Abstract
The initiation of angiogenesis, called the angiogenetic switch, is a crucial early step in tumor progression and propagation, ensuring an adequate oxygen supply. The rapid growth of tumors is accompanied by a reduced microvessel density, resulting in chronic hypoxia that often leads to necrotic areas within the tumor. These hypoxic and necrotic regions exhibit increased expression of angiogenetic growth factors, eg, vascular endothelial growth factor, and may also attract macrophages, which are known to produce a number of potent angiogenetic cytokines and growth factors. A group of molecules that may act as mediators of angiogenesis are the so-called high-mobility group proteins. Recent studies showed that HMGB1, known as an architectural chromatin-binding protein, can be extracellularly released by passive diffusion from necrotic cells and activated macrophages. To examine the angiogenetic effects of HMGB1 on endothelial cells an in vitro spheroid model was used. The results of the endothelial-sprouting assay clearly show that exogenous HMGB1 induced endothelial cell migration and sprouting in vitro in a dose-dependent manner. Thus, this is the first report showing strong evidence for HMGB1-induced sprouting of endothelial cells.
Cell death mediated by hypoxia is a frequent event during the proliferation of tumor cell populations.1 Hypoxia may induce apoptosis of areas of the growing tumor but it can also lead to necrotic death of the corresponding cells.2,3 Tumor propagation and progression depends on the induction of tumor vascularization, ie, the angiogenetic switch.4 Although it is now well documented that tumor cells have the ability to induce angiogenesis by secretion of extracellular molecules promoting the outgrowth of small vessels, the stimulation of angiogenesis mediated by the necrotic cells themselves may be a very efficient mechanism by which tumors can escape growth limiting because of hypoxia.5 A group of molecules that may act as mediators of angiogenesis released by necrotic cells are members of the so-called high-mobility group protein family. High-mobility group proteins are small DNA-binding proteins playing an important role in transcriptional regulation.6 In addition, there is now increasing evidence that besides their role as regulators of transcription at least some members of that group of proteins can also exert extracellular functions. Of these proteins, HMGB1 currently has been investigated most intensively.7,8 It can be secreted by certain cells and plays an important role in inflammation, cell migration, differentiation, and tumorigenesis and has been identified as one of the ligands binding to the receptor for advanced glycation end products (RAGE).9,10 HMGB1 binding to RAGE activates key cell-signaling pathways such as MAP kinases and nuclear factor-κB.11 In vivo, two potential sources of circulating HMGB1 exist: HMGB1 released from damaged or necrotic cells and HMGB1 secreted from activated macrophages in response to, eg, oxygen stress, endotoxin, tumor necrosis factor-α, or interleukin-1β.12–14 Scaffidi and colleagues15 showed that HMGB1 rapidly leaked out from permeabilized necrotic cells, but not from permeabilized apoptotic cells. Through its secretion by activated macrophages HMGB1 again activates macrophages, resulting in secretion of angiogenetic factors, eg, vascular endothelial growth factor (VEGF), tumor necrosis factor-α, and interleukin-8.16,17
It is well documented that advanced glycation end products (AGEs) can promote angiogenesis.18,19 Okamoto and colleagues19 have cultured skin microvascular endothelial cells with AGE resulting in a stimulated growth and tube formation. This effect was enlarged with increased expression of RAGE. Similar results were obtained by Yonekura and colleagues20 who were able to show that the formation of the cord-like structure of endothelial cells induced by AGE was completely abolished by soluble RAGE. As for the molecular mechanism of AGE-induced angiogenesis the up-regulation of VEGF because of signaling via nuclear factor-κB seems to be the key effect.19
Nevertheless, Taguchi and colleagues11 have attempted to exclude an angiogenetic effect of that protein by placing basic fibroblast growth factor-laden pellets into a corneal pocket. In these experiments, no differences in capillary outgrowth from the corneal limbus compared to that after treatment with the competitive inhibitor sRAGE were observed. sRAGE is a truncated variant of the RAGE receptor that binds HMGB1 and blocks the interaction with its receptor. Based on their results Taguchi and colleagues11 have concluded that RAGE blockade does not impair the process of neovascularization at all. However, the latter experimental design does not allow drawing a general conclusion about possible angiogenetic effects of HMGB1. Therefore we used a spheroid model to re-examine the effects of HMGB1 on human endothelial cells.
Materials and Methods
Expression and Purification of HMGB1
The full-length HMGB1 cDNA-coding region was inserted into the glutathione S-transferase (GST) fusion protein expression vector pGEX-6P1 (Amersham Biosciences, Freiburg, Germany) by ligation to SmaI and NotI restriction sites. Escherichia coli BL21, transformed with the recombinant plasmid, were grown in LB medium supplemented with 100 μg/ml of ampicillin for 6 hours at 37°C as preparatory culture and 17 hours at 18°C as main culture. Expression of the GST-HMGB1 fusion protein was induced by incubation with 0.1 mmol/L IPTG for 2 hours at 18°C. The bacterial pellet was resuspended in phosphate-buffered saline (PBS) and lysed by nitrogen and lysozyme. A crude extract was separated by centrifugation and added to a 50% slurry of glutathione-Sepharose 4B equilibrated with PBS. After gentle agitation at 6°C for 45 minutes the matrix was sedimented and washed with PBS. To obtain HMGB1 fragments without GST, fusion protein was cleaved with PreScission Protease (Amersham Biosciences) at 6°C overnight with gentle agitation. The cleaved GST, bonded to the slurry, was then removed by centrifugation. The identity of HMGB1 was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. As a control to exclude any sprouting activity from contaminating proteins obtained during the purification process, proteins were isolated using GST fusion protein expression vector pGEX-6P1 (Amersham Biosciences) without any cloned insert. Purification was performed as mentioned above.
Cell Culture
Human umbilical vein endothelial cells (Promocell, Heidelberg, Germany) were cultured according to the manufacturer’s instructions at 37°C using endothelial cell growth media and endothelial cell growth supplement. Only human umbilical vein endothelial cells cultured from passages 4 to 5 were used for experiments. Endothelial cell growth medium, endothelial cell growth supplement, and endothelial cell basal medium were purchased from Promocell. Fetal calf serum was obtained from Biochrom (Berlin, Germany).
Preparation of a Collagen Stock Solution
A collagen stock solution was prepared from rat tail by isolating the tendons without attached connective tissue. The tendons were transferred into 0.1% acetic acid (v/v in H2O) and stored for 48 hours at 4°C. The final solution was centrifuged at 17,000 × g, 4°C, for 1 hour and the clear supernatant was diluted to an OD 280 nm of 0.25.
In Vitro Angiogenesis Assay
Endothelial cells were harvested and a defined cell number (400 cells/100 μl) was suspended in endothelial cell growth medium containing 0.25% (w/v) methylcellulose (Sigma, Taufkirchen, Germany) for the generation of spheroids. One hundred μl/well of the cell suspension was seeded into nonadherent round-bottom 96-well plates (Greiner, Frickenhausen, Germany). Nearly all cells per well contributed to the formation of a single spheroid (400 cells/spheroid) during the 24-hour culture at 37°C. The spheroids were harvested and embedded in collagen.21
In brief, at room temperature 48 spheroids were suspended in 0.5 ml of endothelial cell basal medium containing 20% fetal calf serum and 1% (w/v) methylcellulose to prevent sedimentation of spheroids before polymerization of the collagen gel. The ice-cold collagen stock solution (8 vol) was mixed with 10× M199 (1 vol; Sigma) and 0.1 N of NaOH (∼1 vol) to adjust the pH to 7.4. Then 0.5 ml of the neutralized collagen solution was rapidly mixed with 0.5 ml of spheroid suspension and transferred into prewarmed 24-well plates. After polymerization 100 μl of basal medium with 10× concentrated test substance was added on top of each gel. The gels were incubated at 37°C in 5% CO2 at 100% humidity. After 24 hours and fixation with 1 ml of 10% paraformaldehyde, in vitro angiogenesis was digitally quantitated by measuring the length of the sprouts that had grown out of each spheroid (ocular grid at ×40 magnification, cumulative sprout length) using the digital imaging software analySIS (Soft Imaging System, Muenster, Germany). The mean and SD of the cumulative sprout length from 10 randomly selected spheroids per gel was determined (corresponding to one test substance). All experiments were performed twice.
Statistics
Statistical significance was tested using the t-test and was set at P < 0.001.
Tissue Samples
In the present study breast cancer samples taken directly after surgery were immunohistochemically analyzed. All patients signed an informed consent.
Immunohistochemistry
Immunohistochemical examination of 5-μm sections of human breast cancer was performed with a goat polyclonal antibody raised against a peptide mapping within an internal region of the HMGB1 protein of human origin (sc-12523; Santa Cruz Biotechnology, Santa Cruz, CA). Immunostaining was achieved by a three-step procedure (primary antibody, secondary antibody, and avidin-biotin-complex) using the Vectastain ABC method (Vector Laboratories, Burlingame, CA). Briefly, sections of paraffin-embedded tissue were dewaxed in xylene and rehydrated in an ethanol series and finally resuspended in PBS (pH 7.4). Endogenous peroxidase activity was quenched by incubation in 0.75% H2O2 in methanol followed by a microwave pretreatment for 15 minutes with citric acid buffer (pH 6.0). After washing two times in tap water and PBS, tissue sections were incubated for 20 minutes in rabbit serum followed by incubation with the goat polyclonal HMGB1 antibody (1:100 diluted in 2% bovine serum albumin in PBS). After incubation overnight at 4°C, tissue sections were washed three times in PBS and incubated with biotin-conjugated rabbit anti-goat IgG for 30 minutes at room temperature. After further washing in PBS, they were incubated for 30 minutes with ABComplex and freshly prepared according to the manufacturer’s instructions. After final washing in PBS, the peroxidase reaction was initiated by application of diaminobenzidine solution (prepared according to the manufacturer’s instructions). The reaction was stopped after 10 minutes by washing in tap water and tissue sections were counterstained with Meyer’s hematoxylin, dehydrated, cleared, and mounted. Immunohistochemistry was evaluated using a digital camera (AxioCam; Zeiss, Göttingen, Germany).
Results
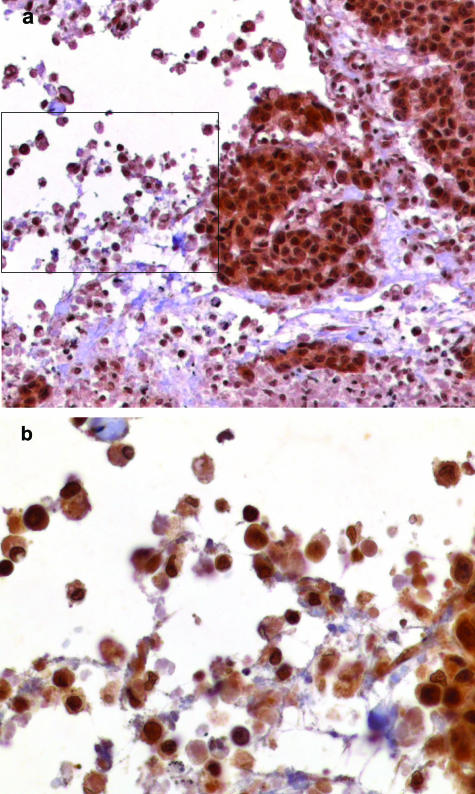
Immunohistochemical analysis confirmed HMGB1 expression in all macrophages within necrotic areas of the tumors tested (Figure 1). To check the specificity of secondary antibody, staining controls without the primary antibody were performed. No immunostaining for HMGB1 was visible in these controls. Furthermore, we examined possible angiogenetic effects of HMGB1 in a three-dimensional spheroid model of endothelial cell differentiation. For a better comparison of the HMGB1 sprouting effects the results were compared to the effects of VEGF, which is known to be one of the key regulators of angiogenesis. Endothelial cell spheroids of defined cell number (400 cells/spheroid) were seeded in collagen gels, stimulated with HMGB1 in different concentrations (2 μg/ml, 0.4 μg/ml, and 0.08 μg/ml) and compared to a VEGF stimulation (25 ng/ml). The cumulative length of outgrowing capillary-like sprouts was quantitated after 24 hours. For every concentration the mean ± SD measuring the average cumulative sprout lengths of 10 randomly selected spheroids per experimental group were determined.
Figure 1.
Immunohistochemical analysis of a necrotic area of breast cancer sample using an antibody raised against the human HMGB1 protein. a: HMGB1 is present in the nuclei and cytoplasm of the breast cancer cells next to the necrotic area. b: Magnification of boxed area of a. HMGB1 is detectable in the nuclei as well as in the cytoplasm of macrophages. Original magnifications: ×100 (a); ×400 (b).
The results clearly point out that HMGB1 induces endothelial cell migration and sprouting in vitro in a dose-dependent manner. In detail, there is no endothelial cell sprouting activity without HMGB1 measurable, reflecting the quiescent phenotype of the human umbilical vein endothelial cells. In contrast, sprouting activity can be stimulated strongly by exogenous HMGB1. A concentration of 2 μg/ml of HMGB1 induced the outgrowth of capillary-like sprouts with an average length of 394 μm. HMGB1 (0.4 μg/ml) induced an average sprouting activity of 242 μm and 0.08 μg/ml HMGB1 an average sprouting of 221 μm, respectively (Figure 2).
Figure 2.
Quantitative three-dimensional in vitro angiogenesis assay based on collagen gel-embedded endothelial cell spheroids treated with VEGF and HMGB1, respectively. Capillary sprouting originating from the spheroids was quantified after 24 hours using digital imaging software analysis. The average cumulative length of 10 randomly selected sprouts was calculated.
In general, relative potency in sprouting formation produced by HMGB1 was lower than that produced by VEGF (average sprouting of 552 μm), but highly significant (P < 0.0001) compared to the negative control. Comparing the HMGB1 angiogenetic effects with those of VEGF, 2 μg/ml of HMGB1 induced an endothelial cell sprouting of 70%, 0.4 μg/ml HMGB1 44%, and 0.08 μg/ml HMGB1 40% activity, respectively. Additionally, to exclude any sprouting activity from contaminating proteins obtained during the purification process, proteins were isolated using a pGEX-6P1 vector without any cloned insert. These proteins did not show sprouting activity at all (data not shown).
Discussion
The initiation of angiogenesis, called the angiogenetic switch, is a crucial early step in tumor progression and propagation, ensuring an adequate oxygen supply.4,5 The rapid growth of tumors is accompanied by a reduced microvessel density, resulting in chronic hypoxia, often leading to necrotic areas within the tumor.22 These hypoxic and necrotic regions correspond to an increased expression of angiogenetic growth factors, eg, VEGF, which are capable to turn what is called the angiogenetic switch, ie, to stimulate endothelial cell growth as a step in angiogenesis.1,23 Recent studies showed that necrotic cells may attract macrophages into the tumor, which then contribute to the angiogenetic process.2 Macrophages have been shown to produce a number of potent angiogenetic cytokines and growth factors, eg, VEGF, tumor necrosis factor-α, and interleukin-8 and constitute a key type of angiogenetic effector cells.16,24 Necrotic cells as well as activated macrophages are a source for a novel type of chemokines, the high-mobility group proteins. As to its proinflammatory activity HMGB1 is the currently best-investigated high-mobility group protein.17
Recent studies showed that HMGB1, known as an architectural chromatin-binding protein, can be extracellularly released by passive diffusion from necrotic cells and is secreted by activated macrophages.8,15,25 Further studies revealed that extracellular HMGB1 may act as a strong macrophage-activating factor when binding to the receptor for advanced glycation end products (RAGE).17 RAGE is a multiligand receptor with advanced glycation end products (AGEs) constituting a major group of ligands. Among the effects of an engagement of RAGE by AGEs are proangiogenetic effects on vascular endothelial cells as for example, their tube formations. This effect is mediated by the induction of VEGF via nuclear factor-κB signaling.19 Accordingly, it seems well reasonable to assume that yet another ligand of RAGE, HMGB1, can also act as an angiogenetic switch molecule.
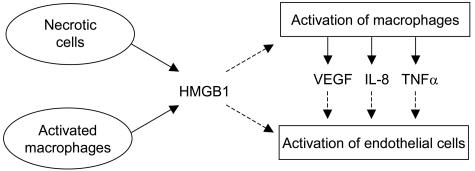
This is the first report showing that HMGB1 can induce sprouting of endothelial cells. To examine the angiogenetic effects of HMGB1 on endothelial cells a spheroid model of endothelial cells in vitro was used. The results of the endothelial-sprouting assay clearly show that exogenous HMGB1 induces endothelial cell migration and sprouting in vitro in a dose-dependent manner. In vivo two sources of HMGB1 exist: necrotic cells and macrophages. Two different ways for the angiogenetic function of HMGB1 can be postulated. One mechanism could be an indirect effect via the activation of macrophages, resulting in secretion of angiogenetic factors as, for example, VEGF, tumor necrosis factor-α, and interleukin-8.16,17 Secondly, HMGB1, acting as an angiogenetic factor itself, can actively be released by activated macrophages and necrotic cells (Figure 3).8,25
Figure 3.
A proposed model for the angiogenetic effects of HMGB1. Solid lines, released factors; dashed lines, their effects.
Our knowledge of the signaling mechanism by which HMGB1 activates endothelial cells and macrophages is still incomplete.17 One possible signaling way could be through its receptor for advanced glycation end products, RAGE, which is expressed, for example, in endothelial cells.26,27 Apart from HMGB1-receptor interactions, it has been demonstrated that HMGB1 binds to many membrane molecules, possibly stimulating angiogenetic effects.28,29
Our results, revealing an angiogenetic effect of HMGB1, are in contrast to an interpretation by Taguchi and colleagues11 placing basic fibroblast growth factor-laden pellets into a corneal pocket, and observing capillary growth from the corneal limbus compared to that after treatment with sRAGE. Taguchi and colleagues11 showed that the interaction between HMGB1 and its receptor RAGE has no effect on neovascularization in the fibroblast growth factor-mediated angiogenesis pathway. Either it could be that the RAGE/HMGB1 interaction does not play a significant role in fibroblast growth factor-mediated angiogenesis or the angiogenetic effect of HMGB1 is masked in this case by stronger effects of other angiogenetic factors not examined in that study. Nevertheless this is the first report that shows an angiogenetic effect of HMGB1. It remains to be investigated if this is solely because of the induction of VEGF or if there are other effects supporting the role HMGB1 as an angiogenetic switch molecule.
Footnotes
Address reprint requests to Dr. J. Bullerdiek, Center for Human Genetics, University of Bremen, Leobenerstr. ZHG, D-28359 Bremen, Germany. E-mail: bullerdiek@uni-bremen.de.
Supported by the Deutsche Forschungsgemeinschaft (grant Bu 592/4-3).
C.S. and H.W. contributed equally to this work.
References
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999;79:991–995. doi: 10.1038/sj.bjc.6690158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Bustin M, Lehn DA, Landsman D. Structural features of the HMG chromosomal proteins and their genes. Biochim Biophys Acta. 1990;1049:231–243. doi: 10.1016/0167-4781(90)90092-g. [DOI] [PubMed] [Google Scholar]
- Muller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, Beltrame M, Bianchi ME. New EMBO members’ review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20:4337–4340. doi: 10.1093/emboj/20.16.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guazzi S, Strangio A, Franzi AT, Bianchi ME. HMGB1, an architectural chromatin protein and extracellular signalling factor, has a spatially and temporally restricted expression pattern in mouse brain. Gene Expr Patterns. 2003;3:29–33. doi: 10.1016/s1567-133x(02)00093-5. [DOI] [PubMed] [Google Scholar]
- Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of RAGE and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Wang H, Vishnubhakat JM, Bloom O, Zhang M, Ombrellino M, Sama A, Tracey KJ. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 1999;126:389–392. [PubMed] [Google Scholar]
- Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Ono M, Torisu H, Fukushi J, Nishie A, Kuwano M. Biological implications of macrophage infiltration in human tumor angiogenesis. Cancer Chemother Pharmacol. 1999;43:S69–S71. doi: 10.1007/s002800051101. [DOI] [PubMed] [Google Scholar]
- Andersson U, Erlandsson-Harris H, Yang H, Tracey KJ. HMGB1 as a DNA-binding cytokine. J Leukoc Biol. 2002;72:1084–1091. [PubMed] [Google Scholar]
- Treins C, Giorgetti-Peraldi S, Murdaca J, Van Obberghen E. Regulation of vascular endothelial growth factor expression by advanced glycation end products. J Biol Chem. 2001;276:43836–43841. doi: 10.1074/jbc.M106534200. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Yamagishi S, Inagaki Y, Amano S, Koga K, Abe R, Takeuchi M, Ohno S, Yoshimura A, Makita Z. Angiogenesis induced by advanced glycation end products and its prevention by cerivastatin. FASEB J. 2002;16:1928–1930. doi: 10.1096/fj.02-0030fje. [DOI] [PubMed] [Google Scholar]
- Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, Okamoto H, Watanabe T, Yamamoto H. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097–1109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112:3249–3258. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- Hemmerlein B, Kugler A, Ozisik R, Ringert RH, Radzun HJ, Thelen P. Vascular endothelial growth factor expression, angiogenesis, and necrosis in renal cell carcinomas. Virchows Arch. 2001;439:645–652. doi: 10.1007/s004280100464. [DOI] [PubMed] [Google Scholar]
- Kim I, Kim HG, Moon SO, Chae SW, So JN, Koh KN, Ahn BC, Koh GY. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res. 2000;86:952–959. doi: 10.1161/01.res.86.9.952. [DOI] [PubMed] [Google Scholar]
- Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, Nishioka Y, Sone S, Kuwano M. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer. 2000;85:182–188. [PubMed] [Google Scholar]
- Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauvala H, Huttunen HJ, Fages C, Kaksonen M, Kinnunen T, Imai S, Raulo E, Kilpelainen I. Heparin-binding proteins HB-GAM (pleiotrophin) and amphoterin in the regulation of cell motility. Matrix Biol. 2000;19:377–387. doi: 10.1016/s0945-053x(00)00084-6. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- Salmivirta M, Rauvala H, Elenius K, Jalkanen M. Neurite growth-promoting protein (amphoterin, p30) binds syndecan. Exp Cell Res. 1992;200:444–451. doi: 10.1016/0014-4827(92)90194-d. [DOI] [PubMed] [Google Scholar]
- Bianchi ME. Interaction of a protein from rat liver nuclei with cruciform DNA. EMBO J. 1988;7:843–849. doi: 10.1002/j.1460-2075.1988.tb02883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]