Abstract
Axonal damage in multiple sclerosis (MS) lesions is associated with failure of fibrinolysis because of the inhibition of the plasminogen activator system. Plasma membrane receptors for tissue plasminogen activator (tPA) and plasminogen concentrate proteolytic activity on the cell surface and provide protection from inhibitors that in turn may locally enhance the fibrinolytic response. Therefore, we have investigated expression of two of these receptors in MS lesions, annexin II tetramer (AIIt) and low-density lipoprotein receptor-related protein (LRP). In acute MS lesions both AIIt and LRP were immunolocalized on macrophages and astrocytes while LRP was additionally found on neuronal cells in cortical gray matter. Western blot analysis confirmed a significant increase in AIIt in MS lesions and in a proportion of normal-appearing white matter samples, with a highly significant correlation between annexin II levels and factors associated with impeded fibrinolysis, such as plasminogen activator inhibitor-1. Immunoblotting analysis of plasmin(ogen) revealed increased levels of lysine-plasminogen in samples expressing high AIIt protein levels. Our results suggest that limited availability of tPA in MS lesions because of formation of tPA-plasminogen activator inhibitor-1 complexes reduces capability of tPA receptors to generate plasmin, which further diminishes fibrinolytic capacity in active MS lesions and possibly leads to axonal damage.
In neuroinflammatory disorders failure of fibrinolysis occurs because of inhibition of the plasminogen activator system.1 A subsequent accumulation of fibrin, which correlates with disease severity,2 gives rise to axonal damage as elegantly shown in a recently reported study of sciatic nerve crush in tPA-deficient animals in which fibrin deposition was found to exacerbate axonal injury.3 An approach to attenuate axonal damage would be to stimulate local pericellular fibrinolytic activity and/or to remove inhibitory influence of plasminogen activator inhibitors (PAIs) and anti-plasmins. Surface binding of tissue plasminogen activator (tPA) and plasminogen to plasma membrane proteins such as annexin II and low-density lipoprotein receptor-related protein (LRP) is an intrinsic mechanism that increases the catalytic efficiency of plasmin generation and provides protection from serpins.4–7
Annexin II belongs to a family of calcium-dependent phospholipid-binding proteins8 that are expressed at low levels in the adult human brain9 but are abundant in pathological conditions such as brain tumors.10 In endothelial cells and macrophages annexin II is expressed as a plasma membrane-bound heterotetramer of two annexin II subunits that are dynamically linked by two p11 (annexin light chain, S100A10) subunits.11 The tetramer (AIIt) provides a surface for tPA and plasminogen interaction resulting in an ∼60-fold increase in plasmin generation12,13 but also promotes plasmin autodegradation14 and reduction15 thus creating short pulses of plasminolytic activity.6 Through links with fibrin and the extracellular matrix protein tenascin C, AIIt can direct plasminolytic activity resulting in fibrinolysis, remodeling of the extracellular matrix, and release of matrix-bound angiogenic factors.16,17 This is particularly exploited by macrophages and tumor cells through loss of focal adhesion contacts and an increase in invasive potential.18,19 In the central nervous system (CNS) annexin II has been implicated in tPA-mediated activation of microglia20 and is required for assembly of glial fibrillary acidic protein filaments in astrocytes.21
LRP is a scavenger receptor that binds a variety of biologically diverse ligands including the broad spectrum protease inhibitor α2-macroglobulin (α2M) and free and PAI-complexed plasminogen activators.22 Cell surface-expressed LRP protein consists of an extracellular ligand-binding heavy chain (515 kd) and a transmembrane light chain (85 kd) and in the adult brain is primarily involved in lipoprotein metabolism23 as well as maintaining protease-inhibitor homeostasis.24 In the CNS LRP is highly expressed by neuronal cells25 but is also found in microglia and astrocytes26 and in a shed form in the cerebrospinal fluid.27 LRP regulates extracellular proteolysis by internalizing proteases and/or protease-inhibitor complexes thus decreasing the overall protease load in the pericellular space.5,28 Receptor-associated protein (RAP) is a chaperon that prevents a premature binding of ligands to the newly synthesized LRP during its export to the cell surface.29
Possible effects of plasminogen activation during the inflammatory process in multiple sclerosis (MS) include fibrinolysis, increase in migration/invasiveness of glial and immune cells, remodeling of the extracellular matrix, and damage to the blood-brain barrier (BBB) and myelin/axon unit.30 The local concentration of plasminogen activators, inhibitors, and their receptors will be the factors determining the final outcome. In two recent studies we have shown that a marked increase in PAI-1 reduces fibrinolytic activity in MS lesions hence contributing to fibrin deposition and axonal injury.1,31 However, small amounts of active tPA may remain in protected proteolytic pockets between macrophages and fibrin-coated axons where AIIt and LRP could play a pivotal role in enhancing fibrin dissolution or removal of enzyme-inhibitors complexes. The aims of the present study were to identify potential targets for enhancing fibrinolysis in the CNS by immunolocalizing and quantitating the expression of AIIt and LRP in normal and MS brain and spinal cord and to establish their relationship to the tPA-plasmin axis and fibrinolytic capacity in MS lesions.
Materials and Methods
Tissue
Snap-frozen CNS tissue samples from a histopathologically well-characterized cluster of 20 postmortem cases of MS and 15 normal controls (NC, Table 1) were obtained from the NeuroResource Tissue Bank, Institute of Neurology, London. All MS cases were classified as secondary progressive MS with characteristic relapsing-remitting course and increasing disability. A total of 58 snap-frozen blocks (0.5 to 1 cm3) of brain- and spinal cord-containing lesions and/or macroscopically normal-appearing white matter (NAWM) and gray matter from MS cases and 19 blocks of white and gray matter from brain and spinal cord of NC cases were homogenized for protein extraction. MS lesions were classified into acute (AL), subacute, and chronic on the basis of the numbers and distribution of oil red-O-positive cells and cellularity in the borders and parenchyma of lesions.32 Histological criteria were supplemented by immunohistochemical staining with specific antibody markers for glial, neuronal, and inflammatory cells.33 Expression of plasminogen activators/inhibitors and fibrinolytic activity have been previously fully characterized in this set of samples.1,31
Table 1.
Summary of Clinical Data for NC and MS Cases
| Sample type | Age and sex (years) | PM time (hours) | MS duration (years) | Cause of death (number of cases) |
|---|---|---|---|---|
| Normal control, n = 15 | 64 (34–80) M:F = 12:3 | 26 (10–52) | N/A | Heart failure (6) |
| Myocardial infarction | ||||
| (4) Bronchopneumonia (2) | ||||
| Pulmonary embolism (1) | ||||
| Liver carcinoma (1) | ||||
| Renal failure (1) | ||||
| Multiple sclerosis, n = 20 | 57 (29–77) M:F = 9:11 | 23 (4–64) | 23 (8–43) | Bronchopneumonia (14) |
| Septicemia (3) | ||||
| Renal failure (1) | ||||
| Heart failure (1) | ||||
| Bowel carcinoma (1) |
Antibodies and Immunohistochemistry
Methanol-fixed cryostat sections were stained using a nickel-enhanced three-step peroxidase method as previously described.33 The primary antibodies used were raised against annexin II (1:2000; Neomarkers, Fremont, CA), p11 (1:1000; Transduction Laboratories, Lexington, KY), LRP heavy chain (1:1000; Calbiochem, Nottingham, UK), LRP light chain (1:100, Calbiochem), RAP (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) and plasminogen (1:1000; DAKO, Ely, UK). Routine immunohistochemical controls included omission of primary antibodies as well as the application of affinity-purified goat (Sigma, Poole, UK) and mouse IgG (Sigma) at the same protein concentrations as primary antibodies.
Protein Extraction and Western Blotting
Protein extraction from snap-frozen blocks of brain and spinal cord of MS and NC cases was performed as previously described and protein concentrations determined by the Lowry method.31 For Western blot analysis 40 μg of supernatant protein was resolved on (5%, 10%, or 15%) sodium dodecyl sulfate-polyacrylamide gels and transferred overnight to Immobilon-P polyvinylidene difluoride membrane.31 The blots were probed with annexin II (1:2000), p11 (1:1000), LRP heavy chain (1:1000), RAP (1:100), or plasminogen (1:1000) antibody for 2 hours at room temperature and developed by enhanced chemiluminescence. Extracts of THP-1 and endothelial cells were used as protein standards for annexin II and p11 blots, respectively. To ensure equal loading of protein membranes were stripped with Gelstrip (Chemicon, Aarhus, Denmark) according to the manufacturers’ instructions and probed with anti-β-actin antibody (1:1000, Sigma). Specificity of immunostaining was ensured by replacing primary antibodies with appropriate species-specific affinity-purified IgG fraction. All blots were scanned using the Gel-Pro analyzer (Media Cybernetics, Wokingham, UK) and the results expressed in densitometry units.
Statistical Analysis
Statistical analysis was performed using Fisher’s exact test suitable for a small sample size with 100% cumulative frequency of the control group as a cutoff point and a significance level set at P < 0.05 (SPSS Software; SPSS Inc., Chicago, IL). The nonparametric Spearman rank correlation test was used for the regression analysis and the effects of age and postmortem time were checked for all data. Partial correlation correcting for these effects was performed when indicated and the r value of the Spearman rank test reported where appropriate.
Results
Distribution and Protein Levels of Annexin II and p11 in Control and MS Brains
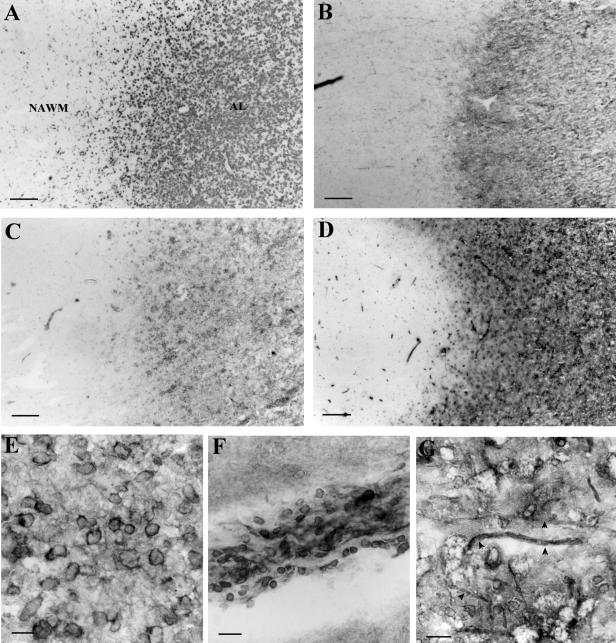
In normal control brains annexin II was immunolocalized to endothelial cells in blood vessels whereas in acute MS lesions (AL) annexin II was found on membranes of foamy macrophages concentrated in lesion borders (Figure 1, C and E) and in the cytoplasm of a small number of hypertrophic astrocytes in lesion parenchyma. A good correlation was observed between the numbers of plasmin(ogen)-positive (Figure 1B) and annexin II-positive macrophages (Figure 1C) on serially stained section. An increase in astrocyte staining was found in subacute and chronic lesion in parallel with an increase in glial fibrillary acidic protein-positive cells (not shown). Adjacent and far normal-appearing white and gray matter were characterized by strong expression of annexin II on blood vessel endothelial cells and infiltrating mononuclear cells (Figure 1F) but glial cells and neurons were not stained.
Figure 1.
Immunohistochemistry of annexin II in MS lesions. Low-power photomicrograph of lesion parenchyma and adjacent NAWM stained with EBM-11, a microglia/macrophage marker (A), anti-plasminogen antibody (B), anti-annexin II antibody (C), and anti-p11 antibody (D). E: Plasma membrane of foamy macrophages in an acute MS lesion stained with anti-annexin II antibody. F: Perivascular inflammatory infiltrate stained with annexin II antibody showing positive mononuclear cells. G: Foamy macrophages and thickened axons (arrowheads) in an acute MS lesion stained with anti-p11 antibody. Scale bars: 320 μm (A–D); 20 μm (E–G).
Staining with anti-p11 antibody revealed similar distribution to that of annexin II with strongly positive membranes of foamy macrophage in acute MS lesions (Figure 1G) and absence of p11 on glia (Figure 1D) and neurones in normal-appearing white and gray matter, respectively. In actively demyelinating lesions p11 also highlighted a subset of thickened axons apposed to annexin II/p11-positive foamy macrophages (Figure 1G) and was immunolocalized to mononuclear cells in perivascular inflammatory infiltrates in NAWM and acute MS lesions.
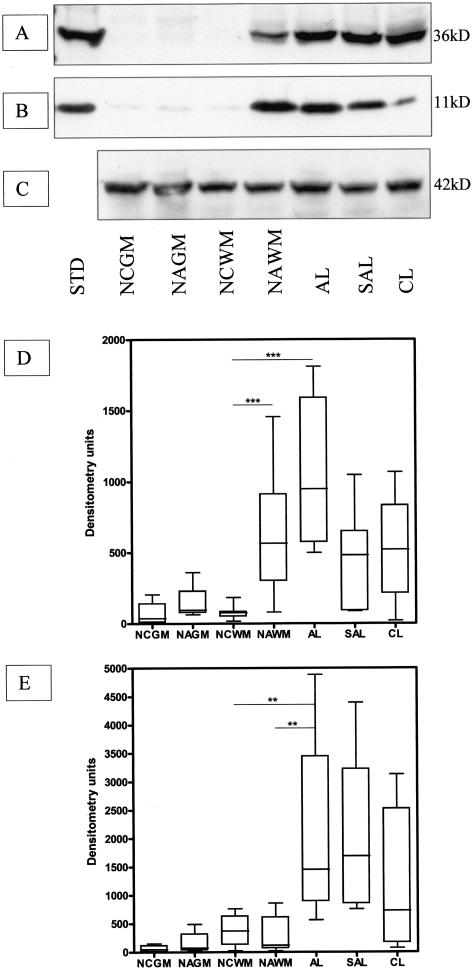
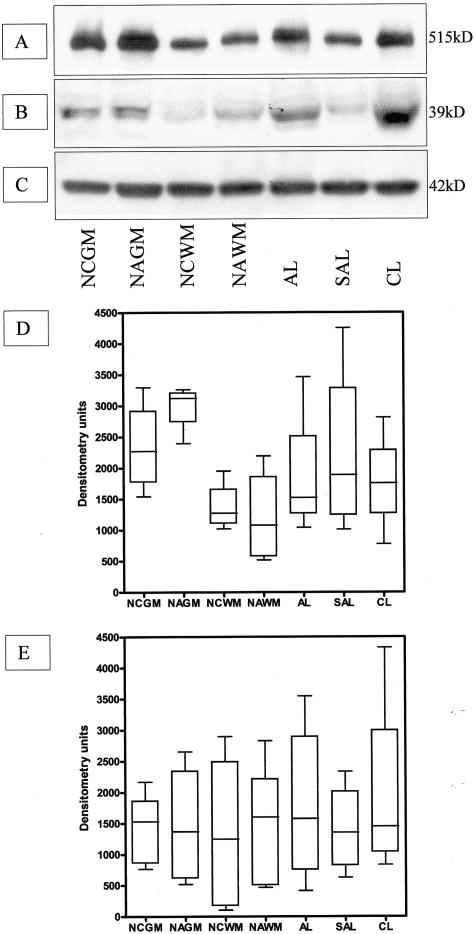
Immunohistochemical findings were reflected in Western blots of annexin II and p11 with both proteins barely detectable in extracts of control brain (Figure 2, A and B) and spinal cord. A significant increase in annexin II [NAWM versus normal control white matter (NCWM) and AL versus NCWM, P < 0.001] and p11 (AL versus NCWM, P < 0.01) content by densitometry was observed in MS tissue samples with comparable protein levels between lesions of differing stages of development (Figure 2, D and E). However, variable levels of expression were found in NAWM samples with values ranging from low, similar to control samples, to high values comparable to those observed in acute MS lesions. Comparison with the protein levels of fibrinolytic agents and clot lysis time reflecting fibrinolytic capacity of this set of samples31 revealed a statistically significant correlation of high annexin II protein with factors associated with impeded fibrinolysis, high PAI-1 protein levels (r = 0.54, P < 0.001) and prolonged clot lysis time (r = 0.54, P < 0.001) as well as low tPA protein levels (r = −0.33, P = 0.015).
Figure 2.
Immunoblots of annexin II (A) and p11 (B) in brain tissue extracts from control and MS cases. C: Blot shown in A was reprobed with the anti-β-actin antibody to ensure equal loading of the gel. D and E: Densitometric analysis of the annexin II (D) and p11 (E) immunoblots. Statistically significant differences between groups are indicated where appropriate. ***, P < 0.001; **, P < 0.01; n = 7 for all sample groups. STD, protein standard; NCGM, normal control gray matter; NAGM, normal-appearing gray matter; SAL, subacute lesion; and CL, chronic lesion.
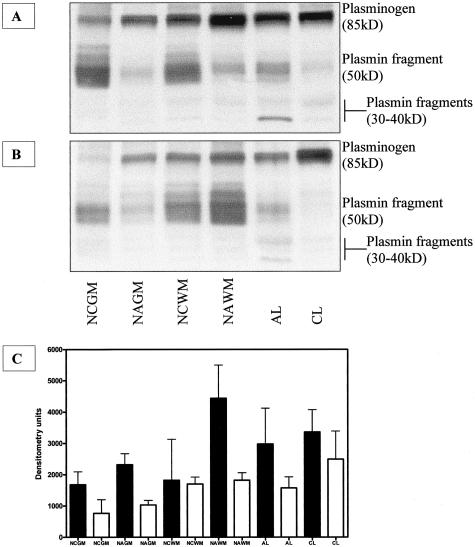
To examine whether there was a link between annexin II protein concentrations and plasmin(ogen) content and processing in control and MS, tissue samples with high and low annexin II content were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and stained with an antibody against human plasminogen. Samples with high annexin II content showed higher levels of the 85-kd cell surface-bound form of plasminogen (lysine-plasminogen)34,35 compared to those with low annexin II (Figure 3; A to C). Plasmin fragments migrated as a larger 50-kd band and two smaller bands between 30 to 40 kd predominantly visible in acute MS lesions (Figure 3, A and B). There were no indications of increased plasminogen turnover in samples expressing high annexin II levels because the relative amounts of plasmin fragments were comparable between two sets of samples.
Figure 3.
Plasminogen/plasmin content in control and MS samples. Equivalent amount of tissue protein extracts (40 μg) from samples expressing high levels (A) and low levels (B) of annexin II were subjected to nonreducing 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stained with anti-plasminogen antibody. Four major bands are visible: lysine-plasminogen (85 kd), binding fragment of plasmin (∼50 kd), and two smaller plasmin fragments between 30 kd and 40 kd. C: Densitometric analysis of the plasminogen immunoblots. Black and white bars represent high and low annexin II-expressing samples, respectively. n = 4 for all sample groups.
Distribution and Protein Levels of LRP and RAP in Control and MS Brains
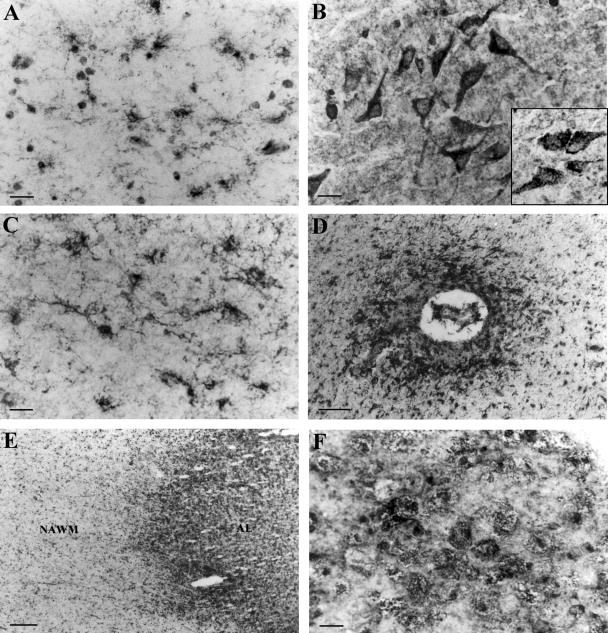
In contrast to annexin II, LRP was ubiquitously expressed on glial cells and neurones in both control and MS brains. In normal periventricular white matter LRP heavy and light chains were immunolocalized in resting microglia (Figure 4A) and astrocyte end-feet-surrounding blood vessels whereas an antibody against RAP showed punctuate staining of glial cell cytoplasm. In control gray matter strong LRP (Figure 4B) and RAP (Figure 4B, inset) staining was visualized in cytoplasm of cortical neurons. NAWM was characterized by a higher expression of LRP on activated microglia (Figure 4C) especially those located in the vicinity of blood vessels (Figure 4D). In the borders of acute MS lesions activated microglia, foamy macrophages, and hypertrophic astrocyte cell bodies were strongly LRP-positive whereas in the lesion center macrophages were predominantly stained (Figure 4, E and F). There were minor differences in distribution and intensity of RAP staining between control and MS tissue although RAP was primarily found in hypertrophic astrocytes in acute MS lesions (data not shown). Immunohistochemical controls, including omission of the primary antibody or application of affinity-purified goat and mouse IgG, were negative. Western blots showed slight variations in LRP and RAP protein levels between control white matter, NAWM, and MS lesions at different stages of development (Figure 5, A and B). However, markedly higher expression of LRP heavy chain was found in control gray matter with further increases in normal-appearing gray matter (Figure 5D).
Figure 4.
Immunolocalization of LRP in control and MS brains. Staining of control white matter showing positive microglia (A) and control gray matter showing positive neuronal perikarya (B). Inset in B shows RAP-positive neurons in control gray matter. C and D: LRP-positive activated microglia in NAWM parenchyma (C) and clustered around a blood vessel (D) in NAWM. E: Low-power photomicrograph of an AL and adjacent NAWM stained with anti-LRP antibody. F: High magnification of the lesion border showing staining of foamy macrophages. Scale bars: 20 μm (A–C, F); 320 μm (E); 50 μm (D).
Figure 5.
Western blots of LRP (A) and RAP (B) in brain tissue extracts from control and MS cases. C: Blot shown in B was reprobed with the anti-β-actin antibody to ensure equal loading of the gel. Densitometric analysis of the LRP (D) and RAP (E) immunoblots. n = 4 for all sample groups.
Discussion
Our results showed that AIIt was highly expressed in acute MS lesions on macrophages and astrocytes whereas LRP was prominent on neuronal cells in cortical gray matter. Both proteins were immunolocalized either on BBB endothelium and infiltrating mononuclear cells or surrounding glial cells. Western blot analysis confirmed a significant increase in AIIt in MS lesions and a proportion of NAWM samples as well as increased lysine-plasmin(ogen) concentrations in samples expressing high annexin II levels. Moreover, statistically significant correlation was found between AIIt levels and markers of impeded fibrinolysis, PAI-1 concentration, and prolonged clot lysis time.
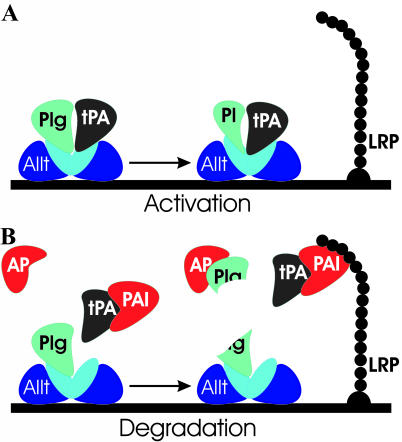
The highly significant increase in AIIt in acute MS lesions suggests that plasmin generation should be enhanced. This can be extrapolated from patients with leukemia that were more prone to bleeding because of increased plasminolytic and fibrinolytic activity resulting from annexin II overexpression.36 However, we have previously shown that the fibrinolytic capacity of acute MS lesions is minimal because of a marked increase in PAI-1 and inhibition of tPA.31 It is evident from the immunoblots that in high annexin II-expressing samples there was an accumulation of membrane-bound lysine-plasminogen (85-kd preactivation intermediate) but plasmin(ogen) turnover was not enhanced in comparison with samples having low annexin II content. In the absence of tPA, plasminogen bound to macrophage plasma membrane undergoes autoproteolysis generating a 48-kd inactive fragment with surface-binding capacity and small soluble fragments with catalytic capacity37 that are rapidly inhibited in the fluid phase by anti-plasmins (Figure 6).38,39 The timing of AIIt involvement in fibrin degradation is also a crucial factor in promoting or inhibiting fibrinolysis because the presence of AIIt during fibrin polymerization inhibits fibrinolysis.40 Therefore, limited availability of tPA in MS lesions because of formation of tPA-PAI-1 complexes as previously shown by nonreducing immunoblots of the same set of samples31,41 reduces the ability of AIIt to generate plasmin and further diminishes the fibrinolytic capacity possibly resulting in increased axonal fibrin deposition and neurodegeneration. Localization of LRP on foamy macrophages in MS lesions can further decrease the protease load in the pericellular space by removing plasminogen activator-inhibitor complexes (Figure 6).
Figure 6.
A diagram depicting interactions between tPA/plasmin(ogen) receptors and their ligands. A: Plasminogen is activated into plasmin in the presence of AIIt-bound tPA. B: In the absence of AIIt-bound tPA a degradation of plasminogen occurs resulting in a release of small catalytic fragments that are inhibited in the fluid phase by anti-plasmins. Plg, plasminogen; Pl, plasmin; AP, anti-plasmin.
The overall outcome of AIIt and LRP ligation in MS tissue may be directed more toward enhancing cell migration and invasiveness rather than fibrinolysis. Binding of annexin II to tenascin C and proteolytic modification of the latter results in mitogenesis, loss of focal adhesions, and cell migration.42 Tenascin C, which is found in NAWM, forms an inhibitory barrier that surrounds acute MS lesions.43 Breaking the tenascin barrier would allow annexin II/plasminogen-expressing macrophages in the lesion border to migrate into adjacent NAWM thus contributing to the radial extension of the active lesion. Likewise, LRP may further support macrophage and immune cell invasion of the NAWM by acting as a motogenic receptor for PAI-144 and by scavenging uPA-PAI-1 complexes that facilitates faster recycling of chemotactic urokinase receptor.5,45
Formation of new MS lesions, most typically in the form of Dawson’s finger, is preceded by changes in BBB permeability and integrity. Both AIIt and LRP, which were immunolocalized either to BBB endothelial cells or surrounding microglia, have the capacity to be part of this process by enhancing proteolytic and nonproteolytic effects of tPA. Brain endothelium expresses high levels of AIIt46 but lower levels of tPA47,48 in comparison to other endothelia. During the early stages of lesion development at the BBB when the deposition of fibrin, a major tPA co-factor,49 is minimal tPA may use AIIt to generate plasminolytic activity16 as well as LRP, in a proteolysis-independent manner, to increase BBB permeability.50 Increased expression of AIIt in some but not other NAWM samples may designate those NAWM areas in which there is a higher likelihood of developing a new lesion. Immunostaining suggests that this increase in AIIt represents expression by activated endothelial and mononuclear cells in and around BBB that may through plasminolytic activity facilitate the initial opening of the BBB leading to formation of Dawson’s finger.
A strong association of LRP with cortical neurons and increased expression in MS gray matter may highlight a neuroprotective role for LRP. We have previously reported high tPA and low PAI-1 expression in control and MS gray matter.1 Unchecked tPA activity is the major factor in generation of excitotoxic brain injury hence the availability of this enzyme in the gray matter needs to be tightly controlled.51 Neuroprotective effects of LRP during the inflammatory response in the CNS are mediated either directly or through its ligand α2M by removing proteases and protease-inhibitor complexes from the extracellular space,22 modulating neuronal responses to excitatory neurotransmitters,52 and targeting neurotrophic factors to neuronal cells.53,54
It is evident from these findings that AIIt and LRP are components of the complex signaling mechanism that may take part in pathways leading to either tissue damage or neuroprotection during MS lesion development. One inference of our results is that both receptors may contribute to initial lesion formation and radial extension which in context of our previous findings further highlights the importance of PAI-1 in inflammatory axonal damage.
Acknowledgments
We thank Dr. Jia Newcombe of NeuroResource for supplying the human CNS tissue; and Dr. Philippe Gasque of the Brain Inflammation and Immunity Group, University of Wales, for the gift of the THP-1 cell line.
Footnotes
Address reprint requests to Dr. Djordje Gverić, Department of Neuroinflammation, Institute of Neurology, UCL, 1 Wakefield St., London WC1N 1PJ, UK. E-mail: d.gueric@ion.ucl.ac.uk.
Supported by the Multiple Sclerosis Society of Great Britain (to the Department of Neuroinflammation at the Institute of Neurology) and AIMS2CURE.
References
- Gveric D, Hanemaaijer R, Newcombe J, van Lent NA, Sier CFM, Cuzner ML. Plasminogen activators in multiple sclerosis lesions—implications for the inflammatory response and axonal damage. Brain. 2001;124:1978–1988. doi: 10.1093/brain/124.10.1978. [DOI] [PubMed] [Google Scholar]
- Inoue A, Koh CS, Yamazaki M, Yanagisawa N, Ishihara Y, Kim BS. Fibrin deposition in the central nervous system correlates with the degree of Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Neuroimmunol. 1997;77:185–194. doi: 10.1016/s0165-5728(97)00072-6. [DOI] [PubMed] [Google Scholar]
- Akassoglou K, Kombrinck KW, Degen JL, Strickland S. Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury. J Cell Biol. 2000;149:1157–1166. doi: 10.1083/jcb.149.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis V. Cellular receptors for plasminogen activators—recent advances. Trends Cardiovasc Med. 1997;7:227–234. doi: 10.1016/S1050-1738(97)00084-4. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Sakthivel R, Kniss D, Graham CH, Strickland DK, McCrae KR. The low density lipoprotein receptor-related protein/alpha(2)-macroglobulin receptor regulates cell surface plasminogen activator activity on human trophoblast cells. J Biol Chem. 1998;273:32273–32280. doi: 10.1074/jbc.273.48.32273. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SL, Kassam G, Choi KS, Kang HM, Fogg DK, Waisman DM. Regulation of plasmin activity by annexin II tetramer. Biochemistry. 2000;39:1021–1028. doi: 10.1021/bi991411z. [DOI] [PubMed] [Google Scholar]
- Lee AYY, Fredenburgh JC, Stewart RJ, Rischke JA, Weitz JI. Like fibrin, (DD)E, the major degradation product of cross-linked fibrin, protects plasmin from inhibition by alpha (2)-antiplasmin. Thromb Haemostasis. 2001;85:502–508. [PubMed] [Google Scholar]
- Hajjar KA, Krishnan S. Annexin II: a mediator of the plasmin/plasminogen activator system. Trends Cardiovasc Med. 1999;9:128–138. doi: 10.1016/s1050-1738(99)00020-1. [DOI] [PubMed] [Google Scholar]
- Reeves SA, Chavez-Kappel C, Davis R, Rosenblum M, Israel MA. Developmental regulation of annexin-II (lipocortin-2) in human brain and expression in high-grade glioma. Cancer Res. 1992;52:6871–6876. [PubMed] [Google Scholar]
- Nygaard ST, Haugland HK, Kristoffersen EK, Lundjohansen M, Laerum OD, Tysnes OB. Expression of annexin II in glioma cell lines and in brain tumor biopsies. J Neuro-Oncol. 1998;38:11–18. doi: 10.1023/a:1005953000523. [DOI] [PubMed] [Google Scholar]
- Nilius B, Gerke V, Prenen J, Szucs G, Heinke S, Weber K, Droogmans G. Annexin II modulates volume-activated chloride currents in vascular endothelial cells. J Biol Chem. 1996;271:30631–30636. doi: 10.1074/jbc.271.48.30631. [DOI] [PubMed] [Google Scholar]
- Cesarman GM, Guevara CA, Hajjar KA. An endothelial cell receptor for plasminogen tissue plasminogen activator (t-PA). 2. Annexin II-mediated enhancement of t-PA-dependent plasminogen activation. J Biol Chem. 1994;269:21198–21203. [PubMed] [Google Scholar]
- Kassam G, Le BH, Choi KS, Kang HM, Fitzpatrick SL, Louie P, Waisman DM. The p11 subunit of the annexin II tetramer plays a key role in the stimulation of t-PA-dependent plasminogen activation. Biochemistry. 1998;37:16958–16966. doi: 10.1021/bi981713l. [DOI] [PubMed] [Google Scholar]
- Choi KS, Fitzpatrick SL, Filipenko NR, Fogg DK, Kassam G, Magliocco AM, Waisman DM. Regulation of plasmin-dependent fibrin clot lysis by annexin II heterotetramer. J Biol Chem. 2001;276:25212–25221. doi: 10.1074/jbc.M101426200. [DOI] [PubMed] [Google Scholar]
- Kwon M, Caplan JF, Filipenko NR, Choi KS, Fitzpatrick SL, Zhang LB, Waisman DM. Identification of annexin II heterotetramer as a plasmin reductase. J Biol Chem. 2002;277:10903–10911. doi: 10.1074/jbc.M111219200. [DOI] [PubMed] [Google Scholar]
- Siever DA, Erickson HP. Extracellular annexin II. Int J Biochem Cell B. 1997;29:1219–1223. doi: 10.1016/s1357-2725(97)00057-5. [DOI] [PubMed] [Google Scholar]
- Ling Q, Jacovina AT, Deora A, Febbraio M, Simantov R, Silverstein RL, Hempstead B, Mark WH, Hajjar KA. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J Clin Invest. 2004;113:38–48. doi: 10.1172/JCI200419684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone DJ, Borth W, Khan KMF, Hajjar KA. Plasminogen-mediated matrix invasion and degradation by macrophages is dependent on surface expression of annexin II. Blood. 2001;97:777–784. doi: 10.1182/blood.v97.3.777. [DOI] [PubMed] [Google Scholar]
- Brownstein C, Deora AB, Jacovina AI, Weintraub R, Gertler M, Khan KMF, Falcone DJ, Hajjar KA. Annexin II mediates plasminogen-dependent matrix invasion by human monocytes: enhanced expression by macrophages. Blood. 2004;103:317–324. doi: 10.1182/blood-2003-04-1304. [DOI] [PubMed] [Google Scholar]
- Siao CJ, Tsirka SE. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J Neurosci. 2002;22:3352–3358. doi: 10.1523/JNEUROSCI.22-09-03352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuglia M, Bianchi R, Verzini M, Giambanco I, Donato R. Annexin II(2)-p11(2) (calpactin-I) stimulates the assembly of GFAP in a calcium-dependent and pH-dependent manner. Biochem Biophys Res Commun. 1995;208:901–909. doi: 10.1006/bbrc.1995.1420. [DOI] [PubMed] [Google Scholar]
- Moestrup SK. The alpha(2)-macroglobulin receptor and epithelial glycoprotein-330-2 giant receptors mediating endocytosis of multiple ligands. BBA-Rev Biomembranes. 1994;1197:197–213. doi: 10.1016/0304-4157(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Narita M, Bu GJ, Holtzman DM, Schwartz AL. The low-density lipoprotein receptor-related protein, a multifunctional apolipoprotein E receptor, modulates hippocampal neurite development. J Neurochem. 1997;68:587–595. doi: 10.1046/j.1471-4159.1997.68020587.x. [DOI] [PubMed] [Google Scholar]
- Makarova A, Mikhailenko I, Bugge TH, List K, Lawrence DA, Strickland DK. The low density lipoprotein receptor-related protein modulates protease activity in the brain by mediating the cellular internalization of both neuroserpin and neuroserpin-tissue-type plasminogen activator complexes. J Biol Chem. 2003;278:50250–50258. doi: 10.1074/jbc.M309150200. [DOI] [PubMed] [Google Scholar]
- Tooyama I, Kawamata T, Akiyama H, Kimura H, Moestrup SK, Gliemann J, Matsuo A, McGeer PL. Subcellular localization of the low density lipoprotein receptor-related protein (a2-macroglobulin receptor) in human brain. Brain Res. 1995;691:235–238. doi: 10.1016/0006-8993(95)00735-9. [DOI] [PubMed] [Google Scholar]
- Marzolo MP, von Bernhardi R, Bu GJ, Inestrosa NC. Expression of alpha(2)-macroglobulin receptor/low density lipoprotein receptor-related protein (LRP) in rat microglial cells. J Neurosci Res. 2000;60:401–411. doi: 10.1002/(SICI)1097-4547(20000501)60:3<401::AID-JNR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Grimsley PG, Quinn KA, Owensby DA. Soluble low-density lipoprotein receptor-related protein. Trends Cardiovasc Med. 1998;8:363–368. doi: 10.1016/s1050-1738(98)00029-2. [DOI] [PubMed] [Google Scholar]
- Hahn-Dantona E, Ruiz JF, Bornstein P, Strickland DK. The low density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J Biol Chem. 2001;276:15498–15503. doi: 10.1074/jbc.M100121200. [DOI] [PubMed] [Google Scholar]
- Bu GJ, Rennke S. Receptor-associated protein is a folding chaperone for low density lipoprotein receptor-related protein. J Biol Chem. 1996;271:22218–22224. doi: 10.1074/jbc.271.36.22218. [DOI] [PubMed] [Google Scholar]
- Cuzner ML, Opdenakker G. Plasminogen activators and matrix metalloproteases, mediators of extracellular proteolysis in inflammatory demyelination of the central nervous system. J Neuroimmunol. 1999;94:1–14. doi: 10.1016/s0165-5728(98)00241-0. [DOI] [PubMed] [Google Scholar]
- Gveric D, Herrera B, Petzold A, Lawrence DA, Cuzner ML. Impaired fibrinolysis in multiple sclerosis: a role for tissue plasminogen activator inhibitors. Brain. 2003;126:1590–1598. doi: 10.1093/brain/awg167. [DOI] [PubMed] [Google Scholar]
- Li H, Newcombe J, Groome NP, Cuzner ML. Characterization and distribution of phagocytic macrophages in multiple sclerosis plaques. Neuropathol Appl Neurobiol. 1993;19:214–223. doi: 10.1111/j.1365-2990.1993.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Gveric D, Cuzner ML, Newcombe J. Insulin-like growth factors and binding proteins in multiple sclerosis plaques. Neuropathol Appl Neurobiol. 1999;25:215–225. doi: 10.1046/j.1365-2990.1999.00187.x. [DOI] [PubMed] [Google Scholar]
- Hajjar KA, Nachman RL. Endothelial cell-mediated conversion of Glu-plasminogen to Lys-plasminogen—further evidence for assembly of the fibrinolytic system on the endothelial cell surface. J Clin Invest. 1988;82:1769–1778. doi: 10.1172/JCI113790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein RL, Friedlander RJ, Nicholas RL, Nachman RL. Binding of Lys-plasminogen to monocytes/macrophages. J Clin Invest. 1988;82:1948–1955. doi: 10.1172/JCI113814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menell JS, Cesarman GM, Jacovina AT, McLaughlin MA, Lev EA, Hajjar KA. Annexin II and bleeding in acute promyelocytic leukemia. New Engl J Med. 1999;340:994–1004. doi: 10.1056/NEJM199904013401303. [DOI] [PubMed] [Google Scholar]
- Falcone DJ, Borth W, McCaffrey TA, Mathew J, McAdam K. Regulation of macrophage receptor-bound plasmin by autoproteolysis. J Biol Chem. 1994;269:32660–32666. [PubMed] [Google Scholar]
- Akenami FT, Koskiniemi M, Farkkila M, Vaheri A. Cerebrospinal fluid plasminogen, plasmin and protease inhibitors in multiple sclerosis. Fibrinolysis Proteol. 1999;13:99–103. [Google Scholar]
- Chandler WL, Alessi MC, Aillaud MF, Vague P, Juhan-Vague I. Formation, inhibition and clearance of plasmin in vivo. Haemostasis. 2000;30:204–218. doi: 10.1159/000054136. [DOI] [PubMed] [Google Scholar]
- Choi KS, Ghuman J, Kassam G, Kang HM, Fitzpatrick SL, Waisman DM. Annexin II tetramer inhibits plasmin-dependent fibrinolysis. Biochemistry. 1998;37:648–655. doi: 10.1021/bi971005k. [DOI] [PubMed] [Google Scholar]
- Simon DI, Xu H, Vaughan DE. Cathepsin D-like aspartyl protease activity mediates the degradation of tissue-type plasminogen-activator plasminogen activator inhibitor-1 complexes in human monocytes. BBA-Mol Cell Res. 1995;1268:143–151. doi: 10.1016/0167-4889(95)00063-x. [DOI] [PubMed] [Google Scholar]
- Chung CY, Murphy-Ullrich JE, Erickson HP. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell. 1996;7:883–892. doi: 10.1091/mbc.7.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutowski NJ, Newcombe J, Cuzner ML. Tenascin-R and C in multiple sclerosis lesions: relevance to extracellular matrix remodelling. Neuropathol Appl Neurobiol. 1999;25:207–214. doi: 10.1046/j.1365-2990.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- Degryse B, Neels JG, Czekay RP, Aertgeerts K, Kamikubo Y, Loskutoff DJ. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J Biol Chem. 2004;279:22595–22604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- Czekay RP, Kuemmel TA, Orlando RA, Farquhar MG. Direct binding of occupied urokinase receptor (uPAR) to LDL receptor-related protein is required for endocytosis of uPAR and regulation of cell surface urokinase activity. Mol Biol Cell. 2001;12:1467–1479. doi: 10.1091/mbc.12.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaan HC, Wang J, Weiss I. Expression of receptors for plasminogen activators on endothelial cell surface depends on their origin. J Thromb Haemost. 2004;2:306–312. doi: 10.1111/j.1538-7933.2004.00593.x. [DOI] [PubMed] [Google Scholar]
- Shatos MA, Orfeo T, Doherty JM, Penar PL, Collen D, Mann KG. Alpha-thrombin stimulates urokinase production and DNA-synthesis in cultured human cerebral microvascular endothelial cells. Arterioscler Thromb Vasc. 1995;15:903–911. doi: 10.1161/01.atv.15.7.903. [DOI] [PubMed] [Google Scholar]
- Levin EG, Santell L, Osborn KG. The expression of endothelial tissue plasminogen activator in vivo: a function defined by vessel size and anatomic location. J Cell Sci. 1997;110:139–148. doi: 10.1242/jcs.110.2.139. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen W. Fibrin-mediated plasminogen activation. Fibrinogen. 2001;936:237–246. doi: 10.1111/j.1749-6632.2001.tb03512.x. [DOI] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S. Tissue plasminogen activator in nervous system function and dysfunction. Thromb Haemost. 2001;86:138–143. [PubMed] [Google Scholar]
- Qiu ZH, Strickland DK, Hyman BT, Rebeck GW. Alpha 2-macroglobulin exposure reduces calcium responses to N-methyl-d-aspartate via low density lipoprotein receptor-related protein in cultured hippocampal neurons. J Biol Chem. 2002;277:14458–14466. doi: 10.1074/jbc.M112066200. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Bu GJ, Sun YL, Daugherty A, Holtzman DM. Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J Biol Chem. 1996;271:30121–30125. doi: 10.1074/jbc.271.47.30121. [DOI] [PubMed] [Google Scholar]
- Bu GJ, Sun YL, Schwartz AL, Holtzman DM. Nerve growth factor induces rapid increases in functional cell surface low density lipoprotein receptor-related protein. J Biol Chem. 1998;273:13359–13365. doi: 10.1074/jbc.273.21.13359. [DOI] [PubMed] [Google Scholar]