Abstract
Tissue inhibitor of metalloproteinases-3 (TIMP-3) is known to inhibit matrix metalloproteinases, aggrecanases, and tumor necrosis factor (TNF)-α-converting enzyme (TACE, ADAM17). These metalloproteases participate in different aspects of joint destruction in inflammatory arthritis. To determine the relative importance of this inhibitor in joint pathology, wild-type and Timp3−/− mice were immunized with methylated bovine serum albumin followed by arthritis induction by intra-articular injection of the same antigen. Animals were monitored for up to 14 days after challenge, and joint tissues were analyzed by routine and Safranin O staining and for the presence of aggrecan neoepitopes produced by metalloprotease cleavage. Serum TNF-α was measured by immunoassay. Compared to wild-type animals, Timp3−/− mice showed a dramatic increase in the initial inflammatory response to intra-articular antigen injection, and serum TNF-α levels were greatly elevated in the Timp3−/− animals after immunization. However, these differences in clinical features disappeared by days 7 to 14. No difference in Safranin O staining or aggrecan cleavage site neoepitope abundance was seen. Thus, in inflammatory joint disease TIMP-3 likely dampens the inflammatory response of TNF-α by reducing ADAM17 activity.
The matrix metalloproteinases (MMPs) consist of a large family of proteolytic enzymes that mediate the degradation of many different components of the extracellular matrix.1 The expression and activity of these enzymes, which are thought to be important in the pathological destruction of joint tissue, are controlled at a number of key points. Various cytokines, growth factors, and other agents can stimulate the synthesis and secretion of these proteases2 that are expressed as inactive proenzymes. The pro-MMPs require activation through proteolytic processing of the proregion,3 and the active enzymes can be inhibited by tissue inhibitors of metalloproteinases (TIMPs), which bind to all known active MMPs with a 1:1 stoichiometry. All connective tissues produce members of the TIMP family4 and these molecules play an important role in controlling connective tissue breakdown by blocking the action of active MMPs. Four members of the TIMP family have been described and these share similar secondary and tertiary structures, and are able to inhibit MMPs, although with different potencies.
TIMP-1 is produced in response to a variety of external stimuli, such as growth factors and cytokines whereas expression of TIMP-2 is mostly constitutive.5,6 However, TIMP-3 is unique in many ways. Unlike TIMP-1 and TIMP-2, which are present in soluble forms, TIMP-3 is bound within the extracellular matrix.7,8 It is an inhibitor of several ADAM’s family members such as ADAM-12S,9 and tumor necrosis factor (TNF)-α convertase (TACE, ADAM-17)10,11 that are not inhibited by other TIMPs. In addition, it is an inhibitor of aggrecanase-1 (ADAMTS-4) and aggrecanase-2 (ADAMTS-5).12,13 TIMP-3 has also been shown to inhibit cell shedding of l-selectin,14 syndecan-1, and syndecan-4,15 and interleukin-616 and M-CSF receptors.17 It is also the only member of the TIMP family in which mutations are implicated in human pathology, namely Sorby’s fundus dystrophy, a degenerative eye disease.18 TIMP-3 has also been shown to be proapoptotic in both normal and cancer cell lines.19,20 Although TIMP-3-deficient mice appear phenotypically normal and are fertile, they were found to develop alveolar air space enlargement with a concomitant impaired capacity for gas exchange,21 accelerated mammary gland apoptosis,22 and cardiac dysfunction.23 Very recently, in line with the observations reported in the present study, they also show increased susceptibility to hepatic inflammation.24
These data suggest that TIMP-3 plays important roles in physiological and pathological processes. The special characteristics of TIMP-3 invite a more thorough focus on its biological roles. Because TIMP-3 is the only member of the TIMP family that is retained by the extracellular matrix, possesses a unique ability to inhibit aggrecanases-1 and -2 as well as TACE, and is present in cartilage and synovium,25,26 it seemed likely that it might play a role in arthritis development. Also, it has been shown recently that TIMP-3 inhibits the breakdown of aggrecan in stimulated cartilage cultures, through inhibition of aggrecanase, the synthesis of which is induced by catabolic factors.27 The loss of aggrecan is considered to be a crucial early event in the pathogenesis of arthritis.28,29 As an approach to better understand the functions of TIMP-3 in joint disease, we used an antigen-induced arthritis model in Timp3 knockout animals. In the present study we investigated the histochemical and immunohistological differences between null and matching wild-type mice and show that the major effect of TIMP-3 deficiency is increased production of TNF-α and concomitant joint inflammation, presumably due to the enhanced TACE activity.
Materials and Methods
Animals
The production of Timp3−/− mice in the C57BL/6 background has been described previously.21 Wild-type C57BL/6 mice, used as controls, were from Jackson Laboratories (Bar Harbor, ME). Animals were given a standard laboratory diet and tap water ad libitum. Protocols for use of animals were approved by the local and McGill University animal care committees.
Arthritis Induction
Mice were injected subcutaneously with 100 μl of an emulsion containing 100 μg of methylated bovine serum albumin (mBSA), prepared as described by Mandell and Hershey,30 in normal saline and an equal volume of Freund’s complete adjuvant (Life Technologies, Inc., Gaithersburg, MD) over two sites in the flank. Heat-killed Bordetella pertussis (2 × 109 cells i.p.; Lee Laboratories, Grayson, GA) was administered as an additional adjuvant. Seven days later, booster injections of 100 μg of mBSA in Freund’s incomplete adjuvant were administered subcutaneously, divided over two sites in the neck region, with an additional 2 × 109 killed B. pertussis intraperitoneally. Arthritis was induced on day 21 by an intra-articular injection of 60 μg of mBSA in 6 μl of normal saline into the right knee. The left knee, which received 6 μl of normal saline, was used as a control. Mice were sacrificed at days 3, 7, and 14 after intra-articular injection.
Histochemistry
Knee joints were dissected and then placed in periodate-lysine-paraformaldehyde fixative31 and decalcified, either by treatment in 10% formic acid for 24 hours followed by paraffin embedding (Oxford Labware, St. Louis, MO), or in 10% ethylenediamine tetraacetic acid (w/v in 0.1 mol/L Tris-HCl, pH 7.4) for 3 weeks, followed by rinsing in phosphate-buffered saline (PBS), infiltration in a 2:1 v/v of sucrose (20% w/v in PBS) and OCT compound (Sakura, Torrance, CA), then embedding in OCT alone, followed by snap-freezing.32 In the case of OCT-embedded tissues, sections were cut with a cryostat at −20°C and were stored at −20°C for further use. In this study, we focused on the tibio-femoral compartment.
Safranin O Staining
Paraffin sections were deparaffinized with xylene and solutions of decreasing ethanol percentage. Staining was with 0.5% hematoxylin for 10 minutes, 0.02% Fast Green for 4 minutes (Chroma-Gesellschaft Schmid GMBH and Co., Stuttgart-Unterturkheim, Germany), acetic acid for 1 minute, and Safranin O (BDH Laboratory Supplies, Poole, UK) for 6 minutes. Proteoglycan depletion was determined by the decreased uptake of Safranin O dye. The slides were scored in a blinded manner by two different observers.
Histological Scoring
Each slide was graded for three different parameters: 1) hyperplasia of the lining layer of the synovium (mostly due to proliferation of the fibroblast-like synoviocytes) and infiltration of the synovial membrane with predominantly mononuclear cells; 2) pannus formation and synovial invasion of the cartilage and cartilage destruction; and 3) Safranin O loss, each on a scale of 0 to 3.33,34 Safranin O loss was assessed (where 0, 1, 2, and 3 represent no loss, 30% loss, 60% loss, and 100% loss of red staining, respectively) by the method described by Van Meurs and colleagues.35 A final score was assigned for each animal by calculating the sum of the above values.
Arthritis Scoring
Each animal was scored at days 3, 7, and 14, after intra-articular injection, for joint swelling and difficulty in moving the joint, on a scale of 0 to 3. The sum of these scores was used as an arthritis score for each animal where 0 represents the normal joint and 6 the most severe arthritis.
Immunohistochemistry
Decalcified sections were fixed with 4% formaldehyde and then digested with proteinase-free chondroitinase ABC (0.25 U/ml Tris acetate, pH 7.3; ICN Biomedicals, Aurora, OH) for 1 hour at 37°C to remove glycosaminoglycan chains. Subsequently, sections were treated with 0.3% H2O2 for 30 minutes followed by incubation with 1.5% normal goat or normal donkey serum (depending on the antibody being used) for 20 minutes. The sections were then incubated with primary antibody raised against either VDIPEN or NITEGE36 peptides for 24 hours. Sections were then incubated with biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) or donkey anti-goat (Jackson ImmunoResearch, West Grove, PA) and antigens were detected using avidin-peroxidase staining (Vectastain Elite ABC kit and DAB kits, Vector Laboratories) and counterstained with hematoxylin. Staining was scored in a blinded manner and graded on a scale of 0 to 3: 0, no staining; 1, minor staining; 2, marked staining; and 3, maximal staining defined as staining of ∼50% of the total cartilage layer as previously described by Van Meurs and colleagues.35
Serum TNF-α Assay
Mice were bled 1 hour after bacterial injection and serum-assayed for mouse TNF-α using the OptEIASet ELISA kit (Pharmingen, San Diego, CA).
Statistics
Statistical significance between scores was determined using the Mann-Whitney test. TNF-α levels were analyzed using the Student’s t-test to compare mean values.
Results
Joint Inflammation and Clinical Symptoms of Arthritis
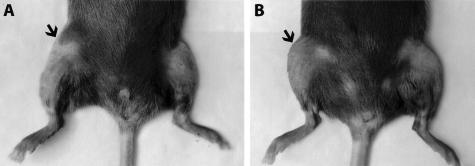
A general inflammatory arthritis model involving direct injection of antigen (mBSA) into the joint of the previously sensitized animal was used because the type II collagen-37 and aggrecan38-induced models, which do not require such direct intervention, are ineffective in the C57BL/6 mouse strain. After intra-articular injection of mBSA, the joints of both Timp3−/− and wild-type mice showed arthritic changes, while the contralateral joints showed no swelling or other pathology. In the wild-type animals clinical symptoms tended to be at their highest between days 2 to 3 after intra-articular injection with joint swelling, difficulty using the affected limb, and occasional redness and warmth over the joint as reported by others.39 By day 7, these symptoms begin to resolve. In Timp3−/− mice, swelling was much more apparent than for age- and sex-matched wild-type mice at days 2 and 3 (Figure 1). Differences in the use of the affected knee were also noticed in the same period. When the clinical scores were combined, a clear difference was seen between the two groups in the first few days of arthritis induction, however with time these differences became much less prominent, with the inflammation resolving and the clinical scores for both wild-type and Timp3−/− declining to almost normal values (Figure 2).
Figure 1.
Gross appearance of Timp3−/− (A) and wild-type (B) mouse knees at day 3 after intra-articular injection of mBSA. The injected limb is on left. Arrowheads indicate swollen areas.
Figure 2.
Arthritis score during first 2 weeks after intra-articular injection of mBSA. Arthritis severity in Timp3−/− mice was significantly higher than in wild-type mice at day 3 (n = 32, range 2 to 6 for Timp3−/− and 2 to 5 for wild-type; *P = 0.001) but not at days 7 (n = 25, range 1 to 4 for Timp3−/− and 1 to 4 for wild-type) and 14 (n = 13, range 0 to 2 for Timp3−/− and 0 to 1 for wild-type).
Histological Differences
Analysis of histological sections demonstrated higher scores in Timp3−/− mice than wild-type on days 3 and 7 after arthritis induction (Figure 3). There was a significant difference in synovial inflammation especially on days 3 and 7 (Figure 4). When the scores for synovial infiltration with mononuclear cells and invasion, and loss of Safranin O staining are compared individually, the difference is mainly in synovial infiltration (Table 1). The differences in loss of Safranin O staining and invasion were not statistically significant, so it seems that the higher sum of scores in Timp3−/− was mainly due to increases in synovial inflammation.
Figure 3.
Total histological score in Timp3−/− and wild-type mice. Note the significant difference on day 3 (n = 7, range 3 to 6 for Timp3−/− and 1 to 2 for wild-type; *P = 0.031) and day 7 (n = 12, range 4 to 9 for Timp3−/− and 1 to 6 for wild-type, *P = 0.016).
Figure 4.
Histological analysis of arthritic joints of wild-type (A, C, and E) and Timp3−/− (B, D, and F) mice on day 3 (A and B), day 7 (C and D), and day 14 (E and F). Safranin O and hematoxylin staining. Original magnifications, ×48.
Table 1.
Histological Grading—Individual Components
| Day 3 | Day 7 | Day 14 | ||
|---|---|---|---|---|
| Synovial inflammation | Timp-3−/− | 3 (3) P = 0.018 | 3 (3) P = 0.014 | 1.86 (1–3) |
| Wild type | 1.33 (1–2) | 1.83 (1–2) | 2.5 (2–3) | |
| Safranin O loss | Timp-3−/− | 1 (0–2) | 2.16 (1–3) P = 0.12 | 2 (0–3) |
| Wild type | 0 (0) | 0.83 (0–3) | 1.8 (0–3) | |
| Synovial invasion | Timp-3−/− | 0.25 (0–1) | 1.5 (0–3) P = 0.25 | 1.42 (1–3) |
| Wild type | 0 (0) | 0.33 (0–1) | 1.66 (0–2) |
Values represent averages and ranges of values obtained. Only results for synovial inflammation on days 3 and 7 showed significant differences between the Timp-3−/− and wild type animals. n = 7, 12, and 13 for days 3, 7, and 14, respectively.
The degradation of the major cartilage proteoglycan, aggrecan, can be evaluated by immunohistochemical analysis using anti-neoepitope antibodies specific for the cleavage products remaining in the cartilage matrix after protease activity. Antibodies recognizing two different, well-characterized C-terminal epitopes were used. The epitope VDIPEN is produced by MMPs40 but can also be generated in a multicleavage mechanism by the lysosomal cysteine protease cathepsin B41 and as a minor cleavage product by ADAMTS-4,42 whereas the epitope NVTEGE is only generated by aggrecanase action.43 Because these antibodies recognize the new C-termini present on the G1 region of aggrecan that remains localized in the extracellular matrix, and the NVTEGE epitope is located downstream of the VDIPEN epitope, the latter is expected to accumulate at the expense of the former if both activities are present. Although cleavage products were observed in Timp3−/− and wild-type animals, no significant difference was seen between the two groups for the VDIPEN epitope (Figure 5) or for the NVTEGE epitope, where much less staining was observed (data not shown). These findings agree with the lack of significant differences in loss of Safranin O staining. As shown previously by van Meurs and colleagues,35 production of the VDIPEN epitope was closely related to loss of Safranin O staining.
Figure 5.
Immunohistochemical localization of aggrecan degradation products in wild-type (A and C) and Timp3−/− (B and D) mice using the anti-VDIPEN antibody, showing the extent of degradation on day 7 (A and B) and day 14 (C and D). Note the intense staining of the growth plate seen in both wild-type and Timp3−/− samples (A and D).
TNF-α Levels
Circulating TNF-α levels were measured in the serum using an enzyme-linked immunosorbent assay. TNF-α levels in the blood are known to rise immediately after subcutaneous injection of inflammatory stimuli, returning to near base line after 3 hours.44 One hour after subcutaneous injection of mBSA and intraperitoneal injection with killed B. pertussis we found a dramatic difference in TNF-α levels in sera (Figure 6). Timp3−/− mice had significantly higher TNF-α levels than wild-type animals (7500 compared to 2600 pg/ml, respectively).
Figure 6.
Serum TNF-α levels in Timp3−/− and wild-type mice 1 hour after the initial immunization with mBSA in Freund’s complete adjuvant and 2 × 109 heat-killed B. pertussis.
Discussion
Compared to the other members of the TIMP family, TIMP-3 has several unique properties. In particular, it is the only member of TIMP family that is bound to the extracellular matrix, and is able to inhibit members of the ADAM and ADAMTS families. A protective role of TIMP-3 against aggrecan degradation in cartilage cultured, in vitro, was recently shown by Gendron and colleagues27 where the effect of TIMP-1, -2, and -3 on degradation of aggrecan, as monitored by GAG release in bovine nasal and porcine articular cartilage stimulated by interleukin-1α or retinoic acid, was investigated. Only treatment with TIMP-3 [using the N-terminal inhibitory domain (N-TIMP-3)], showed a decrease in aggrecan release in a dose-dependent manner, whereas both TIMP-1 and TIMP-2 failed to show such an effect. Similar results would be expected in cartilage cultured under TNF-α stimulation but substantially higher levels of TNF-α are required to obtain comparable anabolic effects to those of interleukin-145 so it is not clear whether the levels of TNF-α present in the mouse joints in the present study would have had a major influence on aggrecan degradation.
Timp3−/− mice develop various pathologies with development.21–24,46 Although no changes in the musculoskeletal system were reported originally21 and no changes were observed in animals used for the present study (∼8 weeks), recent detailed studies have demonstrated increased evidence for articular cartilage catabolism in older animals (Sabebjam et al, submitted). This may be due to a shift in TIMP/MMP balance in favor of extracellular matrix degradation. Thus it is quite possible that the imbalance between MMPs and the TIMPs may lead to enhanced extracellular matrix degradation in various physiological or pathological situations. To investigate such possibility in an arthritis setting an antigen-induced arthritis model was used. The unique properties of TIMP-3 relative to the other members of this protease inhibitor family were expected to have an impact on the severity of joint pathology. Of the several potential TIMP-3 targets: matrix metalloproteinase such as collagenases and stromelysin, aggrecanases (ADAMTS-4 and -5), and TACE (TNF-α-converting enzyme), only inhibition of the latter seemed to be of major consequence in this acute arthritis model.
In these studies no major difference in aggrecan cleavage neoepitopes was observed between the Timp3−/− and wild-type animals although substantial staining for the VDIPEN epitope was seen. These results suggest that under the acute conditions of inflammatory arthritis, TIMP-3 levels in the joint are not adequate to control the activity of aggrecan-degrading proteases. Although these data neither implicate nor exclude TIMP-3 as a controlling inhibitor of cartilage aggrecan degradation, the importance of TIMP-3 in such processes in normal tissue where increased aggrecanase action is evident in older Timp3−/− animals (Sahebjam et al, submitted).
We were able to identify a significant difference in joint size between Timp3−/− mice and wild types in the first few days after arthritis induction. These findings were supported by evidence of increased synovial proliferation and cell infiltration in histological slides of the joints at days 3 and 7 after arthritis induction. By day 14, there was no visible difference in joint size or any difference in synovial inflammation. We were also able to detect significantly higher levels of TNF-α in the Timp3−/− animals 1 hour after the first injection. This is consistent with the increase in joint swelling seen in these mice. Considering our observation of uncoupling of joint swelling and proteoglycan loss, it seems unlikely that these symptoms are due to lack of MMP inhibition but rather because of its other functions, most probably through lack of inhibition of TACE and resulting higher TNF-α levels, although slower turnover of the circulating TNF-α in the Timp3−/− animals is also a possibility. The fact that we did not detect any difference in matrix degradation between the wild-type and TIMP-3-null mice (despite the increased synovial involvement in the TIMP-3 knockout) suggests that the extent of degradation in the wild-type was so advanced that TIMP-3 ablation could not be expected to alter the process.
It is now generally accepted that TNF-α and interleukin-1 are master cytokines in the process of joint inflammation and the concomitant erosive changes in cartilage and bone.47 Studies in animal models of arthritis make it clear that TNF-α is involved early in joint swelling although slight variations exist between different models.48,49 In collagen-induced arthritis, administration of anti-TNF-α can reduce both joint swelling and proteoglycan loss only if administered immediately at the onset of arthritis. If it is started shortly after onset, reduction in swelling is still seen but cartilage damage is unaffected.48 When TNF-α is blocked in streptococcal cell wall arthritis, marked dose-dependent reduction in joint swelling is found. In marked contrast, inhibition of chondrocyte proteoglycan synthesis and total proteoglycan loss appears to be unchanged. In antigen-induced arthritis in rabbits neutralization of TNF-α was found to inhibit inflammatory changes in the joint during the acute phase of the disease but had little effect on the loss of proteoglycan from the cartilage in long term.39,48 All of these results indicate a lack of connection between the early TNF-α-induced inflammatory response and matrix degradation.
Our observations agree with the above results in the sense that increased amounts of TNF-α would result in increased joint swelling and cell infiltration. As for proteoglycan loss, because significant uncoupling is observed between the roles of TNF-α and interleukin-1β in antigen-induced arthritis and streptococcal cell wall arthritis; it appears that increased TNF-α does not necessarily result in increased proteoglycan loss in certain arthritis models. In addition, although TNF-α generation by the action of TACE is inhibitable by TIMP-3, generation of interleukin-1β from its precursor is dependent on the action of the cysteine protease caspase-1 (ICE, interleukin-1-converting enzyme), which would be unaffected by the TIMP composition of the tissue.
The identification of TACE as the mediator of soluble TNF-α production has stimulated major efforts to develop specific inhibitors as therapeutic agents. TIMP-3 has been shown to inhibit TACE in vitro but evidence for TIMP-3 acting as a physiological regulator of this enzyme has been lacking50 until recently.24 The present results are in agreement with this proposal and the development of TACE inhibitors as anti-arthritic drugs.
Acknowledgments
We thank Mia Esser and Louise Marineau for technical assistance and Guylaine Bédard and Mark Lepik for preparation of the illustrations.
Footnotes
Address reprint requests to John S. Mort, Joint Diseases Laboratory, Shriners Hospital for Children, 1529 Cedar Ave., Montreal, Quebec, Canada H3G 1A6. E-mail: jmort@shriners.mcgill.ca.
Supported by the Canadian Arthritis Network of Centres of Excellence, the Canadian Institutes of Health Research, and the Shriners of North America.
Present address of M.M.: Drexel University College of Medicine, Department of Pathology and Laboratory Medicine, Mail Stop 435, 245 N. 15th St., Philadelphia, PA 19102-1192.
References
- Cawston T. Matrix metalloproteinases and TIMPs: properties and implications for the rheumatic diseases. Mol Med Today. 1998;4:130–137. doi: 10.1016/s1357-4310(97)01192-1. [DOI] [PubMed] [Google Scholar]
- Mort JS, Poole AR. Proteases and their inhibitors. Klippel JH, Crofford LJ, Stone JH, Weyland CM, editors. Atlanta: Arthritis Foundation,; Primer of the Rheumatic Diseases. 2001:pp 72–81. [Google Scholar]
- Springman EB, Angleton EL, Birkedal-Hansen H, Van Wart HE. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc Natl Acad Sci USA. 1990;87:364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G, Willenbrock F. Tissue inhibitors of matrix metalloendopeptidases. Methods Enzymol. 1995;248:496–510. doi: 10.1016/0076-6879(95)48032-3. [DOI] [PubMed] [Google Scholar]
- Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- Overall CM. Regulation of tissue inhibitor of matrix metalloproteinase expression. Ann NY Acad Sci. 1994;732:51–64. doi: 10.1111/j.1749-6632.1994.tb24724.x. [DOI] [PubMed] [Google Scholar]
- Leco KJ, Khokha R, Pavloff N, Hawkes SP, Edwards DR. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinct pattern of expression in mouse cells and tissues. J Biol Chem. 1994;269:9352–9360. [PubMed] [Google Scholar]
- Yu WH, Yu SC, Meng Q, Brew K, Woessner JF. TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000;275:31226–31232. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]
- Loechel F, Fox JW, Murphy G, Albrechtsen R, Wewer UM. ADAM 12-S cleaves IGFBP-3 and IGFBP-5 and is inhibited by TIMP-3. Biochem Biophys Res Commun. 2000;278:511–515. doi: 10.1006/bbrc.2000.3835. [DOI] [PubMed] [Google Scholar]
- Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, Stephens PE, Shelley C, Hutton M, Knäuper V, Docherty AJP, Murphy G. TNF-α converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998;435:39–44. doi: 10.1016/s0014-5793(98)01031-x. [DOI] [PubMed] [Google Scholar]
- Lee MH, Knäuper V, Becherer JD, Murphy G. Full-length and N-TIMP-3 display equal inhibitory activities toward TNF-α convertase. Biochem Biophys Res Commun. 2001;280:945–950. doi: 10.1006/bbrc.2000.4192. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of ADAM-TS4 (aggrecanase 1) and ADAM-TS5 (aggrecanase 2). J Biol Chem. 2001;276:12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- Hashimoto G, Aoki T, Nakamura H, Tanzawa K, Okada Y. Inhibition of ADAMTS4 (aggrecanase-1) by tissue inhibitors of metalloproteinases (TIMP-1, 2, 3 and 4). FEBS Lett. 2001;494:192–195. doi: 10.1016/s0014-5793(01)02323-7. [DOI] [PubMed] [Google Scholar]
- Borland G, Murphy G, Ager A. Tissue inhibitor of metalloproteinases-3 inhibits shedding of L-selectin from leukocytes. J Biol Chem. 1999;274:2810–2815. doi: 10.1074/jbc.274.5.2810. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves PG, Wang F, Antcliff J, Murphy G, Lawry J, Russell RG, Croucher PI. Human myeloma cells shed the interleukin-6 receptor: inhibition by tissue inhibitor of metalloproteinase-3 and a hydroxamate-based metalloproteinase inhibitor. Br J Haematol. 1998;101:694–702. doi: 10.1046/j.1365-2141.1998.00754.x. [DOI] [PubMed] [Google Scholar]
- Rovida E, Paccagnini A, Del Rosso M, Peschon J, Dello Sbarba P. TNF-α-converting enzyme cleaves the macrophage colony-stimulating factor receptor in macrophages undergoing activation. J Immunol. 2001;166:1583–1589. doi: 10.4049/jimmunol.166.3.1583. [DOI] [PubMed] [Google Scholar]
- Weber BHF, Vogt G, Pruett RC, Stöhr H, Felbor U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby’s fundus dystrophy. Nat Genet. 1994;8:352–356. doi: 10.1038/ng1294-352. [DOI] [PubMed] [Google Scholar]
- Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest. 1998;101:1478–1487. doi: 10.1172/JCI1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen M, Baker AH, Kahari VM. Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res. 1998;58:2310–2315. [PubMed] [Google Scholar]
- Leco KJ, Waterhouse P, Sanchez OH, Gowing KLM, Poole AR, Wakeham TW, Mak TW, Khokha R. Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3). J Clin Invest. 2001;108:817–829. doi: 10.1172/JCI12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Leco KJ, Voura EB, Yu HY, Waterhouse P, Murphy G, Moorehead RA, Khokha R. Accelerated apoptosis in the Timp-3-deficient mammary gland. J Clin Invest. 2001;108:831–841. doi: 10.1172/JCI13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedak PWM, Smookler DS, Kassiri Z, Ohno N, Leco KJ, Verma S, Mickle DAG, Watson KL, Hojilla CV, Cruz W, Weisel RD, Li RK, Khokha R. TIMP-3 deficiency leads to dilated cardiomyopathy. Circulation. 2004;110:2401–2409. doi: 10.1161/01.CIR.0000134959.83967.2D. [DOI] [PubMed] [Google Scholar]
- Mohammed FF, Smookler DS, Taylor SE, Fingleton B, Kassiri Z, Sanchez OH, English JL, Matrisian LM, Au B, Yeh WC, Khokha R. Abnormal TNF activity in Timp3−/− mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet. 2004;36:969–977. doi: 10.1038/ng1413. [DOI] [PubMed] [Google Scholar]
- Su S, Grover J, Roughley PJ, DiBattista JA, Martel-Pelletier J, Pelletier JP, Zafarullah M. Expression of the tissue inhibitor of metalloproteinases (TIMP) gene family in normal and osteoarthritic joints. Rheumatol Int. 1999;18:183–191. doi: 10.1007/s002960050083. [DOI] [PubMed] [Google Scholar]
- Apte SS, Hayashi K, Seldin MF, Mattei M-G, Hayashi M, Olsen BR. Gene encoding a novel murine tissue inhibitor of metalloproteinases (TIMP), TIMP-3, is expressed in developing mouse epithelia, cartilage, and muscle, and is located on mouse chromosome 10. Dev Dyn. 1994;200:177–197. doi: 10.1002/aja.1002000302. [DOI] [PubMed] [Google Scholar]
- Gendron C, Kashiwagi M, Hughes C, Caterson B, Nagase H. TIMP-3 inhibits aggrecanase-mediated glycosaminoglycan release from cartilage explants stimulated by catabolic factors. FEBS Lett. 2003;555:431–436. doi: 10.1016/s0014-5793(03)01295-x. [DOI] [PubMed] [Google Scholar]
- Arner EC. Aggrecanase-mediated cartilage degradation. Curr Opin Pharmacol. 2002;2:322–329. doi: 10.1016/s1471-4892(02)00148-0. [DOI] [PubMed] [Google Scholar]
- Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5:94–103. doi: 10.1186/ar630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell FD, Hershey AD. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Davoli MA, Lamplugh L, Beauchemin A, Chan K, Mordier S, Mort JS, Murphy G, Docherty AJ, Leblond CP, Lee ER. Enzymes active in the areas undergoing cartilage resorption during the development of the secondary ossification center in the tibiae of rats aged 0–21 days: II. Two proteinases, gelatinase A and collagenase-3, are implicated in the lysis of collagen fibrils. Dev Dyn. 2001;222:71–88. doi: 10.1002/dvdy.1160. [DOI] [PubMed] [Google Scholar]
- Petrow PK, Thoss K, Katenkamp D, Brauer R. Adoptive transfer of susceptibility to antigen-induced arthritis into severe combined immunodeficient (SCID) mice: role of CD4+ and CD8+ T cells. Immunol Invest. 1996;25:341–353. doi: 10.3109/08820139609059316. [DOI] [PubMed] [Google Scholar]
- Ospelt C, Neidhart M, Gay RE, Gay S. Synovial activation in rheumatoid arthritis. Front Biosci. 2004;9:2323–2334. doi: 10.2741/1399. [DOI] [PubMed] [Google Scholar]
- van Meurs JBJ, van Lent PLEM, Holthuysen AEM, Singer II, Bayne EK, van den Berg WB. Kinetics of aggrecanase- and metalloproteinase-induced neoepitopes in various stages of cartilage destruction in murine arthritis. Arthritis Rheum. 1999;42:1128–1139. doi: 10.1002/1529-0131(199906)42:6<1128::AID-ANR9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Sztrolovics R, Alini M, Roughley PJ, Mort JS. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;326:235–241. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, David CS. Immunogentics of type II collagen-induced arthritis in mice. Farid NR, editor. Boca Raton: CRC Press,; 1991:pp 119–134. [Google Scholar]
- Mikecz K, Glant TT, Poole AR. Immunity to cartilage proteoglycans in Balb/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis Rheum. 1987;30:306–318. doi: 10.1002/art.1780300310. [DOI] [PubMed] [Google Scholar]
- Lewthwaite J, Blake S, Hardingham T, Foulkes R, Stephens S, Chaplin L, Emtage S, Catterall C, Short S, Nesbitt A. Role of TNF alpha in the induction of antigen induced arthritis in the rabbit and the anti-arthritic effect of species specific TNF alpha neutralising monoclonal antibodies. Ann Rheum Dis. 1995;54:366–374. doi: 10.1136/ard.54.5.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang AJ, Neame PJ, Last K, Hardingham TE, Murphy G, Hamilton JA. The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B. J Biol Chem. 1992;267:19470–19474. [PubMed] [Google Scholar]
- Mort JS, Magny M-C, Lee ER. Cathepsin B: an alternative protease for the generation of an aggrecan “metalloproteinase” cleavage neoepitope. Biochem J. 1998;335:491–494. doi: 10.1042/bj3350491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westling J, Fosang AJ, Last K, Thompson VP, Tomkinson KN, Hebert T, McDonagh T, Collins-Racie LA, LaVallie ER, Morris EA, Sandy JD. ADAMTS4 cleaves at the aggrecanase site (Glu373-Ala374) and secondarily at the matrix metalloproteinase site (Asn341-Phe342) in the aggrecan interglobular domain. J Biol Chem. 2002;277:16059–16066. doi: 10.1074/jbc.M108607200. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Neame PJ, Boynton RE, Flannery CR. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991;266:8683–8685. [PubMed] [Google Scholar]
- Chorinchath BB, Kong LY, Mao L, McCallum RE. Age-associated differences in TNF-α and nitric oxide production in endotoxic mice. J Immunol. 1996;156:1525–1530. [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor α stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322:547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SE, Pape MC, Khokha R, Watson AJ, Leco KJ. A null mutation for tissue inhibitor of metalloproteinases-3 (Timp-3) impairs murine bronchiole branching morphogenesis. Dev Biol. 2003;261:313–323. doi: 10.1016/s0012-1606(03)00318-x. [DOI] [PubMed] [Google Scholar]
- van den Berg WB. Anti-cytokine therapy in chronic destructive arthritis. Arthritis Res. 2001;3:18–26. doi: 10.1186/ar136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg WB, Joosten LA, Kollias G, De Loo FA. Role of tumour necrosis factor alpha in experimental arthritis: separate activity of interleukin 1beta in chronicity and cartilage destruction. Ann Rheum Dis. 1999;58(Suppl 1):I40–I48. doi: 10.1136/ard.58.2008.i40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RO, Feldmann M, Maini RN. Cartilage destruction and bone erosion in arthritis: the role of tumour necrosis factor alpha. Ann Rheum Dis. 2000;59(Suppl 1):I75–I80. doi: 10.1136/ard.59.suppl_1.i75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RA. Tumor necrosis factor-α converting enzyme. Int J Biochem Cell Biol. 2002;34:1–5. doi: 10.1016/s1357-2725(01)00097-8. [DOI] [PubMed] [Google Scholar]