Abstract
Ischemic injury is invoked as a mechanism contributing to end-organ damage and other complications of sickle cell disease (SCD). However, the intrinsic sensitivity of tissues in SCD to ischemic insults has never been addressed. We examined the effect of renal ischemia in a transgenic mouse expressing human sickle hemoglobin. Twenty-four hours after bilateral, total renal artery occlusion for 15 minutes, transgenic sickle mice exhibited worse renal function and more marked histological injury. With bilateral renal ischemia of greater duration (22.5 minutes), and after 6 hours, transgenic sickle mice exhibited massive vascular congestion, sickling of red blood cells, more marked histological injury in the kidney, and more prominent congestion in the capillary beds in the lungs and heart. Additionally, serum amyloid P-component, the murine homologue of C-reactive protein, was markedly increased in transgenic sickle mice as compared to wild-type mice. Twenty-four hours after bilateral renal ischemia for 22.5 minutes, transgenic sickle mice exhibited 28% mortality, with no mortality observed in any other group. With bilateral renal ischemia of short or long duration, renal expression of caspase-3 was most prominent in transgenic sickle mice subjected to ischemia. Thus, renal ischemia in this murine model induces more severe renal injury and extrarenal complications. We conclude that tissues in SCD exhibit heightened vascular congestion and sensitivity to ischemia and that clinically apparent or silent episodes of ischemia may contribute to the complications of SCD.
Vaso-occlusive disease contributes dominantly to the morbidity and mortality of sickle cell disease (SCD).1–6 The basis for such vaso-occlusion involves such processes as intracellular polymerization of hemoglobin S, altered erythrocyte rigidity and rheology, endothelial activation, enhanced endothelial adhesiveness of erythrocytes, procoagulant processes, the elaboration of vasoactive species, and neurogenic processes.1–6 In its most florid form, as occurs during acute sickle crisis, vaso-occlusive disease impairs, abruptly and severely, the perfusion of macrocirculatory and microcirculatory beds; and although these crisis episodes eventually resolve and perfusion of these circulatory beds returns, ischemic injury and tissue infarction are not infrequently left in their wake.1–6 Even in patients with SCD in steady state, smoldering, low-grade vaso-occlusion likely occurs in a periodic, subclinical, and self-remitting manner, thereby incurring a modicum of ischemia in dependent circulatory beds. For example, studies of the microcirculation by computer-assisted intravital microscopy, undertaken in patients with SCD during steady state, demonstrate diminished red blood cell (RBC) velocity along with sludging and compaction of RBCs (boxcar phenomenon) in the microvasculature, the latter often distended and obstructed.5 The notion has thus appeared that the end-organ damage and other complications of SCD may reflect, at least in part, the cumulative effects of recurrent cycles of ischemic and ischemia-reperfusion injury imposed by episodic, clinically apparent or silent, vaso-occlusive disease.1–6
Although clearly appealing as a pathogenetic pathway in SCD, this paradigm fails to take cognizance of the phenomenon of ischemic preconditioning. Occurring in diverse tissues, ischemic preconditioning refers to the remarkable resistance of tissues to ischemic insults that is conferred by previous, less severe or comparable episodes of ischemia.7–14 This phenomenon was first described some 2 decades ago by Zager and collaborators7,8 in studies undertaken in the kidney, and subsequently was demonstrated in tissues that include the heart, liver, brain, gastrointestinal tract, and skeletal muscle.7–14 The cellular basis for this phenomenon includes alterations in the MAP kinase system, as described in the kidney,9,10 and in alterations in the KATP channel, as described in the heart.11 Ischemic preconditioning is clinically evident as reflected by the fact that adverse myocardial remodeling after myocardial infarction is diminished in patients with episodes of angina before myocardial infarction.13 Elucidating the biological basis for such resistance to ischemia may suggest strategies that reduce ischemic damage sustained in clinically defined settings.
This phenomenon of ischemic preconditioning, in conjunction with the occurrence of subclinical and clinical episodes of ischemia imposed by recurrent vaso-occlusive disease in SCD, raise the possibility that tissues in SCD may be resistant, rather than sensitive, to an ischemic insult. Impaired perfusion of microcirculatory beds and attendant ischemia occurring in a periodic and self-remitting manner in patients with SCD, and documented not only during sickle crisis but also during the crisis-free, steady state, may condition tissues in these patients to withstand subsequent and more severe ischemic insults. The present study thus addresses the hitherto unexplored question regarding the innate sensitivity of tissues in the sickle state to an ischemic event. Our study used a well-established, transgenic murine model that faithfully recapitulates the critical features of SCD; even in the absence of stressors that provoke sickle crisis, this model exhibits vaso-occlusion in microcirculatory beds,15 thereby raising the possibility that ischemic preconditioning of tissues may occur in the natural course of this model. The kidney was used as the organ subjected to ischemia (induced by the clamp model) for the following reasons: ischemic preconditioning is well established in this organ;7–10 ischemic insults to the kidney in SCD have long been recognized;6,16–21 and acute and chronic involvement of the kidney contributes substantially to morbidity and mortality in SCD.6,16–21
Materials and Methods
Model of Ischemia-Reperfusion Injury in Mice
The transgenic murine model of SCD, used in the current study and in our previous publications,15,22 is on a C57Bl/6 background, and is homozygous for the murine β-globin deletion and carries two transgenes, αHβS and αHβS-Antilles.23 Renal ischemia was imposed in wild-type and transgenic sickle mice by bilateral and total occlusion of the renal artery under pentobarbital anesthesia.24,25 The procedure was performed on a heated surface to ensure maintenance of normal body temperature. The kidneys were exposed via a midline abdominal incision and the renal pedicles carefully dissected. Depending on the protocol, the pedicles were clamped bilaterally for 15 or 22.5 minutes using nontraumatic sterile clamps (RS5426, Micro Aneurysm clip, straight, 10 mm, 125 g pressure; Roboz Surgical Instruments, Rockville, MD). A sham procedure in the wild-type and transgenic sickle mice included anesthesia and laparotomy but omitted dissection or clamping of the renal pedicle. After removal of the renal clamps (applied for 15 or 22.5 minutes), the abdominal wall was sutured. After 6 or 24 hours, mice were sacrificed for harvesting of renal or other tissues. Thus, the main protocols in this study included observations undertaken 24 hours after renal ischemia for 15 minutes, and observations undertaken at 6 and 24 hours after renal ischemia for 22.5 minutes. In one additional protocol, transgenic sickle and wild-type mice underwent unilateral clamping of the left renal pedicle for 22.5 minutes, and the contralateral, nonclipped kidneys were examined 6 hours after unilateral renal ischemia.
Assessment of Renal Injury
In some studies, renal function was assessed after ischemia by measurement of plasma creatinine and blood urea nitrogen (BUN) using a Creatinine Analyzer 2 and a BUN Analyzer 2, respectively (Beckman Instruments, Fullerton, CA).
Western Analysis for Expression of Active Caspase-3
Kidney tissue was homogenized in a buffer consisting of phosphate-buffered saline containing 1% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (Bio-Rad, Hercules, CA), and a cocktail of protease inhibitors (Complete; Roche, Indianapolis, IN). Homogenates were incubated on ice with occasional agitation for 90 minutes. Western blot analyses were performed as described in detail in our previous publications.26,27 Briefly, 100 μg protein aliquots were separated on 15% Tris-HCl gels and transferred to polyvinylidene difluoride membranes. A mouse monoclonal primary antibody for caspase-3 (catalogue no. C76920-150; BD Transduction, San Diego, CA) was used and followed by a horseradish peroxidase-conjugated, goat anti-mouse polyclonal secondary antibody (StressGen, Victoria, BC, Canada). Equivalency of loading of Western blot analyses was assessed using a rabbit polyclonal β-actin antibody (A-2006; Sigma, St. Louis, MO). Detection was achieved using a chemiluminescence method (Amersham Pharmacia, Piscataway, NJ).
Measurement of Serum Amyloid P-Component (SAP)
Measurement of SAP was performed using an enzyme-linked immunosorbent assay method.28 Briefly, SAP protein in plasma from mice was captured onto a 96-well microplate previously coated with a sheep anti-mouse polyclonal antibody (catalogue no. 565194; Calbiochem, La Jolla, CA). For detection of captured SAP protein, a rabbit anti-mouse SAP antibody (catalogue no. 565192, Calbiochem), was used as a primary antibody followed by a horseradish peroxidase-conjugated, goat anti-rabbit IgG antibody (catalogue no. DC03L, Calbiochem) as a secondary antibody, and tetramethylbenzidine as the peroxidase substrate (catalogue no. 555214; BD Pharmingen, San Diego, CA). Developed color was detected at 450 nm using an absorbance plate reader and quantified against a reference curve constructed using a mouse SAP standard (catalogue no. 565192, Calbiochem).
Statistics
Results are expressed as means ± SEM. For comparisons involving more than two groups, analysis of variance was used followed by Student’s-Neumann-Keuls test. Mortality was assessed by Fisher’s exact test. Results are considered significant for P < 0.05.
Results
Studies Undertaken 24 Hours after Bilateral Renal Ischemia for 15 Minutes
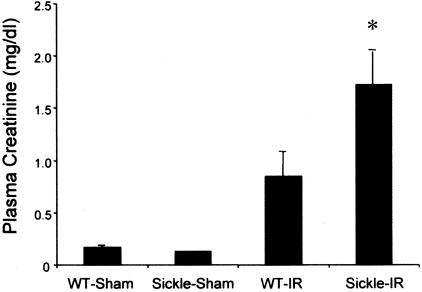
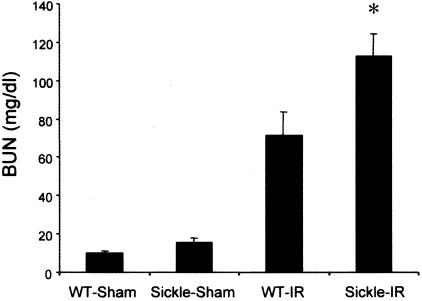
Renal ischemia induced a rise in plasma creatinine and BUN in both wild-type and sickle mice (Figures 1 and 2). However, concentrations of plasma creatinine and BUN after renal ischemia were significantly greater in sickle mice as compared with wild-type mice. Additionally, these markers of renal function were quite congruent because the correlation coefficient between plasma creatinine and BUN was 0.94 (P < 0.0001). Thus, after identical duration of ischemia, renal dysfunction, as assessed by BUN and plasma creatinine, is worse in sickle mice as compared with wild-type mice.
Figure 1.
Plasma creatinine in wild-type (WT) and sickle mice subjected to bilateral renal ischemia for 15 minutes (IR) or sham-ischemia (sham), and measured 24 hours after the ischemic episode. In the sham groups n = 4 whereas n = 7 in WT-IR and n = 8 in sickle-IR. *P < 0.05 versus all other groups.
Figure 2.
BUN in wild-type (WT) and sickle mice subjected to bilateral renal ischemia for 15 minutes (IR) or sham-ischemia (sham), and measured 24 hours after the ischemic episode. In the sham groups n = 4 whereas n = 7 in WT-IR and n = 8 in sickle-IR. *P < 0.05 versus all other groups.
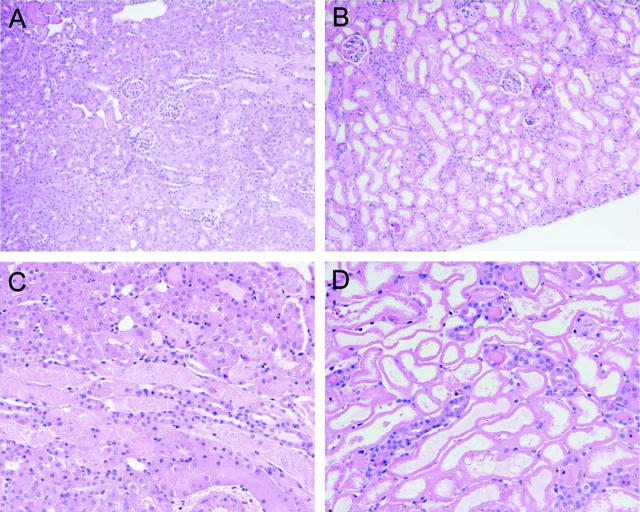
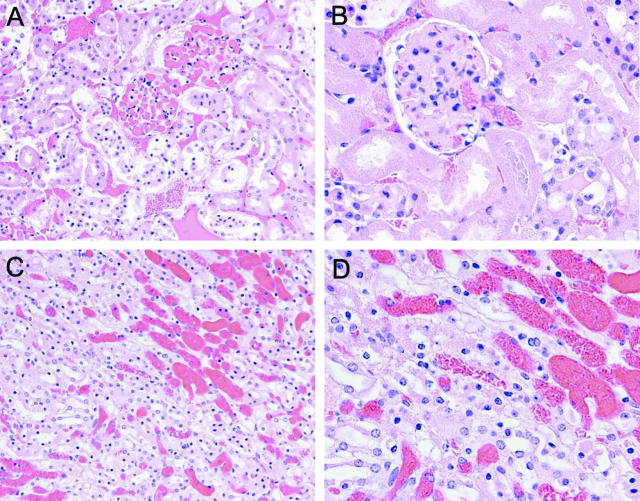
This exacerbation of ischemic damage to the kidney in sickle mice, as reflected by functional markers, was corroborated by assessment of renal histological injury. The kidney in sham-operated wild-type mice showed no histological injury whereas the kidney in the sham-operated sickle mouse showed, as we have previously described,15 congestion in the renal medulla (data not shown). Histological injury induced by renal ischemia, as shown in Figure 3, was dramatically worse in sickle mice as compared to wild-type mice. Renal ischemia in wild-type mice induced occasional foci of acute tubular necrosis, tubular dilatation, and tubular cast formation, and these foci were observed mainly in the deep cortex and outer medulla (Figure 3, A and C). After renal ischemia, histological injury was markedly worse in sickle mice (Figure 3, B and D): acute tubular necrosis in sickle mice was diffuse and extended across the entire width of the cortex; large segments of the cortex in sickle mice exhibited cortical necrosis with occasional islands of viable distal nephron segments (Figure 3, B and D). Thus, after renal ischemia, histological injury is exacerbated in sickle mice and corroborates the findings obtained with markers of renal function.
Figure 3.
Histological appearance of the kidney 24 hours after 15 minutes of bilateral renal ischemia in wild-type mice (A and C) and in sickle mice (B and D) All sections are stained by H&E. Original magnifications: ×100 (A, B); ×200 (C, D).
Studies Undertaken in Sickle Mice after Renal Ischemia for 22.5 Minutes
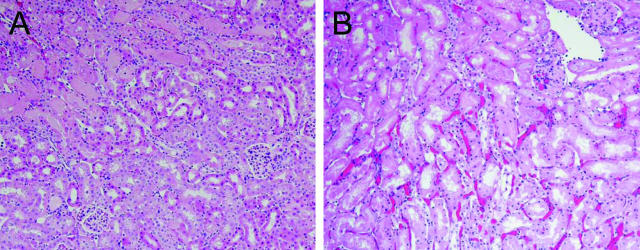
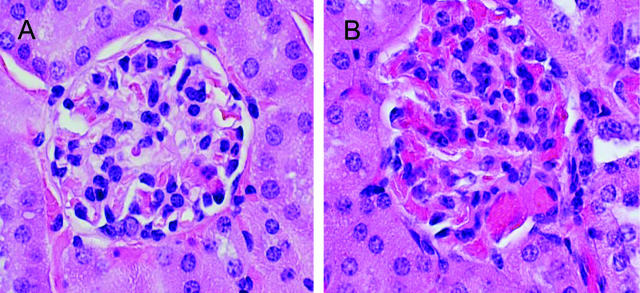
Six hours after bilateral renal ischemia for 22.5 minutes, renal histological injury was markedly greater in sickle mice. Unlike the patchy tubular necrosis observed at the corticomedullary junction in wild-type mice, sickle mice demonstrated extensive tubular necrosis that extended from the corticomedullary junction to involve the full thickness of the cortex, along with marked congestion in peritubular capillaries (Figure 4). The additional and striking findings observed at this time point, and with this greater degree of renal ischemia, were marked vascular congestion and glomerular abnormalities (Figure 5). Capillaries in the glomerular microcirculation, peritubular cortical capillaries, and capillaries in the medulla were all markedly congested, and sickling of RBCs was observed especially in the medulla (Figure 5). After ischemia, sickle mice also exhibited an acute glomerulopathy consisting of endothelialitis and mesangiolysis (Figure 5B). Neither the prominent vascular congestion nor the acute glomerulopathic changes were observed in sham-operated sickle mice or wild-type mice subjected to sham operation or ischemia.
Figure 4.
Histological appearance of the kidney 6 hours after 22.5 minutes of bilateral renal ischemia in wild-type mice (A) and in sickle mice (B). All sections are stained by H&E. Original magnifications, ×100.
Figure 5.
Histological appearance of the kidney in sickle mice 6 hours after 22.5 minutes of bilateral renal ischemia. A: Cortical section demonstrating marked vascular congestion in glomerular and peritubular capillaries. B: Cortical section demonstrating marked vascular congestion and sickled RBCs in the afferent glomerular arteriole, glomerular capillaries, and peritubular capillaries; the central glomerulus shows endothelialitis and mesangiolysis, and is surrounded by necrotic proximal tubules. C: Medullary section demonstrating marked vascular congestion. D: Medullary section demonstrating marked vascular congestion and sickled RBCs as shown in the capillary in the center of the field. All sections are stained by H&E. Original magnifications: ×200 (A, C); ×400 (B, D).
We also subjected transgenic sickle mice and wild-type mice to unilateral renal ischemia for 22.5 minutes, and examined the contralateral nonclipped kidney after 6 hours. The contralateral, nonclipped kidney in the transgenic sickle mouse subjected to unilateral ischemia commonly demonstrated prominent vascular congestion and vaso-occlusion in the glomerular microcirculation unlike the occasional RBCs seen in the glomeruli and capillaries in the contralateral kidney of the wild-type mouse subjected to unilateral ischemia (Figure 6). Such increased vascular congestion, as observed in the contralateral, nonclipped kidney in transgenic sickle mice subjected to unilateral ischemia, was not exhibited by glomeruli in transgenic sickle mice subjected to sham-ischemia (data not shown).
Figure 6.
Marked vascular congestion in glomerular and peritubular capillaries in the contralateral, nonclipped right kidney 6 hours after unilateral clamping of the left renal pedicle for 22.5 minutes in transgenic sickle mice (B), while vascular congestion was mild in the contralateral, nonclipped right kidney 6 hours after unilateral clamping of the left renal pedicle for 22.5 minutes in wild-type mice (A). All sections are stained by H&E. Original magnifications, ×600.
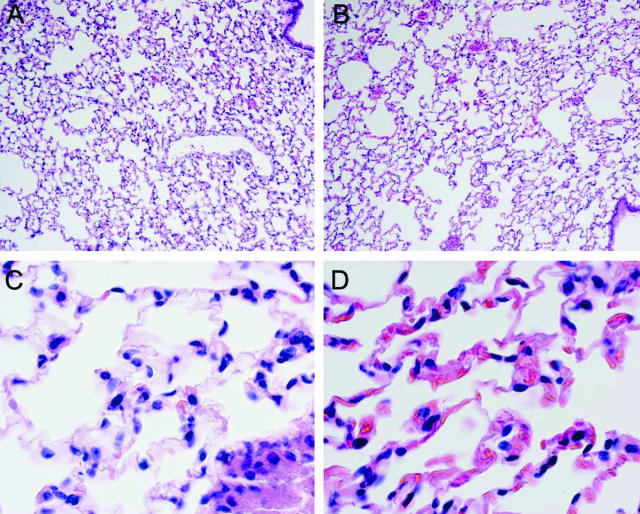
In extrarenal organs, bilateral renal ischemia induced capillary congestion in the lungs and heart in wild-type mice, and such vascular congestion was worse in sickle mice. For example as shown in Figure 7, congestion in alveolar capillaries was accentuated in sickle mice and, as shown in the high-power view, sickling of RBCs was discernible in the lungs of sickle mice subjected to bilateral renal ischemia.
Figure 7.
Histological appearance of the lung 6 hours after 22.5 minutes of bilateral renal ischemia in wild-type mice (A and C) and in sickle mice (B and D). All sections are stained by H&E. Original magnifications: ×100 (A, B); ×600 (C, D).
After bilateral renal ischemia of this duration, mounting mortality was observed in sickle mice but not in wild-type mice. Within 24 hours after bilateral renal ischemia, mortality in sickle mice subjected to ischemia was 28% whereas no mortality occurred in wild-type mice subjected to ischemia, or in wild-type or sickle mice subjected to sham operation (P < 0.05).
Concentration of SAP after Bilateral Renal Ischemia for 22.5 Minutes
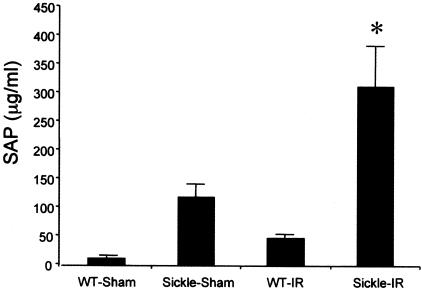
In an attempt to gain insight into possible mechanisms whereby the sickle state magnifies renal and extrarenal injury after bilateral renal ischemia, SAP, the murine homologue to C-reactive protein,28 was measured in plasma in wild-type and sickle mice after 6 hours. As demonstrated in Figure 8, SAP was elevated 10-fold in sham-operated sickle mice as compared with sham-operated wild-type mice. Ischemia increased SAP in both groups, with markedly greater elevation occurring in sickle mice, the latter concentrations achieving values more than sixfold higher than those occurring in wild-type mice subjected to ischemia.
Figure 8.
SAP in wild-type (WT) and sickle mice subjected to bilateral renal ischemia for 22.5 minutes (IR) or sham-ischemia (sham) and measured 6 hours after the ischemic episode. In the sham groups, n = 3 whereas n = 6 in WT-IR and n = 5 in sickle-IR. *P < 0.05 versus all other groups.
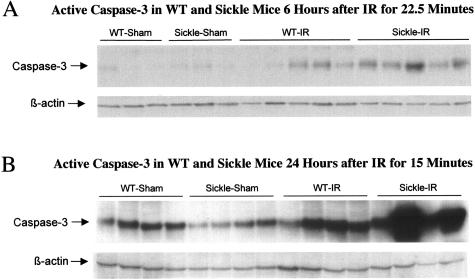
Expression of Active Caspase-3 in the Kidney after Bilateral Renal Ischemia
As a critical participant in cell death pathways, and as added evidence of worsened injury in the kidney of sickle mice, we examined expression of active caspase-3 in the kidney at 24 hours after bilateral renal ischemia for 15 minutes, and at 6 hours after bilateral renal ischemia for 22.5 minutes. As shown in Figure 9, active caspase-3 was induced in wild-type mice by ischemia, and this was markedly enhanced in sickle mice subjected to ischemia.
Figure 9.
Expression of active caspase-3 in wild-type (WT) and sickle mice at 6 hours after bilateral renal ischemia (IR) or sham-ischemia (sham) for 22.5 minutes (A), and at 24 hours after bilateral renal ischemia (IR) or sham-ischemia (sham) for 15 minutes (B). Equivalency of loading of the Western analysis was assessed by β-actin.
Discussion
As assessed by renal histological injury, markers of renal function, and renal expression of cell death-related proteins such as caspase-3, we demonstrate that sickle mice exhibit increased, rather than decreased, sensitivity to renal ischemia. This sensitivity of sickle mice to ischemia imposed on the kidney extended beyond the confines of the kidney: after bilateral renal ischemia of 22.5 minutes in duration, sickle mice exhibited more marked vascular congestion in extrarenal organs such as the lungs and heart, greater increments in the acute phase reactant, SAP, and mounting mortality. In aggregate, these findings demonstrate that in response to renal ischemia, renal damage and extrarenal complications arising from such ischemia were all accentuated in sickle mice.
Vascular congestion in wild-type mice after ischemia was quite mild and confined to the peritubular capillaries. In contrast, sickle mice subjected to ischemia exhibited dramatic congestion in the microcirculation that involved not only the medullary and cortical capillaries but also the glomerular microcirculation. Such marked vaso-occlusion in sickle mice may delay the recovery of renal blood flow after the removal of the renal clamp, and indeed, the dusky cyanotic appearance of the kidney that developed during the ischemic period resolved more slowly in transgenic sickle mice as compared with wild-type mice after the removal of the clamp. Such vaso-occlusion in the postischemic period in transgenic sickle mice may contribute to increased sensitivity to an ischemic insult observed in SCD. Quite remarkably, this marked glomerular congestion and cortical infarction in sickle mice subjected to ischemia recapitulate the appearance of the glomerulus and cortical infarction observed in patients with SCD presenting with acute renal insufficiency or failure.29–33 Congestion in the renal microcirculation of sickle mice after ischemia is also noteworthy in view of the established role of regional vascular congestion as a determinant of sensitivity of nephron segments to ischemic insults,34,35 and the recently appreciated significance of vascular congestion and other microvascular alterations in the initiation and propagation of acute renal injury.36–38 For example, when renal blood flow is interrupted in the mammalian kidney, the S3 segment of the proximal tubule and the medullary portion of the thick ascending limb are the segments of the nephron that are most readily damaged. The vulnerability of these nephron segments to ischemic injury reflects, at least in part, the impairment in oxygenation and delivery of nutrients to these segments incurred by marked congestion and stasis in the neighboring microcirculation supplying these nephron segments. Moreover, the importance of disturbances in the microcirculation in general—through such effects as vascular congestion and sludging, endothelial activation and injury, loss of microcirculatory barrier function, and endothelial production of cytokines, reactive oxygen and nitrogen species, and autacoids—in the initiation and pathogenesis of acute renal failure has been recently emphasized. Thus, the dramatic congestion in the microcirculation in all compartments of the kidney in sickle mice after renal ischemia likely contributes to the heightened sensitivity of the kidney in SCD to acute ischemic insults.
The acute changes observed in the glomerular, vascular, and tubulointerstitial compartments of the kidney in sickle mice may also be relevant to chronic nephropathy that develops in SCD, the pathogenesis of which remains primarily unexplained. For example, a significant number of patients with SCD develop chronic kidney disease and end-stage kidney disease; histological findings in the kidney include glomerular abnormalities such as focal sclerosis, or a membranoproliferative-type lesion, along with chronic tubulointerstitial disease. Speculations regarding the pathogenesis of these glomerulopathic lesions include such mechanisms as hyperfiltration, the effects of relative hypoxia, and adverse effects of heightened renal growth that occurs in SCD.6,16–21 In the present study, sickle mice but not wild-type mice, in response to ischemia, exhibit acute glomerulopathic changes which include endothelialitis and mesangiolysis. It is possible that these latter changes along with accompanying reparative responses, especially if repetitively incurred through recurrent cycles of renal ischemia, may lead to a chronic glomerulopathy. The marked congestion observed in the peritubular microcirculation may also be relevant to chronic sickle cell nephropathy. Loss of peritubular capillaries is recognized in assorted chronic nephritides,39,40 and recent studies in rodents have demonstrated that acute ischemia of sufficient duration leads to loss of peritubular capillaries, inflammation, and chronic injury.41–43 Such loss of capillary beds may predispose to chronic hypoxia and other adverse effects, thereby contributing to chronic injury observed in such settings. Finally, an acute ischemic insult may up-regulate matrix-related genes (such as TGF-β1, MCP-1, and extracellular matrix proteins) in the tubulointerstitium that contribute to chronic tubulointerstitial scarring;44,45 accordingly, the heightened tubulointerstitial injury in the kidney in sickle mice after acute ischemia may accentuate the expression of proinflammatory and fibrogenic genes with attendant chronic tubulointerstitial disease. We thus suggest that the worsened histological injury occurring in the vascular, glomerular, and tubulointerstitial compartments of the kidney in sickle mice after ischemia may be germane to the pathogenesis of chronic sickle cell nephropathy.
Recent studies have shown that acute renal ischemia in rodents may lead to adverse effects in the lungs and heart, and our findings demonstrate that congestion in the lungs and heart, triggered by renal ischemia, was exacerbated in sickle mice. As shown in genetically unaltered rodents, ischemic injury to the kidney leads to increased pulmonary vascular permeability through macrophage-dependent mechanisms, and down-regulation of salt and water channels in the lungs.46,47 Renal ischemia increases expression of tumor necrosis factor-α and interleukin (IL)-1 in the heart, impairs cardiac function as assessed by echocardiography, and induces cardiac apoptosis.48 The relay of information from the ischemic kidney to these vital extrarenal organs likely involves cytokines, and in this regard, attention is directed to tumor necrosis factor-α, IL-1, and IL-6.48,49 Indeed, the extent to which systemic concentrations of cytokines (such as IL-6) are elevated in human acute renal failure correlates with mortality observed in this condition.50 Thus, ischemia to the kidney provokes the appearance of proinflammatory cytokines in the systemic circulation, and adverse effects on vital organs such as the lungs and heart.
Cytokines such as IL-6 are potent stimuli to C-reactive protein (CRP),51 and CRP, in turn, stimulates the cellular production of cytokines including IL-6.52 CRP is considered not only an important biomarker for atherosclerosis but, because of its proinflammatory, procoagulant, and proapoptotic actions,53,54 CRP is increasingly considered an initiator and/or contributor to vascular and other types of tissue injury.53,54 Additionally, in critically ill patients, plasma levels of CRP predict renal and respiratory failure, and overall mortality.55 CRP stimulates the cellular generation of oxidants, increases the activity of MAP kinases, and activates transcription factors such as nuclear factor-κB and AP-1;51,52 these cellular effects may underlie the inductive effect of CRP on a range of cytokines (such as IL-6 and MCP-1) that are quite relevant to SCD.56,57 Our present findings confirm previous observations that SAP, the murine homologue to CRP, is markedly increased in sickle mice.28 Moreover, our findings demonstrate that whereas ischemia increased plasma levels of SAP in both groups, SAP in sickle mice almost tripled after ischemia, and was increased more than sixfold as compared with the wild-type mice subjected to ischemia. Extrapolating from the recognized biological effects of CRP, we speculate that the marked augmentation of SAP in sickle mice after ischemia may be germane to the heightened injury observed in the kidney in general, and to increased congestion observed in such vital organs as the heart and lungs, in particular. As such, CRP may be considered another and related mechanism, along with assorted cytokines (for example, tumor necrosis factor-α, IL-1, and IL-6), whereby ischemic insults to the kidney provoke adverse and aberrant responses in organs such as the lung and heart.
In summary, we demonstrate remarkably increased vascular congestion and sensitivity of the kidney in SCD to ischemic insults. Accumulation of injury—accrued from such heightened sensitivity, and after cycles of overt or clinically silent tissue ischemia—may thus provide a pathway for progressive organ damage and other complications of SCD. Additionally, the exaggeration in the rise in SAP and vascular congestion in organs such as the lungs and heart, and the emergence of mortality in sickle mice subjected to ischemia, indicate that the adverse systemic and extrarenal sequelae of localized renal ischemia are also magnified in SCD. Finally, these adverse systemic effects may feedback locally to the kidney, and acting in concert with the direct harmful effects of renal ischemia, may thereby accentuate overall renal damage.
Acknowledgments
We thank Dr. Hamid Rabb for advice regarding ischemic injury in mice and Mrs. Sharon Heppelmann for secretarial expertise in the preparation of this work.
Footnotes
Address reprint requests to Dr. Karl A. Nath, Mayo Clinic, 200 First St., SW, Guggenheim 542, Rochester, MN 55905. E-mail: nath.karl@mayo.edu.
Suported by Program Project Grant (HL-55552 to K.A.N., R.P.H., Z.S.K.).
References
- Embury SH, Hebbel RP, Steinberg MH, Mohandas N. Pathogenesis of vasoocclusion. Embury SH, Hebbel RP, Mohandas N, Steinberg MH, editors. New York: Raven,; Sickle Cell DiseaseBasic Principles and Clinical Practice. 1994:pp 311–326. [Google Scholar]
- Kaul DK, Fabry ME, Nagel RL. The pathophysiology of vascular obstruction in the sickle syndromes. Blood Rev. 1996;10:29–44. doi: 10.1016/s0268-960x(96)90018-1. [DOI] [PubMed] [Google Scholar]
- Hebbel RP. Blockade of adhesion of sickle cells to endothelium by monoclonal antibodies. N Engl J Med. 2000;342:1910–1912. doi: 10.1056/NEJM200006223422512. [DOI] [PubMed] [Google Scholar]
- Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, Paller MS, Hebbel RP. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96:314–320. [PubMed] [Google Scholar]
- Cheung AT, Chen PC, Larkin EC, Duong PL, Ramanujam S, Tablin F, Wun T. Microvascular abnormalities in sickle cell disease: a computer-assisted intravital microscopy study. Blood. 2002;99:3999–4005. doi: 10.1182/blood.v99.11.3999. [DOI] [PubMed] [Google Scholar]
- Nath KA, Katusic ZS, Gladwin MT. The perfusion paradox and vascular instability in sickle cell disease. Microcirculation. 2004;11:179–193. doi: 10.1080/10739680490278592. [DOI] [PubMed] [Google Scholar]
- Zager RA, Baltes LA, Sharma HM, Jurkowitz MS. Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int. 1984;26:689–700. doi: 10.1038/ki.1984.204. [DOI] [PubMed] [Google Scholar]
- Zager RA, Jurkowitz MS, Merola AJ. Responses of the normal rat kidney to sequential ischemic events. Am J Physiol. 1985;249:F148–F149. doi: 10.1152/ajprenal.1985.249.1.F148. [DOI] [PubMed] [Google Scholar]
- Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem. 2002;277:2040–2049. doi: 10.1074/jbc.M107525200. [DOI] [PubMed] [Google Scholar]
- Bonventre JV. Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens. 2002;11:43–48. doi: 10.1097/00041552-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, Miki T, Seino S, Terzic A. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol. 2003;284:H2106–H2113. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- Eisen A, Fisman EZ, Rubenfire M, Freimark D, McKechnie R, Tenenbaum A, Motro M, Adler Y. Ischemic preconditioning: nearly two decades of research. A comprehensive review. Atherosclerosis. 2004;172:201–210. doi: 10.1016/S0021-9150(03)00238-7. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Anavekar NS, Greaves S, Rouleau JL, Hennekens C, Pfeffer MA, HEART Investigators Angina pectoris prior to myocardial infarction protects against subsequent left ventricular remodeling. J Am Coll Cardiol. 2004;43:1511–1514. doi: 10.1016/j.jacc.2003.09.069. [DOI] [PubMed] [Google Scholar]
- Pong K. Ischaemic preconditioning: therapeutic implications for stroke? Expert Opin Ther Targets. 2004;8:125–139. doi: 10.1517/14728222.8.2.125. [DOI] [PubMed] [Google Scholar]
- Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, Hebbel RP. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol. 2001;158:893–903. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powars DR, Elliott-Mills DD, Chan L, Niland J, Hiti AL, Opas LM, Johnson C. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med. 1991;115:614–620. doi: 10.7326/0003-4819-115-8-614. [DOI] [PubMed] [Google Scholar]
- de Jong PE, Statius van Eps LW. Sickle cell nephropathy: new insights into its pathophysiology. Kidney Int. 1985;27:711–717. doi: 10.1038/ki.1985.70. [DOI] [PubMed] [Google Scholar]
- Falk RJ, Jennette JC. Sickle cell nephropathy. Adv Nephrol Necker Hosp. 1994;23:133–147. [PubMed] [Google Scholar]
- Saborio P, Scheinman JI. Sickle cell nephropathy. J Am Soc Nephrol. 1999;10:187–192. doi: 10.1681/ASN.V101187. [DOI] [PubMed] [Google Scholar]
- Pham PT, Pham PC, Wilkinson AH, Lew SQ. Renal abnormalities in sickle cell disease. Kidney Int. 2000;57:1–8. doi: 10.1046/j.1523-1755.2000.00806.x. [DOI] [PubMed] [Google Scholar]
- Wesson DE. The initiation and progression of sickle cell nephropathy. Kidney Int. 2002;61:2277–2286. doi: 10.1046/j.1523-1755.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- Nath KA, Shah V, Haggard JJ, Croatt AJ, Smith LA, Hebbel RP, Katusic ZS. Mechanisms of vascular instability in a transgenic mouse model of sickle cell disease. Am J Physiol. 2000;279:R1949–R1955. doi: 10.1152/ajpregu.2000.279.6.R1949. [DOI] [PubMed] [Google Scholar]
- Fabry ME, Sengupta A, Suzuka SM, Costantini F, Rubin EM, Hofrichter J, Christoph G, Manci E, Culberson D, Factor SM. A second generation transgenic mouse model expressing both hemoglobin S (HbS) and HbS-Antilles results in increased phenotypic severity. Blood. 1995;86:2419–2428. [PubMed] [Google Scholar]
- Rabb H, Ramirez G, Saba SR, Reynolds D, Xu J, Flavell R, Antonia S. Renal ischemic-reperfusion injury in L-selectin-deficient mice. Am J Physiol. 1996;271:F408–F413. doi: 10.1152/ajprenal.1996.271.2.F408. [DOI] [PubMed] [Google Scholar]
- Zager RA, Johnson A, Hanson S, dela Rosa V. Altered cholesterol localization and caveolin expression during the evolution of acute renal failure. Kidney Int. 2002;61:1674–1683. doi: 10.1046/j.1523-1755.2002.00316.x. [DOI] [PubMed] [Google Scholar]
- Nath KA, Haggard JJ, Croatt AJ, Grande JP, Poss KD, Alam J. The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. Am J Pathol. 2000;156:1527–1535. doi: 10.1016/S0002-9440(10)65024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath KA, Vercellotti GM, Grande JP, Miyoshi H, Paya CV, Manivel JC, Haggard JJ, Croatt AJ, Payne WD, Alam J. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int. 2001;59:106–117. doi: 10.1046/j.1523-1755.2001.00471.x. [DOI] [PubMed] [Google Scholar]
- Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, Hebbel RP, Vercellotti GM. Transgenic sickle mice have vascular inflammation. Blood. 2003;101:3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- Kimmelstiel P. Vascular occlusion and ischemic infarction in sickle cell disease. Am J Med Sci. 1948;216:11–19. [PubMed] [Google Scholar]
- Femi-Pearse D, Odunjo EO. Renal cortical infarcts in sickle-cell trait. Br Med J. 1968;3:34. doi: 10.1136/bmj.3.5609.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WA, Peck D, Lowman RM. Perirenal hematoma in association with renal infarction in sickle-cell trait. Radiology. 1969;92:351–352. doi: 10.1148/92.2.351. [DOI] [PubMed] [Google Scholar]
- Sickles EA, Korobkin M. Perirenal hematoma as a complication of renal infarction in sickle-cell trait. A case report. Am J Roentgenol Radium Ther Nucl Med. 1974;122:800–803. doi: 10.2214/ajr.122.4.800. [DOI] [PubMed] [Google Scholar]
- al Ani O, Baqi N. Clinical quiz. Cortical infarction secondary to intravascular sickling in the renal cortex. Pediatr Nephrol. 1999;13:267–268. [PubMed] [Google Scholar]
- Mason J, Welsch J, Torhorst J. The contribution of vascular obstruction to the functional defect that follows renal ischemia. Kidney Int. 1987;31:65–71. doi: 10.1038/ki.1987.10. [DOI] [PubMed] [Google Scholar]
- Olof P, Hellberg A, Kallskog O, Wolgast M. Red cell trapping and postischemic renal blood flow. Differences between the cortex, outer and inner medulla. Kidney Int. 1991;40:625–631. doi: 10.1038/ki.1991.254. [DOI] [PubMed] [Google Scholar]
- Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, Goligorsky MS. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am J Physiol. 2002;282:F1140–F1149. doi: 10.1152/ajprenal.00329.2001. [DOI] [PubMed] [Google Scholar]
- Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol. 2003;285:F191–F198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- Bohle A, von Gise H, Mackensen-Haen S, Stark-Jakob B. The obliteration of the postglomerular capillaries and its influence upon the function of both glomeruli and tubuli. Functional interpretation of morphologic findings. Klin Wochenschr. 1981;59:1043–1051. doi: 10.1007/BF01747747. [DOI] [PubMed] [Google Scholar]
- Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol. 2001;281:F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- Basile DP, Donohoe DL, Roethe K, Mattson DL. Chronic renal hypoxia after acute ischemic injury: effects of L-arginine on hypoxia and secondary damage. Am J Physiol. 2003;284:F338–F348. doi: 10.1152/ajprenal.00169.2002. [DOI] [PubMed] [Google Scholar]
- Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004;13:1–7. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Basile DP, Martin DR, Hammerman MR. Extracellular matrix-related genes in kidney after ischemic injury: potential role for TGF-beta in repair. Am J Physiol. 1998;275:F894–F903. doi: 10.1152/ajprenal.1998.275.6.F894. [DOI] [PubMed] [Google Scholar]
- Sung FL, Zhu TY, Au-Yeung KK, Siow YL, O K. Enhanced MCP-1 expression during ischemia/reperfusion injury is mediated by oxidative stress and NF-kappaB. Kidney Int. 2002;62:1160–1170. doi: 10.1111/j.1523-1755.2002.kid577.x. [DOI] [PubMed] [Google Scholar]
- Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;55:2362–2367. doi: 10.1046/j.1523-1755.1999.00460.x. [DOI] [PubMed] [Google Scholar]
- Rabb H, Wang Z, Nemoto T, Hotchkiss J, Yokota N, Soleimani M. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int. 2003;63:600–606. doi: 10.1046/j.1523-1755.2003.00753.x. [DOI] [PubMed] [Google Scholar]
- Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:1549–1558. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- Kielar ML, Rohan Jeyarajah D, Lu CY. The regulation of ischemic acute renal failure by extrarenal organs. Curr Opin Nephrol Hypertens. 2002;11:451–457. doi: 10.1097/00041552-200207000-00013. [DOI] [PubMed] [Google Scholar]
- Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, Soroko S, Freedman S, Becker K, Spratt D, Shyr Y, Ikizler TA, PICARD Study Group Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004;65:1357–1365. doi: 10.1111/j.1523-1755.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- Calabro P, Willerson JT, Yeh ET. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108:1930–1932. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Matsumura M, Kasai K. Vascular smooth muscle cell activation by C-reactive protein. Cardiovasc Res. 2003;58:186–195. doi: 10.1016/s0008-6363(02)00855-6. [DOI] [PubMed] [Google Scholar]
- Verma S, Yeh ET. C-reactive protein and atherothrombosis—beyond a biomarker: an actual partaker of lesion formation. Am J Physiol. 2003;285:R1253–R1258. doi: 10.1152/ajpregu.00170.2003. [DOI] [PubMed] [Google Scholar]
- Yeh ET, Palusinski RP. C-reactive protein: the pawn has been promoted to queen. Curr Atheroscler Rep. 2003;5:101–105. doi: 10.1007/s11883-003-0080-4. [DOI] [PubMed] [Google Scholar]
- Lobo SM, Lobo FR, Bota DP, Lopes-Ferreira F, Soliman HM, Melot C, Vincent JL. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest. 2003;123:2043–2049. doi: 10.1378/chest.123.6.2043. [DOI] [PubMed] [Google Scholar]
- Bourantas KL, Dalekos GN, Makis A, Chaidos A, Tsiara S, Mavridis A. Acute phase proteins and interleukins in steady state sickle cell disease. Eur J Haematol. 1998;61:49–54. doi: 10.1111/j.1600-0609.1998.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Perelman N, Selvaraj SK, Batra S, Luck LR, Erdreich-Epstein A, Coates TD, Kalra VK, Malik P. Placenta growth factor activates monocytes and correlates with sickle cell disease severity. Blood. 2003;102:1506–1514. doi: 10.1182/blood-2002-11-3422. [DOI] [PubMed] [Google Scholar]