Abstract
Previously, we and others showed that broad spectrum pharmaceutical inhibition of matrix metalloproteinase (MMP) activity reduces intraosseous tumor burden and bone degradation in animal models of bone metastasis. Herein, we used specific assays to measure net enzymatic activities of individual MMPs during colonization of bone by prostate cancer cells. PC3 cells were injected into the marrow of human fetal femurs previously implanted in SCID mice. Net MMP-9 activity in bone tissues peaked 2 weeks after injection, coinciding with a wave of osteoclast recruitment. In contrast, MMP-2 and MT1-MMP activity did not change. In vitro, co-culture of PC3 cells with bone tissue led to activation of pro-MMP-9 and increases in secreted net MMP-9 activity. Activation of pro-MMP-9 was prevented by metalloprotease inhibitors but not by inhibitors of other classes of proteases. Ribozyme suppression of MMP-9 expression in PC3 cells did not affect pro-MMP-9 activation or net MMP-9 activity and did not affect the phenotype of bone tumors. siRNA targeting of MMP-9 expression in preosteoclasts in vitro demonstrated that tumor-induced preosteoclast motility was dependent on MMP-9 expression. These data suggest that osteoclast-derived MMP-9 may represent a potential therapeutic target in bone metastasis and provide a rationale for the development of MMP-9-specific inhibitors.
Prostate cancer is the most common cancer and second leading cause of cancer death in American males.1 The overwhelming majority of prostate cancer deaths occurs in patients with metastases, and up to 90% of prostate cancer metastases occurs at skeletal sites.2 Patients with bone metastasis frequently suffer from pain, pathological fractures, spinal cord compression, hypercalcemia, and bone marrow suppression.3,4 Although prostate cancer metastases typically appear osteosclerotic on radiographical imaging studies, multiple lines of evidence demonstrate clearly that both bone degradation and bone formation are present within the metastatic deposits. Importantly, there is now evidence that therapies that specifically target skeletal metastases, as opposed to general metastases, may extend survival in patients with prostate cancer.5
The matrix metalloproteinases (MMPs) comprise a family of zinc-dependent endopeptidases that have the capacity to cleave extracellular matrix. Recently, other tumor-promoting activities, such as activation of latent growth factors, have been ascribed to MMPs.6 Indeed, MMP activity has been shown to be required for tumor cell invasion and angiogenesis.7 From a clinical standpoint, we and others demonstrated expression of gelatinases (MMP-2 and MMP-9), and the membrane-type 1 metalloproteinase (MT1-MMP, also called MMP-14) in human prostate cancer tissues.8–11 Enhanced expression of active MMP-2 has been correlated with prostate cancer grade and disease progression.12,13
MMPs are also likely to be involved in the establishment and growth of metastatic cancer in bone. Certain studies imply that some MMPs may be particularly relevant to the bone microenvironment and the bone metastatic process. Collagen I is the most abundant extracellular matrix protein in bone, and MT1-MMP is a potent type I collagenase. Indeed, mice lacking MT1-MMP develop severe skeletal defects.14–16 The MMP-9-deficient mouse also displays defects in bone development, intraosseous angiogenesis, and fracture repair,17–19 and MMP-9 is known to be required for osteoclast migration in developing bones.20
Recently, we demonstrated MMP-2, MMP-9, and MT1-MMP expression in prostate cancer cells and stromal cells in clinical prostate cancer bone metastasis tissue.21 We showed a similar pattern of MMP expression in a predominantly osteolytic animal model of bone metastasis and further demonstrated that broad-spectrum inhibition of MMP activity reduced bone matrix degradation and bone tumor growth.21 Similar results have been achieved in other models of bone metastasis involving breast cancer cells.22–24 Thus, MMP-mediated proteolysis contributes to the so-called vicious cycle25 linking bone matrix turnover and tumor expansion in bone. However, the contribution of individual proteases and the particular cell types responsible for their production are not well understood.
Although preclinical studies suggest that MMP activity plays an important role in metastasis, in vivo enzymatic activities (ie, substrate cleavage rates) of individual MMPs have not been described. Part of the reason for the difficulty in establishing the role of particular MMPs in vivo is the lack of specific assays for measuring activity of individual proteases in tissues. Instead, previous investigations have centered on the detection of MMP mRNA or protein using in situ hybridization, immunohistochemistry, zymography, or immunoblotting. These techniques are semiquantitative in nature. Moreover, they do not take into account the presence of enzymatic inhibitors in the tissues and thus may be lacking in clinical relevance. For example, high molar levels of tissue inhibitors of metalloproteinases (TIMPs) might negate high molar levels of proteases with respect to net enzymatic activity. In this study, we used specific enzymatic assays designed to measure net activity of MT1-MMP, MMP-2, and MMP-9 individually in experimental prostate cancer bone metastasis tissue. Herein, we showed a significant up-regulation of net tissue MMP-9 activity emanating primarily from newly recruited osteoclasts during the early colonization of the bone marrow spaces by tumor cells. In contrast, net MMP-2 and MT1-MMP activities in the tissues remained low and unchanged. We further found that metalloproteinase activity, but not the activity of other classes of proteases, is responsible for activation of pro-MMP-9, and that MMP-9 is necessary for tumor-induced osteoclast recruitment.
Materials and Methods
Cell Lines
PC3, an androgen-independent cell line derived from a bone metastasis of a high-grade prostatic adenocarcinoma,26 was purchased from American Type Culture Collection (Manassas, VA). It was maintained in RPMI 1640 (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS). PC3 ML 200-2 GFP (200-2) and PC3 ML GFP (ML) were generous gifts from Dr. Ruth Muschel (University of Pennsylvania, Philadelphia, PA). Both cell lines were derived from PC3; the former was transfected with GFP and MMP-9 ribozyme genes, and the latter only with a GFP gene. 200-2 was maintained in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 400 μg/ml of hygromicin (Invitrogen), 0.5 μg/ml puromycin (Sigma, St. Louis, MO), and 10% FBS, whereas ML was maintained in Dulbecco’s modified Eagle’s medium with 400 μg/ml of G418 (Invitrogen) and 10% FBS. BMA3.1A7 (BMA), a macrophage cell line derived from mouse bone marrow (preosteoclast), was a generous gift from Dr. Kenneth Rock (Dana-Farber Cancer Institute, Boston, MA).27 This cell line was maintained in RPMI 1640 supplemented with 10% FBS.
Mice
Five-week-old male C.B.-17.scid mice were purchased from Taconic Farms (Germantown, NY) and allowed to acclimate to their housing for 1 week. Mice were maintained under aseptic conditions according to the NIH standards established in the Guidelines for the Care and Use of Experimental Animals, and the Animal Investigation Committee of Wayne State University approved all of the experimental protocols.
Cancer Cell-Bone Co-Cultures
For consistency, we used human male fetal femurs (20 to 22 weeks of gestation) for both the in vitro and in vivo models of bone metastasis. The bone tissue was obtained from Advanced Bioscience Resources (Alameda, CA). This nonprofit organization obtains fetal tissues from elective pregnancy terminations for biomedical research using informed consent procedures according to regulations issued by each state involved and the federal government. For prostate cancer cell-bone co-cultures, the bones were cut longitudinally and then transversely into four fragments and maintained in a 24-well plate in serum-free RPMI 1640 medium until use. At the same time, PC3 cells were seeded onto a 24-well plate (1 × 105/well) in RPMI 1640 medium supplemented with 10% FBS. Twenty-four hours later, the media were discarded and PC3 cells were washed once with phosphate-buffered saline (PBS). Serum-free RPMI 1640 medium (500 μl) was added to each well containing PC3 cells. Each piece of bone fragment was further cut into five equal size pieces (∼2 mm × 2 mm × 3 mm) and designated wells with PC3 cells were overlaid with five such pieces of bone fragments. In other wells, PC3 cells or bone fragments were cultured alone in 500 μl of serum-free RPMI 1640 medium. Cell-bone co-culture studies with PC3 cells included four groups: PC3 alone, bone alone, PC3 + bone, and PC3 + bone + batimastat (BB-94). Cell-bone co-culture studies with ML and 200-2 cells included five groups: ML alone, 200-2 alone, bone alone, ML + bone, and 200-2 + bone. Cell-bone co-culture studies with different protease inhibitors included five groups: 200-2 + bone, and 200-2 + bone plus one of the following inhibitors: aprotinin, E-64, GM-6001, and pepstatin A. All of the protease inhibitors were purchased from Calbiochem (San Diego, CA), except BB-94, which was a generous gift from Dr. Shariar Mobashery (University of Notre Dame, Notre Dame, IN). The final concentrations of the protease inhibitors used in these studies were 2 μmol/L for BB-94, 10 μg/ml for aprotinin, 10 μmol/L for E64, 5 μmol/L for GM-6001, and 1 μmol/L for pepstatin A. According to previous studies by our group and other groups, these concentrations were neither cytotoxic nor cytostatic and were higher than the IC50 for the proteases.21,28–32 All cultures and co-cultures were maintained at 37°C for 48 hours. Conditioned media (CM) were collected, clarified by centrifugation, and subjected to zymography or MMP activity assay.
Cell-Cell Co-Cultures and CM Treatment
In a 24-well plate, BMA and 200-2 cells were seeded alone or together (105/well/cell type) in RPMI 1640 medium supplemented with 10% FBS. Twenty-four hours later, the media were discarded and the cells were washed once with PBS. Serum-free RPMI 1640 medium (500 μl) was added to each well and the cells were cultured at 37°C for 48 hours. CM were collected, clarified by centrifugation, and subjected to gelatin zymography.
200-2 cells were seeded into a T-75 flask and cultured until ∼80% confluence and incubated with serum-free RPMI for 48 hours. The CM was collected and clarified by centrifugation. BMA cells were seeded onto a 24-well plate (105/well) for 24 hours and then treated with the CM collected from 200-2 cells for 48 hours. The medium was collected for zymography and total RNA was prepared from the cells for reverse transcriptase (RT)-polymerase chain reaction (PCR).
Establishment of PC3 Human Bone Tumors and Subcutaneous Tumors
There were three groups in this experiment: control mice implanted with human bones later injected with culture medium, mice implanted with human bones later injected with PC3 cells, and mice injected subcutaneously with PC3 cells. Each group consisted of nine animals. Implantation of mice with human fetal bone fragments was performed as described previously.21,33 Briefly, human fetal femurs were cut longitudinally and transversely into four fragments, each ∼1 cm × 0.3 cm × 0.2 cm. Under isoflurane inhalational anesthesia, a single bone fragment was implanted subcutaneously into the flank with the opened marrow cavity placed against the mouse muscle and the cortical bone surface against the underside of the mouse skin. After 4 weeks of engraftment, the SCID-human mice were ready for intraosseous injection. PC3 cells were trypsinized and resuspended in serum-free RPMI 1640 medium. A 27-gauge needle was used to inject 1 × 105 cells in a volume of 20 μl directly into the marrow of the previously implanted bone. The control SCID-human mice received an injection of 20 μl of serum-free medium containing no PC3 cells into the implanted bone fragment. PC3 subcutaneous tumors were established by injecting 2.5 × 106 cells in a volume of 50 μl subcutaneously into the flank of mice of the same age. Consistent with previous experience,33 a higher number of cells was required at the mouse subcutaneous site compared with the implanted human bone site to achieve similar tumor sizes at equivalent time points.
Preparation of Extracts from Control Bones and PC3 Tumors
Two, 4, and 6 weeks after the intraosseous and subcutaneous injections, implanted control bone fragments, bone tumors, and subcutaneous tumors were harvested and immediately homogenized individually with 500 μl of tissue lysis buffer (25 mmol/L Tris-HCl, 100 mmol/L NaCl, 1% IGEPAL, pH 7.3) in a mortar (CoorsTek, Golden, CO). A serine and cysteine protease inhibitor cocktail (Roche, Indianapolis, IN) was added to the lysis buffer according to the manufacturer’s instruction (see below); the buffer contained no inhibitors of MMP activity. The extracts were centrifuged at 3000 rpm at 4°C for 10 minutes. The supernatants were collected and stored at −80°C.
Gelatin Zymography
Presence or absence of latent or active species of MMP-2 and -9 were monitored by gelatin zymography. Briefly, equal volumes of CM obtained from in vitro experiments or equal amounts (30 μg) of sample protein of tissue lysates were mixed with 4× sample buffer (0.25 mol/L Tris-HCl, pH 6.8, 0.4% sodium dodecyl sulfate, 40% glycerol, and bromophenol blue) and loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel containing 1 mg/ml gelatin (Sigma Chemical Co.). The gel was run under nonreducing conditions at constant voltage of 125 V for 2 hours and 30 minutes. After electrophoresis, the gel was incubated in renaturing buffer (2.5% Triton X-100) at room temperature for 30 minutes, washed twice with distilled water (20 minutes each time), and then incubated with the developing buffer (50 mmol/L Tris, pH 8.0, 5 mmol/L CaCl2, 200 mmol/L NaCl, and 0.02% Brij-35) for 30 minutes. Finally, the gel was developed in fresh developing buffer at 37°C overnight, stained in 0.5% Coomassie Blue solution for ∼2 hours, and destained in the destaining buffer (5% acetic acid, 10% methanol in distilled water) until bands of gelatinolytic activity were visualized.
Net Enzymatic Activity Assays for MMP-2, MMP-9, and MT1-MMP
Substrate-linked enzyme-linked immunosorbent assay (ELISA) techniques were used to quantify enzymatic activity of individual MMPs. The Fluorokine E kit (R&D Systems, Minneapolis, MN) was used to quantitate the specific, net, enzymatic activity of human MMP-9 in experimental samples (tissue extracts from in vivo experiments and CM from in vitro experiments). The samples were thawed on ice and all reagents needed for the assay were brought to room temperature. The MMP-9 activity assay was performed according to the manufacturer’s instructions with some modifications. The specificity for the assay was provided by the anti-MMP-9 antibody as described below.
Briefly, serial dilutions of recombinant (r)-pro-MMP-9 standard were incubated in individual wells of a 96-well plate coated with monoclonal anti-MMP-9 antibody. After washing away unbound substances, 4-aminophenylmercuric acetate (APMA) was added (final concentration of 3 mmol/L) to process the now bound r-pro-MMP-9 to active r-MMP-9. After several additional washes, a fluorogenic MMP substrate linked to a quencher was added. In this system, r-MMP-9 bound to the plate cleaved the peptide linker between the fluorophore and the quencher molecule releasing fluorescent signals that were proportional to the enzymatic substrate cleavage rate. Because there were no inhibitors present, the molar amount of r-MMP-9 bound to the plate was directly proportional to the enzymatic activity. Fluorescent signals were read by a MAXline microplate reader (Molecular Devices, Sunnyvale, CA) and a standard curve was developed relating fluorescence to the amount of r-MMP-9.
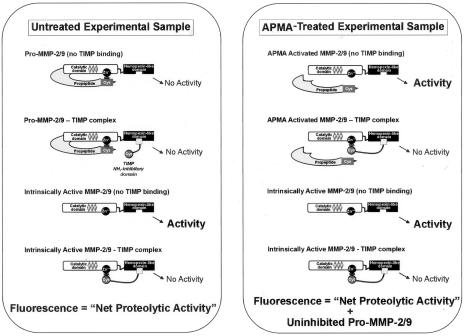
Preliminary experiments were done to determine the amount of sample necessary to ensure that all enzymatic activity measurements fell well above the lower limits of detection and within the linear range of the assay. To measure net enzymatic activity, experimental samples were assayed without initial APMA activation. The samples were incubated in the wells, and the wells were then washed to remove any unbound substances. Importantly, any active MMP-9 enzyme bound to the plate but inhibited by MMP inhibitors (eg, TIMPs) would not cleave the substrate. Similarly, pro-MMP-9 bound to the plate would also fail to cleave the substrate (Figure 1). Thus, the assay measured only net MMP-9 activity in the samples. The net MMP-9 activity was expressed, as per the standard curve, as the amount of activity that would have been produced by an equivalent concentration of inhibitor-free r-MMP-9 (ng equivalent r-MMP-9/100 μl CM or ng equivalent r-MMP-9/100 μg tissue sample total protein).
Figure 1.
Schematic representation of the different forms of MMP-2 and MMP-9 secreted in PC3 bone co-cultures or found in implanted human fetal bone tissues colonized with PC3 cells. In the untreated samples, only the uninhibited form of the active enzyme would yield a fluorescent signal. APMA treatment of the samples promotes the autocatalytic cleavage of the N-terminal propeptide domain of pro-MMP-2/9 to yield an artificially activated species. Binding of pro- or active MMP-2/9 to TIMPs would result in complexes that cannot generate enzymatic activity because of interaction of TIMP’s inhibitory N-terminal domain with the enzyme’s catalytic site. However, these MMP-2/9-TIMP complexes can dissociate in a zymogram gel and are thus evident as gelatinolytic MMP-2/9 bands.
APMA-activated MMP-9 (comprising both inhibitor-free zymogen and inhibitor-free active enzyme) was determined by adding APMA (final concentration, 3 mmol/L) to the wells for 2 hours. Again, any inhibitors bound to pro-MMP-9 in the samples would suppress cleavage of the quenched fluorescent substrate even after APMA activation. For example, a TIMP can bind to an MMP zymogen by its C-terminal domain. After APMA activation, the inhibitory N-terminal domain of the TIMP would bind to the Zn2+ atom in the MMP catalytic site when the propeptide is released (Figure 1).
Net MMP-2 and MT1-MMP activities were measured in a similar way using Biotrak kits purchased from Amersham Pharmacia Biotech (Piscataway, NJ), in which the specificity of each assay depended on an antibody-capture step. These assays used a secondary detection enzyme that was activated by the antibody-captured MMP. The detection enzyme cleaved a chromogenic substrate, and the intensity of the developed color was read by a Bio-Rad microplate reader (Bio-Rad Laboratories, Hercules, CA). The color intensity was proportional to the amount of MMP activity. For the MMP-2 assay, a standard curve was generated using recombinant pro-MMP-2 that was activated by APMA. The MT1-MMP assay measured only the active species; a standard curve was generated using recombinant active MT1-MMP.
Immunostaining of PC3 Bone Tumors
PC3 bone tumor and control bone tissues harvested at different time points were immediately fixed with 4% paraformaldehyde overnight and then treated with decalcification buffer (7.5% ethylenediamine tetraacetic acid and 1% paraformaldehyde) for 2 weeks. The decalcified tissues were dehydrated, infiltrated, and paraffin-embedded. Tissue sections (5 μm) were prepared and the paraffin slides were deparaffinized and rehydrated. To retrieve antigens, the slides were treated with 20 μg/ml of proteinase K (Sigma) for 20 minutes at 37°C. After quenching with 3% hydrogen peroxide to remove endogenous peroxidase activity, the slides were incubated with Super Block (ScyTek Laboratories, Logan, UT). Primary antibodies recognizing cytokeratin (clone C-11+PCK-26+CY-90+KS-1A3+M20+A53-B/A2, 1:50; Sigma), MMP-9 (1:50; Chemicon, Temecula, CA), or TRAcP (clone Zy 9C5, 1:50; Zymed Laboratories, South San Francisco, CA) were applied to localize and co-localize respective human target protein expression in PC3 bone tumors and/or control bone tissues. For single chromogenic immunostaining, a secondary antibody conjugated with peroxidase was used, and 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA) was used as the substrate for the peroxidase-mediated reaction. The reaction was stopped when the best contrast of brown granules was achieved. After the color development, the slides were counterstained with Mayer’s hematoxylin and examined under a light microscope. For double-immunofluorescence staining, biotinylated secondary antibodies, and fluorescein avidin DCS and Texas Red avidin DCS (Vector Laboratories) were used. The slides were observed under a LSM 310 confocal microscope (Carl Zeiss MicroImaging, Thornwood, NY). Negative staining controls of the sections were obtained by omitting the primary antibody in each case. Additionally, hematoxylin and eosin staining was performed to study the histological changes of PC3 bone tumors in those tissue sections analyzed for cytokeratin expression.
Semiquantitative RT-PCR
RT-PCR was performed as previously described.34 Briefly, reverse transcription was fashioned in 0.65-ml RNase-free tubes under optimized conditions in a DNA Thermal Cycler 480 (Perkin Elmer, Boston, MA). Equal amounts of total RNA (500 ng) from each sample were used for this reaction. The whole product of reverse transcription in each tube was amplified by PCR. Cycle parameters were a 1-minute melting step at 95°C, a 1-minute annealing step at 55°C, and a 2-minute extension step at 72°C. Twenty-five cycles were selected for amplification of all target genes based on experiments that tested the linear range of amplification with different cycles. The housekeeping gene GAPDH was also amplified and used as an internal control. The primers of mouse MMP-9 (5′-ACC ACC ACA ACT GAA CCA CA-3′ and 5′-ACC AAC CGT CCT TGA AGA AA-3′) amplified a 304-bp product. The primers of mouse GAPDH (5′-ACC CAG AAG ACT GTG GAT GG-3′ and 5′-CAC ATT GGG GGT AGG AAC AC-3′) amplified a 171-bp product. The amplified genes were resolved on 1% agarose gels and revealed by ethidium bromide staining.
Cell Motility Assay
In a 12-well plate, 200-2 cells were seeded (105/well) with RPMI 1640 medium supplemented with 10% FBS in triplicate. The next day, the medium was removed and the cells were washed once with PBS. Then 500 μl of serum-free RPMI 1640 medium were added to each well. Cell culture inserts with 8.0-μm pores (BD Falcon, Treyburn, NC) containing 105 BMA cells in 1 ml of serum-free RPMI 1640 were placed in the wells. Controls for spontaneous transmigration included inserts with BMA cells but no tumor cells in the bottom well. After incubation at 37°C for 16 hours, the upper face of the insert membrane was wiped using a cotton swab to remove the cells and the lower face of the membrane was stained with Diff-Quik kit (Dade Behring Inc., Newark, DE). The migrated cells in the center of the membrane were counted in 10 consecutive fields (each one with an area of 300 × 200 μm2) under a microscope at ×400 power. The mean number of traversing cells was calculated, and expressed in relative units, considering the control as 100%.
RNA Interference Study
We designed a specific small interfering RNA (siRNA) targeting mouse MMP-9 mRNA based on a published siRNA against human MMP-9.35 Briefly, by alignment analysis, we identified a corresponding sequence targeting mouse MMP-9 that was 90% homologous with the human MMP-9 sequence. The mouse target sequence for siRNA was 5′-AAC ATC ACA TAC TGG ATC CAA-3′ (GenBank accession number, NM 013599; coding region 358 to 379). According to this sequence, a pair of primers were designed and synthesized by Invitrogen. Using Silencer siRNA construction kit (Ambion Inc., Austin, TX), the specific siRNA targeting mouse MMP-9 was synthesized via in vitro transcription. The sense siRNA sequence was 5′-CAU CAC AUA CUG GAU CCA AUU-3′ and the anti-sense sequence was 5′-UUG GAU CCA GUA UGU GAU GUU-3′.
BMA cells were seeded into T-75 culture flasks. When the cells reached ∼70% confluence, they were transiently transfected with 30 nmol/L of the control siRNA (Ambion) and siRNA against mouse MMP-9, respectively, for 48 hours. The cell motility assay was performed using the treated cells, and mouse MMP-9 expression was monitored by zymography.
Statistical Analysis
The data obtained from MMP enzymatic activity assays were statistically analyzed using one-way analysis of variance, which tests whether the mean of a single variable differs among three or more groups. Whenever statistical significance was obtained with this test, the Tukey-Kramer posttest was used to further determine which specific pair/pairs were statistically different. The data obtained from the cell motility assays were analyzed using Student’s t-test, which compares whether the mean of a single variable differs between two groups. GraphPad InStat (GraphPad Software, San Diego, CA) was used to perform the statistical tests. Values of P ≤ 0.05 were considered statistically significant.
Results
Up-Regulation of MMP-9 Protein and Activity in Co-Cultures of PC3 Cells with Bone Tissue
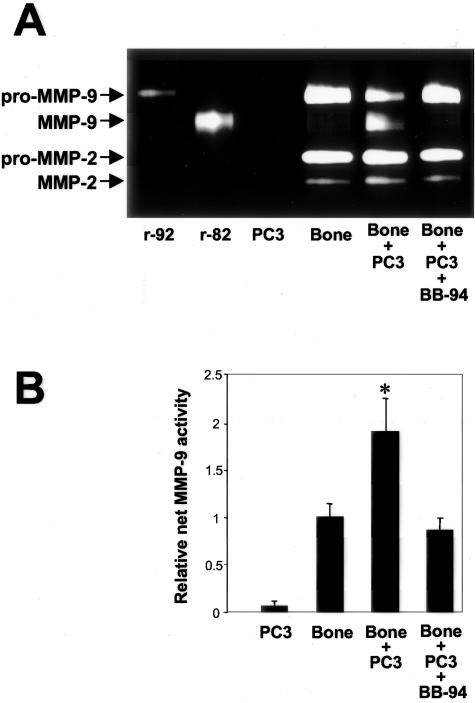
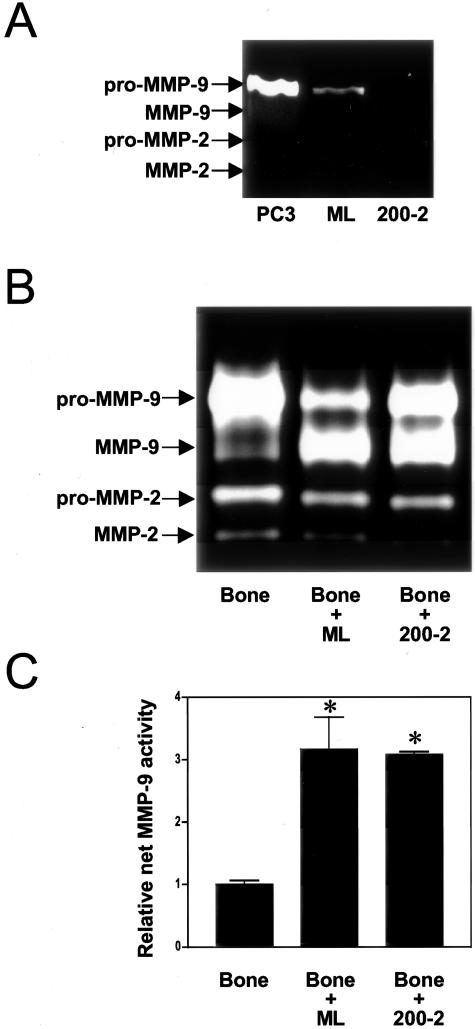
To study the effect of prostate cancer-bone interactions on MMP secretion, zymogen activation, and enzymatic activity, we examined the CM from PC3 cell-bone tissue co-cultures. Zymography was used to detect gelatinase expression, and the MMP substrate-linked ELISA techniques were used to quantitate net, secreted activity for MMP-2 and MMP-9. Previously, we demonstrated increased synthesis of the pro-form of MMP-9 when PC3 cells were co-cultured with isolated fibroblasts.34 In contrast, when PC3 cells were cultured with intact bone tissue, a synergistic increase in secretion of the active form of the enzyme was detected (Figure 2A). Regarding MMP-2, no significant changes in secretion were observed for either the latent or active forms. Inclusion of BB-94 (final concentration, 2 μmol/L) in the cell-bone co-culture significantly reduced the secreted amount of the active species of MMP-9. Previously, we showed that this concentration of BB-94 does not affect cell proliferation or viability.21
Figure 2.
Secreted MMP forms and net MMP activity in co-cultures of PC3 cells with bone tissues. Co-cultures of PC3 cells and human fetal bone tissues were performed, and CM were collected after 48-hour incubation periods as described in Materials and Methods. Where indicated, BB-94 was included in the co-culture, but not in the fluorescent assay. A: Gelatin zymography was performed on CM to assess for the presence of MMP species. Recombinant human pro-MMP-9 and MMP-9 were used as markers. B: Net secreted MMP-9 activity in the CM was assessed using the substrate-linked ELISA technique. Values depicted are means ± SE (n = 6, except for the co-culture with BB-94 with n = 3). The activity was normalized to that produced by bone alone to account for differences in mass of bone tissue used in the co-culture. *, P < 0.05 bone + PC3 compared to PC3, bone, and bone + PC3 + BB-94 (Tukey-Kramer multiple comparisons test).
Using the enzymatic activity assay, we found a synergistic increase in net, secreted MMP-9 activity when bone tissue and PC3 cells were cultured together (Figure 2B). Inclusion of BB-94 in the cell-bone co-culture abrogated the increase in net, secreted MMP-9 activity. Secreted, net MMP-2 activity levels remained low with no change during co-culture of bone with PC3 cells (data not shown). MT1-MMP was not measured in the cell-bone co-culture system because it is membrane-bound and not secreted into the medium. Together, these data demonstrated that interaction of prostate cancer cells with an intact bone environment led to secretion of MMP-9 protein and increases in net MMP-9 activity. Moreover, matrix metalloprotease activity appears to be required for the activation of MMP-9 from zymogen to active enzyme.
Time Course of Bone Matrix Degradation and Osteoclast Recruitment in Experimental PC3 Bone Tumors
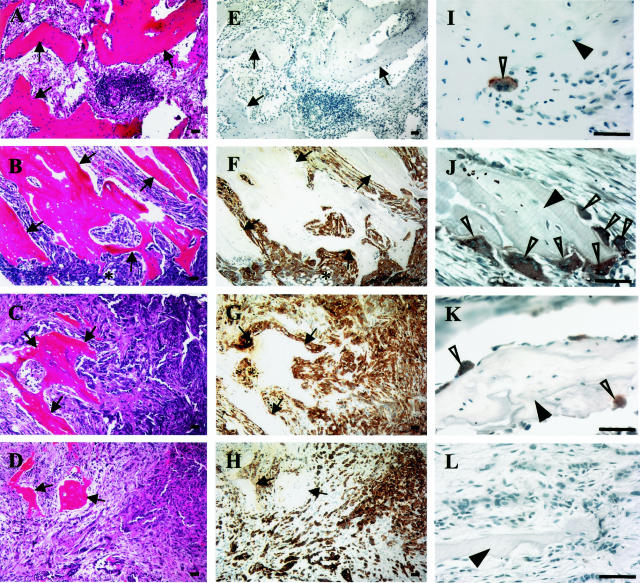
To correlate net tissue MMP activity measurements with progression of experimental bone metastases, PC3 bone tumors were established using the SCID-human mouse model.21,33 H&E staining and immunostaining with anti-cytokeratin antibody showed that PC3 cells were easily detectable in the marrow spaces 2 weeks after intraosseous inoculation (Figure 3, B and F). At this time point, the gross appearance of the excised bone fragment was unchanged compared with excised control bone fragments. Under low-power examination, cortical bone was still intact, trabeculae were unchanged, and some residual hematopoietic marrow could be identified. As expected, 4 weeks after PC3 cell injection (Figure 3, C and G), virtually all of the marrow space was occupied by tumor cells, and hematopoietic marrow could no longer be identified. The trabeculae were markedly thinned, occupying a decreased proportional area of the tissue section. The cortical bone was still intact and the gross appearance and size of the excised tissue was unchanged. Six weeks after tumor cell injection (Figure 3, D and H), the trabeculae were almost completely destroyed, the cortical bone had been breached by tumor cells, and the gross size of the excised bone tumor tissues had increased significantly compared with the control bone tissues.
Figure 3.
PC3 cell colonization of fetal human bones implanted in SCID mice, degradation of marrow trabeculae, and osteoclast recruitment. H&E staining (A–D) and immunostaining for cytokeratin (E–H) in adjacent, serial paraffin sections obtained from control bone tissues (A and E) and PC3 bone tumors at 2 (B and F), 4 (C and G), and 6 (D and H) weeks. PC3 cells in bone tissues were specifically recognized by a positive immunoreaction with a monoclonal antibody against cytokeratin (cytoplasmic brown granules). Arrows point to bone trabeculae and an asterisk indicates area of hematopoiesis. Immunostaining for TRAcP, a specific osteoclast marker, was performed on paraffin slides prepared from control bones (I) and PC3 bone tumors at 2 (J), 4 (K), and 6 weeks (L). TRAcP-positive cells (open arrowheads) were revealed by dark brown granules in their cytoplasm. Filled arrowheads indicate bone trabeculae. Scale bars, 50 μm.
Tissue sections prepared from control bone tissues and PC3 bone tumor tissues at 2, 4, and 6 weeks were immunostained with an antibody recognizing TRAcP, a specific marker for osteoclasts. Very few osteoclasts could be identified in the control bones (Figure 3I). In contrast, a large number of osteoclasts was found associated with trabeculae in PC3 bone tumors at 2 weeks (Figure 3J). The number of osteoclasts declined at 4 weeks, and very few were found at 6 weeks (Figure 3, K and L). These data suggested that osteoclast recruitment was highest at the 2-week time point when the microscopic anatomy of the bone trabeculae was still morphologically intact and the tumor cells were actively populating the marrow spaces.
Up-Regulation of MMP-9 Protein and Activity in Experimental PC3 Bone Tumor Tissue
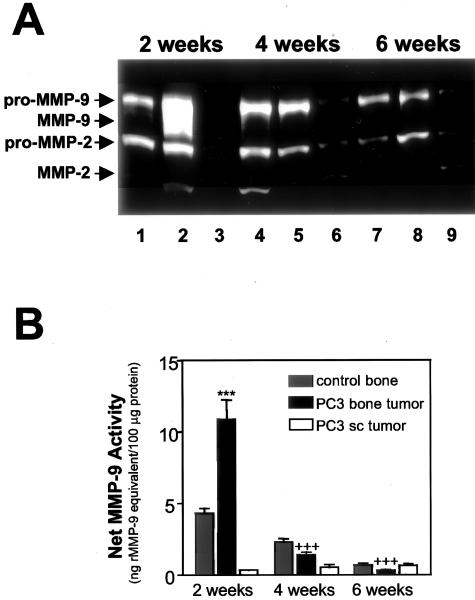
Zymographic analysis of the tissue extracts 2 weeks after intraosseous PC3 cell injections showed a dramatic increase in the active form of MMP-9 in bone tumor tissues compared with control bone implants or subcutaneous tumors (Figure 4A). Conversely, the active MMP-9 species was not detected in any of the tissue extracts obtained 4 or 6 weeks after injection (Figure 4A). The amount of active MMP-2 remained relatively low across all time points and showed no significant differences between control bone implants, bone tumors, or subcutaneous tumors.
Figure 4.
Induction of net MMP-9 activity in implanted bone tissues colonized with PC3 cells. A: Gelatin zymography was performed on tissue extracts obtained from control bones (lanes 1, 4, and 7), PC3 bone tumors (lanes 2, 5, and 8), and PC3 subcutaneous tumors (lanes 3, 6, and 9) at the indicated time points after injection. B: Net tissue MMP-9 activity in the same sets of tissue extracts (in triplicates) was assessed with the quenched fluorescent substrate-linked MMP-9 ELISA assay. Values depicted are means ± SE (n = 3). The net tissue MMP-9 activity was expressed as the amount of activity that would result from an equivalent concentration of inhibitor-free recombinant MMP-9 (ng r-MMP-9 equivalent/100 μg of tissue sample total protein). ***, Statistical difference of P < 0.001 in net tissue MMP-9 activity between PC3 bone tumors and control bones or between PC3 bone tumors and PC3 subcutaneous tumors at 2 weeks. +++, Statistical difference of P < 0.001 in net tissue MMP-9 activity between 2-week bone tumors and both 4-week and 6-week bone tumors.
The MMP-9 substrate-linked ELISA was used to quantify net MMP-9 activity in the same tissue extracts used for zymographic analyses. Two weeks after intraosseous injection, we found much higher net MMP-9 activity in the bone implants injected with PC3 cells than in the control bone implants injected with culture medium alone or in PC3 subcutaneous tumors (Figure 4B, P < 0.001). Net MMP-9 activity in PC3 bone tumors declined precipitously to lower levels (P < 0.001) at 4 and 6 weeks, at which times there were no statistical differences in net MMP-9 activity between bone tumors, control bone implants, and subcutaneous tumors (Figure 4B).
Because the zymogram (Figure 4A) showed an increase in the active MMP-9 species at the 2-week point in the bone tumor tissue versus the control bone tissue, we wished to determine whether there was enhanced activation of the zymogen or de novo synthesis of pro-MMP-9 as a consequence of tumor cell-bone interactions. To this end, we treated the tissue samples collected at the 2-week time point with APMA to activate all of the uninhibited endogenous pro-MMP-9. These studies showed that PC3 bone tumor tissue had twofold higher APMA-activated MMP-9 activity than bone tissue alone (23.1 ± 3.3 versus 10.6 ± 3.1 ng rMMP-9 equivalent/100 μg protein, respectively; P < 0.05, Student’s t-test). Thus, the higher net MMP-9 activity in the bone tumor tissue was not just because of enhanced activation but likely also because of an increase in pro-MMP-9 synthesis, consistent with a role for tumor cell-bone interactions in the expression of pro-MMP-9. No differences in APMA-activated MMP-9 activity were observed between bone tumors and control bones at the 4- and 6-week time points (data not shown).
Net tissue MT1-MMP and MMP-2 activities were measured in bone tumor tissue and control bone tissue using similar ELISA-based assays but with chromogenic substrates. In contrast with net tissue MMP-9 activity, overall net tissue MT1-MMP activity in bone tumor tissues was either no different or lower than MT1-MMP activity in control bone tissues (data not shown). Overall net tissue MMP-2 activity was very low at all time points, and no differences were found between bone tumors and bone controls at any individual time points or across time points (data not shown).
Immunofluorescent Localization of MMP-9 Protein in PC3 Bone Tumors
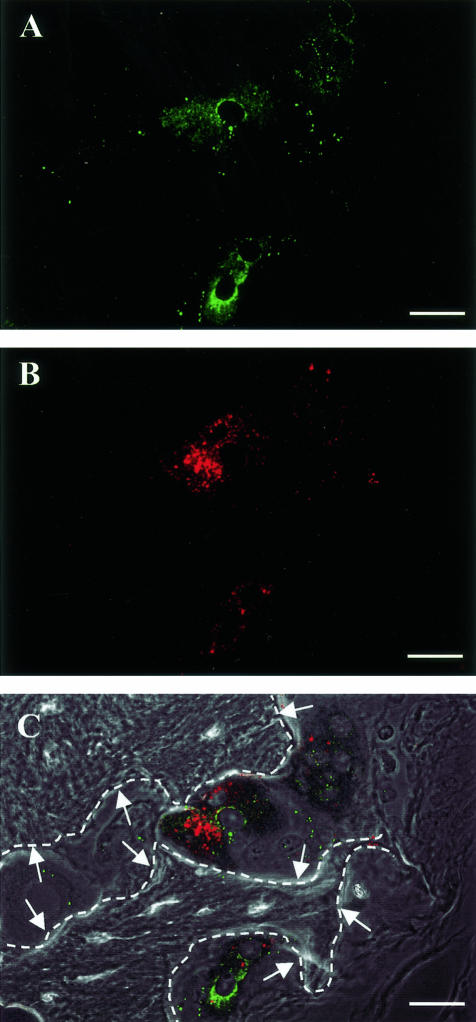
To further understand the source of MMP-9 in PC3 bone tumors, histological sections were subjected to dual-color immunofluorescent staining with antibodies recognizing MMP-9 and TRAcP and analyzed by confocal microscopy. In the 2-week bone tumors, MMP-9 protein localized to osteoclasts (Figure 5). In addition, dual-color immunofluorescence for MMP-9 and cytokeratin demonstrated that MMP-9 protein also localized to tumor cells, consistent with our previous study21 (data not shown). In general, the fluorescent signals for MMP-9 were more intense in osteoclasts than in PC3 cells, however, the number of PC3 cells was vastly higher than the number of osteoclasts. It should be emphasized that immunohistochemical studies do not discriminate between zymogen and active forms of MMP-9 and that quantitative comparisons of the amount of MMP-9 activity emanating from tumor cells versus osteoclasts could not be determined. TRAcP staining and MMP-9 staining were very weak or undetectable in PC3 bone tumors at 4 and 6 weeks and in the implanted control bones at all time points (data not shown).
Figure 5.
Immunolocalization of MMP-9 in osteoclasts in implanted human fetal bone at 2 weeks after PC3 cell injection. Paraffin slides prepared from PC3 bone tumors were double-immunostained with primary antibodies recognizing MMP-9 and TRAcP. MMP-9 localization was recognized as cytoplasmic green fluorescein granules (A), whereas TRAcP immunostaining was identified by cytoplasmic Texas Red granules (B). The color images were merged with the phase contrast image in (C). Arrows point to bone trabeculae visualized by phase contrast imaging. Dashed lines indicate the edge of bone trabeculae. Scale bars, 25 μm.
Specific Suppression of MMP-9 Expression in PC3 Cells Does Not Abrogate the Increase in MMP-9 Activity Resulting from Tumor-Bone Interaction
To assess the relative contribution of prostate cancer cells to the overall production and activation of MMP-9 during tumor-bone interactions, we used the 200-2 cell line, a PC3 derivative obtained by stable transfection of PC-3 ML cells with a ribozyme directed against MMP-9 mRNA. Zymography confirmed that the ribozyme significantly diminished MMP-9 expression by 200-2 cells compared with parental ML cells (Figure 6A). When the parental ML cells were co-cultured with bone fragments, the active MMP-9 species was secreted into the media and net, secreted MMP-9 activity increased. Secretion of active MMP-9 and net MMP-9 activity was not reduced when 200-2 cells were used (Figure 6, B and C). These observations suggest that bone tissue is the primary source of MMP-9, and that interaction of tumor cells with bone leads to MMP-9 activation and a net increase in MMP-9 activity.
Figure 6.
MMP-9 activation and MMP-9 activity in co-cultures of bone tissue and non-MMP-9-expressing PC3 cells. A: Gelatin zymographic analysis of CM from the different variants of PC3 cells cultured alone. Compared with Figure 2, larger volumes of CM were necessary to see any expression of pro-MMP-9 in PC3 cells cultured alone. Expression of pro-MMP-9 was suppressed in 200-2 cells. B: Active MMP-9 was released into the CM when either MMP-9-expressing (ML) or non-MMP-9-expressing (200-2) cells were co-cultured with bone tissue. C: The relative net MMP-9 activity in the same samples of CM was assessed using the quenched fluorescent substrate-linked ELISA assay. Values depicted are means ± SE (n = 3), and secreted net enzymatic activity is shown normalized to the amount secreted by bone alone. *, P = 0.05 (Tukey-Kramer multiple comparisons test).
To further determine the role of prostate cancer-derived MMP-9 in vivo, 200-2 cells were injected into previously implanted human bone xenografts as described in Material and Methods. Two weeks after intraosseous cancer cell inoculation, the net MMP-9 enzymatic activity found in tissue extracts from 200-2 bone tumors did not differ statistically from that in tissue extracts from ML bone tumors (mean ± SE, 17.9 ± 3.9 versus 18.0 ± 2.7 ng equivalent r-MMP-9/100 μg tissue sample total protein, respectively). Histological examination of bone tumors generated by 200-2 or ML cells showed no obvious differences in tumor burden or bone degradation between the two groups (data not shown). These data suggested that prostate cancer cell-derived MMP-9 does not contribute significantly to overall net tissue MMP-9 activity or to any obvious phenotypic changes during the early phases of bone marrow colonization by tumor cells.
Mechanism of Pro-MMP-9 Activation
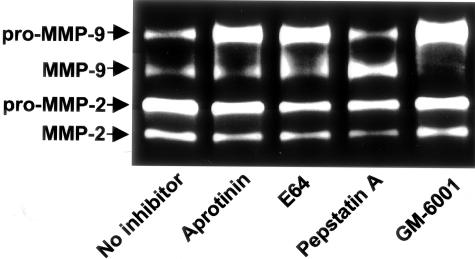
In Figure 2, we demonstrated that inclusion of BB-94 in the co-culture prevented the activation of MMP-9, suggesting that metalloproteinase activity is necessary for MMP-9 activation. To determine which class(es) of enzymes catalyze the conversion of pro-MMP-9 to MMP-9 during tumor-bone interactions, 200-2 cell-bone co-culture was fashioned in the presence of nontoxic doses of inhibitors for serine (aprotinin), matrix metallo (GM-6001), aspartic (pepstatin A), or cysteine (E-64) proteases. Only GM-6001 was effective in diminishing the band corresponding to active MMP-9 in zymographic analyses of CM (Figure 7), confirming that the activation of MMP-9 is accomplished via the activity of metalloproteinases, but not other classes of proteases. Although gelatin zymography is not a quantitative assay, we noticed an apparent increase in the level of active MMP-9 after exposure of the bone/PC3 co-culture to pepstatin A (Figure 7). This could be explained by the participation of some aspartic-like protease in the degradation of a pro-MMP-9 activator. This was not further explored because it was out of the scope of this study.
Figure 7.
Involvement of metalloproteinases in the activation of pro-MMP-9 induced by interaction of prostate cancer cells with the bone microenvironment. Co-cultures of 200-2 cells and human bone tissues were performed in the presence of nontoxic doses of inhibitors for different classes of proteases. Analysis of the CM by gelatin zymography showed that the activation of pro-MMP-9 was inhibited by GM-6001, a broad-spectrum inhibitor of metalloproteinases.
Induction of Pro-MMP-9 Expression and Motility in Preosteoclasts
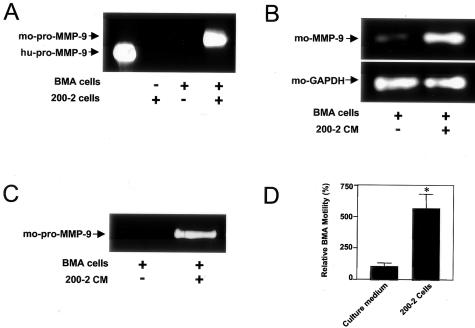
Because tissue MMP-9 activity correlated temporally with maximal osteoclast presence, we hypothesized that MMP-9 expression was necessary for tumor-induced osteoclast recruitment. To test this hypothesis, we exposed mouse BMA preosteoclast cells to human 200-2 cells or 200-2 CM to investigate whether MMP-9 expression in these cells is affected by prostate cancer cells. Zymographic analysis of CM obtained from co-culture revealed the presence of secreted mouse pro-MMP-9 protein, whereas no gelatinolytic bands were observed when CM came from either BMA or 200-2 cells cultured alone (Figure 8A). By RT-PCR, enhanced mouse MMP-9 gene expression was observed in BMA cells incubated with 200-2 CM (Figure 8B), which also induced protein expression of the zymogen, as shown in the gelatin zymogram (Figure 8C). To evaluate whether prostate cancer-released factors may be critical for the motility of BMA cells, migration assays were performed. The presence of confluent 200-2 cells in the lower chamber significantly increased the migration of BMA cells through the filter (Figure 8D). MMP-9 silencing by RNA interference in BMA preosteoclasts (Figure 9A) significantly inhibited migratory activity enhanced by prostate cancer cells (Figure 9B). Together, these results indicated that prostate cancer cells can induce pro-MMP-9 expression and motility in osteoclast precursor cells, and that the cell movement is dependent on pro-MMP-9 expression.
Figure 8.
Induction of pro-MMP-9 expression and motility in preosteoclasts. A: Gelatin zymography was performed using CM obtained from co-cultures of BMA murine preosteoclast cells and 200-2 cells, as described in Materials and Methods. A gelatinolytic band corresponding to mouse (mo) pro-MMP-9 (∼105 kd) was only detected by zymography after co-culture of both cell types. Recombinant human (hu) pro-MMP-9 (92 kd) was used as a marker in lane 1. RT-PCR (B) and gelatin zymography (C) were performed to detect MMP-9 mRNA and protein expression, respectively, in BMA cells after exposure to 200-2 CM. A marked induction of MMP-9 mRNA and pro-MMP-9 protein was evident. D: Transmigration of BMA cells in a transwell system toward 200-2 cells or culture medium (control). A significant increase in relative preosteoclast cell motility was observed when 200-2 cells were used in the lower chamber. Values represent mean ± SE (n = 3). *, P = 0.02, Student’s t-test.
Figure 9.
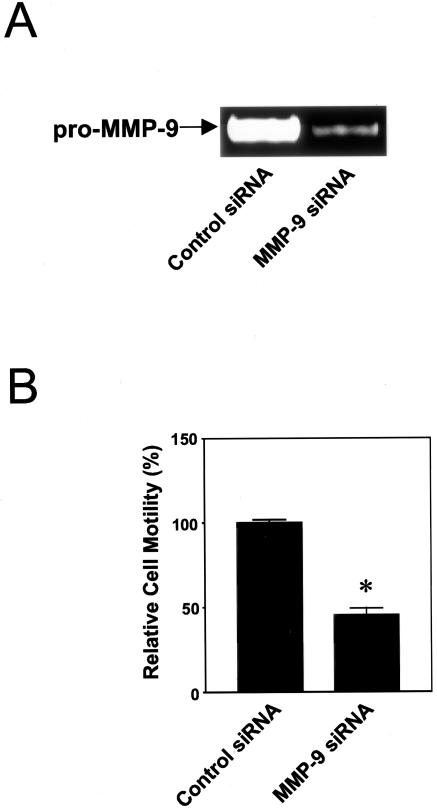
Inhibition of the migratory activity of preosteoclasts by silencing of MMP-9 expression using siRNA. A: Gelatin zymogram showed a reduction of MMP-9 expression in BMA cells treated with siRNA against mouse MMP-9. B: Transmigration of BMA cells toward 200-2 cells in the lower chamber was significantly reduced when MMP-9 was silenced in preosteoclasts. Values represent mean ± SE (n = 3). *, P = 0.002, Student’s t-test.
Discussion
We and others previously showed that pharmaceutical inhibition of MMP activity diminished bone tumor growth and tumor-induced bone remodeling in in vivo models of breast and prostate cancer.21–24 Because these studies used broad-spectrum MMP inhibitors, the relative contribution of individual MMPs could not be determined. In addition, the cellular source of MMP activity remained undefined. Herein, we hypothesized that particular individual MMPs may contribute to promotion of intraosseous tumor growth and tumor-induced bone remodeling.
In this study, we demonstrated an increase in net tissue MMP-9 enzymatic activity during colonization of marrow spaces by PC3 cells. To our knowledge, this is the first direct quantitation of enzymatic activity of individual MMPs in cancer tissues. Interestingly, MMP-9 activity increased during a time in which there was no change in MMP-2 or MT1-MMP activity, suggesting some specificity with regard to the role of individual proteases in bone metastasis. The data suggest that MMP-9, but not MMP-2 or MT1-MMP, is a relevant target for pharmaceutical inhibition. As we measured enzymatic activities of only three MMPs, the relative importance of other proteases remains to be determined.
In previous studies, we showed that co-cultures of PC3 cells with various fibroblasts induced pro-MMP-9 expression in cancer cells.34 In experimental bone metastasis tissues, we also showed that various MMP proteins, including MMP-9, immunolocalized to tumor cells and stromal cells.21 In the current study, we sought to determine the primary cellular source of MMP-9 activity. In the in vivo model, maximal tissue MMP-9 activity occurred 2 weeks after intraosseous injection of tumor cells. This time point correlated with a peak in osteoclastic activity, as judged by the number of osteoclasts lining marrow trabeculae. Throughout the next few weeks, tumor cells continued to proliferate but osteoclast numbers declined. These data suggested that the early increase in net tissue MMP-9 activity was because of a transient wave of osteoclast recruitment and activity. Because we could not directly quantify the relative amount of MMP-9 emanating from osteoclasts versus tumor cells, and because the number of cancer cells was vastly greater than the number of osteoclasts, we designed additional experiments using a PC3 cell line derivative that had been transfected with a MMP-9 ribozyme to suppress MMP-9 expression. When these cells were co-cultured with bone tissue, we again found activation of pro-MMP-9 and enhanced MMP-9 enzymatic activity in the culture medium. Enhancement of net tissue MMP-9 activity was also found when these cells were injected into bones in vivo. Also, we found no obvious differences in intraosseous tumor burden or bone degradation between bone tumors created using tumor cells with and without MMP-9 expression. Together, these data indicated that bone cells (most likely osteoclasts) contributed the majority of MMP-9 protein and activity, and that interactions of prostate cancer cells with bone tissue resulted in activation of bone-derived pro-MMP-9.
In the current study, we used a tumor cell-bone tissue co-culture system to simulate a complete bone microenvironment and demonstrated pro-MMP-9 synthesis, secretion, and subsequent activation of the zymogen to the active form in the extracellular environment. In contrast, activation of the zymogen does not occur in a simple cell-cell co-culture system.34 The co-culture studies of bone tissue and prostate cancer cells including inhibitors of different classes of proteases indicated that pro-MMP-9 activation seems to be accomplished mainly via the activity of metalloproteinases. Putative candidates responsible for MMP-9 activation include MMP-3, MMP-13, MMP-7, MMP-2, and MMP-26.36–40 Additional studies are necessary to determine the protease(s) and cellular source(s) of the proteases responsible for activation of the MMP-9 zymogen. These data suggest the presence of particular protease pathways that are active in tumor-induced bone remodeling and tumor expansion in bone.
Prostate cancer bone metastases are usually described as osteoblastic,41 but biochemical and histological studies show clear evidence of both bone resorption and formation.42,43 Osteolysis is important for physical expansion of tumor cells in the marrow, and matrix degradation provides calcium and marrow-derived growth factors for metastatic cancer cell proliferation and osteoblast differentiation.44 In the current study, we used the PC3 prostate cancer cell line, which in most systems produces bone tumors that are predominantly osteolytic.33,45,46 We consider the PC3 bone model to mimic osteolytic processes that are present in all clinical bone metastases; an obvious limitation of this model, however, is that it does not include an element of osteoblastic activity.
In normal bone remodeling, nonmineralized bone matrix overlying trabeculae is first degraded by osteoblasts and/or bone-lining cells secreting MMPs.47 Although not delineated in the current study, it is conceivable that tumor-derived proteases could contribute to degradation of nonmineralized matrix in a similar fashion. Osteoclasts subsequently migrate into bone remodeling areas; in fact, the osteoclast migration process itself is MMP-dependent.20 Osteoclasts attach to the now exposed mineralized matrix and solubilize it using cysteine proteases.48 The organic components in bone are thus made available within the osteoclast lacuna where they are further degraded by MMP activity49,50 as the pH in the lacuna shifts from acidic toward neutral.51 A specific role for MMP-9 in bone remodeling was suggested by examination of MMP-9-deficient mice. During development, these mice have faulty osteoclast recruitment and invasion into cartilage leading to delayed ossification of the growth plate. Endothelial recruitment is also impaired.18,19 In addition, adult MMP-9-deficient mice display impaired fracture healing. The cartilage callus around the fracture is resistant to remodeling, and there is defective ossification of the callus, most likely because of impaired differentiation of osteoblasts.17 The current study suggests that bone-derived MMP-9 also plays an important role in pathophysiological bone remodeling induced by the presence of metastatic tumor cells; the primary pathophysiological defect appears to involve osteoclasts.
In developing bones, MMP activity is involved in osteoclast migration, distinct from its degradative role in the osteoclastic lacuna.20 MMP-9 activity, in particular, has been implicated in this process.52 Osteoclast recruitment associated with the presence of tumor cells also involves metalloprotease activity; we previously showed that broad spectrum MMP inhibitor treatment of mice bearing PC3 bone tumors prevented osteoclast recruitment.21 Consistent with this observation, in the current study, the peak in osteoclast activity coincided with the peak in bone tissue MMP-9 activity, suggesting the pathophysiological defect to be an excess of osteoclast MMP-9 because of tumor-bone interactions. Experimentally, this issue would be best addressed by specific inhibition of MMP-9 in osteoclasts in an in vivo model of bone metastasis. However, methods are lacking for this approach; moreover, it would be extremely difficult to demonstrate unequivocal in vivo inhibition of MMP-9 activity. In vitro, we observed that prostate cancer cells induced motility and expression of pro-MMP-9 in preosteoclasts, and that this migratory activity was abrogated by siRNA-mediated MMP-9 silencing. Interestingly, we found that the active form of MMP-9 was not present during the migration of the preosteoclasts in the cell-cell co-culture system. Similar results have been found for glioblastoma cells53 and osteosarcoma cells.35 In fact, it is hypothesized that pro-MMP-9 may affect the migratory state independent of its enzyme activity.35 Together, these observations suggest that MMP-9 is involved in tumor-induced migration of osteoclasts to sites of bone remodeling, but the exact mechanism remains to be determined. Of course, reduced osteoclast numbers in areas of bone tumor would, by itself, lead to reduced overall osteoclastic activity.
Inhibition of MMP activity has been effective in various animal tumor models, including bone metastasis models. However, results from clinical trials with synthetic MMP inhibitors have been disappointing. There are reasons to believe that the clinical trials did not adequately test the hypothesis that protease inhibition is effective, particularly in bone metastasis.54 First, achievement of a meaningful response was probably impeded by very high tumor burdens present in most of the patients included in the clinical trials. Second, the trials did not address issues specific to bone metastasis (versus general metastasis). Third, proteolytic activity in tumor tissues was not measured initially, nor was it monitored during treatment. Thus it is impossible to know if the drugs sufficiently inhibited their intended target. Finally, the agents used in the trials were mostly broad-spectrum inhibitors with many side effects. Because of side effects, dosages were often reduced, further emphasizing the need to develop methods to monitor protease inhibition during treatment. The primary aim of the current study was to use an assay that might be potentially clinically relevant—an assay that can reveal the potential therapeutic value of targeting individual enzymes. Herein, we demonstrated at least some degree of specificity with regard to the net tissue activity of individual MMPs. The data suggested that assays that measure activities of individual enzymes could be clinically relevant and thus should be included in clinical trials wherever possible. In the future, we expect that molecular imaging55,56 will provide a noninvasive method of measuring of proteolytic activity before and during treatment.
In conclusion, we found a significant induction of net tissue MMP-9 activity during the early phases of intraosseous tumor expansion. In contrast, net tissue activities of MMP-2 and MT1-MMP were unchanged. We further showed that osteoclasts were likely the primary source of MMP-9 activity and that osteoclast recruitment depends on MMP-9. Moreover, metalloproteinase activity seems to be important for the activation of pro-MMP-9. In contrast, tumor cell-derived MMP-9 appears to play little role in intraosseous tumor expansion. Because metalloproteinase inhibition is known to be able to disrupt the vicious cycle of tumor growth and tumor-induced bone remodeling, the data suggest that MMP-9-specific inhibitors might have a better benefit:risk profile than broad spectrum MMP inhibitors. The data also suggest that osteoclasts appear to be the primary target of MMP-9 inhibition. Because the PC3 bone model used here induced only an osteolytic response, additional studies are underway with other cancer cell lines that induce osteoblastic and osteolytic/osteoblastic responses. Based on our studies, future clinical trials using protease inhibitors should include assays that monitor enzymatic inhibition.
Footnotes
Address reprint requests to Michael L. Cher, M.D., Wayne State University School of Medicine, 540 E. Canfield, Room 9105, Detroit, MI 48201. E-mail: mcher@med.wayne.edu.
Supported by the National Institutes of Health (grants RO1 CA88028 to M.L.C. and RO1 CA82298 to R.F.). Confocal microscopy was supported in part by P30 CA22453-20 to the Karmanos Cancer Institute.
References
- Netto GJ, Humphrey PA. Molecular biologic aspects of human prostatic carcinoma. Am J Clin Pathol. 1994;102:S57–S64. [PubMed] [Google Scholar]
- Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- Cheville JC, Tindall D, Boelter C, Jenkins R, Lohse CM, Pankratz VS, Sebo TJ, Davis B, Blute ML. Metastatic prostate carcinoma to bone: clinical and pathologic features associated with cancer-specific survival. Cancer. 2002;95:1028–1036. doi: 10.1002/cncr.10788. [DOI] [PubMed] [Google Scholar]
- Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer. 2000;88:2989–2994. doi: 10.1002/1097-0142(20000615)88:12+<2989::aid-cncr14>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- Tu SM, Millikan RE, Mengistu B, Delpassand ES, Amato RJ, Pagliaro LC, Daliani D, Papandreou CN, Smith TL, Kim J, Podoloff DA, Logothetis CJ. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet. 2001;357:336–341. doi: 10.1016/S0140-6736(00)03639-4. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- Stearns ME, Wang M. Type IV collagenase (M(r) 72,000) expression in human prostate: benign and malignant tissue. Cancer Res. 1993;53:878–883. [PubMed] [Google Scholar]
- Festuccia C, Bologna M, Vicentini C, Tacconelli A, Miano R, Violini S, Mackay AR. Increased matrix metalloproteinase-9 secretion in short-term tissue cultures of prostatic tumor cells. Int J Cancer. 1996;69:386–393. doi: 10.1002/(SICI)1097-0215(19961021)69:5<386::AID-IJC6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Wood M, Fudge K, Mohler JL, Frost AR, Garcia F, Wang M, Stearns ME. In situ hybridization studies of metalloproteinases 2 and 9 and TIMP-1 and TIMP-2 expression in human prostate cancer. Clin Exp Metastasis. 1997;15:246–258. doi: 10.1023/a:1018421431388. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Shekarriz B, Nemeth JA, Dong Z, Cummings GD, Fridman R, Sakr W, Grignon DJ, Cher ML. Membrane type 1-matrix metalloproteinase (MT1-MMP) and MMP-2 immunolocalization in human prostate: change in cellular localization associated with high-grade prostatic intraepithelial neoplasia. Clin Cancer Res. 1999;5:4105–4110. [PubMed] [Google Scholar]
- Lokeshwar BL, Selzer MG, Block NL, Gunja-Smith Z. Secretion of matrix metalloproteinases and their inhibitors (tissue inhibitor of metalloproteinases) by human prostate in explant cultures: reduced tissue inhibitor of metalloproteinase secretion by malignant tissues. Cancer Res. 1993;53:4493–4498. [PubMed] [Google Scholar]
- Stearns M, Stearns ME. Evidence for increased activated metalloproteinase 2 (MMP-2a) expression associated with human prostate cancer progression. Oncol Res. 1996;8:69–75. [PubMed] [Google Scholar]
- Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Chrysovergis K, Yamada S, Birkedal-Hansen H. MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: a critical process in skeletal growth. J Cell Biol. 2003;163:661–671. doi: 10.1083/jcb.200307061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA. Altered fracture repair in the absence of MMP9. Development. 2003;130:4123–4133. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR, Henriksen K, Lenhard T, Foged NT, Werb Z, Delaisse JM. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol. 2000;151:879–889. doi: 10.1083/jcb.151.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blavier L, Delaisse JM. Matrix metalloproteinases are obligatory for the migration of preosteoclasts to the developing marrow cavity of primitive long bones. J Cell Sci. 1995;108:3649–3659. doi: 10.1242/jcs.108.12.3649. [DOI] [PubMed] [Google Scholar]
- Nemeth JA, Yousif R, Herzog M, Che M, Upadhyay J, Shekarriz B, Bhagat S, Mullins C, Fridman R, Cher ML. Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis. J Natl Cancer Inst. 2002;94:17–25. doi: 10.1093/jnci/94.1.17. [DOI] [PubMed] [Google Scholar]
- Weber MH, Lee J, Orr FW. The effect of Neovastat (AE-941) on an experimental metastatic bone tumor model. Int J Oncol. 2002;20:299–303. [PubMed] [Google Scholar]
- Winding B, NicAmhlaoibh R, Misander H, Hoegh-Andersen P, Andersen TL, Holst-Hansen C, Heegaard AM, Foged NT, Brunner N, Delaisse JM. Synthetic matrix metalloproteinase inhibitors inhibit growth of established breast cancer osteolytic lesions and prolong survival in mice. Clin Cancer Res. 2002;8:1932–1939. [PubMed] [Google Scholar]
- Lee J, Weber M, Mejia S, Bone E, Watson P, Orr W. A matrix metalloproteinase inhibitor, batimastat, retards the development of osteolytic bone metastases by MDA-MB-231 human breast cancer cells in Balb C nu/nu mice. Eur J Cancer. 2001;37:106–113. doi: 10.1016/s0959-8049(00)00363-4. [DOI] [PubMed] [Google Scholar]
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- Brown PD. Matrix metalloproteinase inhibitors: a novel class of anticancer agents. Adv Enzyme Regul. 1995;35:293–301. doi: 10.1016/0065-2571(94)00022-u. [DOI] [PubMed] [Google Scholar]
- Devy L, Hollender P, Munaut C, Colige A, Garnotel R, Foidart JM, Noel A, Jeannesson P. Matrix and serine protease expression during leukemic cell differentiation induced by aclacinomycin and all-trans-retinoic acid. Biochem Pharmacol. 2002;63:179–189. doi: 10.1016/s0006-2952(01)00848-6. [DOI] [PubMed] [Google Scholar]
- Sarin A, Adams DH, Henkart PA. Protease inhibitors selectively block T cell receptor-triggered programmed cell death in a murine T cell hybridoma and activated peripheral T cells. J Exp Med. 1993;178:1693–1700. doi: 10.1084/jem.178.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardy RE, Grobelny D, Foellmer HG, Fernandez LA. Inhibition of angiogenesis by the matrix metalloprotease inhibitor N-[2R-2-(hydroxamidocarbonymethyl)-4-methylpentanoyl)]-L-tryptophan methylamide. Cancer Res. 1994;54:4715–4718. [PubMed] [Google Scholar]
- Wolf M, Clark-Lewis I, Buri C, Langen H, Lis M, Mazzucchelli L. Cathepsin D specifically cleaves the chemokines macrophage inflammatory protein-1 alpha, macrophage inflammatory protein-1 beta, and SLC that are expressed in human breast cancer. Am J Pathol. 2003;162:1183–1190. doi: 10.1016/s0002-9440(10)63914-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth JA, Harb JF, Barroso U, Jr, He Z, Grignon DJ, Cher ML. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res. 1999;59:1987–1993. [PubMed] [Google Scholar]
- Dong Z, Nemeth JA, Cher ML, Palmer KC, Bright RC, Fridman R. Differential regulation of matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2 expression in co-cultures of prostate cancer and stromal cells. Int J Cancer. 2001;93:507–515. doi: 10.1002/ijc.1358. [DOI] [PubMed] [Google Scholar]
- Sanceau J, Truchet S, Bauvois B. Matrix metalloproteinase-9 silencing by RNA interference triggers the migratory-adhesive switch in Ewing’s sarcoma cells. J Biol Chem. 2003;278:36537–36546. doi: 10.1074/jbc.M304300200. [DOI] [PubMed] [Google Scholar]
- Okada Y, Gonoji Y, Naka K, Tomita K, Nakanishi I, Iwata K, Yamashita K, Hayakawa T. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells. Purification and activation of the precursor and enzymic properties. J Biol Chem. 1992;267:21712–21719. [PubMed] [Google Scholar]
- Knauper V, Smith B, Lopez-Otin C, Murphy G. Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13). Eur J Biochem. 1997;248:369–373. doi: 10.1111/j.1432-1033.1997.00369.x. [DOI] [PubMed] [Google Scholar]
- von Bredow DC, Cress AE, Howard EW, Bowden GT, Nagle RB. Activation of gelatinase-tissue-inhibitors-of-metalloproteinase complexes by matrilysin. Biochem J. 1998;331:965–972. doi: 10.1042/bj3310965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YG, Xiao AZ, Newcomer RG, Park HI, Kang T, Chung LW, Swanson MG, Zhau HE, Kurhanewicz J, Sang QX. Activation of pro-gelatinase B by endometase/matrilysin-2 promotes invasion of human prostate cancer cells. J Biol Chem. 2003;278:15056–15064. doi: 10.1074/jbc.M210975200. [DOI] [PubMed] [Google Scholar]
- Fridman R, Toth M, Pena D, Mobashery S. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2). Cancer Res. 1995;55:2548–2555. [PubMed] [Google Scholar]
- Charhon SA, Chapuy MC, Delvin EE, Valentin-Opran A, Edouard CM, Meunier PJ. Histomorphometric analysis of sclerotic bone metastases from prostatic carcinoma special reference to osteomalacia. Cancer. 1983;51:918–924. doi: 10.1002/1097-0142(19830301)51:5<918::aid-cncr2820510526>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Percival RC, Urwin GH, Harris S, Yates AJ, Williams JL, Beneton M, Kanis JA. Biochemical and histological evidence that carcinoma of the prostate is associated with increased bone resorption. Eur J Surg Oncol. 1987;13:41–49. [PubMed] [Google Scholar]
- Garnero P, Buchs N, Zekri J, Rizzoli R, Coleman RE, Delmas PD. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br J Cancer. 2000;82:858–864. doi: 10.1054/bjoc.1999.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T. Cellular and molecular mechanisms of breast and prostate cancer metastasis to bone. Eur J Cancer. 1998;34:240–245. doi: 10.1016/s0959-8049(97)10132-0. [DOI] [PubMed] [Google Scholar]
- Soos G, Haas GP, Wang CY, Jones RF. Differential gene expression in human prostate cancer cells adapted to growth in bone in Beige mice. Urol Oncol. 2003;21:15–19. doi: 10.1016/s1078-1439(02)00202-8. [DOI] [PubMed] [Google Scholar]
- Bastide C, Bagnis C, Mannoni P, Hassoun J, Bladou F. A Nod Scid mouse model to study human prostate cancer. Prostate Cancer Prostatic Dis. 2002;5:311–315. doi: 10.1038/sj.pcan.4500606. [DOI] [PubMed] [Google Scholar]
- Chambers TJ, Fuller K. Bone cells predispose bone surfaces to resorption by exposure of mineral to osteoclastic contact. J Cell Sci. 1985;76:155–165. doi: 10.1242/jcs.76.1.155. [DOI] [PubMed] [Google Scholar]
- Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, Lee-Rykaczewski E, Coleman L, Rieman D, Barthlow R, Hastings G, Gowen M. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem. 1996;271:12511–12516. doi: 10.1074/jbc.271.21.12511. [DOI] [PubMed] [Google Scholar]
- Reponen P, Sahlberg C, Munaut C, Thesleff I, Tryggvason K. High expression of 92-kD type IV collagenase (gelatinase B) in the osteoclast lineage during mouse development. J Cell Biol. 1994;124:1091–1102. doi: 10.1083/jcb.124.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka K, Nemoto K, Tezuka Y, Sato T, Ikeda Y, Kobori M, Kawashima H, Eguchi H, Hakeda Y, Kumegawa M. Identification of matrix metalloproteinase 9 in rabbit osteoclasts. J Biol Chem. 1994;269:15006–15009. [PubMed] [Google Scholar]
- Everts V, Delaisse JM, Korper W, Beertsen W. Cysteine proteinases and matrix metalloproteinases play distinct roles in the subosteoclastic resorption zone. J Bone Miner Res. 1998;13:1420–1430. doi: 10.1359/jbmr.1998.13.9.1420. [DOI] [PubMed] [Google Scholar]
- Yu X, Huang Y, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res. 2003;18:1404–1418. doi: 10.1359/jbmr.2003.18.8.1404. [DOI] [PubMed] [Google Scholar]
- Lakka SS, Gondi CS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Rao JS. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23:4681–4689. doi: 10.1038/sj.onc.1207616. [DOI] [PubMed] [Google Scholar]
- Matrisian LM, Sledge GW, Mohla S. Extracellular proteolysis and cancer: meeting summary and future directions. Cancer Res. 2003;63:6105–6109. [PubMed] [Google Scholar]
- Laxman B, Hall DE, Bhojani MS, Hamstra DA, Chenevert TL, Ross BD, Rehemtulla A. Noninvasive real-time imaging of apoptosis. Proc Natl Acad Sci USA. 2002;99:16551–16555. doi: 10.1073/pnas.252644499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]