Abstract
Brain hemorrhage is a severe complication of both neoplastic and nonneoplastic brain disease. Mice deficient in the αvβ8 integrin display defective brain vessel formation resulting in hemorrhage and perinatal death, but the mechanism of brain hemorrhage is unknown. Because the αvβ8 integrin is expressed by astrocytes and not expressed by endothelium, paracrine interactions between astrocytes and endothelial cells could contribute to the maintenance of brain vessel integrity. We have investigated the mechanisms underlying astrocytic-endothelial paracrine signaling and have found that integrin-mediated activation of transforming growth factor (TGF)-β by astrocytes influences endothelial cell function. Thus, we identified the integrin αvβ8 in human perivascular glial cell processes surrounding developing blood vessels. Human astrocytic αvβ8 was a major cell surface receptor for latent TGF-β, and αvβ8-dependent activation of TGF-β was the major mechanism of TGF-β activation in primary cultures of astrocytes or freshly dissociated fetal brain cells. This activation of TGF-β was sufficient to inhibit endothelial migration in fibrin gels and to alter expression of genes affecting proteolytic and angiogenic pathways. Taken together, our data suggest that astrocytic αvβ8 acts as a central regulator of brain vessel homeostasis through regulation of TGF-β activation and expression of TGF-β-responsive genes that promote vessel differentiation and stabilization, most notably plasminogen activator inhibitor-1 and thrombospondin-1.
Brain hemorrhage is a serious and often fatal complication of premature birth, vascular malformations, aneurysms, ischemic brain disease, and brain tumors.1 The mechanisms underlying brain hemorrhage may be brain-specific because of the close relationship that brain endothelial cells have with perivascular astrocytes. Thus, the abluminal aspect of brain capillary endothelial cells form an extensive interface with astrocyte end-feet and the close apposition of these end-feet appear to be key to vascular wall integrity and endothelial differentiation in vivo.2,3 Astrocytes have the potential to interact with endothelial cells either through direct astrocyte-endothelial contact or through paracrine interactions. However, brain endothelial cells are separated from astrocytic end-feet by a thin basal lamina and a discontinuous interposed pericyte layer, suggesting that paracrine interactions may be more important than direct astrocyte-endothelial cell interactions.1 To date, little is known of the paracrine factors that are involved in astrocyte-endothelial interactions.
Transforming growth factor (TGF)-β has been widely implicated as a master regulatory cytokine involved in paracrine regulation of blood vessel development, differentiation, and function and is thus a likely candidate to mediate astrocyte-endothelial interactions.4 TGF-β exists in three isoforms in mammals, TGF-β1, TGF-β2, and TGF-β3. Notably in mice, deficiency of TGF-β1 leads to early embryonic loss due to defective yolk sac vasculogenesis.4,5 No TGF-β isoform-specific brain vessel phenotype has been identified, most likely due to functional redundancy among TGF-β isoforms because mutations of the type I and type III TGF-β receptors ALK-1 and endoglin, respectively, lead to the development of postnatal brain vessel malformations as a component of hereditary hemorrhagic telangectasia, in both mice and humans.5
The mechanisms whereby TGF-β exerts its influence on endothelium are complex and depend both on the tissue microenvironment and the activation-state of the endothelial cells.6 Thus, in vitro, the response of primary cultures of endothelial cells to exogenous TGF-β are highly variable and depend on the composition and complexity of the extracellular environment.7 The protean effects of TGF-β on endothelial function point to a critical role for TGF-β as a central regulator of endothelial cell behavior. Indeed, the exogenous application of TGF-β to endothelial cells in vitro or vessels in vivo can lead to the up- or down-regulation of a variety of critical pro- and anti-angiogenic molecules including collagen-I, matrix-metalloprotease-2, plasminogen activator inhibitor-1, or thrombospondin-1.8–11 In turn, this impacts matrix deposition or degradation, endothelial cell migration, growth, or differentiation.
TGF-β isoforms are ubiquitously expressed but almost completely sequestered in a latent form referred to as the small latent complex by the noncovalent association of the propeptide of TGF-β, known as latency-associated peptide (LAP), with the active TGF-β peptide.12 Thus, a critical regulator of TGF-β function is its activation. Mechanisms that mediate activation of TGF-β can be broadly separated into those that require proteolysis and those that expose the functional domain of the TGF-β peptide, presumably through conformational alterations.12 For example, plasmin-mediated13 or metalloproteolytic cleavage14 have both been shown to mediate activa-tion of TGF-β; the extracellular matrix molecule thrombospondin15 and the integrin αvβ616 have both been shown to bind to the LAP of TGF-β1 (LAP-β1), mediating activation of TGF-β, probably through disrupting the noncovalent association of LAP with the active TGF-β peptide. Recently, we have described a mechanism of TGF-β activation in tumor cell lines whereby the integrin αvβ8 binds to the RGD sequence of LAP-β1 and through a metalloproteolytic mechanism involving the transmembrane protease, MT1-MMP, mediates the activation of TGF-β.17
The integrin αv and β8 subunits have both been knocked out in mice. Individual deficiencies of the αv or the β8 subunits each result in nearly identical lethal phenotypes involving defective vasculogenesis during early development, and in later development, defective brain vessel formation resulting in brain hemorrhage.18,19 The brain vessels of either αv- or β8-deficient embryos show brain region-specific morphological alterations. Thus, the vessels in the ganglionic eminence of mid- to late-gestation embryos show defective anastamotic connections and increased endothelial cell proliferation resulting in glomeruloid vascular malformations, which are often associated with hemorrhage.19 Ultrastructural and immunocytochemical examination of either αv-null or β8-null embryos reveals a primary defect of end-feet association of a major subset of perivascular cells with endothelial cells.19,20 This cellular subset appeared to be in the neuroglial lineage because it expressed the immature neuroglial marker nestin and normal numbers of perivascular smooth muscle cells and pericytes were found surrounding brain vessels of αv- or β8-deficient embryos.19,20 The perivascular cells that failed to associate with endothelial cells are likely to be radial glial cells or their progeny because brain vessels migrate toward the ventricular surface on a radial glial scaffold and radial glia are known to express nestin.21 Furthermore, selective ablation of the αv-integrin subunit in embryonic or postnatal glia in mice results in intracerebral hemorrhage.22
In this study, we address the hypothesis that astrocytic αvβ8 mediates activation of TGF-β and thus drives paracrine interactions between astrocytes and endothelial cells, and as such, acts as an angiogenic control switch. We show that αvβ8 is expressed in perivascular cell processes surrounding developing human cerebral blood vessels. Expression of αvβ8 is maintained in either primary cultures of astrocytes or freshly dissociated immature neuroglial cells. We demonstrate that the binding of latent TGF-β to astrocytic αvβ8 leads to the metalloproteolytic-dependent liberation of active TGF-β, which in turn, inhibits endothelial migration in fibrin gels. Finally, we provide evidence that the molecular mechanisms involved in the astrocytic αvβ8-dependent effect on endothelial cell behavior is through the TGF-β-dependent alteration of expression of anti-angiogenic molecules, most notably plasminogen-activator inhibitor-1 (PAI-1) and thrombospondin-1 (TSP-1).
Materials and Methods
Cell Culture, Constructs, and Antibodies
Human astrocytes were obtained from commercial sources (Clonetics, Walkersville, MD) or were harvested from fetal brain. Briefly, human fetal brain (18 to 22 weeks of gestation) was obtained at the time of elective termination of intrauterine pregnancy from otherwise healthy females. Informed consent was obtained from all participants as part of an approved ongoing research protocol by the University of California San Francisco (UCSF) Committee on Human Research. After removal of the meninges, the tissue was minced and washed in wash medium [Iscove’s modified Dulbecco’s media (UCSF Cell Culture Facility) with penicillin-streptomycin (1000 μg/ml, UCSF Cell Culture Facility), fungizone (2.5 μg/ml, UCSF Cell Culture Facility), gentamicin (50 μg/ml, UCSF Cell Culture Facility), ampicillin (50 μg/ml; Sigma, St. Louis, MO), and erythromycin (100 μg/ml, Sigma)]. After trituration, the tissue was washed in wash medium and then enzymatically treated for 5 to 10 minutes at 37°C in digestion buffer [Blenzyme 3 (0.28 U/ml; Roche, Indianapolis, IN), hyaluronidase (0.1%, Sigma) in wash media]. After washing several times in astrocyte media [wash media with 20% fetal calf serum with 6,7-dimethyl-5,6,7,8-tetrahydropertine hydrochloride (0.5 μg/ml, Sigma), glutathione (2.5 μg/ml, Sigma), and ascorbic acid (50 μg/ml, Sigma)], the cells were passed through a 100-μm cell strainer (Fisher, Pittsburgh, PA) to remove vessels and either counted and directly assayed or plated at a density of ∼30 × 106 cells/150-ml flask coated with poly-l-lysine (0.01%) and placed in a humidified incubator for 5 to 7 days at 37°C with 7.5% CO2. At confluence, contaminating oligodendroglia and microglia were removed by shaking for 2 to 8 hours at 37°C at 180 rpm. The adherent cells were washed extensively and then cultured for an additional week. Confluent cultures were then split and used at the first or second passage. Lineage and differentiation status of the cultures were assessed by immunofluorescence microscopy with anti-GFAP (Sigma), anti-nestin (Chemicon, Temecula, CA), lectin-fluorescein isothiocyanate (Sigma), anti-Map 2 kinase (Chemicon). The cultures were routinely 80 to 85% GFAP-positive, 100% nestin-positive, 0% Map-2c-positive, and 0% lectin-fluorescein isothiocyanate-positive. bEND.3 murine endothelioma cells (American Type Culture Collection, Manassas, VA)23 were maintained in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum with penicillin and streptomycin and were retrovirally transduced with pLEGFP (Clontech, Palo Alto, CA) and selected in Geneticin, (G418; Life Technologies, Inc., Carlsbad, CA), as described.17 Human dermal fibroblasts were cultured from human foreskin as previously described.24 Additional antibodies used were anti-CD34 (DakoCytomation, Carpinteria, CA), anti-β8 (14E5, 37e1,17 G17, and blocking peptide, SC-10817; Santa Cruz Biotechnology, Santa Cruz, CA), anti-αv (L230),25 anti-β1 (P5D2), anti-β3 (AP3, ATCC), anti-β5 (P1F6, Chemicon), anti-β6 (E7P6),25 anti-LAP (VB3A9),16 anti-pan-TGF-β (clone 1D11; R&D Systems, Minneapolis, MN), anti-thrombospondin (Ab-11 and Ab-1; Neomarkers, Fremont, CA), and anti-PAI-1 (H4B3 and H14H7-biotin; Molecular Innovations, Inc., Southtop, MI). The metalloprotease inhibitor GM6001 (Ryss Lab, Inc., Union City, CA) was used as described.17 Simian LAP-β1 was prepared as described.17 Recombinant active TGF-β was from R&D Systems. Additionally, TGF-β-specific peptides used were GRRGDLATIH, TGF-β1; HGRGDLGRLK, TGF-β3.17
Immunocytochemistry, Fluorescence-Activated Cell Sorting, Immunoprecipitation, Affinity Chromatography, Cell Adhesion, and Western Blotting
Fresh brain tissues were fixed in 4% paraformaldehyde overnight at 4°C and then embedded in paraffin. Immunocytochemistry, confocal microscopy, image acquisition, and processing were performed as described with the exception that the secondary reagents used were biotinylated donkey anti-goat, Oregon Green streptavidin conjugate and goat anti-mouse Alexa Fluor 595 conjugate (Molecular Probes, Eugene, OR).26,27 Immunoelectron microscopy was performed exactly as described.27 Flow cytometry, 125I surface labeling, immunoprecipitation, and affinity chromatography were performed as described.28 Cell adhesion assays were performed as described17,28 with the only modification being the addition of LAP-β1 and LAP-β3 peptides, GRRGDLATIH or HGRGDLGRLK, respectively (200 μg/ml). Western blotting was performed as described with the following minor modifications, 3 × 104 bEND.3, 1 × 105 astrocytes, or both were added to 24-well dishes and cultured overnight before lysis or conditioned media harvest.
TGF-β Bioassays
TGF-β bioassays using astrocyte co-cultures with the reporter cell line TMLC29 were performed exactly as described with the following slight modifications.17,29 Briefly, assays to detect active TGF-β in the cell culture supernatant were performed using 7 × 105 cultured astrocytes or 4 × 106 freshly dissociated fetal cortical cells incubated in 250 μl of serum-free Isocove’s MEM for 1 hour at 37°C.
Fibrin Gel Assays
Astrocytes, bEND.3, or human dermal fibroblasts were detached with trypsin and allowed to attach to collagen-coated Cytodex 3 beads (Amersham Biosciences, Piscataway, NJ) in an nontissue-coated Petri dish. After confluence was reached, the beads were embedded into a 0.28% fibrin (Calbiochem, La Jolla, CA) gel suspended in 5% fetal calf serum in Dulbecco’s modified Eagle’s medium in the presence or absence of either 200 μg/ml of 37E1, 10 μg/ml of 1D11, or 1 ng/ml of recombinant active TGF-β1 (R&D Systems). Assays were done in 24-well dishes, in a gel volume of 1 ml, adding 500 μl of 10% fetal calf serum/Dulbecco’s modified Eagle’s medium, in the presence or absence of the above additions, on top of the polymerized gel.
Real-Time Polymerase Chain Reaction (PCR) Analysis
Astrocytes (1 × 105) or bEND.3 cells (3 × 104) were added to individual wells of a 24-well dish in the presence or absence of either 200 μg/ml of 37E1, 10 μg/ml of 1D11, or 1 pmol/L of recombinant active TGF-β1 (R&D Systems) and grown for 16 hours, as above. RNA isolation, DNase treatment, cDNA synthesis, real-time PCR, and gene normalization using geometric averaging of multiple human or mouse internal control genes was performed as described.30,31 Primer sequences can be found at http://www.asthmagenomics.ucsf.edu.
Results
The β8 Integrin Subunit Co-Localizes with Glial Fibrillary Acidic Protein and Nestin in Perivascular Cell Processes
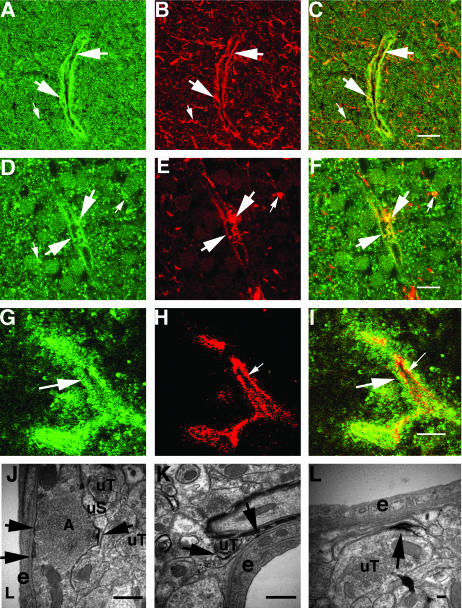
We used immunofluorescence microscopy to localize β8 in fetal brain. We found that β8 was immunolocalized in either a diffuse fine punctate pattern (Figure 1A) or in a fine and coarse punctate pattern (Figure 1; A, D, and G) in the developing cortical and periventricular zones. We have previously reported a very similar pattern of immunoreactivity in the adult mouse and rat brain where we found that β8 was widely expressed in both neuronal dendrites and in glial processes.27 Punctate staining was accentuated around small- and medium-sized vessels throughout the cortices but was particularly apparent surrounding the small vessels of the periventricular region (Figure 1, D and G). The β8 staining pattern was specific because no staining was seen when the antibody was preincubated with the peptide immunogen (data not shown).
Figure 1.
Localization of β8 in perivascular immature astrocytic cell processes in fetal brain and subcellular localization in adult rat brain hippocampus. A–C: Twenty-two week fetal cortex. D–I: Twenty-two week fetal periventricular zone. A, D, and G: Immunofluorescence localization of β8 surrounding blood vessels (large arrows) and in the surrounding developing cortex (small arrows). B: Nestin immunolocalization surrounding blood vessels (large arrows) and in the surrounding developing neuropil (small arrows). C: Co-localization of β8 (green) and nestin (red). Large arrows indicate co-localization (yellow) surrounding a blood vessel and small arrows indicate co-localization (yellow) in the developing neuropil. E: GFAP immunolocalization surrounding blood vessels (large arrows) and in the surrounding developing neuropil (small arrows). F: Co-localization of β8 (green) and GFAP (red). Large arrows indicate co-localization (yellow) surrounding a blood vessel and small arrows indicate co-localization (yellow) in the developing neuropil. H: CD34 immunolocalization in blood vessel endothelium (small arrows). I: Co-localization of β8 (green) and CD34 (red). The large arrow indicates β8 immunoreactivity in perivascular cell processes. The small arrow indicates CD34 immunofluorescence in the endothelium, which does not co-localize with β8. Shown are confocal images. J–L: Immunoelectron microscopy of adult rat brain showing β8 immunolocalization in glial processes. J: Horseradish peroxidase reaction product highlights an immunolabeled astrocytic process (A) adjacent to an endothelial cell (e) of a small blood vessel. Unlabeled terminals (uT) and an unlabeled dendritic spine (uS) are indicated. Arrows indicate patches of immunoperoxidase reaction product in the astrocytic process. K: Arrows indicate an immunolabeled astrocytic process in contact with the basement membrane of an endothelial cell (e), of a small blood vessel in the hippocampus of an adult rat. uT, unlabeled terminal. L: Arrow indicates an immunolabeled portion of an astrocytic process that is in close proximity to an endothelial cell (e) of a small blood vessel. uT, unlabeled terminal. Scale bars: 50 μm (C, F); 20 μm (I); 500 nm (J, K); 100 nm (L).
β8 co-localized with nestin in numerous perivascular cell processes both in the developing cortex (Figure 1; A to C) and in the periventricular zone (data not shown). β8 also co-localized with GFAP in a subset of perivascular cell processes (Figure 1E). β8 co-localized poorly with the endothelial marker CD34 on the luminal surface of blood vessels, which is consistent with previous reports that β8 is not expressed by endothelium (Figure 1; G to I).19,27 To demonstrate the subcellular localization of β8 expression in glial processes surrounding blood vessels we performed immunoelectron microscopy of adult rat brain. We found ultrastructural evidence of β8 expression in the tips of astrocytic processes close to or immediately apposed to vascular basement membrane (Figure 1; J to L).
The Integrin αvβ8 Is Expressed by Human Astrocytes
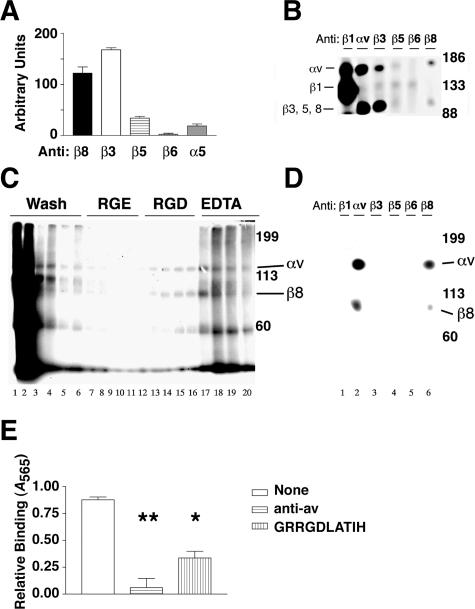
To characterize the repertoire of integrins expressed by astrocytes, primary astrocyte cultures were immunolabeled with anti-integrin antibodies and analyzed by flow cytometry (Figure 2A). The integrin αvβ8 subunit was expressed at easily detectable levels (Figure 2A). When the anti-β8 mean fluorescence intensity was compared to other integrin subunits that recognize the Arg-Gly-Asp (RGD) sequence, αvβ8 was expressed as abundantly as αvβ3 and more abundantly than αvβ5 (Figure 2A). In contrast, no expression of αvβ6 and very little α5 subunit expression were detected. Because no complex specific antibody exists for αvβ1, the expression of αvβ1 was determined by immunoprecipitation. The β1 subunit associates promiscuously with at least 12 α subunits, many of which co-migrate with the αv subunit. The β1 subunit distinctively migrates at 130 kd whereas the β3, β5, β6, and β8 integrin subunits migrate at ∼80 to 95 kd, nonreduced (Figure 2B). Because a 130-kd band was essentially absent from the anti-αv immunoprecipitations, astrocytes do not express αvβ1 (Figure 2B). The immunoprecipitations of 125I cell surface-labeled astrocyte lysates thus confirmed the relative levels of integrin expression identified by flow cytometry (Figure 2, A and B). We conclude that cultured human astrocytes express high cell surface levels of αvβ3 and αvβ8, lower levels of αvβ5, little or no αvβ1, and no αvβ6.
Figure 2.
The integrin αvβ8 is expressed on the cell surface of human astrocytes, binds to and mediates cell adhesion to the LAP of TGF-β1. A: Flow cytometry of astrocytes stained with anti-integrin antibodies recognizing αvβ8, αvβ3, αvβ5, αvβ6, or α5 (n = 3). Shown is SE. Fluorescence intensity is shown in arbitrary units. B: Immunoprecipitation of 125I cell surface-labeled human astrocytes using anti-integrin subunit and complex specific antibodies against β1 (P5D2), αv (L230), β3 (AP3), αvβ5 (P1F6), αvβ6 (E7P6), or αvβ8 (14E5). Samples were resolved by 7.5% SDS-PAGE under nonreducing conditions. Shown are the migration of molecular weight markers on the right and the expected migration of integrin subunits on the left. C: LAP affinity chromatography of 125I surface-labeled human astrocyte lysates. Fractions were resolved by 7.5% SDS-PAGE under nonreducing conditions. Lanes 1 to 6, wash fractions; lanes 7 to 12, GRGESPK (1 mg/ml); lanes 13 to 16, GRGDSPK (1 mg/ml); lanes 17 to 20, ethylenediamine tetraacetic acid (10 mmol/L). Shown on the right are the molecular weight markers and the expected migration of integrin αv and β8 subunits. D: The RGD elution fractions were pooled and immunoprecipitated using anti-integrin subunit and complex specific antibodies against β1 (P5D2), αv (L230), β3 (AP3), αvβ5 (P1F6), αvβ6 (E7P6), or αvβ8 (14E5). Shown on the right are the molecular weight markers and the expected migration of integrin αv and β8 subunits. E: Astrocyte adhesion to immobilized LAP in the presence of no inhibitor (open bar), anti-αv (horizontal lines), or GRRGDLATIH (vertical lines). Shown is SE. *, P < 0.05; **, P < 0.001.
The Astrocytic Integrin αvβ8 Binds to and Mediates Adhesion to the LAP of TGF-β1
To determine whether human astrocytic integrins interact with the latent TGF-β complex, we performed affinity chromatography using lysates of 125I cell surface-labeled human astrocytes and immobilized LAP. Two bands of the appropriate molecular weight for the αv and associating β subunits (150 and 90 kd, respectively) were eluted by RGD (Figure 2C, lanes 13 to 16) but not by the control RGE peptide (Figure 2C, lanes 7 to 12) and also eluted by ethylenediamine tetraacetic acid (Figure 2C, lanes 17 to 20). The 150- and 90-kd bands were identified as the αv and β8 integrin subunits, respectively, because they were immunoprecipitated by anti-αv and anti-β8 antibodies and not by antibodies to the β1, β3, β5, or β6 integrin subunits (Figure 2D). In the RGD and ethylenediamine tetraacetic acid eluates, a 60-kd band, of unknown identity, was co-eluted with the150- and 90-kd bands. However, the 60-kd protein did not co-immunoprecipitate from eluted fractions with anti-αv or anti-β8 (Figure 2D). Taken together, these data demonstrate that the integrin αvβ8 is the major LAP-binding integrin that is expressed by human astrocytes and the binding interaction is both RGD- and cation-dependent.
We next performed cell adhesion assays to determine whether astrocytic αvβ8 was capable of mediating cell adhesion to LAP (Figure 2E). We found that astrocytes attached to LAP and that the attachment was primarily mediated by αvβ8, because 68% of astrocyte adhesion to LAP could be inhibited by GRRGDLATIH, an RGD peptide that selectively inhibits αvβ8-mediated adhesion while not effecting the adhesion of αvβ3 or αvβ5, the other αv-integrins expressed by astrocytes.17 These data demonstrate that primary cultures of human astrocytes express high levels of both αvβ8 and αvβ3, but bind to and adhere to the LAP of TGF-β almost exclusively by an αvβ8-dependent mechanism.
αvβ8 When Expressed by Astrocytes or Disaggregated Fetal Brain Cells Activates TGF-β
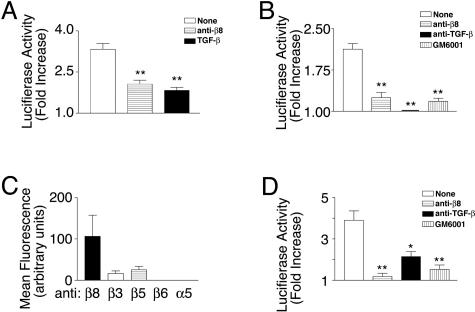
We co-cultured human astrocytes with a TGF-β reporter cell line, TMLC,29 to determine whether astrocytes are capable of supporting αvβ8-mediated activation of TGF-β. The TMLC cell line was created by stably transfecting mink lung epithelial cells with a reporter construct consisting of the TGF-β-responsive fragment of the plasminogen activator inhibitor-1 (PAI-1) promoter fused to the luciferase minigene.29 The TMLC cells produce very little active TGF-β and therefore produce very little background luciferase activity. However, TMLC luciferase activity is strongly induced by exposure to active TGF-β produced either by activating cell types or present in cell-free supernatants.17,29 Co-culture of astrocytes with TMLC cells produced a significant increase in luciferase activity, which was almost entirely due to αvβ8-mediated activation of TGF-β, because an anti-β8-specific neutralizing antibody impaired TMLC luciferase activity almost as well as a pan-TGF-β neutralizing antibody (Figure 3A). Active TGF-β was also released from the astrocyte cell surface because supernatants from astrocytes applied directly to TMLC cells caused a significant increase in luciferase activity (Figure 3B). This release again was primarily αvβ8-dependent, because TGF-β activity in supernatants could be blocked almost completely by an anti-β8 neutralizing antibody (Figure 3B). Finally, the release of active TGF-β from the cell surface was also dependent on metalloprotease activity because GM6001, a broad inhibitor of metalloproteases, could completely inhibit TGF-β release (Figure 3B). Taken together our data demonstrate that human astrocytes in vitro release active TGF-β from the cell surface through an αvβ8- and metalloprotease-dependent mechanism, which is similar to what we have previously reported in carcinoma cell lines.17
Figure 3.
The activation and release of active TGF-β by astrocytes or freshly dissociated fetal brain cells is mediated by the integrin-αvβ8 and is metalloprotease-dependent. A: TGF-β bioassay of active TGF-β produced by astrocytes. Human astrocytes were co-cultured with TMLC TGF-β reporter cells, which stably express a portion of the plasminogen activator inhibitor-1 promoter driving the luciferase minigene, in the presence of no inhibitor (open bar), anti-β8 (horizontal lines), or anti-pan-TGF-β (filled bar). **, P < 0.001. B: TGF-β bioassay of active TGF-β released into the supernatants of human astrocytes. Supernatants from astrocytes in suspension were treated either with no inhibitor (open bar), anti-β8 (horizontal lines), anti-pan-TGF-β (filled bar), or GM6001, a pan-metalloprotease inhibitor (vertical lines) and cell-free supernatants were applied to TMLC cells. **, P < 0.001. C: Integrins known to interact with RGD that are expressed by freshly dissociated fetal brain cells as determined using flow cytometry using integrin heterodimer or subunit-specific antibodies to αvβ8, β3, αvβ5, αvβ6, or α5 (n = 3). Shown is mean fluorescence intensity. D: TGF-β bioassay of active TGF-β released into the supernatants of freshly dissociated fetal brain cells. Supernatants from freshly disaggregated fetal brain cells in suspension were treated either with no inhibitor (open bar), anti-β8 (horizontal lines), anti-pan-TGF-β (filled bar), or GM6001, a pan-metalloprotease inhibitor (vertical lines) and cell-free supernatants were applied to TMLC cells. Shown in A, B, and D are the fold increases of luciferase activity over the TMLC baseline activity. Shown is SE. *, P < 0.05; **, P < 0.001.
Because cultured astrocytes may not accurately reflect their phenotype in vivo, we also evaluated freshly disaggregated fetal brain cells for the expression of αvβ8 and for the ability to support αvβ8-mediated activation of TGF-β. We found that freshly disaggregated fetal brain cells expressed mainly αvβ8 and less αvβ3 and αvβ5 (Figure 3C), whereas astrocytes in culture expressed high levels of both αvβ3 and αvβ8 (Figure 2A). Supernatants taken from freshly disaggregated fetal brain cells, when co-cultured with TMLC cells, also caused a significant increase in luciferase activity, which was both αvβ8- and metalloprotease-dependent, because anti-β8 neutralizing antibodies or GM6001 completely inhibited TGF-β activation (Figure 3D). The anti-TGF-β antibody did not block the induction of luciferase activity from the reporter cells as well as anti-β8 or GM6001, which is most likely due to a lower efficacy of the anti-TGF-β antibody in this assay (Figure 3D). Thus, human astrocytes or freshly dissociated fetal brain cells activate and release TGF-β from the cell surface through an αvβ8- and metalloprotease-dependent mechanism.
Astrocytic Integrin-αvβ8-Mediated Activation of TGF-β Can Inhibit Endothelial Migration in a Three-Dimensional Environment
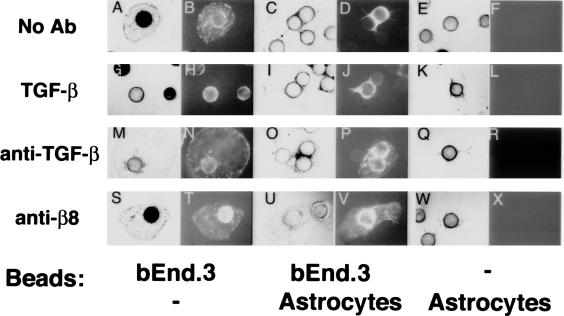
Endothelial responses to TGF-β in vitro are dependent on the complexity of the tissue culture microenvironment.7 Because three-dimensional fibrin gels more closely mimic the in vivo microenvironment compared to two-dimensional culture, we asked if endothelial behavior could be altered by co-culture with astrocytes in fibrin gels. We chose to study microvascular endothelial cells because the co-localization between perivascular cells and endothelial cells in the fetal brain occurred in small vessels (Figure 1) as did the brain hemorrhage seen in β8-deficient mice.19 We chose the polyoma virus middle-T-transformed bEND.3 cell line, because it was derived from murine brain capillaries, was stable phenotypically, was easy to maintain in culture, and was easily transfected.
We used a novel astrocyte-endothelial fibrin gel co-culture system whereby the GFP-labeled murine bEND.3 cells were cultured on collagen-coated microbeads. The endothelial-coated beads were co-cultured with human astrocytes also cultured on collagen-coated microbeads. In this system, we found that the endothelial cells migrated off the beads in large sheets and form cyst-like structures in the fibrin gel, similar to previous reports using this cell line (Figure 4A).23 The GFP-labeled endothelial cells were easily distinguished from astrocytes by fluorescence (Figure 4; A to X). When endothelial-coated beads were co-cultured with astrocyte-coated beads, endothelial cell migration was markedly inhibited (Figure 4, C and D). Similarly, addition of exogenous TGF-β completely inhibited endothelial migration from endothelial-coated beads cultured alone (Figure 4, G and H). To determine whether integrin αvβ8-mediated astrocytic activation of TGF-β was involved in inhibition of endothelial migration, a neutralizing anti-TGF-β1 or anti-β8 blocking antibody was added to endothelial- and astrocyte-coated beads alone or in co-culture. Anti-TGF-β or anti-β8 was able to substantially rescue the astrocyte-mediated inhibition of endothelial migration (Figure 4; O, P, U, and V), while having no effect on endothelial- or astrocyte-coated beads when cultured alone (Figure 4; M, N, Q, R, S, T, W, and X). In the presence of anti-β8 and anti-TGF-β some of the astrocyte-mediated inhibition of endothelial migration remained (Figure 4; O, P, U, and V), either reflecting limitations in the antibodies used or additional uncharacterized mechanisms for astrocyte-mediated inhibition of endothelial cell behavior. The inhibitory effect of astrocytes was specific to astrocytes because beads coated with dermal fibroblasts had no effect on endothelial migration in fibrin gels (data not shown). Taken together, these data demonstrate that αvβ8-mediated activation of TGF-β by astrocytes is physiologically significant, because it is sufficient to inhibit endothelial migration in a complex biological environment.
Figure 4.
αvβ8-mediated activation of TGF-β by astrocytes inhibits endothelial migration in fibrin gels. A polyoma middle T-transformed murine brain endothelial cell line (bEND.3) was retrovirally transduced with a retrovirus encoding the enhanced green fluorescence protein (GFP) and was allowed to form a confluent monolayer on porcine collagen-coated microcarrier beads. Endothelial cell-coated beads alone (A, B, G, H, M, N, S, T), or endothelial cell-coated beads co-cultured with astrocyte-coated beads (C, D, I, J, O, P, U, V), or astrocyte-coated beads alone (E, F, K, L, Q, R, W, X) were embedded into fibrin gels containing either no additions (A–F), recombinant active TGF-β (G–L), anti-TGF-β1 (M–R), or anti-β8 (S–X). The assay was allowed to proceed 48 hours. The endothelial cells are phase and GFP-bright and the astrocytes are phase-bright and GFP-negative. Shown are representative phase (A, C, E, G, I, K, M, O, Q, S, U, W) and fluorescent (GFP) fields (B, D, F, H, J, L, N, P, R, T, V, X).
Gene Profiling of Astrocytes and Endothelial Cells Reveals that Key Angiogenic Genes Are Regulated by TGF-β
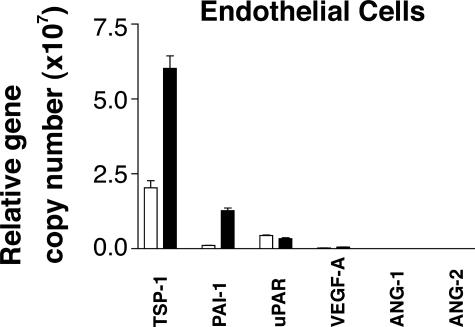
Having shown that astrocytes activate TGF-β in an αvβ8-dependent manner, we sought to determine whether astrocytic αvβ8-mediated activation of TGF-β was sufficient to influence endothelial gene expression of TGF-β-responsive genes through astrocyte-endothelial cell interactions. As a first step in understanding the molecular basis of endothelial responses to astrocytic activation of TGF-β, we surveyed the TGF-β responsiveness of bEND.3 and astrocytes using real-time PCR. We used a panel of primers to molecules implicated in angiogenic pathways that have been reported to be TGF-β responsive (Table 1). TGF-β treatment of bEND.3 cells resulted in significant up-regulation of the anti-angiogenic genes, PAI-1 and thrombospondin-1 (TSP-1). In addition, TGF-β1 treatment of bEND.3 cells caused significant down-regulation of the proangiogenic gene angiopoeitin-2(Ang-2) and a slight but statistically significant down-regulation of urokinase plasminogen activator receptor(uPAR) (Table 1). Only one proangiogenic gene, vascular endothelial growth factor-A, showed a significant increase in expression after treatment with TGF-β (Table 1). When the relative transcript expression levels were examined, TSP-1, PAI-1, and uPAR were the most highly expressed TGF-β-responsive genes by bEND.3 cells (Figure 5). These results demonstrate that a major effect of TGF-β on endothelial gene expression is to induce an anti-angiogenic state through increasing the expression of two key anti-angiogenic genes and decreasing the expression of two key proangiogenic genes.
Table 1.
TGF-β Responsiveness of Anti- and Pro-Angiogenic Genes Expressed by bEND.3 Cells
| Anti-angiogenic genes* | Fold gene induction/repression (P value) | Pro-angiogenic genes† | Fold gene induction/repression (P value) |
|---|---|---|---|
| PAI-1 | 11.5 (P < 0.0001) | ANG-2 | 0.2 (P < 0.001) |
| TSP-1 | 3.0 (P = 0.002) | uPAR | 0.8 (P = 0.002) |
| ALK-5 | 0.9 (ns) | Id1 | 0.7 (ns) |
| PECAM | 1.1 (ns) | ANG-1 | 0.7 (ns) |
| ALK-1 | 0.9 (ns) | ||
| VEGF-A | 2.2 (P = 0.01) |
Plasminogen activator inhibitor-1 (PAI-1), thrombospondin-1 (TSP-1), activin receptor-like kinase 5 (ALK-5), platelet endothelial cell adhesion molecule(PECAM).
Angiopoietin-2 (ANG-2), urokinase plasminogen activator inhibitor receptor (uPAR), inhibitor of differentiation-1 (Id1), angiopoietin-1 (ANG-1), activin receptor-like kinase 1(ALK-1), vascular endothelial growth factor-A(VEGF-A).
Figure 5.
Gene profiling of endothelial cells reveals that key angiogenic genes are regulated by TGF-β. A: Real-time PCR of the murine endothelial bEND.3 cells using mouse-specific primers to thrombospondin-1, plasminogen activator inhibitor-1, urokinase plasminogen activator receptor, vascular endothelial growth factor-A, and angiopoietin-1 and -2, were used for real-time PCR to determine the relative abundance of angiogenic genes in untreated (open bars) or TGF-β1-treated (closed bars) cells. Shown are the relative gene copy numbers calculated using two species-specific housekeeping genes (chosen from five that showed the least variance between treatments) using geNorm.31 Shown is SE (n = 3).
Astrocytic Integrin αvβ8-Mediated Activation of TGF-β Is Sufficient to Influence Endothelial Gene Expression of Key Angiogenic Genes
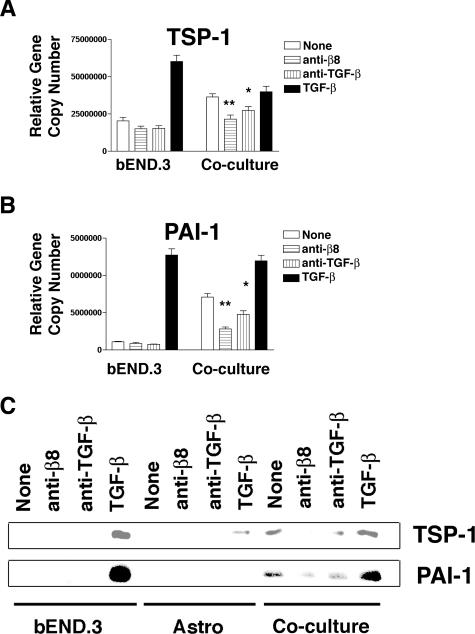
To determine whether astrocyte-mediated activation of TGF-β was sufficient to influence endothelial gene expression, we studied co-cultures of human astrocytes and murine bEND.3 endothelial cells using real-time PCR. We used mouse species-specific primers to TSP-1 and PAI-1to dissect out the endothelial responses to astrocytic αvβ8-mediated TGF-β activation. Human astrocytes co-cultured with mouse endothelial cells significantly increased endothelial TSP-1 or PAI-1 gene expression over solo endothelial cultures (Figure 6, A and B). The induction of endothelial TSP-1 by co-culture with astrocytes appeared to approach the maximal TGF-β-mediated induction of TSP-1, because under co-culture conditions high concentrations of TGF-β did not further increase endothelial TSP-1 expression (Figure 6A). The induction of endothelial PAI-1 expression was significantly increased by co-culture with astrocytes, but did not reach the levels of PAI-1 induction seen when high levels of exogenous TGF-β were added to solo endothelial cell cultures or astrocyte-endothelial cell co-cultures (Figure 6B). The induction of endothelial TSP-1 and PAI-1 seen in co-cultures with astrocytes was due to astrocytic αvβ8-mediated activation of TGF-β, because neutralizing antibodies to αvβ8 or TGF-β were able to almost completely inhibit the TSP-1 and PAI-1 induction (Figure 6, A and B). This effect on endothelial gene expression of TSP-1 and PAI-1 in co-cultures was specific to the astrocytic αvβ8-mediated release of TGF-β because the anti-human β8-neutralizing antibody does not cross-react with mouse and mouse endothelial cells do not express αvβ8.
Figure 6.
Astrocytic integrin αvβ8-mediated activation of TGF-β is sufficient to influence endothelial gene and protein expression of key anti-angiogenic genes. A and B: Real-time PCR of mouse bEND.3 cells alone or in co-culture with human astrocytes using mouse-specific primers to thrombospondin-1 (TSP-1) (A) or to plasminogen activator inhibitor-1 (PAI-1) (B). Cells were treated with nothing (open bars), anti-β8 (horizontal lines), anti-pan-TGF-β (vertical lines), or recombinant active TGF-β (filled bars). Shown are the relative gene copy numbers. Shown is SE (n = 3). *, P < 0.05; **, P < 0.001. C: Detection of thrombospondin-1 (TSP-1, top) or plasminogen activator inhibitor-1 (PAI-1, bottom) by Western blotting of supernatants of bEND.3, astrocytes, or co-cultures of bEND.3 and astrocytes treated with anti-β8, anti-pan-TGF-β, or recombinant active TGF-β. Shown is a representative experiment (n = 3).
Co-culture of astrocytes with endothelial cells did not increase either astrocytic TSP-1 or PAI-1 gene expression (data not shown), consistent with our observation that co-culture of astrocytes with endothelial cells did not significantly increase overall TGF-β activation (relative TMLC luciferase activity in arbitrary units; astrocytes alone: 8747 ± 822; astrocytes and endothelial cells: 6483 ± 1616). We also confirmed that the astrocytic αvβ8-mediated activation of TGF-β-dependent induction of endothelial gene expression resulted in an increase in protein production. The endothelial TSP-1 and PAI-1 genes induced by co-culture with astrocytes resulted in an increase in TSP-1 and PAI-1 protein expression, because TSP-1 and PAI-1 were not detectable by Western blot of solo cultures of endothelial cells, but were clearly detectable on treatment with active TGF-β or co-culture with astrocytes (Figure 6C). Furthermore, this induction of TSP-1 and PAI-1 protein was dependent on astrocytic αvβ8-mediated activation of TGF-β, because the induction could be blocked in co-cultures with an anti-β8 or anti-TGF-β neutralizing antibody (Figure 6C). These data demonstrate that paracrine signaling to endothelial cells occurs through astrocytic αvβ8-mediated activation of TGF-β, which leads to increased mRNA and protein expression of TSP-1 and PAI-1, two key anti-angiogenic genes.
Discussion
Blood vessel integrity is maintained through the complex interplay of multiple cellular programs that control cell adhesion, migration, differentiation, extracellular matrix synthesis, and proteolysis.32 Although much is known of these cellular programs in endothelial cells, relatively little is known of the interplay between endothelial cells and the cells that surround them. In this study, we provide the first evidence that integrins expressed by perivascular astrocytes modulate endothelial cell function through both cell adhesive and paracrine mechanisms, involving binding to and activation of TGF-β.
Our data show that β8 is expressed by a subset of radial glial-like cell processes surrounding blood vessels because β8 immunostaining reveals punctate staining co-localizing with both nestin and GFAP surrounding blood vessels in the fetal brain. Radial glia have been reported to mark with both GFAP and nestin in the mammalian brain33,34 and β8 is expressed in radial glia in embryonic mouse brain.19,22 Although the exact role of radial glia in brain vessel development has not been defined, data demonstrating the close relationship of radial glia and blood vessels in the developing mammalian brain suggests that they may act as a scaffold for vessel development and later may differentiate into perivascular astrocytes.19,35 αvβ8 is expressed in radial glial-like cells surrounding brain vessels during development and αvβ8 can be found in glial processes surrounding blood vessels after birth suggesting a role for astrocytic αvβ8 in both blood vessel development and function.
The close relationship between astrocytes and endothelium suggests direct cell-cell interactions or indirect interactions through an intermediate extracellular matrix ligand. Our data using affinity chromatography demonstrate the RGD- and cation-dependent binding of astrocytic αvβ8 to LAP and suggest that αvβ8 is a major astrocytic cell surface receptor for LAP. In addition, it is likely that LAP is the major ligand for αvβ8 because we have found that LAP is the only known ligand that αvβ8 binds to with sufficiently high affinity to support stable cell adhesion.17,28 Thus, astrocytes could adhere directly to LAP that is a component of either the small latent complex or large latent complex incorporated into the periendothelial cell matrix. Presumably, both forms exist in vivo with the large latent complex being the predominant form.36,37 We have not yet determined whether αvβ8 preferentially binds to and activates the small latent complex or the large latent complex. Interestingly, the integrin αvβ6 has been recently shown to preferentially activate TGF-β in the large latent complex.38 Similarly, αvβ8-mediated cell adhesive interactions could certainly contribute to the alterations in glial-endothelial cell interactions seen in both the αv- and β8-integrin subunit-deficient mice. Evidence in support of a role for αvβ8 in mediating these types of glial-endothelial cell adhesive interactions is the immunocytochemical detection of αvβ8 in glial cell processes surrounding brain endothelium and αvβ8mediated astrocyte adhesion to LAP.
The apposition of αvβ8-expressing astrocyte cell processes and brain endothelium across a thin basal lamina would also allow for reciprocal astrocyte-endothelial paracrine interactions, in addition to stabilizing glial-end-feet association with blood vessels. Indeed, our data show that αvβ8-mediated activation and release of TGF-β is the major mechanism of TGF-β activation in both cultured astrocytes and freshly dissociated fetal brain cells. The involvement of a metalloprotease in astrocytic αvβ8-mediated activation of TGF-β provides a possible mechanism of locally regulating integrin αvβ8-mediated TGF-β activation through the spatial coordination of MMP activity with TGF-β bound to the cell membrane by integrin αvβ8. Although we have not yet definitively identified the metalloprotease or a mechanism of spatial coordination involved in astrocytic αvβ8-mediated activation of TGF-β, it is likely that MT1-MMP can serve this purpose because it is highly expressed by astrocytes and our previous work in cancer cell lines has identified it as a metalloprotease that is required for αvβ8-mediated activation of TGF-β.17,39 Furthermore, MT1-MMP has recently been shown to traffic to caveolae in endothelial cells, raising the possibility that membrane trafficking of MT1-MMP could be a mechanism of regulating αvβ8-mediated activation of TGF-β by locally controlling MT1-MMP activity in astrocytes.39 However, it is likely that multiple metalloproteases also serve this purpose, since to date, no metalloprotease knockout mouse, including the MT1-MMP knockout mouse, exhibits a similar cerebral brain hemorrhage phenotype as β8-deficient mice.
The activation of TGF-β has been reported to be increased by co-culturing rat astrocytes with endothelial cells suggesting reciprocal interactions between these cell types.40 However, we saw no significant change in total TGF activation when total TGF-β activation in solo cultures of astrocytes was compared to the same number of astrocytes cultured with endothelial cells. Thus, there are likely species-specific or developmental stage-specific differences between cultured astrocytes or endothelial cells, because the source of the astrocytes in our study was the mid-gestation human fetus, rather than the neonatal rat and the source of our endothelial cells was the bEND.3 cell line, rather than bovine adrenal capillary endothelial cells.40
TGF-β activation has also been shown to occur in co-cultures of smooth muscle cells and pericytes with endothelial cells.13 However, we have no immunocytochemical data that would suggest that αvβ8 is expressed by cerebral smooth muscle cells or pericytes.27 In addition, αv- and β8-subunit knockout mice do not display any defects in perivascular smooth muscle cells or pericytes.19,22 Thus, any smooth muscle cell or pericyte contribution to TGF-β activation in blood vessel development and function would likely be αvβ8-independent.
The levels of αvβ8 expressed by freshly disaggregated fetal brain cells exceeds that of astrocytes and are similar to the levels achieved by retroviral transduction of human cancer cell lines that potently inhibit cell proliferation in vitro and in vivo.26 Because greater than 90% of freshly disaggregated fetal brain cells express αvβ8 and the majority of cell types in the mid-gestation human fetal brain correspond to immature neuronal lineages including neuronal stem cells and radial glia, it is likely that αvβ8 can mediate TGF-β activation in immature neural cell types other than mature astrocytes. In vitro, αvβ8 has been shown to play a role in oligodendroglial migration and we have shown, using immunoelectron microscopy, that β8 localizes to dendritic spines of mature CNS synapses.27 These findings suggest that αvβ8-mediated activation of TGF-β may play roles in myelination, synapatogenesis or plasticity, or neuronal survival in addition to blood vessel formation. Interestingly, selective ablation of all αv-integrins in neuronal subsets leads to severe neurological defects.22
The activation-state of endothelial cells has recently been suggested to be determined by the relative expression of two TGF-β type I receptors, activin-like kinase-1 and -5 (ALK-1 and ALK-5), which in turn determine the type of response that endothelial cells have to TGF-β.6 Thus, when embryonic mouse cerebral endothelial cells are isolated and cultured they express both ALK-1 and ALK-5, which mediate TGF-β signaling through the SMAD-1/5 and SMAD 2/3 pathways, respectively.6 TGF-β signaling through the ALK-1 pathway may induce a proangiogenic state with induction of Id1 (inhibitor of DNA binding and basic helix-loop-helix transcription factors); TGF-β signaling through the ALK-5 pathway may induce an anti-angiogenic state with induction of PAI-1, the major inhibitor of plasminogen activators.6 We have found that bEND.3 cells also express both ALK-1 and ALK-5. However, bEND.3 cells likely respond to TGF-β primarily through the ALK-5 pathway because on TGF-β treatment, PAI-1 but not Id1, is induced. Thus, astrocytic integrin αvβ8-mediated activation of TGF-β induces an anti-angiogenic phenotype of endothelial cells with associated inhibition of endothelial migration and induction of PAI-1 and TSP-1 consistent with signaling though the ALK-5 pathway.
The mechanism of inhibition of endothelial cell migration in fibrin gels that is dependent on the astrocytic integrin αvβ8-mediated activation of TGF-β may be due to inhibition of plasmin-mediated fibrin proteolysis, because PAI-1 is the major inhibitor of uPA and tPA and can also directly inhibit plasmin.41 However, because PAI-1 can also directly inhibit cell migration by inhibiting uPA bound to uPAR or by interfering with vitronectin-mediated cell migration, PAI-1 has the potential to inhibit endothelial cell migration either through inhibiting fibrinolysis or by disrupting endothelial cell-matrix interactions.42 In addition, the induction of TSP-1 by the astrocytic integrin αvβ8-mediated activation of TGF-β may also play a role in inhibiting endothelial cell migration in fibrin gels through binding of TSP-1 to its cell surface receptor CD36 or binding to and sequestering angiogenic growth factors such as basic fibroblast growth factor.43 Finally, although both TSP-1 and plasmin are implicated in mediating activation of TGF-β in nonneural tissues and other cell-types,13,15 αvβ8 appears to be the main activator of TGF-β in astrocytes because astrocytic activation of TGF-β can be blocked completely by neutralizing antibodies to αvβ8.
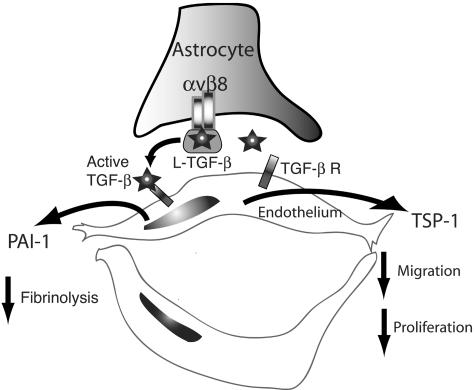
Taken together, our data suggests that αvβ8 acts as a central regulator of brain vessel homeostasis through regulation of TGF-β activation and subsequent modulation of TGF-β-responsive genes in adjacent endothelial cells (Figure 7). These data support a model in which the normal function of astrocytic αvβ8-mediated activation of TGF-β is to promote vessel differentiation and stabilization through the expression of anti-angiogenic genes that inhibit proteolysis, migration, and proliferation. In this model, the inhibition of αvβ8-mediated TGF-β activation would lead to vessel destabilization and dedifferentiation and a proangiogenic response; increasing αvβ8-mediated TGF-β activation could potentially lead to vessel wall thickening as seen in transgenic mice overexpressing active TGF-β in astrocytes.44 This model provides a novel mechanistic pathway for brain hemorrhage and vasculopathies and could explain the mechanisms underlying the destabilized vascular phenotype seen in the cerebral vessels of the αv- and β8-integrin subunit knockout mice.
Figure 7.
Model of astrocytic integrin αvβ8-mediated activation of TGF-β as an angiogenic control switch. Integrin αvβ8 expressed in astrocyte end-feet binds to latent-TGF-β (L-TGF-β) localized in the basal lamina surrounding cerebral brain vessels, potentially stabilizing astrocyte end-feet association with blood vessels. Through a metalloproteolytic cleavage event, L-TGF-β bound to αvβ8 is activated and released allowing active TGF-β to diffuse to the abluminal surface of the endothelial cells where it can bind to TGF-β receptors and stimulate TGF-β signaling, leading to up-regulation of the anti-angiogenic genes, plasminogen activator inhibitor-1 (PAI-1) and thrombospondin-1 (TSP-1), which inhibit local fibrinolysis, cell migration, and proliferation. In this model, the homeostatic function of αvβ8-TGF-β interaction would be to stabilize the cerebral vasculature, and loss or gain of αvβ8 function could potentially lead to destabilization of cerebral vessels and angiogenesis or vascular wall thickening, respectively.
Footnotes
Address reprint requests to Stephen L. Nishimura, M.D., Department of Pathology, Bldg. 3, Rm 207, 1001 Potrero Ave., San Francisco, CA 94110. E-mail: cdog@itsa.ucsf.edu.
Supported by grants from the National Institutes of Health (HL63993, HL70622, and NS44655 to S.N.).
References
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier biology and methodology. J Neurovirol. 1999;5:556–569. doi: 10.3109/13550289909021285. [DOI] [PubMed] [Google Scholar]
- Martin JS, Dickson MC, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Analysis of homozygous TGF beta 1 null mouse embryos demonstrates defects in yolk sac vasculogenesis and hematopoiesis. Ann NY Acad Sci. 1995;752:300–308. doi: 10.1111/j.1749-6632.1995.tb17439.x. [DOI] [PubMed] [Google Scholar]
- van den Driesche S, Mummery CL, Westermann CJ. Hereditary hemorrhagic telangiectasia: an update on transforming growth factor beta signaling in vasculogenesis and angiogenesis. Cardiovasc Res. 2003;58:20–31. doi: 10.1016/s0008-6363(02)00852-0. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri JA, Pratt BM, Tucker AM. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988;106:1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel EG, Shum L, Pompili VJ, Yang ZY, San H, Shu HB, Liptay S, Gold L, Gordon D, Derynck R, Nabel GJ. Direct transfer of transforming growth factor beta 1 gene into arteries stimulates fibrocellular hyperplasia. Proc Natl Acad Sci USA. 1993;90:10759–10763. doi: 10.1073/pnas.90.22.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puyraimond A, Weitzman JB, Babiole E, Menashi S. Examining the relationship between the gelatinolytic balance and the invasive capacity of endothelial cells. J Cell Sci. 1999;112:1283–1290. doi: 10.1242/jcs.112.9.1283. [DOI] [PubMed] [Google Scholar]
- Kim JA, Tran ND, Wang SJ, Fisher MJ. Astrocyte regulation of human brain capillary endothelial fibrinolysis. Thromb Res. 2003;112:159–165. doi: 10.1016/j.thromres.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Loganadane LD, Berge N, Legrand C, Fauvel-Lafeve F. Endothelial cell proliferation regulated by cytokines modulates thrombospondin-1 secretion into the subendothelium. Cytokine. 1997;9:740–746. doi: 10.1006/cyto.1997.0229. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)-beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. Beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, Shani M, Dvorak HF, Wolburg H, Bader BL, Dvorak AM, Hynes RO. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol Cell Biol. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of {alpha}v integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- Montesano R, Pepper MS, Mohle-Steinlein U, Risau W, Wagner EF, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- Hansen SL, Myers CA, Charboneau A, Young DM, Boudreau N. HoxD3 accelerates wound healing in diabetic mice. Am J Pathol. 2003;163:2421–2431. doi: 10.1016/S0002-9440(10)63597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinacker A, Chen A, Agrez M, Cone RI, Nishimura S, Wayner E, Pytela R, Sheppard D. Role of the integrin alpha v beta 6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994;269:6940–6948. [PubMed] [Google Scholar]
- Cambier S, Mu DZ, O’Connell D, Boylen K, Travis W, Liu WH, Broaddus VC, Nishimura SL. A role for the integrin alphavbeta8 in the negative regulation of epithelial cell growth. Cancer Res. 2000;60:7084–7093. [PubMed] [Google Scholar]
- Nishimura SL, Boylen KP, Einheber S, Milner TA, Ramos DM, Pytela R. Synaptic and glial localization of the integrin alphavbeta8 in mouse and rat brain. Brain Res. 1998;791:271–282. doi: 10.1016/s0006-8993(98)00118-8. [DOI] [PubMed] [Google Scholar]
- Nishimura SL, Sheppard D, Pytela R. Integrin alpha v beta 8. Interaction with vitronectin and functional divergence of the beta 8 cytoplasmic domain. J Biol Chem. 1994;269:28708–28715. [PubMed] [Google Scholar]
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- Fjellbirkeland L, Cambier S, Broaddus VC, Hill A, Brunetta P, Dolganov G, Jablons D, Nishimura SL. Integrin alphavbeta8-mediated activation of transforming growth factor-beta inhibits human airway epithelial proliferation in intact bronchial tissue. Am J Pathol. 2003;163:533–542. doi: 10.1016/s0002-9440(10)63681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034.0031–0034.0011. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Frederiksen K, McKay RD. Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J Neurosci. 1988;8:1144–1151. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res. 1995;84:109–129. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Park-Snyder S, Miyazono K, Twardzik D, Mundy GR, Bonewald LF. Characterization and autoregulation of latent transforming growth factor beta (TGF beta) complexes in osteoblast-like cell lines. Production of a latent complex lacking the latent TGF beta-binding protein. J Biol Chem. 1994;269:6815–6821. [PubMed] [Google Scholar]
- Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol. 1994;124:171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez BG, Matias-Roman S, Yanez-Mo M, Vicente-Manzanares M, Sanchez-Madrid F, Arroyo AG. Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol Biol Cell. 2004;15:678–687. doi: 10.1091/mbc.E03-07-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CM, Darland DC, Massingham LJ, D’Amore PA. Endothelial cell-astrocyte interactions and TGF beta are required for induction of blood-neural barrier properties. Brain Res Dev Brain Res. 2004;152:25–38. doi: 10.1016/j.devbrainres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Hekman CM, Loskutoff DJ. Bovine plasminogen activator inhibitor 1: specificity determinations and comparison of the active, latent, and guanidine-activated forms. Biochemistry. 1988;27:2911–2918. doi: 10.1021/bi00408a037. [DOI] [PubMed] [Google Scholar]
- Stefansson S, Petitclerc E, Wong MK, McMahon GA, Brooks PC, Lawrence DA. Inhibition of angiogenesis in vivo by plasminogen activator inhibitor-1. J Biol Chem. 2001;276:8135–8141. doi: 10.1074/jbc.M007609200. [DOI] [PubMed] [Google Scholar]
- Armstrong LC, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 2003;22:63–71. doi: 10.1016/s0945-053x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Sanan DA, Mucke L, Masliah E. Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am J Pathol. 2000;156:139–150. doi: 10.1016/s0002-9440(10)64713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]