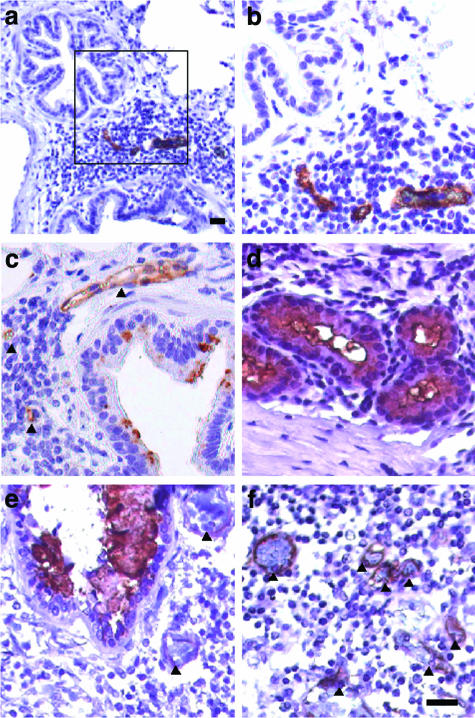
Figure 1.
MECA-79 staining of paraffin sections of allergic sheep lungs (a–d) and human asthmatic lung (e and f). a and b: Lymphoid aggregate between two bronchioles. Three MECA-79+ vessels are present. The box in a is shown at higher magnification in b. c: A bronchus and an associated lymphoid aggregate. The wall of the bronchus shows spotty MECA-79 reactivity that is associated with goblet cells. Two MECA-79+ vessels are present within a lymphoid aggregate and one positive vessel is immediately adjacent (indicated by arrowheads). d: Bronchial glands. Segments of the walls stain with MECA-79. Reactive lumenal contents are present. This field shows only serous glands. Mucous gland segments (not shown) also stain positively. e and f: Sections from the lung of a human asthmatic taken at autopsy. e: A bronchus with MECA-79+ lumenal contents. Bronchioles with positive contents were also seen. In e and f, positively stained blood vessels are indicated by arrowheads. Scale bars, 25 μm.
Unexpectedly, we observed MECA-79 staining of the epithelial lining of bronchioles, bronchi, and bronchial glands (Figures 1 and 2B). Staining was not present with a rat IgM control (not shown). The staining was variable with both positive and negative segments, in many cases within the same epithelium. In the case of bronchi, staining was observed over goblet cell granules. Consistent with the possibility that the MECA-79-reactive material represented a secretory product, lumenal contents of airways and glands stained positively. Airway staining was observed to a varying extent in six allergic sheep. In this set of animals, 31 ± 5% of the conducting airways (bronchi and bronchioles) demonstrated positive staining over all or part of their walls with a range of 13 to 45% for individual animals. In contrast, airway staining was not observed in any of the four control sheep (not shown). To determine the generality of our findings, two specimens of lung from human asthmatics were subjected to MECA-79 staining. Positive venules were present in parenchymal lymphoid aggregates (Figure 1). As in the sheep, segments of airway walls and lumenal contents were reactive with the antibody. Our observations in asthmatic sheep and humans represent the second report of MECA-79 reactivity in epithelial structures. Genbacev and colleagues31 described MECA-79 staining of the surface epithelium and glands of receptive uterine endometrium in humans in a pattern that was suggestive of a secretory product.
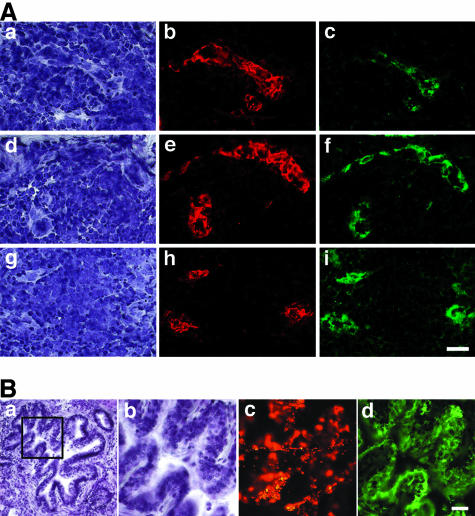
Figure 2.
Dual expression of PNAd and HEC-GlcNAc6ST in vessels of allergic sheep lung and in airway epithelium. The tissue was obtained from an allergic sheep. The animal had not been challenged with antigen for at least 4 weeks. Cryostat sections were double stained for immunofluorescence with MECA-79 (red) and anti-murine HEC-GlcNAc6ST (green). The sections were also counterstained with hematoxylin. A: Three separate regions containing infiltrates. a, d, and g: Bright-field images with hematoxylin staining. b, e, and h: The respective MECA-79 staining. c, f, and i: The respective staining for HEC-GlcNAc6ST. B: Bronchiole. A low magnification under bright-field optics (hematoxylin staining) is seen in a. b–d: The boxed region in a at higher magnification. Shown in b is the bright-field image (hematoxylin stain); in c MECA-79 staining (red); in d HEC-GlcNAc6ST staining (green). In d, it is apparent that HEC-GlcNAc6ST staining is concentrated in the apical regions of the airway epithelium. c demonstrates that some of MECA-79-reactive material is in the lumen of the airway, suggestive of a secretory product. Scale bars, 25 μm.
Expression of HEC-GlcNAc6ST
The MECA-79 epitope, as well as the L-selectin recognition determinant, critically depend on GlcNAc-6-sulfation.15,21 The major enzyme responsible for this modification in lymph node HEVs is HEC-GlcNAc6ST (also known as LSST, GST-3, and GlcNAc6ST-2), a sulfotransferase that is highly restricted to HEVs in lymphoid organs and induced HEV-like vessels at sites of chronic inflammation.24,32,33 Using a peptide-directed antibody against this enzyme,24,34 we looked for its expression in the lungs of the allergic sheep. As anticipated, the MECA-79+ vessels in lymphoid collections expressed HEC-GlcNAc6ST (Figure 2A), whereas MECA-79 unreactive vessels were negative for the enzyme. MECA-79+ segments of airway epithelium also expressed HEC-GlcNAc6ST (Figure 2B). The enzyme was present in the apical region of the epithelial cells in a pattern that was consistent with a Golgi localization. As specificity controls, we demonstrated that the inclusion of the soluble peptide immunogen prevented staining of both vessels and airway epithelium (Figure 3).
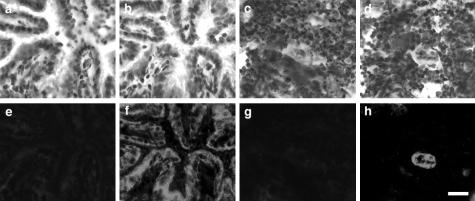
Figure 3.
Specificity of staining with anti-HEC-GlcNAc6ST antibody. a–d: Bright-field images with hematoxylin staining. a and b: Bronchioles. c and d: Lymphoid aggregates with a centrally placed HEV-like vessel. e and f: The respective fields stained for HEC-GlcNAc6ST. In e and g, soluble peptide immunogen was included with the antibody and it blocked staining of the bronchiole wall and the HEV-like vessel, respectively. Scale bar, 25 μm.
Effect of MECA-79 on Antigen-Induced Airway Responses, AHR, and BAL Leukocytes
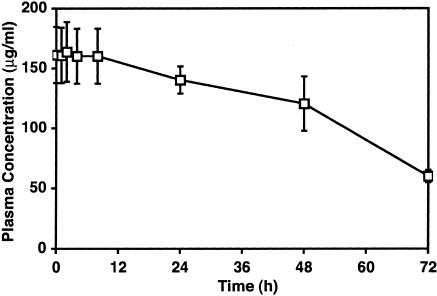
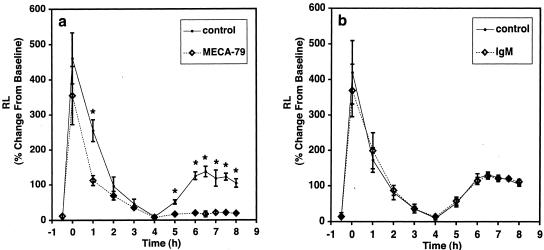
Intravenously administered MECA-79 inhibits short-term homing of lymphocytes to lymph nodes of mice.20 We chose a dose for the sheep studies that approximated the mg/kg range as used in mice.35 Initially, we treated one sheep with MECA-79 at 6 mg/kg, intravenously for 30 minutes before allergen challenge. At this dose there were no effects on EAR, LAR, and AHR (not shown). In a second sheep, we used the same experimental paradigm, but increased the dose to 12 mg/kg, intravenously. Here, we found that the LAR at 6 hours was 18% greater than the baseline value compared to a control value of 131% greater than baseline. Based on these results, we sought to maximize the effects of MECA-79 and therefore for the main study MECA-79 (12.2 mg/kg) was given both 24 hours and 30 minutes before antigen challenge to four sheep. We determined that the plasma level was 160 μg/ml at the time of antigen challenge and declined slowly throughout the next 72 hours to ∼50% of this concentration (Figure 4). Figure 5a shows the time course of the airway responses before and after antigen challenge in animals that were pretreated with MECA-79. The responses were compared to those obtained in control trials in which the same sheep had been injected with saline instead of antibody. In the control trial, RL increased to 461 ± 72% greater than the baseline value of 0.97 ± 0.01 cm H2O/L/second. RL returned to near baseline by 4 hours after challenge, but then began to increase again by 5 hours. The average LAR was 121% greater than baseline between 6 to 8 hours. When these same sheep were treated with MECA-79, there was no protection against the immediate bronchoconstrictor response. RL increased 355 ± 83% immediately after challenge from a baseline value of 0.97 ± 0.01 cm H2O/L/second. However, MECA-79 administration speeded the recovery of RL toward baseline. At 1 hour after antigen, RL was only 112 ± 14% greater than baseline compared to the control trial in which RL was 255 ± 31% greater than baseline. As in the control trial, RL returned to baseline values by 4 hours, but in contrast to the control trial the MECA-79-treated animals did not exhibit a LAR. From 6 to 8 hours, the average LAR was only 20% greater than baseline. Thus MECA-79, in addition to accelerating the recovery of the EAR, significantly inhibited the LAR. To verify that the protective effect of MECA-79 did not result from the nonspecific effects of an intravenous administration of an IgM protein, we repeated the challenge studies in four sheep in which a control rat IgM was used. The airway responses in the control IgM-treated sheep were indistinguishable from those in the control trial (Figure 5b). Neither MECA-79 nor the control antibody had an effect on baseline RL.
Figure 4.
Plasma levels of MECA-79 mAb in treated sheep. Sheep were injected twice with MECA-79 before A. suum challenge. At the indicated times after challenge, plasma samples were taken and analyzed for the concentration of mAb by enzyme-linked immunosorbent assay. The values shown are means ± SEM for four sheep.
Figure 5.
Effect of MECA-79 administration on antigen-induced early and late bronchial responses. MECA-79 (a) or control rat IgM (b) was administered 24 hours and 30 minutes before antigen challenge (T = 0). The changes in lung resistance (RL) were measured throughout time. Values shown are means ± SEM for four sheep in each group. The historical responses of the same four sheep (control) are shown when PBS was injected instead of antibodies. *, Differences relative to control with P ≤ 0.02 by paired t-test.
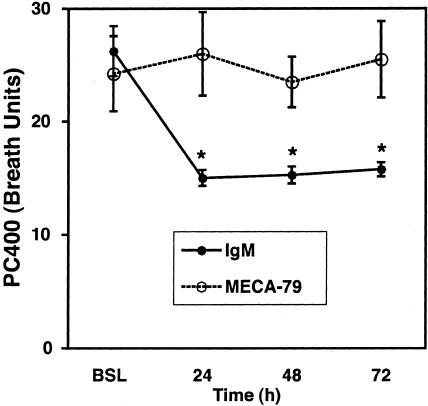
In the sheep model, AHR can last for up to 2 weeks after a single antigen challenge.3 Figure 6 illustrates the effects of MECA-79 and the control IgM antibody on postantigen-induced AHR. As expected with the control antibody, AHR was evident 24 to 72 hours after antigen challenge: PC400 fell from a baseline value of 26 ± 2 breath units to 15 ± 1, 15 ± 1, and 16 ± 1 breath units at 24, 48, and 72 hours after challenge, respectively. In contrast, the PC400 of the sheep treated with MECA-79 remained at the prechallenge levels from 24 through 72 hours: prechallenge PC400: 24 ± 3 breath units; respective PC400 at 24, 48, and 72 hours were 24 ± 3, 26 ± 4, and 26 ± 3 breath units.
Figure 6.
Effect of MECA-79 administration on AHR at the various times after airway antigen challenge. Sheep receiving control antibody showed a decrease in PC400 after antigen challenge, when compared to baseline (BSL) indicative of the development of AHR. There was no change in the PC400 in the MECA-79-treated sheep indicating that antigen-induced AHR was blocked. Means ± SEM for four control IgM-injected and four MECA-79-injected sheep are shown. *, Differences between IgM- and MECA-79-treated sheep where P ≤ 0.03 by an unpaired two-tailed t-test.
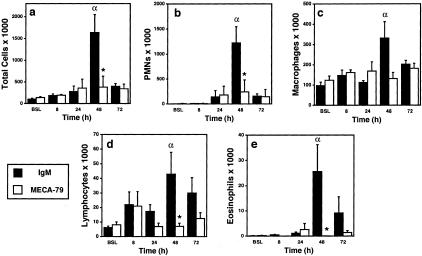
To gain information on leukocyte infiltration into airways, we obtained BAL fluid from the two groups of antibody-treated sheep. Consistent with previous studies performed in this model,3,4 allergen challenge in the IgM-treated sheep led to a time-dependent accumulation of leukocytes in BAL fluid. By 48 hours, there was a significant increase in total leukocytes and in each of the major of total leukocyte classes (PMNs, macrophages, lymphocytes, and eosinophils) relative to the baseline value (Figure 7). As is characteristic of this model, PMNs were the predominant leukocyte found in BAL fluid. Treatment of the sheep with MECA-79 significantly suppressed the total number of leukocytes at 48 hours (76% inhibition) with clear effects on each class, ranging from 60% (macrophages) to 99% (eosinophils).
Figure 7.
Effect of MECA-79 on the accumulation of different leukocytes in BAL fluid. a, total cells; b, PMNs; c, macrophages; d, lymphocytes; e, eosinophils. BAL was performed at the indicated times after antigen challenge on sheep that were preinjected with either MECA-79 or control rat IgM. Leukocytes were enumerated in the BAL fluid. Values indicate means ± SEM for four sheep. α denotes significant increases (P ≤ 0.05 by an unpaired t-test) in the number of BAL leukocytes collected from control antibody-treated sheep at the indicated time relative to the baseline values (BSL). *, Significant differences (P ≤ 0.05 by an unpaired two-tailed t-test) between MECA-79- and IgM-treated sheep.
Discussion
The process of leukocyte recruitment from the blood into tissues occurs in a series of steps involving highly regulated adhesive and signaling interactions between the leukocyte and the blood vessel wall.36,37 For allergic diseases of the airways as well as other inflammatory diseases, there has been considerable interest in defining the molecular underpinnings of these various steps, with a major impetus being the development of new therapeutic interventions. In addition to the involvement of selectins in the earliest stages of airway inflammation (reviewed in the Introduction), other studies have implicated immunoglobulin-type endothelial ligands and their integrin receptors on leukocytes in later stages of the recruitment cascade. In particular, studies with null mice and adhesion-blocking reagents point to the importance of ICAM-1 and the α4β1/VCAM-1 system in the recruitment of eosinophils and T cells to airways after allergen challenge.38 In fact, monoclonal antibody inhibition of α4 integrins and small molecule inhibitors of α4 are highly efficacious at blocking both LAR and AHR in the sheep model used here.3,25 Clinical trials with antagonists of the α4β1/VCAM-1 pathway are currently being performed in asthmatic patients.38
Prompted by the finding that a L-selectin mAbs, as well as low-molecular weight selectin antagonists, are therapeutic in the sheep model of allergic bronchoconstriction,4 we used the MECA-79 mAb for immunohistochemistry and physiological studies in this model. Previous studies have demonstrated that this antibody is active in in vitro adhesion assays and in vivo homing studies.20,22,35 It directly competes with L-selectin by binding to an overlapping determinant on endothelial ligands.21,29,39 Our histological studies demonstrated the presence of MECA-79+ vessels in the lungs of allergic sheep. The vessels occurred within lymphoid collections that were closely associated with bronchioles and bronchi. Our findings with MECA-79 parallel the staining results of Topilla and colleagues19 in peribronchial biopsies of human asthmatics. This similarity could make our observations in this preclinical model relevant to human disease.
The pathophysiological significance of the MECA-79 staining in allergic sheep lungs was evaluated by performing antigen provocation studies. We devised an intravenous injection protocol for MECA-79 that maintained high levels of antibody in the plasma throughout a 72-hour trial period after allergen. MECA-79 pretreatment produced a dramatic protective effect on LAR and AHR. The protection against LAR and AHR seen with MECA-79 was specific for this antibody and not because of nonspecific effects of antibody administration because there was no such protection in the IgM-treated animals. Previous animal studies with MECA-79 have demonstrated inhibitory effects on the short-term accumulation of intravenously injected lymphocytes within lymph nodes and bronchus-associated lymphoid tissue of wild-type mice, as well as in inflammatory foci.20,22,40,41 However, before the present results, therapeutic effects of this antibody have not been reported in a model of inflammation.
Interestingly, we found that the fall-off phase of EAR was accelerated in the presence of MECA-79. A similar affect on EAR was observed with L-selectin mAb treatment.4 EAR depends on the allergen-triggered release of mediators from mast cells and basophils through IgE and other pathways.2 Blood-borne basophils express L-selectin,42 and it is conceivable that MECA-79 inhibits an early phase of their recruitment into lungs of asthmatic sheep.
To examine the effect of MECA-79 on leukocyte recruitment into the lungs, we determined the levels of recoverable cells in BAL fluid. This measurement reflects leukocyte extravasation across lung vasculature but also depends on migration of the cells through the lung parenchyma and across the airway walls.12 In the sheep model the maximum cellular response in the BAL occurs between 24 to 48 hours after allergen provocation with PMNs being the predominant class of leukocyte showing the largest change.3,4 MECA-79 significantly inhibited the BAL levels of PMNs, but in addition had significant effects on lymphocytes and eosinophils as well. These effects were most apparent 48 hours after challenge. In view of the MECA-79 staining of vessels, we strongly suspect that a major component of this inhibitory effect was exerted at the level of leukocyte-endothelial interactions. However, we cannot exclude the possibility that the inhibitory effects on some populations were indirect; for example, by inhibiting the entry of leukocyte subsets that in turn orchestrate the entry of further leukocytes through L-selectin-independent pathways. The potent activity of MECA-79 in blocking the BAL accumulation of all leukocyte classes contrasts with the effects of an α4 integrin mAb in the same model.3 Although the α4 mAb reduced LAR and AHR to a comparable extent as MECA-79, its only notable effect on BAL leukocytes was to diminish eosinophil accumulation throughout the 48-hour period after challenge.3 However, effects on multiple cell classes were observed using an α1β1-specific antibody, which also blocked LAR and AHR.26 Thus, our present findings provide a novel mechanism for inhibiting both the global inflammatory response and the airway dysfunction that follows allergen challenge.
HEC-GlcNAc6ST was discovered as a GlcNAc-6-O-sulfotransferase that is highly localized to HEVs of mouse lymph nodes.32,33 In reconstitution studies, this enzyme together with a set of glycosyltransferases confer L-selectin ligand activity to mucin-like glycoprotein acceptors.43 This enzyme can also contribute to the reconstitution of the MECA-79 epitope in transfected cells.21 Mice that are null for HEC-GlcNAc6ST exhibit impaired ligand function in lymph node HEVs and greatly reduced MECA-79 staining, confirming an essential role for this enzyme.44 A second HEV-localized sulfotransferase, known as GlcNAc6ST-1, is likely to be responsible for the HEC-GlcNAc6ST-independent MECA-79 staining.45,46 The contributions of HEC-GlcNAc6ST to selectin ligand elaboration extend beyond HEVs in secondary lymphoid organs. Thus, the enzyme has been detected at the transcript level and the protein level in MECA-79+-positive vessels at sites of inflammation and within ectopic lymphoid aggregates.14,24,34,39 Utilization of the HEC-GlcNAc6ST-null mice has confirmed the contribution of the enzyme to MECA-79 staining in the vessels of neolymphoid aggregates.24 In the present study, we have extended the correlation to the lungs of allergic sheep by showing co-staining of vessels for the enzyme and MECA-79.
An unanticipated finding of the present study was the MECA-79 reactivity of a subset of airways and glands in allergic sheep. A similar pattern was found in the lungs of human asthmatic patients. The stained material was found in the walls of these structures and frequently in the lumen, suggestive of a secretory product. We also detected the presence of HEC-GlcNAc6ST in the epithelial segments that showed associated MECA-79 staining. Because the known carriers of the MECA-79 epitope are all sialomucins,30 the reactive material in airways and glands is probably mucin in nature as well. A previous structural analysis of respiratory mucins from cystic fibrosis patients revealed 6-sulfo sLex on extended core 1 O-glycans,47 which is predictive of MECA-79 reactivity and L-selectin ligand activity.21 These structures may account for the L-selectin ligand activity demonstrated for a subset of human bronchial mucins.48 Mucins are the primary components of the airway mucus gel and account for its principle rheological properties.49,50 Overproduction of mucus with altered properties is a hallmark and a major contributing factor to the obstruction of airways in several chronic diseases (asthma, cystic fibrosis, and chronic obstructive pulmonary disease).49,50 Furthermore, mucins in the respiratory tract and elsewhere are known to provide carbohydrate-based docking sites for microbial pathogens, which may either promote or impede infection.50 Notably, Pseudomonas aeruginosa, a major airway pathogen in cystic fibrosis patients, possesses receptors for sLex and 6-sulfo sLex.51 Moreover, certain strains of influenza viruses exhibit very high-affinity binding to 6-sulfo sLex and related structures.52 Thus, the MECA-79+ mucins revealed in the present study may provide binding substrates for certain bacteria and viruses. Also deserving attention is the possibility that these airway-associated mucins could interact with extravasated leukocytes via L-selectin and mediate adhesive interactions and/or trigger signaling responses.53 The absence of airway staining in normal sheep together with the partial and variable presence of MECA-79+ secretory products in asthmatic sheep strongly suggest that the expression of these substances is linked to the release of inflammatory mediators likely resulting from the recurrent allergen exposure.6 The correlation that we observed between HEC-GlcNAc6ST in airway epithelial cells and the MECA-79-reactive material indicates that regulation may be exerted through controlling the level of this sulfotransferase. In this regard, it is noteworthy that in vitro treatment of human bronchial mucosal explants with tumor necrosis factor-α, a proinflammatory cytokine, increases the level of GlcNAc-6-O-sulfotransferase activity.54
The primary finding of the present study is that MECA-79 blunts LAR and AHR in a large animal model of asthma. Numerous studies have reported MECA-79 staining of blood vessels in inflammatory lesions from mice to human55,56 including the aforementioned staining in peribronchial biopsies from human asthmatics. Heretofore, the functional significance of such staining has been validated in only two animal models (hyperplastic thymus in AKR mice and inflamed lacrimal glands in NOD mice) by showing that intravenous administration of MECA-79 substantially inhibits the short-term accumulation of injected lymphocytes to the inflamed tissue, as does an antibody to L-selectin.40,41 A recent study establishes that the L-selectin/PNAd system accounts for a major component of lymphocyte homing to bronchus-associated lymphoid tissue in mice.22 The present report also demonstrates effects of MECA-79 on leukocyte recruitment as revealed by the BAL fluid counts but goes beyond previous studies by showing therapeutic effects on clinically relevant airway responses. Future efforts are needed to determine the relevance of these findings to human asthma and to other inflammatory settings where conspicuous MECA-79 staining of blood vessels has been noted.
Acknowledgments
We thank Dr. Walter Finkbeiner, Department of Pathology, University of California, San Francisco, for providing autopsy samples from lungs of human asthmatics and for his assistance in evaluating immunostaining patterns; and Dr. Ted Yednock for helpful advice. Tonsils were provided by the Cooperative Human Tissue Network funded by the NCI.
Footnotes
Address reprint requests to Steven Rosen, Department of Anatomy, University of California, San Francisco, CA 94143-0452. E-mail: sdr@itsa.ucsf.edu.
Supported by the National Institutes of Health (R37GM23547 and R01GM57411 to S.D.R.) and a Sandler Center for Basic Research Grant in Asthma (to S.D.R.).
References
- Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- Abraham WM, Sielczak MW, Ahmed A, Cortes A, Lauredo IT, Kim J, Pepinsky B, Benjamin CD, Leone DR, Lobb RR, Weller PF. Alpha 4-integrins mediate antigen-induced late bronchial responses and prolonged airway hyperresponsiveness in sheep. J Clin Invest. 1994;93:776–787. doi: 10.1172/JCI117032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WM, Ahmed A, Sabater JR, Lauredo IT, Botvinnikova Y, Bjercke RJ, Hu X, Revelle BM, Kogan TP, Scott IL, Dixon RA, Yeh ET, Beck PJ. Selectin blockade prevents antigen-induced late bronchial responses and airway hyperresponsiveness in allergic sheep. Am J Respir Crit Care Med. 1999;159:1205–1214. doi: 10.1164/ajrccm.159.4.9806002. [DOI] [PubMed] [Google Scholar]
- Bentley AM, Kay AB, Durham SR. Human late asthmatic reactions. Clin Exp Allergy. 1997;27(Suppl 1):71–86. doi: 10.1111/j.1365-2222.1997.tb01830.x. [DOI] [PubMed] [Google Scholar]
- Abraham WM, Ahmed A, Lauredo IT, Botvinnikova Y, Sielczak MW. Chronic allergen challenge causes airway remodeling in sheep. Am J Respir Crit Care Med. 2001;163:A415. [Google Scholar]
- Lukacs NW. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol. 2001;1:108–116. doi: 10.1038/35100503. [DOI] [PubMed] [Google Scholar]
- Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- Gundel RH, Wegner CD, Torcellini CA, Clarke CC, Haynes N, Rothlein R, Smith CW, Letts LG. Endothelial leukocyte adhesion molecule-1 mediates antigen-induced acute airway inflammation and late-phase airway obstruction in monkeys. J Clin Invest. 1991;88:1407–1411. doi: 10.1172/JCI115447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sanctis GT, Wolyniec WW, Green FH, Qin S, Jiao A, Finn PW, Noonan T, Joetham AA, Gelfand E, Doerschuk CM, Drazen JM. Reduction of allergic airway responses in P-selectin-deficient mice. J Appl Physiol. 1997;83:681–687. doi: 10.1152/jappl.1997.83.3.681. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, John A, Berlin A, Bullard DC, Knibbs R, Stoolman LM. E- and P-selectins are essential for the development of cockroach allergen-induced airway responses. J Immunol. 2002;169:2120–2125. doi: 10.4049/jimmunol.169.4.2120. [DOI] [PubMed] [Google Scholar]
- Gosset P, Tillie-Leblond I, Janin A, Marquette CH, Copin MC, Wallaert B, Tonnel AB. Expression of E-selectin, ICAM-1 and VCAM-1 on bronchial biopsies from allergic and non-allergic asthmatic patients. Int Arch Allergy Immunol. 1995;106:69–77. doi: 10.1159/000236892. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hashimoto S, Imai K, Amemiya E, Yamaguchi M, Yachi A, Horie T. Elevation of serum soluble intercellular adhesion molecule-1 (sICAM-1) and sE-selectin levels in bronchial asthma. Clin Exp Immunol. 1994;96:110–115. doi: 10.1111/j.1365-2249.1994.tb06239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- Fiscus LC, Van Herpen J, Steeber DA, Tedder TF, Tang ML. L-Selectin is required for the development of airway hyperresponsiveness but not airway inflammation in a murine model of asthma. J Allergy Clin Immunol. 2001;107:1019–1024. doi: 10.1067/mai.2001.114703. [DOI] [PubMed] [Google Scholar]
- Avila PC, Boushey HA, Wong H, Grundland H, Liu J, Fahy JV. Effect of a single dose of the selectin inhibitor TBC1269 on early and late asthmatic responses. Clin Exp Allergy. 2004;34:77–84. doi: 10.1111/j.1365-2222.2004.01831.x. [DOI] [PubMed] [Google Scholar]
- Beeh KM, Beier J, Buhl R, Zahten R, Wolff G. Influence of inhaled bimosiamose (TBC 1269), a synthetic pan-selectin antagonist, on the allergen-induced late asthmatic response (LAR) in patients with mild allergic asthma. Am J Respir Crit Care Med. 2004:169. (Abstr) [Google Scholar]
- Toppila S, Paavonen T, Laitinen A, Laitinen LA, Renkonen R. Endothelial sulfated sialyl Lewis x glycans, putative L-selectin ligands, are preferentially expressed in bronchial asthma but not in other chronic inflammatory lung diseases. Am J Respir Cell Mol Biol. 2000;23:492–498. doi: 10.1165/ajrcmb.23.4.4113. [DOI] [PubMed] [Google Scholar]
- Streeter PR, Rouse BTN, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JC, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, Rabuka D, Hindsgaul O, Marth JD, Lowe JB, Fukuda M. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension beta 1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- Xu B, Wagner N, Pham LN, Magno V, Shan Z, Butcher EC, Michie SA. Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by L-selectin/PNAd, alpha4beta1 integrin/VCAM-1, and LFA-1 adhesion pathways. J Exp Med. 2003;197:1255–1267. doi: 10.1084/jem.20010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst R. Is BALT a major component of the human lung immune system? Immunol Today. 1992;13:119–122. doi: 10.1016/0167-5699(92)90106-H. [DOI] [PubMed] [Google Scholar]
- Bistrup A, Tsay D, Shenoy P, Singer MS, Bangia N, Luther SA, Cyster JG, Ruddle NH, Rosen SD. Detection of a sulfotransferase (HEC-GlcNAc6ST) in high endothelial venules of lymph nodes and in HEV-like vessels within ectopic lymphoid aggregates: relationship to the MECA-79 epitope. Am J Pathol. 2004;164:1635–1644. doi: 10.1016/S0002-9440(10)63722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WM, Gill A, Ahmed A, Sielczak MW, Lauredo IT, Botinnikova Y, Lin KC, Pepinsky B, Leone DR, Lobb RR, Adams SP. A small-molecule, tight-binding inhibitor of the integrin alpha(4)beta(1) blocks antigen-induced airway responses and inflammation in experimental asthma in sheep. Am J Respir Crit Care Med. 2000;162:603–611. doi: 10.1164/ajrccm.162.2.9911061. [DOI] [PubMed] [Google Scholar]
- Abraham WM, Ahmed A, Serebriakov I, Carmillo AN, Ferrant J, de Fougerolles AR, Garber EA, Gotwals PJ, Koteliansky VE, Taylor F, Lobb RR. A monoclonal antibody to alpha1beta1 blocks antigen-induced airway responses in sheep. Am J Respir Crit Care Med. 2004;169:97–104. doi: 10.1164/rccm.200304-543OC. [DOI] [PubMed] [Google Scholar]
- Leckie MJ, ten Brinke A, Khan J, Diamant Z, O’Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, Hansel TT, Holgate ST, Sterk PJ, Barnes PJ. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- Salmi M, Hellman J, Jalkanen S. The role of two distinct endothelial molecules, vascular adhesion protein-1 and peripheral lymph node addressin, in the binding of lymphocyte subsets to human lymph nodes. J Immunol. 1998;160:5629–5636. [PubMed] [Google Scholar]
- Berg EL, Robinson MK, Warnock RA, Butcher EC. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri KD, Finger EB, Gaudernack G, Springer TA. Sialomucin CD34 is the major L-selectin ligand in human tonsil high endothelial venules. J Cell Biol. 1995;131:261–270. doi: 10.1083/jcb.131.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang ZQ, Kiessling LL, Rosen SD, Fisher SJ. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- Bistrup A, Bhakta S, Lee JK, Belov YC, Gunn MD, Zuo F-R, Huang C-C, Kannagi R, Rosen SD, Hemmerich S. Sulfotransferases of two specificities function in the reconstitution of high-endothelial-cell ligands for L-Selectin. J Cell Biol. 1999;145:899–910. doi: 10.1083/jcb.145.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka N, Petryniak B, Nakayama J, Tsuboi S, Suzuki M, Yeh J-C, Izawa D, Tanaka T, Miyasaka M, Lowe JB, Fukuda M. A novel, high endothelial venule-specific sulfotransferase expresses 6-sulfo sialyl Lewis x, an L-selectin ligand displayed by CD34. Immunity. 1999;11:79–89. doi: 10.1016/s1074-7613(00)80083-7. [DOI] [PubMed] [Google Scholar]
- Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197:1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zante A, Gauguet JM, Bistrup A, Tsay D, van Andrian UH, Rosen SD. Lymphocyte-HEV interactions in lymph nodes of a sulfotransferase-deficient mouse. J Exp Med. 2003;198:1289–1300. doi: 10.1084/jem.20030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Bochner BS. Road signs guiding leukocytes along the inflammation superhighway. J Allergy Clin Immunol. 2000;106:817–828. doi: 10.1067/mai.2000.110813. [DOI] [PubMed] [Google Scholar]
- Hiraoka N, Kawashima H, Petryniak B, Nakayama J, Mitoma J, Marth JD, Lowe JB, Fukuda M. Core 2 branching beta1,6-N-acetylglucosaminyltransferase and high endothelial venule-restricted sulfotransferase collaboratively control lymphocyte homing. J Biol Chem. 2004;279:3058–3067. doi: 10.1074/jbc.M311150200. [DOI] [PubMed] [Google Scholar]
- Michie SA, Streeter PR, Butcher EC, Rouse RV. L-selectin and alpha 4 beta 7 integrin homing receptor pathways mediate peripheral lymphocyte traffic to AKR mouse hyperplastic thymus. Am J Pathol. 1995;147:412–421. [PMC free article] [PubMed] [Google Scholar]
- Mikulowska-Mennis A, Xu B, Berberian JM, Michie SA. Lymphocyte migration to inflamed lacrimal glands is mediated by vascular cell adhesion molecule-1/alpha(4)beta(1) integrin, peripheral node addressin/L-selectin, and lymphocyte function-associated antigen-1 adhesion pathways. Am J Pathol. 2001;159:671–681. doi: 10.1016/s0002-9440(10)61738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BS, Schleimer RP. Mast cells, basophils, and eosinophils: distinct but overlapping pathways for recruitment. Immunol Rev. 2001;179:5–15. doi: 10.1034/j.1600-065x.2001.790101.x. [DOI] [PubMed] [Google Scholar]
- Tangemann K, Bistrup A, Hemmerich S, Rosen SD. Sulfation of an HEV-expressed ligand for L-selectin: effects on tethering and rolling of lymphocytes. J Exp Med. 1999;190:935–941. doi: 10.1084/jem.190.7.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerich S, Bistrup A, Singer MS, Zante AV, Lee JK, Tsay D, Peters M, Carminati JL, Brennan TJ, Carver-Moore K, Leviten M, Fuentes ME, Ruddle NH, Rosen SD. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity. 2001;15:237–247. doi: 10.1016/s1074-7613(01)00188-1. [DOI] [PubMed] [Google Scholar]
- Uchimura K, Muramatsu H, Kadomatsu K, Fan QW, Kurosawa N, Mitsuoka C, Kannagi R, Habuchi O, Muramatsu T. Molecular cloning and characterization of an N-acetylglucosamine-6-O-sulfotransferase. J Biol Chem. 1998;273:22577–22583. doi: 10.1074/jbc.273.35.22577. [DOI] [PubMed] [Google Scholar]
- Uchimura K, Kadomatsu K, El-Fasakhany FM, Singer MS, Izawa M, Kannagi R, Takeda N, Rosen SD, Muramatsu T. N-acetylglucosamine 6-O-sulfotransferase-1 regulates lymphocyte homing and expression of abluminal L-selectin ligands in high endothelial venules of lymph nodes. J Biol Chem. 2004;279:35001–35008. doi: 10.1074/jbc.M404456200. [DOI] [PubMed] [Google Scholar]
- Lo-Guidice JM, Wieruszeski JM, Lemoine J, Verbert A, Roussel P, Lamblin G. Sialylation and sulfation of the carbohydrate chains in respiratory mucins from a patient with cystic fibrosis. J Biol Chem. 1994;269:18794–18813. [PubMed] [Google Scholar]
- Crottet P, Kim YJ, Varki A. Subsets of sialylated, sulfated mucins of diverse origins are recognized by L-selectin. Lack of evidence for unique oligosaccharide sequences mediating binding. Glycobiology. 1996;6:191–208. doi: 10.1093/glycob/6.2.191. [DOI] [PubMed] [Google Scholar]
- Rose MC, Nickola TJ, Voynow JA. Airway mucus obstruction: mucin glycoproteins, MUC gene regulation and goblet cell hyperplasia. Am J Respir Cell Mol Biol. 2001;25:533–537. doi: 10.1165/ajrcmb.25.5.f218. [DOI] [PubMed] [Google Scholar]
- Thornton D, Sheehan J. From mucins to mucus. Proc Am Thorac Soc. 2004;1:54–61. doi: 10.1513/pats.2306016. [DOI] [PubMed] [Google Scholar]
- Scharfman A, Delmotte P, Beau J, Lamblin G, Roussel P, Mazurier J. Sialyl-Le(x) and sulfo-sialyl-Le(x) determinants are receptors for P. aeruginosa. Glycoconj J. 2000;17:735–740. doi: 10.1023/a:1011091112884. [DOI] [PubMed] [Google Scholar]
- Gambaryan AS, Tuzikov AB, Pazynina GV, Webster RG, Matrosovich MN, Bovin NV. H5N1 chicken influenza viruses display a high binding affinity for Neu5Acalpha2–3Galbeta1-4(6-HSO(3))GlcNAc-containing receptors. Virology. 2004;326:310–316. doi: 10.1016/j.virol.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Ding Z, Issekutz TB, Downey GP, Waddell TK. L-selectin stimulation enhances functional expression of surface CXCR4 in lymphocytes: implications for cellular activation during adhesion and migration. Blood. 2003;101:4245–4252. doi: 10.1182/blood-2002-06-1782. [DOI] [PubMed] [Google Scholar]
- Delmotte P, Degroote S, Lafitte JJ, Lamblin G, Perini JM, Roussel P. Tumor necrosis factor α increases the expression of glycosyltransferases and sulfotransferases responsible for the biosynthesis of sialylated and/or sulfated Lewis x epitopes in the human bronchial mucosa. J Biol Chem. 2001;277:424–431. doi: 10.1074/jbc.M109958200. [DOI] [PubMed] [Google Scholar]
- Rosen SD. Endothelial ligands for L-selectin: from lymphocyte recirculation to allograft rejection. Am J Pathol. 1999;155:1013–1020. doi: 10.1016/S0002-9440(10)65201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkonen J, Tynninen O, Hayry P, Paavonen T, Renkonen R. Glycosylation might provide endothelial zip codes for organ-specific leukocyte traffic into inflammatory sites. Am J Pathol. 2002;161:543–550. doi: 10.1016/S0002-9440(10)64210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]