Abstract
Intra-amniotic lipopolysaccharide (LPS) causes an acute inflammatory response and cardiac dysfunction in fetal mice. We hypothesized that the placenta protects the fetus against maternally administered bacterial toxins, delaying the onset of a fetal inflammatory response and vascular compromise. At 14 to 15 days of gestation, DBA mice were randomized to receive LPS (2.4 mg/kg) or vehicle intraperitoneally. Doppler ultrasonography of fetal cardiovascular hemodynamics was performed before and 6 hours after maternal LPS. Six hours after the LPS, maternal serum concentrations of tumor necrosis factor-α and interleukin (IL)-6 (P < 0.05) were increased. Placenta showed severe maternal vascular dilatation and congestion. The expressions of tumor necrosis factor-α, IL-1α, and IL-6 (P < 0.05) were increased, and the expression of Toll-like receptor 4 was constitutive in placenta. The expression of Toll-like receptor 2 increased (P < 0.05) and was detected in labyrinthine macrophages. No inflammatory activation was found in fetal tissues, and amniotic fluid revealed no significant increase in cytokines. The ultrasonographic examination demonstrated increased fetal cardiac afterload after LPS, with 65% of the fetuses exhibiting atrioventricular valve regurgitation. In conclusion, maternal inflammatory insult activates placental labyrinthine macrophages leading to an acute increase in placental vascular resistance and fetal cardiac dysfunction without an inflammatory response in fetus.
Endotoxin (lipopolysaccharide, LPS) plays a key role in the myocardial depression seen in adult patients with sepsis or severe infection by gram-negative bacteria.1–3 LPS induces an inflammatory response by binding to CD14 and subsequently activating a transmembrane receptor, Toll-like receptor-4 (TLR4). This leads to the production of cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, and other inflammatory mediators.4,5 Earlier in vitro studies have shown that proinflammatory cytokines, eg, TNF-α and IL-1β, after LPS challenge contribute to the development of cardiac dysfunction.6
Maternal infections have been associated with fetal death or severe fetal compromise, preterm delivery, and premature rupture of fetal membranes.7,8 LPS has been implicated as a cause of preterm labor in humans and is known to cause fetal death or abortion in animals.9,10 Maternal LPS exposure in animals induced cytokine production in the maternal compartment, placental hemorrhage, degradation of the maternal/fetal interface, and resorption or fetal abortion.11–17 Nevertheless, the mechanisms behind the fetal loss induced by maternally administered LPS are not understood. Placental trophoblast, an epithelial cell of fetal origin that forms a physical barrier between the mother and the developing fetus, additionally serves as a component of the host immune system during pregnancy.18 By producing various proinflammatory cytokines and possessing cytokine receptors, placental trophoblasts have some resemblance to macrophages, which are also found in chorionic villous stroma.19,20
We have recently shown that, in mouse, intra-amniotically administered LPS acutely induces cytokine expression in placenta, fetal membranes, and fetal myocardium, leading to severe cardiac dysfunction and arrhythmias.21 Direct injection of LPS into a sheep fetus has been associated with placental injury together with reduced cerebral oxygen supply and placental blood flow.22,23 According to our hypothesis, the placenta protects the fetus delaying the induction of cytokine responses in fetal tissues. Our aim was to investigate the acute inflammatory responses in the placenta and in the fetal compartment after maternal intraperitoneal administration of LPS. A further aim was to find out whether the placental insult has pathophysiological consequences on fetal cardiovascular hemodynamics.
Materials and Methods
Animal Protocol
The protocol was approved by the Animal Research Committee of the University of Oulu. After preliminary experiments with 28 dams and 126 fetuses, 12 timed pregnant DBA/2 mice and 51 fetuses (a mean of five per litter) were included in this randomized study. The length of gestation (±12 hours) was verified by the presence of a vaginal plug (designated as day 0 of pregnancy).
On day 14 of gestation (term 21 days), the mice were anesthetized with a solution containing 50 μg of midazolam, 3.2 μg of fentanyl citrate, and 100 μg of fluanisone per kg of body weight. The depth of anesthesia was monitored by the loss of toe and tail pinch reflexes. After the induction of anesthesia, the first ultrasonographic examination was performed (see below). The dams were randomized to receive either LPS or vehicle [phosphate-buffered saline (PBS)] intraperitoneally. LPS (Escherichia coli 055:B5; Sigma-Aldrich, St. Louis, MO) was solubilized in 0.9% sterile PBS at a concentration of 0.72 mg/ml and used immediately or frozen once. LPS was administered at a dose of 2.4 mg/kg by a single 0.1 ml i.p. injection (∼70 μg/animal). In the vehicle group 0.1 ml of PBS was given intraperitoneally to the dam. Six hours after the injection, the animals were anesthetized again and a second ultrasonographic examination identical to the first one was performed. Thereafter, the dams were sacrificed by cervical dislocation. The placenta, fetal membranes, liver, lungs, and heart from the dam and fetuses were recovered and processed for analysis. In addition, amniotic fluid and maternal serum were collected.
Doppler Echocardiography
The ultrasonographic examination of fetuses was performed by using the Acuson Sequoia 512 equipment (Mountain View, CA) with a 13 MHz linear probe.21,24 The fetuses were localized in each uterine horn, starting from the top of the horn. After identifying the fetal heart by color Doppler, the sample volume of pulsed Doppler was placed over the heart. The length of the sample volume was adjusted to cover the entire heart. The high-pass filter was set at its minimum. The fetal heart was examined from different directions, to minimize the angle between the Doppler beam and the inflow and outflow regions of the heart, to obtain their maximal velocities. The maximal inflow and outflow velocities were recorded using a sweep speed of 100 mm/second. From the sagittal view of the fetus, the descending aorta, the intracranial artery, and ductus venosus were located with the color Doppler technique. Their blood velocity waveforms were obtained by pulsed Doppler ultrasonography. With the same technique, the umbilical cord was identified and the blood velocity waveforms of the umbilical artery were obtained. Immediately after the second ultrasonographic examination, the abdomen was carefully opened, and the fetuses were identified according to their location at the ultrasonography.
The data were videotaped and analyzed afterward off-line, using the cardiac measurement package of the ultrasound equipment. The fetal heart rate and the time-velocity integral of the outflow waveform were measured. The outflow mean velocity (Vmean) was calculated by the formula: Vmean = fetal heart rate × time-velocity integral. The proportions (percent) of isovolumetric relaxation and contraction times of the cardiac cycle were calculated. Isovolumetric relaxation was measured as the period between the closure of the semilunar valve and the opening of the atrioventricular valve, whereas isovolumetric contraction represents the period between the closure of the atrioventricular valve and the opening of the semilunar valve. The presence of atrioventricular or semilunar valve regurgitation was documented. Furthermore, the pulsatility index [PI = (peak systolic velocity − end-diastolic velocity)/time-averaged maximum velocity over the cardiac cycle] values were obtained from the umbilical artery, descending aorta, and intracranial artery blood velocity waveforms. The pulsatility index value for veins (PIV) was calculated from the ductus venosus blood velocity waveforms [PIV = (peak systolic velocity − velocity during atrial contraction)/time-averaged maximum velocity over the cardiac cycle].25 The intraobserver variability with respect to Doppler ultrasonographic parameters was analyzed in 15 fetuses from three different dams. The examination was repeated 30 minutes after the first one under the same anesthesia.
RNA Analysis
Total RNA was isolated using Trizol reagent (Life Technologies Inc., Grand Island, NY) according to the manufacturer’s instructions. The mRNA levels of IL-1α, IL-1β, IL-6, IL-10, TNF-α, IL-1 receptor 1 (IL-1R1), macrophage inflammatory protein (MIP)-2, and IL-10 were analyzed by RNase protection assay using Riboquant multiprobe set (BD Biosciences Pharmingen, San Diego, CA) as described previously.21 In addition, TLR2 and TLR4, macrophage marker F4/80, matrix metalloproteinase-9, and vascular endothelial growth factor mRNAs were analyzed in placenta with the same technique. Constitutive GAPDH and L32 were analyzed as reference. The quantity of protected RNA was determined using a PhosphorImager and associated software (Bio-Rad, Hercules, CA). Cytokine values were expressed as a ratio of the mRNA levels in the LPS/vehicle groups.
Histopathological Examination and Immunostaining
The tissues were fixed in 4% formaldehyde in PBS for 24 hours. Thereafter, they were embedded in paraffin, cut into 5-μm sections and processed for hematoxylin and eosin staining. A duplicate tissue section was also stained for naphthol-ASD-chloroacetate esterase by Leder’s stain to detect granulocytes and to evaluate the inflammatory state. In addition, basic cytokeratin and endothelial cell (CD34) markers were used to differentiate placental structures. After deparaffinization and dehydration, the sections were immunostained for TLR2. Briefly, the sections were incubated for 20 minutes in boiling aqueous 10 mmol/L sodium citrate water (pH 6.0). The sections were allowed to cool for 20 minutes, washed in PBS, and treated with 3% H2O2 in methanol for 15 minutes at room temperature. After washing with PBS, the sections were blocked with 1:5 normal donkey serum in PBS for 30 minutes. For TLR2 immunostaining, the sections were incubated for 1 hour at + 4°C with goat anti-mouse TLR2 antibody (AF 1530; R&D Systems Inc., Minneapolis, MN). The detection was performed with an avidin-biotin peroxidase system using AEC as a substrate. Finally, the sections were counterstained with hematoxylin. The immunohistochemical assay of TLR4 in placenta was attempted using the following antibodies: C-18, L-14, M-16, C-80 (Santa Cruz Biotechnology, Santa Cruz, CA) and MTS-510 (Hycult Biotechnology, Uden, Netherlands). Various antigen retrieval methods were used.
Cytometric Bead Array
Cytometric bead array by BD Biosciences was used to study cytokines and chemokines in amniotic fluid and maternal serum. A mouse inflammation kit (BD Biosciences) was used according to the manufacturer’s instructions to detect IL-6, IL-10, MCP-1, interferon-γ, TNF-α, and IL-12p70. For each sample (25 μl), 50 μl of antibody-bead reagent and 50 μl of antibody phycoerythrin reagent were added. The mixture was incubated for 2 hours at room temperature and washed to remove the unbound detector phycoerythrin reagent before further analysis. Two-color flow cytometric analysis was performed using a FACSCalibur flow cytometer (BD Biosciences). Data were acquired and analyzed using BD cytometric bead array software. The sensitivities of the cytometric bead array assays for IL-6, IL-10, MCP-1, interferon-γ, TNF-α, and IL-12p70 were 5.0, 17.5, 52.7, 2.5, 7.3, and 10.7 pg/ml, respectively.
Statistical Analysis
Categorical data were analyzed using the χ2 test. Continuous data between groups was analyzed using Student’s t-test. The nonparametric Mann-Whitney U-test was chosen if the data were not normally distributed. The level of statistical significance was set at P < 0.05.
Results
Dose Response to LPS
In preliminary experiments, the effects of different doses of LPS (0.6 to 2.4 mg/kg) and different time intervals of LPS challenge (6 to 24 hours) were studied, using fetal survival and premature birth as outcome variables. Regardless of the LPS dosage, the fetal death rate approached 100% at 24 hours after the intraperitoneal injection. The fetal death rate ranged between 30% (LPS dose, 1.2 mg/kg) and 70% (LPS dose, 2.4 mg/kg) at 12 hours after LPS. There were no premature births in any of the groups studied. On the contrary, there is a notable likelihood of spontaneous fetal loss after the intrauterine death. Based on these preliminary experiments, LPS dose of 2.4 mg/kg and a 6-hour interval were chosen to determine the pathophysiological effects of the acute proinflammatory cytokine response in the placenta. At 6 hours, fetal death rate reached 11% with the LPS dose of 2.4 mg/kg (Table 1).
Table 1.
Fetal Cardiovascular Parameters in Study Groups
| LPS
|
PBS
|
|||
|---|---|---|---|---|
| Before (n = 26) | After 6 hours (n = 23) | Before (n = 25) | After 6 hours (n = 25) | |
| FHR (bpm) | 207 (47) | 196 (43) | 200 (19) | 253 (38) |
| OF Vmean (cm/second) | 16.3 (2.9) | 11.1 (3.8)* | 15.5 (2.4) | 16.5 (2.1) |
| IRT % | 8.2 (6.1–10.3) | 8.3 (6.4–10.6) | 8.3 (6.3–10.4) | 7.6 (5.6–9.6) |
| ICT % | 4.9 (2.7–7.7) | 4.9 (1.9–11.1) | 5.7 (3.3–8.8) | 5.2 (1.9–8.8) |
| UA PI | 2.54 (0.56) | 3.47 (1.07)* | 2.44 (0.33) | 2.52 (0.18) |
| DAO PI | 2.77 (0.43) | 3.77 (1.36)* | 2.83 (0.31) | 2.62 (0.27) |
| ICA PI | 2.25 (0.23) | 2.42 (0.34) | 2.48 (0.23) | 2.43 (0.24) |
| DV PIV | 1.09 (0.10) | 1.53 (0.97)* | 1.08 (0.09) | 1.07 (0.14) |
| Regurgitation (%): | ||||
| Yes | 0% | 65% | 0% | 4% |
| Type of regurgitation (n): | ||||
| AVVR | 4 | 1 | ||
| Semilunar | 0 | 0 | ||
| AVVR + Semilunar | 11 | 0 | ||
| Fetal deaths (n) | 3 | 0 | ||
P < .05, versus PBS group, values given are mean (SD) or median (range).
FHR, fetal heart rate; OF, outflow; Vmean, mean velocity; IRT, isovolumetric relaxation time; ICT, isovolumetric contraction time; UA, umbilical artery; DAO, descending aorta; ICA, intracranial artery; DV, ductus venosus; PI, pulsatility index; PIV, pulsatility index for veins; AVVR, atrioventricular valve regurgitation.
Inflammatory Mediators in Maternal Serum and Amniotic Fluid
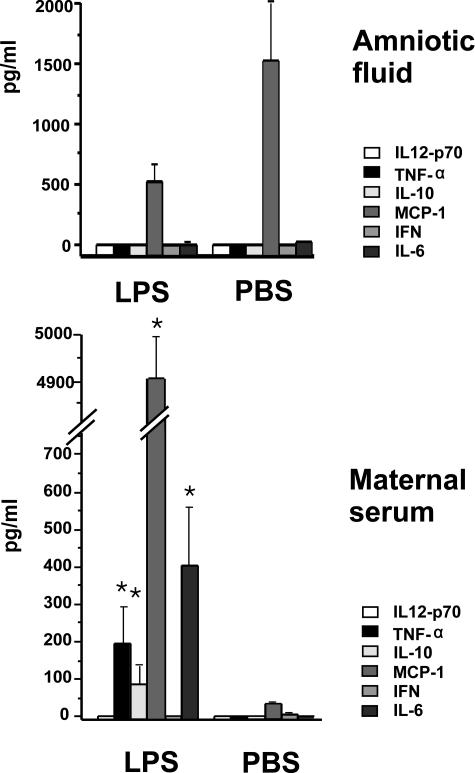
Six hours after LPS the dams showed clinical symptoms of inflammation, such as ruffled hair and diminished activity. None of the dams died spontaneously and the symptoms tended to subside toward 24 hours after LPS. The maternal serum concentrations of TNF-α, MCP-1, IL-10, and IL-6 were significantly increased compared to vehicle (Figure 1). IL-12p70 and interferon-γ were undetectable. Six hours after LPS and vehicle injections, only constitutive MCP-1 was detected in amniotic fluid. The other inflammatory mediators examined were undetectable (Figure 1). In contrast, at the 12-hour time point TNF-α and IL-6 concentrations were significantly increased (data not shown).
Figure 1.
Concentrations of inflammatory mediators in maternal serum and amniotic fluid 6 hours after intraperitoneal LPS (2.4 mg/kg) or vehicle. Results are expressed as mean ± SEM.
Gene Expression of the Inflammatory Mediators
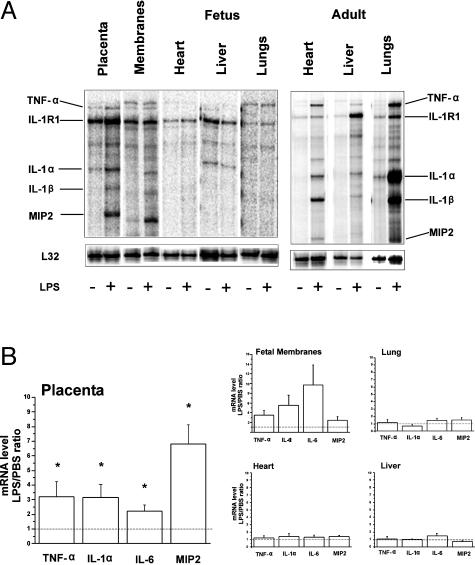
Six hours after LPS, the maternal liver, lung, and heart showed induction of proinflammatory cytokines (Figure 2). The mRNAs of TNF-α, IL-1α, MIP2, and IL-6 (Figure 2) were significantly increased in placenta after LPS compared to the vehicle group. IL-1β and IL-10 were not significantly induced and IL-1R1 remained constitutively expressed. In a separate analysis of the placenta, IL-4 (mean ± SEM, 2.39 ± 0.35) and matrix metalloproteinase-9 (1.9 ± 0.36) mRNAs were found to be significantly increased, whereas the expressions of vascular endothelial growth factor and F4/80 remained constitutive (data not shown). Furthermore, there was a significant increase in the expression of TLR2 in placenta, whereas the expression of TLR4 remained constitutive (Figure 4). In fetal membranes, TNF-α, IL-1α, MIP2, and IL-6 mRNA expression levels were highly variable and did not reach the level of statistical significance. In fetal tissues, there were no significant differences in the mRNA expression levels between the LPS and vehicle groups 6 hours after LPS. At 8 to 12 hours after LPS, the cytokine response in the membranes was more prominent and statistically significant. However, the fetal tissues revealed no significant inflammatory response (data not shown).
Figure 2.
Expressions of inflammatory mediators 6 hours after maternal LPS (2.4 mg/kg) or vehicle. A: Representative gel electrophoresis of multiple mRNAs in fetal and maternal tissues by ribonuclease protection assay. B: Expressions of inflammatory mediators in fetal and gestational tissues. The data are expressed as a fold increase in mRNA levels in the LPS group relative to the vehicle group. The results are mean ± SEM (n = 5 to 12 animals per group). *P < 0.05 versus vehicle group. Only the mediators induced by LPS in the placenta are shown.
Figure 4.
A: Immunostaining of mouse placenta. Highly specific positive staining for TLR2 antibody appears in red in placental macrophages in labyrinthine mesenchyme. B: Control staining without secondary antibody. Bottom: The mRNA expression of TLR2 and TLR4 in placenta 6 hours after intraperitoneal LPS and vehicle. The bars indicate the fold increase compared to vehicle-treated animals. Results are mean ± SEM.
Histopathology and TLR2 Staining
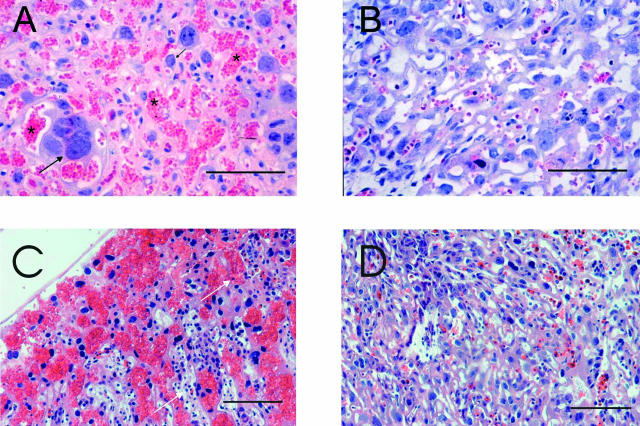
Maternal lung and liver showed normal histology and occasional granulocytes attached to the endothelium, no vascular congestion was seen. In contrast the histology of the placenta was abnormal. The most consistent finding in the LPS group was blood congestion and vascular dilatation in the labyrinthine part of the placenta (10 of 10 in the LPS group versus 1 of 10 in the vehicle group) (Figure 3). The most dilated vessels with stasis were located in the basal part of the placenta, in close connection to the thin muscular layer of uterus. Six hours after LPS, no placental thrombosis was observed and there was minimal evidence of margination or tissue accumulation of inflammatory cells. In contrast, 12 hours after LPS margination of lymphocytes and granulocytes was evident and congestion was more prominent (Figure 3). No differences in the extent or position of calcium deposits and necrosis could be detected. The immunostaining of placenta with TLR2 antibody revealed intense constitutive cytoplasmic immunoreactivity in the macrophage-like cells of the labyrinth (Figure 4). Using the C-18 antibody, TLR4 was localized in the placental spongiotrophoblast cells. However, because the spongiotrophoblast cells of TLR4-null mice also stained positively, the specificity of the antibody could not be confirmed.
Figure 3.
Histopathology of placenta by Leder’s staining. A: Six hours after intraperitoneal LPS administration, severe vascular congestion in the labyrinthine part of the placenta is seen. Cytotrophoblast (black arrow) and maternal red blood cells as shown with asterisk. B: Placenta 6 hours after vehicle administration (PBS) shows no congestion. Placenta 12 hours after LPS (C) and PBS (D). Note the accumulation of lymphocytes and granulocytes (white arrow) after LPS. Scale bars, 50 μm.
Ultrasonographic Examination
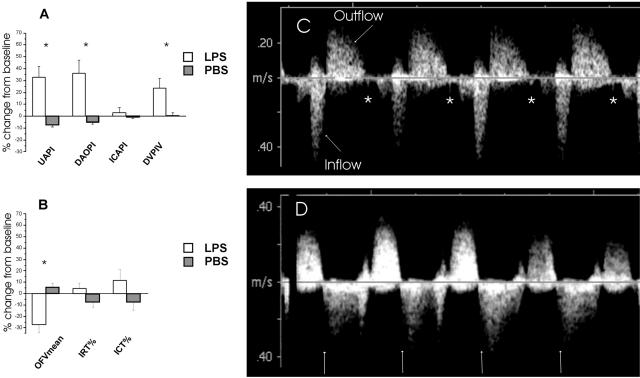
The mean intraobserver variability of the outflow time-velocity integral calculations was 6.0% (95% confidence interval, 4.0 to 8.4%). In the PI and PIV calculations, mean intraobserver variability ranged from 3.6% to 6.7% (95% confidence interval, 2.3 to 8.8%). The corresponding variability in the time interval measurements was from 2.5 to 15.9% (95% confidence interval, 2.0 to 21.8%). The baseline values were similar in both groups. After 6 hours, outflow Vmean was lower (P < 0.005) in the LPS group than in the vehicle group. Percentage of isovolumetric contraction and isovolumetric relaxation did not differ between the groups. In the LPS group, 15 fetuses (65%) had valve regurgitations (Figure 5, Table 1). Eleven fetuses had both atrioventricular (AV) and semilunar valve regurgitations, while four had only AV valve regurgitation. The PI values of umbilical artery (P < 0.005) and descending aorta (P < 0.005) and the ductus venosus PIV (P < 0.05) values were higher in the LPS group than in the vehicle group. There was no difference in the PI of intracranial artery between the groups (Figure 5).
Figure 5.
Left: Ultrasonographic parameters of fetal circulation (A) and cardiac function (B) 6 hours after LPS or vehicle (PBS) administration. Data are presented as change (%) from the baseline values. Right: C: Inflow and outflow blood velocity waveforms of the fetal heart obtained 6 hours after vehicle, semilunar valve closure is shown as a valve click (*). D: After LPS administration, semilunar valve regurgitation is seen. The valve click is missing, and regurgitation starts immediately after the end of ejection (arrow). PI, pulsatility index; PIV, pulsatility index for veins; UA, umbilical artery; DAO, descending aorta; ICA, intracranial artery; DV, ductus venosus; OFVmean, outflow mean velocity; IRT, isovolumetric relaxation time; ICT, isovolumetric contraction time. Data are expressed as mean ± SEM.
Discussion
We investigated the mechanism of fetal hemodynamic deterioration after an acute inflammatory stress in the maternal compartment. The introduction of endotoxin intraperitoneally into pregnant mice induced a substantial increase of cytokine production in maternal organs and placental tissue. Despite the significant increase of cytokines and clinical signs of systemic disease in the dam, the symptoms were transient, and there was no histopathological evidence of shock or vascular congestion, as studied in the maternal liver and lung. The placenta, however, showed severe congestion after LPS administration. Despite the lack of the cytokine response in the fetal myocardium and other tissues, the endotoxin challenge led to the development of severe abnormalities in fetal cardiovascular function.
Severe vascular congestion was evident 6 hours after LPS in the labyrinthine part of the placenta that is analogous to the human villous placenta consisting of chorionic trophoblast, allantoic mesenchyme, and vasculature. Most of the dilated vessels were maternal, as judged from the paucity of nucleated fetal red blood cells. We suggest that the fetal vessels in the labyrinth were severely engorged due to external compression by edematous extravascular tissue, resulting in an inappropriate increase of placental vascular resistance. The increased production of proinflammatory mediators, such as TNF-α and IL-1, together with other inflammatory agents may lead to increased permeability in gestational tissues, resulting in acute congestion.
Even though placental congestion was severe, the placental cytokine response was modest, suggesting involvement of a specific cell type. The cells responding to LPS were further investigated. The LPS used in the present study may contain bacterial lipoproteins, and it has been suggested to serve as a ligand for both TLR4 and TLR2.26 In human placenta, TLR4 and TLR2 have been localized in villous and intermediate trophoblasts and placental macrophages, also known as Hofbauer cells. Holmlund and colleagues27 and Kumazaki and colleagues28 have shown some evidence of increased immunoreactivity of TLR4 in preterm human placentas with chorionamnionitis. In the present study, the expression of TLR4 in mouse placenta remained constitutive after LPS administration, whereas the expression of TLR2 was significantly increased. We localized TLR2, a signaling receptor for gram-positive bacteria, lipoproteins, and glycolipids, known to be induced by LPS-induced activation of TLR4.29 TLR2 immunoreactivity was highly specific in the macrophages of mouse labyrinth mesenchymal stroma, similar to Hofbauer cells in human placenta. The cytotrophoblast in the labyrinth, which reportedly contains TLR4 in human placenta, did not show any detectable TLR2. Interestingly, this compartment of the placenta is the same area where the acute effects of endotoxin have been documented in previous rodent studies.11,17 The present data suggest a key role of immune cells in the labyrinth and the importance of TLRs in the inflammatory response to pathogens.
The lack of any detectable induction in cytokine or chemokine expression in the fetal heart, lung, or liver suggests that there was no direct inflammatory insult to the fetus. Our previous study revealed that, when given intra-amniotically, LPS acutely induces the inflammatory response in the fetal myocardium, leading to severe cardiovascular compromise.21 Unlike after intra-amniotic LPS, amniotic fluid revealed no increase in cytokine concentrations, nor was there any significant induction in cytokine expression in fetal membranes 6 hours after maternal LPS administration. At a later time point (8 hours), the induction in the membranes was significant and toward 12 hours the concentrations of proinflammatory cytokines in the amniotic fluid tended to increase, suggesting spreading of the acute inflammation. However no signs of fetal inflammation could be detected 8 hours after LPS. Despite the lack of a fetal inflammatory response, the PI values of the descending aorta and the umbilical artery were elevated 6 hours after LPS administration. The PI values describe downstream vascular impedance that is resistance to pulsatile flow. In contrast the PI values in intracranial artery were unchanged 6 hours after LPS. In case of hemodynamic compromise, redistribution of fetal circulation in favor of the brain takes place as the fetus attempts to maintain adequate blood supply to developing brain by decreasing blood flow in lower body. This in turn reflects to systemic circulation, seen here as elevated PI of descending aorta. A significant correlation exists between umbilical artery PI values and placental vascular resistance.30 Because a substantial proportion of the descending aorta volume blood flow is directed to placenta, the increase in placental vascular resistance is also reflected in the descending aorta vascular impedance. Thus, the results indicate an acute increase in the placental vascular resistance and fetal cardiac afterload. The cardiac outflow mean velocity was significantly reduced in the LPS group indicating a drop in cardiac output. According to the present results, these signs may be due to severe vascular congestion in the maternal compartment of the placenta. Severe fetal hypoxemia with acidemia depresses cardiac output.31 In the present study this cannot be excluded although the placental vascular endothelial growth factor expression revealed no increase after LPS injection. In human fetuses with severe placental insufficiency and increased vascular resistance, a shift in the fetal cardiac output toward the left ventricle has been shown. In the final state of placental insufficiency, both ventricular outputs decrease.32 In a human fetus, the increased afterload and altered cardiac function may finally lead to elevated venous pressure.33 In the present study, PIV in the ductus venosus was increased, suggesting a rise in systemic venous pressure.34
Within 6 hours, 3 of the 26 fetuses in the LPS group were dead. Approximately 65% of the surviving fetuses had valve regurgitations present after LPS administration. An acute increase in the afterload has been shown to induce tricuspid regurgitation in sheep fetuses with ductal closure and in human pregnancies with ductal constriction.35 The presence of atrioventricular valve regurgitation in this setting shows that the fetal myocardium was still able to generate pressure. This is noteworthy, as in our previous study with intra-amniotically administered LPS, valve leaks were not present, and the time intervals describing cardiac systolic and diastolic functions were abnormal.21 In addition, arrhythmias, such as II degree AV blocks were evident after intra-amniotic LPS, whereas no arrhythmias after intraperitoneal LPS were seen in the present study. These findings suggest that without inflammation in the myocardium, the fetal heart is able to maintain cardiac performance to generate sufficient blood pressure and perfusion. On the other hand, in the presence of myocardial inflammation, the fetal heart is unable to meet the demands of an increased afterload.
Because of the small cardiac size of the fetal mouse, it is impossible to distinguish between the right and left ventricular inflow and outflow areas with this technique. However, fetal circulation is characterized by open ductus arteriosus and foramen ovale. Thus, the fetal ventricles function in parallel and the pressure faced by both ventricles is equal. On this basis, the pathophysiological effects would be similar in both ventricles. Our previous study on fetal mice demonstrated good reproducibility of the Doppler parameters, proving the validity of this methodology.21 Similar results concerning reproducibility have also been reported previously.24
Our model of acute systemic inflammation mimics maternal sepsis or endotoxemia in pregnancy. A similar rapid increase of circulating proinflammatory cytokines can be seen in systemic inflammatory response syndrome, which may be a consequence of infection or noninfectious causes. An analogous experimental setting has been used to model preterm birth in mice.13,15,17 In our study, however, no premature births took place in any of the groups. In mice of the C3H/HeN strain impregnated with males of the B6D2F1 strain, intrauterine fetal death has been described even at such low doses of LPS as 5 μg per animal. On the other hand, concentrations as high as 50 μg/dam have been used in models of premature delivery.13,17 According to our findings, however, it is more likely that, at least in an acute model with a high LPS dose (∼70 μg/dam) given to mice of the DBA/2, strain, fetal demise, often followed by spontaneous abortion are inevitable. One limitation of the present model is that we could not monitor invasively the effects of LPS on maternal cardiovascular performance or vasoactive agents, which could have an adverse impact on placental function.
In conclusion, despite the lack of a cytokine response in the fetal heart or other tissues, the endotoxin challenge led to the development of severe fetal cardiovascular dysfunction. Thus, the placental barrier did protect the fetus against the inflammatory insult, but the acute placental lesions led to fetal compromise. This study provides new insights into a mechanism leading to cardiac failure that may precede fetal death.
Footnotes
Address reprint requests to Samuli Rounioja, M.D., Department of Pediatrics and Biocenter Oulu, P.O. Box 5000, FIN-90014 University of Oulu, Finland. E-mail: samuli.rounioja@oulu.fi.
Supported by the Academy of Finland, Biocenter Oulu, the Alma and K.A. Snellman Foundation (Oulu, Finland), the Sigrid Juselius Foundation, and Emil Aaltonen Foundation.
References
- Natanson C, Eichenholz PW, Danner RL, Eichacker PQ, Hoffman WD, Kuo GC, Banks SM, MacVittie TJ, Parrillo JE. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med. 1989;169:823–832. doi: 10.1084/jem.169.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273:117–123. [PubMed] [Google Scholar]
- Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- Beutler B. Endotoxin, Toll-like receptor 4, and the afferent limb of innate immunity. Curr Opin Microbiol. 2000;3:23–28. doi: 10.1016/s1369-5274(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- Romero R, Roslansky P, Oyarzun E, Wan M, Emamian M, Novitsky TJ, Gould MJ, Hobbins JC. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol. 1988;158:1044–1049. doi: 10.1016/0002-9378(88)90216-5. [DOI] [PubMed] [Google Scholar]
- Cox SM, MacDonald PC, Casey ML. Assay of bacterial endotoxin (lipopolysaccharide) in human amniotic fluid: potential usefulness in diagnosis and management of preterm labor. Am J Obstet Gynecol. 1988;159:99–106. doi: 10.1016/0002-9378(88)90501-7. [DOI] [PubMed] [Google Scholar]
- Silver RM, Edwin SS, Trautman MS, Simmons DL, Branch DW, Dudley DJ, Mitchell MD. Bacterial lipopolysaccharide-mediated fetal death. Production of a newly recognized form of inducible cyclooxygenase (COX-2) in murine decidua in response to lipopolysaccharide. J Clin Invest. 1995;95:725–731. doi: 10.1172/JCI117719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay DG, Wong T-C. The effect of bacterial endotoxin on the placenta of the rat. Am J Pathol. 1963;42:357–377. [PMC free article] [PubMed] [Google Scholar]
- Leazer TM, Barbee B, Ebron-McCoy M, Henry-Sam GA, Rogers JM. Role of the maternal acute phase response and tumor necrosis factor alpha in the developmental toxicity of lipopolysaccharide in the CD-1 mouse. Reprod Toxicol. 2002;16:173–179. doi: 10.1016/s0890-6238(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Fidel PL, Jr, Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton DB. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- Davies JK, Shikes RH, Sze CI, Leslie KK, McDuffie RS, Jr, Romero R, Gibbs RS. Histologic inflammation in the maternal and fetal compartments in a rabbit model of acute intra-amniotic infection. Am J Obstet Gynecol. 2000;183:1088–1093. doi: 10.1067/mob.2000.108888. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Blanchard R, Mehta SP. Differential fetal and maternal contributions to the cytokine milieu in a murine model of infection-induced preterm birth. Am J Obstet Gynecol. 1999;180:429–434. doi: 10.1016/s0002-9378(99)70227-9. [DOI] [PubMed] [Google Scholar]
- Zahl PA, Bjerknes C. Induction of decidual-placental hemorrhage in mice by the endotoxins of certain gram-negative bacteria. Proc Soc Exp Biol Med. 1943;54:329–332. [Google Scholar]
- Kaga N, Katsuki Y, Obata M, Shibutani Y. Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol. 1996;174:754–759. doi: 10.1016/s0002-9378(96)70460-x. [DOI] [PubMed] [Google Scholar]
- Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med. 2000;6:589–593. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- Hunt JS, Pollard JW. Macrophages in the uterus and placenta. Curr Top Microbiol Immunol. 1992;181:39–63. doi: 10.1007/978-3-642-77377-8_2. [DOI] [PubMed] [Google Scholar]
- Guilbert L, Robertson SA, Wegmann TG. The trophoblast as an integral component of a macrophage-cytokine network. Immunol Cell Biol. 1993;71:49–57. doi: 10.1038/icb.1993.5. [DOI] [PubMed] [Google Scholar]
- Rounioja S, Rasanen J, Glumoff V, Ojaniemi M, Makikallio K, Hallman M. Intra-amniotic lipopolysaccharide leads to fetal cardiac dysfunction—a mouse model for fetal inflammatory response. Cardiovasc Res. 2003;60:156–164. doi: 10.1016/s0008-6363(03)00338-9. [DOI] [PubMed] [Google Scholar]
- Duncan JR, Cock ML, Rees S, Harding R. The effects of repeated endotoxin exposure on placental structure in sheep. Placenta. 2003;24:786–789. doi: 10.1016/s0143-4004(03)00104-8. [DOI] [PubMed] [Google Scholar]
- Dalitz P, Harding R, Rees SM, Cock ML. Prolonged reductions in placental blood flow and cerebral oxygen delivery in preterm fetal sheep exposed to endotoxin: possible factors in white matter injury after acute infection. J Soc Gynecol Invest. 2003;10:283–290. doi: 10.1016/s1071-5576(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Gui YH, Linask KK, Khowsathit P, Huhta JC. Doppler echocardiography of normal and abnormal embryonic mouse heart. Pediatr Res. 1996;40:633–642. doi: 10.1203/00006450-199610000-00020. [DOI] [PubMed] [Google Scholar]
- Hecher K, Campbell S, Doyle P, Harrington K, Nicolaides K. Assessment of fetal compromise by Doppler ultrasound investigation of the fetal circulation. Arterial, intracardiac, and venous blood flow velocity studies. Circulation. 1995;91:129–138. doi: 10.1161/01.cir.91.1.129. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, Ekstrom ES, Scheynius A. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 2002;107:145–151. doi: 10.1046/j.1365-2567.2002.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazaki K, Nakayama M, Yanagihara I, Suehara N, Wada Y. Immunohistochemical distribution of Toll-like receptor 4 in term and preterm human placentas from normal and complicated pregnancy including chorioamnionitis. Hum Pathol. 2004;35:47–54. doi: 10.1016/j.humpath.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Lee HK, Lee J, Tobias PS. Two lipoproteins extracted from Escherichia coli K-12 LCD25 lipopolysaccharide are the major components responsible for Toll-like receptor 2-mediated signaling. J Immunol. 2002;168:4012–4017. doi: 10.4049/jimmunol.168.8.4012. [DOI] [PubMed] [Google Scholar]
- Acharya G, Erkinaro T, Makikallio K, Lappalainen T, Rasanen J. Relationships among Doppler-derived umbilical artery absolute velocities, cardiac function, and placental volume blood flow and resistance in fetal sheep. Am J Physiol. 2004;286:H1266–H1272. doi: 10.1152/ajpheart.00523.2003. [DOI] [PubMed] [Google Scholar]
- Block BS, Parer JT, Court DJ, Llanos AJ. Continuous measurement of oxygen consumption in fetal sheep. Am J Obstet Gynecol. 1984;150:406–408. doi: 10.1016/s0002-9378(84)80148-9. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Arduini D. Fetal cardiac function in intrauterine growth retardation. Am J Obstet Gynecol. 1991;165:876–882. doi: 10.1016/0002-9378(91)90431-p. [DOI] [PubMed] [Google Scholar]
- Makikallio K, Vuolteenaho O, Jouppila P, Rasanen J. Association of severe placental insufficiency and systemic venous pressure rise in the fetus with increased neonatal cardiac troponin T levels. Am J Obstet Gynecol. 2000;183:726–731. doi: 10.1067/mob.2000.106753. [DOI] [PubMed] [Google Scholar]
- Makikallio K, Vuolteenaho O, Jouppila P, Rasanen J. Ultrasonographic and biochemical markers of human fetal cardiac dysfunction in placental insufficiency. Circulation. 2002;105:2058–2063. doi: 10.1161/01.cir.0000015505.24187.fa. [DOI] [PubMed] [Google Scholar]
- Tulzer G, Gudmundsson S, Rotondo KM, Wood DC, Yoon GY, Huhta JC. Acute fetal ductal occlusion in lambs. Am J Obstet Gynecol. 1991;165:775–778. doi: 10.1016/0002-9378(91)90327-n. [DOI] [PubMed] [Google Scholar]