Abstract
Interleukin (IL)-27 is a novel heterodimeric cytokine of the IL-12 family that is composed of two subunits, Epstein-Barr virus (EBV)-induced gene 3 (EBI3) and p28. EBI3 is expressed at high levels in EBV-transformed B-cell lines and is induced in vitro by the EBV oncogene LMP1 in a nuclear factor (NF)-κB-dependent manner. We show here that EBI3 expression is up-regulated in human T-cell leukemia virus type 1 (HTLV-1)-infected cell lines and IL-2-dependent leukemic cells from adult T-cell leukemia/lymphoma (ATL) patients, compared to normal activated T cells. EBI3 expression was decreased in HTLV-1-transformed cells after treatment with the NF-κB inhibitor BAY11-7082 and was induced in Jurkat cells by expression of HTLV-1 wild-type Tax oncoprotein, but not by the Tax mutant M22, which is defective for NF-κB activation. In situ analysis of EBI3 and p28 expression in Hodgkin’s lymphomas (HLs), in various EBV-associated lymphoproliferative disorders (LPDs) (including post-transplant LPDs and nasal-type NK/T-cell lymphomas), and in ATL showed that EBI3 was expressed by neoplastic cells in all cases of HL and of LMP1-positive EBV-associated LPD, at variable levels in ATL cases, but rarely in control T-cell lymphomas. In contrast, in all lymphomas tested, no or few tumoral cells expressed p28. Consistent with these data, no significant p28 or IL-27 expression was detected in HL-derived cell lines, or in EBV- or HTLV-1-transformed cell lines. This selective overexpression of EBI3 by transformed cells suggests that EBI3 may play a role, independently from its association to p28, in regulating anti-viral or anti-tumoral immune responses.
In humans, two transforming viruses, Epstein-Barr virus (EBV) and human T-cell leukemia virus type 1 (HTLV-1), are causally associated with the development of lymphomas. EBV, a human γ-herpesvirus, efficiently transforms primary B cells, in vitro, into continuously growing lymphoblastoid cell lines (LCLs), and has been closely associated with the development of several lymphoid malignancies, including endemic Burkitt’s lymphoma (BL), Hodgkin’s lymphoma (HL), and lymphoproliferative disorders (LPDs) arising in immunocompromised individuals.1,2 HTLV-1 is a retrovirus and is the etiological agent of adult T-cell leukemia/lymphoma (ATL), an aggressive malignancy of mature CD4-positive T lymphocytes.3
Previously, we reported the characterization of a novel EBV-induced gene, EBI3.4 EBI3 was cloned by substractive hybridization from an EBV-infected BL cell line and was found to be highly expressed in LCLs. It codes for a soluble type 1 cytokine receptor, homologous to the p40 subunit of interleukin (IL)-12. Recently, EBI3 has been shown to associate with a new IL-12 p35-related subunit, p28, to form a novel noncovalently linked heterodimeric cytokine (EBI3/p28), named IL-27.5 The receptor complex for IL-27 is composed of TCCR (also called WSX-1 or IL-27R) and gp130.5,6 Initial studies have suggested that IL-27 may play an important role in initiation of Th1 responses. This role was based on: 1) the in vitro expression profile of EBI3 and p28, as defined by quantitative reverse transcriptase-polymerase chain reaction analysis, showing that co-expression of EBI3 and p28 was mainly observed in activated macrophages and dendritic cells;5 2) the ability of IL-27 to induce T-bet and IL-12Rβ2 expression in naive CD4-positive T cells,7–9 to stimulate their proliferation, and to synergize with IL-12 for interferon-γ production5,7; 3) the phenotype of IL-27R-deficient mice showing a defect in Th1 response initiation on infection with Listeria monocytogenes or Leishmania major.10,11 Consistent with this model, we observed that EBI3 and p28 were co-expressed by macrophages and macrophage-derived cells at the site of disease in human Th1-associated granulomatous diseases.12 However, subsequent studies led to reconsider this model and a possible role of IL-27 as a suppressor of T-cell activation has emerged.13 Indeed, two recent studies showed that IL-27R-deficient mice were capable of mounting a Th1 response to infection by Toxoplasma gondii and Trypanosoma cruzi, but exhibited increased mortality because of dysregulated T-cell activation and hyperproduction of proinflammatory cytokines.14,15 Taken together, these findings have suggested that IL-27 functions may be complex, and that IL-27 may, depending on the context, function either as a Th1 response co-inducer or as a negative regulator of T-cell and inflammatory responses.
Previously we showed that EBI3 is induced in vitro by the EBV oncogene LMP1, and that its induction by LMP1 depends on nuclear factor (NF)-κB activation.4,16 LMP1 is one of the nine EBV-encoded proteins expressed in latently infected B cells and plays a key role in EBV-mediated growth transformation.17 LMP1 is also expressed in vivo in many EBV-associated LPDs, including HL, post-transplant LPD, and nasal type NK/T-cell lymphoma. In a previous study, we found that EBI3 was not expressed in EBV-positive BL, consistent with the absence of LMP1 expression in this type of lymphoma, but was expressed by Hodgkin and Reed-Sternberg (HRS) tumoral cells in most cases of HL.18 The expression of EBI3 in other lymphomas has not been reported. The expression profile of p28 in tumor tissues has also not been investigated.
Activation of the NF-κB pathway is a common feature of transforming virus.19 Indeed, activation of NF-κB by viral proteins leads to transactivation of numerous cellular genes, including cytokines, involved in cell survival and evasion of immune response. Thus, similar to EBV-transformed B cells, HTLV-1-transformed T cells exhibit high constitutive NF-κB activity.20,21 The HTLV-1 Tax oncoprotein plays an important role in T-cell transformation through its ability to induce constitutive NF-κB activation and to dysregulate cellular gene expression.
To investigate the potential role of IL-27 in viral lymphomagenesis, we further analyzed the expression of both subunits of IL-27 in EBV-associated lymphoid malignancies and extended our study to ATL. Both in situ and in vitro analyses indicated that EBI3, but no or low p28 and IL-27, is expressed by tumoral cells. This dissociated expression of EBI3 and p28 suggests that EBI3 may play a role, independently from its association with p28, to regulate anti-viral and anti-tumoral responses.
Materials and Methods
Primary Cell Culture, Cell Lines, and Transfection
KMH2, L428, and HDLM2 are EBV-negative cell lines derived from HL patients with nodular sclerosis (L428 and HDLM2) or mixed cellularity (KMH2) subtype. NC-37 is an EBV-positive BL cell line. IB4 and LCL-1, -2, -3, -4 (gift from Ellen Cahir-McFarland, Harvard Medical School, Boston, MA) are in vitro EBV-transformed lymphoblastoid cell lines. Jurkat, MOLT-4, and CEM are HTLV-1-negative T-cell lines. MT-2 and HUT-102 are Tax-positive HTLV-1-infected T-cell lines. ATL1 and ATL2 are IL-2-dependent leukemic cells derived from two different patients with acute ATL. All cell lines were maintained in RPMI 1640-Glutamax media (Invitrogen Corp.) supplemented with 10 to 20% fetal bovine serum and antibiotics. Media for leukemic cells was further supplemented with IL-2 (20 U/ml; Roche Diagnostics). To analyze fresh leukemic cells, peripheral blood mononuclear cells from five patients diagnosed with either acute (n = 4) or chronic (n = 1) ATL were purified by Ficoll-Hypaque (Amersham Biosciences) gradient centrifugation. Samples analyzed contained >70% of leukemic cells. For two of these ATL patients, tissue biopsies were available and were studied by immunohistochemistry for IL-27 expression (cases 23 and 26 in Table 3). CD3-positive T cells, CD4-positive or CD8-positive T-cell subsets were isolated from peripheral blood mononuclear cells from HTLV-1-negative donors by negative selection using magnetic beads (Miltenyi Biotec). Purity was >99.5% for CD3-positive cell selection, >97.5% for CD4-positive cell selection, and >94% for CD8-positive cell selection, as assessed by fluorescence-activated cell sorting (FACS) analysis and/or immunocytostaining. All blood samples were obtained after informed consent. Purified T cells and T-cell subsets were cultured in RPM1 1640 media supplemented with 10% fetal bovine serum, l-glutamine, and antibiotics and were stimulated for various times with phytohemagglutinin (4 μg/ml, Roche Diagnostics) and IL-2 (20 U/ml). BAY11-7082 was purchased from Calbiochem and reconstituted in dimethyl sulfoxide. Jurkat cells (5 × 106 cells per transfection) were transfected with pJFE control plasmid or pJFE plasmid encoding wild-type Tax or M22 mutant (gift from Françoise Bex, Université libre de Bruxelles, Brussels, Belgium) by electroporation with a Bio-Rad Gene Pulser Xcell electroporation system at 250 V and 500 μF at room temperature in 200 μl of Optimem media (Gibco BRL) containing DNA.
Table 3.
Immunohistochemical Analysis of IL-27 Expression in Involved Tissues from ATL Patients and Control T-Cell Lymphomas
| Case no. | Diagnosis | Site | % of positive tumoral cells
|
|
|---|---|---|---|---|
| EBI3 | p28 | |||
| Controls | ||||
| 1 | AITL | Lymph node | + | − |
| 2 | AITL | Lymph node | − | − |
| 3 | PTL | Lymph node | + | − |
| 4 | PTL | Lymph node | − | − |
| 5 | PTL | Lymph node | − | − |
| 6 | PTL | Salivary gland | − | − |
| 7 | PTL | Lymph node | − | − |
| 8 | PTL | Lymph node | + | − |
| 9 | ALCL | Lymph node | − | − |
| 10 | ALCL | Lymph node | − | − |
| 11 | ALCL | Lymph node | − | − |
| 12 | ALCL | Lymph node | − | ND |
| 13 | MF | Skin | − | ND |
| 14 | MF | Skin | − | ND |
| 15 | MF | Skin | − | ND |
| 16 | MF | Skin | − | ND |
| ATL | ||||
| 17 | ATL, leuk | Lymph node | − | − |
| 18 | ATL, leuk | Skin | + | + |
| 19 | ATL, leuk | Lymph node | − | ND |
| 20 | ATL, leuk | Skin | − | − |
| 21 | ATL, leuk | Lymph node | ++++ | + |
| 22 | ATL, leuk | Lymph node | +++ | NA |
| 23 | ATL, leuk | Lymph node | + | − |
| 24 | ATL, leuk | Skin | + | − |
| 25 | ATL, leuk | Skin | ++++ | + |
| 26 | ATL, leuk | Skin | − | − |
| 27 | ATL, leuk | Lymph node | ++++ | − |
| 28 | ATL, leuk | Lymph node | ++ | NI |
| 29 | ATL, lymp | Lymph node | + | ND |
| 30 | ATL, lymp | Lymph node | + | ND |
| 31 | ATL, lymp | Lymph node | − | ND |
| 32 | ATL, lymp | Lymph node | + | − |
| 33 | ATL, lymp | Abdomen | + | − |
| 34 | ATL, lymp | Lymph node | + | − |
| 35 | ATL, lymp | Lymph node | − | − |
AITL, angioimmunoblastic T-cell lymphoma; PTL, peripheral T-cell lymphoma, unspecified; ALCL, anaplastic large cell lymphoma; MF, mycosis fungoides; ATL, adult T-cell lymphoma/leukemia; leuk, leukemic form; lymp, lymphomatous form; ND, not done; NI, not interpretable; NA, not available.
, Virtually all cells negative;
, 1 to 5% positive cells;
, 10 to 30% positive cells;
, >30 to 80% positive cells;
, >80% positive cells.
Tissues
Most tissues analyzed were retrieved from the files of the Department of Pathology of Necker Hospital. Lymphomas were classified according to the World Health Organization classification. We studied paraffin-embedded tissues from 14 cases of HL (7 nodular sclerosis cases, 2 lymphocyte depletion cases, 2 mixed cellularity cases, 1 unclassified case, and 2 nodular lymphocyte predominant cases), 16 cases of EBV-associated LPD (12 cases of B- or T-cell LPD, 10 of which from transplanted patients; 1 case of lymphomatoid granulomatosis; and 3 cases of NK/T-cell lymphoma, nasal type), 19 cases of ATL (12 cases of leukemic form and 7 cases of lymphomatous form), and 16 cases of control T-cell lymphomas (2 cases of angioimmunoblastic T-cell lymphoma; 6 cases of peripheral T-cell lymphoma, unspecified; 4 cases of anaplastic large cell lymphoma; and 4 cases of mycosis fungoides). All cases of HL- and of EBV-associated LPD were tested for LMP1 expression by immunohistochemistry, and when negative were further tested for EBERs by in situ hybridization to determine their EBV status. Among HLs, eight cases were negative for EBV (LMP1− and EBER−), and six cases were EBV-positive (LMP1+). Among EBV-associated LPDs, 13 cases were LMP1+, and 3 cases were LMP1− but EBER+. Diagnosis of ATL was made based on previously proposed criteria,22 including clinical and biological features, the presence of anti-HTLV-1 antibodies in the serum, and the detection of HTLV-1 genome in DNA of leukemic cells. ATL patients originated from West Africa, French Guiana, and French West Indies. Seven cases of nonneoplastic lymph nodes exhibiting follicular hyperplasia of unknown origin were included as controls. All tissues analyzed were collected for histological examination and diagnosis purposes. Therefore, this study complies with the French ethical law for studies on human tissues.
Immunostaining
Immunostaining was performed on either paraffin-embedded tissue sections or acetone/methanol (1:1)-fixed cytospin preparation. For immunostaining on cytospin preparation, slides were rehydrated in Tris-buffered saline and saturated by incubation with Tris-buffered saline containing 20% normal human serum or 5% human veinoglobulins for 30 minutes. They were then incubated with the primary antibody diluted in Tris-buffered saline-0.3% bovine serum albumin for 1 hour. Binding of primary antibodies was detected using peroxidase-conjugated EnVision+ reagent (DakoCytomation). The peroxidase reaction was developed with 3′-diaminobenzidine and slides were counterstained with Harris hematoxylin. Immunostaining of paraffin sections was performed as previously described,12 by an indirect avidin-biotin peroxidase technique using ChemMate detection reagents (DakoCytomation). In double-immunostaining experiments, binding of the primary antibody in the first label was detected using peroxidase-conjugated EnVision+ reagent and diaminobenzidine (DakoCytomation) as a chromogenic substrate. Binding of the primary antibody in the second label was detected using an indirect avidin-biotin-alkaline phosphatase kit (BioGenex) and Fast blue (Sigma) as a chromogene, and slides were counterstained with methyl green. To ensure the absence of cross-reactivity between the first and second labeling steps, primary antibody was omitted or isotype-matched control antibody was used in the second label.
EBI3 was detected using 2G4H6 mouse monoclonal antibody (mAb) (IgG2a)23 at 2 μg/ml, in parallel with an isotype-matched control mAb (RPC5, IgG2a; Cappel Durham). p28 was detected using affinity-purified rabbit polyclonal anti-p28 antibodies (DNAX)12 at 1 to 3 μg/ml and normal rabbit IgG (Sigma) were used as a negative control. In some experiments, rat anti-p28 29B5 mAb (DNAX)12 was used at 10 μg/ml, in parallel to normal rat IgG (Sigma) as a negative control. LMP1 was detected using CS1 to CS4 mouse mAbs (DakoCytomation) at 4 μg/ml. CD3 mAb (clone F7.2.38) and CD8 mAb (clone C8/144B), both from DakoCytomation, were used at 10 μg/ml and 0.25 μg/ml, respectively. CD25 mAb (clone 4C9; Novocastra Laboratories Ltd.) was used at a 1:100 dilution. For immunocytostaining, the following antibodies were used: CD3 mAb (clone UCHT1, DakoCytomation) at 1.5 μg/ml, CD4 mAb (clone SK3; BD Biosciences) at 0.15 μg/ml, and CD8 mAb (clone DK25, DakoCytomation) at 0.25 μg/ml. The detection of EBERs was performed by in situ hybridization using EBER PNA probe and the PNA in situ hybridization detection system (DakoCytomation).
Western Blot Analysis and Enzyme-Linked Immunosorbent Assay (ELISA)
Cells were washed in cold phosphate-buffered saline (PBS) and lysed for 1 hour on ice in lysis buffer (1% Nonidet P-40, 50 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 3% glycerol, 1.5 mmol/L ethylenediaminetetraacetic acid) supplemented with protease inhibitors (1 mmol/L phenylmethyl sulfonyl fluoride, 1 μg/ml pepstatin, 1 μg/ml leupeptin). Cell lysates were centrifuged for 15 minutes at 13,000 × g to remove cell debris and protein concentration was determined using the bicinchoninic acid protein assay reagent (Pierce). Lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose for immunoblotting with anti-EBI3 2G4H6 mAb, mouse anti-Tax mAb (provided by John Brady, NCI, NIH, Bethesda, MD), or rat anti-p28 mAbs (clones 29B5 or 18C5, DNAX). Binding of mouse or rat mAbs was detected with horseradish peroxidase-conjugated sheep anti-mouse antibodies (Amersham Biosciences) or horseradish peroxidase-conjugated goat anti-rat antibodies (Santa Cruz), respectively. Peroxidase reaction was developed with chemiluminescence reagents (Pierce). EBI3 and IL-27 ELISA were previously described.5,23 EBI3 ELISA detects both free EBI3 and IL-27 (detection limit, 1 ng/ml), whereas IL-27 ELISA is specific for EBI3/p28 heterodimer (detection limit, 0.3 ng/ml). For ELISA, cells (5 to 10 × 105 per ml) were grown in RPM1 1640 complete media or in RPMI 1640 media supplemented with 1% Nutridoma (Roche Diagnostics) for 24 to 48 hours. Supernatants were then collected, spun to remove debris, and stored at −80°C. In some experiments, cell culture supernatants were concentrated using Centriprep 10 (Millipore) or Ultrafree-4 centrifugal filter unit (Millipore), before being tested by ELISA.
Cell Surface Immunofluorescence and FACS Analysis
Cells were washed in FACS buffer (PBS, 2% fetal bovine serum, 0.01% sodium azide), and incubated for 30 minutes on ice with isotype control (MOPC 141, IgG2b; Sigma) or anti-IL-27R mAb (anti-TCCR, IgG2b; R&D Systems) at 10 μg/ml in FACS buffer. Binding of mouse antibodies was detected using phycoerythrin-conjugated F(ab′)2 fragment goat anti-mouse IgG (Coulter) and a minimum of 5000 gated cells were analyzed on a FACScan. In binding assays, cells were washed in FACS buffer, and incubated with recombinant Flag-tagged IL-27 (DNAX) for 1 hour at 1 μg/ml in FACS buffer followed by incubation with M2 anti-Flag antibody (Sigma), and goat anti-mouse IgG as above. As a control, Flag-tagged IL-27 was omitted in the first step. Anti-TCCR mAb was first verified to detect cell surface IL-27R by transfection of COS7 cells with an expression vector coding for human IL-27R (gift from Hugues Gascan’s laboratory, Institut National de la Santé et de la Recherche Medicale U564, Angers, France).
Results
Immunohistochemical Analysis of IL-27 Expression in HL and Other EBV-Associated LPDs
To further investigate the expression of EBI3 and IL-27 in EBV-associated lymphoid neoplasia, 14 cases of HL (6 EBV+ and 8 EBV−) and 16 cases of various EBV-associated LPDs were analyzed by immunohistochemistry for expression of the two subunits of IL-27, EBI3 and p28, in parallel to LMP1 expression (Tables 1 and 2, and Figure 1).
Table 1.
Immunohistochemical Analysis of IL-27 Expression in HL
| Case no. | Diagnosis | Site | LMP1 status | % of EBI3+ HRS cells | % of p28+ HRS cells |
|---|---|---|---|---|---|
| 1 | NS | Lymph node | pos | ++++ | − |
| 2 | NS | Lymph node | pos | ++++ | + |
| 3 | NS | Lymph node | neg | ++++ | − |
| 4 | NS | Lymph node | neg | ++++ | + |
| 5 | NS | Lymph node | neg | ++++ | + |
| 6 | NS | Mediastinum | neg | ++++ | − |
| 7 | NS | Mediastinum | neg | ++++ | − |
| 8 | LD | Lymph node | pos | ++++ | + |
| 9 | LD | Lymph node | neg | ++++ | − |
| 10 | MC | Lymph node | pos | ++++ | − |
| 11 | MC | Lymph node | pos | ++++ | + |
| 12 | uc | Lymph node | pos | ++++ | − |
| 13 | NLP | Lymph node | neg | ++++ | − |
| 14 | NLP | Lymph node | neg | ++++ | − |
NS, nodular sclerosis; LD, lymphocyte depletion; MC, mixed cellularity; uc, unclassified; NLP, nodular lymphocyte predominant.
, virtually all cells negative;
, 1 to 5% positive cells;
, >90% positive cells.
Table 2.
Immunohistochemical Analysis of IL-27 Expression in Various EBV-Associated LPD
| Case no. | Diagnosis | Site | % of positive tumoral cells
|
||
|---|---|---|---|---|---|
| LMP1 | EBI3 | p28 | |||
| 1 | post-transplant B-cell LPD | Lymph node | +++ | ++++ | − |
| 2 | post-transplant B-cell LPD | Small Bowel | +/+++ | ++++ | +++/+ |
| 3 | post-transplant B-cell LPD | Retroperitoneum | ++ | +++ | − |
| 4 | post-transplant B-cell LPD | Kidney | +++ | ++++ | − |
| 5 | post-transplant B-cell LPD | Skin | − | − | ND |
| 6 | post-transplant B-cell LPD | Lymph node | ++ | ++++ | − |
| 7 | post-transplant B-cell LPD | Small bowel | ++/++++ | ++/++++ | − |
| 8 | post-transplant B-cell LPD | Gingiva | − | − | ND |
| 9 | post-transplant B-cell LPD | Tonsil | +++ | +++ | − |
| 10 | post-transplant T-cell LPD | Lymph node | +++ | +++ | − |
| 11 | B-cell LPD | Lymph node | ++++ | ++++ | − |
| 12 | B-cell LPD | Retroperitoneum | ++ | ++++ | − |
| 13 | Lymphomatoid granulomatosis | Lymph node | +++ | ++++ | − |
| 14 | NK/T-cell lymphoma, nasal type | Lymph node | +++ | ++++ | − |
| 15 | NK/T-cell lymphoma, nasal-type | Nose | + | ++ | − |
| 16 | NK/T-cell lymphoma, nasal-type | Nose | − | + | ND |
, Virtually all cells negative;
, 1 to 5% positive cells;
, 10 to 30% positive cells;
, >30 to 80% positive cells;
, >80% positive cells. ND, not done.
LMP1-negative cases were positive for EBV, as assessed by EBER in situ hybridization.
In two cases (cases 2 and 7), the percentage of positive cells was highly heterogeneous and the lower and upper values are indicated.
Figure 1.
Immunohistochemical analysis of IL-27 expression in HL and various EBV-associated LPDs. a–d: Staining of sections from two cases of HL (a, b: mixed cellularity; c, d: nodular lymphocyte predominant) with anti-EBI3 (a, c) or anti-p28 (b, d) antibodies, showed that HRS cells express EBI3, but no detectable p28. Of note (c, d), macrophages (arrows) that exhibit weaker staining than tumoral cells (arrowhead) for EBI3 are also stained with anti-p28 antibodies, whereas strongly EBI3-positive tumoral cells remain negative for p28. e: Numerous tumoral cells from an LMP1-positive (inset) post-transplant B-cell LPD express EBI3. f: Similarly, numerous tumoral cells from a nasal type NK/T-cell lymphoma that express LMP1 (inset) are strongly labeled with anti-EBI3 antibody. In one case of post-transplant LPD (case 2 in Table 2), a specific population of tumoral cells with a plasmacytic differentiation (arrowheads) express both EBI3 (g) and p28 (h). In all cases, no signal was observed with control antibodies used in parallel (data not shown). Original magnifications: ×20 (a, b); ×40 (c–h). Original zoom factors: 1.1 (a, b); 1.3 (c, d); 1 (e–h).
Consistent with previous work,18 EBI3 was expressed by HRS cells in all cases of HL, independently of LMP1 expression and of the histological subtype (Table 1). In each case, the percentage of EBI3-positive HRS cells was >90%, with nearly all tumoral cells positive for EBI3 in most cases (12 of 14). In contrast, p28 expression by HRS cells was detected in only five cases and in these cases only a minority of HRS cells (ranging from 2 to 5%) was positive for p28 (Table 1). In addition to tumoral cells, variable numbers of cells were positive for EBI3. Among these, cells with a morphology consistent with dendritic cells were strongly labeled with EBI3, whereas macrophages, endothelial cells, and rare plasma cells showed weak EBI3 staining. Macrophages, endothelial cells, plasma cells, as well as fibroblasts, also stained with anti-p28 antibodies (Figure 1, a to d; and data not shown). This staining pattern of nontumoral cells was similar to the one we previously observed in reactive lymph nodes.12
Similarly, a dissociated expression of EBI3 and p28 by neoplastic cells was observed in the 16 cases of EBV-associated LPD (Table 2). In these cases, expression of EBI3 by tumoral cells was heterogeneous, ranging from 0 to >90% of tumoral cells positive for EBI3. Interestingly, the percentage of EBI3-positive neoplastic cells correlated with that of LMP1-positive cells in most cases. Indeed, in the three LMP1-negative cases, no or few tumoral cells (2% at the most) expressed EBI3. In contrast, in all cases positive for LMP1, we always observed EBI3 expression by tumoral cells (Figure 1, e and f). Also, heterogeneity of LMP1 expression within a given case was associated with heterogeneity in tumor cell expression of EBI3, and we observed an overlapping distribution of EBI3- and LMP1-positive tumoral cells. However, although a positive correlation was observed between EBI3 and LMP1 expression by tumoral cells, the number of EBI3-positive tumoral cells was, in most cases, slightly more than that of LMP1-positive cells (Table 2). This may be because of the higher sensitivity of EBI3 detection by immunohistochemistry, compared to that of LMP1. Also, the substantial overexpression of EBI3 compared to LMP1 observed in one case (case 6) suggests that other mechanisms than LMP1 induction may result in EBI3 expression by tumoral cells. Concerning p28, no significant expression by tumor cells was observed in 15 of 16 cases. In only one case (case 2), a significant fraction of tumoral cells expressed p28. In this case, we observed two patterns of staining, corresponding to two different populations of neoplastic cells. One population consisting of large cells showed strong EBI3 reactivity in most cells (>90%positive cells), as well as LMP1 reactivity in a fraction of them, but rarely expressed p28 (∼1% positive cells). The second population consisted of smaller cells with a plasmacytic differentiation, many of which were positive for both EBI3 and p28 (>70% positive cells for both subunits), but rarely expressed LMP1 (∼1% positive cells) (Figure 1, g and h). Expression of p28 by this specific tumoral cell population may be related to its peculiar stage of differentiation, because normal plasma cells and cells with a plasmacytic differentiation present in follicles of reactive lymph nodes expressed p28 (Ref. 12 and this study). The pattern of EBI3 and p28 staining of nontumoral cells was similar to the one observed in HL.
Taken together, these data indicate that HLs and LMP1-positive LPDs are characterized by strong EBI3 expression by neoplastic cells. In contrast, we failed to detect a significant production of p28 by tumoral cells in most cases.
Analysis of IL-27 and IL-27R Expression in HL-Derived Cell Lines and LCLs
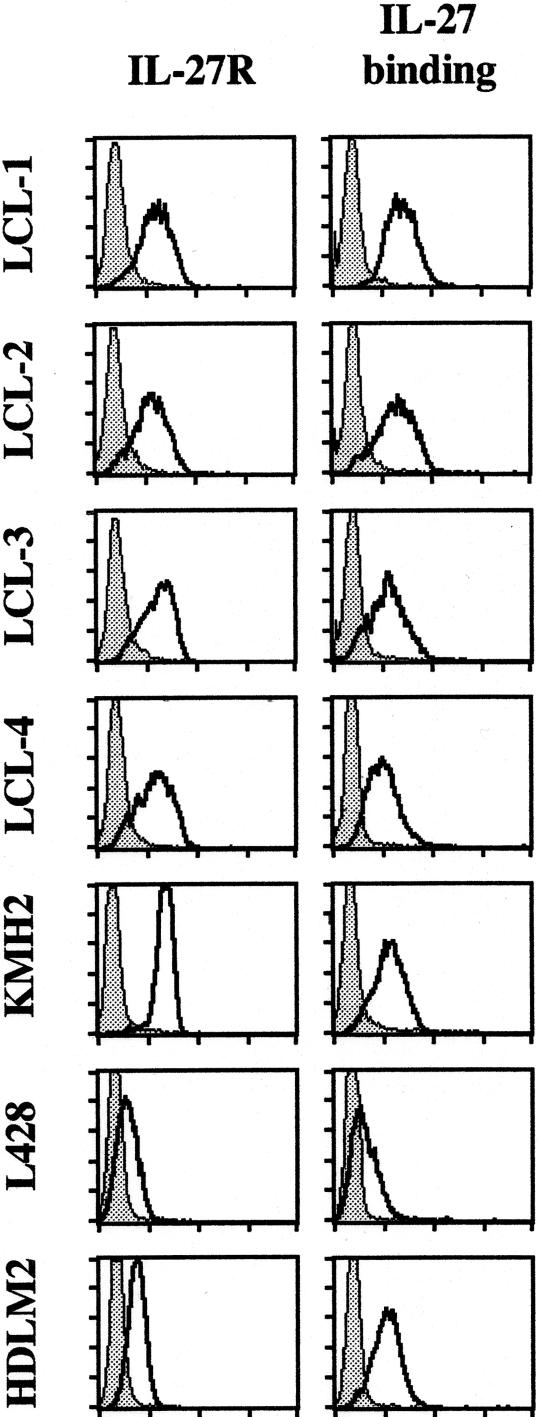
To further analyze IL-27 expression, three HL-derived cell lines, L428, KMH2, and HDLM2, and four LCLs were tested for IL-27 expression. These cell lines expressed EBI3 as tested by Western blot analysis of the cell lysate (Ref. 18 and data not shown). By ELISA of the cell culture supernatant, HL-derived cell lines secreted from 1.9 to 35.3 ng/ml of EBI3,18 and LCLs from 1.3 to 5.7 ng/ml of EBI3. By Western blot, no p28 could be detected in their cell lysates and no p28 induction was observed after LMP1 transient or stable transfection (limit of detection, 0.5 ng per lane) (data not shown). By ELISA, no IL-27 was detected in cell culture supernatants (limit of detection, 0.3 ng/ml), unless they were concentrated by 20-fold to 40-fold. Under these latter conditions, low levels of IL-27 were detected (∼1 ng/ml). Consistent with a low level of p28 expression, no signal or a very faint p28 signal was detected by immunocytostaining of both HL-derived cell lines and LCLs (data not shown). We next examined whether these cell lines express IL-27R. All seven cell lines expressed IL-27R and bound IL-27, as assessed by FACS analysis (Figure 2). Thus, consistent with the in situ analysis, in vitro studies indicated that HL-derived cell lines and LCLs express high levels of EBI3, and IL-27R, but only a very low amount, if any, of p28.
Figure 2.
HL-derived cell lines and LCLs express IL-27R. Four LCLs (LCL-1, -2, -3, -4) and three HL-derived cell lines (KMH2, L428, HDLM2) were tested for IL-27R cell surface expression and IL-27 binding by FACS analysis (x axis, log fluorescence intensity; y axis, cell number) as indicated in Materials and Methods. The filled gray line corresponds to controls.
HTLV1-Infected T-Cell Lines Constitutively Express EBI3
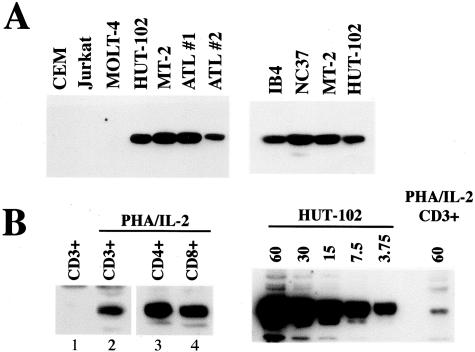
Previous studies in LCLs16,24 or in mouse B cells25,26 have shown that EBI3 is a NF-κB-induced gene. Because HTLV-1-positive T cells exhibit high constitutive NF-κB activity,20,21 we hypothesized that EBI3 may be up-regulated in these cells. To test this hypothesis, two HTLV-1-infected cell lines, HUT-102 and MT2, as well as IL-2-dependent leukemic cells derived from two ATL patients, were tested by Western blot for EBI3 expression. Three HTLV-1-negative T-cell lines, Jurkat, CEM, and MOLT-4, were tested in parallel as negative controls. As shown in Figure 3A, left, no signal was detected in the cell lysates from the three control T-cell lines. In contrast, EBI3 was constitutively expressed in IL-2-dependent leukemic cells from ATL patients and in the two HTLV-1-infected cell lines (Figure 3A, left). In these latter, EBI3 levels were similar to those observed in two EBV-positive B cell lines, NC37 and IB4 (Figure 3A, right). By ELISA, MT2 and HUT-102 were found to secrete 3.9 and 8 ng/ml of EBI3, respectively, and IL-2-dependent leukemic cells secreted 6.3 and 2.2 ng/ml. These values are in the range of that observed in LCLs. Next, we investigated whether normal primary T cells express EBI3. To this end, CD3-positive T cells were purified from peripheral blood from HTLV-1-negative individuals, and cultured for 2 to 7 days with phytohemagglutinin and IL-2. In unstimulated T cells, no EBI3 could be detected by either Western blot analysis of the cell lysate or immunocytostaining (Figure 3B and data not shown). After 2 days of stimulation, weak EBI3 signal could be detected (Figure 3B) and this expression was maintained for up to 7 days (data not shown). Similar experiments performed with purified CD4-positive or CD8-positive T cells indicated that both T-cell subsets expressed EBI3 after stimulation (Figure 3B). However, although EBI3 expression could be detected in normal activated T cells, levels were much lower than those observed in HTLV-1-infected cells (Figure 3C). Indeed, titration experiment showed that EBI3 signal observed in the cell lysate from HUT-102 cells was at least 16-fold higher than that observed in the cell lysate from stimulated normal T cells. Consistent with this low level of intracellular EBI3 in stimulated normal T cells, no EBI3 could be detected in neat cell culture supernatants by ELISA, and levels were just above the limit of detection when supernatants were concentrated by 25-fold. Altogether, these data indicate that EBI3 is specifically up-regulated in HTLV-1-infected cells.
Figure 3.
Immunoblot analysis of EBI3 expression in HTLV-1-infected cells and normal T cells. A: Cell lysates (1% Nonidet P-40 extracts, 10 μg per lane) from three HTLV-1-negative T-cell lines (CEM, Jurkat, MOLT-4), two HTLV-1-positive T-cell lines (HUT-102, MT-2), and IL-2-dependent leukemic cells from two ATL patients (ATL 1, ATL 2) (left), as well as two EBV-positive B cell lines, IB4 and NC37 (right), were analyzed by Western blot with anti-EBI3 antibodies. B (left): EBI3 immunoblot analysis of cell lysates (1% Nonidet P-40 extracts, 50 μg per lane) from purified CD3-positive T cells (lanes 1 and 2), purified CD4-positive T cells (lane 3), and purified CD8-positive T cells, either unstimulated (lane 1) or stimulated for 40 to 42 hours with phytohemagglutinin and IL-2 (lanes 2 to 4) were analyzed by immunoblotting with anti-EBI3 antibodies. The low level of EBI3 expression detected was unlikely due to contaminating cell populations because the numbers of EBI3-positive cells as tested by immunocytostaining (at least 20%) were clearly more than that of contaminating cells (from 0.5 to 6%) (data not shown). B (right): The amounts of cell lysates (Nonidet P-40 extracts) indicated at top of the blot (in μg) from HUT-102- or phytohemagglutinin/IL-2-activated primary CD3-positive T cells were analyzed by immunoblotting with anti-EBI3 antibodies. In B, a more sensitive chemiluminescent substrate was used than in A, because of the low level of EBI3 expressed in normal T cells.
Next, we examined whether normal T cells or HTLV-1-positive T-cell lines expressed IL-27. As observed in HL-derived cell lines and LCLs, no signal or a barely detectable p28 signal was detected by immunocytostaining in HTLV-1-infected cells (HUT-102 and MT-2) or in activated normal T cells (both CD4- and CD8-positive subsets) (data not shown). No IL-27 could be detected in the supernatant from HUT-102, even after a 30-fold concentration. Similar to EBV-positive cell lines, HTLV1-positive cell lines (MT-2 and HUT-102) expressed IL-27R, as tested by FACS analysis (data not shown).
Role of HTLV-1 Tax and NF-κB Activation in EBI3 Expression
Tax is a viral transactivator that has been shown to transactivate the expression of numerous cellular genes, including many cytokine or cytokine receptor genes,3 in part through its ability to activate NF-κB. To determine whether Tax induces EBI3 expression and to evaluate the role of Tax-induced NF-κB activation, Jurkat cells were transiently transfected with increasing amounts of expression plasmid coding for wild-type Tax or for a mutant of Tax, M22, previously shown to be defective for NF-κB activation,27 and cell lysates were analyzed for EBI3 by immunoblotting. Transfection of 0.5 μg of pJFE-Tax resulted in significant induction of EBI3 (Figure 4B, lane 2), and transfection of 1 and 2 μg resulted in higher EBI3 levels (Figure 4B, lanes 3 and 4). In contrast, transfection of 15 or 25 μg of pJFE-M22 (Figure 4B, lanes 5 and 6) failed to induced EBI3 expression, although levels of mutant Tax protein were comparable to the ones observed with wild-type Tax protein, as assessed by Tax immunoblot (Figure 4B, compare lanes 3 and 4 to lanes 5 and 6, respectively). By Western blot, we were not able to detect p28 induction by Tax (data not shown).
Figure 4.
Role of Tax and NF-κB activation on EBI3 expression by HTLV-1-infected cells. A: Jurkat cells were transfected with pJFE control vector (lane 1) or the indicated amount (in μg) of pJFE-Tax (lanes 2 to 4) or pJFE-M22 (lanes 5 and 6). The total amount of plasmid DNA transfected was kept constant in each transfection by addition of pJFE vector. Cells were lysed 22 hours after transfection in Nonidet P-40 lysis buffer and cell lysates (106 cells per lane) were analyzed by immunoblotting with anti-EBI3 and anti-Tax antibodies. B: HUT-102 cells were treated with 5 μmol/L BAY 11-7082 (+) or with dimethyl sulfoxide (−). At time indicated at the top of the blot, cells were lysed in Nonidet P-40 lysis buffer and cell lysates (60 μg per lane) were analyzed by Western blot for EBI3 and Tax expression.
To further examine to which extent the high constitutive NF-κB activity observed in HTLV-1-transformed cells contributes to the high level of EBI3 expression, HUT-102 cells were treated with BAY11-7082, an inhibitor of NF-κB. BAY11-7082 has been previously shown to inhibit NF-κB activation in HUT-102 (effective dose, 5 μmol/L),28 and to down-regulate EBI3 expression in LCLs.24 Incubation of HUT-102 with 5 μmol/L BAY11-7082 resulted in a small decrease of EBI3 expression after 24 hours, and in a more pronounced decrease at 48 hours, as assessed by Western blot analysis of the cell lysate (Figure 4B). This effect was specific because Tax levels were not affected by BAY11-7082 treatment (Figure 4B). Thus, as observed in EBV-transformed B cells, expression of EBI3 in HTLV-1-transformed T cells is dependent on NF-κB activation.
In Situ Analysis of IL-27 Expression in Involved Tissues from ATL Patients
The observation that EBI3 is highly expressed, in vitro, in HTLV-1-transformed T cells led us to investigate whether tumoral cells from involved tissues from ATL patients express EBI3. Therefore, the in situ expression of EBI3 was analyzed by immunohistochemistry in 19 tissue specimens (lymph nodes and skin lesions) from ATL patients, in parallel to p28 expression. As controls, 16 cases of non-HLTV-1-associated mature T-cell lymphomas were analyzed (Table 3 and Figure 5). Among control lymphomas, three cases (cases 2, 3, and 4) were positive for EBER in 0.5 to 3% of tumor cells. However, these cells did not express LMP1.
Figure 5.
Immunohistochemical analysis of EBI3 expression in ATL. a–c: Sections from involved lymph nodes from different ATL patients (all with leukemic form) were stained with anti-EBI3 antibody. a and b: Numerous EBI3-positive neoplastic cells are observed. In contrast, reactive lymphocytes (a, arrows) infiltrating the tumor are negative. In case shown in c, tumoral cells are negative for EBI3, but numerous cells strongly labeled with anti-EBI3 antibodies are observed. Higher magnification (inset) shows that these cells have extended cytoplasmic processes and are morphologically consistent with dendritic cells. d: EBI3 immunostaining in a case of anaplastic large cell lymphoma shows that tumoral cells are negative for EBI3. Staining of a dendritic-appearing cell is observed. e: Double staining for EBI3 (brown) and CD3 (blue) in a nonneoplastic reactive lymph node. Whereas a cell morphologically consistent with a dendritic cell is strongly positive for EBI3 (and CD3-negative), all CD3-positive T cells are negative for EBI3. f: Double staining for EBI3 (brown) and CD8 (blue) in an ATL case (same case as in a) showed that all reactive CD8+ T cells (arrows) surrounding an EBI3+ tumoral cell (arrowhead) are negative for EBI3. Tumoral cells were identified based on their morphology and their immunophenotype including the expression of CD25 (inset: double staining for EBI3 (brown) and CD25 (blue)). In all cases, no signal was observed with control antibodies used in parallel (data not shown). Original magnifications, ×40. Zoom factors: 1 (a); 0.8 (b–d); 1.3 (e–f).
In control T-cell lymphomas, expression of EBI3 by tumoral cells was detected in only three cases (one case of angioimmunoblastic T-cell lymphoma and two cases of peripheral T-cell lymphomas, unspecified). In these cases, only few tumoral cells (less than 5%) were positive for EBI3. Of note, although anaplastic large cell lymphomas share many features with HL, tumoral cells did not express EBI3 in the four cases tested (Figure 5d). In ATL cases, EBI3-positive neoplastic cells were detected in 13 of 19 cases. Their percentage was highly heterogeneous (ranging from 1 to >90% positive tumoral cells) and did not correlate with the site of involvement (lymph node or skin). Interestingly all cases showing the highest scores (five cases ranging from >10 to >90%) were found among patients with leukemic form of ATL (Table 3), whereas patients with lymphomatous form had only low frequency of EBI3-positive tumoral cells (ranging from 1 to 5% in the five of seven cases that contained positive tumoral cells). However, although expressed at low levels in some cases, expression of EBI3 was specific to tumoral T cells, because we did not detect the expression of EBI3 by normal T cells in lymphoid tissues. Indeed, double-staining experiments with anti-EBI3 and anti-CD3 antibodies performed in nonneoplastic lymph nodes (n = 7) showed that normal T cells were consistently negative for EBI3 (Figure 5e). In line with these data, reactive CD8+ lymphocytes infiltrating ATL tumor were negative for EBI3, whereas CD25+ tumoral cells were strongly labeled with anti-EBI3 mAb (Figure 5f). Expression of p28 by neoplastic cells was not detected in the control T-cell lymphomas, and was observed in only three ATL cases. In these latter, less than 5% of tumoral cells were positive for p28. Thus, as observed in HL and other EBV-associated LPD, EBI3 and p28 expression by neoplastic cells was dissociated and characterized by the selective overexpression of EBI3. As previously observed, variable numbers of macrophages and endothelial cells stained with EBI3 and p28 antibodies in both control and ATL cases. In addition, variable numbers of dendritic cells strongly expressing EBI3 were observed. Whereas these cells were usually scarce in the previously analyzed lymphomas, they were abundant in many of the T-cell lymphomas (both control and ATL lymphomas) (Figure 5, c and d; and data not shown). EBI3 expression was also investigated in fresh leukemic cells from patients with acute (n = 4) or chronic (n = 1) ATL. Surprisingly, although these cells have been reported to exhibit high constitutive NF-κB activation,20,21 no EBI3 expression was detected by either immunoblotting or immunocytostaining (data not shown).
Discussion
This study provides additional information on the in vivo expression profile of IL-27 in human pathologies. In a previous study of IL-27 expression in three types of Th1-associated granulomatous diseases, sarcoidosis, tuberculosis, and Crohn’s disease, we observed that EBI3 and p28 were co-expressed by macrophages and macrophage derived-cells (epithelioid cells and multinucleated giant cells) of granulomas. Also, in both granulomatous diseases and control reactive lymph nodes, sinus macrophages, endothelial cells of postcapillary venules, and plasma cells were found to co-express both subunits of IL-27.12 Consistent with these data, in all types of lymphomas analyzed, we noticed the presence of variable numbers of macrophages, endothelial cells, and plasma cells that expressed EBI3 and p28. Also as previously observed,12 dendritic cells that infiltrate the tumor expressed EBI3, but no detectable levels of p28.
In contrast to the coordinated expression of IL-27 subunits observed within epithelioid granulomas, expression of EBI3 and p28 by tumoral cells was dissociated and characterized in many cases by the selective overexpression of EBI3. In all cases of HL and of LMP1-positive EBV-associated LPD, as well as a subset of ATL cases, we observed strong EBI3 expression by tumoral cells, without significant expression of p28 by these cells. Our failure to detect in situ expression of p28 by tumor cells was unlikely to be because of a poor sensitivity of our p28 immunohistochemical technique, because other cell types such as macrophages or endothelial cells were stained with anti-p28 antibodies in the tissues analyzed. Also, in the cell culture supernatant from in vitro-cultured HL-derived cell lines and EBV- or HTLV-1-infected cell lines, we were not able to detect significant amounts of IL-27, whereas under the same conditions we could detect IL-27 secretion from supernatant of in vitro-derived dendritic cells.29
The pattern of EBI3 expression observed in situ in EBV-associated LPD is consistent with our previous in vitro studies, showing that LMP1 induces EBI3 expression in B lymphoblasts.4 In LMP1-negative cases of post-transplant LPD and of nasal-type NK/T-cell lymphoma, no or rare EBI3 expression was detected. These data are in line with the absence of tumor cell expression of EBI3 observed in endemic BL.18 In contrast, in all LMP1-positive EBV-associated LPDs, EBI3 was expressed by tumoral cells. However, although LMP1 expression was always associated with EBI3 expression, we also detected EBI3 expression by tumoral cells in the absence of LMP1, as observed in HL (this study and Ref. 18). EBI3 is a NF-κB up-regulated gene, and in HRS cells other mechanisms than LMP1 expression have been proposed to result in constitutive NF-κB activation, including expression and engagement of TNF-R family members such as CD30 or CD40, or activating mutations in molecules of the NF-κB pathway.30,31 Also, in mouse primary B cells, EBI3 has been shown to be induced on activation25,26 and expression of EBI3 by tumoral B cells may be related to their specific stage of activation and differentiation.
Our study also shows that EBI3 is overexpressed in HTLV-1-infected T-cell lines and IL-2-dependent leukemic cells from ATL patients, compared to normal activated peripheral T cells. Immunohistochemical analyses showed that EBI3 is expressed at variable levels by tumoral cells in involved tissues from ATL patients, but not by normal T cells and very rarely in the control mature T-cell lymphomas analyzed. The factors underlying the heterogeneity of EBI3 expression in ATL tissues remain to be determined, but interestingly, the highest levels were found among patients with the leukemic form. In vitro, we demonstrated that EBI3 expression by HTLV-1-transformed cells is dependent on NF-κB activation, and can be induced by Tax in a NF-κB-dependent manner. The in situ activation of NF-κB in lymphomas from ATL patients is not known, and the in situ expression of Tax in these lymphomas has been difficult to evidence. Indeed, no or low levels of Tax mRNA have been detected by in situ hybridization or reverse transcriptase-polymerase chain reaction/in situ hybridization in skin lesions or lymph nodes of ATL patients,32,33 and the sensitivity of Tax antibody was too low to allow Tax protein detection by immunohistochemistry (F.L., unpublished data).
Although HL and anaplastic large cell lymphoma share common features, including the expression of CD30, no tumoral cell expression of EBI3 was observed in the latter. In anaplastic large cell lymphomas, the NPM-ALK oncoprotein has been shown to abrogate CD30-induced NF-κB activation,34 which may contribute to their lack of EBI3 expression. More surprisingly, tumoral T cells from mycosis fungoides also did not express EBI3, although constitutive NF-κB activation is assumed to be a characteristic feature of these lymphomas.35 This suggests that specific NF-κB complexes may be required for induction of EBI3. Indeed, studies using mice deficient for specific NF-κB components showed that CD40-mediated EBI3 induction in mouse B cells requires specific NF-κB subunits.26 In the same line, we did not detect EBI3 expression in fresh leukemic cells from ATL patients, although these cells exhibit constitutive NF-κB activity. These cells express little or no Tax, and their NF-κB complexes are different from the one observed in Tax-positive HTLV-1-infected cell lines,20 which may explain the differential expression of EBI3 in fresh leukemic cells and HTLV-1-positive cell lines.
The biological significance for the selective expression of EBI3 in virus-associated lymphomas remains to be established. LMP1 and Tax are both major targets of specific cytotoxic T lymphocytes and the survival of LMP1- or Tax-positive cells depends on their positive effects on tumoral cell growth and their ability to down-regulate the immune response. Indeed, both proteins have been shown to play an important role in lymphomagenesis by up-regulating the expression of different cytokines, which act as autocrine growth factors or regulate the anti-tumoral immune response.3,36,37 Because IL-27 had been shown to stimulate the proliferation of CD4-positive T lymphocytes and had been suggested to suppress T-cell activation, we initially speculated that IL-27 may be expressed by tumoral cells and play a role in tumor progression, either as an autocrine growth factor as has been demonstrated for IL-2 and IL-15 in ATL, or as a suppressor of T-cell activation to dampen anti-viral or anti-tumoral responses. Our data do not support this hypothesis, because in most cases, EBI3 expression by tumoral cells was not associated with that of p28, suggesting that no or very little IL-27 is produced by tumoral cells. Also, although HL-derived cell lines, LCLs, and HTLV-1-infected cell lines expressed IL-27R, we could not observe a role for IL-27 on their growth either by using anti-IL-27 neutralizing antibodies or by adding recombinant IL-27 (F.L., E.B., O.D., unpublished data). Very recently, IL-27 was shown to display potent in vivo anti-tumoral activity in a murine tumor model and to function as an adjuvant on the induction of hepatitis C virus-specific cytotoxic T lymphocytes.38,39 In view of these novel roles for IL-27, our failure to detect significant production of IL-27 by tumoral cells may not be surprising.
An initial study of EBI3 and p28 expression profile in various in vitro cultured cells and in tissues demonstrated that the expression of both subunits of IL-27 overlaps only partially, and that EBI3 displays a broader expression profile than that of p28. Also, the kinetics of expression of both subunits within a given cell type was not coordinated.5 These observations lead to the hypothesis that EBI3 may play a role independently from its association to p28. The dissociated expression pattern of EBI3 and p28 we observed in this study further supports this hypothesis. At present no function has been reported for EBI3 alone, although a role as an IL-27 antagonist has been suggested.5 If so, EBI3 overexpression by tumoral cells may be part of a strategy to antagonize IL-27 anti-tumoral activity. EBI3 associates not only with p28, but also with the p35 subunit of IL-12,40 but a biological function for EBI3/p35 heterodimer remains to be established. The existence of another partner for EBI3 has also been suggested by studies of EBI3 knockout mice.41 Elucidation of the respective roles of the different forms of EBI3 (EBI3, EBI3/p28, EBI3/p35) will allow a better comprehension of the role played by this molecule in the regulation of immune responses and in anti-tumoral immunity.
Footnotes
Address reprint requests to Odile Devergne, CNRS UMR 8147, Hôpital Necker, Bâtiment Sèvres, 161 rue de Sèvres, 75 015 Paris, France. E-mail: devergne@necker.fr.
Supported by the Association de Recherche Contre le Cancer (grants no. 4416 and 7711 to O.D.) and CNRS/Assistance Publique-Hôpitaux de Paris (fellowship to F.L.).
Present address of S.P.: Micromet AG, München, Germany.
References
- Rickinson A, Kieff E. Epstein-Barr virus. Knipe DM, Howley PM, editors. Philadelphia: Lippincott/Williams & Wilkins Co.; Fields Virology. (4th ed.) 2001:pp 2575–2628. [Google Scholar]
- Küppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3:801–812. doi: 10.1038/nri1201. [DOI] [PubMed] [Google Scholar]
- Yoshida M. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu Rev Immunol. 2001;19:475–496. doi: 10.1146/annurev.immunol.19.1.475. [DOI] [PubMed] [Google Scholar]
- Devergne O, Hummel M, Koeppen H, Le Beau MM, Nathanson EC, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, Xia M, Wakeham AC, Nishina H, Potter J, Saris CJ, Mak TW. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- Larousserie F, Pflanz S, Coulomb-L’Herminé A, Brousse N, Kastelein R, Devergne O. Expression of IL-27 in human Th1-associated granulomatous diseases. J Pathol. 2004;202:164–171. doi: 10.1002/path.1508. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, Zhang M, Hisaeda H, Mak TW, Yoshimura A, Yoshida H. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- Devergne O, Cahir McFarland ED, Mosialos G, Izumi KM, Ware CF, Kieff E. Role of the TRAF binding site and NF-κB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J Virol. 1998;72:7900–7908. doi: 10.1128/jvi.72.10.7900-7908.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahir McFarland ED, Izumi KM, Mosialos G. Epstein-Barr virus transformation: involvement of latent membrane protein 1-mediated activation of NF-κB. Oncogene. 1999;18:6959–6964. doi: 10.1038/sj.onc.1203217. [DOI] [PubMed] [Google Scholar]
- Niedobitek G, Pazolt D, Teichmann M, Devergne O. Frequent expression of the Epstein-Barr virus (EBV)-induced gene, EBI3, an IL-12 p40-related cytokine, in Hodgkin and Reed-Sternberg cells. J Pathol. 2002;198:310–316. doi: 10.1002/path.1217. [DOI] [PubMed] [Google Scholar]
- Hiscott J, Kwon H, Genin P. Hostile takeovers: viral appropriation of the NF-κB pathway. J Clin Invest. 2001;107:143–151. doi: 10.1172/JCI11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N, Fujii M, Ikeda S, Yamada Y, Tomonaga M, Ballard DW, Yamamoto N. Constitutive activation of NF-κB in primary adult-T-cell leukemia cells. Blood. 1999;93:2360–2368. [PubMed] [Google Scholar]
- Arima N, Matsushita K, Obata H, Ohtsubo H, Fujiwara H, Arimura K, Kukita T, Suruga Y, Wakamatsu S, Wakamastu S, Hidaka S, Tei C. NF-κB involvement in the activation of primary adult-T-cell leukemia cells and its clinical implications. Exp Hematol. 1999;27:1168–1175. doi: 10.1016/s0301-472x(99)00053-3. [DOI] [PubMed] [Google Scholar]
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol. 1991;79:428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- Devergne O, Coulomb-L’Herminé A, Capel F, Moussa M, Capron F. Expression of Epstein-Barr virus-induced gene 3, an interleukin-12 p40-related molecule, throughout human pregnancy: involvement of syncytiotrophoblasts and extravillous trophoblasts. Am J Pathol. 2001;159:1763–1776. doi: 10.1016/S0002-9440(10)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahir-McFarland ED, Carter K, Rosenwald A, Giltnane JM, Henrickson SE, Staudt LM, Kieff E. Role of NF-κB in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J Virol. 2004;78:4108–4119. doi: 10.1128/JVI.78.8.4108-4119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Peet GW, Balzarano D, Li X, Massa P, Barton RW, Marcu KB. Novel NEMO/IκB kinase and NK-κB target genes at the pre-B to immature B cell transition. J Biol Chem. 2001;276:18579–18590. doi: 10.1074/jbc.M100846200. [DOI] [PubMed] [Google Scholar]
- Dadgostar H, Zarnegar B, Hoffmann A, Qin XF, Truong U, Rao G, Baltimore D, Cheng G. Cooperation of multiple signaling pathways in CD40-regulated gene expression in B lymphocytes. Proc Natl Acad Sci USA. 2002;99:1497–1502. doi: 10.1073/pnas.032665099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Greene WC. Identification of HTLV-1 tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- Mori N, Yamada Y, Ikeda S, Yamasaki Y, Tsukasaki K, Tanaka Y, Tomonaga M, Yamamoto N, Fujii M. Bay 11-7082 inhibits transcription factor NF-κB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood. 2002;100:1828–1834. doi: 10.1182/blood-2002-01-0151. [DOI] [PubMed] [Google Scholar]
- Nagai T, Devergne O, Mueller TF, Perkins DL, van Seventer JM, van Seventer GA. Timing of IFN-β exposure during human dendritic cell maturation and naive Th cell stimulation has contrasting effects on Th1 subset generation: a role for IFN-β-mediated regulation of IL-12 family cytokines and IL-18 in naive Th cell differentiation. J Immunol. 2003;171:5233–5243. doi: 10.4049/jimmunol.171.10.5233. [DOI] [PubMed] [Google Scholar]
- Staudt LM. The molecular and cellular origins of Hodgkin’s disease. J Exp Med. 2000;191:207–212. doi: 10.1084/jem.191.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99:4283–4297. doi: 10.1182/blood-2002-01-0099. [DOI] [PubMed] [Google Scholar]
- Setoyama M, Fujiyoshi T, Mizoguchi S, Katahira Y, Yashiki S, Tara M, Kanzaki T, Sonoda S. HTLV-1 messenger RNA is expressed in vivo in adult-T-cell leukemia/lymphoma patients: an in situ hybridization study. Int J Cancer. 1994;57:760–764. doi: 10.1002/ijc.2910570525. [DOI] [PubMed] [Google Scholar]
- Ohshima K, Suzumiya J, Izumo S, Mukai Y, Tashiro K, Kikuchi M. Detection of human T-lymphotropic virus type-I DNA and mRNA in the lymph nodes using polymerase chain reaction in situ hybridization (PCR/ISH) and reverse transcription (RT-PCR/ISH). Int J Cancer. 1996;66:18–23. doi: 10.1002/(SICI)1097-0215(19960328)66:1<18::AID-IJC4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Horie R, Watanabe M, Ishida T, Koiwa T, Aizawa S, Itoh K, Higashihara M, Kadin ME, Watanabe T. The NPM-ALK oncoprotein abrogates CD30 signaling and constitutive NF-κB activation in anaplastic large cell lymphoma. Cancer Cell. 2004;5:353–364. doi: 10.1016/s1535-6108(04)00084-4. [DOI] [PubMed] [Google Scholar]
- Izban KF, Ergin M, Qin JZ, Martinez RL, Pooley RJ, Jr, Saeed S, Alkan S. Constitutive expression of NF-κB is a characteristic feature of mycosis fungoides: implications for apoptosis resistance and pathogenesis. Hum Pathol. 2000;12:1482–1490. doi: 10.1053/hupa.2000.20370. [DOI] [PubMed] [Google Scholar]
- Mosialos G. Cytokine signaling and Epstein-Barr virus-mediated cell transformation. Cytokine Growth Factor Rev. 2001;12:259–270. doi: 10.1016/s1359-6101(00)00035-6. [DOI] [PubMed] [Google Scholar]
- Matsuoka M. Human T-cell leukemia virus type I and adult T-cell leukemia. Oncogene. 2003;22:5131–5140. doi: 10.1038/sj.onc.1206551. [DOI] [PubMed] [Google Scholar]
- Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, Mizuguchi J, Yoshimoto T. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci USA. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis EE, Neurath MF, Corazza N, Iijima H, Trgovcich J, Wirtz S, Glickman J, Bailey D, Yoshida M, Galle PR, Kronenberg M, Birkenbach M, Blumberg RS. Disruption of T helper 2-immune responses in Epstein-Barr virus-induced gene 3-deficient mice. Proc Natl Acad Sci USA. 2002;99:16951–16956. doi: 10.1073/pnas.252648899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Moriya O, Belladonna ML, Kamiya S, Lemonnier FA, Yoshimoto T, Akatsuka T. Adjuvant activities of novel cytokines, interleukin-23 (IL-23) and IL-27, for induction of hepatitis C virus-specific cytotoxic T lymphocytes in HLA-A*0201 transgenic mice. J Virol. 2004;78:9093–9104. doi: 10.1128/JVI.78.17.9093-9104.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]