Abstract
Autoimmune pancreatitis (AIP), a recently defined disease of unknown etiology, is characterized by inflammatory infiltrates in the pancreas with conspicuous involvement of the ducts. The disease clinically manifests in humans as epigastric pain, weight loss, and jaundice. This report describes the development of a novel animal model of this disease in the rat, which we have termed experimental autoimmune pancreatitis. Adoptive transfer of amylase-specific CD4+ T cells was able to confer pancreatitis to naïve syngeneic recipient animals. No treatments before the adoptive transfer of T cells were necessary for disease to ensue, and the severity of disease was proportional to the number of T cells administered. The pancreatic lesions of rats with experimental autoimmune pancreatitis were characterized histologically as overwhelmingly lymphocytic with occasional plasma cells, neutrophils, and mast cells. Acinar tissue destruction and ductular inflammation were common features, with less frequent involvement of larger ducts. Immunohistochemical analysis revealed the presence of CD4+ T cells in large numbers as well as CD8+ T cells, macrophages, and dendritic cells. Expression of MHC I and MHC II also increased at the site of the lesion. Clinically, the disease manifested as either failure to gain weight at a rate concomitant with control animals or as outright weight loss. Thus, administration of activated CD4+ T cells specific for the pancreatic enzyme amylase can induce pancreatitis in the rat in a manner that is reminiscent of human AIP.
Autoimmune pancreatitis (AIP) is a rare, recently defined clinical condition.1,2 Disease entities such as idiopathic chronic pancreatitis, idiopathic duct-centric chronic pancreatitis, sclerosing pancreatitis, lymphoplasmacytic sclerosing pancreatitis, or a certain subset of tumefactive chronic pancreatitis are now thought to belong to this more recently identified disease entity.2 Subsuming these entities under the term AIP is because of, in large part, histological and immunohistological findings that strongly suggest an autoimmune mechanism.3 Histologically, lesions are characterized by lymphocytic and plasmacytic infiltrates in the pancreas with the conspicuous involvement of ducts and variable degrees of destruction of the parenchyma.2,3 The inflammation often leads to edema, narrowing of the duct lumen, thickening of the duct wall, and parenchymal scarring. Immunophenotypic analysis of these lesions reveals that the infiltrating lymphocytes are predominantly CD4+ T cells, although CD8+ T cells and B cells can also be observed. Recent literature has also demonstrated that high levels of IgG4 are associated with and may be involved in the pathogenesis of the disease.4
The clinical symptoms associated with this disease can be vague, but often include jaundice, slight discomfort in the epigastrum or back, and weight loss. Jaundice is associated with inflammation and narrowing of the distal common bile duct. Gross examination will reveal the pancreas to be firm or hard and possibly enlarged. Diagnostic evaluation of such patients will often lead to surgical resection of the pancreas because of a presumed diagnosis of carcinoma.5 Laboratory findings will often show increased levels of pancreatic enzymes and hypergammaglobulinemia. Autoantibodies, such as anti-nuclear antibodies or antibodies directed against pancreatic enzymes such as carbonic anhydrase II (CA-II) or lactoferrin (LF), can also often be detected, further suggesting an autoimmune mechanism.6
The rat has long served as a valuable model for the study of autoimmunity. Experimentally induced autoimmune diseases, such as experimental autoimmune encephalomyelitis, can be induced in rats by active immunization with proteins or peptides emulsified in adjuvants, or by adoptive transfer of activated T cells specific for autoantigenic determinants. The resulting autoimmune disease often recapitulates many important aspects of the human disease that it is modeling. In this regard, the animal model can be used to study specific aspects of the pathogenic mechanism of the human disease, and might further be used to evaluate potential therapies.
Several models of pancreatitis in the rat currently exist, but rely on chemically or surgically induced pancreatic injury.7 None are induced in otherwise unmanipulated animals by a purely immunological challenge. Thus, in these models the resulting inflammation cannot be termed “autoimmune” because the inflammation is not specifically targeting an autoantigenic epitope. This report documents the development of a model of AIP in normal rats. CD4+ T cell lines that specifically recognize the pancreatic enzyme amylase were generated. Adoptive transfer of activated anti-amylase T cells resulted in pancreatitis that was typified by mononuclear cell infiltrates and destruction of lobular tissue. The disease model is not restricted to a single strain of rat because both DA(RP) and Lewis rats were susceptible. Clinically, the disease manifests as either failure to gain weight at a rate concordant with control animals, or as outright weight loss. Thus, the adoptive transfer of activated T cells specific for amylase is sufficient for induction of AIP in the rat.
Materials and Methods
Antigens
α-Amylase (lot no. 121K7657, catalog no. A-6255) was obtained from Sigma Chemical Co. (St. Louis, MO). It was derived from a porcine source and contained a dominant band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Carbonic anhydrase II (lot no. 013K9299, catalog no. C-2522) was derived from bovine erythrocytes and was purchased from Sigma Chemical Co. Lactoferrin (LF) (lot no. 011K7405, catalog no. L4765) was derived from bovine colostrum and was purchased from Sigma Chemical Co.
Rats
Two rat strains were used during the course of these studies. Mature female Lewis rats, MHC type RT1l, were purchased from Charles River Laboratories (Wilmington, MA). DA(RP) rats8 are maintained in the Borwell Animal Resource Center of Dartmouth Medical School according to institutional guidelines. These rats had been previously shown to be more susceptible than Lewis rats to autoimmune insulitis, suggesting that they might have a more permissive genetic composition for the development of inflammatory conditions of the pancreas. Spontaneous pancreatitis is not seen in the pancreata of rats from either of these strains (unpublished observations).
Creation of Autoreactive T Cell Lines
In brief, rats were injected in the foot pad and at one site on each lateral thigh with 0.1 ml of an antigen-adjuvant emulsion. The total amount of peptide injected per site was 50 to 100 μg. Nine days thereafter, the rat was killed and the draining popliteal and inguinal lymph nodes were removed for tissue culture. The nodes were disrupted to obtain a single cell suspension that was washed repeatedly with Dulbecco’s modified phosphate-buffered saline. Cells were then cultured for 72 hours in a RPMI 1640-based medium containing 5% NCTC-109, glutamine, antibiotics, 2-mercaptoethanol, and 50 μg/ml of the specific antigen. For the first 72 hours of culture this medium contained 1% syngeneic rat serum. After 72 hours and thereafter, the medium contained 10% fetal bovine serum, and as an interleukin-2 source, 5% supernatant of splenocytes cultured in a similar medium with 5 μg/ml concanavalin A. Every 7 to 10 days the cells were restimulated with antigen in the presence of irradiated syngeneic accessory cells. The same procedure was used to generate CD4+ T cell lines specific for amylase, carbonic anhydrase II, and LF.
Adoptive Transfer of Disease
Anti-amylase, anti-carbonic anhydrase II, or anti-LF T cells were activated in vitro with 5 μg/ml concanavalin A in the presence of irradiated (1500 rads) syngeneic splenocytes for 72 hours. The cells were collected, washed twice, and adjusted to the appropriate concentration in Dulbecco’s modified phosphate-buffered saline. Cells were administered to previously unmanipulated, anesthetized animals in a volume of 2 ml via tail vein injection. Administration of 2 ml of saline served as a negative control. The total number of cells administered varied according to the particular experiment. Each group consisted of at least three rats, unless specifically indicated otherwise. The animals were weighed daily, and examined for any symptoms of disease; such as wasting, hunched posture, piloerection, or obvious behavioral abnormalities.
Immunohistochemistry
Cryostat sections (6 μm thick) of the pancreas were cut and mounted on glass slides for immunohistochemical staining. The staining procedure has been detailed elsewhere.9 After a 30-second exposure to absolute methanol at −20°C and extensive rinsing in 0.5 mol/L Tris buffer (pH 7.6), the sections were incubated with appropriate primary antibodies at 4°C overnight. After further buffer washes, the slides were incubated with biotinylated secondary anti-murine IgG antibodies (Vector Laboratories, Burlingame, CA) for 40 minutes, washed in buffer, and then incubated with avidin-biotinylated horseradish peroxidase complex (Vector Laboratories) for 2 hours. The chromatic reaction was developed by incubating slides with 3,3′-diaminobenzidine in the presence of H2O2. After dehydration and mounting of coverslips, the slides were assessed histologically.
Antibodies
Antibodies used in immunohistochemistry include R7.3 (anti-α/β T cell receptor), W3/25 (anti-CD4), OX-8 (anti-CD8), 3.2.3 (anti-NK cell), OX-6 (anti-MHC II), OX-18 (anti-MHC I), OX-42 (anti-CD11b), and TLD-1F5 (anti-macrophage, equivalent to ED2, anti-CD163) and were all grown in our laboratory and used in the form of exhausted culture supernatant. RLN.9D3 (anti-B cell; Serotec, Raleigh, NC) was used at a dilution of 1:200, 8A2 (anti-CD11c, Serotec) was used at a dilution between 1:50 and 1:100.
Results
Generation of Amylase-Specific T Cell Lines
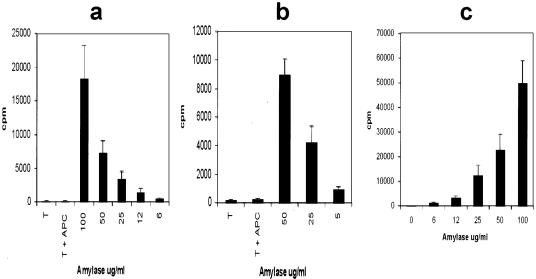
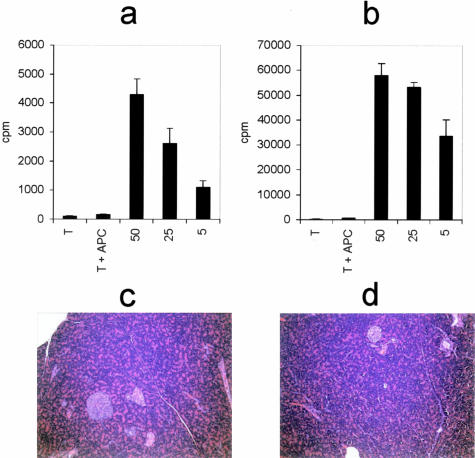
T cells specific for amylase were developed in both the DA(RP)8 and Lewis rat strains by immunization with porcine amylase emulsified in Freund’s complete adjuvant. Nine days after immunization cells were obtained from the draining lymph nodes and cultured in vitro with antigen. After expansion, the cells were allowed to return to resting phase at which point they were assayed for their ability to recognize and proliferate in response to antigen. Cells from both the DA(RP) and Lewis rat strains were specific for amylase and responded in a dose-dependent manner (Figure 1, a and b, respectively).
Figure 1.
DA(RP) (a) or Lewis (b) anti-amylase T cell lines were evaluated for antigen specificity in a proliferation assay using syngeneic antigen-presenting cells and varying concentrations of antigen. c: DA(RP), RT1.AaB/Dl, were stimulated with varying concentrations of amylase in the presence of Lewis, RT1.AlB/Dl, antigen-presenting cells to confirm MHC II restriction of the T cells. Vertical axes represent 3H-thymidine incorporation in dividing cells. Results are expressed as cpm ± SD.
DA(RP) and Lewis rats express identical MHC II molecules (RT-1Bl), but different MHC I molecules.8 Therefore, to ascertain that the T cell lines were MHC II restricted, DA(RP) T cells were stimulated with Lewis antigen-presenting cells along with increasing concentrations of antigen. A concentration-dependent proliferative response was observed with only background levels of proliferation in the absence of antigen, indicating that the cells were MHC II restricted and were not responding to the alloantigen (Figure 1c).
Induction of Pancreatitis
We initially attempted to induce an AIP in rats by an active immunization strategy involving immunization with amylase emulsified in complete Freund’s adjuvant. Animals were immunized with amylase in complete Freund’s adjuvant subcutaneously at multiple sites and examined daily for any symptoms of disease. These animals displayed neither clinical symptoms nor histological signs of pancreatitis when examined 9 days after immunization. Likewise, animals immunized with amylase in complete Freund’s adjuvant and subsequently boosted with amylase in Freund’s incomplete adjuvant on day 14 also developed no signs of clinical symptoms. Histological examination of pancreata on day 22 showed no evidence of inflammation.
The ability of adoptively transferred, activated, CD4+ T cells to induce an AIP was next assessed. Amylase-specific T cells from either the DA(RP) or Lewis rat strains were activated by culture with irradiated syngeneic antigen-presenting cells in the presence of 5 μg/ml concanavalin A for 72 hours. The cells were then injected intravenously at various concentrations into naïve syngeneic animals. Animals injected with PBS alone served as a negative control. The animals were weighed daily and observed for any signs indicative of illness. At various time points animals were sacrificed and pancreata examined histologically.
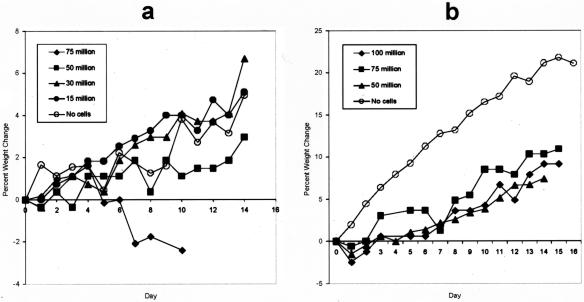
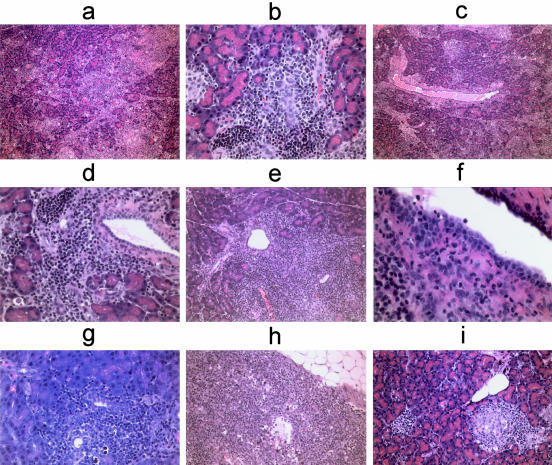
DA(RP) rats that received either 15 million or 30 million activated anti-amylase T cells gained weight during a 14-day course in a manner similar to control animals. However, DA(RP) rats that received 50 million cells failed to gain weight at the same pace as control animals. Finally, DA(RP) rats that received 75 million T cells steadily lost weight throughout the course of 10 days (Figure 2a). Histological examination of pancreata revealed that all animals given anti-amylase T cells had a diffuse pancreatitis at all doses of cells tested. The inflammation was typified by mononuclear cell infiltration and destruction of acinar tissue (Figure 3a). Although the infiltrate was overwhelming lymphocytic, plasma cells, and neutrophils were occasionally observed (Figure 3, a and f). Mast cells were sporadically present at the site of inflammation, but were frequently observed in the pancreas-associated lymph nodes (Figure 3, c and h). Periductal inflammation with infrequent lymphocytes or neutrophils infiltrating the duct wall was a common feature (Figure 3; c, d, and f), but ducts were not the sole target of inflammation. The smaller ductules appeared to be a more favored site of inflammation and tissue destruction than the larger ducts (Figure 3b). Inflammation could also be occasionally noted surrounding the islets of Langerhans (Figure 3i). The significance of this inflammation is not known, however none of animals with experimental autoimmune pancreatitis (EAP) became diabetic.
Figure 2.
DA(RP) (a) or Lewis (b) rats received syngeneic activated anti-amylase T cells at the indicated dosages and were followed daily for weight change. Data are presented as percent weight change as compared to the initial weight on day 0.
Figure 3.
H&E staining of pancreata from animals given activated anti-amylase T cells. a: DA(RP) given 75 × 106 T cells and sacrificed on day 10. Section demonstrates diffuse lobular tissue destruction. b: DA(RP) given 30 × 106 T cells and sacrificed on day 32. Section demonstrates an inflamed ductule. c: Same animal as depicted in a demonstrating acinar tissue destruction as well as periductal mast cells. d: DA(RP) given 75 × 106 T cells and sacrificed on day 10 (different animal from that depicted in a). Section demonstrates periductal inflammation. e: Lewis rat given 75 × 106 T cells and sacrificed on day 15. Section demonstrates periductal inflammation in the Lewis rat. f: Lewis rat given 100 × 106 T cells and sacrificed on day 15. Section demonstrates neutrophils infiltrating the duct wall. g: Lewis rat given 50 × 106 T cells and sacrificed on day 28. Section demonstrates apoptotic bodies at the site of the lesion. h: Same animal as depicted in g. Section demonstrates mast cells in the pancreas-associated lymph node. i: Same animal depicted in d. Sample depicts inflammation surrounding an islet. Original magnifications: ×10 (a, c); ×40 (b, d, g); ×20 (e, h, i); ×60 (f).
To determine whether this disease could be induced in other strains, Lewis animals were given escalating doses of activated Lewis rat-derived anti-amylase T cells and followed for signs of pancreatitis. Although no animals of this strain lost weight, even when given 100 million cells per animal, all showed a greatly reduced rate of weight gain throughout the course of 2 weeks (Figure 2b). Histological examination of pancreata harvested from these animals showed inflammatory infiltrates and tissue destruction indistinguishable from that seen when DA(RP) animals were used.
Lewis animals were next given either 25 or 50 million activated anti-amylase T cells and followed for 28 days to determine the persistence of disease. Throughout the course of this experiment these animals gained weight, but at a pace significantly slower than control animals. Histological examination of pancreas tissue harvested from these animals revealed focal areas of inflammation often centered in periductal areas, although acinar infiltrates could also be observed. The overall severity of inflammation was greatly reduced as compared to lesions sampled at earlier time points. Moreover, no discernable fibrosis had developed in the pancreata within the 28-day period (Figure 3, g and h).
Amylase is also produced by the salivary gland. To determine whether the anti-amylase T cells were also targeting salivary gland-derived amylase, the salivary glands from animals with pancreatitis were examined histologically for signs of inflammation. The salivary glands from all animals used in this study were examined in this way. In no instance was inflammation present, indicating that in this model the inflammation specifically targets the pancreas (data not shown).
Sera from all animals with pancreatitis were evaluated for circulating amylase and lipase levels. Conspicuously, there was no statistically significant deviation in the levels for either enzyme between animals with pancreatitis and control animals (data not shown). Furthermore, despite widespread pancreatic tissue destruction including inflammation of pancreatic islets (Figure 3i), none of the animals developed signs of diabetes, such as elevated blood glucose levels, polydipsia, or polyuria.
Immunohistochemical Analysis of Pancreatitis Lesions
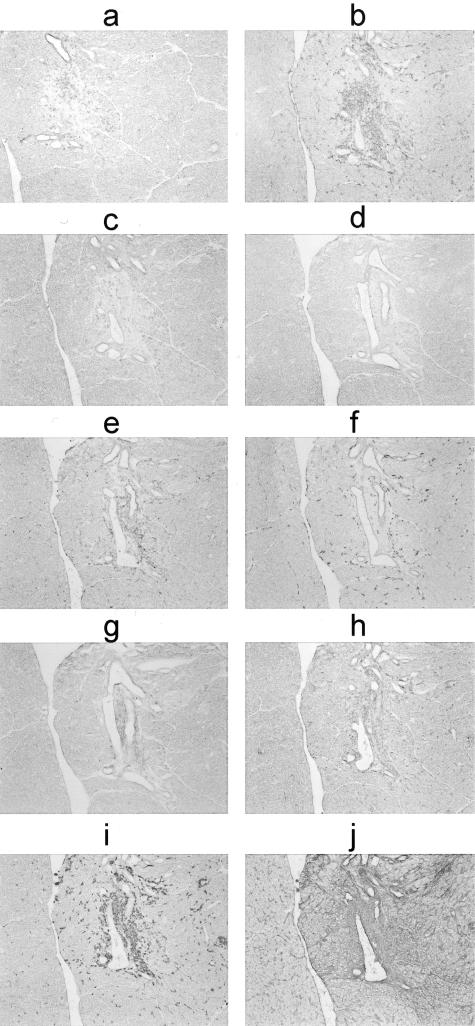
Inflamed pancreata from Lewis animals sacrificed 16 days after transfer of activated anti-amylase T cells were examined by immunohistochemistry to determine the nature of the inflammatory infiltrates. As expected, T cells were the primary inflammatory cell type present in the lesions (Figure 4; a to c). Of these, the vast majority was CD4+ (Figure 4b), although some CD8+ cells could also be observed (Figure 4c). OX-42 and TLD-1F5-positive macrophages were also present (Figure 4, e and f). The lesions also contained 3.2.3-positive NK cells (Figure 4h). There was no staining for RLN.9D3-positive B cells (Figure 4d). There appeared to be increased expression of CD11c, particularly in the duct wall, although there were no apparent infiltrating dendritic cells (Figure 4g). There was greatly increased expression of MHC I (Figure 4j) and MHC II (Figure 4i), the former molecule was not confined to any particular cell type, whereas the latter was confined primarily to infiltrating macrophages.
Figure 4.
Immunohistochemical analysis of serial sections of an inflamed pancreas from a Lewis rat given activated anti-amylase T cells 16 days previously. This series of sections demonstrates an inflammatory lesion focused on a pancreatic duct. a: Anti-αβ TCR, R7.3; b: anti-CD4, W3/25; c: anti-CD8, OX-8; d: anti-B cell, RLN.9D3; e: anti-CD11b, OX-42; f: anti-CD163, TLD-1F5; g: anti-CD11c, 8A2; h: anti-NK cell, 3.2.3; i: anti-MHC II, OX-6; j: anti-MHC I, OX-18.
T Cell Lines Specific for Either Carbonic Anhydrase or LF Fail to Induce AIP
Human patients with AIP will frequently have autoantibodies directed against either carbonic anhydrase (CA-II) or LF.2 It is therefore possible that immune responses directed against these molecules would also elicit AIP. T cell lines specific for either CA-II or LF were generated in the DA(RP) rat strain to test this hypothesis. Both cell lines responded to their respective antigen in a dose-dependent manner (Figure 5, a and b). The cell lines were activated with concanavalin A as before, and adoptively transferred into naïve, syngeneic recipients at 75 × 106 cells per animal. The animals were weighed daily, yet their rate of weight gain did not deviate from their control counterparts (data not shown). Seventeen days after adoptive transfer the animals were sacrificed and pancreata examined by histology for signs of pancreatitis. Neither the animals receiving anti-CAII T cells, nor the animals receiving anti-LF T cells had any histological signs of pancreatitis (Figure 5, c and d).
Figure 5.
DA(RP) anti-CAII (a) and anti-LF (b) T cells were evaluated for antigen specificity in a proliferation assay using syngeneic antigen-presenting cells and varying concentrations of antigen. Vertical axes represent the 3H-thymidine incorporation in dividing cells. Results are displayed as cpm ± SD. c: Histological section of a pancreas from a DA(RP) rat given anti-CAII cells. d: Histological section of a DA(RP) rat given anti-LF cells. Original magnifications, ×20.
Discussion
In this report we demonstrate that activated T cells specific for the pancreatic enzyme amylase are able to induce AIP in rats. Adoptive transfer of the T cells alone is sufficient to induce disease because the animals required no additional manipulation, either before or after receiving the cells, for disease to develop. Although autoantibodies are a commonly observed feature of AIP,2 we found that administration of amylase antiserum was not required for the development of EAP. Although many existing models of pancreatitis require chemically induced pancreatic injury,7 the model described here does not require any such insult to facilitate disease. Thus, the T cells are able to enter the pancreas, recognize their cognate antigen, recruit additional inflammatory cells, and ultimately cause widespread and chronic inflammation and destruction of pancreatic tissue.
The amylase used in these studies was purified from porcine pancreas. The T cells obtained by immunization with this antigen were able to specifically induce inflammation in the rat pancreas. Amylase is also produced by the salivary glands, however the salivary glands from animals with pancreatitis were uniformly devoid of inflammation (data not shown). It has been shown that in rodents the salivary gland and pancreas produce unique isozymes of amylase that differ at both the mRNA and protein level.10 Overall, there is a 12% difference in the amino acid composition of the two isoforms that, although apparent throughout the lengths of the molecules, tend to be more focused approximately between residues 110 and 170. One notable difference between the two isoforms that lies within this particular area is the deletion of a three amino acid stretch in the pancreatic form relative to the salivary form. There is also a four amino acid substitution at residues 254 to 257. These two segments represent attractive candidate regions for the epitopes recognized by the T cells that allow immunological distinction between the two isoforms. Obviously, assignment of such an epitope can certainly not be made on this basis alone. Suffice it to say that there are ample differences at the amino acid level between the pancreatic and salivary forms of amylase that the ability of the immune system to distinguish between the two forms is not surprising. Furthermore, Takeuchi and colleagues11 have demonstrated that antisera prepared against pancreatic and parotid amylase have minimal, if any, cross reactivity, highlighting the fact that the two molecules are antigenically distinct. It appears likely that the T cells generated by immunization with porcine pancreatic amylase recognize an epitope specific for the pancreatic isozyme, and not an epitope shared by salivary and pancreatic isozymes.
The amylase used in these studies ran as a single band on polyacrylamide gel electrophoresis (data not shown). However, this simple test is not sufficient to guarantee purity. Given that the antigen was purified biochemically, there may still exist a trace pancreas-specific contaminant that serves as the antigen that induces EAP. It is difficult with these data alone to conclusively exclude this possibility. Identification of an immunogenic epitope within the amylase molecule itself will be necessary to resolve these concerns.
The use of a xenogeneic source of immunogen may be an important factor for the induction of pathogenic T cells. Such a phenomenon has been well documented for the induction of encephalitogenic T cells in the rat, in which immunization with guinea pig myelin is far more pathogenic than is immunization with rat myelin.12 It may be that in EAP, as it is in experimental autoimmune encephalomyelitis, immunization with a xenogeneic antigen is necessary, or at least far more potent at breaking tolerance and inducing autoimmunity.
AIP in humans appears to be heterogeneous both clinically and histopathologically. Until recently many patients with AIP have been assigned a diagnosis of idiopathic chronic pancreatitis because their disease does not have distinctive symptoms or clinical course. At presentation, only 8 to 25% of such patients have other recognized autoimmune diseases such as Sjogren sialadenitis, inflammatory bowel disease, or sclerosing cholangitis.13 Because of pancreatic enlargement that may be confined to the head, many patients have undergone pancreatic resection because pancreatic carcinoma was suspected.5 Histopathological examination of resection specimens has allowed the recognition of characteristic duct-centric inflammation characterized by the presence of lymphocytes and plasma cells within the duct wall and periductal connective tissue. Venulitis is commonly observed in involved portions of the pancreas. Ductal involvement ranges from the main pancreatic duct to small intralobular branches. The amount of inflammation and atrophy of acinar tissue is also quite variable in human cases, as is the degree of fibrosis. The inflammatory infiltrate may contain granulocytes including both neutrophils and eosinophils, or with predominance of either, but the presence of granulocytes is variable. Neutrophils may be present in duct lumens, sometimes associated with destruction of the duct epithelium. These lesions (granulocytic epithelial lesions) have been likened to the crypt abscesses seen in ulcerative colitis, and are most likely to be seen in patients that also have inflammatory bowel disease.13 The disease may be localized or less commonly diffuse within the pancreas. Part of the variability in humans is attributed to the duration and severity of the process, but it is possible that part of the variability between cases reflects involvement of distinct epitopes.
The histopathological changes in EAP clearly mimic a segment of the changes seen in human cases, but are less variable than the spectrum seen in humans. Notably, ductal involvement in EAP is less conspicuous than in humans and involves small- and medium-size ducts rather than larger intralobular ducts and the main duct. In the rat model there is overwhelming involvement of lymphocytes while plasma cells, neutrophils, and eosinophils are not as easily observed. Inflammation in EAP, at least within the first 28 days of disease, appears to be more widespread and includes a greater degree of lobular tissue destruction than in the human, but fibrosis and scarring are less. Amylase is expressed by acinar cells in the lobular tissue of the pancreas, as well as by periductal cells,14 and indeed this is the area in which the inflammation is localized. Although a few neutrophils were present in the inflammatory infiltrate, none of the ductal lesions qualifies as a granulocytic epithelial lesion. Thus, EAP is a better match for the AIP cases in humans that have been called lymphoplasmacytic sclerosing pancreatitis or AIP without granulocytic epithelial lesions.
These discrepancies may be due to of the introduction into the rat of millions of antigen-specific T cells at one point in time, and the histological examination of the pancreas after only a few weeks. In humans, lower numbers of pathogenic cells may exert their effect throughout a much more extended period. It may be that throughout a much greater period of time that the histological features of pancreatitis in the rat would change to become more similar to those of the human, to include scarring and inflammation more focused on the duct wall. Clinically, human patients with AIP experience epigastric pain, jaundice, and weight loss that is frequently of protracted duration. Of the symptoms comparable between humans and rodents, weight is the most easily measured parameter. Thus, decreasing body mass, or failure to gain weight at a rate coinciding to control animals, offers a tractable means of following the progression and course of EAP.
Immunohistochemcial analysis reveals that the majority of the infiltrating inflammatory cells are CD4+ T cells, with fewer, but still observable numbers of CD8+ T cells, macrophages, and even B cells; all features reminiscent of the human condition. We also demonstrate that MHC I and MHC II are greatly up-regulated at the site of the lesion, bespeaking widespread immunological activation of the pancreatic parenchyma.
The disease does not appear to be strain-specific because both DA(RP) and Lewis rat strains were susceptible. However, the DA(RP) strain did appear to be more sensitive. At the greatest dosage of cells given to DA(RP) rats, 75 million, the animals showed progressive weight loss. In contrast, Lewis animals given as many as 100 million cells per animal did not have overt weight loss. Yet, all Lewis rats given anti-amylase T cells failed to gain weight at a rate coinciding with control animals.
It must be noted that we did not test numerous rat strains for EAP sensitivity, so no broad claims regarding strain or species resistance or susceptibility can be made. As in so many autoimmune models, there may be differing degrees of susceptibility to target organ inflammation, as opposed to autoimmunity in general. For example, the Lewis rat is highly susceptible to several models of autoimmunity, such as experimental autoimmune encephalomyelitis and experimental autoimmune myocarditis,15 whereas the DA(RP) is less susceptible to these conditions (unpublished observations). However, the DA(RP) rat is more susceptible to insulitis than is the Lewis rat.8 This raises the possibility that the cellular, molecular, and genetic elements controlling pancreatitis in these two strains may differ. Such differences may in part explain why the clinical course of disease between the DA(RP) and Lewis rat strains was not identical.
Despite the presence of autoantibodies against CA-II and LF in AIP patients,2 T cells specific for these enzymes were unable to induce EAP in rats. One possible explanation for this finding is that the autoantibodies against these enzymes in human patients represent a consequence, perhaps a late consequence, of tissue destruction, as opposed to representing the fundamental pathogenic mechanism. In such a scenario, autoantibodies against CA-II and LF would arise via epitope spreading. Although we have not addressed the topic of epitope spreading in this report, the issue becomes an interesting consideration for future investigations. The data regarding the immunogenicity of CA-II and LF stand in contrast to a previous report showing that immunization with either of these two enzymes in thymectomized mice leads to pancreatitis.16 The pathogenicity of immunization with amylase was not tested in Uchida and colleague’s16 groundbreaking work so it is not known if amylase can be used to induce pancreatitis in immunologically crippled animals. It is known that thymectomy greatly increases the rate and sensitivity to autoantigens17 and as a result, both our work and possibly the work of Uchida and colleagues16 point to a T cell etiology of AIP. Thus it appears that in immunologically normal animals LF and CA-II are unable to induce EAP, whereas amylase can serve as a sufficient antigen. These data do not rule out the possibility that additional pancreas-specific antigens may remain that are capable of inducing pancreatitis.
The observation that an immune response directed against amylase is sufficient to induce pancreatitis in rats potentially provides new insight into the pathogenic mechanism of the human disease. Such an immune response against a constitutive, common organ product has not been evaluated in the human condition and thus it is not known if immune responses directed against amylase are involved in the pathogenesis of AIP. In light of the findings presented here, an investigation along these lines may be warranted.
The ability to induce EAP in an immunologically intact animal model presents the opportunity to dissect basic immunological mechanisms responsible for a complex and rare human disease. Furthermore, this model represents a novel means for the study of organ-specific autoimmunity in general. Thus, new opportunities now exist to investigate topics ranging from basic scientific interest to clinical intervention of a previously enigmatic, poorly defined illness.
Acknowledgments
We thank Dr. Brent Harris for assistance in imaging and Dr. Jerry Yeo for assistance with clinical laboratory data.
Footnotes
Address reprint requests to William F. Hickey, M.D., Department of Pathology, Dartmouth Medical School, Dartmouth Hitchcock Medical Center, Lebanon, NH 03756. E-mail: william.f.hickey@dartmouth.edu.
References
- Pearson RK, Longnecker DS, Chari ST, Smyrk TC, Okazaki K, Frulloni L, Cavallini G. Controversies in clinical pancreatology: autoimmune pancreatitis: does it exist? Pancreas. 2003;27:1–13. doi: 10.1097/00006676-200307000-00001. [DOI] [PubMed] [Google Scholar]
- Kloppel G, Luttges J, Lohr M, Zamboni G, Longnecker D. Autoimmune pancreatitis: pathological, clinical, and immunological features. Pancreas. 2003;27:14–19. doi: 10.1097/00006676-200307000-00002. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Egawa N, Nakajima H. Autoimmune pancreatitis is a systemic autoimmune disease. Am J Gastroenterol. 2003;98:2811–2812. doi: 10.1111/j.1572-0241.2003.08758.x. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Kamata N. Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2694–2699. doi: 10.1111/j.1572-0241.2003.08775.x. [DOI] [PubMed] [Google Scholar]
- Bartolome MJ, de las Heras G, Lopez-Hoyos M. Low-avidity antibodies to carbonic anhydrase-I and -II in autoimmune chronic pancreatitis. Sci World J. 2002;2:1560–1568. doi: 10.1100/tsw.2002.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Mufti RA, Williamson RC. Experimental models of pancreatitis. Ann Acad Med Singapore. 1999;28:133–140. [PubMed] [Google Scholar]
- Griffin AC, Zhao W, Wegmann KW, Hickley WF. Experimental autoimmune insulitis. Induction by T lymphocytes specific for a peptide of proinsulin. Am J Pathol. 1995;147:845–857. [PMC free article] [PubMed] [Google Scholar]
- Davidson TS, Hickey WF. Distribution and immunoregulatory properties of antisecretory factor. Lab Invest. 2004;84:307–319. doi: 10.1038/labinvest.3700036. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O, Bovey R, Young RA. Tissue-specific expression of mouse-alpha-amylase genes: nucleotide sequence of isozyme mRNAs from pancreas and salivary gland. Cell. 1980;21:179–187. doi: 10.1016/0092-8674(80)90125-7. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Mura M, Sasaki R, Matsushima T, Sugimura T. Comparative studies on electrophoretic mobility and immunogenicity of pancreatic and parotid amylases of rat. Biochim Biophys Acta. 1975;403:456–460. doi: 10.1016/0005-2744(75)90073-x. [DOI] [PubMed] [Google Scholar]
- Kibler RF, Fritz RB, Chou F, Jen Chou CH, Peacocke NY, Brown NM, McFarlin DE. Immune response of Lewis rats to peptide C1 (residues 68–88) of guinea pig and rat myelin basic proteins. J Exp Med. 1977;146:1323–1331. doi: 10.1084/jem.146.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, Leins A, Longnecker D, Klöppel G. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;495:552–563. doi: 10.1007/s00428-004-1140-z. [DOI] [PubMed] [Google Scholar]
- Bendayan M, Ito S. Immunohistochemical localization of exocrine enzymes in normal rat pancreas. J Histochem Cytochem. 1979;27:1029–1034. doi: 10.1177/27.6.110872. [DOI] [PubMed] [Google Scholar]
- Ratcliffe NR, Hutchins J, Barry B, Hickey WF. Chronic myocarditis induced by T cells reactive to a single cardiac myosin peptide: persistent inflammation, cardiac dilatation, myocardial scarring and continuous myocyte apoptosis. J Autoimmun. 2000;15:359–367. doi: 10.1006/jaut.2000.0432. [DOI] [PubMed] [Google Scholar]
- Uchida KO, Okazaki K, Nishi K, Uose T, Nakase S, Ohana H, Matsushima M, Omori Y, Chiba T. Experimental immune-mediated pancreatitis in neonatally thymectomized mice immunized with carbonic anhydrase II and lactoferrin. Lab Invest. 2002;82:411–424. doi: 10.1038/labinvest.3780435. [DOI] [PubMed] [Google Scholar]
- Smith H, Chen IM, Kubo R, Tung KS. Neonatal thymectomy results in a repertoire enriched in T cells deleted in adult thymus. Science. 1989;245:749–752. doi: 10.1126/science.2788921. [DOI] [PubMed] [Google Scholar]