Abstract
The activity of protein phosphatase-2A (PP2A) is compromised and is believed to be a cause of the abnormal hyperphosphorylation of tau in Alzheimer’s disease (AD) brain. We investigated in AD the role of the two known endogenous PP2A inhibitors, called I1PP2A and I2PP2A, which regulate the intracellular activity of PP2A in mammalian tissues. We found a significant increase in the neocortical levels of I1PP2A and I2PP2A in AD as compared to control cases by in situ hybridization. The immunohistochemical studies revealed that I2PP2A was translocated from neuronal nuclei to cytoplasm in AD. The 39-kd full-length I2PP2A was selectively cleaved into an ∼20-kd fragment in AD brain cytosol. Digestion of the recombinant human I2PP2A with AD brain extract showed an increase in the generation of the ∼20 kd and other fragments of the inhibitor as compared to control brain extract. Double-immunohistochemical studies revealed co-localization of PP2A with PP2A inhibitors in neuronal cytoplasm and co-localization of the inhibitors with abnormally hyperphosphorylated tau. These studies suggest the possible involvement of I1PP2A and I2PP2A in the abnormal hyperphosphorylation of tau in AD.
Neurofibrillary degeneration of the abnormally hyperphosphorylated tau is one of the hallmarks of Alzheimer’s disease (AD) and tauopathies.1–3 Unlike normal microtubule-associated protein (MAP) tau, which stimulates assembly and stabilizes microtubules,4 the hyperphosphorylated tau sequesters normal tau, MAP1 and MAP2 and inhibits assembly, and depolymerizes microtubules.5–7
The activities of protein phosphatase (PP) 2A and PP1 are compromised in AD brain,8,9 and the inhibition of PP2A activity by okadaic acid produces in metabolically active brain slices from adult rats the abnormal hyperphosphorylation of tau that inhibits its binding and the promotion of microtubule assembly in vitro.10 Injection of calyculin A, a potent and specific inhibitor of PP2A and PP1, into rat hippocampus bilaterally induces the tau hyperphosphorylation and develops deficit in spatial memory retention in Morris water maze.11 In the CA3 area of the AD hippocampus the expression of mRNAs of the catalytic subunit PP2A Cα and the regulatory subunits PR55γ and PR61ε of PP2A, a trimeric holoenzyme, are reduced.12 The expression of PP2Ac gene13,14 and of PP2A ABαC protein subunits have been found to be down-regulated in the affected area of AD brain.15 Furthermore, the level of methyltransferase, which promotes PP2A activity by methylating its catalytic subunit PP2Ac and the association of the PP2Ac with the scaffolding subunit A and regulatory subunit Bα to form the trimeric holoenzyme in the brain are decreased in AD.16 Thus, it is strongly suspected that PP2A, which also regulates the activity of PP1 through dephosphorylation of PP1 inhibitors, I-1/DARPP-32, might be involved in the abnormal hyperphosphorylation of tau and that the abnormally phosphorylated tau can indeed be responsible for the AD neurofibrillary degeneration.
The intracellular PP2A activity is regulated by two inhibitor proteins, called I1PP2A and I2PP2A, in mammalian tissues.17–19 They inhibit PP2A in a noncompetitive manner with the substrate and exhibit apparent Ki values in the nanomolar range.17 I1PP2A has been found to be the same protein as PHAP I,20 LANP,21 pp32,22 and mapmodulin.23 Proteins homologous to I2PP2A have also been isolated and described as human SET,19,24 PHAP II,20 and template-activating factor-1β (TAF-1β).25 Except for mapmodulin,23 it has been reported that these proteins could inhibit the PP2A activity in vitro.
In this study we show 1) an increase in the mRNA expressions of I1PP2A and I2PP2A in temporal and in entorhinal cortices of AD as compared to age-matched control cases; 2) a shift in the intracellular distribution of I2PP2A from its primarily nuclear location to the cytoplasm in neurons and as well as a cleavage of the full-length 39-kd protein to a ∼20-kd fragment in AD brains; 3) an increase in the I2PP2A cleavage activity in AD brain; and 4) a co-localization of the two inhibitors with PP2A in neuronal cytoplasm and with the abnormally hyperphosphorylated tau in neurons with early- to middle-stage neurofibrillary degeneration.
Materials and Methods
Case Demographics and Tissue
Postmortem human hippocampus, temporal cortex (middle temporal gyrus), and cerebellum were dissected from seven age- and postmortem-matched cases of AD and seven cases of controls without neurological disease. The AD cases were diagnosed clinically by National Institute of Neurological and Communicative Diseases and Stroke—Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria26 and after death histopathologically by The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) criteria.27 The case demographics including postmortem intervals are reported in Table 1. For histochemical studies, serial sections (40 μm thickness) were cut and stored in an anti-freezing solution28 at −20°C until used. Paraffin-embedded sections (5 μm thickness) were used only for double-fluorescent immunohistochemistry between PP2A catalytic subunit and PP2A inhibitors.
Table 1.
Profiles of Alzheimer’s Disease and Control Cases
| Age (years) | Sex | PMI (hour) | Braak score | ||
|---|---|---|---|---|---|
| AD | 01–02* | 89 | F | 3.0 | V |
| 01–05* | 80 | F | 2.2 | VI | |
| 01–07* | 85 | F | 1.7 | V | |
| 01–10* | 78 | F | 1.8 | VI | |
| 01–11* | 95 | F | 3.2 | VI | |
| 01–12 | 86 | M | 2.3 | VI | |
| 01–13 | 91 | F | 3.0 | V | |
| Control | 00–34* | 85 | M | 3.2 | II |
| 00–49* | 86 | F | 2.5 | III | |
| 01–31* | 81 | M | 2.8 | III | |
| 01–37* | 88 | F | 3.0 | II | |
| 01–46* | 90 | F | 3.0 | III | |
| 01–51 | 88 | F | 3.5 | III | |
| 02–01 | 88 | F | 3.0 | IV |
PMI, post mortem interval.
These cases were used only for in situ hybridization and immunohistochemistry.
In Situ Hybridization
Five cases from AD and five cases from control group were examined (Table 1). Generation of probes for I1PP2A and I2PP2A and in situ hybridization were performed as previously described.28 Digoxigenin-labeled cRNA probes (anti-sense and sense probe) were made by in vitro transcription using the human I1PP2A or I2PP2A cDNA29 subcloned into pGEM-T vector (Promega, Madison, WI) as template in the presence of digoxigenin-labeled dUTP. For control study, pTRI-GAPDH-human (Ambion, Austin, TX) was used for template. Sections (40 μm) were postfixed for 20 minutes in 4% formaldehyde, followed by a 5-minute wash in 0.1 mol/L phosphate buffer, pH 7.2. Sections were treated with 0.001% proteinase K (Promega), and subsequently for 10 minutes in 0.1 mol/L triethanolamine and 0.225% acetic acid anhydrous solution. After washing with 0.1 mol/L phosphate buffer, sections were dehydrated through a series of increasing concentrations of ethanol and air-dried.
The sections were prehybridized for 30 minutes at 50°C in hybridization buffer (10% sodium dextran sulfate, 20 mmol/L Tris-HCl, pH 8.0, 0.3 mol/L NaCl, 0.2% sarcosyl, 0.02% heat-denatured salmon sperm DNA, 1× Denhardt’s solution, 50% formamide), and then hybridized overnight at 50°C in hybridization solution with 100 ng/ml of cRNA probes. After rinsing in 5× standard saline citrate at 60°C, the sections were washed in 50% formamide/2× standard saline citrate at 60°C for 30 minutes (high stringency wash). The sections were subjected to 30 minutes of RNase digestion at 37°C with 1 μg/ml of RNase A (Roche, Indianapolis, IN) in 10 mmol/L Tris-HCl, pH 7.5, 1 mmol/L ethylenediamine tetraacetic acid, 0.5 mol/L NaCl, and then washed at high stringency. For detection of digoxigenin-labeled cRNA probes, anti-digoxigenin antibody conjugated to alkaline phosphatase (Roche) was reacted at a dilution of 1:500 and color was developed by incubation with 4-nitro blue tetrazolium chloride and 5-bromo-4 chloro-3 indolylphosphate solution (Roche).
Quantitative Analysis of in Situ Hybridization
To determine and compare the mRNA expression of PP2A inhibitors between AD and control, three images at ×20 magnification were captured from the entorhinal cortex, temporal cortex, and cerebellum. The intensity of the signals in stained neurons was determined by the program Simple PCI (C Imaging System, Cranberry Township, PA) and normalized per pixel in the circumscribed area. In situ hybridizations were performed on serial sections for I1PP2A, I2PP2A, and GAPDH. The levels of I1PP2A and I2PP2A mRNA intensities were normalized to the level of GAPDH mRNA intensity in the corresponding tissue. Mean values for each individual were analyzed by t-test between the AD and control cases for I1PP2A and I2PP2A. Differences with P < 0.05 were considered significant. All quantification and analysis were performed blind to the disease status.
Antibodies
The following antibodies were used: anti-I1PP2A (R-42089), a rabbit affinity-purified polyclonal antibody to a synthetic peptide corresponding to amino acid residues 10 to 23 of I1PP2A (rat/human); anti-I2PP2A (R-42187), a rabbit affinity-purified polyclonal antibody to a synthetic peptide corresponding to amino acid residues 18 to 29 of human I2PP2A; anti-I2PP2A (R1482), a rabbit affinity-purified polyclonal antibody to a synthetic peptide corresponding to amino acid residues 161 to 177 of human I2PP2A; anti-I1PP2A (5G6), a monoclonal antibody (mAb) to human recombinant I1PP2A; mAb to PP2A catalytic α subunit (BD Science, San Diego, CA); several phospho-dependent antibodies to tau phosphorylated at various sites: mAb PHF-1 to tau pSer396/pSer404;30,31 mAb M4 to pThr231/pSer 235;32 mAb 12E8 to tau pSer262/pSer356;33 mAb β-actin (Sigma, St. Louis, MO); and rabbit polyclonal antibody to topoisomerase I (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Immunohistochemistry
Both frozen sections and paraffin-embedded sections were used. In the case of paraffin sections (5 μm), they were dewaxed and antigen retrieval was performed by microwave irradiation in 10 mmol/L sodium citrate buffer, pH 6.0, 5 minutes at 850 W, twice. After washing in Tris-buffered saline, frozen sections were treated with 0.3% H2O2 plus 5% bovine serum albumin in Tris-buffered saline for 30 minutes. After this treatment, frozen sections and paraffin sections were subjected to the following same protocol. Sections were blocked with 5% normal goat serum in bovine serum albumin/Tris-buffered saline for 10 minutes and then treated with primary antibodies. Polyclonal antibodies to I1PP2A and I2PP2A28 were used at the concentration of 5 μg/ml. In the case of double-labeled immunohistochemical staining, PHF-1 diluted to 1/200, or M4 diluted to 1/1000, or 12E8 diluted to 1/250, or 5 μg/ml of mouse antibody to PP2A catalytic subunit was mixed with I1PP2A or I2PP2A antibody at this step. After overnight incubation at 4°C, sections were washed with Tris-buffered saline and incubated with biotinylated anti-rabbit IgG diluted to 1/200 for 1 hour at room temperature. After washing, the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine were used to visualize the I1PP2A and I2PP2A antibody staining. In the case of double-immunohistochemical staining for PP2A inhibitors and phosphorylated tau, the sections were developed with Oregon green goat anti-mouse IgG (Molecular Probes, Eugene, OR) diluted to 1/1000. For double-fluorescent immunohistochemistry of PP2A inhibitors and PP2A catalytic subunit, Cy-3 goat anti-rabbit F (ab′)2 (Jackson Immuno-Research Laboratories, West Grove, PA) diluted to 1/1000 and biotinylated anti-mouse IgG diluted to 1/200 were used as secondary antibodies and avidin fetal calf serum (Vector Laboratories) was used for green fluorescent visualization. Finally sections were subjected to Sudan Black B staining to inhibit the lipofuscin auto-fluorescence.
Counts of Neurons with Redistributed I2PP2A
A total of more than 150 neurons with I2PP2A immunoreactivity were counted in five to seven random fields at ×20 magnification (Zeiss Axioscope) which covered ∼80% of each temporal cortex section. The immunopositive neurons were divided into two groups based on the positive or negative nuclear immunoreactivity. The ratio of neurons with negative nuclei to those with positive nuclei were calculated for each AD and control case. Mean values for each brain were analyzed by t-test between the five AD and five control cases. Differences with P < 0.05 were considered significant.
Preparation of Nuclear and Cytosolic Fractions from AD and Control Brains
For preparation of nuclear and cytosolic fractions, temporal cortex from seven cases of each group was homogenized at 4°C in 10 vol of 0.32 mol/L sucrose, 1 mmol/L KH2PO4, pH 6.5, 1 mmol/L MgCl2, 1 mmol/L AEBSF, 2 μg/ml of leupeptin, 2 μg/ml of aprotinin, 1 μg/ml of pepstatin A, and 0.1 mmol/L EGTA using a glass-Teflon homogenizer with 20 strokes. The homogenate was centrifuged at 850 × g for 10 minutes at 4°C and the supernatant (S1) was saved. The pellet was resuspended with 5 vol of the same buffer, then centrifuged as above. The pellet was separated from supernatant (S2) and resuspended with the same buffer (N1). S1 and S2 were pooled and centrifuged at 100,000 × g for 20 minutes at 4°C to obtain supernatant (S3). N1 was used as the nuclear fraction and S3 was used as the cytosol fraction for Western blots.
Western Blots
Nuclear and cytosol fractions from AD and control brains (40 μg/gel lane for I1PP2A blots, and 75 μg/gel lane for I2PP2A blots) were subjected to 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). The protein bands were transferred on Immobilon-P membrane (Millipore, Bedford, MA) and detected by incubation with 2.5 μg/ml of 5G6 mouse mAb to I1PP2A or 2.5 μg/ml of R1482 rabbit polyclonal antibody to I2PP2A, followed by incubation with anti-mouse or -rabbit horseradish peroxidase-conjugated antibody (Jackson ImmunoResearch Laboratory), development with ECL Western blotting detection reagents (Amersham Biosciences Corp., Piscataway, NJ) for 1 minute and visualization by exposing to Hyperfilm ECL (Amersham Biosciences Corp). The ECL films of the blots were scanned and analyzed using TINA 2.0 software (Raytest, Straubenhardt, Germany). I1PP2A and I2PP2A signal intensities were normalized to signal intensity of topoisomerase I for nuclear fraction and of β-actin for cytosol fraction. Mean values for each individual were analyzed by t-test between the seven AD and seven control cases for I1PP2A and I2PP2A. Differences with P < 0.05 were considered significant.
In Vitro Digestion of Recombinant I2PP2A with Brain Extract
Three cases of control (case numbers 0034, 0049, 0201; see Table 1) and three cases of AD brains (case numbers 0102, 0105, 0110; see Table 1) were studied. Middle temporal gyrus of each case was homogenized at 4°C in 5 vol of 25 mmol/L Tris (pH 7.2) without any proteinase inhibitors using glass-Teflon homogenizer, 20 up and down strokes. The homogenate was centrifuged at 16,100 × g for 10 minutes at 4°C, then the supernatant was collected. This supernatant (20% brain extract) was used as a source of the I2PP2A hydrolase activity.
Recombinant human brain I2PP2A, 10 μg,29 was incubated with or without 700 μg of 20% brain extract at 37°C for 0, 7.5, 15, 30, 60, and 90 minutes, and 24 hours. At 24 hours a second dose of the same brain extract was added and incubation continued for another 15, 30, and 60 minutes. The incubated samples containing 100 ng of recombinant I2PP2A plus 7 μg of brain extract per gel lane was subjected to 10% SDS-PAGE, followed by transfer of the separated proteins to Immobilon-P membrane and development of the Western blots with antibody R1482 to I2PP2A.
Mass Spectrometric Analysis of I2PP2A and Its Cleavage Products
For mass spectrometry, 7 μg of recombinant I2PP2A was digested with 7 μg of AD brain extract at 37°C for 90 minutes. Reaction mixture was subjected to 10% SDS-PAGE. The brain extract incubated in the absence of the recombinant I2PP2A was used as a control. Several bands were cut out from Coomassie Brilliant Blue-stained gel and analyzed by matrix-assisted laser desorption ionization tandem time-of-flight mass spectrometry at the Stanford PAN Facility (Palo Alto, CA).
Results
Increase in the mRNA Levels of I1PP2A and I2PP2A in AD Temporal and Entorhinal Cortices
To study the role of PP2A inhibitors in neurofibrillary pathology, the distribution and expression of mRNAs of these inhibitors were determined by in situ hybridization histochemistry in AD and control brains. The expression of the two inhibitors was found mainly in neurons with high intensity in temporal cortex, entorhinal area of hippocampus, and granular cell layer of cerebellum (Figure 1; a to c). A strong hybridization signal was detected in pyramidal cell layers of CA1 to CA4 and granular cell layer of dentate gyrus in the hippocampus (data not shown). In the cerebellum Purkinje cells, and basket and stellate cells in molecular layer also showed strong hybridization (data not shown). No hybridization signals were detected in the control experiments using sense RNA probes, indicating the specificity of the two anti-sense RNA probes (data not shown).
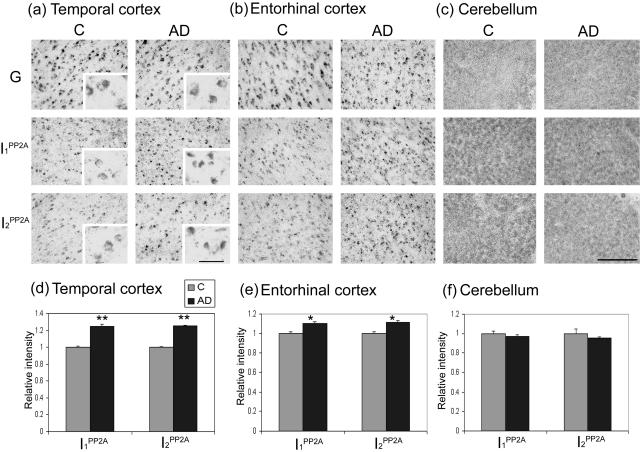
Figure 1.
Expression of I1PP2A and I2PP2A mRNAs in AD and control brains. a–c: Expression of I1PP2A and I2PP2A mRNAs in temporal cortex, entorhinal cortex, and cerebellum from AD and control (C) brains determined by digoxigenin-labeled in situ hybridization. I1PP2A and I2PP2A signals from five AD and five control cases were quantitated using PCI software and normalized by GAPDH (G) signal. d–f: Mean ± SEM of normalized data relative to control. Differences between AD and control cases were analyzed statistically by t-test. The levels of both I1PP2A and I2PP2A mRNAs were elevated in AD temporal cortex (**P < 0.001) and entorhinal cortex (*P < 0.05). Scale bars: 200 μm; 50 μm (insets).
A comparison of the levels of I1PP2A and I2PP2A mRNAs between AD and control cases revealed a disease-associated increase of neuronal mRNA in temporal and entorhinal cortices. To account for any variability among the cases, and accurately evaluate differences between AD and control, all quantitative analyses were normalized to the intensity of GAPDH mRNA for each serial tissue section. The relative expression of I1PP2A and I2PP2A mRNAs after normalization with GAPDH mRNA was ∼25% (P < 0.001) and 10% (P < 0.05) higher in AD temporal and entorhinal cortices, respectively (Figure 1; a, b, d, e) than in the corresponding areas of the control cases. In granule cell layers and Purkinje cells in the cerebellum, no significant differences were found in the levels of I1PP2A and I2PP2A mRNAs between AD and control cases (Figure 1, c and f).
Redistribution of I2PP2A from Neuronal Nucleus to Cytoplasm in AD Brain
Subcellular localization of I1PP2A and I2PP2A were reported previously in various cultured cells. I1PP2A, which is homologous to PHAP-I/LANP/mapmodulin/pp32, is localized in both cytoplasm and nucleus20,21,23,34 and I2PP2A, which is the same as SET/TAF-1, is mainly localized in the nucleus.24,35,36 TAF-1 has been previously shown to be cleaved and the N-terminal cleaved half, which like the full-length protein has PP2A inhibitory activity, is localized in the cytoplasm.36
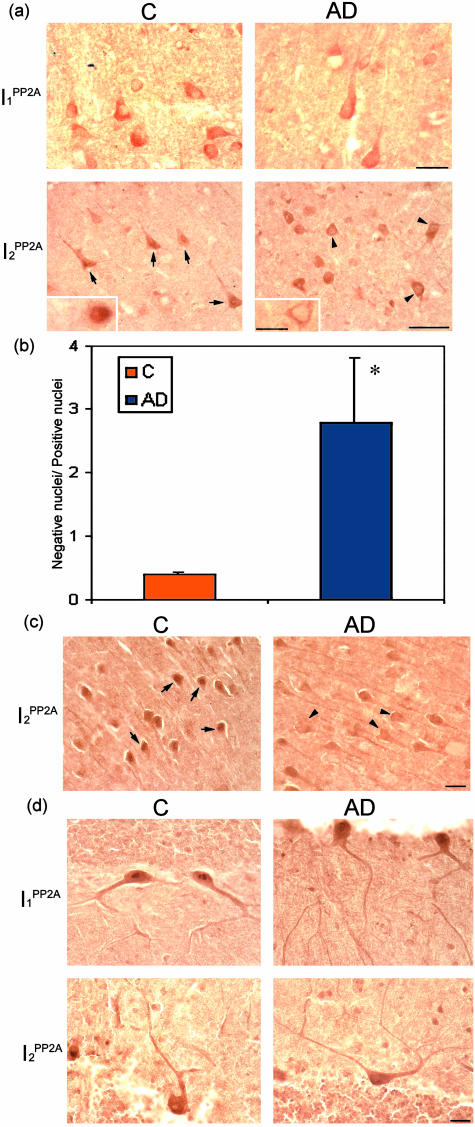
We investigated the subcellular distribution of both I1PP2A and I2PP2A in AD and control brains immunohistochemically. Consistent with the previous studies, subcellular localization of I1PP2A was in the cytoplasm and/or nucleus and of I2PP2A was mainly in the nucleus in human temporal cortex from control brains (Figure 2a). Although the subcellular localization of I1PP2A was similar in AD and control brains, I2PP2A was, with increased frequency, translocated from the nucleus to the cytoplasm in AD temporal cortex (Figure 2a). The number of cells that showed the translocation in AD temporal cortex were counted and compared to the translocated neurons in control. The ratio of neurons with immunonegative nuclei to those with immunopositive nuclei for I2PP2A was more than sixfold greater in AD than control brains (Figure 2b; P < 0.05). The same shift from the nuclear to cytoplasmic compartment was also seen in AD hippocampus (Figure 2c). In cerebellum, both PP2A inhibitors in AD had similar subcellular localization compared to that in control cases (Figure 2d).
Figure 2.
Subcellular localization of I1PP2A and I2PP2A in AD and control brains. I1PP2A and I2PP2A expression was studied by immunohistochemistry using affinity-purified polyclonal antibodies in temporal cortex (TC), hippocampus, and cerebellum. a: I1PP2A was expressed in the cytosol and/or nucleus in both AD and control (C) brains in TC. I2PP2A was predominantly expressed in the nucleus (arrows) in TC from control brain, but was translocated from nucleus to cytosol (arrowheads) in AD brain. b: The ratio (mean ± SEM) of neurons with immunonegative nuclei to those with immunopositive nuclei. In AD the number of neurons in temporal cortex showing the translocation of I2PP2A from nuclear to cytoplasmic localization increased markedly (*P < 0.05). c: I2PP2A in AD hippocampus (CA1) was also translocated from nucleus (arrows) to cytoplasm (arrowheads) as compared to control. d: Subcellular localizations of I1PP2A and I2PP2A were similar between AD and control in the cerebellum. Scale bars: 25 μm [a (I1PP2A and insets), b, d]; 50 μm [a (I2PP2A)].
Cleavage of I2PP2A in AD Temporal Cortex
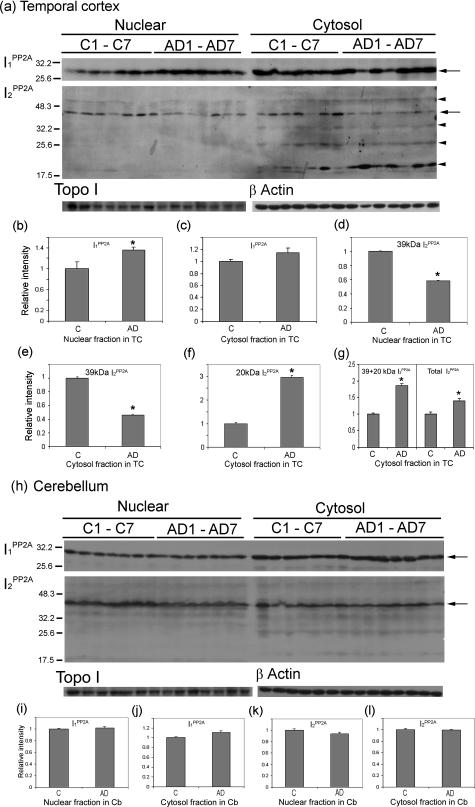
To biochemically confirm the immunohistochemical results, Western blots were performed using nuclear and cytosol fractions prepared from AD and control temporal cortices. The levels of I1PP2A were found to be increased both in nuclear and cytosol fractions in AD but reached significance (P < 0.05) only in the former (Figure 3; a to c). On the other hand, consistent with immunohistochemical findings, the signal of I2PP2A in nuclear fraction was reduced (P < 0.05) in AD compared to control (Figure 3, a and d). In the cytosol, the 39-kd I2PP2A was cleaved and the levels of the fragments were higher in AD than in controls. The signal of I2PP2A at 39 kd in the cytosolic fraction was decreased in AD (Figure 3, a and e; P < 0.05). A major cleavage product, the ∼20-kd I2PP2A polypeptide, which was seen in cytosolic and not in nuclear fraction, appeared in few control and most AD cases (Figure 3a). In addition to the ∼20-kd fragment, weak signals of ∼56-kd, ∼34-kd, and ∼25-kd I2PP2A immunoreactivities were also seen in both control and AD cytosols. The level of 20-kd polypeptide was significantly higher in AD cytosol compared to that in control (Figure 3f; P < 0.05). The combined levels of the I2PP2A-39-kd and -20-kd polypeptides and as well as of the 39-kd I2PP2A plus all of its cleavage product polypeptides were increased (P < 0.05) in AD compared to control (Figure 3, a and g).
Figure 3.
Cleavage and distribution of I2PP2A in nuclear and cytosolic fractions in AD and control brains. Subcellular fractions from temporal cortex (TC) and cerebellum (Cb) of control (C) and AD cases were analyzed by Western blots. I1PP2A and I2PP2A signals were quantitated using TINA 2.0 software and normalized by topoisomerase I (Topo I) for nuclear fraction and β-actin for cytosol fraction. a–c: In temporal cortex, the level of I1PP2A in AD was increased significantly in nuclear fraction (a, b; *P < 0.05) and insignificantly in the cytosol (a, c) as compared to control cases. In contrast, the level of I2PP2A in the nuclear fraction was decreased in AD as compared to the control cases (a, d; *P < 0.05). The 39-kd I2PP2A in the cytosol fraction was decreased in AD (a, e; *P < 0.05), but an ∼20-kd fragment of I2PP2A was significantly increased in AD as compared to controls (a, f; *P < 0.05). Total relative intensity of both 39-kd plus 20-kd I2PP2A and as well as of the 39-kd plus all its cleavage product polypeptides (total I2PP2A) were increased in AD as compared to control (a, g; *P < 0.05). h: In cerebellum, expression levels of both I1PP2A and I2PP2A were similar in AD and control cases (h–l), and only background level of 20-kd fragment of I2PP2A was seen in cerebellum. b–g, i–l: Mean ± SEM of normalized data relative to control. The left of a and h indicates the molecular weight markers, and on the right of a and h, full-length I1PP2A (30 kd) and I2PP2A (39 kd) is indicated by arrows, and the cleavage products of I2PP2A by arrowheads (from top to bottom, the ∼56-, ∼34-, ∼25-, and ∼20-kd polypeptides).
We also performed the same study using nuclear and cytosol fraction from cerebellum. Expression levels of both PP2A inhibitors in AD were not significantly different between AD and control cases (Figure 3; h to l). In addition, only background level of ∼20-kd I2PP2A was seen in the cerebellum (Figure 3h), suggesting that this cleavage of I2PP2A was selective to areas of the brain that develop neurofibrillary pathology.
Cleavage of Recombinant I2PP2A by Brain Extract
To confirm whether ∼56-kd, ∼34-kd, ∼25-kd, and ∼20-kd polypeptides of I2PP2A are generated from I2PP2A full-length protein (277 amino acids), recombinant I2PP2A was digested by brain extracts from AD and control cases. Digestion of recombinant I2PP2A by both AD and control brain extracts resulted in at least five to six strong signals (Figure 4a). In AD cases, the 39-kd signal was reduced in a time-dependent manner with a simultaneous appearance of ∼56-, ∼34-, and ∼25-kd signals within 7.5 minutes of digestion. This was followed by the appearance of a ∼22-kd fragment during 15 to 30 minutes of digestion.
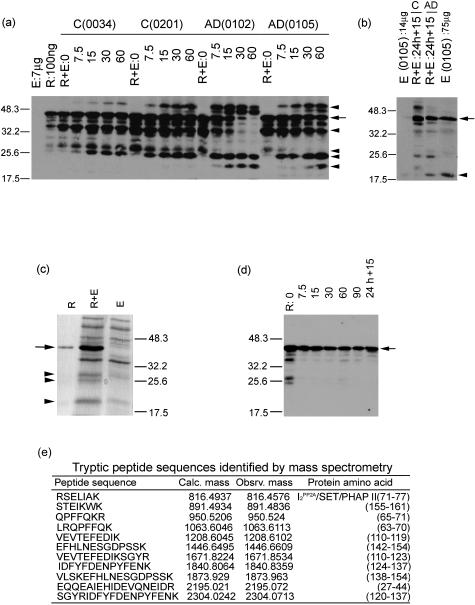
Figure 4.
Digestion of recombinant human brain I2PP2A with AD and control brain extracts and identification of the cleavage products by Western blots and by mass spectrometry. a: Ten μg of recombinant I2PP2A was digested with 700 μg of temporal cortex extract from AD brains no. 0102 and no. 0105 and control (C) brain no. 0034 and no. 0201 for 0, 7.5, 15, 30, and 60 minutes, and 24 hours at 37°C. The digest containing 100 ng of recombinant I2PP2A and 7 μg of brain extract/gel lane was subjected to SDS-PAGE, followed by Western blots developed with antibody R1482 to I2PP2A. The left of a indicates the molecular weight markers, and on the right, the I2PP2A full-length 39 kd is indicated by an arrow, and the cleavage products generated by arrowheads (from top to bottom, the ∼56-, ∼34-, ∼28-, ∼25-, and ∼22-kd polypeptides). The cleavage products were generated significantly earlier on digestion with AD than control brain extract. The I2PP2A signal was undetectable in 7 μg of brain extract (E) used as a control. R, recombinant human brain I2PP2A. b: After 24 hours of digestion, a second dose of the brain extract was added to the digest and incubated for another 15 minutes. The digestion with AD but not control brain extract generated a 20-kd I2PP2A polypeptide that co-migrated with the I2PP2A cleavage product seen in 75-μg AD brain extract (right lane, arrowhead); the 20-kd signal was undetectable in 14 μg of the AD brain extract (left lane), arrow indicates the 39-kd I2PP2A. c: Coomassie Blue-stained gel of 700 ng of recombinant I2PP2A (R), 7 μg AD brain extract (E), and digestion of 7 μg of recombinant I2PP2A with 7 μg of AD brain extract (R + E) for 90 minutes at 37°C. The polypeptide bands corresponding to ∼28, ∼25, and ∼22 kd (arrowheads) were cut out and subjected to tryptic digest and mass spectrometry. Arrow shows the 39-kd I2PP2A. d: Recombinant of I2PP2A without brain extract was incubated at 37°C for 7.5, 15, 30, 60, and 90 minutes, and 24 hours 15 minutes. One hundred ng of recombinant I2PP2A/lane was subjected to SDS-PAGE, followed by Western blots developed with antibody R1482 to I2PP2A. The degradative products that were seen at the time 0 point disappeared as early as during 7.5 minutes of digestion. Arrow shows the 39-kd I2PP2A. e: The mass spectrometric analysis of ∼25-kd polypeptide cut out from (R + E) showed it to be the amino terminal fragment of I2PP2A. Not shown in this figure, the ∼28-kd polypeptide also was identified an amino terminal fragment of I2PP2A; the ∼22-kd protein band, because of other proteins with the similar molecular weight from brain extract, could not be identified.
On the other hand, in control cases the appearance of these products was apparently delayed and the ∼22-kd fragment was not seen during digestion. We did not detect any ∼28-kd fragment in AD cases, but an ∼56-kd signal could be seen in both AD and control cases, which was probably a dimer of the ∼28-kd fragment, suggesting that the ∼28-kd fragment in AD cases formed the dimer (within 7.5 minutes) earlier than in control (Figure 4a). Identically treated brain extract alone did not generate these signals (data not shown). These in vitro digestion conditions used were not sufficient to generate the I2PP2A-immunopositive ∼20-kd polypeptide that we had found increased in AD brain extract in Figure 3a. We found that the addition of a second dose of the brain extract after 24 hours of incubation as the source of the proteolytic activity generated a ∼20-kd I2PP2A-immunopositive polypeptide during 15, 30, and 60 minutes of incubation. However, digestion with the AD brain extract generated this ∼20-kd polypeptide markedly more than the control brain extract (Figure 4b). Similar results were obtained by 30 or 60 minutes of further digestion (data not shown). AD or control brain extracts alone when treated identically showed only a background signal at ∼20-kd position (Figure 4b). Similarly, incubation of recombinant I2PP2A alone did not result in the generation of the ∼20-kd polypeptide (Figure 4d).
To further confirm that the cleavage products were from I2PP2A, digested products were subjected to SDS-PAGE, stained with Coomassie blue (Figure 4c), and the ∼28-kd, ∼25-kd, and ∼22-kd polypeptide bands generated were sliced out and used for analysis by mass spectrometry. The mass spectrometric analysis of the tryptic digest of the ∼28-kd and ∼25-kd polypeptides revealed a complete match to that of I2PP2A/SET/PHAPII/TAF1 and showed that these polypeptides were from the amino terminal half or greater half of I2PP2A (Figure 4e). These tryptic peptide sequences lacked the C-terminal acidic region, indicating that these peptides were from the amino terminal region. The ∼22-kd polypeptide, which was a mixture of the inhibitor and some unrelated brain proteins, could not be positively identified by mass spectrometry. All cleavage products were, however, positively identified with several antibodies to I2PP2A (data not shown). Amino terminal sequencing of the digest of the recombinant I2PP2A by AD brain extract revealed the presence of an ∼20-kd amino terminal fragment (Tanimukai et al, in preparation).
Co-Localization of PP2A Inhibitors with PP2A Catalytic Subunit and with Hyperphosphorylated Tau in Neuronal Cytoplasm
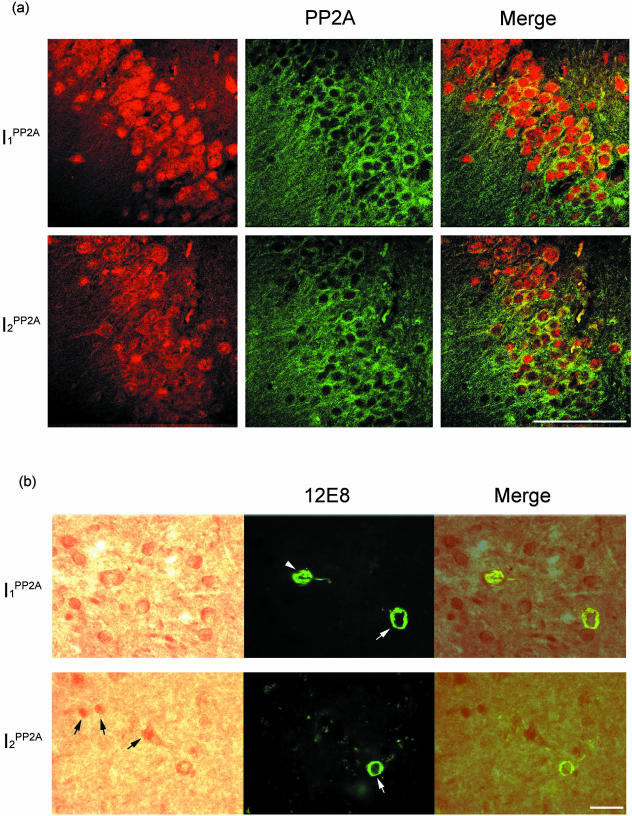
Increase in the levels of mRNAs of I1PP2A and I2PP2A, and cleavage and translocation of I2PP2A from the nuclear to the cytoplasmic compartment in neurons in AD prompted us to investigate whether the PP2A inhibitors were involved in the hyperphosphorylation of tau in AD. We performed double-labeled immunohistochemical studies using specific antibodies against I1PP2A, I2PP2A, PP2A catalytic subunit, and to tau abnormally hyperphosphorylated at serine 396/404 (PHF1), Thr231/Ser 235 (M4), and Ser262/356 (12E8). Both PP2A inhibitors co-localized with PP2A catalytic subunit in neuronal cytoplasm in AD brains (Figure 5a). In addition, PP2A inhibitors were also co-localized with what appeared to be early- to middle-stage neurofibrillary tangles of the abnormally hyperphosphorylated tau in neuronal cytoplasm (Figure 5b, data not shown for mAb PHF1 and mAb M4). The neurons that expressed I1PP2A and I2PP2A mainly in the nucleus did not co-localize with phosphorylated tau immunoreactivity (Figure 5b), indicating that PP2A inhibitors in cytoplasm were probably responsible for tau hyperphosphorylation.
Figure 5.
Co-localization of I1PP2A and I2PP2A with PP-2A and with phosphorylated tau in AD brain. a: Subcellular localizations of I1PP2A, I2PP2A, and PP-2A in AD hippocampus. Both I1PP2A and I2PP2A co-localized with PP-2A in neuronal cytoplasm. b: Both I1PP2A and I2PP2A were co-localized with mostly early-stage (white arrows) to middle-stage (white arrowhead) neurofibrillary changes as seen with phospho-dependent antibodies (12E8) to abnormally hyperphosphorylated tau in AD temporal cortex. The neurons that expressed I2PP2A mainly in the nucleus did not co-localize with phosphorylated tau (black arrows). Scale bars: 100 μm (a); 25 μm (b).
Discussion
Neurofibrillary degeneration of abnormally hyperphosphorylated tau is a primary and a pivotal lesion of AD and several tauopathies.37 The present study demonstrates an intraneuronal increase in the AD neocortex in the mRNA expressions of I1PP2A and I2PP2A, the two major regulators of the intracellular PP2A activity, and the co-localization of these two inhibitors with PP2A and with the abnormally hyperphosphorylated tau and the early- to middle-stage neurofibrillary tangles. Selective cleavage and translocation of I2PP2A from its primary nuclear location to the cytoplasm in neurons of the neocortex in AD that undergoes neurofibrillary degeneration, shows a novel mechanism by which this PP2A inhibitor might contribute to the abnormal hyperphosphorylation of tau. These findings reveal for the first time the possible involvement of I1PP2A and I2PP2A in Alzheimer neurofibrillary pathology through the inhibition of PP2A activity and the resulting abnormal hyperphosphorylation of tau.
Neurofibrillary pathology in AD brain is known to start from entorhinal cortex in stages I/II of Braak, then spreads to the hippocampal formation, amygdala, and thalamus in stages III/IV. The pathology further develops in temporal proneocortex, and gradually invades the extended neocortical association areas in stage V, until finally the pathological process even progresses through the border fields into the primary areas in stage VI.38 In this study, we used the tissue from the late stage of AD ie, stage V to VI. At this late stage, the majority of neurons in the hippocampus, especially the entorhinal cortex, are at advanced stages of neurofibrillary degeneration. The neuronal loss in the medial temporal cortex and other areas of the neocortex is relatively small at Braak stage V to VI of AD. As expected, we found that the temporal cortex had relatively mild tau pathology compared to entorhinal cortex and hippocampal formation (data not shown). In the present study, a statistically significant increase in the mRNA expressions of I1PP2A and I2PP2A was found both in the temporal cortex and the entorhinal cortex in AD. A relatively less marked increase in the expression of mRNAs of I1PP2A and I2PP2A in the entorhinal cortex as compared to temporal cortex in AD found in the present study is probably related to advanced stage of neurofibrillary degeneration in the former. In the AD cases at Braak stages V and VI, which were used for the present study, a very large number of neurons are at advanced stages of neurofibrillary degeneration as compared to a relatively much smaller number in the temporal cortex of the same brains. We believe a smaller increase in the expression of I1PP2A and I2PP2A mRNAs seen in the entorhinal cortex than the temporal cortex in the AD cases found in the present study reflect a larger number of neurons with advanced stages of neurofibrillary degeneration in the AD cases studied. Consistent with the present study, microarray analyses have also shown an up-regulation of the expression of acidic nuclear p32 and SET genes in AD hippocampus.14
AD is a slow but progressive neurodegenerative disorder with an average progression of 7 to 10 years. Thus, the 15 to 25% increase in the PP-2A inhibitors found in the AD neocortex could have a considerable accumulative effect with time on the hyperphosphorylation of tau and the consequent neurofibrillary degeneration. In the present study the cerebellum, which is unaffected by neurofibrillary pathology in AD and was used as an internal control, did not show any significant changes in the mRNA expressions of I1PP2A or I2PP2A.
The immunohistochemical data in control brains showed that I1PP2A was localized in the nucleus and/or cytoplasm. On the other hand, I2PP2A was localized mainly in the nucleus, with a weak signal in the neuronal cytoplasm. These results are consistent with previous studies that showed that I1PP2A works as a shuttle protein between nucleus and cytoplasm39 and that I2PP2A localized mainly in the neuronal nuclei in the mammalian brain.28
The subcellular localization of I1PP2A was similar to that in control brain, whereas that of I2PP2A was changed with increased frequency from nucleus to the cytoplasmic dominant localization in neurons in AD temporal cortex. Similar translocation of I2PP2A was seen in AD hippocampus but not in AD cerebellum, indicating that this translocation was region-specific and was seen in the areas of the brain affected by neurofibrillary pathology. The corresponding biochemical data obtained from Western blots showed that I2PP2A immunoreactivity in nuclear fraction from AD temporal cortex was significantly decreased compared to that from the corresponding control tissue. In addition, the level of the 39-kd full-length I2PP2A in cytosol was reduced but at the same time, ∼20-kd fragment of I2PP2A appeared and was significantly increased in AD compared to control cases. Consistent with these findings, previously it has been reported that the 20 kd of I2PP2A isolated from bovine kidney, which has the inhibitory activity, is derived from the cleavage of the 39-kd I2PP2A/SET/TAF1.19 Furthermore, in HeLa cells transfected with the amino terminal half of TAF1, the inhibitor is localized in the cytoplasm.36 We postulated that the cleavage of the 39-kd I2PP2A and its translocation from the neuronal nucleus to the cytoplasm in the affected brain areas in AD might involve an increase in the activity of a specific hydrolase(s) in the diseased tissue. We found that the recombinant human brain I2PP2A was digested faster with AD than control brain extract, generating similar proteolytic fragments of the inhibitor.
The generation of the 20-kd I2PP2A from the full-length protein appears to take place in several steps. The 20-kd I2PP2A appeared to be the final product. It might have been cleaved from 22-kd I2PP2A because this polypeptide disappeared when the 20-kd polypeptide appeared during the digestion (Figure 4, a and b). In AD and control brain cytosols, the 22-kd I2PP2A was undetectable (Figure 3a). Although at present we do not know the identity of the protease(s) involved in the cleavage of I2PP2A in AD brain, PHAP-II (I2PP2A/SET/TAF1) has been shown to be cleaved in K562 cells by granzyme A.40 Interestingly, Beresford and colleagues41 have also shown that recombinant SET protein is cleaved by granzyme A in vitro and produces 25-kd, 22-kd, and 20-kd polypeptides. Granzyme A, a serine protease, is abundant in cytotoxic granules. Although there is no evidence of granzyme A in neurons, we speculate that some serine protease(s) in human brain might have similar role for this cleavage.
The cleavage and the translocation of I2PP2A might play a significant role in tau hyperphosphorylation because the 20-kd N-terminal fragment also has the PP2A inhibitory activity42 and PP2A is mainly localized in the neuronal cytoplasm, as shown in the present study and previously.43 It has been reported that PP2A inhibitors inhibit PP2A activity by binding directly to the PP2A catalytic subunit.44,45 It remains to be investigated whether the cleaved I2PP2A binds easier than full-length I2PP2A to PP2A. In this study, we have observed the co-localization of PP2A inhibitors and PP2A catalytic subunit in the neuronal cytoplasm, suggesting that both PP2A inhibitors could bind and inhibit PP2A in the neuronal cytoplasm.
The present study showed the co-localization of PP2A inhibitors and tau abnormally hyperphosphorylated at Ser396/404, Thr231/Ser 235, and Ser262/356 in the neuronal cytoplasm. Most neurons that showed the co-localization between PP2A inhibitors and phosphorylated tau appeared to be in the early to middle stage of neurofibrillary pathology. These results and increase in the levels of mRNAs of PP2A inhibitors and redistribution of I2PP2A from neuronal nucleus to cytoplasm suggest that PP2A inhibitors might inhibit PP2A activity at the relatively early stage of neurofibrillary changes in AD brain. This speculation of the possible involvement of PP2A inhibitors in the hyperphosphorylation of tau is supported by a previous report in which I1PP2A and I2PP2A inhibited 50 to 80% of PP2A activity toward tau at the PHF1, 12E8, and M4 sites in vitro.29
In conclusion, I1PP2A and I2PP2A, the two major regulators of intracellular PP2A activity, are selectively up-regulated in the areas of the AD brain affected with neurofibrillary pathology. In addition, I2PP2A, which is mainly localized in the nucleus as the full-length protein, is cleaved and its amino terminal half and/or greater halves, which are known to inhibit PP2A, are translocated to the cytoplasm in neurons in the affected areas of the brain in AD. I1PP2A and translocated I2PP2A co-localize both with PP2A and the early- to middle-stage neurofibrillary changes of abnormally hyperphosphorylated tau. These findings lead us to speculate a scenario in which the increase in the messages of I1PP2A and I2PP2A, plus the translocation of I2PP2A from the neuronal nucleus to the cytoplasm, results in an inhibition of PP2A activity in AD brain. The inhibition of PP2A activity probably leads to the abnormal hyperphosphorylation of tau not only by a decrease in the dephosphorylation but also by an increase in the phosphorylation of tau by tau protein kinases that are regulated by PP2A. Inhibition of PP2A has been shown to increase the activities of calcium calmodulin-dependent protein kinase II,46 cyclic AMP-dependent protein kinase A,47 and various members of the mitogen-activated protein kinase family.48–50 Thus, I1PP2A and I2PP2A offer previously unknown therapeutic targets for AD and tauopathies.
Acknowledgments
We thank Dr. Ichiro Tsujio for providing the human brain I1PP2A and I2PP2A cDNAs; Dr. Ezzat EI-Akkad for preparing the recombinant I2PP2A; Janet Biegelson and Sonia Warren for secretarial assistance including the preparation of the manuscript; Drs. Thomas Beach and Lucia Sue, Sun Health Research Institute, Brain Donation Program, for human brain tissue; and Drs. Peter Davies (Albert Einstein College of Medicine, Bronx, NY), Yasuo Ihara (University of Tokyo, Tokyo, Japan), and Dale Schenk (Elan Corp., San Francisco, CA) for their kind gifts of monoclonal antibodies, PHF-1, M4, and 12E8, respectively.
Footnotes
Address reprint requests to Khalid Iqbal, Ph.D., Chairman, Department of Neurochemistry, New York State Institute for Basic Research in Developmental Disabilities, 1050 Forest Hill Rd., Staten Island, New York 10314-6399. E-mail: iqbalk@worldnet.att.net.
Supported in part by the New York State Office of Mental Retardation and Developmental Disabilities; the Institute for the Study of Aging, New York, NY; Alzheimer’s Association, Chicago, IL; and the National Institutes of Health/National Institute on Aging (grant AG19158).
References
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I, Smith AJ, George L, Tung YC, Zaidi T. Identification and localization of tau peptide to paired helical filaments of Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:5646–5650. doi: 10.1073/pnas.86.14.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Balin BJ, Otvos Jr L, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A del C, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A del C, Grundke-Iqbal I, Iqbal K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- Alonso A del C, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of MAP1 and MAP2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem. 1993;61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- Gong CX, Shaikh S, Wang JZ, Zadi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem. 1995;65:732–738. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J Biol Chem. 2000;275:5534–5544. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- Sun L, Liu SY, Zhou XW, Wang XC, Liu R, Wang Q, Wang JZ. Inhibition of protein phosphatases 2A and protein phosphatases 1 induced tau phosphorylation and impairment of spatial memory retention in rats. Neuroscience. 2003;118:1175–1182. doi: 10.1016/s0306-4522(02)00697-8. [DOI] [PubMed] [Google Scholar]
- Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VM. PP2A mRNA expression is quantitatively decreased in Alzheimer’s disease hippocampus. Exp Neurol. 2001;168:402–412. doi: 10.1006/exnr.2001.7630. [DOI] [PubMed] [Google Scholar]
- Loring JF, Wen X, Lee JM, Seilhamer J, Somogyi R. A gene expression profile of Alzheimer’s disease. DNA Cell Biol. 2001;20:683–695. doi: 10.1089/10445490152717541. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci USA. 2004;101:2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, White CL., III Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- Sontag E, Hladik C, Montgomery L, Luangpirom A, Mudrak I, Ogris E, White CL., III Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J Neuropathol Exp Neurol. 2004;63:1080–1091. doi: 10.1093/jnen/63.10.1080. [DOI] [PubMed] [Google Scholar]
- Li M, Guo H, Damuni Z. Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry. 1995;34:1988–1996. doi: 10.1021/bi00006a020. [DOI] [PubMed] [Google Scholar]
- Li M, Makkinje A, Damuni Z. Molecular identification of I1PP2A, a novel potent heat-stable inhibitor protein of protein phosphatase 2A. Biochemistry. 1996;35:6998–7002. doi: 10.1021/bi960581y. [DOI] [PubMed] [Google Scholar]
- Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271:11059–11062. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- Vaesen M, Barnikol-Watanabe S, Gotz H, Awni LA, Cole T, Zimmermann B, Kratzin HD, Hilschmann N. Purification and characterization of two putative HLA class II associated proteins: PHAPI and PHAPII. Biol Chem Hoppe Seyler. 1994;357:113–126. doi: 10.1515/bchm3.1994.375.2.113. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Taoka M, Satozawa N, Nakayama H, Ichimura T, Takahashi N, Yamakuni T, Song SY, Isobe T. A nuclear factor containing the leucine-rich repeats expressed in murine cerebellar neurons. Proc Natl Acad Sci USA. 1994;91:9670–9674. doi: 10.1073/pnas.91.21.9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TH, Brody JR, Romantsev FE, Yu JG, Kayler AE, Voneiff E, Kuhajda FP, Pasternack GR. Structure of pp32, an acidic nuclear protein which inhibits oncogene-induced formation of transformed foci. Mol Biol Cell. 1996;7:2045–2056. doi: 10.1091/mbc.7.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur N, Rancano C, Pfeffer SR. Biochemical characterization of mapmodulin, a protein that binds microtubule-associated proteins. J Biol Chem. 1997;272:30577–30582. doi: 10.1074/jbc.272.48.30577. [DOI] [PubMed] [Google Scholar]
- von Linden M, van Baal S, Wiegant J, Raap A, Hagemeijer A, Grosveld G. Can, a putative oncogene associated with myeloid leukemogenesis, may be activated by fusion of its 3′ half to different genes: characterization of the set gene. Mol Cell Biol. 1992;12:3346–3355. doi: 10.1128/mcb.12.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Kawase H, Handa H, Yano K, Yamasaki M, Ishimi Y, Okuda A, Kikuchi A, Matsumoto K. Replication factor encoded by a putative oncogene, set, associated with myeloid leukemogenesis. Proc Natl Acad Sci USA. 1995;92:4279–4283. doi: 10.1073/pnas.92.10.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlar EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish A Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Tanimukai H, Grundke-Iqbal I, Iqbal K. Inhibitors of protein phosphatases-2A: topology and subcellular localization. Mol Brain Res. 2004;126:146–156. doi: 10.1016/j.molbrainres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Tsujio I, Zaidi T, Xu J, Kotula L, Grundke-Iqbal I, Iqbal K: Inhibitors of protein phosphatase-2A from human brain: structures, immunocytological localization and activities towards dephosphorylation of the Alzheimer type hyperphosphorylated tau. FEBS Lett (in press) [DOI] [PubMed] [Google Scholar]
- Greenberg SG, Davies P, Schein JD, Binder LI. Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J Biol Chem. 1992;267:564–569. [PubMed] [Google Scholar]
- Otvos L, Jr, Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;39:669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Watanabe A, Takio K, Suzuki M, Arai T, Titani K, Ihara Y. Characterization of two distinct monoclonal antibodies to paired helical filaments: further evidence for fetal-type phosphorylation of the tau in paired helical filaments. J Neurochem. 1993;60:2068–2077. doi: 10.1111/j.1471-4159.1993.tb03491.x. [DOI] [PubMed] [Google Scholar]
- Seubert P, Mawal-Dewan M, Barbour R, Jakes R, Goedert M, Johnson GV, Litersky JM, Schenk D, Leiberburg I, Trojanowski JQ, Lee VM. Detection of phosphorylated Ser262 in fetal tau, adult tau and paired helical filament tau. J Biol Chem. 1995;270:18917–18922. doi: 10.1074/jbc.270.32.18917. [DOI] [PubMed] [Google Scholar]
- Malek SN, Katumuluwa AI, Pasternack GR. Identification and preliminary characterization of two related proliferation-associated nuclear phosphoproteins. J Biol Chem. 1990;265:13400–13409. [PubMed] [Google Scholar]
- Adachi Y, Pavlakis GN, Copeland TD. Identification and characterization of SET, a nuclear phosphoprotein encoded by the translocation break point in acute undifferentiated leukemia. J Biol Chem. 1994;269:2258–2262. [PubMed] [Google Scholar]
- Nagata K, Saito S, Okuwaki M, Kawase H, Furuya A, Kusano A, Hanai N, Okuda A, Kikuchi A. Cellular localization and expression of template-activating factor I in different cell types. Exp Cell Res. 1998;240:274–281. doi: 10.1006/excr.1997.3930. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Alonso A del C, El-Akkad E, Gong CX, Haque N, Khatoon S, Tanimukai H, Tsujio I, Grundke-Iqbal I. Pivotal role of neurofibrillary degeneration in Alzheimer disease and therapeutic targets. Takeda M, Tanaka T, Cacabelos R, editors. Basel: Karger,; Molecular Neurobiology of Alzheimer Disease and Related Disorders. 2004:pp 42–51. [Google Scholar]
- Braak H, Griffing K, Braak E. Neuroanatomy of Alzheimer’s disease. Alzheimer’s Res. 1997;3:235–247. [Google Scholar]
- Brennan CM, Gallouzi IE, Steitz JA. Protein ligands to HuR modulate its interaction with target mRNAs in vivo. J Cell Biol. 2000;151:1–14. doi: 10.1083/jcb.151.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford PJ, Kam CM, Powers JC, Lieberman J. Recombinant human granzyme A binds to two putative HLA-associated proteins and cleaves one of them. Proc Natl Acad Sci USA. 1997;94:9285–9290. doi: 10.1073/pnas.94.17.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford PJ, Zhang D, Oh DY, Fan Z, Greer EL, Russo ML, Jaju M, Lieberman J. Granzyme A activates an endoplasmic reticulum-associated caspase-independent nuclease to induce single-stranded DNA nicks. J Biol Chem. 2001;276:43285–43293. doi: 10.1074/jbc.M108137200. [DOI] [PubMed] [Google Scholar]
- Saito S, Miyaji-Yamaguchi M, Shimoyama T, Nagata K. Functional domains of template-activating factor-I as a protein phosphatases 2A inhibitor. Biochem Biophys Res Commun. 1999;259:471–475. doi: 10.1006/bbrc.1999.0790. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Sersen E, Iqbal K, Grundke-Iqbal I. Expression of protein phosphatases (PP-1, PP2A, PP-2B and PTP-1B) and protein kinases (MAP kinase and P34cdc2) in the hippocampus of patients with Alzheimer disease and normal aged individuals. Brain Res. 1994;655:70–76. doi: 10.1016/0006-8993(94)91598-9. [DOI] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signaling. Biochem J. 2001;353:417–439. [Google Scholar]
- Yu LG, Packman LC, Weldon M, Hamlett J, Rhodes JM. Protein phosphatase 2A, a negative regulator of the ERK signaling pathway, is activated by tyrosine phosphorylation of putative HLA class II-associated protein I (PHAPI)/pp32 in response to the antiproliferative lectin, jacalin. J Biol Chem. 2004;279:41377–41383. doi: 10.1074/jbc.M400017200. [DOI] [PubMed] [Google Scholar]
- Bennecib M, Gong CX, Grundke-Iqbal I, Iqbal K. Inhibition of PP-2A upregulates CaMKII in rat forebrain and induces hyperphosphorylation of tau at Ser 262/356. FEBS Lett. 2001;490:15–22. doi: 10.1016/s0014-5793(01)02127-5. [DOI] [PubMed] [Google Scholar]
- Li L, Sengupta A, Haque N, Grundke-Iqbal I, Iqbal K. Memantine inhibits and reverses the Alzheimer type abnormal hyperphosphorylation of tau and associated neurodegeneration. FEBS Lett. 2004;566:261–269. doi: 10.1016/j.febslet.2004.04.047. [DOI] [PubMed] [Google Scholar]
- An WL, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K, Grundke-Iqbal I, Winblad B, Pei JJ. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer’s disease. Am J Pathol. 2003;163:591–607. doi: 10.1016/S0002-9440(10)63687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kins S, Kurosinski P, Nitsch RM, Gotz J. Activation of the ERK and JNK signaling pathways caused by neuron-specific inhibition of PP-2A in transgenic mice. Am J Pathol. 2003;163:833–843. doi: 10.1016/S0002-9440(10)63444-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei JJ, Gong CX, An WL, Winblad B, Cowburn RF, Grundke-Iqbal I, Iqbal K. Okadaic-acid-induced inhibition of protein phosphatase 2A produces activation of mitogen-activated protein kinases ERK1/2, MEK1/2, and p70 S6, similar to that in Alzheimer’s disease. Am J Pathol. 2003;163:845–858. doi: 10.1016/S0002-9440(10)63445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]