Abstract
To investigate the therapeutic potential of bone marrow transplantation in Duchenne muscular dystrophy, green fluorescent protein-positive (GFP+) bone marrow cells were transplanted into irradiated wild-type and dystrophin-deficient mdx mice. Tibialis anterior muscles showed fivefold to sixfold more GFP+ mononucleated cells and threefold to fourfold more GFP+ myofibers in mdx than in wild-type mice. In contrast, dystrophin expression in mdx mice remained within the level of nontransplanted mdx mice, and co-expression with GFP was rare. Longitudinal sections of 5000 myofibers showed 160 GFP+ fibers, including 9 that co-expressed dystrophin. GFP was always visualized as full-length sarcoplasmic fluorescence that exceeded the span of sample length (up to 1500 μm), whereas dystrophin expression was restricted to 11 to 28% of this length. Dystrophin expression span was much shorter in GFP+ fibers (116 ± 46 μm) than in revertant fibers (654 ± 409 μm). These data suggest that soluble GFP diffuses far from the fusion site with a pre-existing dystrophin− myofiber whereas dystrophin remains mainly expressed close to the site of fusion. Because restoration of dystrophin in whole muscle fiber length is required to expect functional improvement and clinical benefits for Duchenne muscular dystrophy, future applications of cell therapies to neuromuscular disorders could be more appropriately envisaged for replacement of defective soluble sarcoplasmic proteins.
Duchenne muscular dystrophy (DMD) is due to the absence in muscle fibers of a large submembrane protein called dystrophin. There are three potential treatments to restore the expression of dystrophin, pharmacological methods,1 gene therapy,2,3 and cell therapy,4 of which future success is uncertain because of puzzling preclinical results. Clinical trials of cell therapy using direct myoblast transplantation into muscle have produced limited results due to massive cell death, limited diffusion, and poor fusion of the transplanted cells. Evidence that bone marrow (BM)-derived cells can give rise to skeletal muscle fibers5–8 and restore dystrophin in vivo9 has suggested a possible benefit of BM-derived cell transplantation in the treatment of DMD in the future.10 However, efficiency of fusion of circulating BM-derived cells with pre-existing muscle fibers appears somewhat discrepant in the literature. On the one hand, experiments using transplantation of green fluorescent protein (GFP)-expressing BM cells in normal irradiated mice have convincingly demonstrated the presence of GFP+ muscle fibers assessing prior fusion with BM-derived cells. Up to 5% of total muscle fibers expressed GFP in some muscle groups in steady state conditions.7,8,11 This rate of fusion remains too low to expect clinical benefits in DMD, but evidence that chronic exercise can induce a 12-fold increase of GFP+ muscle fiber density7 suggests that homing of BM-derived cells into muscle could well be manipulated to improve the phenomenon for therapeutic purposes. On the other hand, dystrophin restoration after BM transplantation was always <1% of total muscle fibers during the life span of transplanted mdx dystrophin-deficient mice.12 Taken together these studies may suggest some discrepancy between GFP and dystrophin expressions the significance of which has not been investigated. The mechanisms by which circulating cells settle muscle tissue remain elusive. Moreover, the number of fusion events necessary to induce detectable expression of a circulating cell-derived gene by a pre-existing myofiber deficient for this gene has not been investigated. This point, however, is crucial to appreciate feasibility of treating deficiencies of dystrophin and other muscle membrane-associated proteins using BM-derived cells because membrane protein restoration along the whole muscle fiber length is likely required to expect functional improvement and clinical benefits after cell therapy. In the present study, we compared spatial distribution of GFP and dystrophin expression using both cross and longitudinal muscle sections in dystrophin-deficient mdx mice transplanted with GFP+ and dystrophin+ BM-derived cells.
Materials and Methods
Mouse Strain
B6 (C57BL/6) mice and mdx (Dmdmdx-4Cv) were used. The Dmdmdx-4Cv mutant in which a C-to-T nucleotide transition generates a stop codon in exon 53 of the dystrophin gene has been said to have almost no background of revertant fibers in skeletal muscle.12 Both mouse strains were transplanted with BM-derived cells from B6TgGFP transgenic mice [C57BL/6TgN(actEGFP)Osb YO1] in which the GFP transgene is expressed under the control of a nontissue-specific promoter, chicken β-actin with cytomegalovirus enhancer, as a cytoplasmic protein.13 In B6TgGFP mice, both BM cells and muscle fibers constitutively express GFP. Therefore, after BM transplantation, GFP served as an unambiguous marker for donor-derived cells in host muscle. B6, mdx, and B6TgGFP mice were housed in our level 2 biosafety animal facility, and received food and water ad libitum. Before manipulations, animals were anesthetized using intraperitoneal injection of chloral hydrate. This study was conducted in accordance with the EC guidelines for animal care (Journal Officiel des Communautés Européennes, L358, December 18, 1986).
BM Transplantation
Briefly, donor BM cells were obtained by flushing femurs of B6TgGFP mice with Dulbecco’s modified Eagle’s medium (Invitrogen, Paisley, UK), and washed twice in cold phosphate-buffered saline (PBS). Retro-orbital injection of 3 to 5 × 107 BM cells in 0.1 ml of mouse serum and PBS (1:1), was done in 9.0 Gy-irradiated, 4-week-old mdx and B6 mice (60Co γ rays within 1 day before BM transplantation). After transplantation, mice received ciprofloxacin, 10 mg/kg/day, for 4 weeks to prevent infection during the aplastic phase.
Flow Cytometry Analysis
To quantify the amount of engraftment, the peripheral blood mononuclear cells of transplanted mice were analyzed by flow cytometry using a XL cytometer (Beckman-Coulter, Hialeah, FL) before sacrifice, ie, at 1, 3, and 6 months after transplantation. Leukocytes were gated on, and GFP fluorescence was measured under the fluorescein isothiocyanate channel. All analyses and quantitation were performed using the System II software from Beckman-Coulter.
Tissue Preparation
Paraformaldehyde fixation is necessarily used to retain GFP within cells, rapid loss of the GFP signal being observed in fresh-frozen sections.8 At sacrifice time mice were anesthetized and sequentially transcardially perfused with PBS and buffered 4% paraformaldehyde. Glutaraldehyde was avoided to minimize autofluorescence.14 Whole muscles were then carefully dissected and pinned at each myotendinous extremity in slightly stretched position, then postfixed in 4% paraformaldehyde for 2 hours, and soaked in 10% sucrose in PBS for 2 hours and then in 30% sucrose overnight at 4°C. Whole muscle samples were snap-frozen in embedding medium (Tissue-Tek; Sakura, Japan) and serial 7-μm-thick cuts on both longitudinal and cross sections were performed. All sections were then coverslipped with Vectashield mounting medium for fluorescence (Vector Laboratories, Burlingame, CA) with or without a nuclear counterstaining by 4,6-diamidino-2-phenylindole (DAPI). The oxidative NADH tetrazolium reductase (NADH-TR) was performed using the conventional procedure.
Immunohistochemistry
GFP immunostaining was done using a primary rabbit polyclonal antibody (1:100; Molecular Probes Inc., Eugene, OR) and biotinylated anti-rabbit antibody revealed by peroxidase-conjugated streptavidin (1:400, Vector Laboratories) and AEC substrate (DAKO, Glostrup, Denmark) after gentle trypsinization for 10 minutes at 37°C for antigen retrieval. Collagen IV immunostaining was done using a primary rabbit polyclonal antibody (1:200; Chemicon, Temecula, CA) and Cy3 secondary anti-rabbit antibody (1:100; BD Pharmingen, San Diego, CA) without antigen retrieval procedure. We also used biotinylated rat anti-mouse antibodies to CD11b (1:100, BD Pharmingen) revealed by tetramethyl-rhodamine isothiocyanate-conjugated streptavidin (1:400, Vector Laboratories). Mouse monoclonal antibodies were used at the following concentrations: anti-dystrophin-2, 1:20 (Novocastra, Newcastle on Tyne, UK); anti-laminin-1, 1:100 (Sigma-Aldrich); anti-M-cadherin, 1:100 (NanoTools, Teningen, Germany); anti-NCAM, 1:100 (BD Pharmingen); and anti-Pax7, 1:100 (Developmental Studies Hybridoma Bank) with M.O.M. kit (Vector Laboratories) allowing the use of mouse monoclonal antibodies for mouse tissues. The secondary antibody used was tetramethyl-rhodamine isothiocyanate-conjugated goat anti-mouse (1:200; Jackson Laboratory, Bar Harbor, ME) or biotinylated rat anti-mouse antibody (1:100, BD Pharmingen) revealed by tetramethyl-rhodamine isothiocyanate-conjugated streptavidin (1:400, Vector Laboratories). In all these experiments, muscle sections were pretreated for antigen retrieval. To obtain dystrophin immunostaining on fixed tissue, muscle sections were incubated for 20 minutes in 95°C acid citrate buffer. For all others immunostainings, antigen retrieval was done using gentle trypsinization for 10 minutes at 37°C.
Fluorescence Microscopy
Immunostained sections were examined using both a LSM 410M and 510M confocal microscope and a Zeiss Axiophot microscope (Carl Zeiss Inc., Germany). Quantitative studies were done using an Orca ER digital camera (Hamamatsu Photonics, Japan) and a Simple PCI software (C-Imaging; Compix, Inc.), as previously described.8 We used a double-fluorescence analysis procedure including detection of green fluorescence through a very narrow bandpass (emission, 505 to 530 nm) and control of specificity through highpass (emission, 515 to 680 nm). Using the narrow bandpass, specific GFP fluorescence and autofluorescence exhibit a similar green shade but markedly differ by both fluorescence intensity and pattern of expression. As shown in Figure 1A, autofluorescent fibers, in addition to being less fluorescent, showed marginal enhancement not observed in GFP+ muscle fibers. Through highpass, GFP fluorescence and autofluorescence could be definitely distinguished, autofluorescent fibers showing weak, marginal fluorescence with a yellow-green shade easy to distinguish from the bright, diffuse and pure green fluorescence of GFP+ fibers, as shown in Figure 1I. Of course, autofluorescent muscle fibers and macrophages were not taken into account in the study. This procedure was supported by spectral analysis of regions of interest into both GFP+ and autofluorescent fibers using the Meta system on a LSM 510 Carl Zeiss confocal microscope. This analysis confirmed clearly different spectral emission profiles due to scattered emission of autofluorescence, in keeping with the yellow-green appearance of autofluorescent fibers detected through highpass IF (data not shown).
Figure 1.
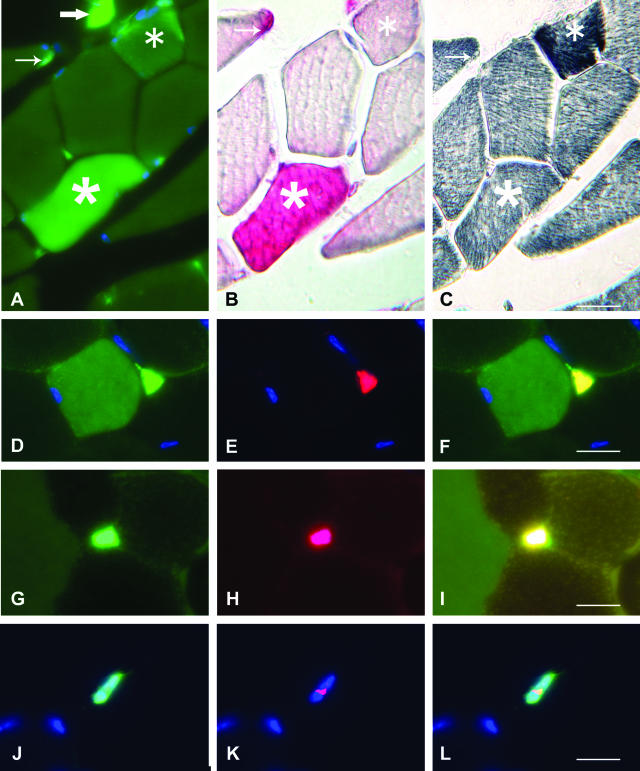
A–C: Alternate TA muscle cross-sections from B6 mice, 6 months after transplantation with GFP+ BM cells, showing fluorescent structures through narrow bandpass; GFP immunoreactivity is revealed in red by the peroxidase-AEC reagent and the NADH-TR oxidative enzymatic reaction. The GFP+ muscle fiber (five-branch star) shows diffuse cytoplasmic fluorescence, positive GFP immunoreactivity, and mild oxidative activity. The autofluorescent fiber (six-branch star) shows marginal fluorescence, negative GFP immunoreactivity, and strong oxidative activity. Please note the presence of a GFP+ interstitial mononucleated cell (large arrow) and a GFP+ satellite cell (small arrow). D–L: Satellite cell markers expressed by GFP+ mononucleated cells associated with TA muscle fibers, 6 months after transplantation. D shows a GFP+ mononucleated cell associated with a GFP+ myofiber, E shows M-cadherin expression, and F is a merge image assessing the satellite cell nature of the GFP+ mononucleated cell. G shows a GFP+ mononucleated cell associated with a GFP− myofiber adjacent to a GFP+ myofiber, H shows NCAM expression, and I is a merge image assessing the satellite cell nature of the GFP+ mononucleated cell; please note the different pattern of fluorescence of the GFP+ muscle fiber (left) and autofluorescent fiber (right) (merged picture corresponding to high-pass immunofluorescence microscopy). J shows a GFP+ mononucleated cell in the absence of GFP+ myofibers, K shows expression of the nuclear transcription factor Pax7, and L is a merge image assessing the satellite cell nature of the GFP+ mononucleated cell. Nuclei are labeled with DAPI (blue). Scale bars, 10 μm.
Results
Fifteen mdx mice and twelve B6 mice transplanted with GFP+ BM cells were included in the study. All had a proportion of GFP+ peripheral blood mononucleated cells >95% as compared to donor values.
GFP+ Mononuclear Cells and Muscle Fibers Are Found in Both Mdx and B6 Mice
Nine transplanted mdx mice and nine B6 mice were sacrificed at 1, 3, and 6 months after transplantation to evaluate GFP expression in tibialis anterior muscle cross-sections. Both GFP+ muscle fibers and GFP+ mononucleated cells were detected. The presence of cytoplasmic GFP was assessed by both green fluorescence and chromogenic immunolabeling as shown in Figure 1, A and B. GFP+ muscle fibers could be easily distinguished from autofluorescent muscle fibers that were slightly atrophic and markedly oxidative as previously reported,14 in addition of being punctate in appearance and yellowish at immunofluorescence microscopy through highpass (Figure 1; A to C and I). GFP+ mononucleated cells included interstitial cells and cells tightly associated to muscle fibers (Figure 1, A and B). Interstitial cells were mainly CD11b+ macrophages, whereas muscle fiber-associated cells were CD11b−, appeared sublaminal after laminin-1 or collagen IV immunostaining, and expressed the canonical satellite cell markers M-cadherin (Figure 1; D to F), N-CAM (Figure 1; G to I), and the nuclear transcription factor Pax7 (Figure 1; J to L). Sublaminal location was used to recognize satellite cells for quantitative evaluations.
GFP+ Myofibers Are More Numerous in Mdx Than in B6 BM Recipient Mice
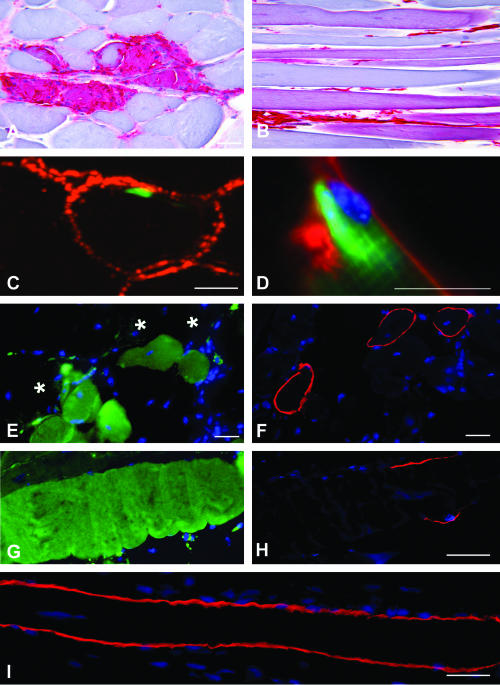
GFP+ mononucleated cells were found in TA muscle cross-sections in higher numbers in mdx than in B6 mice at each time point: 100 ± 25.4% muscle fibers versus 14.5 ± 2.7% at 1 month (P < 0.001), 115.5 ± 19% versus 15 ± 4% at 3 months (P < 0.001), and 101.5 ± 8.2% versus 20.5 ± 3.6% at 6 months (P < 0.001). Most of these cells were CD11b+ macrophages (Table 1). The proportion of GFP+ myofibers at each time point was significantly higher in mdx than in B6 mice: 1.5 ± 0.2% muscle fibers versus 0% at 1 month, 1.8 ± 0.6% versus 0.6 ± 0.2% at 3 months (P < 0.05), and 4.1 ± 1.1% versus 1 ± 0.2% at 6 months (P < 0.01). Of note, strong GFP positivity was observed in foci of muscle fiber necrosis and myophagocytosis typical of the muscle dystrophic process (Figure 2A). Such a GFP expression was not taken into account. However, in contrast to B6 mice in which GFP+ muscle fibers were usually found in isolation, mdx mice frequently showed clusters of nonnecrotic GFP+ muscle fiber profiles (Figure 2, B and E). Such clusters of nonnecrotic GFP+ muscle fibers suggested a relationship of GFP expression with the necrosis/regeneration process. Some GFP+ muscle fibers showed centronucleation and others did not (Figure 2B), suggesting either local GFP expression at the site of previous muscle fiber repair or GFP diffusion from the necrotic areas along nonnecrotic portions of focally damaged fibers. In contrast to GFP+ muscle fibers, density of GFP+ satellite cells was similar in mdx and B6 mice (Table 1) and increased with time in both groups (P < 0.05). As B6 mice, mdx mice showed a proportion of isolated GFP+ muscle fibers and rare pictures suggestive of individual satellite cell fusion with the underlying muscle fiber (Figure 2, C and D).
Table 1.
GFP+ Mononucleated Cells in Muscle Cross Sections in Wild-Type and mdx Mice at Various Time Points after BM Transplantation
| Time after transplant | Transplanted B6
|
Transplanted mdx
|
||||||
|---|---|---|---|---|---|---|---|---|
| (n) | Myofibers evaluated | GFP+CD11b+ cells (for 100 myofibers) | GFP+ sat cells (for 100 myofibers) | (n) | Myofibers evaluated | GFP+CD11b+ cells (for 100 myofibers) | GFP+ sat cells (for 100 myofibers) | |
| 1 month | 3 | 389–531 | 6.9 ± 1.4 | 0.6 ± 0.1 | 3 | 542–697 | 84.3 ± 35.3 | 0.6 ± 0.1 |
| 3 months | 3 | 423–689 | 6.4 ± 0.3 | 0.8 ± 0.2 | 3 | 562–778 | 88.1 ± 21.6 | 0.9 ± 0.5 |
| 6 months | 3 | 571–701 | 7.1 ± 1.5 | 1.1 ± 0.3 | 3 | 754–901 | 93.4 ± 5.9 | 1.3 ± 0.4 |
Figure 2.
TA muscle of mdx mice transplanted with GFP+ BM cells. A and B: GFP immunodetection revealed in red by the peroxidase-AEC reagent. A: A cross-section shows strong immunoreactivity in a necrotic/regenerating area characteristic of muscular dystrophy. Immunoreactivity is found in both inflammatory cells and sarcoplasm of damaged muscle fibers. B: A longitudinal section shows several immunoreactive profiles mainly corresponding to nonnecrotic muscle fibers. C and D: Merged images of green GFP fluorescence and red fluorescence corresponding to basal lamina antigens. One BM-derived cell in a sublaminal satellite cell niche (C merges confocal microscopic images of GFP and collagen IV). A picture suggestive of an ongoing fusion between a sublaminal BM-derived satellite cell and the underlying muscle fiber (D merges immunofluorescence microscopic image of GFP and laminin-1). E and F: Consecutive cross-sections show no co-localization of GFP (E) and dystrophin (F). G and H: Consecutive longitudinal sections show a muscle fiber with extensive GFP sarcoplasmic expression (G) but short sarcolemmal dystrophin expression suspended between dystrophin-negative fiber portions (H). I: Longitudinal muscle section of nontransplanted mdx mouse used as control showing a revertant fiber extensively expressing dystrophin (fluorescence microscopy). Nuclei are labeled with DAPI (blue). Scale bars, 10 μm.
BMT Does Not Increase Dystrophin Expression in mdx Muscle Cross-Sections
Unlike GFP, dystrophin immunocytochemical expression did not increase in muscle cross-sections of mdx mice muscle from 1 to 6 months after transplantation, and accounted for less than 1% of myofibers at each time point (Table 2). Evaluation of GFP expression on alternate cross-sections showed little co-localization, most dystrophin+ myofibers being GFP− (Figure 2, E and F). Taken together with the similar proportion of dystrophin+ fibers observed in transplanted and nontransplanted age-matched mdx mice (n = 3 in both groups at each time point, Table 2), this data suggested some background reversion, a previously reported but poorly acknowledged finding in mdx 4 cv mice.15
Table 2.
GFP and Dystrophin Expression in Muscle Cross Sections of Wild-Type and mdx Mice at Various Time Points after BM Transplantation and in Age-Matched mdx Controls
| Time after transplant | Transplanted mice
|
Age-matched controls
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B6
|
Mdx | mdx | |||||||||
| (n) | Myofibers evaluated | % GFP+ myofibers | % Dys expression | (n) | Myofibers evaluated | % GFP+ myofibers | % Dys expression | (n) | Myofibers evaluated | % Dys expression | |
| 1 month | 3 | 862–921 | 0 ± 0 | 100 | 3 | 521–1135 | 1.5 ± 0.2 | 0.8 ± 0.3 | 3 | 896–987 | 0.6 ± 0.1 |
| 3 months | 3 | 721–823 | 0.6 ± 0.2 | 100 | 3 | 655–927 | 1.8 ± 0.6 | 0.8 ± 0.2 | 3 | 706–1011 | 0.8 ± 0.05 |
| 6 months | 3 | 877–983 | 1 ± 0.2 | 100 | 3 | 607–1306 | 4.1 ± 1.1 | 0.7 ± 0.4 | 3 | 763–906 | 0.9 ± 0.2 |
Extensive Sarcoplasmic Expression of GFP Contrasts with Restricted Sarcolemmal Expression of Dystrophin
Because complete lack of dystrophin expression at the level of BM-derived cell fusion with individual mdx muscle fibers appeared unlikely, we analyzed serial longitudinal sections of 5000 TA muscle fibers collected from three additional mdx mice 6 months after transplantation alternately immunostained for dystrophin and examined for GFP fluorescence, compared to 2500 muscle fibers from one nontransplanted mdx mouse of the same age, immunostained for dystrophin. In the mdx control, 0.48% of fibers expressed dystrophin (12 of 2500). Fifty percent of these revertant fibers showed extensive sarcolemmal expression of dystrophin exceeding the span of sample length (ie, 334 to 1435 μm; mean, 673 ± 409 μm) (Figure 2I). The remaining 50% showed partial sarcolemmal expression ranging from 161 to 1235 μm in length (mean , 635 ± 451 μm) corresponding to 55 to 91% of the analyzed muscle fiber length (Table 3). The mean distance of dystrophin expression was 654 ± 409 μm. Of note, dystrophin positivity in revertant fibers never appeared as suspended between two negative sarcolemmal portions suggesting a larger extent of dystrophin expression than that observed.
Table 3.
GFP and Dystrophin Co-Expression in Serial Longitudinal Sections of 9 of 5000 Myofibers from Three mdx Mice 6 Months after Transplantation, and Dystrophin Expression in 12 of 2500 Myofibers from One Nontransplanted mdx Mouse of the Same Age Used as a Control for Revertant Fibers
| No. | BM-transplanted mdx mice
|
Nontransplanted mdx mouse
|
||||
|---|---|---|---|---|---|---|
| Analyzed myofiber length in μm | Length of GFP expression in μm (% of analyzed length) | Length of dystrophin expression in μm (% of analyzed length) | No. | Analyzed myofiber length in μm | Length of dystrophin expression in μm (% of analyzed length) | |
| 1 | 417 | 417 (100%) | 110 (26%) | 1 | 402 | 402 (100%) |
| 2 | 393 | 392 (100%) | 83 (21%) | 2 | 228 | 161 (71%) |
| 3 | 397 | 397 (100%) | 45 (11%) | 3 | 334 | 334 (100%) |
| 4 | 482 | 482 (100%) | 77 (16%) | 4 | 1442 | 1442 (100%) |
| 5 | 873 | 873 (100%) | 148 (17%) | 5 | 899 | 497 (55%) |
| 6 | 721 | 721 (100%) | 138 (19%) | 6 | 540 | 540 (100%) |
| 7 | 1252 | 1252 (100%) | 193 (15%) | 7 | 525 | 525 (100%) |
| 8 | 835 | 834 (100%) | 152 (18%) | 8 | 1350 | 1236 (92%) |
| 9 | 342 | 342 (100%) | 97 (28%) | 9 | 1407 | 1160 (82%) |
| 10 | 798 | 798 (100%) | ||||
| 11 | 464 | 319 (69%) | ||||
| 12 | 665 | 441 (66%) | ||||
In transplanted mdx mice, GFP expression always appeared as a full-length sarcoplasmic fluorescence exceeding the span of sample length (up to 1500 μm). Only a minority of GFP+ fibers (9 of 160) co-expressed dystrophin. Unlike revertant fibers, dystrophin expression by GFP+ fibers was restricted to a very limited sarcolemmal distance ranging from 45 to 194 μm (mean, 116 ± 47 μm) corresponding to 11 to 28% (mean, 19%) of the analyzed muscle fiber length. Six of nine fibers showed dystrophin expression suspended between two negative portions of sarcolemma (Figure 2, G and H) and seven showed centronucleation at the level of dystrophin expression. The GFP− and dystrophin+ fibers were more numerous (31 of 40) and showed a pattern similar to revertant fibers observed in the control. Taken together, these data strongly suggest that the soluble cytoplasmic protein GFP diffuses far away from the site of BM-derived cell fusion with a pre-existing myofiber whereas dystrophin remains mainly expressed close to the site of fusion.
Discussion
In this study, GFP+ BM-derived cells fused with adult myofibers at a higher rate in mdx than in wild-type mice, as assessed by an increase of GFP+ myofibers. However, fusion of dystrophin+ GFP+ BM-derived cells was not associated with significant restoration of dystrophin expression in mdx mice when assessed on muscle cross-sections, due to marked contrast between extensive sarcoplasmic expression of GFP and short sarcolemmal expression of dystrophin along myofibers.
These results reconcile data from the literature using GFP expression6–8 or dystrophin restoration9,12 as a marker of fusion of BM-derived cells with pre-existing myofibers. Discrepancy between GFP and dystrophin expression along myofibers may be theoretically due to two different mechanisms, limited nuclear reprogramming events and different diffusion properties of GFP and dystrophin: 1) unlike GFP, which is constitutively expressed by all cells independently from their lineage in the model used,16 dystrophin is only expressed by cells undergoing terminal muscle differentiation. The exact type of circulating cells able to fuse with pre-existing muscle fibers (ie, hematopoietic stem cells or mesenchymal stem cells or both) is still fiercely debated.12,17–20 It is most likely that hematopoietic cells of the myeloid lineage can fuse to muscle fibers and acquire myogenic properties.18–20 In this situation, myogenic transactivation of nuclei from newly fused hematopoietic cells result in their myogenic reprogrammation. If incomplete, such reprogramming events may well account for restricted or absent dystrophin expression. We emphasize that participation of hematopoietic cells to muscle fiber formation has been exclusively demonstrated in the setting of marked muscle regeneration induced by repeated bouts of cardiotoxin-induced myonecrosis.18–20 Conspicuous muscle necrosis/regeneration is characteristic of the dystrophic process in mdx mice, and stochastic fusion events of inflammatory cells with regenerating muscle fibers likely occurred in this setting. This was suggested by strong GFP positivity observed in foci of muscle fiber necrosis and myophagocytosis, and frequent detection of GFP+ muscle fiber clusters remote from necrotic areas. In addition, however, sublaminal BM-derived cells and isolated GFP+ muscle fibers were similarly found in mdx and B6 mice in the present study. This suggested a more conventional myogenic differentiation process previously reported in steady state conditions,6–8 involving formation of bona fide satellite cells and subsequent accretion of these cells to the underlying muscle fiber. Finally, it seems likely that the higher rate of GFP+ muscle fiber formation observed in mdx mice as compared to B6 mice, resulted from a combination of natural satellite cell accretion found in both strains, and stochastic fusions of inflammatory cells directly related to the dystrophic process. 2) Expression domain of membrane-bound dystrophin may differ from that of cytosolic GFP. It is widely accepted that some muscle proteins are present only within a limited distance from the nucleus containing the relevant activated gene, forming the so-called nuclear domain.21 Both existence and length of dystrophin nuclear domains may depend on the type of muscle fiber growth. During in vitro myogenesis, dystrophin mainly appears after fusion in myotubes containing at least three nuclei.22 Hybrid myotubes formed in vitro by the fusion of normal rat and dystrophic mdx mouse myoblasts showed that dystrophin was present over the entire membrane of all hybrid myotubes even when nuclei ratio normal/dystrophic was low (as low as 1 of 12).22 In vivo transplantation of normal myoblasts into mdx mouse muscle23 in which myogenic cells fuse with pre-existing fibers, showed that the dystrophin nuclear domain is ∼300 to 400 μm.24,25 These results are very similar to those we observed after BM transplantation (116 μm). In a previous study using direct myoblast injection in mdx muscle, the dystrophin domain was shown to be twofold to threefold smaller than that of soluble cytoplasmic β-galactosidase used as a reporter gene.25 The domain of GFP expression has not been previously determined but seems very extensive as demonstrated in the present study. In fact, GFP seems to be a highly diffusible cytosolic protein as assessed by rapid leakage of GFP from cryosectioned myofibers in the absence of prior fixation observed by us and others.7
In the present study, the level of revertant background of transplanted and nontransplanted mdx 4 cv mice was significant, as previously reported.15 It is not surprising that revertant fibers often showed extensive continuous longitudinal expression of dystrophin since similar lengths have been previously reported.26 Continuous increase of longitudinal revertant dystrophin expression, attributed to clonal expansion of revertant myoblasts from developmental stages, has been observed until 18 months in mdx mice.26
We conclude that: 1) muscle settlement by BM-derived cells and fusion of these cells with pre-existing myofibers occur at higher rate in mdx mice than in wild-type mice; 2) the fusion rate in mdx mice should not be overestimated, GFP being seemingly able to diffuse long distances away from the point of fusion of BM-derived cells with the myofiber whereas dystrophin expression remains apparently restricted to the nuclear domain of newly fused nuclei; 3) considering the thousands of nuclear domains per muscle fiber, future applications of stem cell therapies to neuromuscular disorders could be more appropriately envisaged for replacement of defective soluble sarcoplasmic proteins than for dystrophin deficiency.
Footnotes
Address reprint requests to Pr. Romain K. Gherardi, EMI0011, Faculté de Médecine, 8 rue du Général Sarrail, 94000 Créteil, France. E-mail: romain.gherardi@hmn.ap-hop-paris.fr.
Supported by the Association Française contre les Myopathies and the Fond d’étude et de recherche du corps médical des Hôpitaux de Paris.
F.C. and P.A.D. have equally contributed to this work.
References
- Wagner KR, Hamed S, Hadley DW, Gropman AL, Burstein AH, Escolar DM, Hoffman EP, Fischbeck KH. Gentamicin treatment of Duchenne and Becker muscular dystrophy due to nonsense mutations. Ann Neurol. 2001;49:706–711. [PubMed] [Google Scholar]
- Kapsa R, Kornberg AJ, Byrne E. Novel therapies for Duchenne muscular dystrophy. Lancet Neurol. 2003;2:299–310. doi: 10.1016/s1474-4422(03)00382-x. [DOI] [PubMed] [Google Scholar]
- Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan JC, Garcia L, Danos O. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science. 2004;306:1796–1799. doi: 10.1126/science.1104297. [DOI] [PubMed] [Google Scholar]
- Torrente Y, Belicchi M, Sampaolesi M, Pisati F, Meregalli M, D’Antona G, Tonlorenzi R, Porretti L, Gavina M, Mamchaoui K, Pellegrino MA, Furling D, Mouly V, Butler-Browne GS, Bottinelli R, Cossu G, Bresolin N. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest. 2004;114:182–195. doi: 10.1172/JCI20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Fukada S, Miyagoe-Suzuki Y, Tsukihara H, Yuasa K, Higuchi S, Ono S, Tsujikawa K, Takeda S, Yamamoto H. Muscle regeneration by bone marrow or fetal liver cells from green fluorescent protein-gene transgenic mice. J Cell Sci. 2002;115:1285–1293. doi: 10.1242/jcs.115.6.1285. [DOI] [PubMed] [Google Scholar]
- LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- Dreyfus PA, Chretien F, Chazaud B, Kirova Y, Caramelle P, Garcia L, Butler-Browne G, Gherardi RK. Adult bone marrow-derived stem cells in muscle connective tissue and satellite cell niches. Am J Pathol. 2004;164:773–779. doi: 10.1016/S0002-9440(10)63165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Bennett RR, Muskiewicz KR, Meyerrose T, Nolta JA, Gilgoff I, Stein J, Chan YM, Lidov HG, Bonnemann CG, Von Moers A, Morris GE, Den Dunnen JT, Chamberlain JS, Kunkel LM, Weinberg K. Long-term persistence of donor nuclei in a Duchenne muscular dystrophy patient receiving bone marrow transplantation. J Clin Invest. 2002;110:807–814. doi: 10.1172/JCI16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton TR, Nystrom M, Blau HM. Significant differences among skeletal muscles in the incorporation of bone marrow-derived cells. Dev Biol. 2003;262:64–74. doi: 10.1016/s0012-1606(03)00357-9. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Stornaiuolo A, Mavilio F. Failure to correct murine muscular dystrophy. Nature. 2001;441:1014–1015. doi: 10.1038/35082631. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Snyder DS, Goodell MA. Skeletal muscle fiber-specific green autofluorescence: potential for stem cell engraftment artifacts. Stem Cells. 2004;22:180–187. doi: 10.1634/stemcells.22-2-180. [DOI] [PubMed] [Google Scholar]
- Danko I, Chapman V, Wolff JA. The frequency of revertants in mdx mouse genetic models for Duchenne muscular dystrophy. Pediatr Res. 1992;32:128–131. doi: 10.1203/00006450-199207000-00025. [DOI] [PubMed] [Google Scholar]
- Manfra DJ, Chen SC, Yang TY, Sullivan L, Wiekowski MT, Abbondanzo S, Vassileva G, Zalamea P, Cook DN, Lira SA. Leukocytes expressing green fluorescent protein as novel reagents for adoptive cell transfer and bone marrow transplantation studies. Am J Pathol. 2001;158:41–47. doi: 10.1016/S0002-9440(10)63942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti S, Strazzer S, Del Bo R, Salani S, Bossolasco P, Fortunato F, Locatelli F, Soligo D, Moggio M, Ciscato P, Prelle A, Borsotti C, Bresolin N, Scarlato G, Comi GP. A subpopulation of murine bone marrow cells fully differentiates along the myogenic pathway and participates in muscle repair in the mdx dystrophic mouse. Exp Cell Res. 2002;277:74–85. doi: 10.1006/excr.2002.5543. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Green R, Capetenaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med. 2003;9:1520–1527. doi: 10.1038/nm963. [DOI] [PubMed] [Google Scholar]
- Corbel SY, Lee A, Yi L, Duenas J, Brazelton TR, Blau HM, Rossi FM. Contribution of hematopoietic stem cells to skeletal muscle. Nat Med. 2003;9:1528–1532. doi: 10.1038/nm959. [DOI] [PubMed] [Google Scholar]
- Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- Pavlath GK, Rich K, Webster SG, Blau HM. Localization of muscle gene products in nuclear domains. Nature. 1989;337:570–573. doi: 10.1038/337570a0. [DOI] [PubMed] [Google Scholar]
- Huard J, Labrecque C, Dansereau C, Robitaille L, Tremblay JP. Dystrophin expression in myotubes formed by the fusion of normal and dystrophin myoblasts. Muscle Nerve. 1991;14:178–182. doi: 10.1002/mus.880140213. [DOI] [PubMed] [Google Scholar]
- Smith HK, Maxwell L, Rodgers CD, McKee NH, Plyley MJ. Exercise-enhanced satellite cell proliferation and new myonuclear accretion in rat skeletal muscle. J Appl Physiol. 2001;90:1407–1414. doi: 10.1152/jappl.2001.90.4.1407. [DOI] [PubMed] [Google Scholar]
- Karpati G, Pouliot Y, Zubrzycka-Gaarn E, Carpenter S, Ray PN, Worton RG, Holland P. Dystrophin is expressed in mdx skeletal muscle fibers after normal myoblast implantation. Am J Pathol. 1989;135:27–32. [PMC free article] [PubMed] [Google Scholar]
- Kinoshita I, Vilquin JT, Asselin I, Chamberlain J, Tremblay JP. Transplantation of myoblasts from a transgenic mouse overexpressing dystrophin produced only a relatively small increase of dystrophin-positive membrane. Muscle Nerve. 1998;21:91–103. doi: 10.1002/(sici)1097-4598(199801)21:1<91::aid-mus12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lu QL, Morris GE, Wilton SD, Ly T, Artem’yeva OV, Strong P, Partridge TA. Massive idiosyncratic exon skipping corrects the nonsense mutation in dystrophic mouse muscle and produces functional revertant fibers by clonal expansion. J Cell Biol. 2000;148:985–991. doi: 10.1083/jcb.148.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]