Abstract
Liver cirrhosis is characterized by hepatic dysfunction with extensive accumulation of fibrous tissue in the liver. In response to chronic hepatic injury, hepatic portal myofibroblasts and activated hepatic stellate cells (HSCs) play a role in liver fibrosis. Although administration or gene expression of hepatocyte growth factor (HGF) leads to improvement in hepatic fibrosis/cirrhosis, the related mechanisms are not fully understood. We investigated mechanisms involved in resolution from liver cirrhosis by HGF, focusing on growth regulation and apoptosis in portal myofibroblasts. Cultured rat HSCs could not proliferate, were withdrawn after passage, and were replaced by proliferating portal myofibroblasts during the passages. In quiescent HSCs, c-Met receptor expression was undetected whereas c-Met receptor expression was detected in activated HSCs and liver myofibroblasts expressing α-smooth muscle actin (α-SMA), suggesting that activated HSCs and portal myofibroblasts are targets of HGF. For cultured rat portal myofibroblasts, HGF counteracted phosphorylation of extracellular signal-regulated kinase (Erk) 1/2 and mitogenic stimulus induced by platelet-derived growth factor, induced c-jun N-terminal kinase (JNK) 1 phosphorylation, and promoted apoptotic cell death. In the dimethylnitrosamine rat model of liver cirrhosis, administration of HGF suppressed proliferation while promoting apoptosis of α-SMA-positive cells in the liver, events that were associated with reduced hepatic expressions of α-SMA and histological resolution from liver cirrhosis. Growth inhibition and enhanced apoptosis in portal myofibroblasts by HGF are newly identified mechanisms aiding resolution from liver fibrosis/cirrhosis by HGF.
Liver cirrhosis, which usually is as a long-term consequence of chronic hepatic injury caused by alcohol abuse or hepatitis virus infection, is characterized by extensive fibrous scarring of the liver.1 Advanced cirrhosis is generally irreversible and is often associated with variceal hemorrhage or development of hepatocellular carcinoma. Hence, liver cirrhosis is a major cause of morbidity and mortality worldwide. Approaches to promote the remodeling of the excess extracellular matrix (ECM) associated with reorganization of the hepatic structure are critical to establish a therapeutic base.
In the liver, two different cell populations play a key role in the pathogenesis of liver cirrhosis as major sources of hepatic ECM. Quiescent hepatic stellate cells (HSCs) (also known as lipocytes, fat-storing cells, or Ito cells) synthesize low levels of ECM proteins, whereas in response to chronic hepatic injury, HSCs undergo phenotypic change into myofibroblast-like cells, a process termed “activation.”2,3 In addition to activated HSCs, liver myofibroblasts, which are located in periportal and perivenous areas in the normal liver, migrate to the site of hepatic injury and are involved in fibrotic change of the liver. Distinct from HSCs, portal myofibroblasts maintain proliferative ability and can be expanded in vitro and in vivo,4–7 while portal myofibroblasts also express ECM proteins involved in fibrotic change of the liver. Although phenotypic change and cell proliferation in HSCs and portal myofibroblasts are a pathogenic cause of hepatic fibrosis, recent approaches indicated that apoptotic cell death in activated HSCs and liver myofibroblasts may be involved in the resolution from hepatic fibrosis in rats.7–11 Thus, changes in cell fate and behavior of activated HSCs and portal myofibroblasts affect the pathogenesis and recovery from hepatic fibrosis/cirrhosis.
Hepatocyte growth factor (HGF), originally identified and cloned as a mitogenic protein for hepatocytes,12,13 exerts diverse cellular responses through c-Met receptor tyrosine kinase in a wide variety of cells.14,15 Multiple biological events of HGF include mitogenic, motogenic, morphogenic, and anti-cell death activities. Likewise, HGF stimulates expression and activity of proteases involved in breakdown of ECM proteins, including urokinase-type plasminogen activator and matrix metalloproteinases.16–19 Physiologically, HGF plays a role in regeneration and protection of organs, including the liver.15 Based on the trophic roles of HGF in tissue regeneration and protection, approaches to address the therapeutic potential of HGF have been made using a variety of experimental models. Importantly, HGF has therapeutic effects on liver cirrhosis, decreasing both ECM deposition and mortality in distinct models of liver cirrhosis in rats.20–24 However, the molecular and cellular mechanisms by which supplements of HGF leads to recovery from cirrhosis are not fully understood.
We now report evidence that the c-Met receptor is expressed in hepatic portal myofibroblasts and that HGF suppressed DNA synthesis and stimulated apoptotic cell death in portal myofibroblasts, and these processes were associated with recovery from liver cirrhosis in rats. We propose that newly identified mechanisms may well lead the way to therapeutic approaches for patients with liver cirrhosis.
Materials and Methods
Growth Factors
Human recombinant HGF was purified from conditioned medium of Chinese hamster ovary cells transfected with an expression vector containing cDNA for human HGF deleted with five amino acid residues, as described.20,25 Recombinant human transforming growth factor (TGF)-β1 and platelet-derived growth factor (PDGF)-BB were obtained from R&D Systems (Minneapolis, MN).
Animal Treatment
Five-week-old male Sprague-Dawley rats were used. All animal experiments were done in accordance with National Institutes of Health guidelines, as dictated by the animal care facility at Osaka University Graduate School of Medicine. For development of experimental hepatic cirrhosis, rats were intraperitoneally administered dimethylnitrosamine (DMN) dissolved in saline at 10 mg per kg of body weight, on 3 consecutive days a week for 5 continuous weeks, then these rats were intraperitoneally given saline alone or HGF daily in a dose of 300 μg per kg of body weight for 1 week. Seven or eight rats were included in each experimental group.
Histological Procedures
Tissues were fixed in 10% neutralized formaldehyde or 70% ethanol and embedded in paraffin. For immunohistochemical detection of desmin and α-smooth muscle actin (α-SMA), the tissue sections were, respectively, incubated with monoclonal anti-mouse desmin antibody (EPOS system; DAKO, Carpinteria, CA) and monoclonal anti-human α-SMA antibody (EPOS system) for 1 hour. The reaction was visualized with use of 0.025% diaminobenzidine and 0.003% hydrogen peroxide. For detection of the c-Met receptor in cultured cells, cells were washed with phosphate-buffered saline (PBS) and fixed with 70% ethanol for 10 minutes at room temperature. After washing three times with PBS, these cells were incubated for 1 hour with rabbit anti-mouse c-Met antibody (SP260; Santa Cruz Biotechnology, Santa Cruz, CA) and Alexa Fluor 546 goat anti-rabbit IgG (Molecular Probes, Eugene, OR) for 1 hour. Nuclei were stained with TO-PRO-3 iodide (642/661) (Molecular Probes). In the control specimen, the c-Met primary antibody was preabsorbed with an excess of c-Met blocking peptide (SP260P, Santa Cruz Biotechnology). For double immunohistochemistry of desmin and c-Met, tissue sections were incubated overnight with anti-desmin antibody and polyclonal anti-mouse c-Met antibody (SP260; Santa Cruz Biotechnology). Tissue sections were sequentially incubated with fluorescein isothiocyanate-conjugated anti-mouse IgG and rhodamine-conjugated anti-rabbit IgG (ICN Pharmaceuticals Inc., Aurora, OH). Fibrous tissue area was quantitated in hepatic tissue sections stained for α-SMA, using a computerized morphometric analysis using the NIH image software (Wayne Rasband Analytics, NIH, Bethesda, MD).
For detection of cells undergoing DNA synthesis, 5′-bromo-2′-deoxyuridine (BrdU, 100 mg per kg) was intraperitoneally injected into rats, 1 hour before killing the animals. Tissues were fixed in 70% ethanol and sections were denatured in 2 mol/L HCl for 30 minutes at room temperature, followed by neutralization with 0.1 mol/L borate buffer for 30 minutes. After washing in phosphate-buffered saline (PBS), tissue sections were incubated with monoclonal anti-BrdU antibodies (1:500 dilution, DAKO) for 1 hour, followed by sequential incubation with biotinylated anti-mouse IgG (Vector Laboratories, Burlingame, CA) and peroxidase-labeled avidin-biotin complex, using the Vectastain Elite ABC kit (Vector Laboratories). The reaction was detected in substrate solution containing diaminobenzidine. For detection of cells undergoing apoptosis, tissue sections were subjected to terminal dUTP nick-end labeling (TUNEL), using an In Situ Apoptosis Detection kit (MK-500; Takara Shuzo Co., Tokyo, Japan). After BrdU or TUNEL staining, sections were successively incubated with monoclonal anti-human α-SMA antibody and alkaline phosphatase-conjugated anti-mouse IgG, and the reaction was detected using a commercial kit (Vectastain ALP ABC, Vector Laboratories). The number of cells double positive for BrdU and α-SMA or TUNEL and α-SMA was counted in the stained sections at 400-fold field magnification under a microscope, using at least 10 randomly selected microscopic fields per each section.
Western Blots for Desmin and α-SMA in Hepatic Tissues
Hepatic tissues were homogenized in buffer composed of 50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1% Nonidet P-40, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mmol/L ethylenediamine tetraacetic acid, 1 mmol/L phenylmethyl sulfonyl fluoride, and 5 μg/ml leupeptin. The homogenates were centrifuged and the supernatants were subjected to SDS-polyacrylamide gel electrophoresis (PAGE), electroblotted onto polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA), and probed with monoclonal anti-mouse desmin (DAKO) or α-SMA antibodies (DAKO). Proteins reacting with these antibodies were detected using an enhanced chemiluminescence reagent (Amersham Life Science Inc., Arlington, IL).
Cultivation of HSCs and Liver Myofibroblasts
HSCs were isolated according to Knook and colleagues.26 Briefly, the liver was digested in situ with solutions containing collagenase type I. The digested liver was filtrated and parenchymal hepatocytes were removed after centrifugation at 50 × g for 1 minute. Nonparenchymal cells obtained after centrifugation at 1400 × g were resuspended and subjected to a Nycodenz density gradient centrifugation. Cells in the top layer were recovered. The cells were cultured in proline-free Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. Portal myofibroblasts were obtained after serial passages (three to five passages) of the culture. Populations of different cell types in cultures were analyzed by vitamin A autofluorescence and immunocytochemistry using antibodies against fibulin-2 (for portal myofibroblasts), Mac-1 (for macrophages), and von Willebrand factor (for endothelial cells).
Western Blot Analysis of c-Met Receptor and α-SMA in Cultured Cells
To detect c-Met receptors using Western blots, cells were lysed in the sample buffer for SDS-PAGE. The cell lysate was subjected to SDS-PAGE and proteins were electroblotted onto polyvinylidene difluoride membranes. After blocking with PBS containing 5% skim milk, the membrane was incubated with rabbit anti-mouse c-Met antibody (SP260, Santa Cruz Biotechnology). For analysis of α-SMA expression, the membrane was successively incubated with mouse monoclonal anti-human α-SMA antibody, biotinylated anti-mouse IgG (Vector Laboratories), and peroxidase-labeled avidin-biotin complex (Vectastain, Vector Laboratories). Immunoreactive proteins were visualized using an enhanced chemiluminescence reagent.
Measurement of DNA Synthesis, Apoptosis, and Lactate Dehydrogenase (LDH) Activity
Cells were seeded at a density of 2 × 104 cells per well in 24-well plates and cultured for 24 hours. After being serum-starved in DMEM supplemented with 0.2% fetal bovine serum for 24 hours, the cells were incubated in the absence or presence of varying concentrations of HGF with or without 10 ng/ml of PDGF-BB for 20 hours, then pulse-labeled with 1.0 μCi per ml [methyl-3H] thymidine (Amersham Life Science Inc.) for 6 hours. The number of viable cells was determined using trypan blue dye extrusion assays. To detect apoptotic cells, cells were fixed in 70% ethanol and apoptotic cells were stained using the TUNEL reaction, and In Situ Apoptosis Detection kits (MK-500; Takara Shuzo Co). LDH activity in the culture media was determined using a kit (Wako Pure Chemicals, Co., Osaka, Japan).
Analysis of Extracellular Signal-Regulated Kinase (ERK)-1/2 and c-jun N-Terminal Kinase (JNK)
For analysis of ERK-1/2 phosphorylation, cells were serum-starved in DMEM supplemented with 0.2% fetal bovine serum for 24 hours and treated with 10 ng/ml of PDGF in the absence or presence of HGF for 24 hours. For analysis of JNK, serum-starved cells were treated with 10 ng/ml of HGF then were lysed with sample buffer for SDS-PAGE and the lysate was subjected to SDS-PAGE, followed by electroblotting on polyvinylidene difluoride membrane. After blocking, the membrane was sequentially incubated with anti-phospho-p44/p42 mitogen-activated protein kinase (ERK-1/2) antibody (E10; New England BioLabs Inc., Beverly, MA) or an anti-phosphorylated JNK antibody (Phosphoplus SAPK/JNK antibody kit; Cell Signaling Technology, Beverly, MA), biotinylated anti-mouse IgG (Vector Laboratories), and horseradish peroxidase-conjugated streptavidin (Amersham Pharmacia Biotech UK, Little Chalfont, UK). Immunoreactive proteins were detected using an enhanced chemiluminescence reagent. To detect total ERK1/2 or JNK1 proteins, the cells lysate were subjected to Western blots as described above except for use of an anti-ERK-1 antibody (K-23; Santa Cruz Biotechnology) or an anti-JNK antibody (Phosphoplus SAPK/JNK antibody kit).
Data Analysis
Data represent the mean ± SD. Statistical analyses were made using unpaired Student’s t-test (two-tailed) or analysis of variance. Differences were considered to be statistically significant at the P < 0.05 levels.
Results
Resolution of Liver Cirrhosis by HGF
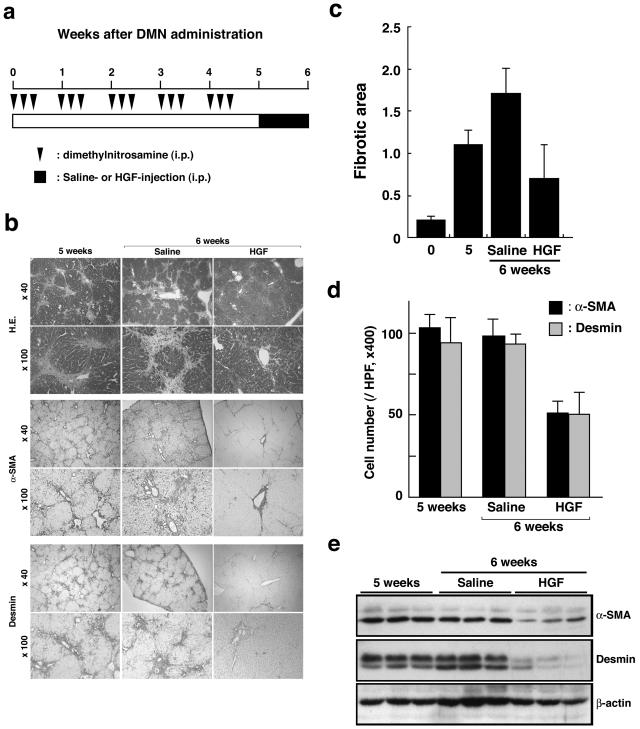
DMN was given to rats intraperitoneally for 3 consecutive days each week for 5 weeks (Figure 1a), and liver specimens were subjected to histological analysis (Figure 1b). Formation of fibrotic septa joining the central area and the nodular pattern of parenchymal cells separated by fibrous septa were evident and reticulin fibers had spread radially throughout the liver. These pathological changes are characteristic of liver cirrhosis. To evaluate expansion of activated HSCs and liver myofibroblasts, hepatic sections were subjected to immunostaining for α-SMA and desmin (Figure 1b). Desmin and α-SMA are expressed in activated HSCs and liver myofibroblasts.7 Cells positive for α-SMA and desmin localized in the fibrous area spread throughout the liver, indicating that expansion of activated HSCs and portal myofibroblasts is associated with fibrotic changes in the liver. Associated with these changes, TGF-β1 levels in the cirrhotic liver tissues increased to 5.1-times higher level than in the normal liver (not shown).
Figure 1.
Enhanced recovery from liver cirrhosis by HGF. a: Experimental schedule for development of liver cirrhosis by DMN administration and treatment with HGF. b: Histological analysis of hepatic sections. Hepatic sections were subjected to staining with H&E and immunohistochemical staining for α-SMA or desmin. c: Change in fibrotic area in rat livers untreated or treated with HGF. Fibrotic area was quantitated by computer-aided image analysis in hepatic tissue sections stained for α-SMA. Each value represents the mean ± SD (n = 8). d: Changes in the number of cells positive for α-SMA and desmin in the livers of rats untreated or treated with HGF. Each value represents the mean ± SD (n = 8). e: Changes in expression of hepatic α-SMA and desmin in livers of rats untreated or treated with HGF. Expression of α-SMA and desmin was analyzed using Western blots.
To determine whether HGF affects resolution from liver cirrhosis, rats subjected to DMN treatment for 5 weeks were daily given HGF or saline for 1 week. In control rats given saline alone, there was little change in the histopathology of the liver: fibrous areas still spread radially throughout the liver and cells positive for α-SMA and desmin were entirely localized in fibrous areas (Figure 1b). In contrast, in rats treated with HGF, fibrotic septa joining the central area and reticulin fibers either disappeared or were markedly decreased. Likewise, cells positive for α-SMA and desmin were remarkably decreased by HGF treatment (Figure 1b). When expansion of fibrous tissue areas was quantitated by image analysis in hepatic tissue sections stained for α-SMA, fibrous area markedly increased by DMN treatment (Figure 1c). The fibrous area in DMN-treated rats given HGF for 1 week decreased to 41% of DMN-treated rats given saline alone.
The remarkable decrease in fibrous tissue expansion and hepatic expression of α-SMA by HGF suggested that HGF directly or indirectly affects the fate of activated HSCs and/or portal myofibroblasts. One potential mechanism for reduction in α-SMA expression might be that HGF facilitates a phenotypic reversal of activated HSCs to a quiescent phenotype. Therefore, the number of cells that expressed α-SMA or desmin was quantitated in hepatic sections subjected to immunohistochemistry (Figure 1d). The number of α-SMA- and desmin-positive cells, respectively, reached 98.4 ± 12.3 and 87.6 ± 10.3 cells per microscopic field at 6 weeks in control rats not given HGF treatment. In rats subjected to a 1-week treatment with HGF, the number of α-SMA-positive cells decreased to 51.4 ± 7.6 cells per microscopic field at 6 weeks. If HGF facilitates a reversal of activated HSCs (positive for both α-SMA and desmin) to a quiescent phenotype (desmin-positive but α-SMA-negative), decrease in the number of α-SMA-positive cells would be associated with an increase in the number of desmin-positive cells. However, the number of desmin-positive cells was also decreased by HGF to 51.2 ± 13.5 cells, a value being the same as that of α-SMA-positive cells at 6 weeks in HGF-treated rats. Consistently, when hepatic tissue extracts were subjected to Western blots, hepatic expressions of both α-SMA and desmin were decreased by HGF treatment (Figure 1e). These findings strongly suggested that HGF did not facilitate a reversal of activated HSCs to the quiescent phenotype.
c-Met Receptor Expression in Activated Liver Myofibroblasts
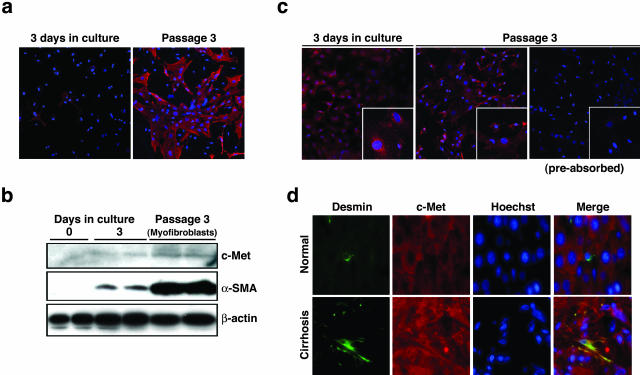
To determine how HGF directly or indirectly affects the fate of α-SMA-positive cells, c-Met receptor expression was analyzed in HSCs and portal myofibroblasts isolated from the rat liver. In agreement with previous findings, HSCs had vitamin A-containing vacuoles characterized by autofluorescence and had a star-like appearance within a few days after plating (not shown). Three days after plating, cells with a fibroblast-like appearance appeared. After passage of the culture, most of cells had a fibroblast-like appearance. Previous studies indicated that HSCs in culture had little potential to proliferate, were withdrawn because of cell death, and could be maintained in culture at maximum until passage 2 only.7 Instead, portal myofibroblasts showed rapid proliferative ability and could be expanded up to the 10th passage. Taken together, we speculated that portal myofibroblasts present in primary culture of HSCs proliferated and HSCs were replaced by portal myofibroblasts. Because fibulin-2 is specifically expressed in portal myofibroblasts but not in HSCs,7 we analyzed fibulin-2 expression in cultures of HSCs (Figure 2a). In primary culture of HSCs (3 days after plating), there were contaminating portal myofibroblasts positive for fiblulin-2, whereas 75 to 85% of cells were positive for fibulin-2 after serial passages of the culture (at passage 3). The result indicated that HSCs were replaced by proliferating portal myofibroblasts during serial passages and cultivation. Knittel and colleagues4 reported that 50 to 100% of rat portal myofibroblasts were positive for fibulin-2.
Figure 2.
c-Met receptor expression in cultures of HSCs and portal myofibroblasts, and in normal and cirrhotic livers. a: Expression of fibulin-2 in cultures of HSCs and portal myofibroblasts. Fiblin-2 expression was analyzed by immunocytochemistry in primary cultures of HSCs on day 3 after plating and the cells after three passages. b: Changes in expression of c-Met receptor and α-SMA in cultured HSCs and portal myofibroblasts. Expressions of c-Met receptor and α-SMA were analyzed using Western blots. c: c-Met receptor expression in HSCs and activated liver myofibroblasts as detected immunocytochemically. C-Met expression was analyzed in primary culture of HSCs on day 9 after plating and the culture of cells after three passages. d: Localization of c-Met receptor expression in normal and cirrhotic livers. Hepatic sections were subjected to immunohistochemical staining for desmin (green) and c-Met receptor (red). Nuclei were detected by staining with Hoechst 33342. Note the merged image of desmin and c-Met receptor staining. Original magnifications: ×200; ×400 (insets).
In quiescent HSCs in primary culture (day 0), α-SMA was undetectable in Western blots, but was detectable in cells cultured for 3 days (Figure 2b). Expression of α-SMA remarkably increased in cells expanded after serial passages and cultured in the presence of 10 ng/ml of TGF-β1 for 3 days. When c-Met receptor expression was analyzed in these cells using Western blots, the c-Met receptor was undetectable in quiescent HSCs, whereas it was weakly detectable in the cells cultured for 3 days. This result suggests that the c-Met receptor in HSCs may be inducible during activation toward myofibroblast-like cells or that the weak expression of c-Met receptor and α-SMA may by because of proliferating portal myofibroblasts. In the cells mostly composed of proliferating liver myofibroblasts, c-Met receptor was significantly detectable. Likewise, expression of the c-Met receptor was analyzed by immunocytochemistry (Figure 2c). In primary culture of HSCs (9 days after plating), c-Met receptor expression was detected in cells with two different appearances (Figure 2c, inset). One cell type showed cell enlargement and spread radically, suggesting that these cells may be activated HSCs, whereas the other type showed a fibroblast-like appearance, suggesting that these cells may be portal myofibroblasts. In the passaged culture (at three passages), c-Met receptor was expressed in most of cells with the myofibroblast-like appearance. Together with fibulin-2 expression in these cells, we concluded that portal myofibroblasts were positive for the c-Met receptor.
To determine whether induction of c-Met receptor expression in HSCs/portal myofibroblasts would be seen during the pathogenesis of liver cirrhosis, expressions of the c-Met receptor and desmin were analyzed using hepatic sections of normal and cirrhotic rat livers (Figure 2d). In normal rat livers, the c-Met receptor was expressed in hepatocytes, however, c-Met expression was not detectable in desmin-positive cells, as was evident in the merged image. In contrast, in the cirrhotic liver, desmin-positive cells underwent morphological changes characterized by multiple cellular processes. The merged image of desmin and c-Met staining indicated that the c-Met receptor was co-localized in desmin-positive cells. Thus, the c-Met receptor is expressed in portal myofibroblasts and/or activated HSCs, at least in the cirrhotic liver.
Although HSCs underwent phenotypic change into myofibroblast-like cells, lack of any potential to proliferate raised the question as to the pivotal role of activated HSCs in cirrhotic change of the liver.7 On the other hand, portal myofibroblasts retain rapidly proliferating ability in vitro. It is notable that there is a proliferation of periportal and perivenous liver myofibroblasts in cirrhotic rat livers in distinct models,5,6 suggesting a pivotal role of portal myofibroblasts in fibrotic change of the liver. Together with our finding that the c-Met receptor is expressed in proliferating liver myofibroblasts, we focused on biological actions of HGF on portal myofibroblasts.
Anti-Proliferative Responses Induced by HGF in Liver Myofibroblasts
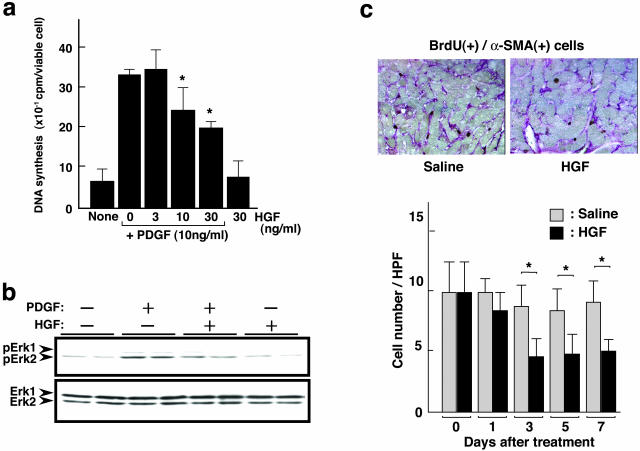
In terms of cell growth regulation, HGF bidirectionally regulates cell growth in a cell type-dependent manner. HGF inhibits cell growth in distinct types of cells and apoptotic cell death is involved in HGF-induced cell growth inhibition.27–32 Taken together with the above finding that portal myofibroblasts are potential targets of HGF, we investigated biological actions of HGF on portal myofibroblasts, focusing on proliferation and apoptosis of portal myofibroblasts. Because PDGF is a potent mitogen for portal myofibroblasts and is involved in pathogenesis of hepatic fibrosis,33,34 we examined effects of PDGF and HGF on DNA synthesis of portal myofibroblasts. To prepare portal myofibroblasts, the cells were obtained after serial passages (three to five passages) of HSC culture and subjected to measurement of DNA synthesis. Addition of PDGF to cultures of portal myofibroblasts increased DNA synthesis to a fivefold higher level greater than that in control cultures (Figure 3a). On the other hand, addition of HGF alone had no significant effect on DNA synthesis of portal myofibroblasts. However, the simultaneous addition of HGF in the presence of PDGF dose dependently inhibited the DNA synthesis induced by PDGF. Fifty percent inhibition was seen in the case of 30 ng/ml of HGF.
Figure 3.
Effect of PDGF and HGF on DNA synthesis and ERK1/2 phosphorylation in portal myofibroblasts, and changes in α-SMA-positive cells undergoing DNA synthesis in cirrhotic livers in rats untreated or treated with HGF. a: Changes in DNA synthesis. Portal myofibroblasts were cultured in the absence or presence of PDGF, HGF, and their combinations for 20 hours and DNA synthesis was measured by pulse-labeling with [methyl-3H] thymidine. Each value represents the mean ± SD (n = 4). *, P < 0.05 versus cells incubated with PDGF alone. b: Changes in ERK1/2 phosphorylation in portal myofibroblasts by stimuli with PDGF, HGF, and their combination. Cells were cultured in the absence or presence of PDGF, HGF, and their combination for 24 hours and cell lysates were subjected to SDS-PAGE. Phosphorylated ERK1/2 and total ERK1/2 proteins were sequentially detected using Western blots with antibodies against anti-phosphorylated ERK1/2 and ERK1/2, respectively. c: Distribution and changes in α-SMA-positive cells undergoing DNA synthesis in cirrhotic livers in rats untreated or treated with HGF. Top: Photographs show distribution of α-SMA-positive cells undergoing DNA synthesis in liver sections obtained from cirrhotic livers on day 3 after HGF treatment. Expression of α-SMA was detected by immunostaining (red) and cells undergoing DNA synthesis were detected by BrdU incorporation and subsequent immunostaining (brown). Bottom: Graph shows change in the number of α-SMA-positive cells undergoing DNA synthesis after HGF. The number of cells double positive for α-SMA and BrdU incorporation was determined in liver sections subjected to immunostaining for α-SMA and BrdU incorporation. Rats were subjected to DMN administration for 5 weeks, followed by daily administration of HGF. Each value represents the mean ± SD (n = 8). *, P < 0.05. Original magnifications, ×200.
Because ERK1/2 plays a definitive role in proliferation of portal myofibroblasts downstream of the PDGF receptor, we next analyzed changes in activation of ERK1/2 (Figure 3b). Portal myofibroblasts were cultured in the absence or presence of PDGF, HGF, or PDGF + HGF for 24 hours and cell extracts were subjected to Western blots. In serum-starved cells, ERK1/2 was marginally phosphorylated, whereas it was phosphorylated in the presence of PDGF. In contrast, no significant change in the marginal phosphorylation of ERK1/2 was seen in the presence of HGF alone. On the other hand, in cells stimulated with PDGF and HGF, the level of phosphorylated ERK1/2 was higher than that in control cultures but was lower than that in cells stimulated with PDGF alone. These results suggested that HGF stimulus is suppressive on PDGF-induced ERK1/2 phosphorylation in portal myofibroblasts and that the suppression of PDGF-induced ERK1/2 phosphorylation by HGF may be involved in inhibitory effects of HGF on PDGF-induced DNA synthesis in portal myofibroblasts.
We therefore asked if the suppressive effect of HGF on PDGF-induced DNA synthesis in portal myofibroblasts is associated with the decrease in expansion of α-SMA-positive cells (portal myofibroblasts and activated HSCs) in cirrhotic livers. When hepatic tissue PDGF levels were determined using enzyme-linked immunosorbent assay, the PDGF level increased from 95 ng/g tissue in normal liver to 315 ng/ml in cirrhotic liver. Previous studies indicated that hepatic macrophages and activated HSCs express PDGF,35,36 suggesting that these cells may be major sources of PDGF in fibrotic liver. Consistently, infiltration of hepatic macrophages as detected by immunohistochemistry was markedly increased in cirrhotic liver compared to findings in the normal liver (not shown). On the other hand, expression of PDGF receptor was noted in α-SMA-positive portal myofibroblasts.37 Together with the potent mitogenic actions of PDGF on portal myofibroblasts, PDGF increased in the cirrhotic liver might be involved in proliferation of portal myofibroblasts in cirrhotic livers. To determine changes in the proliferation of portal myofibroblasts in cirrhotic livers by HGF treatment, the number of portal myofibroblasts undergoing DNA synthesis was quantitated in hepatic tissue sections subjected to immunohistochemistry for α-SMA and BrdU (Figure 3c). In control livers of rats not given HGF treatment, the number of cells double positive for α-SMA and BrdU reached 9.9 ± 2.5 cells per 100 nuclei in microscopic fields and remained at similar levels for 7 days. In rat treated with HGF, the number of α-SMA-positive cells undergoing DNA synthesis decreased to 5.3 ± 1.6 on day 3 after HGF treatment and a similar suppressive effect was seen for up to day 7. These findings indicate that HGF treatment suppresses proliferation of portal myofibroblasts in cirrhotic livers and that the counteractive effect of HGF on mitogenic action of PDGF in portal myofibroblasts may possibly be involved in the growth suppression of portal myofibroblasts.
Apoptosis of Activated Liver Myofibroblasts Enhanced by HGF
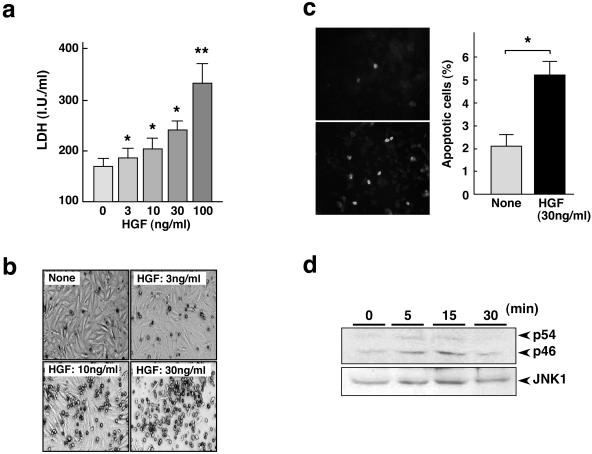
Of note, apoptotic cell death contributes to resolution of hepatic fibrosis.7–11 Together with previous findings that HGF is proapoptotic in distinct types of cells,30–32 we hypothesized a potential involvement of HGF in the apoptosis of liver myofibroblasts. To determine changes in the apoptosis of portal myofibroblasts, the cells were cultured in the absence or presence of HGF for 48 hours and enzymatic activity of LDH released from the dying cells was measured (Figure 4a). LDH is released from necrotic cells and from those in later stages of apoptosis.38–40 LDH activity was dose dependently increased by addition of HGF, suggesting that HGF enhances cell death in portal myofibroblasts. Consistently, the increase in cell death of liver myofibroblasts by HGF was obvious based on appearances. Cultivation with HGF for 5 days dose dependently increased the number of cells detached from culture plates (Figure 4b). To determine whether the increases in LDH release and detached cells were because of apoptotic cell death, the cells were cultured in the absence or presence of HGF for 48 hours then subjected to TUNEL staining (Figure 4c). In control cultures, the number of apoptotic cells reached 2.1 ± 0.5%, whereas HGF increased the number of apoptotic cells to 5.2 ± 0.6%, which means that HGF facilitates the apoptosis of portal myofibroblasts.
Figure 4.
Apoptosis and JNK1 phosphorylation promoted by HGF in portal myofibroblasts. a: Change in LDH activity in culture media. LDH activity was measured in conditioned media obtained after cultivation of portal myofibroblasts for 48 hours in the absence or presence of HGF. Each value represents the mean ± SD (n = 6). *, P < 0.05 and **, P < 0.01 versus nontreated control cells. b: Appearances of portal myofibroblasts cultured in the absence or presence of 3 ng/ml, 10 ng/ml, and 30 ng/ml HGF for 5 days. c: Apoptosis in activated liver myofibroblasts as detected by TUNEL staining. Cells were cultured in the absence or presence of HGF for 48 hours and subjected to TUNEL staining (left). The number of TUNEL-positive cells is shown at the right. Each value represents the mean ± SD. *, P < 0.01. d: Phosphorylation of JNK-1 enhanced by HGF in portal myofibroblasts. Phosphorylation of two forms of JNK-1 (p54 and p46) was detected using Western blots.
Because the JNK1 pathway is critical in mediating apoptosis, and Conner and colleagues32 showed the close involvement of JNK1 in HGF-mediated apoptosis, we investigated the activation status of JNK1 in portal myofibroblasts. Portal myofibroblasts were treated with HGF and phosphorylated/activated JNK was detected using Western blots (Figure 4d). There are two known forms of JNK1, p54/JNK1 and p46/JNK1 and these isoforms are biosynthesized by alternative splicing. In control cultures, p46/JNK was weakly detected, but p54/JNK1 was mostly undetectable. When cells were treated with HGF, both phosphorylated p54/JNK1 and p46/JNK1 increased at 5 minutes after HGF stimulus. Phosphorylation of p54/JNK1 and p46/JNK1 was seen for up to 15 minutes and then this phosphorylation state decreased 30 minutes after HGF treatment.
In Vivo Enhancement of HSC Apoptosis by HGF
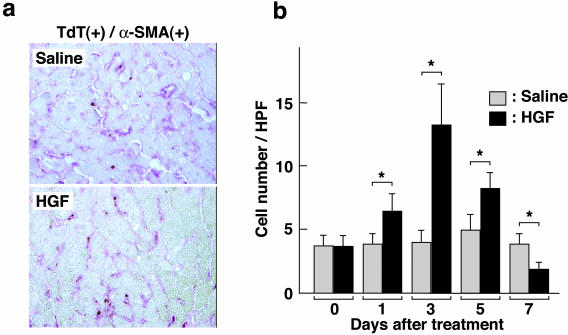
Based on the finding that HGF induces apoptotic responses in portal myofibroblasts, we analyzed effects of HGF administration on apoptotic cell death in α-SMA-positive cells in cirrhotic livers (Figure 5). Distribution and the number of α-SMA-positive cells undergoing apoptosis in the cirrhotic liver were determined by immunohistochemical analysis of hepatic sections. Five weeks after DMN treatment, the number of apoptotic cells double positive for both α-SMA and TUNEL reached 3.5 ± 1.0 per 100 nuclei as seen microscopically. In control rats treated with saline alone, the number of apoptotic cells did not change significantly during the 7 subsequent days. On the other hand, when rats were daily administered HGF, the number of apoptotic cells increased from day 1 and reached a peak on day 3. The number of apoptotic cells reached 13.3 ± 3.4, the value being approximately threefold greater than control values. Increased apoptosis by HGF treatment was seen on day 5 but on day 7 decreased to a level lower than that of controls. Thus HGF administered to rats with liver cirrhosis facilitated apoptotic cell death in α-SMA-positive cells that are likely to be portal myofibroblasts.
Figure 5.
Distribution and changes in apoptotic liver myofibroblasts in cirrhotic livers in rats untreated and treated with HGF. a: Distribution of apoptotic α-SMA-positive cells in liver sections obtained from cirrhotic livers on day 3 after HGF treatment. α-SMA-positive cells were detected by immunostaining (red) and apoptotic cells were detected using TUNEL staining (brown). b: Change in the number of apoptotic α-SMA-positive cells after HGF treatment. The number of cells double-positive for α-SMA and TUNEL staining was determined in liver sections. Rats were subjected to DMN administration for 5 weeks, followed by daily administration of HGF. Each value represents the mean ± SD (n = 8). *, P < 0.05. Original magnifications, ×200.
Discussion
As a result of hepatic injury and/or inflammation, activated HSCs and portal myofibroblasts play a role as a major cellular source of ECM deposited in liver fibrosis/cirrhosis. On the other hand, during spontaneous recovery from liver fibrosis, activated HSCs and portal myofibroblasts underwent apoptotic cell death.7–11 Thus apoptosis of activated HSCs and portal myofibroblasts are closely involved in recovery from liver fibrosis/cirrhosis. In the present study, we found that portal myofibroblasts and activated HSCs express the c-Met receptor, HGF inhibits DNA synthesis and enhanced apoptotic cell death for portal myofibroblasts. All these biological effects of HGF on portal myofibroblasts were associated with recovery from liver cirrhosis in rats.
Growth inhibitory effect of HGF was initially noted in an unexpected finding that a tumor cytotoxic factor that inhibits growth of several types of tumor cells was identified to be HGF.27 Although subsequent studies revealed that HGF inhibits proliferation of several types of neoplastic cell lines,28–30 intracellular signaling events by which HGF inhibits cell proliferation are not understood, except in a restricted numbers of studies. In a HepG2 human hepatoma cell line, hyperphosphorylation or a longer phosphorylation of ERK in the presence of HGF was associated with growth inhibitory effects of HGF,41,42 findings which seem to be inconsistent with our present results. In portal myofibroblasts, a lesser degree of ERK phosphorylation in cells treated with PDGF plus HGF than that seen in PDGF alone was associated with suppressive effects of HGF on PDGF-dependent growth. Perhaps, the phosphorylation status of ERK modulates growth inhibitory effects of HGF, but may possibly be cell type-dependent, eg, normal versus tumor cells. Because proliferation of liver myofibroblasts on PDGF stimulus is tightly associated with the phosphorylation status of ERK,33,34 decreased ERK phosphorylation by HGF seems to be partly involved in the suppressive effect of HGF on PDGF-induced cell proliferation in portal myofibroblasts.
For a variety of normal and neoplastic cells, including hepatocytes, renal epithelial cells, and cardiomyocytes, HGF has potent anti-apoptotic actions, while HGF has the potential to induce apoptotic cell death in some neoplastic cell lines.30–32 Conner and colleagues32 suggested that HGF-mediated apoptosis involves activation of JNK1. JNKs (JNK1 and JNK2) are activated by various extracellular stimuli, including growth factors, and play a role in determining whether cells survive or undergo apoptosis.43,44 Likewise, Ron/Stk that belongs to the c-Met receptor tyrosine kinase family mediates apoptotic cell death, as well as cell proliferation, and activation of JNK is involved in Ron/Stk-mediated apoptosis.45 Activation of JNK1 by HGF may possibly be involved in c-Met receptor-mediated apoptosis in portal myofibroblasts.
To search for potential mechanisms involved in the apoptosis of HSCs, expression of receptors that mediate apoptosis was analyzed.10,46 Fas receptor and Fas-ligand became increasingly expressed during in vitro activation of HSCs and Fas-mediated apoptosis is involved in spontaneous apoptosis of HSCs.10 On the other hand, p75 nerve growth factor receptor expression increased during activation of HSCs and nerve growth factor-stimulation increased apoptosis in cultured HSCs.46 We have newly identified that HGF and the c-Met receptor system is involved in the apoptosis of portal myofibroblasts. Although the contribution of each receptor-ligand system in resolution of liver fibrosis/cirrhosis remains to be further addressed, these distinct systems may cooperatively regulate apoptosis of activated HSCs and portal myofibroblasts toward resolution. In addition to c-Met receptor expression in portal myofibroblasts, HGF was expressed in the cirrhotic liver, and hepatic HGF level changed from 263 to 145 ng/g tissue in the normal and cirrhotic livers, respectively. The decrease in hepatic HGF level seems to somewhat increase susceptibility to fibrotic change of the liver and it may possibly be because of the increased expression of TGF-β1 in the cirrhotic rat liver,21,23 because TGF-β1 is a potent inhibitor for expression of HGF.47 On the other hand, we propose that apoptosis enhanced by administration of HGF is likely to be a mechanism by which HGF facilitates resolution of liver fibrosis/cirrhosis.
Previous approaches using distinct animal models of tissue fibrosis provide evidence that HGF has anti-fibrotic actions on tissue fibrosis, including liver cirrhosis,20–24 renal fibrosis,48,49 lung fibrosis,18,50 and cardiomyopathy.51 These studies addressed mechanisms involved in anti-fibrotic actions of HGF, focusing on cell proliferation, expression of TGF-β, and induction of proteases involved in the breakdown of ECM. However, little attention has been directed toward potential mechanisms to directly regulate growth and apoptosis of myofibroblasts, the principle cell type responsible for tissue fibrosis. Taking it into consideration that expansion and apoptosis in myofibroblasts are, respectively, associated with tissue fibrosis and its resolution in distinct tissues, our findings may provide a better understanding of pathogenic as well as therapeutic mechanisms involved in the enhanced resolution from fibrotic disorders by HGF.
Advanced liver fibrosis and cirrhosis are generally considered to be irreversible conditions even after removal of the hepatic injury; however, histopathological assessments of biopsy tissues from patients with liver fibrosis, who have been effectively treated, indicate that recovery with remodeling of excess ECM is feasible.8,52,53 These results suggest that a capacity for fibrous tissue remodeling toward resolution exists in liver fibrosis/cirrhosis. If pathological changes of liver fibrosis/cirrhosis are regulated by a counterbalance between an endogenous capacity for fibrous tissue remodeling and fibrogenic events, augmentation of the capacity for fibrous tissue remodeling could be considerable for treatment of liver fibrosis/cirrhosis.
In summary, we obtained evidence that the c-Met receptor was expressed in liver myofibroblasts, and HGF suppressed proliferation and stimulated apoptotic cell death in liver myofibroblasts, all these events being involved in a rapid resolution from liver cirrhosis by HGF. These results may provide a rationale for the potential therapeutic value of HGF for treatment of patients with liver fibrosis/cirrhosis, as based on augmentation of the capacity for fibrous tissue remodeling.
Acknowledgments
We thank M. Ohara (Fukuoka) for comments and for language assistance.
Footnotes
Address reprint requests to Dr. Toshikazu Nakamura, Division of Molecular Regenerative Medicine, Course of Advanced Medicine, Osaka University Graduate School of Medicine, Yamada-oka 2-2-B7, Suita, Osaka 565-0871, Japan. E-mail: nakamura@onbich.med.osaka-u.ac.jp.
Supported by the Ministry of Education, Science, Technology, Sports, and Culture of Japan.
Present address of W.-H.K.: Department of Surgery, Ajou University School of Medicine, San 5, Wonchon-Dong, Paldal-Gu, Suwon, 442-721, Korea.
References
- Friedman SL. The cellular basis of hepatic fibrosis. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- Alcolado R, Arther MJP, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci. 1997;92:103–112. doi: 10.1042/cs0920103. [DOI] [PubMed] [Google Scholar]
- Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665–672. doi: 10.1007/s005350070045. [DOI] [PubMed] [Google Scholar]
- Knittel T, Kobold D, Saile B, Grundmann A, Neubauer K, Piscaglia F, Ramadori G. Rat liver myofibroblasts and hepatic stellate cells: different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology. 1999;117:1205–1221. doi: 10.1016/s0016-5085(99)70407-5. [DOI] [PubMed] [Google Scholar]
- Bhunchet E, Wake K. Role of mesenchymal cell populations in porcine serum-induced rat liver fibrosis. Hepatology. 1992;16:1452–1473. doi: 10.1002/hep.1840160623. [DOI] [PubMed] [Google Scholar]
- Tuchweber B, Desmouliere A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest. 1996;74:265–278. [PubMed] [Google Scholar]
- Ramadori G, Saile B. Mesenchymal cells in the liver—one cell type or two? Liver. 2002;22:283–294. doi: 10.1034/j.1600-0676.2002.01726.x. [DOI] [PubMed] [Google Scholar]
- Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJP. Mechanisms of spontaneous resolution of rat liver fibrosis: hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427–436. doi: 10.1055/s-2001-17557. [DOI] [PubMed] [Google Scholar]
- Saile B, Knittel T, Matthes N, Schott P, Ramadori G. CD95/CD95L-mediated apoptosis of the hepatic stellate cell. A mechanism terminating uncontrolled hepatic stellate cell proliferation during hepatic tissue repair. Am J Pathol. 1997;151:1265–1272. [PMC free article] [PubMed] [Google Scholar]
- Saile B, Matthes N, Neubauer K, Eisenbach C, El-Armouche H, Dudas J, Ramadori G. Rat liver myofibroblasts and hepatic stellate cells differ in CD95-mediated apoptosis and response to TNF-α. Am J Physiol. 2002;283:G435–G444. doi: 10.1152/ajpgi.00441.2001. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8:404–409. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119:591–600. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Matsumoto K, Nakamura T, Montesano R. Hepatocyte growth factor increases urokinase-type plasminogen activator (u-PA) and uPA receptor expression in Madin-Darby canine kidney epithelial cells. J Biol Chem. 1992;267:20493–20496. [PubMed] [Google Scholar]
- Dunsmore SE, Rubin JS, Kovacs SO, Chedid M, Parks WC, Welgus HG. Mechanisms of hepatocyte growth factor-stimulation of keratinocyte metalloproteinase production. J Biol Chem. 1996;271:24567–24582. doi: 10.1074/jbc.271.40.24576. [DOI] [PubMed] [Google Scholar]
- Dohi M, Hasegawa T, Yamamoto K, Marshall BC. Hepatocyte growth factor attenuates collagen accumulation in a murine model of pulmonary fibrosis. Am J Respir Crit Care Med. 2000;162:2302–2307. doi: 10.1164/ajrccm.162.6.9908097. [DOI] [PubMed] [Google Scholar]
- Ozaki I, Zhao G, Mizuta T, Ogawa Y, Hara T, Kajihara S, Hisatomi A, Sakai T, Yamamoto K. Hepatocyte growth factor induces collagenase (matrix metalloproteinase-1) via the transcription factor Ets-1 in human hepatic stellate cell line. J Hepatol. 2002;36:169–178. doi: 10.1016/s0168-8278(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Matsumoto K, Ichida T, Nakamura T. Hepatocyte growth factor suppresses the onset of liver cirrhosis and abrogates lethal hepatic dysfunction in rats. J Biochem. 1995;118:643–649. doi: 10.1093/oxfordjournals.jbchem.a124958. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Imai E, Shiota A, Fujise N, Morinaga T, Higashio K. Anti-fibrogenic effect of a deletion variant of hepatocyte growth factor on liver fibrosis in rats. Hepatology. 1996;24:636–642. doi: 10.1053/jhep.1996.v24.pm0008781336. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Matsumoto K, Yamada A, Ichida T, Asakura H, Komoriya Y, Nishiyama E, Nakamura T. Preventive and therapeutic effects in rats of hepatocyte growth factor infusion on liver fibrosis/cirrhosis. Hepatology. 1997;26:81–89. doi: 10.1053/jhep.1997.v26.pm0009214455. [DOI] [PubMed] [Google Scholar]
- Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E, Fujimoto J. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- Sato M, Kakubari M, Kawamura M, Sugimoto J, Matsumoto K, Ishii T. The decrease in total collagen fibers in the liver by hepatocyte growth factor after formation of cirrhosis induced by thioacetamide. Biochem Pharmacol. 2000;59:681–690. doi: 10.1016/s0006-2952(99)00359-7. [DOI] [PubMed] [Google Scholar]
- Seki T, Ihara I, Sugimura A, Shimonishi M, Nishizawa T, Asami O, Hagiya M, Nakamura T, Shimizu S. Isolation and expression of cDNA for different forms of hepatocyte growth factor from human leukocyte. Biochem Biophys Res Commun. 1990;172:321–327. doi: 10.1016/s0006-291x(05)80212-8. [DOI] [PubMed] [Google Scholar]
- Knook DL, Seffelaar AM, de Leeuw AM. Fat-storing cells of the rat liver. Their isolation and purification. Exp Cell Res. 1982;139:468–471. doi: 10.1016/0014-4827(82)90283-x. [DOI] [PubMed] [Google Scholar]
- Higashio K, Shima N, Goto M, Itagaki Y, Nagao M, Yasuda H, Morinaga T. Identity of a tumor cytotoxic factor from human fibroblasts and hepatocyte growth factor. Biochem Biophys Res Commun. 1990;170:397–404. doi: 10.1016/0006-291x(90)91287-3. [DOI] [PubMed] [Google Scholar]
- Tajima H, Matsumoto K, Nakamura T. Hepatocyte growth factor has potent anti-proliferative activity in various tumor cell lines. FEBS Lett. 1991;291:229–232. doi: 10.1016/0014-5793(91)81291-f. [DOI] [PubMed] [Google Scholar]
- Shiota G, Rhoads DB, Wang TC, Nakamura T, Schmidt EV. Hepatocyte growth factor inhibits growth of hepatocellular carcinoma cells. Proc Natl Acad Sci USA. 1992;89:373–737. doi: 10.1073/pnas.89.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner EA, Wirth PJ, Kiss A, Santoni-Rugiu E, Thorgeirsson SS. Growth inhibition and induction of apoptosis by HGF in transformed rat liver epithelial cells. Biochem Biophys Res Commun. 1997;236:396–401. doi: 10.1006/bbrc.1997.6938. [DOI] [PubMed] [Google Scholar]
- Arakaki N, Kazi JA, Kazihara T, Ohnishi T, Daikuhara Y. Hepatocyte growth factor/scatter factor activates the apoptosis signaling pathway by increasing caspase-3 activity in sarcoma 180 cells. Biochem Biophys Res Commun. 1998;245:211–215. doi: 10.1006/bbrc.1998.8397. [DOI] [PubMed] [Google Scholar]
- Conner EA, Teramoto T, Wirth PJ, Kiss A, Garfield S, Thorgeirsson SS. HGF-mediated apoptosis via p53/bax-independent pathway activating JNK1. Carcinogenesis. 1999;20:583–590. doi: 10.1093/carcin/20.4.583. [DOI] [PubMed] [Google Scholar]
- Pinzani M. PDGF and signal transduction in hepatic stellate cells. Front Biosci. 2002;7:1720–1726. doi: 10.2741/A875. [DOI] [PubMed] [Google Scholar]
- Marra F, Arrighi MC, Fazi M, Caligiuri A, Pinzani M, Romanelli RG, Efsen E, Laffi G, Gentilini P. Extracellular signal-regulated kinase activation differentially regulates platelet-derived growth factor’s actions in hepatic stellate cells, and is induced by in vivo liver injury in the rat. Hepatology. 1999;30:951–958. doi: 10.1002/hep.510300406. [DOI] [PubMed] [Google Scholar]
- Ikura Y, Morimoto H, Ogami M, Jomura H, Ikeoka N, Sakurai M. Expression of platelet-derived growth factor and its receptor in livers of patients with chronic liver disease. J Gastroenterol. 1997;32:496–501. doi: 10.1007/BF02934089. [DOI] [PubMed] [Google Scholar]
- Marra F, Valente AJ, Pinzani M, Abboud HE. Regulation of platelet-derived growth factor secretion and gene expression in human liver fat-storing cells. Gastroenterology. 1994;107:1110–1117. doi: 10.1016/0016-5085(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Kinnman N, Francoz C, Barbu V, Rey C, Hultcranz R, Poupon R, Housset C. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163–173. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]
- Chen Z, Chua CC, Ho Y, Hamdy R, Chua BHL. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- Crenesse C, Laurens M, Gugenheim J, Heurteaux C, Cursio R, Rossi B, Schmid-Alliana S. Intermittent ischemia reduces warm hypoxia-reoxygenation-induced JNK1/SAPK1 activation and apoptosis in rat hepatocytes. Hepatology. 2001;34:972–978. doi: 10.1053/jhep.2001.28709. [DOI] [PubMed] [Google Scholar]
- Gressner AM, Polzar B, Lahme B, Mannherz H-G. Induction of rat liver parenchymal cell apoptosis by hepatic myofibroblasts via transforming growth factor-β. Hepatology. 1996;23:571–581. doi: 10.1002/hep.510230324. [DOI] [PubMed] [Google Scholar]
- Shima N, Stolz DB, Miyazaki M, Gohda E, Higashio K, Michalopoulos GK. Possible involvement of p21/waf1 in the growth inhibition of HepG2 cells induced by hepatocyte growth factor. J Cell Physiol. 1998;177:130–136. doi: 10.1002/(SICI)1097-4652(199810)177:1<130::AID-JCP14>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tsukada Y, Miyazawa K, Kitamura N. High intensity ERK signal mediates hepatocyte growth factor-induced proliferation inhibition of the human hepatocellular carcinoma cell line HepG2. J Biol Chem. 2001;276:40968–40976. doi: 10.1074/jbc.M010890200. [DOI] [PubMed] [Google Scholar]
- Dunn C, Wiltshire C, MacLaren A, Gillespie DA. Molecular mechanism and biological functions of c-Jun N-terminal kinase signaling via the c-Jun transcription factor. Cell Signal. 2002;14:585–593. doi: 10.1016/s0898-6568(01)00275-3. [DOI] [PubMed] [Google Scholar]
- Lin A. Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays. 2003;25:17–24. doi: 10.1002/bies.10204. [DOI] [PubMed] [Google Scholar]
- Iwama A, Yamaguchi N, Suda T. STK/RON receptor tyrosine kinase mediates both apoptotic and growth signals via the multifunctional docking site conserved among the HGF receptor family. EMBO J. 1996;15:5866–5875. [PMC free article] [PubMed] [Google Scholar]
- Trim N, Morgan S, Evans M, Issa R, Fine D, Afford S, Wilkins B, Iredale J. Hepatic stellate cells express the low affinity nerve growth factor receptor p75 and undergo apoptosis in response to nerve growth factor stimulation. Am J Pathol. 2000;156:1235–1243. doi: 10.1016/S0002-9440(10)64994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Tajima H, Okazaki H, Nakamura T. Negative regulation of hepatocyte growth factor gene expression in human lung fibroblasts and leukemic cells by transforming growth factor-β1 and glucocorticoids. J Biol Chem. 1992;267:24917–24120. [PubMed] [Google Scholar]
- Mizuno S, Kurosawa T, Matsumoto K, Horikawa Y, Okamoto M, Nakamura T. Hepatocyte growth factor prevents renal fibrosis and dysfunction in a mouse model of chronic renal disease. J Clin Invest. 1998;101:1827–1834. doi: 10.1172/JCI1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Dai C, Liu Y. Systemic administration of naked plasmid encoding hepatocyte growth factor ameliorates chronic renal fibrosis in mice. Gene Ther. 2001;8:1470–1479. doi: 10.1038/sj.gt.3301545. [DOI] [PubMed] [Google Scholar]
- Yaekashiwa M, Nakayama S, Ohnuma K, Sakai T, Abe T, Satoh K, Matsumoto K, Nakamura T, Takahashi T, Nukiwa T. Simultaneous or delayed administration of hepatocyte growth factor equally represses the fibrotic changes in murine lung injury induced by bleomycin. A morphologic study. Am J Respir Crit Care Med. 1997;156:1937–1944. doi: 10.1164/ajrccm.156.6.9611057. [DOI] [PubMed] [Google Scholar]
- Taniyama Y, Morishita R, Aoki M, Hiraoka K, Yamasaki K, Hashiya N, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Angiogenesis and antifibrotic action by hepatocyte growth factor in cardiomyopathy. Hypertension. 2002;40:47–53. doi: 10.1161/01.hyp.0000020755.56955.bf. [DOI] [PubMed] [Google Scholar]
- Rojkind M, Dunn MA. Hepatic fibrosis. Gastroenterology. 1979;76:849–863. [PubMed] [Google Scholar]
- Hunt J. Long-term follow-up of patients with hepatitis B treated with interferon. Infections Cytokines. 1992;20:6–9. [Google Scholar]