Abstract
Cutaneous leishmaniasis (CL), caused by the intracellular protozoan Leishmania major, is characterized by lesion formation and ulceration at the site of infection. The mechanism of ulcer formation during CL is not fully understood. The expression of Fas and FasL and the levels of apoptosis in skin biopsies and in restimulated blood mononuclear cells from patients with 1 to 7 months of L. major-induced CL were analyzed using immunohistochemistry and fluorescence-activated cell sorting analysis. The levels of soluble Fas and FasL were also analyzed by enzyme-linked immunosorbent assay. A substantial number of apoptotic keratinocytes were observed mainly in the superficial epidermis of morphologically active and healing CL skin samples. Fas expression was increased on epidermis in active CL, whereas Fas expression was similar in healing and healthy epidermis. FasL-expressing macrophages and T cells were found in subepidermal infiltrate, mainly in active disease. When CL peripheral blood mononuclear cells were restimulated with L. major, Fas was up-regulated on effector T cells, and high levels of sFasL were secreted. Supernatants from restimulated cultures induced apoptosis in human keratinocytes (HaCaT), possibly through Fas/FasL interactions. Our results indicate that FasL-expressing effector T cells and macrophages may act to induce apoptosis and ulcer formation in Fas-expressing keratinocytes during L. major infection.
Cutaneous leishmaniasis (CL) in Iran is caused by the intracellular protozoan Leishmania major (in rural areas) and Leishmania tropica (mainly in urban areas) and is spread by sand flies. CL is characterized by a lesion at the site of infection that usually ulcerates and typically heals within a year of onset. Upon infection, L. major promastigotes immediately infect histiocytes and neutrophils and transform into amastigotes. Infection induces a local inflammatory reaction in the subcutis with histamine secretion and subsequent infiltration of neutrophils and monocytes, resulting in skin induration and lesion formation. Ulceration appears a few weeks after lesion development. During early ulceration interleukin (IL-4), IL-10, and interferon (IFN)-γ are reported to be expressed in the central part of the ulcer,1 whereas the epidermis surrounding the ulcer is infiltrated with Langerhan’s cells. Keratinocytes surrounding the ulcer up-regulate ICAM-1 and HLA-DR. Several histological profiles of ulcers have been described, possibly reflecting different stages of healing. Inflammatory cells surrounding the lesion or ulcer typically consist of T cells (CD4+ and CD8+ cells), B cells (mainly plasma cells), and macrophages.1 Focal macrophage granulomas, containing infected and destructed macrophages as well as extracellular parasites and necrotic material, may surround the ulcer or may be found in the midst of nonorganized inflammation or in the absence of other inflammatory processes. Local high expression of IFN-γ, IL-12, and tumor necrosis factor-α in the lesion has been correlated to healing (Th1-type response) and IL-4 and IL-10 to chronic infection (Th2-type response).1 However, the mechanisms of ulcer formation during CL are not fully understood.
Alterations of receptor-mediated apoptosis have been described in several parasitic diseases2–4mainly as a direct consequence of parasite pathogenic mechanisms.5,6 One important receptor-mediated apoptotic pathway is the Fas/FasL pathway. Fas is a member of the tumor necrosis factor receptor superfamily7 and ubiquitously expressed on most cells in the body. On binding of soluble8 or membrane-bound FasL,9 most activated Fas-expressing cells undergo apoptosis. T cells, although they express Fas ubiquitously, need to be activated to become susceptible to Fas-mediated apoptosis.10,11 Fas-expressing keratinocytes are sensitive to Fas/FasL-mediated apoptosis.12
Intact Fas/FasL signaling has been proposed to be important for healing in mouse models of L. major.13–15 Mouse strains resistant to L. major (C57BL/6) show early up-regulation of Fas and high levels of activation-induced lymphocyte apoptosis on L. major infection. Lpr mutant (Fas-defective) mice are more susceptible compared to wild-type mice to L. major infection.13,14 Similarly, gld/gld (FasL-deficient mice) are more susceptible to L. major but eradicate infection upon sFasL treatment.13 In the context of apoptosis during CL, it was suggested that Leishmania delay spontaneous apoptosis in infected neutrophils for 2 to 3 days (both in mice and man) during the first phase of infection, allowing parasites to enter resting macrophages upon neutrophil phagocytosis.6 In man, up to 30% apoptotic T cells (both CD4+- and CD8+-positive) were described in L. braziliensis-induced active CL and the level of apoptosis was suggested to decrease during healing.16 However, low levels of FasL-expressing cells have been reported in skin biopsies from L. aethiopica-induced local and diffuse CL.17
We propose that ulceration during L. major-induced CL is caused by FasL-expressing Leishmania-primed T cells and macrophages, through induction of apoptosis in Fas-expressing keratinocytes. To test this hypothesis, we investigated skin, plasma/serum, and peripheral blood mononuclear cells (PBMCs) from a group of CL patients with 1 to 7 months history of CL. Furthermore, in vitro experiments were performed to modulate apoptosis of keratinocytes.
Materials and Methods
Samples
Plasma, PBMCs, and skin biopsies were donated by CL patients and healthy Iranian volunteers. CL was diagnosed clinically and parasitologically by direct smears and/or culture. Some of the isolates were cultured and identified as L. major by isoenzyme technique and monoclonal antibodies. The CL patients were all male military recruits who moved from nonendemic areas to L. major hyperendemic foci before the onset of disease. CL patients had a 1 to 7 months history of ulceration. Informed consent was obtained from all sample donors for the usage of biological material. The controls (14 male and 1 female) were selected from nonendemic areas and had no signs of exposure to Leishmania antigens (no response to leishmanin skin test antigen) and were otherwise healthy. This study has received ethical approval from both Swedish and Iranian ethical committees. Biopsies were taken under sterile conditions and in local anesthesia from the indurations lining the ulcers in eight CL patients. The biopsies were split and either frozen in OCT (TissueTek, Zoeterwoude, Netherlands) or fixed in 4% formalin and paraffin embedded. Control skin was obtained from three healthy Iranian volunteers undergoing cosmetic surgery and processed in the same way as the biopsies from CL patients. Venous blood from 15 healthy volunteers and 19 CL patients was obtained and plasma and PBMCs were prepared as previously described.18
Giemsa Staining of Embedded Skin Biopsies
The morphology of the lesions was evaluated in Giemsa-stained sections and designated as active, active to healing, or healing depending on the presence of inflammatory cells, epidermal hyperplasia, and fibrotic tissue.
Immunohistochemical Staining of Paraffin-Embedded Skin Biopsies
Paraffin-embedded skin biopsies were sectioned in 5-μm sections not more than a week before immunohistochemical stainings. Deparaffination and rehydration were performed as previously described.19 Sections were incubated with mouse anti-Fas monoclonals (Dakopatts, Stockholm, Sweden) at 10 μg/ml, mouse anti-FasL monoclonals (20 μg/ml) (BD, Stockholm, Sweden), or isotype controls (20 μg/ml) (Dakopatts) for 15 minutes at room temperature. Streptavidin-avidin enhancement was performed according to the manufacturer’s instruction (Dakopatts). The antigens were visualized with diaminobenzidine (Vector Laboratories Inc., Burlingame, CA) and hematoxylin (Sigma-Aldrich, Stockholm, Sweden) counterstaining was performed. Three sections from the same sample were processed on two different occasions with similar results.
Double Staining of FasL and CD3 or CD68
Frozen biopsies were sectioned in 12-μm-thick sections, briefly dried, and fixed in acetone. Sections from three donors displaying active lesion properties (CL11, CL30, and CL31) were processed in duplicates with similar results. The sections were incubated in protein block (DAKO) for 10 minutes, followed by a 1-hour incubation with the primary antibody FasL (BD) 5 μl/ml, rabbit anti-CD3 (AH Diagnostics, Stockholm, Sweden) 1:300, followed by a 1-hour incubation with Cy3-conjugated goat anti-mouse (Jackson ImmunoResearch, West Grove, PA) at 1:400 and Alexa-conjugated goat anti-rabbit (Molecular Probes, Eugene, OR) at 1:250. Isotype controls were prepared in parallel. Fluorescein isothiocyanate (FITC)-conjugated mouse anti-CD68 (DAKO) was added (1:20) at room temperature for 1 hour after the secondary staining of FasL had been performed. The sections were washed, mounted in 20% DABCO (Sigma) in glycerol, and analyzed with a ×40 oil immersion lens in an Nikon Optiphot 2 UV microscope.
Terminal dUTP Nick-End Labeling (TUNEL) Staining and Visualization of Caspase Activity in Paraffin-Embedded Skin
Apoptosis was assessed through visualizing fragmented DNA using the TUNEL kit according to the manufacturer’s instructions (Roche, Penzberg, Germany). Positive controls were obtained by addition of DNase (Life Technologies, Sweden) and negative controls by omission of the TdT enzyme. Caspase activity was visualized using M30 CytoDeath (Roche), which detects caspase-specific cleavage of cytokeratin 18, and visualized with diaminobenzidine. Cytokeratins, in particular cytokeratin 18, are affected in very early events of apoptosis. Sections were stained with isotype controls in parallel to M30 as negative controls.
Evaluation of Immunohistochemistry
The sections were analyzed using a Nikon Optiphot 2 microscope. Fas was analyzed using a ×6.5 or ×10 lens, whereas FasL, M30, and TUNEL were analyzed using ×40 or ×100 oil immersion lens. Expression of Fas was assessed using an arbitrary scale from − to +++. All sections were assessed blind, and independent of morphological designation. Levels of FasL-expressing cells, apoptotic cells, and caspase activity were quantified by counting the number of positive cells in 9 to 13 fields at ×40 magnification. The number of cells per mm2 was then calculated from the known diameter of the field at ×40 magnification. One experiment representative of duplicates are shown. Differences in numbers in between duplicates were typically less than 15%.
Detection of Membrane-Bound Fas, FasL, and Apoptosis in PBMCs
Approximately 0.5 × 106 cells were incubated on ice with FITC-conjugated mouse anti-human Fas monoclonal IgG (Dakopatts), phycoerythrin (PE)-conjugated mouse anti-human FasL monoclonal IgG (Alexis, KeLab, Gothenburg, Sweden; and RnD, Amersham, UK), FITC- and PE-conjugated mouse isotype control IgG (Dakopatts) in fluorescence-activated cell sorting buffer [phosphate-buffered saline (PBS) containing 2% bovine serum albumin] for 15 minutes on ice. The following antibodies were used to determine different cell subsets: CD56-PE or APC, CD3-APC, CD4-FITC or PE, CD8-Cy3 or PE, CD19-FITC or PE (BD). The Annexin V assay was performed according to the manufacturer’s instructions on fresh, untreated, or L. major-stimulated PBMCs. Nonviable cells were excluded through scattering properties on forward/side scatter. Apoptosis was defined as Annexin V-positive, propidium iodide-negative cells.
Stimulation of PBMCs with Live L. major Promastigotes
Thawed PBMCs were plated at 1 × 106 cells/ml. L. major was propagated as previously described.20 Promastigotes were used at stationary growth phase, washed three times in sterile PBS, and resuspended in PBS. Promastigotes were added at 1 × 106 cells/ml (cell:parasite ratio, 1:1). Killed L. major (heat killed) were used in parallel to live promastigotes during the first set of experiments. Because live L. major proved to be a more effective inducer of Fas expression, apoptosis, and sFasL in CL PBMCs, live promastigotes were used in all donors tested. As a control, an irrelevant antigen, HIV-2-inactivated virus (kindly provided by Dr. Charlotta Nilsson, Smittskyddinstitutet, Karolinska Institute, Sweden), was used to stimulate CL PBMCs at 5 μg/ml in two donors and two controls. The HIV-2 control antigen did not induce enhanced expression of Fas, FasL, or apoptosis in these cultures (results not shown). The PBMCs were stimulated for 5 days. Supernatants were collected after centrifugation at 400 × g for 10 minutes and immediately frozen at −70°C.
Measurement of sFas and sFasL in Plasma/Serum or Culture Supernatants
Levels of sFas and sFasL were assessed in duplicates in serum/plasma and supernatants from stimulated cultures using commercial enzyme-linked immunosorbent assays (sFas: R&D, Amersham, UK; sFasL: Nordic BioSite, Täby, Sweden).
Induction of Apoptosis in Human Keratinocytes
HaCat, immortalized human keratinocytes,21 kindly provided by Prof. Fusenig, Heidelberg, Germany, were plated on sterile culture slides (Nunc, Nalgene, Hereford, UK) and incubated at 37°C until confluence was reached (4 to 5 days). Apoptosis was assessed after 40 hours of incubation with 1 μg/ml of Fas-activating monoclonal22 (CH11; MBL, Nagoya, Japan23,24) or supernatants obtained from PBMCs (diluted 1:4 in Dulbecco’s modified Eagle’s medium). In an attempt to block Fas-mediated apoptosis of keratinocytes induced by supernatants from L. major-stimulated CL PBMCs, the Fas-blocking monoclonal antibody ZB4 (MBL) was added to the cultures at 1 to 2 μg/ml.24 The samples were washed twice in Annexin V binding buffer and incubated in the dark for 25 minutes with Annexin V/propidium iodide. After incubation, the cells were fixed in 1% paraformaldehyde (Sigma) for 10 minutes at room temperature. The cells were mounted with anti-fading solution (Vector) and immediately analyzed.
Statistical Analysis
Mann-Whitney signed or unsigned rank test, the Wilcoxon signed rank test, or linear regression test using Statview 2.3 was applied for statistical analysis.
Results
Morphology of Skin Biopsies Investigated
All biopsies investigated were taken at the periphery of apparently active lesions. Morphology was assessed in Giemsa-stained sections under the supervision of a consultant of dermatohistopathology. In all eight lesions epidermal hyperplasia was noted and in one of these there was in addition central necrosis and ulceration (CL11) (Table 1). Four lesions were defined as active because they contained massive subepidermal, perivascular infiltration of mononuclear inflammatory cells, mainly histiocytes and plasma cells but also lymphocytic cells. In samples with active lesion, no fibrotic tissue was noted. Two lesions were designated active/healing. In these samples, a clear gradient was noted in the biopsies, in which the inflammatory reaction present in the central part of the lesion confined with fibrotic scar tissue and healthy skin in the periphery of the biopsy. Two lesions were designated healing and consisted of scar tissue and sparse mononuclear inflammation. No granuloma was noted in the lesions and in addition no L. major amastigote was found in these biopsies (Table 1). However all biopsies were from patients with CL confirmed by the demonstration of Leishmania by smear or culture.
Table 1.
Clinical Background of Biopsies Collected and Morphological Assessment of Biopsies
| Sample | No. of ulcers | Duration of symptom | Morphology | Classification |
|---|---|---|---|---|
| CL11 | 2 | 1.5 months | Epidermal hyperplasia. Ulceration. Lymphocytic perivascular infiltration. Mainly histiocytes | Active |
| CL31 | 8 | 1 month | Small biopsy. Epidermal hyperplasia. Mainly subepidermal inflammation of lymphocytoid small cells. | Active |
| CL30 | 1 | Not known | Epidermal hyperplasia. Massive infiltration of lymphocytoid small cells. | Active |
| CL9 | 2 | 1 month | Epidermal hyperplasia. Lymphocytic perivascular infiltration. Mainly histiocytes and plasma cells. | Active |
| CL25 | 1 | 2 to 3 months | Epidermal hyperplasia. Component of scar tissue. Scanty lymphocytoid small cells, mainly subepidermal. | Active-healing |
| CL26 | 1 | 7 months | Epidermal hyperplasia. Inflammatory diffuse infiltration, mainly histiocytes. | Active-healing |
| CL1 | 1 | 2 to 3 months | Epidermal hyperplasia. Scarring and fibrosis. Scantly perivascular infiltration of inflammatory cells. | Healing |
| CL8 | 2 | 2 months | Epidermal hyperplasia. Scarring and fibrosis. Scantly inflammatory areas. | Healing |
The morphological assessment was performed blindly on Giemsa-stained sections. Hypertrophic epidermis and varying levels of subepidermal inflammation was noted in all CL biopsies. Scar formation, in terms of subepidermal fibrotic tissue and dampened inflammatory reactions, was noted in ulcers with a history of more than 2 to 3 months.
Fas Expression in Hypertrophic Epidermis
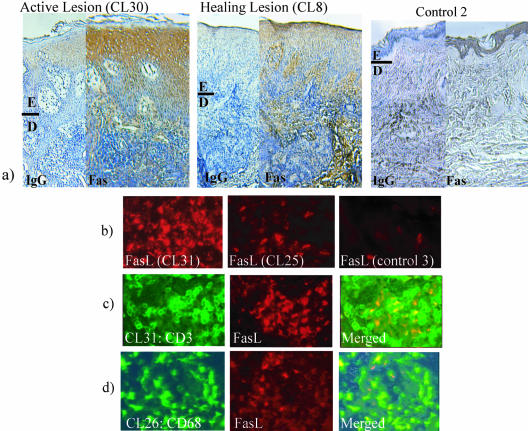
In the epidermis surrounding active lesions, high levels of Fas were expressed mainly in the superficial hypertrophic epidermis, whereas Fas was only slightly expressed or absent in the basal layer of the epidermis, close to the basal membrane (Figure 1a and Table 2). In healing lesions, the expression of Fas was similar to healthy skin throughout the epidermis (Figure 1a and Table 2). Fas was evenly expressed at a low level in healthy, normal epidermis (Figure 1a and Table 2). Low or no unspecific binding was noted in healthy or CL skin sections incubated with isotype control antibodies for primary anti-Fas or anti-FasL antibodies (Figure 1a).
Figure 1.
Visualization of Fas and FasL in skin biopsies from CL. a: Fas expression in epidermis was visualized using diaminobenzidine (brown) and counterstained with hematoxylin. Sections were incubated with an isotype control (IgG) to detect background or with an anti-Fas monoclonal (clone DX2). In active CL Fas is highly expressed on the keratinocytes in superficial epidermis. In healing CL less and more even expression of Fas was noted. As a comparison, the keratinocyte expression of Fas in healthy skin was evaluated and showed a similar pattern to healing CL. Both active and healing CL show hypertropic epidermis (E) compared to healthy skin. Dermis (D) contains inflammatory cells and fibrosis in the active and healing lesion. b: FasL was visualized using Cy3 (red). FasL-expressing cells were mainly present in aggregations of up to 30 cells/group. The FasL cells were only present in the dermis, both adjacent to the epidermis and in deep dermis. A few FasL-positive cells were found in one of the controls (control 3). More FasL-positive cells were found in active lesions compared to healing lesions. Virtually no FasL-positive cells were present in healthy skin. c: T cells (green) were visualized using rabbit anti-CD3 in combination with goat anti-rabbit (Alexa, green). FasL-positive cells (red) were found in areas of both CD3-positive and -negative cells (FasL+CD3+ area shown). d: Macrophages (green) were visualized by incubating the sections with a FITC-conjugated mouse anti-CD68 after FasL staining (red) had been performed. FasL-positive cells were found in both CD68+ and CD68− cells (FasL+CD68+ area shown). Original magnifications: ×10 (a); ×40 (b).
Table 2.
Expression of Fas and FasL as Well as Occurrence of Apoptosis in Skin Biopsies from CL
| Sample | Morphology | Fas (intensity of staining)
|
FasL (cells/mm2) connective tissue | Apoptosis, TUNEL (M30) (cells/mm2)
|
||||
|---|---|---|---|---|---|---|---|---|
| Superficial epidermis | Basal epidermis | Dermis | Connective tissue | Epidermis | Connective tissue | |||
| CL11 | Active | ++ | ++ | + | ++ | 18 | 20 (9.2) | 10 (13) |
| CL31 | Active | +++ | − | + | 9.5 | 16 (9.3) | 10 (13) | |
| CL30 | Active | +++ | + | + | + | 5.3 | 6 (27) | 6.6 (7) |
| CL9 | Active | ++ | − | + | + | 5.3 | 8.5 (2.2) | 13 (1.3) |
| CL25 | Active-healing | +++ | + | + | + | 4 | 10 (nd) | 5.5 (nd) |
| CL26 | Active-healing | + | − | − | + | 1 | 5 (5.1) | 2 (4.8) |
| CL1 | Healing | + | + | + | ++ | 1.3 | 13 (1.6) | 3.6 (5.5) |
| CL8 | Healing | + | + | + | + | 1.4 | 2.5 (1.8) | 1.2 (1.3) |
| Control 1 | Healthy skin | + | + | ++ | ++ | 0 | 1 (0.6) | 0.01 (0.7) |
| Control 2 | Healthy skin | + | + | + | + | 0 | 1 (0.5) | 3.29 (2.6) |
| Control 3 | Scar | ++ | + | + | + | 0.1 | 1.5 (nd) | 1.4 (nd) |
Expression of Fas was assessed using an arbitrary scale from − to +++. FasL, M30, and TUNEL results were quantified by counting the number of FasL-expressing cells in 9 to 13 fields at ×40 magnification. The number of cells per mm2 was then calculated from the known diameter of the field at ×40 magnification.
High expression of Fas was consistently noted in the peripheral part of epidermis in active CL. FasL-expressing cells were present in subepidermal tissue in all CL biopsies, but at higher density in active versus healing disease. Apoptotic cells (both measured as TUNEL-positive and as M30-positive cells) were found both in epidermis and subepidermally in all patients. However more apoptotic cells were noted in subepidermal tissue in active versus healing disease. All sections were assessed blindly and all four analyses were performed independently from each other and morphological assessment. One experiment representative of duplicates are shown in the case of Fas, FasL, and TUNEL. M30 was repeated in three donors with similar results.
nd, not done.
FasL-Expressing Cells in Active CL Compared to Healing CL or Control Biopsies
FasL is normally not expressed on keratinocytes and no FasL-expressing keratinocytes were present in the epidermis of the CL or healthy skin investigated. However, FasL-expressing inflammatory cells were found in subepidermal CL tissue (Table 2 and Figure 1b). More FasL-expressing cells were found in inflammatory areas adjacent to active lesions (mean, 9.4 FasL+cells/mm2) compared to healthy skin (0 FasL+ cells/mm2) or healing lesions (1.9 FasL+ cells/mm2) (Table 2 and Figure 1b). Typically, groups of up to 30 FasL-expressing cells were found in dermis, both adjacent to the epidermis and in deep dermis. At the site of epidermal breaking in one of the active lesions (CL11), epidermis was infiltrated with FasL-expressing cells. In the same area, high expression of Fas and many apoptotic cells were noted (Table 2).
Both T Cells and Macrophages Expressed FasL
In all three donors investigated, FasL-expressing cells were shown to be both of T-cell origin (Figure 1c) and macrophage origin (Figure 1d). When staining for FasL-CD3 and FasL-CD68, areas of CD3+FasL− and FasL+CD3− and CD68+FasL−, and FasL+CD68− cells were present in all biopsies investigated, strengthening the result that several cell types can express FasL during CL infection.
Apoptotic Cells in the Hypertrophic Epidermis and Connective Tissue in Active and Healing CL
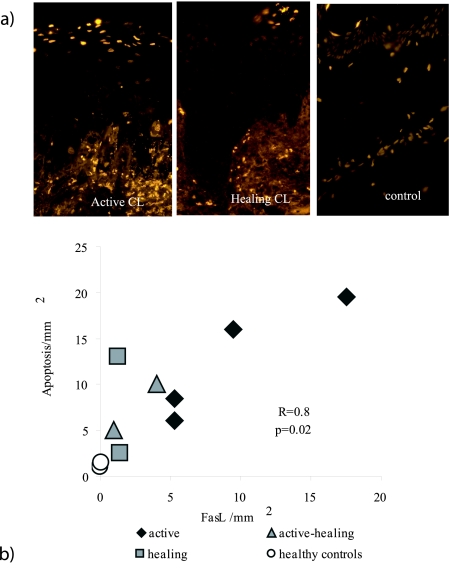
Similar amounts of apoptotic cells were visualized in the epidermis of active (Figure 2a) and healing (Figure 2a) skin biopsies (Table 2). Compared to healthy skin (Figure 2a and Table 2) there was a clear increase in the number of apoptotic keratinocytes during CL. Very few or no apoptotic cells were present in subepidermal tissue in normal skin. Interestingly, more apoptotic cells were found in the inflammatory areas surrounding active lesions (9.9) compared to active/healing or healing lesions (3.1). This may indicate that the inflammatory process is more active during active disease. In the inflammatory areas, the occurrence of both FasL and apoptotic cells was higher in active lesions compared to healing or healthy controls (Table 2). Interestingly, the numbers of FasL-expressing macrophages and T cells in the dermis correlated to the levels of apoptotic cells in the epidermis in the CL biopsies (r = 0.8, P = 0.02, n = 8). The TUNEL results were verified by staining for a product of caspase activity, cytokeratin 18. Although lower levels of caspase activity was shown as compared to number of TUNEL-positive cells, biopsies from active lesions had more positive cells as compared to healing or control sections (Table 2).
Figure 2.
Apoptotic cells were present in epidermis and correlated with the number of FasL-expressing cells found in dermis. a: Apoptosis was visualized by staining for TUNEL-positive cells (TMR) in paraffin-embedded sections. Apoptotic cells are seen as bright yellow. More apoptosis was measured in active lesions as compared to healthy or controls. Nonapoptotic nuclei are seen as orange-brown. b: The occurrence of FasL-expressing cells in inflammatory areas in dermis correlated with the occurrence of apoptotic cells in epidermis in CL biopsies (r = 0.8, P < 0.02, n = 8).
Alteration of the Fas/FasL Pathway Are Not Reflected in Serum and PBMCs during CL
The levels of sFas and sFasL in serum were similar in CL (n = 19) and healthy controls (n = 15, Table 3). Furthermore, there was no difference in the overall expression of Fas or FasL on freshly obtained PBMCs from CL or controls (Table 4). However, there was a significant reduction of Fas expression on CD4+ cells in CL (MFI 12 versus 22, P < 0.02). The levels of Fas and FasL were similar in CD8+- and CD56+-positive cells. CL CD19+ cells expressed less FasL compared to controls (5 versus 7.6, P = 0.02; Table 4). The number of CD4+ cells (44% and 49%), CD8+ cells (28% and 29%), CD56+cells (19% versus 22%) and CD19+ cells (5.1 versus 3.8) were similar in CL and controls.
Table 3.
Levels of sFas and sFasL in Serum and Expression of Fas/FasL on PBMCs during CL
| Sample | Median sFas (pg/ml) (IQR) | Median sFasL (pg/ml) (IQR) | |
|---|---|---|---|
| Serum | CL | 4818 (1316) | 128 (46) |
| Control | 5728 (2192) | 90 (43) |
Serum levels of sFas and sFasL remain normal during CL (controls, n = 15; CL = 19).
Table 4.
Levels of sFas and sFasL in Serum and Expression of Fas/FasL on PBMCs during CL
| Sample | % Cells | Median Fas MFI (IQR) | Median FasL MFI (IQR) | % Apoptosis (IQR) | |
|---|---|---|---|---|---|
| Total PBMC | CL | 12 (3.6) | 5.6 (4.3) | 13* (5.8) | |
| Control | 16 (9.4) | 6.6 (1.8) | 10* (2.0) | ||
| CD19 | CL | 5.1 (2.8) | 7 (3.1) | 5.0* (2.4) | |
| Control | 3.8 (3.0) | 8.8 (6.6) | 7.6* (3.2) | ||
| CD8 | CL | 28 (8.6) | 12 (2.2) | 9.9 (7.1) | |
| Control | 29 (8.8) | 13 (10) | 8.2 (5.2) | ||
| CD4 | CL | 44 (4.0) | 12* (3.7) | 6.0 (5.8) | |
| Control | 49 (5.8) | 22* (11) | 5.8 (0.9) | ||
| CD56 | CL | 19 (6.2) | 11 (3.3) | 6.3 (10) | |
| Control | 22 (11) | 12 (8.8) | 7.6 (2.8) |
Expression of Fas and FasL in total and different subtypes of PBMCs in CL (n = 9 except in apoptosis where n = 7) and controls (n = 7, except in apoptosis where n = 6).
There was a significant reduction of Fas expression on CD4+ cells in CL, although the number of CD4+ cells in patients and controls were similar.
P = 0.02.
Fas/FasL Expression, Release, and Apoptosis in PBMCs Restimulated with Live L. major
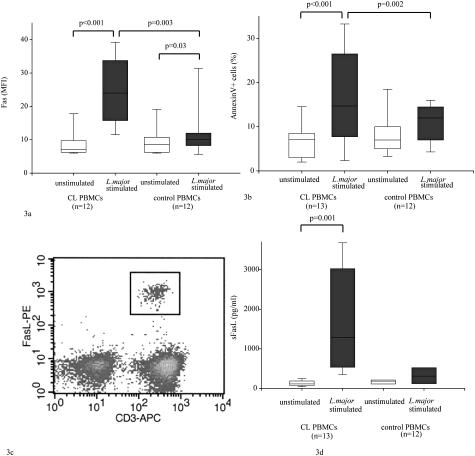
The expression of Fas was up-regulated in CL PBMC specimens 3.4 times upon L. major stimulation after 5 days in culture [median MFI, 7.0 (IQR 3) versus 24 (IQR 17); P < 0.001, n = 12]. Control PBMCs slightly up-regulated Fas on L. major stimulation [median MFI, 8.5 (IQR 4) versus 10 (IQR 3.5); P < 0.03, n = 12]. Unstimulated CL and control PBMCs expressed similar levels of Fas (median MFI, 7.0 versus 8.5; Figure 3a). CL PBMCs up-regulated Fas significantly more than control PBMCs on L. major stimulation (P < 0.003).
Figure 3.
Fas/FasL expression, release, and apoptosis in PBMCs restimulated with live L. major. PBMCs were restimulated 1:1 with L. major promastigotes for 5 days. Fas expression and levels of apoptosis were assessed using fluorescence-activated cell sorting. White boxes: Unstimulated cultures. Gray boxes: L. major-stimulated cultures. The horizontal and vertical lines in each box represent the median and the range (95% confidence interval), respectively. Differences were calculated by the signed or unsigned Mann-Whitney test. a: Fas expression on PBMCs after L. major restimulation. In CL (n = 12), PBMCs stimulated with L. major up-regulate Fas compared to healthy controls (n = 12) and unstimulated PBMCs. b: Levels of apoptotic cells in L. major-stimulated PBMCs. Apoptosis was assessed using Annexin V/propidium iodide stainings and Annexin V-positive, propidium iodide-negative cells were designated as apoptotic cells. In CL PBMCs stimulated with L. major (n = 13), more apoptosis was measured compared to control PBMCs (n = 12). c: The origin of sFasL was assessed in one donor. Intracellular staining of Fas revealed 2% FasL-expressing cells in L. major-stimulated CL PBMCs. All FasL+ cells were T cells, both CD8+ and CD4+. d: sFasL is secreted by L. major-stimulated CL PBMCs. Levels of sFasL were assessed using enzyme-linked immunosorbent assay. In CL PBMCs (n = 12) stimulated with L. major, there was a significant increase in the levels of sFasL compared to unstimulated cultures or L. major-stimulated control CL (n = 6, P < 0.01).
To investigate if Fas up-regulation was a feature of all cell types in PBMCs in the presence of L. major, different subtypes of PBMCs were analyzed for Fas expression. On L. major restimulation of CL PBMCs, Fas was up-regulated 2.7 times in CD8+ and 3.2 times in CD4+ cells compared to unstimulated CL PBMCs [median MFI, 17.5 (IQR 12.5) versus 6.5 (IQR 3.5), P = 0.008; and median, MFI 26 (IQR 22) versus 8 (IQR 4.5), P = 0.008, n = 8]. No up-regulation of Fas on CD8+ was measured in control PBMCs on stimulation [MFI, 7 (IQR 1.0) versus 8 (IQR 1.0); n = 4]. Fas expression was up-regulated 4.2 times on CL CD56+ (n = 4) and 2 times on control CD56+ (n = 4) on L. major stimulation compared to unstimulated controls. This difference was however not significant. More apoptosis was measured in L. major-stimulated CL PBMCs compared to unstimulated CL PBMCs [17% (IQR 5.2) versus 7% (IQR 16); P < 0.001, n = 13] and stimulated control PBMCs [17% (IQR 5.2) versus 12% (IQR 7); P < 0.004, n = 13 and 12, Figure 3b]. Furthermore, in stimulated CL PBMCs (n = 10), the expression of Fas and the levels of apoptosis were correlated (n = 10, r = 0.6, P < 0.05).
FasL could only be measured on cells of one of four L. major-restimulated CL PBMCs on intracellular staining of FasL (Figure 3c and Figure 4e). In this sample all FasL-expressing cells (2% of T cells) were CD3+, both CD8+ dim and CD4+. No FasL could be measured on L. major-stimulated control PBMCs (n = 4). In live L. major-stimulated CL PBMCs, the levels of sFasL in supernatants were more than 10 times higher compared to unstimulated CL PBMCs [median, 1282 (IQR 2148) versus 114 (IQR 106); P = 0.001, n = 12, Figure 3d, individual differences between stimulated and unstimulated supernatants 3 to 20 times]. In control PBMCs the levels of sFasL were not significantly higher compared to unstimulated cultures [median, 310 (IQR 353) versus 184 (IQR 80) pg/ml; n = 6]. The levels of sFas in supernatants did not differ in stimulated or unstimulated CL PBMCs (median, 2043 versus 1900 pg/ml; n = 6) or stimulated or unstimulated control PBMCs (median, 1595 versus 1436 pg/ml; n = 6).
Figure 4.
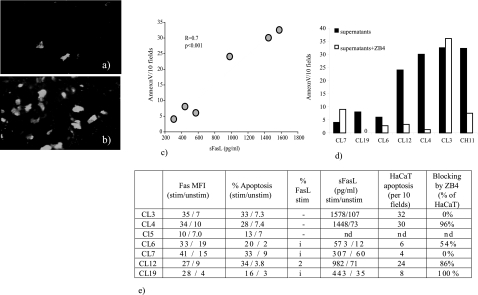
sFasL secreted by L. major-stimulated CL PBMCs induce apoptosis in Fas-expressing keratinocytes. a and b: Supernatants from L. major-stimulated CL PBMCs induced apoptosis in human keratinocytes. Keratinocytes (HaCaT) were incubated with medium alone (a) or supernatants from stimulated CL PBMCs for 40 hours. Annexin V/propidium iodide stainings were performed to assess apoptosis. Typically apoptosis (reflecting cell turnover in the culture) in control cultures was ∼15 to 20 cells/10 fields. The number of Annexin V+/propidium iodide− cells were evaluated with a ×25 fluorescence lens in a Nikon Optiphot 2 UV microscope equipped with suitable barrier filters. The results shown in b are apoptosis exceeding the turnover apoptosis. Annexin V-positive cells are seen in white. c: Levels of sFasL in supernatants from stimulated CL PBMCs influenced the level of apoptosis induced in keratinocytes. Supernatants from six different CL PBMCs were incubated with HaCaT. All samples were performed in duplicates. Two samples were repeated on two different occasions with similar results. There was a strong correlation between the levels of sFasL in the supernatants used (x axis) and the observed levels of apoptosis in keratinocytes (y axis) (r = 0.7, P < 0.001). sFasL was measured by enzyme-linked immunosorbent assay. d: Adding Fas-blocking antibodies (ZB4, open bars) to stimulated supernatants and HaCaT blocked keratinocyte apoptosis in two of three supernatants (CL12 and CL4) containing high levels of sFasL. In one case, CL3, apoptosis was not blocked. The positive control CH11 induced apoptosis at 1 μg/ml and was used as a positive control. CH11 was effectively blocked in the presence of ZB4 at 1 μg/ml. e: Individual data for the supernatants included in the HaCaT experiments. The expression of Fas or levels of apoptosis in restimulated PBMCs did not correlate with the levels of HaCaT apoptosis induced. CL5 was not included in the HaCaT experiments. The FasL staining in some of the donors were performed with an inefficient batch of FasL antibody and thus inconclusive (i).
Supernatants from L. major-Stimulated CL PBMCs Induce Apoptosis in Human Keratinocytes
Our next step was to investigate if high levels of sFasL in the microenvironment surrounding the L. major infection could cause the increased levels of keratinocyte apoptosis noted in CL lesions. Thus, supernatants from L. major-stimulated CL PBMCs were incubated with a spontaneously immortalized human keratinocyte cell line (HaCaT). Three of six supernatants from L. major-stimulated CL PBMCs tested induced substantial levels of apoptosis in HaCaT after 40 hours of incubation (Figure 4; a to d). The numbers of apoptotic keratinocytes correlated with the levels of sFasL in the supernatants added (Figure 4c). Adding Fas-blocking antibodies (ZB4) to supernatants from stimulated PBMCs and HaCaT blocked keratinocyte apoptosis in two of three supernatants tested in which apoptosis had been induced by supernatants. All samples were tested in duplicates and repeated at two different occasions with similar results. In the case of one supernatant apoptosis was not blocked by the anti-Fas antibody (Figure 4d). Substantial amounts of apoptosis were induced in HaCaT after 40 hours of incubation with the positive control CH11 at 1 μg/ml. The apoptotic effect of CH11 was effectively blocked in the presence of ZB4 at 1 μg/ml (Figure 4d). Supernatants from unstimulated CL supernatants did not induce apoptosis in HaCaT.
Discussion
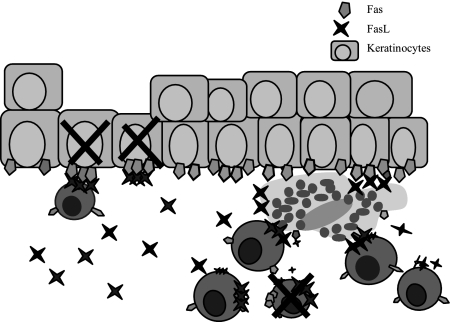
L. major-induced CL is a self-healing infection, which nonetheless causes suffering in patients and leaves an unsightly scar. The mechanisms behind the ulcer formation during CL remain unclear. We proposed that L. major-primed, FasL-expressing effector T cells and macrophages induce apoptosis of Fas-expressing keratinocytes during CL lesion development, leading to ulcer formation (Figure 5). The biopsies investigated in this study originated from intact skin surrounding established ulcers. High levels of keratinocyte apoptosis were measured in the epidermis of both active and healing CL compared to healthy controls. This was evident mainly in the superficial epidermis. In active lesions Fas was highly expressed on epidermis, co-localizing with the majority of apoptotic cells. In healing lesions Fas expression did not differ from healthy skin, although the level of apoptosis was similar to the active lesions. FasL was not expressed in keratinocytes in the biopsies analyzed. T cells, that up-regulated Fas on encounter with the Leishmania infection, probably undergo Fas/FasL-mediated bystander apoptosis at the site of infection, explaining why apoptotic cells were found in the inflammatory areas surrounding the biopsies (Figure 5).
Figure 5.
Possible mechanism for Fas/FasL-mediated apoptosis leading to keratinocyte death and ulceration during CL. Keratinocytes up-regulate Fas as a result of the inflammatory reaction surrounding L. major-infected macrophages. FasL-expressing T cells and macrophages accumulate at the site of L. major infection. The Fas-FasL or Fas-sFasL interaction will lead to keratinocyte apoptosis and ulceration as well as to activation-induced T-cell apoptosis. Macrophages are quite resistant against FasL-mediated apoptosis.42
Apoptosis has been suggested to be important during keratinocyte homeostasis25 and keratinocytes constitutively express functional Fas12,26 whereas FasL-expressing cells are scarce in normal skin.27 Up-regulation of Fas is a general sign of activation making cells prone to undergo apoptosis.10,11,28 When cultured with IFN-γ, keratinocytes up-regulate their expression of Fas and become more susceptible to Fas-mediated apoptosis.26 The same phenomena is described in T cells 5 to 7 days on activation and leads to activation-induced cell death and contraction of immune responses.11 The presence of IFN-γ at the site of infection together with the increased expression of HLA-DR1 suggest that this inflammatory milieu may lead to activation of keratinocytes followed by up-regulation of Fas on these cells. In addition, the inflammatory infiltrate of the dermis and subcutis investigated in this study contained more FasL-expressing cells, both T cells and macrophages, and more apoptotic cells in the biopsies from active disease compared to healing disease. Furthermore, the number of FasL-expressing inflammatory cells correlated with the Fas expression on keratinocytes in epidermis, suggesting that the Fas/FasL pathway may be involved in the increased levels of keratinocyte apoptosis observed.
In our material, no major alterations of the Fas/FasL-signaling pathway were observed ex vivo in PBMCs or sera from CL patients, probably reflecting the fact that CL is a localized disease with a low infectious burden. However, when CL PBMCs were restimulated with L. major, Fas was highly up-regulated on effector T cells (CD3+ and CD8+), high levels of sFasL were secreted, and increased levels of PBMC apoptosis were measured in restimulated CL cultures. FasL, however, could only be measured on CD3+ cells (both CD4+ and CD8+ origin) in one patient sample, probably due to the fact that FasL is only transiently expressed on the cell surface before it is cleaved by unknown metalloproteinases.29 sFasL has been suggested to be able to both induce8,30,31 and to inhibit32 Fas-mediated apoptosis. Thus, we assayed if the high levels of sFasL measured in the restimulated CL PBMCs could induce apoptosis in keratinocytes in vitro in an attempt to mimic the microenvironment at the site of L. major infections. Supernatants containing high levels of sFasL (more than 1000 pg/ml) induced substantial amounts of apoptosis in human keratinocytes (HaCaT) after 40 hours of incubation, indicating that L. major-primed CL T cells and macrophages may induce apoptosis in keratinocytes and lead to ulcer formation during CL. In 33% of the samples, blocking the Fas/FasL system did not inhibit keratinocyte apoptosis. One possible explanation is that other receptor-mediated apoptotic pathways, such as TRAIL, were up-regulated in parallel to the Fas/FasL system and more so in the donor PBMCs where keratinocyte apoptosis could not be blocked.
Alteration of Fas and FasL in the T-cell compartment leading to keratinocyte death and ulceration has been described in other cutaneous diseases such as toxic epidermal necrolysis and Steven-Johnson syndrome,27,33 in which the epidermis is shed on up to 90% of the body surface. During toxic epidermal necrolysis, high serum levels of sFasL have been measured at the time of diagnosis and high levels of FasL have been measured in the dermis at the site where the epidermis has been shed. Furthermore, in one publication it was reported that FasL-expressing CD8+ cells caused keratinocyte apoptosis.27 On treatment with intravenous administration of immunoglobulins containing Fas-blocking antibodies, it was suggested that this limited the ulcerative process. However, further studies have shown varying success using such a regimen, probably because of the different levels of Fas-blocking antibodies in different preparations of immunoglobulins.34–37 In atopic dermatitis and allergic contact dermatitis, keratinocyte apoptosis has been correlated to FasL-expressing, activated infiltrating T cells.38 Dysregulation of Fas/FasL is involved in the pathogenesis of several infectious diseases, one example being HIV, in which bystander apoptosis in CD4 cells have been suggested to be Fas-FasL-mediated.39
In CL, it remains unclear if ulceration is necessary to clear infection, or appears as a side effect of the strong local inflammatory reaction induced by L. major. In the context of CTL’s cytoxicity against L. major-infected cells both the perforin/granzyme system and functional FasL have been suggested to be important for the control of parasite replication in murine models of the disease.40,41 We propose that during CL, T cells of both CD4+ and CD8+ origin, as well as macrophages, up-regulate FasL when primed toward L. major. At the site of infection, in a microenvironment with high levels of IFN-γ and high Fas expression on epidermal keratinocytes, FasL+ effector cells will induce apoptosis in keratinocytes and activated T cells. These findings improve our knowledge on apoptosis of bystander cells during parasitic infection and pave the way for new interventions based on the possibility of modulating Fas-mediated apoptosis.
Acknowledgments
We thank Ki Curra, Dermatologic Diagnostic Center, Karolinska University Hospital, and RosMari Johansson, the Microbiology and Tumor Biology Center, Karolinska Institutet, for help with method development visualizing skin Fas and FasL; and Dr. Malin Haeggström, the Microbiology and Tumor Biology Center, Karolinska Institutet, for help in double staining of skin tissue.
Footnotes
Address reprint requests to Liv Eidsmo, MTC, Karolinska Institutet, Box 280, 171 77 Stockholm, Sweden. E-mail: liv.eidsmo@mtc.ki.se.
Supported by Sida/Department for Research Co-operation (grant SW-2000-152); United Nations Development Programme/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases (grant A10349); the Swedish Medical Research Council; and the Swedish Society of Medicine (travel grant 2001-28).
References
- Gaafar A, Veress B, Permin H, Kharazmi A, Theander TG, el Hassan AM. Characterization of the local and systemic immune responses in patients with cutaneous leishmaniasis due to Leishmania major. Clin Immunol. 1999;91:314–320. doi: 10.1006/clim.1999.4705. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Makala L, Otsuka H, Mikami T, Nagasawa H. Mechanisms of apoptosis in murine fibroblasts by two intracellular protozoan parasites, Toxoplasma gondii and Neospora caninum. Parasite Immunol. 2002;24:347–354. doi: 10.1046/j.1365-3024.2002.00476.x. [DOI] [PubMed] [Google Scholar]
- Debrabant A, Lee N, Bertholet S, Duncan R, Nakhasi HL. Programmed cell death in trypanosomatids and other unicellular organisms. Int J Parasitol. 2003;33:257–267. doi: 10.1016/s0020-7519(03)00008-0. [DOI] [PubMed] [Google Scholar]
- Pino P, Vouldoukis I, Kolb JP, Mahmoudi N, Desportes-Livage I, Bricaire F, Danis M, Dugas B, Mazier D. Plasmodium falciparum-infected erythrocyte adhesion induces caspase activation and apoptosis in human endothelial cells. J Infect Dis. 2003;187:1283–1290. doi: 10.1086/373992. [DOI] [PubMed] [Google Scholar]
- Freire-de-Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- Aga E, Katschinski DM, van Zandbergen G, Laufs H, Hansen B, Muller K, Solbach W, Laskay T. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J Immunol. 2002;169:898–905. doi: 10.4049/jimmunol.169.2.898. [DOI] [PubMed] [Google Scholar]
- Cascino I, Fiucci G, Papoff G, Ruberti G. Three functional soluble forms of the human apoptosis-inducing Fas molecule are produced by alternative splicing. J Immunol. 1995;154:2706–2713. [PubMed] [Google Scholar]
- Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Rosen D, Ronen D, Behrens CK, Krammer PH, Clark WR, Berke G. The regulation of CD95 ligand expression and function in CTL. J Immunol. 1998;161:3943–3949. [PubMed] [Google Scholar]
- Klas C, Debatin KM, Jonker RR, Krammer PH. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993;5:625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- Krueger A, Fas SC, Baumann S, Krammer PH. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunol Rev. 2003;193:58–69. doi: 10.1034/j.1600-065x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Sung KJ, Paik EM, Jang KA, Suh HS, Choi JH. Apoptosis is induced by anti-Fas antibody alone in cultured human keratinocytes. J Dermatol. 1997;24:427–434. doi: 10.1111/j.1346-8138.1997.tb02816.x. [DOI] [PubMed] [Google Scholar]
- Conceicao-Silva F, Hahne M, Schroter M, Louis J, Tschopp J. The resolution of lesions induced by Leishmania major in mice requires a functional Fas (APO-1, CD95) pathway of cytotoxicity. Eur J Immunol. 1998;28:237–245. doi: 10.1002/(SICI)1521-4141(199801)28:01<237::AID-IMMU237>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Huang FP, Xu D, Esfandiari EO, Sands W, Wei XQ, Liew FY. Mice defective in Fas are highly susceptible to Leishmania major infection despite elevated IL-12 synthesis, strong Th1 responses, and enhanced nitric oxide production. J Immunol. 1998;160:4143–4147. [PubMed] [Google Scholar]
- Desbarats J, Stone JE, Lin L, Zakeri ZF, Davis GS, Pfeiffer LM, Titus RG, Newell MK. Rapid early onset lymphocyte cell death in mice resistant, but not susceptible to Leishmania major infection. Apoptosis. 2000;5:189–196. doi: 10.1023/a:1009601200580. [DOI] [PubMed] [Google Scholar]
- Bertho AL, Santiago MA, Da-Cruz AM, Coutinho SG. Detection of early apoptosis and cell death in T CD4+ and CD8+ cells from lesions of patients with localized cutaneous leishmaniasis. Braz J Med Biol Res. 2000;33:317–325. doi: 10.1590/s0100-879x2000000300010. [DOI] [PubMed] [Google Scholar]
- Mustafa T, Bjune TG, Jonsson R, Pando RH, Nilsen R. Increased expression of Fas ligand in human tuberculosis and leprosy lesions: a potential novel mechanism of immune evasion in mycobacterial infection. Scand J Immunol. 2001;54:630–639. doi: 10.1046/j.1365-3083.2001.01020.x. [DOI] [PubMed] [Google Scholar]
- Eidsmo L, Wolday D, Berhe N, Sabri F, Satti I, El Hassan AM, Sundar S, Chiodi F, Akuffo H. Alteration of Fas and Fas ligand expression during human visceral leishmaniasis. Clin Exp Immunol. 2002;130:307–313. doi: 10.1046/j.1365-2249.2002.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strater J, Walczak H, Hasel C, Melzner I, Leithauser F, Moller P. CD95 ligand (CD95L) immunohistochemistry: a critical study on 12 antibodies. Cell Death Differ. 2001;8:273–278. doi: 10.1038/sj.cdd.4400813. [DOI] [PubMed] [Google Scholar]
- Maasho K, Akuffo HO. Cells from healthy non-exposed individuals produce cytokines to selected fractions of Leishmania promastigotes. Scand J Immunol Suppl. 1992;11:179–184. doi: 10.1111/j.1365-3083.1992.tb01647.x. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon M, Bong AB, Drewniok C, Herz J, Geilen CC, Reifenberger J, Benninghoff B, Slade HB, Gollnick H, Schon MP. Tumor-selective induction of apoptosis and the small-molecule immune response modifier imiquimod. J Natl Cancer Inst. 2003;95:1138–1149. doi: 10.1093/jnci/djg016. [DOI] [PubMed] [Google Scholar]
- Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadeel B, Thorpe CJ, Yonehara S, Chiodi F. Anti-Fas IgG1 antibodies recognizing the same epitope of Fas/APO-1 mediate different biological effects in vitro. Int Immunol. 1997;9:201–209. doi: 10.1093/intimm/9.2.201. [DOI] [PubMed] [Google Scholar]
- Arnold R, Seifert M, Asadullah K, Volk HD. Crosstalk between keratinocytes and T lymphocytes via Fas/Fas ligand interaction: modulation by cytokines. J Immunol. 1999;162:7140–7147. [PubMed] [Google Scholar]
- Matsue H, Kobayashi H, Hosokawa T, Akitaya T, Ohkawara A. Keratinocytes constitutively express the Fas antigen that mediates apoptosis in IFN gamma-treated cultured keratinocytes. Arch Dermatol Res. 1995;287:315–320. doi: 10.1007/BF01105085. [DOI] [PubMed] [Google Scholar]
- Abe R, Shimizu T, Shibaki A, Nakamura H, Watanabe H, Shimizu H. Toxic epidermal necrolysis and Stevens-Johnson syndrome are induced by soluble Fas ligand. Am J Pathol. 2003;162:1515–1520. doi: 10.1016/S0002-9440(10)64284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyaizu N, McCloskey TW, Than S, Hu R, Kalyanaraman VS, Pahwa S. Cross-linking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon-gamma and tumor necrosis factor-alpha secretion. Blood. 1994;84:2622–2631. [PubMed] [Google Scholar]
- Vargo-Gogola T, Crawford HC, Fingleton B, Matrisian LM. Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch Biochem Biophys. 2002;408:155–161. doi: 10.1016/s0003-9861(02)00525-8. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Hershkoviz R, Hecht I, Cahalon L, Lider O. Fibronectin-associated Fas ligand rapidly induces opposing and time-dependent effects on the activation and apoptosis of T cells. J Immunol. 2003;171:5882–5889. doi: 10.4049/jimmunol.171.11.5882. [DOI] [PubMed] [Google Scholar]
- Aoki K, Kurooka M, Chen JJ, Petryniak J, Nabel EG, Nabel GJ. Extracellular matrix interacts with soluble CD95L: retention and enhancement of cytotoxicity. Nat Immunol. 2001;2:333–337. doi: 10.1038/86336. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- Paul C, Wolkenstein P, Adle H, Wechsler J, Garchon HJ, Revuz J, Roujeau JC. Apoptosis as a mechanism of keratinocyte death in toxic epidermal necrolysis. Br J Dermatol. 1996;134:710–714. doi: 10.1111/j.1365-2133.1996.tb06976.x. [DOI] [PubMed] [Google Scholar]
- Bachot N, Revuz J, Roujeau JC. Intravenous immunoglobulin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis: a prospective noncomparative study showing no benefit on mortality or progression. Arch Dermatol. 2003;139:33–36. doi: 10.1001/archderm.139.1.33. [DOI] [PubMed] [Google Scholar]
- Prins C, Kerdel FA, Padilla RS, Hunziker T, Chimenti S, Viard I, Mauri DN, Flynn K, Trent J, Margolis DJ, Saurat JH, French LE. Treatment of toxic epidermal necrolysis with high-dose intravenous immunoglobulins: multicenter retrospective analysis of 48 consecutive cases. Arch Dermatol. 2003;139:26–32. doi: 10.1001/archderm.139.1.26. [DOI] [PubMed] [Google Scholar]
- Stella M, Cassano P, Bollero D, Clemente A, Giorio G. Toxic epidermal necrolysis treated with intravenous high-dose immunoglobulins: our experience. Dermatology. 2001;203:45–49. doi: 10.1159/000051702. [DOI] [PubMed] [Google Scholar]
- Trent JT, Kirsner RS, Romanelli P, Kerdel FA. Analysis of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis using SCORTEN: The University of Miami Experience. Arch Dermatol. 2003;139:39–43. doi: 10.1001/archderm.139.1.39. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, Noll M, Brocker EB, Blaser K, Akdis CA. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106:25–35. doi: 10.1172/JCI9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badley AD, Pilon AA, Landay A, Lynch DH. Mechanisms of HIV-associated lymphocyte apoptosis. Blood. 2000;96:2951–2964. [PubMed] [Google Scholar]
- Chakour R, Guler R, Bugnon M, Allenbach C, Garcia I, Mauel J, Louis J, Tacchini-Cottier F. Both the Fas ligand and inducible nitric oxide synthase are needed for control of parasite replication within lesions in mice infected with Leishmania major whereas the contribution of tumor necrosis factor is minimal. Infect Immun. 2003;71:5287–5295. doi: 10.1128/IAI.71.9.5287-5295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenares M, Kima PE, Samoff E, Soong L, McMahon-Pratt D. Perforin and gamma interferon are critical CD8+ T-cell-mediated responses in vaccine-induced immunity against Leishmania amazonensis infection. Infect Immun. 2003;71:3172–3182. doi: 10.1128/IAI.71.6.3172-3182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman H, Pagliari LJ, Georganas C, Mano T, Walsh K, Pope RM. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to Fas-mediated apoptosis. J Exp Med. 1999;190:1679–1688. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]