Abstract
Distal myopathy with rimmed vacuoles (DMRV), is an autosomal recessive disorder with early adult onset, displays distal dominant muscular involvement and is characterized by the presence of numerous rimmed vacuoles in the affected muscle fibers. The pathophysiology of DMRV has not been clarified yet, although the responsible gene was identified as that encoding UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase involved in the biosynthesis of sialic acids. To identify defective carbohydrate moieties of muscular glycoproteins from DMRV patients, frozen skeletal muscle sections from seven patients with DMRV, as well as normal and pathological controls, were treated with or without sialidase or N-glycosidase F followed by lectin staining and lectin blotting analysis. The sialic acid contents of the O-glycans in the skeletal muscle specimens from the DMRV patients were also measured. We found that Arachis hypogaea agglutinin (PNA) lectin reacted strongly with sarcolemmal glycoproteins in the DMRV patients but not with those in control subjects. α-Dystroglycan from the DMRV patients strongly associated with PNA lectin, although that from controls did not. The sialic acid level of the O-glycans in the DMRV muscular glycoproteins with molecular weights of 30 to 200 kd was reduced to 60 to 80% of the control level. The results show that impaired sialyl O-glycan formation in muscular glycoproteins, including α-dystroglycan, occurs in DMRV.
Distal myopathy with rimmed vacuoles (DMRV), also known as Nonaka distal myopathy (OMIM 605820), is an autosomal recessive muscular disease that was delineated in Japan. The disease is characterized by early-adult onset with weakness of the tibialis anterior muscles, sparing of the quadriceps muscles until the late stage, and the presence of numerous rimmed vacuoles in affected muscle fibers without infiltration by inflammatory cells.1 Previous linkage studies2,3 indicated that DMRV is allelic to hereditary inclusion body myopathy (HIBM), also known as IBM2 (OMIM 600737),4 a similar autosomal recessive disorder that was delineated among Jews originating from Middle Eastern countries.3,5,6 This disease is the most common form of ethnic-related familial degenerative myopathy in Israel, with a prevalence of 1:1500 in the Jewish-Persian community.7 Recently, Eisenberg and colleagues8 identified mutations in the UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (UDP-GlcNAc 2-epimerase) (EC 5.1.3.14/2.7.1.60) gene (GNE) in HIBM families. Then, mutations were identified in the same gene in Japanese patients with DMRV, as expected.9–12
UDP-GlcNAc 2-epimerase has been shown to be the key enzyme in the sialic acid biosynthetic pathway.13 This enzyme is bifunctional, having epimerase activity, in the amino-terminal region, and kinase activity, in the carboxy-terminal region,14 and is involved in the first two steps of the biosynthesis of N-acetylneuraminic acid (NeuAc).15 NeuAc is a common sialic acid in humans and the precursor of sialic acid compounds. Sialic acids are carbohydrates found on the cell surface, where they mediate a lot of cellular functions, eg, cell-cell or cell-matrix interactions. As the modification of sialic acid-containing glycoproteins and glycolipids on the cell surface is essential for biological functions, a genetic defect in the enzyme might cause a myopathy such as DMRV/HIBM. Mutations associated with DMRV/HIBM have been identified in both the epimerase and kinase domains.8 Recent studies revealed that GNE mutations including a common one for Japanese DMRV patients, V572L, cause reduction of UDP-GlcNAc 2-epimerase/ManNAc kinase activity.16 This enzyme is known to be a key enzyme in the sialic acid biosynthetic pathway. However, what the enzyme defect causes is obscure.
In this study, we examined the carbohydrate moieties of muscular glycoproteins from DMRV and control cases, using lectins and glycosidases to elucidate what a genetic defect in GNE results in.
Materials and Methods
Patients
Seven affected individuals from five unrelated Japanese families with DMRV [DMRV-1: a 34-year-old male; DMRV-2: a 31-year-old male; DMRV-3: a 53-year-old female; DMRV-4: an 18-year-old female; and DMRV-5 brother (B): a 31-year-old male; DMRV-5 sister-1 (S-1): a 30-year-old female; and DMRV-5 sister-2 (S-2): a 29-year old female; the latter three being siblings from the same family], a patient with facioscapulo humeral muscular dystrophy (FSHD, a 59-year-old male), a patient with oculopharyngeal muscular dystrophy (OPMD, a 50-year-old female), a patient with Emery-Dreifuss muscular dystrophy (EDMD, a 41-year-old male), a patient with myotonic dystrophy (MD, a 45-year-old male), and four normal controls (N1, a 42-year-old female; N2, a 35-year-old female; N3, a middle-aged female; and N4, a 61-year-old male) were studied, with their informed consent and the approval of our local ethics committee. The five DMRV families were diagnosed based on the following findings: 1) weakness of the tibialis anterior muscles beginning after the age of 20; 2) sparing of the quadriceps muscles until the late stage; 3) the presence of numerous rimmed vacuoles in affected muscle fibers; and 4) identification of mutations in GNE; patients DMRV-1 to DMRV-4 are homozygous for the V572L mutation,11,17 and the three siblings, DMRV-5B, DMRV-5S-1, and DMRV-5S-2, are compound heterozygous for G295D/A631V.17 The clinical manifestations are more severe in DMRV-1 to DMRV-4 than in DMRV-5B, DMRV-5S-1, and DMRV-5S-2.
Antibodies
The primary antibodies used in these studies were a mouse monoclonal antibody to α-dystroglycan (α-DG) (250-fold diluted, clone IIH6 kindly provided by Dr. K.P. Campbell, Howard Hughes Medical Institute, University of Iowa, Iowa City, IA),18 an affinity-purified sheep polyclonal antibody directed against the 20-amino acid C-terminal sequence of chick α-DG (500-fold diluted; kindly provided by Dr. S. Kröger, University of Mainz, Mainz, Germany),19 a mouse monoclonal antibody against β-dystroglycan (β-DG) (clone 8D5, 100-fold diluted), a mouse monoclonal anti-dystrophin antibody (clone 6C5, 100-fold diluted; Novacastra Laboratories, Newcastle, UK), a mouse monoclonal anti-laminin α2 chain antibody (clone 5H2, 100-fold diluted; Chemicon, Temecula, CA), a mouse monoclonal anti-CD63 antibody (200-fold diluted) (Immunotech, Marseille, France),20 a rabbit polyclonal anti-LIMP 2 antibody (200-fold diluted; kindly provided by Dr. Y. Tanaka, Kyushu University, Kyushu, Japan),21 and mouse monoclonal anti-endolyn/CD164 antibodies (clone N6B6.2, 100-fold diluted; BD-PhaMingen, San Diego, CA; clones 103B2 and 105A5, 100-fold diluted, kindly provided by Dr. A.C.W. Zannettino, University of Adelaide, Adelaide, Australia).22
Immunohistochemical Analysis and Lectin Staining
Immunohistochemical analysis was performed on frozen tissue cryosections of biopsied skeletal muscle specimens from normal controls (N1 and N2), pathological controls (OPMD, EDMD, and MD), and the DMRV patients (DMRV-1 to DMRV-5S-2). In brief, frozen sections (8 μm) were mounted on glass slides, dried at room temperature for 1 hour, fixed in cold acetone or 4% paraformaldehyde in phosphate-buffered saline (PBS) for 1 minute at room temperature, and then incubated with 1% bovine serum albumin-PBS. The sections were then incubated with the above primary antibodies overnight at 4°C, followed by incubation with fluorescein isothiocyanate-labeled (200-fold diluted), Cy3-labeled (300-fold diluted) (Jackson ImmunoResearch, West Grove, PA), Alexa Fluor 488-labeled (1000-fold), or Alexa Fluor 594-labeled (1000-fold) secondary antibodies (Molecular Probes, Eugene, OR) as described previously.23
For lectin staining, frozen skeletal muscle tissue sections from the patients with DMRV and the normal controls were incubated with the following fluorescein isothiocyanate-labeled or rhodamine isothiocyanate-labeled lectins: Wheat germ agglutinin (WGA, 1 μg/ml), Arachis hypogaea (peanut) agglutinin (PNA, 1 μg/ml), Glycine max (soybean) agglutinin (SBA, 1 μg/ml), Datura stramonium agglutinin (DSA, 1 μg/ml), Ricinus communis agglutinin120 (RCA120, 1 μg/ml) (Vector Laboratories Inc., Burlingame, CA), and Maackia amurensis agglutinin (MAA, 1 μg/ml) (EY Laboratories, San Mateo, CA) in 1% bovine serum albumin-PBS for 1 hour at room temperature.26 The stained sections were observed under a microscope (Axiovert 1000M; Carl Zeiss, Oberkochen, Germany) equipped with a laser-scanning confocal imaging system, LSM510 (Carl Zeiss). Digitized images were captured under identical conditions.
Western Blotting and Lectin Blotting
Frozen skeletal muscle sections from the DMRV patients and controls including normal subjects were sonicated in 100 μl of Triton X lysis buffer (1% Triton X, 150 mmol/L NaCl, and 20 mmol/L Tris-HCl, pH 7.5). The tissue extracts were centrifuged at 10,000 × g for 5 minutes at 4°C to remove the insoluble debris. Then, the supernatants were treated with trichloroacetic acid, followed by centrifugation at 10,000 × g for 5 minutes at 4°C. The trichloroacetic acid pellets were resuspended in 20 μl of Triton X lysis buffer, and then neutralized with 1 mol/L Tris-HCl (pH 9.0). Sample protein concentration was performed using micro BCA protein assay reagent (Pierce, Rockford, IL). Twenty μg aliquots of protein of the tissue extracts were solubilized with an equal volume of sodium dodecyl sulfate (SDS) sample buffer (62.5 mmol/L Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, and 5% β-mercaptoethanol). Then, they were separated on a 4 to 20% Tris-glycine polyacrylamide gel (PAG mini; Daiichi Pure Chemical Co., Ltd., Tokyo, Japan), and then electrotransferred to polyvinylidene difluoride (PVDF) Immobilon-P membranes (Millipore Corp., Bedford, MA). For Western blotting, the blots were blocked in Tris-buffered saline (20 mmol/L Tris-HCl, pH 7.5, 100 mmol/L NaCl) containing 5% skim milk for 15 minutes at room temperature. Then, they were probed with an anti-α-DG antibody (clone IIH6) at a dilution of 1:500 in Tris-buffered saline containing 5% skim milk for 1 hour at room temperature. After the blots had been washed with Tris-buffered saline three times, then probed with a peroxidase-conjugated donkey anti-mouse secondary antibody (Amersham Pharmacia Biotech, Arlington Heights, IL) for 1 hour at room temperature. Then, the blots were washed with Tris-buffered saline three times, and then developed with ECL (Amersham Pharmacia Biotech) as the chemiluminescent substrate on Hyperfilm ECL (Amersham Pharmacia Biotech) for varying times (1 to 10 minutes).
Specific binding of lectins was used for identification of the carbohydrate moieties in extracts of the frozen skeletal muscle tissues, according to the manufacturer’s specifications (DIG glycan differentiation kit; Roche Molecular Biochemicals). PVDF membranes with the extracts that had been separated by SDS-polyacrylamide gel electrophoresis (PAGE) were incubated for 1 hour with PNA and MAA lectins conjugated to the steroid hapten digoxigenin (DIG). After washing the membranes, glycoproteins were detected with anti-DIG Fab fragments conjugated with alkaline phosphatase, followed by detection with CDP-star (Roche Molecular Biochemicals) as the chemiluminescent substrate.
Chemical Analysis of Oligosaccharide Chains on Glycoproteins Blotted on PVDF Membranes
The sialic acid content was determined by fluorometric analysis using the α-keto acid-specific reagent 1,2-diamino-3,4-methylenedioxybenzene (DMB).24 Skeletal muscle glycoproteins from the DMRV patients and controls including normal controls blotted onto PVDF membranes were analyzed by the mild acid hydrolysis-fluorometric HPLC method, as described previously.25
Enzyme Treatments
Sections were treated with 50 mU sialidase from Clostridium perfringens (Roche, Mannheim, Germany) in 50 mmol/L sodium acetate (pH 5.0) for 6 hours at 37°C or with 5 U N-glycosidase F (Roche) in PBS for 12 hours at 37°C, respectively. Then, samples were analyzed by means of lectin staining and lectin blotting.
Lectin Precipitation
Frozen skeletal muscle sections were solubilized in 100 μl of Triton X lysis buffer. The extracted proteins were added to 20 μl of PNA-agarose (Wako Pure Chemical Industries, Ltd., Tokyo, Japan) overnight with shaking at 4°C. The precipitated proteins were washed extensively in Triton X lysis buffer and then boiled in SDS sample buffer. The supernatant (not binding PNA-agarose) was treated with trichloroacetic acid, followed by centrifugation at 10,000 × g for 5 minutes at 4°C, and then solubilization in SDS sample buffer. The proteins were separated and blotted as described above, and then detected with anti-α-DG antibody IIH6.
Statistical Analysis
The data in this study were presented as means ± SD. Statistical comparisons were conducted by means of Student’s t-test under the conditions of two-tailed distribution and two-sample equal variance.
Results
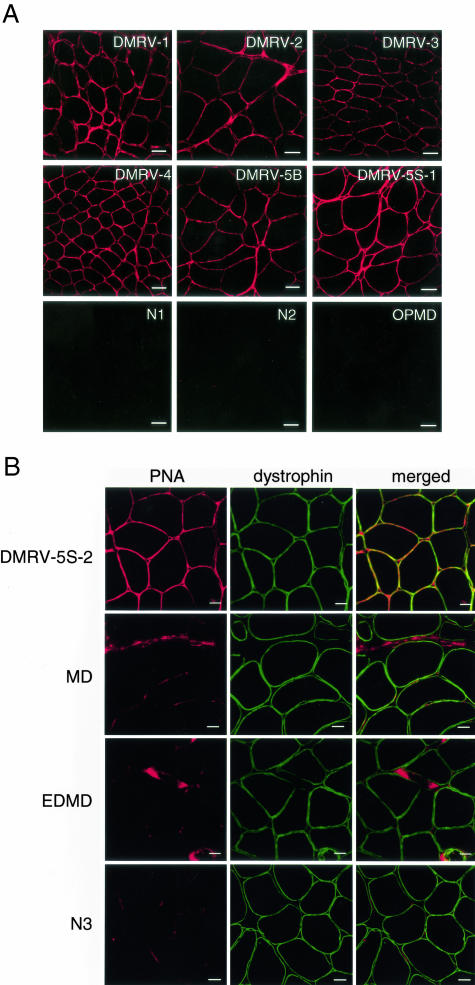
To identify carbohydrate moieties in muscular glycoproteins of both the DMRV patients and control subjects, lectin-staining of frozen sections from normal controls (N1 and N2), pathological controls (OPMD, EDMD, and MD), and the DMRV patients (DMRV-1 to DMRV-5S-2) was analyzed. We used five lectins in this study, ie, PNA (specific for Galβ1-3GalNAc), SBA (specific for GalNAcα1-3Gal), RCA120 (specific for Gal), WGA (specific for GlcNAc and Neu5Ac), and MAA (specific for Neu5Acα2-3Gal). PNA lectin reacted strongly with the sarcolemma and connective tissues in skeletal muscle sections from all seven DMRV patients (Figure 1), whereas it hardly stained those in normal controls and the OPMD patient under the described conditions (Figure 1A). Furthermore, we double-stained skeletal muscle sections with PNA lectin and an antibody against dystrophin, a classical sarcolemma marker, to determine the localization of PNA reactivity in the DMRV muscles. The merged images clearly show that there is co-localization of PNA-reactive materials and dystrophin in the DMRV muscle, although weak PNA reactivity is also present in the connective tissues (Figure 1B). In contrast, PNA stained the connective tissues of the MD, EDMD, and normal control muscles (Figure 1B). These results indicate an increase in the disaccharide Galβ1-3GalNAc unit in the plasma membranes of the DMRV skeletal muscles.
Figure 1.
PNA histochemical analysis of biopsied skeletal muscle tissues. A: Transverse cryosections of muscle biopsy specimens from normal controls (N1 and N2), an OPMD patient, and DMRV patients (DMRV-1, DMRV-2, DMRV-3, DMRV-4, DMRV-5B, and DMRV-5S-1) were stained with rhodamine isothiocyanate-labeled PNA. PNA-reactive glycoconjugates are localized in the sarcolemma in DMRV skeletal muscles but not in normal skeletal muscles. B: Transverse cryosections of muscle biopsy specimens from a DMRV patient (DMRV-5S-2), a MD patient, an EDMD patient, and a normal control (N3) were co-stained with PNA lectin and an antibody against dystrophin. The merged images show that PNA-reactive materials were co-localized with dystrophin in the DMRV patient, although PNA reactivity was also observed in connective tissues of the DMRV, MD, EDMD, and normal control. Scale bars: 50 μm (A); 20 μm (B).
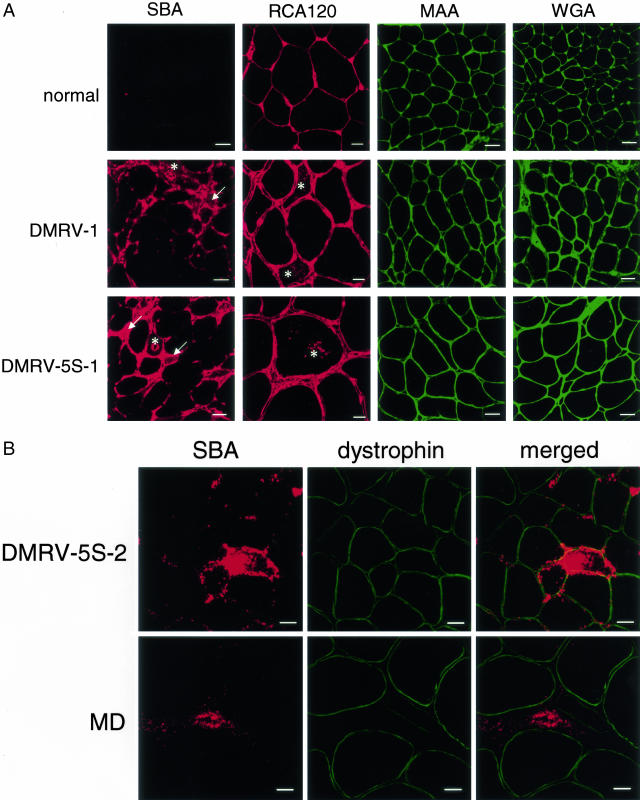
SBA lectin stained the connective tissues in the DMRV muscles stronger than those in a control (Figure 2A). Rimmed vacuoles, which were confirmed by means of phase contrast microscopy, showed increased reactivity with SBA (Figure 2A). The sarcoplasm of several atrophic fibers in DMRV sections also showed increased reactivity with SBA (Figure 2A). The SBA reactivity was stronger in the rimmed vacuoles and sarcoplasm of the atrophic fibers in DMRV than in connective tissues. We next double stained the DMRV muscles with SBA lectin and an antibody against dystrophin. The localization of SBA-reactive materials was apparently different from that of dystrophin (Figure 2B). This suggested that the SBA-reactive materials are not localized in the sarcolemma. RCA120 lectin reacted with the sarcolemma, connective tissues, and blood vessels in the DMRV skeletal muscles, although it also reacted with those in controls. The rimmed vacuoles and sarcoplasm of the atrophic fibers in DMRV were stained strongly (Figure 2A). These results indicate that terminal Gal and GalNAcα1-3Gal carbohydrate moieties are increased in the rimmed vacuoles and sarcoplasm of atrophic fibers in DMRV. In contrast, MAA and WGA stained the sarcolemma, connective tissues, and blood vessels in both the control and DMRV patient muscles (Figure 2). These results indicate the presence of Neu5Acα2-3Gal and (GlcNAc)n carbohydrate moieties in both the DMRV and control skeletal muscles.
Figure 2.
Lectin histochemical analyses of biopsied skeletal muscle tissues. A: Transverse cryosections of muscle biopsy specimens from normal controls (N1 or N2) and DMRV patients (DMRV-1 and DMRV-5S-2) were stained with rhodamine isothiocyanate-labeled SBA, RCA120, and fluorescein isothiocyanate-labeled MAA and WGA. Increased reactivity for SBA was observed mainly in the connective tissue, the rimmed vacuoles (asterisks), and the cytoplasm of the DMRV atrophic fibers (arrows). RCA120 reactivity was found in the sarcolemmal region, the connective tissue, and the rimmed vacuoles (asterisks) of the DMRV skeletal muscles. B: Transverse cryosections of muscle biopsy specimens from a DMRV patient (DMRV-5S-2) and a MD patient were co-stained with SBA lectin and an antibody against dystrophin. The merged images show that the SBA reactivity was not co-localized with dystrophin, indicating that the SBA-reactive materials were not co-localized with the sarcolemma in the DMRV and MD patients. The most intense SBA reactivity was found in the cytoplasm around rimmed vacuoles of the DMRV patient. SBA reactivity was also found in the connective tissues of both the DMRV and MD patients. Scale bars: 50 μm (A: SBA, MAA, and WGA); 20 μm (A: RCA120, B).
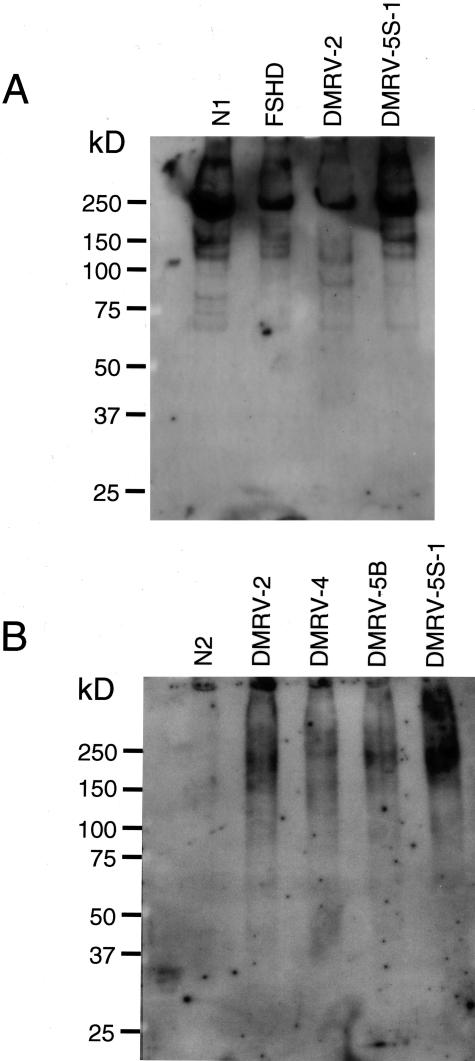
To confirm the results of lectin staining, we performed lectin blotting analysis with a highly specific DIG-labeled lectin-CDP-star ECL system. MAA reacted strongly with a major band at 250 kd and several minor bands for both control and DMRV muscle cell extracts (Figure 3A). In contrast, PNA reacted with highly glycosylated broad bands at ∼200 kd for cell extracts of the DMRV skeletal muscle specimens, but not in control specimens (Figure 3B).
Figure 3.
Lectin blot analysis of Triton X solubilized cryosections of muscle biopsy specimens from control subjects including normal ones (N1, N2, and FSHD) and DMRV patients (DMRV-2, DMRV-4, DMRV-5B, and DMRV-5S-1). The samples were electrophoresed and detected with either MAA (A) or PNA (B) lectin.
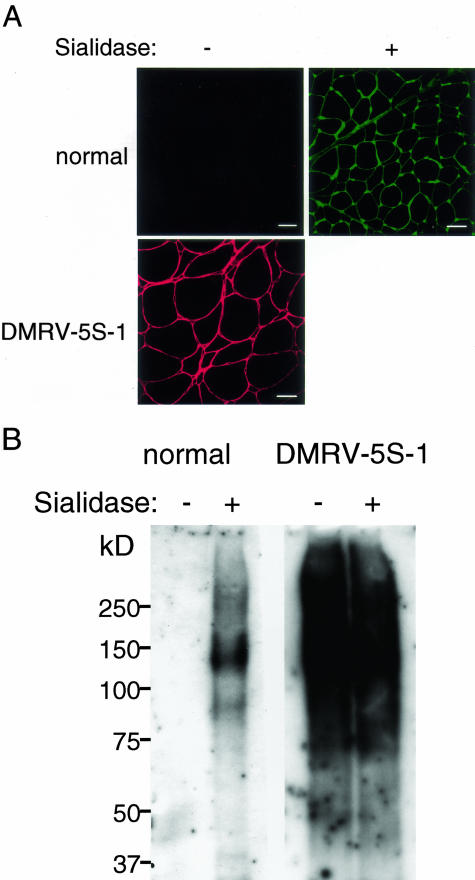
The increase in PNA reactivity to DMRV skeletal muscle glycoproteins could be because of an increase in Galβ1-3GalNAc moieties. Alternatively, subterminal Galβ1-3GalNAc residues on skeletal muscle glycoproteins might be prevented from interacting with PNA by further modification with terminal sialic acids in normal controls. To address this possibility, we examined the ability of sialidase to expose the cryptic PNA-binding sites on normal skeletal muscle glycoproteins. Digestion of frozen sections from normal controls with C. perfringens sialidase resulted in strong reactivity with PNA on both lectin staining (Figure 4A) and lectin blotting analysis (Figure 4B). This indicates exposure of latent binding sites for PNA in the sialidase-treated normal muscle sections as well as in the nontreated DMRV skeletal muscle sections.
Figure 4.
Effect of sialidase treatment on reactivity of PNA. Normal skeletal muscle glycoproteins react strongly with PNA after digestion with C. perfringens sialidase. A: Lectin staining of transverse cryosections of muscle biopsy specimens from a normal control (N1) and a DMRV patient (DMRV-5S-1) with PNA. Cryosections were treated with (+) or without (−) C. perfringens sialidase. B: A blot of muscle biopsy extracts from a control and a DMRV patient (DMRV-5S-1) was probed with PNA. The extracts were incubated with (+) or without (−) C. perfringens sialidase before electrophoresis and blotting. Scale bars, 50 μm.
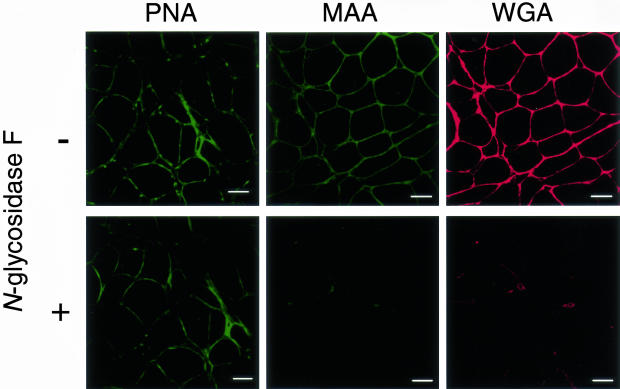
To characterize the glycoforms that reacted with PNA, MAA, and WGA in the DMRV skeletal muscle specimens, DMRV skeletal muscle sections were treated with N-glycosidase F. N-Glycosidase F is known to cleave N-linked high mannose and hybrid and complex oligosaccharides. Digestion of frozen skeletal muscle sections from the DMRV patients with N-glycosidase F did not alter the reactivity with PNA (Figure 5), whereas it reduced the reactivity with MAA and WGA (Figure 5). These results indicate that the oligosaccharides that reacted with PNA in the DMRV skeletal muscle specimens were mainly O-linked glycans, whereas those that reacted with MAA and WGA were mainly N-linked glycans.
Figure 5.
Effect of N-glycosidase F treatment on staining with PNA, MAA, and WGA. DMRV skeletal muscle glycoproteins react strongly with PNA but not with MAA or WGA after digestion with N-glycosidase F. Cryosections from a DMRV patient (DMRV-5S-1) were treated with (+) or without (−) N-glycosidase F before lectin staining. Scale bars, 50 μm.
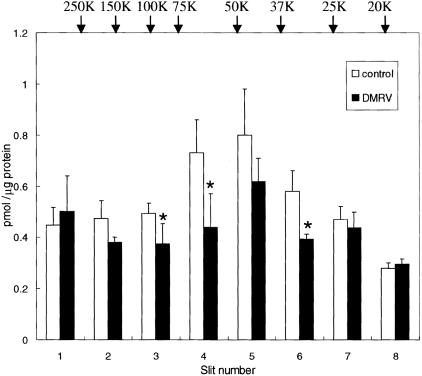
In DMRV patients, mutation of the GNE gene reduces the enzymatic activity of UDP-GlcNAc 2-epimerase, according to a report by Noguchi and colleagues.16 Therefore, we measured the sialic acid contents of skeletal muscle specimens from the DMRV patients. Homogenates prepared from the skeletal muscle specimens were subjected to SDS-PAGE before transfer to PVDF membranes. The membranes were cut into eight equal pieces according to decreasing Mr, and then analyzed for sialic acid content by the mild hydrolysis-fluorometric HPLC method. No difference was detected in each membrane slice (Mr 20,000 to >250,000) between the patients and normal controls without glycosidase treatment (data not shown). Then, the skeletal muscle extracts were treated with N-glycosidase F to detect O-linked sialic acid components. A 20% reduction in the total sialic acid content (calculated as sum of the sialic acid contents of membrane slices 1 to 8) was observed in the DMRV muscles on treatment with N-glycosidase F (controls, 4.27 ± 0.46 pmol/μg versus DMRV patients, 3.51 ± 0.26 pmol/μg). Furthermore, a 20 ∼ 40% reduction was observed for each of membrane slice 2 (Mr, 120,000 to 200,000; P = 0.13), slice 3 (Mr, 80,000 to 120,000; P < 0.05), slice 4 (Mr, 60,000 to 80,000; P < 0.05), and slice 6 (Mr, 30,000 to 40,000; P < 0.05) (Figure 6).
Figure 6.
O-Glycan sialylation of skeletal muscle specimens from DMRV patients and controls. The sialic acid contents of skeletal muscle specimens from DMRV patients (n = 4; DMRV-2, DMRV-4, DMRV-5B, and DMRV-5S-1) and controls (n = 3) were determined by the fluorometric HPLC method. Glycoproteins in skeletal muscle homogenates treated with N-glycosidase F were subjected to SDS-PAGE, followed by blotting onto a PVDF membrane, as described in Materials and Methods. The membrane was cut into eight equal pieces according to Mr. Each column represents the means ± SD. *, P < 0.05.
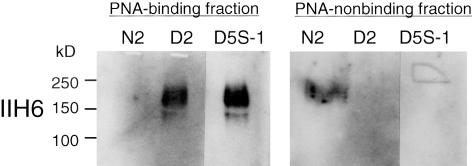
Because the reactivity of PNA was significantly increased in the DMRV patients, we tried to identify the sarcolemmal glycoproteins that reacted with PNA. We obtained two fractions for the skeletal muscle extracts according to PNA-binding activity by means of PNA agarose; PNA-binding and PNA-nonbinding fractions. The PNA fraction of the DMRV muscle extracts reacted with a monoclonal antibody, IIH6, that recognizes the carbohydrate epitope of α-DG, but that of a normal control extract did not (Figure 7). By contrast, the PNA-nonbinding fraction of the normal control extract reacted with IIH6 (Figure 7). These data suggest that α-DG contains PNA-reactive sugar chains in the DMRV patients. Furthermore, we examined the expression of glycosylated α-DG in the biopsied muscle specimens from the DMRV patients with IIH6. Positive staining was demonstrated in all of the DMRV patients as well as the normal controls. This suggests that the basic structure of the α-DG sugar chain recognized by the IIH6 antibody was not altered in the DMRV patients (data not shown). Immunostaining with antibodies to β-DG and laminin α2 revealed normal expression of these substances on the sarcolemmal membrane in the DMRV patients (data not shown).
Figure 7.
Characterization of PNA-reactive skeletal muscle glycoproteins from DMRV patients by PNA lectin precipitation, followed by Western blotting. Triton X lysis buffer extracts of DMRV-2 (D2), DMRV-5S-1 (D5S-1), and normal control (N2) muscles, each including 100 μg of protein, were subjected to precipitation with PNA agarose. The precipitated proteins (PNA-binding fraction) were blotted with an antibody to α-DG (IIH6). Furthermore, the supernatant (PNA-nonbinding fraction) of the extract with PNA agarose was subjected to precipitation with trichloroacetic acid and then blotted with IIH6.
Discussion
In the current study, we obtained evidence that the hyposialylation of O-linked glycoproteins including α-DG occurs in DMRV skeletal muscles. Enhanced staining with PNA lectin, which binds core 1 oligosaccharides (disaccharide Galβ1-3GalNAc), was observed in the sarcolemma of DMRV skeletal muscles but not in normal controls or the pathological controls (Figure 1, A and B). This suggested that sialylated core 1 oligosaccharides having the structure of Siaα2-3Galβ1-3GalNAc were decreased. In contrast, normal reactivity with MAA and WGA, that mainly bind N-linked sialylated glycans, was observed in the DMRV muscles (Figures 2A and 3). This finding suggested that at least sialylation of N-linked glycans was not so severely affected in DMRV. Furthermore, we have shown that digestion of skeletal muscles from normal patients with sialidase exposes the latent binding sites for PNA (Figure 4). Lectin blotting of the DMRV skeletal muscle extracts with PNA suggested that the major PNA-reactive bands are those of highly glycosylated 150- to 200-kd proteins (Figure 3), and precipitation studies showed that one of the major bands was α-DG (Figure 7).
Stäsche and colleagues15 reported that the level of UDP-GlcNAc 2-epimerase activity was very low in many organs including skeletal muscles in rat, although the secreting organs (liver, salivary glands, and intestinal mucosa) exhibited high specific activities. We tried to directly determine the enzyme activity in the biopsied skeletal muscles from the patients with DMRV and the control subjects. But the level of the enzyme activity was very low, being below the detection level, in both control and DMRV muscles, as expected. We are currently developing a more sensitive assay method for determining the enzyme activity. In a recent article, Noguchi and colleagues16 reported that the UDP-GlcNAc 2-epimerase activity in expressed mutants C13S, H132Q, D176V, D177C, V331A, and D378Y was reduced to less than 20% of the control level. The ManNAc kinase activity in expressed mutants I472T, V572L, A630T, A631V, and G703S showed dramatic reductions.16 Hinderlich and colleagues27 also showed that the ManNAc kinase activity of the M712T mutant was reduced by ∼30% compared to that of the wild-type enzyme. All of the mutations identified in DMRV patients caused partial reduction of the enzymatic activity of either UDP-GlcNAc 2-epimerase or ManNAc kinase. Schwarzkopf and colleagues28 reported that complete inactivation of UDP-GlcNAc 2-epimerase by gene targeting in mice caused early embryonic lethality, thereby emphasizing the fundamental roles of the enzyme and sialylation during development. These reports strongly suggest that DMRV is caused by a partial loss of function of the gene product.
The total sialic acid contents in skeletal muscles, cultured myoblasts, and lymphoblastoid cell lines from DMRV patients have been measured. Hinderlich and colleagues27 could not find any influence of the M712T mutation on the expression of total sialic acids on the lymphoblastoid cell lines, whereas Noguchi and colleagues16 showed that the sialic acid levels in skin fibroblasts and myotubes from DMRV patients were significantly decreased to ∼60 to 74% of those in the control cells when the cells were cultured in serum-free medium. They showed that a 25% reduction in the sialic acid content was observed in the DMRV skeletal muscles.16 However, we could not find any difference between the total sialic acid contents in the DMRV and control muscles (data not shown). MAA and WGA lectin staining for specific sialic acids was performed in this study, and the results showed no significant differences between the DMRV and control muscles. Because MAA and WGA mainly react with N-linked sialic acids (Figure 5), we tried to determine the O-linked sialic acid content in skeletal muscles from the DMRV patients after N-glycosidase F treatment. The results revealed that it was reduced to 80% of that in the control muscles. In particular, a 40% reduction was observed in membrane slice 4 (Mr, 60,000 to 80,000) and a 32% reduction in slice 6 (Mr, 30,000 to 40,000) (Figure 6). Our data suggested that the V572L and G295D/A631V mutations mainly affect the expression of O-linked sialic acids in muscular glycoproteins.
Despite the increasing number of studies on the genetics of HIBM/DMRV, little is known about the pathophysiologies of these diseases. In particular, the carbohydrate chain structures of the glycoproteins and their role have been obscure. It is indispensable to know the changes in glycosylation of the sarcolemmal glycoproteins including the dystrophin-glycoprotein complex (DGC), which is thought to play an important role as a transmembrane linker between the intracellular cytoskeleton and extracellular components.29 This will lead to a better understanding of the roles of carbohydrate structures in the pathophysiological processes. A candidate glycoprotein is α-DG, which has a predominantly O-linked glycan, including Siaα2-3Galβ1-3GalNAc-Ser/The (sialyl core 1 glycan),30 and rare O-mannosyl glycans consisting of Siaα2-3Galβ1-4GlcNAcβ1-2Man-Ser/Thr.31 Fukuyama congenital muscular dystrophy (FCMD) (OMIM 253800), muscle-eye-brain disease (MEB) (OMIM 253280), and Walker-Warburg syndrome (WWS) (OMIM 236670) are congenital muscular dystrophies with similar defects in muscular and brain development. Recent studies have shown that the hypoglycosylation of α-DG, which was detected as a lack of reactivity with monoclonal antibodies, VIA4–1 and IIH6, recognizing the carbohydrate epitope, is the underlying biochemical defect in these congenital muscular dystrophies.32 They have been called “α-dystroglycanopathies.” Recently, Huizing and colleagues33 reported that HIBM muscles showed aberrant glycosylation of α-DG similar to in α-dystroglycanopathies, whereas several other groups reported that the IIH6 monoclonal antibody showed a normal pattern of expression on the sarcolemmal membrane in DMRV patients.16,34 Also, we could not find any significant changes in the immunoreaction of IIH6 with DMRV muscles. Clinically, DMRV usually seems to be moderate when compared with α-dystroglycanopathies, and patients with this disease do not have any apparent brain disorder. This might be because of the fact that the lesion in DMRV is located at the termini of sugar chains of glycoproteins including α-DG and thus the basic structure would be maintained. Furthermore, the residual activity of the GNE gene product would determine the clinical features. Although unique O-linked oligosaccharides on α-DG appear to be critical for interaction with laminin, agrin, perlecan, and neurexin,32 treatment of muscle α-DG with sialidase did not affect laminin binding or IIH6 reactivity.35 These findings suggested that the indicated laminin binding to muscle α-DG is insensitive to changes in the terminal sialic acid residues. The issues of why O-linked sarcolemmal glycoproteins are predominantly affected and how their hyposialylation results in rimmed vacuole formation and muscular weakness must be clarified. In this study, we revealed that SBA and RCA120 strongly reacted with the rimmed vacuoles and atrophic fibers besides the sarcolemma and connective tissue in the DMRV muscles. The affected glycans demonstrated by the abnormal staining might be related to the development of DMRV. Detailed analysis of the functions of generic carbohydrate structures of α-DG would help.
We conclude that the core 1 glycans recognized by PNA lectin are increased in DMRV muscles, probably because of impaired sialyl O-glycan formation in sarcolemmal glycoproteins including α-DG. The functional consequence of this finding remains to be determined. Unlike other α-dystroglycanopathies, DMRV/HIBM involve intracellular vacuoles, suggesting that a different pathophysiological mechanism may be involved. Further understanding of the modification of glycoproteins should lead to the development of diagnostic and therapies for DMRV/HIBM.
Acknowledgments
We thank Drs. Kevin Campbell (Howard Hughes Medical Institute, University of Iowa), Stephan Kröger (University of Mainz), Yoshitaka Tanaka (Kyushu University), and A.C.W. Zannettino (University of Adelaide) for the gifts of the antibodies used in this study; and Drs. Takahiko Hara, Yuki Nakayama, Koji Kasahara, Ikuo Wada, and Tamao Endo for the valuable comments.
Footnotes
Address reprint requests to Dr. Hitoshi Sakuraba, Department of Clinical Genetics, The Tokyo Metropolitan Institute of Medical Science, Tokyo Metropolitan Organization for Medical Research, 3-18-22 Honkomagome, Bunkyo-ku, Tokyo 113-8613, Japan. E-mail: sakuraba@rinshoken.or.jp.
Supported by grants from the Ministry of Education, Science, Sports, and Culture of Japan (to H.S.); the Ministry of Health, Labor, and Welfare of Japan (to H.S.); the Japan Society for the Promotion of Science [to H. S. and grants-in-aid for scientific research (C) to Y.T., M.K., and E.U.]; the Tokyo Metropolitan Government (to H.S. and Y.S.); and the Ichiro Kanehara Foundation (to Y.T.).
References
- Nonaka I, Sunohara N, Ishiura S, Satoyoshi E. Familial distal myopathy with rimmed vacuole and lamellar (myeloid) body formation. J Neurol Sci. 1981;51:141–155. doi: 10.1016/0022-510x(81)90067-8. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T, Asaka T, Saito M, Tanaka H, Higuchi S, Tanaka K, Saida K, Uyama E, Mizusawa H, Fukuhara N, Nonaka I, Takamori M, Tsuji S. Gene locus for autosomal recessive distal myopathy with rimmed vacuoles maps to chromosome 9. Ann Neurol. 1997;41:432–437. doi: 10.1002/ana.410410405. [DOI] [PubMed] [Google Scholar]
- Mitrani-Rosenbaum S, Argov Z, Blumenfeld A, Seidman CE, Seidman JG. Hereditary inclusion body myopathy maps to chromosome 9p1–q1. Hum Mol Genet. 1996;5:159–163. doi: 10.1093/hmg/5.1.159. [DOI] [PubMed] [Google Scholar]
- Asaka T, Ikeuchi K, Okino S, Takizawa Y, Satake R, Nitta E, Komai K, Endo K, Higuchi S, Oyake T, Yoshimura T, Suenaga A, Uyama E, Saito T, Konagaya M, Sunohara N, Namba R, Takada H, Honke K, Nishina M, Tanaka H, Shinagawa M, Tanaka K, Matsushima A, Tsuji S, Takamori M. Homozygosity and linkage disequilibrium mapping of autosomal recessive distal myopathy (Nonaka distal myopathy). J Hum Genet. 2001;46:649–655. doi: 10.1007/s100380170016. [DOI] [PubMed] [Google Scholar]
- Argov Z, Yarom R. “Rimmed vacuole myopathy” sparing the quadriceps. A unique disorder in Iranian Jews. J Neurol Sci. 1984;64:33–43. doi: 10.1016/0022-510x(84)90053-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg I, Hochner H, Shemesh M, Levi T, Potikha T, Sadeh M, Argov Z, Jackson CL, Mitrani-Rosenbaum S. Physical and transcriptional map of the hereditary inclusion body myopathy locus on chromosome 9p12–p13. Eur J Hum Genet. 2001;9:501–509. doi: 10.1038/sj.ejhg.5200665. [DOI] [PubMed] [Google Scholar]
- Argov Z, Eisenberg I, Mitrani-Rosenbaum S. Genetics of inclusion body myopathies. Curr Opin Rheumatol. 1998;10:543–547. doi: 10.1097/00002281-199811000-00006. [DOI] [PubMed] [Google Scholar]
- Eisenberg I, Avidan N, Potikha T, Hochner H, Chen M, Olender T, Barash M, Shemesh M, Sadeh M, Grabov-Nardini G, Shmilevich I, Friedmann A, Karpati G, Bradley WG, Baumbach L, Lancet D, Asher EB, Beckmann JS, Argov Z, Mitrani-Rosenbaum S. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001;29:83–87. doi: 10.1038/ng718. [DOI] [PubMed] [Google Scholar]
- Kayashima T, Matsuo H, Satoh A, Ohta T, Yoshiura K, Matsumoto N, Nakane Y, Niikawa N, Kishino T. Nonaka myopathy is caused by mutations in the UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase gene (GNE). J Hum Genet. 2002;47:77–79. doi: 10.1007/s100380200004. [DOI] [PubMed] [Google Scholar]
- Tomimitsu H, Ishikawa K, Shimizu J, Ohkoshi N, Kanazawa I, Mizusawa H. Distal myopathy with rimmed vacuoles: novel mutations in the GNE gene. Neurology. 2002;59:451–454. doi: 10.1212/wnl.59.3.451. [DOI] [PubMed] [Google Scholar]
- Arai A, Tanaka K, Ikeuchi T, Igarashi S, Kobayashi H, Asaka T, Date H, Saito M, Tanaka H, Kawasaki S, Uyama E, Mizusawa H, Fukuhara N, Tsuji S. A novel mutation in the GNE gene and a linkage disequilibrium in Japanese pedigrees. Ann Neurol. 2002;52:516–519. doi: 10.1002/ana.10341. [DOI] [PubMed] [Google Scholar]
- Nishino I, Noguchi S, Murayama K, Driss A, Sugie K, Oya Y, Nagata T, Chida K, Takahashi T, Takusa Y, Ohi T, Nishimiya J, Sunohara N, Ciafaloni E, Kawai M, Aoki M, Nonaka I. Distal myopathy with rimmed vacuoles is allelic to hereditary inclusion body myopathy. Neurology. 2002;59:1689–1693. doi: 10.1212/01.wnl.0000041631.28557.c6. [DOI] [PubMed] [Google Scholar]
- Keppler OT, Hinderlich S, Langner J, Schwartz-Albiez R, Reutter W, Pawlita M. UDP-GlcNAc 2-epimerase: a regulator of cell surface sialylation. Science. 1999;284:1372–1376. doi: 10.1126/science.284.5418.1372. [DOI] [PubMed] [Google Scholar]
- Hinderlich S, Stasche R, Zeitler R, Reutter W. A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. Purification and characterization of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase. J Biol Chem. 1997;272:24313–24318. doi: 10.1074/jbc.272.39.24313. [DOI] [PubMed] [Google Scholar]
- Stäsche R, Hinderlich S, Weise C, Effertz K, Lucka L, Moormann P, Reutter W. A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. Molecular cloning and functional expression of UDP-N-acetyl-glucosamine 2-epimerase/N-acetylmannosamine kinase. J Biol Chem. 1997;272:24319–24324. doi: 10.1074/jbc.272.39.24319. [DOI] [PubMed] [Google Scholar]
- Noguchi S, Keira Y, Murayama K, Ogawa M, Fujita M, Kawahara G, Oya Y, Imazawa M, Goto Y, Hayashi YK, Nonaka I, Nishino I. Reduction of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase activity and sialylation in distal myopathy with rimmed vacuoles. J Biol Chem. 2004;279:11402–11407. doi: 10.1074/jbc.M313171200. [DOI] [PubMed] [Google Scholar]
- Uyama E, Hino H, Obayashi K, Arai A, Tanaka K, Tsuji S, Uchino M. Distal myopathy with rimmed vacuoles: phenotype/genotype relationship in Japanese families. Ann Neurol. 2003;54(suppl 7):S73. [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Herrmann R, Straub V, Blank M, Kutzick C, Franke N, Jacob EN, Lenard HG, Kroger S, Voit T. Dissociation of the dystroglycan complex in caveolin-3-deficient limb girdle muscular dystrophy. Hum Mol Genet. 2000;9:2335–2340. doi: 10.1093/oxfordjournals.hmg.a018926. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis HK, van Oosterhout JJ, Rozemuller E, van Iwaarden F, Sixma JJ. Studies with a monoclonal antibody against activated platelets: evidence that a secreted 53,000-molecular weight lysosome-like granule protein is exposed on the surface of activated platelets in the circulation. Blood. 1987;70:838–845. [PubMed] [Google Scholar]
- Kuronita T, Eskelinen EL, Fujita H, Saftig P, Himeno M, Tanaka Y. A role for the lysosomal membrane protein LGP85 in the biogenesis and maintenance of endosomal and lysosomal morphology. J Cell Sci. 2002;115:4117–4131. doi: 10.1242/jcs.00075. [DOI] [PubMed] [Google Scholar]
- Zannettino AC, Buhring HJ, Niutta S, Watt SM, Benton MA, Simmons PJ. The sialomucin CD164 (MGC-24v) is an adhesive glycoprotein expressed by human hematopoietic progenitors and bone marrow stromal cells that serves as a potent negative regulator of hematopoiesis. Blood. 1998;92:2613–2628. [PubMed] [Google Scholar]
- Kotani M, Osanai T, Tajima Y, Kato H, Imada M, Kaneda H, Kubo H, Sakuraba H. Identification of neuronal cell lineage-specific molecules in the neuronal differentiation of P19 EC cells mouse central nervous system. J Neurosci Res. 2002;67:595–606. doi: 10.1002/jnr.10150. [DOI] [PubMed] [Google Scholar]
- Hara S, Yamaguchi M, Takemori Y, Nakamura M, Ohkura Y. Highly sensitive determination of N-acetyl- and N-glycolylneuraminic acids in human serum and urine and rat serum by reversed-phase liquid chromatography with fluorescence detection. J Chromatogr. 1986;377:111–119. doi: 10.1016/s0378-4347(00)80766-5. [DOI] [PubMed] [Google Scholar]
- Sato C, Fukuoka H, Ohta K, Matsuda T, Koshino R, Kobayashi K, Troy FA, II, Kitajima K. Frequent occurrence of pre-existing alpha 2−>8-linked disialic and oligosialic acids with chain lengths up to 7 Sia residues in mammalian brain glycoproteins. Prevalence revealed by highly sensitive chemical methods and anti-di-, oligo-, and poly-Sia antibodies specific for defined chain lengths. J Biol Chem. 2000;275:15422–15431. doi: 10.1074/jbc.275.20.15422. [DOI] [PubMed] [Google Scholar]
- Pena SD, Gordon BB, Karpati G, Carpenter S. Lectin histochemistry of human skeletal muscle. J Histochem Cytochem. 1981;29:542–546. doi: 10.1177/29.4.6166659. [DOI] [PubMed] [Google Scholar]
- Hinderlich S, Salama I, Eisenberg I, Potikha T, Mantey LR, Yarema KJ, Horstkorte R, Argov Z, Sadeh M, Reutter W, Mitrani-Rosenbaum S. The homozygous M712T mutation of UDP-N-acetylglucosamine 2-epimerase/ N-acetylmannosamine kinase results in reduced enzyme activities but not in altered overall cellular sialylation in hereditary inclusion body myopathy. FEBS Lett. 2004;566:105–109. doi: 10.1016/j.febslet.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Schwarzkopf M, Knobeloch KP, Rohde E, Hinderlich S, Wiechens N, Lucka L, Horak I, Reutter W, Horstkorte R. Sialylation is essential for early development in mice. Proc Natl Acad Sci USA. 2002;99:5267–5270. doi: 10.1073/pnas.072066199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Yamada H, Matsumura K, Shimizu T, Kobata A, Endo T. Detection of O-mannosyl glycans in rabbit skeletal muscle alpha-dystroglycan. Biochim Biophys Acta. 1998;1425:599–606. doi: 10.1016/s0304-4165(98)00114-7. [DOI] [PubMed] [Google Scholar]
- Chiba A, Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, Kanazawa I, Kobata A, Endo T. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve alpha-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of alpha-dystroglycan with laminin. J Biol Chem. 1997;272:2156–2162. doi: 10.1074/jbc.272.4.2156. [DOI] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Huizing M, Rakocevic G, Sparks SE, Mamali I, Shatunov A, Goldfarb L, Krasnewich D, Gahl WA, Dalakas MC. Hypoglycosylation of alpha-dystroglycan in patients with hereditary IBM due to GNE mutations. Mol Genet Metab. 2004;81:196–202. doi: 10.1016/j.ymgme.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Saito F, Tomimitsu H, Arai K, Nakai S, Kanda T, Shimizu T, Mizusawa H, Matsumura K. A Japanese patient with distal myopathy with rimmed vacuoles: missense mutations in the epimerase domain of the UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE) gene accompanied by hyposialylation of skeletal muscle glycoproteins. Neuromuscul Disord. 2004;14:158–161. doi: 10.1016/j.nmd.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Burwell AL, Geissler AL. Tissue-specific heterogeneity in alpha-dystroglycan sialoglycosylation. Skeletal muscle alpha-dystroglycan is a latent receptor for Vicia villosa agglutinin b4 masked by sialic acid modification. J Biol Chem. 1997;272:22315–22321. doi: 10.1074/jbc.272.35.22315. [DOI] [PubMed] [Google Scholar]